Abstract
Background and purpose
Venous thromboembolism (VTE) is a serious postoperative complication after total knee arthroplasty (TKA). Use of a tourniquet has shown conflicting results for risk of VTE after TKA. We aimed to investigate the associated risk of VTE after TKA using tourniquet in a fast-track set-up as no previous data exists.
Patients and methods
We performed an observational cohort study from 9 fast-track centers including unilateral primary TKA from 2010–2017 with prospective collection of preoperative risk-factors and complete 90-day follow-up. Use of a tourniquet was registered in the Danish Knee Arthroplasty Register. Postoperative VTE was identified from health records. We performed risk analyses using a mixed-effects logistic regression model adjusting for previously identified risk factors.
Results
Of the 16,250 procedures (39% males, mean age 67.9 [SD 10.0] years, median LOS 2 [interquartile range 2–3]) 12,518 (77%) were performed with a tourniquet. The annual tourniquet usage varied greatly between departments from 0% to 100%, but also within departments from 0% to 99%. There was no significant difference between the 2 groups with 52 (0.42%) VTEs in the tourniquet group vs. 25 (0.67%) in the no-tourniquet group (p = 0.06 for cumulative 90-day incidence of VTE). This association remained statistically insignificant for VTE using tourniquet after adjustment for previously identified risk factors.
Conclusion
We found no association between the use of a tourniquet and increased risk of 90-day VTE after primary fast-track TKA, irrespective of the length of time for which the tourniquet was applied.
Venous thromboembolism (VTE) is a serious postoperative complication after total knee arthroplasty (TKA). Traditional international guidelines recommend prolonged chemical thromboprophylaxis for up to 35 days postoperatively but Danish national guidelines recommend in-hospital only thromboprophylaxis if LOS ≤ 5 days and adhering to a fasttrack protocol [1]. Recent investigations have questioned the need for thromboprophylaxis as a one-size-fits-all and also emphasized the potential benefit of identifying high-risk patients [2,3].
A tourniquet has traditionally been used during TKA, to reduce blood loss, and improve cementation and surgical field visualization. The use of a tourniquet has shown conflicting results regarding risk of VTE after TKA. Theoretically, the subsequent venous stasis, restricted and turbulent blood flow, and endothelial damage following the use of an intraoperative tourniquet could increase the risk of VTE as per the Triad of Virchow. This hypothesis is further backed by a prospective cohort of 154 TKAs displaying elevated D-dimer, increased coagulopathy on thromboelastographic measurement, and increased asymptomatic DVTs [4]. The latest review concluded that use of a tourniquet was associated with an increased overall risk of postoperative VTE [5]. While improved cementation and implant fixation is one of the proposed benefits of tourniquet in primary TKA, this has been disputed in several studies using radiostereometric analysis that have found similar cemented fixation with and without a tourniquet [6,7]. This is supported by a large nationwide UK register study showing that the use of a tourniquet did not have any effect on prothesis survival [8].
The introduction of fast-track or enhanced recovery protocols has shown favorable outcomes with regards to risk of VTE with a 90-day risk of VTE of around 0.40% for fast-track TKA despite in-hospital only thromboprophylaxis if postoperative length of stay (LOS) ≤ 5 days [9].
Consequently, we hypothesized that use of a tourniquet in an unselected prospective multicenter fast-track TKA setting with in-hospital only thromboprophylaxis if LOS ≤ 5 days was not associated with increased risk of 90-day symptomatic VTE.
Patients and methods
Study design
The study was an observational study on a prospective cohort of unselected consecutive primary unilateral TKAs from the Centre for Fast-track Hip and Knee Replacement (www.fthk.dk) with data on the use of a tourniquet from the Danish Knee Register (DKR) [10]. Reporting of the study was done according to the STROBE guidelines.
