Abstract
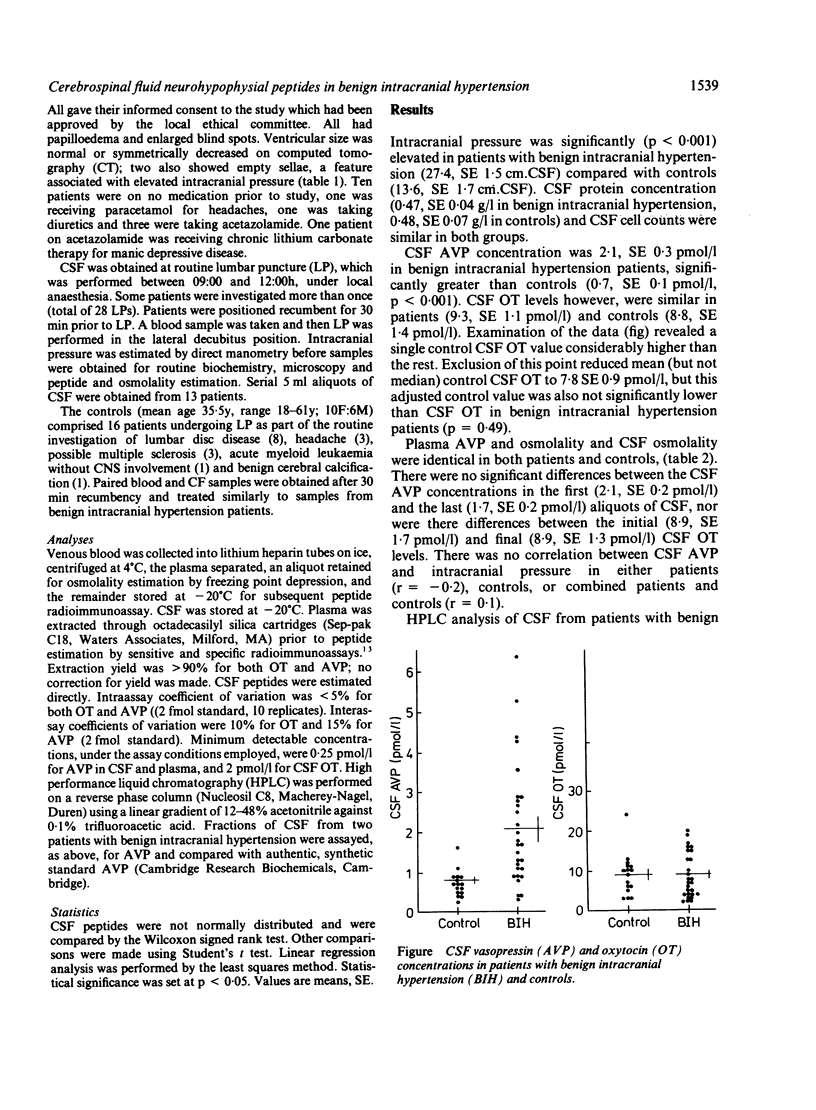
The cerebrospinal fluid (CSF) concentrations of arginine vasopressin (AVP) and oxytocin (OT) were investigated both in patients with benign intracranial hypertension and in age and sex matched controls. Twenty eight lumbar punctures were performed on 15 patients with benign intracranial hypertension as part of their routine investigation and therapy. All patients had raised intracranial pressure (27.4, SE 1.7 cm.CSF). CSF AVP levels were significantly elevated in benign intracranial hypertension (2.1, SE 0.3 pmol/l) compared with controls (0.7, SE 0.1 pmol/l, p less than 0.001) but CSF OT concentrations were similar in both groups. CSF osmolality and plasma AVP and osmolality were identical in patients and controls. There was no correlation between CSF AVP concentration and intracranial pressure. The selective elevation of AVP in CSF may be of importance in the pathogenesis of raised intracranial pressure in benign intracranial hypertension.
Full text
PDF