Setting and data sources
We included unselected unilateral procedures from January 2010 to August 2017 from the Centre for Fast-track Hip and Knee Replacement (FTHK). The FTHK consist of 9 centers that adhere to similar protocols including multimodal opioidsparing analgesia, early mobilization (< 6 hours postoperatively), routine use of tranexamic acid (TXA), with at least one dose (1 g) of intravenous TXA intraoperatively, discharge to own home based on functional criteria, and in-hospital only thromboprophylaxis in case of LOS < 5 days without routine use of pneumatic compression devices or compression stockings. Thromboprophylaxis was administered 6 to 8 hours postoperatively and consisted of either rivaroxaban (Xarelto, Bayer Pharma, Berlin, Germany) 10 mg/day, enoxaparin (Klexane, Sanofi-Aventis, Paris, France) 4,000 IU/day, dalteparin (Fragmin, Pfizer Health Care, New York, United States) 5,000 IU/day, or fondaparinux (Arixtra, GlaxoSmithKline, London, UK) 2.5 mg/day. In the case of LOS > 5 days thromboprophylaxis was administered according to local guidelines for a maximum of 14 days [9].
The centers within FTHK prospectively collected patient-reported questionnaires on demographics and comorbidities ≤ 30 days from surgery [11] for all arthroplasties performed. Data on tourniquet utilization was obtained after crosslinking data with DKR where tourniquet use and duration are postoperatively reported by the surgeon. Validation of tourniquet use within the DKR is hindered by the lack of systematic data collection of tourniquet use elsewhere. LOS and 90-day readmissions were obtained from the Danish National Patient Registry (DNPR) with > 99% completeness for somatic admission [12]. Finally, data on causes leading to LOS > 4 days, 90-day readmission, or death was based on review of discharge summaries from medical records. All cases with suspected thromboembolism from either diagnostic codes or discharge summaries had their complete medical records, including radiological examinations reviewed. Review of health records was done by PBP and CCJ and cases of doubt were discussed with HK until consensus was achieved. PBP, CCJ, and HK were blinded regarding utilization of a tourniquet at the time of health record review.
Participants
We included all patients ≥ 18 years with a Danish Civil Registration number undergoing an elective unilateral TKA. We excluded primary procedures due to fracture surgery less than 90 days prior to the TKA. Furthermore, surgery due to severe congenital disorders, cancer, and simultaneous or staged bilateral procedures less than 90 days apart were excluded.
Variables
The primary objective was to investigate the independent association between use of a tourniquet and cumulative 90-day incidence of VTE (90-day VTE), adjusted for BMI, age, and history of VTE as these have previously been associated with elevated risk of 90-day VTE in a fast-track setting [9]. VTE was a priori defined as medical records reporting a sonography verified DVT or a PE diagnosed by computer tomography or ventilation perfusion scintigraphy. Thus, data on VTE relies on clinical data from review of complete medical records and not only diagnostic coding, which is why data is supposed to be valid and not further validated. LOS was calculated as postoperative nights in hospital, including transfers to other departments/hospitals. 90-day readmission required at least 1 night in hospital and being potentially related to the surgery, thus excluding planned unrelated hospitalization such as cataract surgery etc.
Statistics
We included all eligible procedures with combined data from the FTHK and DKR. Therefore, no pre-study power calculation was performed. Continuous data are reported as mean (SD) or median (IQR) as appropriate after evaluation of normality with Q–Q plots and histograms. Categorical data is reported as count (%) and using Fisher’s exact test for statistical evaluation. Comparisons were considered statistically significant with 2-tailed P < 0.05. Investigation of tourniquet
use and duration of tourniquet (per 10 minutes) as independent risk factors was done with 2 binomial mixed-effects logistic regression models including use of tourniquet or duration of tourniquet and BMI, age, and history of VTE as fixed factors and hospital as a random factor. Selection of potential confounders was based on previously found risk factors for VTE within a fast-track setting [9]. Model fit was evaluated based on visual analysis of Q–Q plot residuals and statistically evaluated by the DHARMa package for R (Florian Hartig 2022; R Foundation for Statistical Computing, Vienna, Austria). Confidence intervals for use of tourniquet in Figure 2 are based on the normal approximation method. Finally, we performed a post-hoc analysis of 2 comparable centers with regards to volume, but opposite usage of tourniquet 0% vs. 100% from 2015 and onwards. Statistical analysis was conducted in R v3.6.1 (R Core Team, 2019) using RStudio v1.2.1335 (RStudio Team, 2018). We assumed that missing data was missing at random and thus cases with missing data on use of torniquet was excluded from the study. Regarding the logistic regression models complete-case analysis was performed with only up to 2% missing data and complete data on primary outcome.
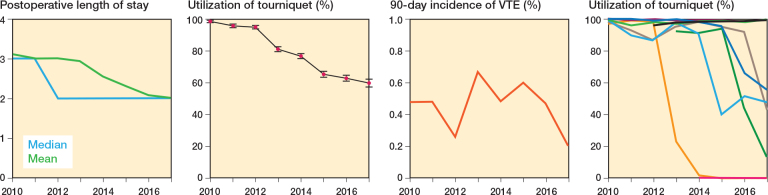
Figure 2.
Development in postoperative length of stay, utilization of tourniquet, 90-day incidence of VTE, and Utilization of tourniquet from the 9 departments within LCDB from 2010 to 2017.
Ethics, registration, data sharing plan, funding, and disclosures
Due to Danish legislation, the non-interventional nature of the study did not require ethical approval. Collection of health records without informed consent was approved by the Danish National Board of Health (3-3013-56/2/EMJO) and storage of data was approved by the Danish Data Protection Agency (P-2019-709). The LCDB is registered on ClinicalTrials.gov (NCT01515670).
Limited data (deidentified preoperative questionnaire on comorbidities and specific complications), due to data retrieval of most variables from third parties, is available for sharing upon reasonable request after approval from the Capital Region of Denmark. Data collection was supported by an unconditional PhD grant to PBP by the Lundbeck Foundation (R230-2017-166).
None of the authors reports intellectual or financial conflict of interest regarding the current study. PBP and AT have received payments from entities unrelated to the current study.
Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.13793
Results
We included 16,250 TKAs in 14,539 patients (Figure 1). 9,912 (61%) of the procedures were performed in women. Mean age was 67.9 years (SD 10.0) and mean BMI 29.6 (SD 5.5) (Table 1). Median LOS was 2 days (IQR 2–3), mean LOS 2.6 days (SD 2.6), and 763 (4.7%) had LOS > 5 days. The cumulative 90-day incidence of VTE was 77 (0.47%) with 32 (0.20%) DVT, 43 (0.26%) PE, and 2 (0.01%) having both and DVT and PE.
Figure 1.
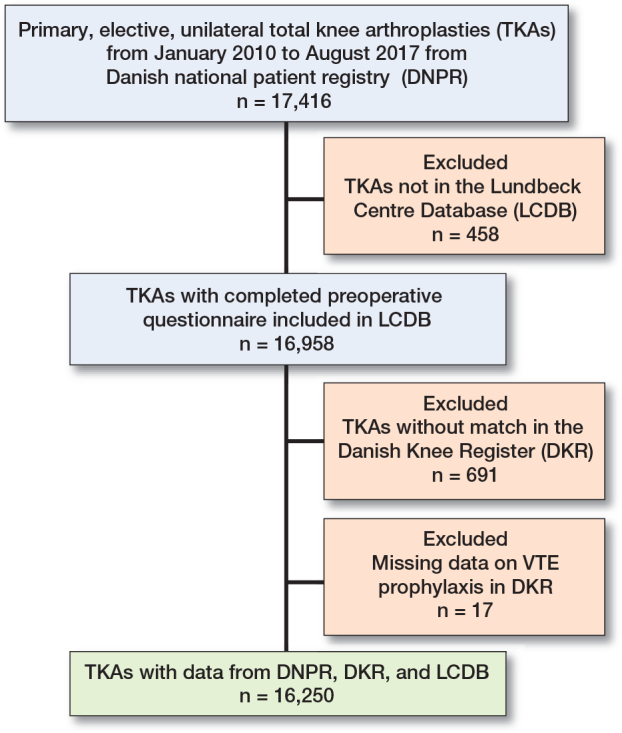
Flowchart of study population. TKA = total knee arthroplasty. VTE = venous thromboembolism.
Table 1.
Patient characteristics of 16,250 total knee arthroplasties. Values are count (%) unless otherwise specified
| Variable | No tourniquet n = 3,732 | Tourniquet n = 12,518 | P value | Total n = 16,250 |
|---|---|---|---|---|
| LOS, median (IQR) | 2 (2–3) | 2 (2–3) | < 0.001 | 2 (2–3) |
| VTE | 25 (0.7) | 52 (0.4) | 0.1 | 77 (0.5) |
| BMI mean (SD) | 30.1 (5.7) | 29.4 (5.4) | < 0.001 | 29.6 (5.5) |
| Missing | 17 | 57 | 74 | |
| Age, mean (SD) | 67.7 (10) | 67.9 (9.9) | 0.2 | 67.9 (10) |
| History of VTE | 306 (8.4) | 858 (7.0) | 0.02 | 1,165 (7.3) |
| Missing | 75 | 199 | 274 | |
| Female | 2,298 (62) | 7,604 (61) | 0.6 | 9,912 (61) |
| Cardiovascular disease | 561 (15.3) | 1767 (14.2) | 0.2 | 2,332 (15) |
| Missing | 55 | 118 | 173 | |
| Pulmonary disease | 382 (10) | 1,049 (8.4) | 0.002 | 1,433 (8.9) |
| Missing | 28 | 67 | 95 | |
| Hereditary VTE | 460 (14) | 1484 (14) | 0.9 | 1,946 (14) |
| Missing | 378 | 1554 | 1,932 | |
| Potent anticoagulative | ||||
| treatment | 291 (7.8) | 848 (6.8) | 0.01 | 1,140 (7.0) |
| Smoking | 535 (15) | 1,513 (12) | < 0.001 | 2,049 (13) |
| Missing | 39 | 80 | 119 | |
| Alcohol | 283 (7.7) | 859 (6.9) | 0.2 | 1,144 (7.1) |
| Missing | 47 | 92 | 13 | |
| Living with others | 2,308 (62) | 8,461 (68) | 10,781 (67) | |
| Living alone | 1,375 (37) | 3,955 (32) | 5,335 (33) | |
| Nursing home | 17 (0.5) | 57 (0.5) | < 0.001 | 74 (0.5) |
| Missing | 32 | 45 | 77 | |
| Walking aid | 902 (25) | 2,405 (20) | < 0.001 | 3,312 (21) |
| Missing | 90 | 238 | 328 | |
| Anemia | 940 (25) | 2,943 (24) | 0.09 | 3,889 (24) |
| Missing | 19 | 151 | 170 | |
| Hypertension | 2,227 (60) | 6,262 (50) | < 0.001 | 8,501 (53) |
| Missing | 8 | 53 | 61 | |
| Hypercholesterolemia | 1,271 (35) | 4,894 (39) | < 0.001 | 6,172 (38) |
| Missing | 52 | 52 | 104 | |
| Psychiatric disorder | 380 (11) | 997 (12) | 0.3 | 1,378 (11) |
| Missing | 164 | 3,913 | 4,081 | |
| Stroke | 251 (6.9) | 749 (6.1) | 0.2 | 1,001 (6.2) |
| Missing | 78 | 153 | 231 | |
| IDDM | 93 (2.5) | 346 (2.8) | 439 (2.7) | |
| NIDDM | 403 (11) | 1,030 (8.3) | 1,434 (8.9) |
LOS = postoperative length of stay, VTE = venous thromboembolism, IQR = interquartile range, SD = standard deviation, IDDM = insulin-dependent diabetes mellitus, NIDDM = non-insulin-dependent diabetes mellitus.
12,518 (77%) procedures were performed using a tourniquet. Annual tourniquet usage decreased from 99% in 2010 to about 60% in 2017 (Figure 2) but varied greatly between departments from 0% to 100%, but also within departments from 0% to 99% (Figure 3). Data on duration of tourniquet inflation was available in 8,020 procedures (64%) with a median duration of 60 minutes (IQR 51–70). In 749 (9.3%) procedures duration of inflation was < 30 minutes compared with 3,416 (42%) procedures with 30–60 minutes, 3,619 (45%) procedures with 61–90 minutes, and 271 (3.4%) procedures with > 90 minutes of inflated tourniquet.
There was no significant difference between the 2 groups with 52 (0.42%) VTEs after median 7 (IQR 4–22) days in the tourniquet group vs. 25 (0.67%) after 13 (IQR 6–19) days in the no-tourniquet group (P = 0.06 for cumulative 90-day incidence of VTE). The association remained statistically insignificant after adjustment for previously shown risk factors (BMI, age, and history of VTE) with OR 0.62 (CI 0.38–1.01, P = 0.054) for VTE within 90 days with utilization of tourniquet (Table 2). Additionally, no significant difference was found in the analysis on association between duration of tourniquet use and 90-day incidence of VTE with 0 (0%) for duration of tourniquet < 30 minutes, 14 (0.4%) for 30–60 minutes, 19 (0.5%) for 61–90 minutes, and 2 (0.7%) for duration of tourniquet > 90 minutes, P = 0.2 (Table 3).
Table 2.
Associations between use of tourniquet and cumulative 90-day incidence of VTE in 16,250 procedures from 2010 to 2017
| OR (CI) | P value | |
|---|---|---|
| Fisher’s exact test | 0.62 (0.38–1.0) | 0.056 |
| Mixed Model 1: Use of tourniquet a | 0.62 (0.38–1.0) | 0.054 |
| Mixed Model 2: Duration of inflation, per 10 minutes a | 1.1 (0.97–1.1) | 0.2 |
Mixed model adjusting for BMI, age, and history of VTE as fixed factors and hospital as a random factor, reference level = no tourniquet.
Table 3.
Associations between duration of tourniquet use and cumulative 90-day incidence of VTE in 16,250 procedures from 2010 to 2017. Values are count (%)
| Minutes of tourniquet use | No VTE n = 16,173 | VTE n = 77 | Total n = 16,250 | P value |
|---|---|---|---|---|
| < 30 | 749 | 0 (0.00) | 749 | |
| 30–60 | 3,402 | 14 (0.41) | 3,416 | |
| 60–90 | 3,600 | 19 (0.53) | 3,619 | |
| > 90 | 269 | 2 (0.74) | 271 | 0.2 a |
| Missing | 8,153 | 42 | 8,195 |
Unadjusted logistic regression.
The post-hoc analysis from hospitals 1 and 7 from 2015–2017 comparing 0 and 100% utilization of tourniquet found no significant differences with 90-day incidences of VTE of 6 (0.49%) and 5 (0.31%), P = 0.7, for hospitals 1 and 7, respectively.
Discussion
The aim of the study was to identify a potential association between the use of a tourniquet and postoperative VTE after fast-track TKA. We were unable to find a significant association between the use of a tourniquet and an increased risk of VTE within 90 days (irrespective of the length of time the tourniquet was inflated). However, there was an insignificant trend for fewer VTEs with regards to use of tourniquet and the duration thereof, suggesting a potential power problem in our study to reach a more valid conclusion.
A recent meta-analysis including 41 RCTs and 2,819 patients concluded that TKA with a tourniquet was associated with an increased risk of serious adverse events, pain, and marginally longer hospital stays [13]. More specifically, the meta-analysis found that use of a tourniquet was associated with a statistically non-significant increase in risk of both DVT (RR 1.8) and PE (RR 4.5). This mimics the conclusion of a previous meta-analysis, which found a 5-fold increased risk for a thrombotic event when a tourniquet was used during primary TKA surgery [14].
The previous reports stand in contrast to the findings in our study, which found non-significant lower risk of VTE following TKAs performed with a tourniquet. This may be explained by the fast-track concepts reducing the surgical stress response and re-establishing normal routines through early mobilization. Early mobilization is a crucial part of a fast-track set-up, which has been shown to have a favorable profile regarding VTEs compared with more conventional pathways, even with short-term VTE prophylaxis [9]. Thus, early mobilization has previously been shown to significantly reduce the risk of VTE [15]. The meta-analysis by Ahmed et al. included studies dating as far back as 1995, where much later mobilization and increased LOS was common [13]. Thus, inclusion of older studies with utilization of outdated standards of perioperative care may reduce the generalizability for modern fast-track protocols. Unfortunately, very few recent studies describe the perioperative treatment and mobilization regimes in detail, making direct comparison difficult. The overall risk for VTE of 1.5–3.4% reported in previous meta-analyses [4,14] supports this theory, as such VTE rates are much higher compared with the VTE rate reported in our study.
We were unable to find a difference in VTE rates, irrespective of the length of time for which the tourniquet was applied. However, due to the lower number of events, there is a risk for type 2 error.
Low risk of events is a challenge when investigating potential risk factors for VTE following TKA surgery, with an RCT design being virtually impossible, as it would require tens of thousands of patients to provide sufficient power to provide any meaningful conclusion on VTE rates. Therefore, the use of a meta-analysis is justified when investigating rare events but may introduce risk of bias if the included studies do not represent current clinical practice. Consequently, large observational cohort studies are of value when investigating rare events following TKA.
A recent RCT performed in a fast-track set-up did not find any differences regarding postoperative pain, morphine consumption, or LOS between TKA performed with and without a tourniquet [16], which is in line with our results.
We can only speculate on why we found a near significant increased risk of VTE following TKA performed without a tourniquet—which contradicts our study hypothesis. Residual confounding such as different perioperative treatment in centers with low utilization of a tourniquet as well as the undocumented use of a tourniquet in selected patients may explain this difference.
We found that while the use of a tourniquet varied greatly between departments, there was an overall trend toward reduced use of a tourniquet during the study period from close to 100% in 2010 to 60% by 2017. The above-mentioned meta-analysis [14] reporting a higher risk of adverse events, as well as recent RSA data suggesting equal fixation [6], may be the most likely explanation for the decreased utilization within our departments.
It is important to stress that our study investigated only the rate of symptomatic VTE and does not provide any information regarding other complications or functional outcomes. Some reviews have suggested, that use of tourniquet leads to increased pain and reduced functional outcome [5,13], while others have disputed this claim [17-19].
The study design is the main limitation of our study, as a non-randomized design allows for residual confounders and bias, which might influence the results. Therefore, pragmatic observations or registry-based studies may provide important insight, especially when including complete follow-up and detailed demographic data in order to adjust for potential confounding [20]. Furthermore, we did not include pressure during tourniquet use in our analysis. We chose not to include it as this data is not validated and can depend greatly on patient characteristics, and may be changed during surgery, making conclusions rather unreliable in our study design.
The strength of this study is 100% follow-up for all patients, with all complications verified by chart review, thus not relying on administrative diagnosis codes. Also, prospective recording of comorbidities limits potential confounding. Finally, while perioperative treatment could have changed during the study period, alongside the use of tourniquet, thus affecting the results, all the participated hospitals followed the same fast-track protocol with early mobilization and TE prophylaxis during hospital stay only for the entire study period.
Conclusion
We found no association between the use of a tourniquet and an increase in risk of VTE within 90 days after primary TKA performed in a fast-track set-up, irrespective of the length of time for which the tourniquet was applied.
KG, AT, AK, and HK planned the study. All authors collected data. PBG, MM, an CCJ analyzed the data. PBP and KG wrote the manuscript, which was revised by all the authors.
Handling co-editors: Li Felländer-Tsai and Philippe Wagner
Acta thanks Michael Clarius and Rikard Wedin help with peer review of this manuscript.
References
- 1.Falck-Ytter Y, Francis C W, Johanson N A, Curley C, Dahl O E, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 Suppl.): e278S-325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemeth B, Nelissen R, Arya R, Cannegieter S. Preventing VTE following total hip and knee arthroplasty: is prediction the future? J Thromb Haemost 2021; 19(1): 41-5. doi: 10.1111/jth.15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jørgensen C C, Petersen P B, Reed M, Kehlet H. Recommendations on thromboprophylaxis in major joint arthroplasty: many guidelines, little consensus? J Thromb Haemost 2019; 17(2): 250-3. doi: 10.1111/jth.14362 [DOI] [PubMed] [Google Scholar]
- 4.Huang C R, Pan S, Li Z, Ruan R X, Jin WY, Zhang X C, et al. Tourniquet use in primary total knee arthroplasty is associated with a hypercoagulable status: a prospective thromboelastography trial. Int Orthop 2021; 45(12): 3091-100. doi: 10.1007/s00264-021-05126-x. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed I, Chawla A, Underwood M, Price A J, Metcalfe A, Hutchinson C, et al. Tourniquet use for knee replacement surgery. Cochrane Database Syst Rev 2020; 12: CD012874. doi: 10.1002/14651858.CD012874.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejaz A, Laursen A C, Jakobsen T, Rasmussen S, Nielsen P T, Laursen M B. Absence of a tourniquet does not affect fixation of cemented TKA: a randomized RSA study of 70 patients. J Arthroplasty 2015; 30(12): 2128-32. doi: 10.1016/j.arth.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 7.Molt M, Harsten A, Toksvig-Larsen S. The effect of tourniquet use on fixation quality in cemented total knee arthroplasty: a prospective randomized clinical controlled RSA trial. Knee 2014; 21(2): 396-401. doi: 10.1016/j.knee.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Farhan-Alanie M M, Lee Y, Underwood M, Metcalfe A, Wilkinson M J, Price A J, et al. Effect of tourniquet use on the risk of revision in total knee replacement surgery: an analysis of the National Joint Registry Data Set. BMJ Open 2021; 11(6): e045353. doi: 10.1136/bmjopen-2020-045353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen P B, Kehlet H, Jørgensen C C. Safety of in-hospital only thromboprophylaxis after fast-track total hip and knee arthroplasty: a prospective follow-up study in 17,582 procedures. Thromb Haemost 2018; 118(12): 2152-61. doi: 10.1055/s-0038-1675641. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen A B, Mehnert F, Odgaard A, Schrøder H M. Existing data sources for clinical epidemiology: the Danish knee Arthroplasty register. Clin Epidemiol 2012; 4(1): 125-35. doi: 10.2147/CLEP.S30050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jørgensen C C, Kehlet H. Role of patient characteristics for fast-track hip and knee arthroplasty. Br J Anaesth 2013; 110(6): 972-80. doi: 10.1093/bja/aes505. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M, Schmidt S A J, Sandegaard J L, Ehrenstein V, Pedersen L, Sørensen H T. The Danish National patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7: 449-90. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed I, Chawla A, Underwood M, Price A, Metcalfe A, Hutchinson C, et al. Time to reconsider the routine use of tourniquets in total knee arthroplasty surgery. Bone Joint J 2021; 103-B(5): 830-9. doi: 10.1302/0301-620X.103B.BJJ-2020-1926.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Li N, Chen S, Tan Y, Al-Aidaros M, Chen L. The effects of a tourniquet used in total knee arthroplasty: a meta-analysis. J Orthop Surg Res 2014; 9(1): 13. doi: 10.1186/1749-799X-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearse E O, Caldwell B F, Lockwood R J, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of postoperative venous thromboembolism. J Bone Joint Surg Br 2007; 89(3): 316-22. doi: 10.1302/0301-620X.89B3.18196. [DOI] [PubMed] [Google Scholar]
- 16.Palanne R, Rantasalo M, Vakkuri A, Madanat R, Olkkola K T, Lahtinen K, et al. Effects of anaesthesia method and tourniquet use on recovery following total knee arthroplasty: a randomised controlled study. Br J Anaesth 2020; 125(5): 762-72. doi: 10.1016/j.bja.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Smith A F, Usmani R H, Wilson KD, Smith L S, Malkani A L. Effect of tourniquet use on patient outcomes after cementless total knee arthroplasty: a randomized controlled trial. J Arthroplasty 2021; 36(7): 2331-4. doi: 10.1016/j.arth.2021.01.053. [DOI] [PubMed] [Google Scholar]
- 18.Harsten A, Bandholm T, Kehlet H, Toksvig-Larsen S. Tourniquet versus no tourniquet on knee-extension strength early after fast-track total knee arthroplasty: a randomized controlled trial. Knee 2015; 22(2): 126-30. doi: 10.1016/j.knee.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy Deering E, Hu S Y, Abdulkarim A. Does tourniquet use in TKA increase postoperative pain? A systematic review and meta-analysis. Clin Orthop Relat Res 2019; 477(3): 547-58. doi: 10.1097/CORR.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frieden T R. Evidence for health decision making: beyond randomized, controlled trials. N Engl J Med 2017; 377(5): 465-75. doi: 10.1056/NEJMra1614394. [DOI] [PubMed] [Google Scholar]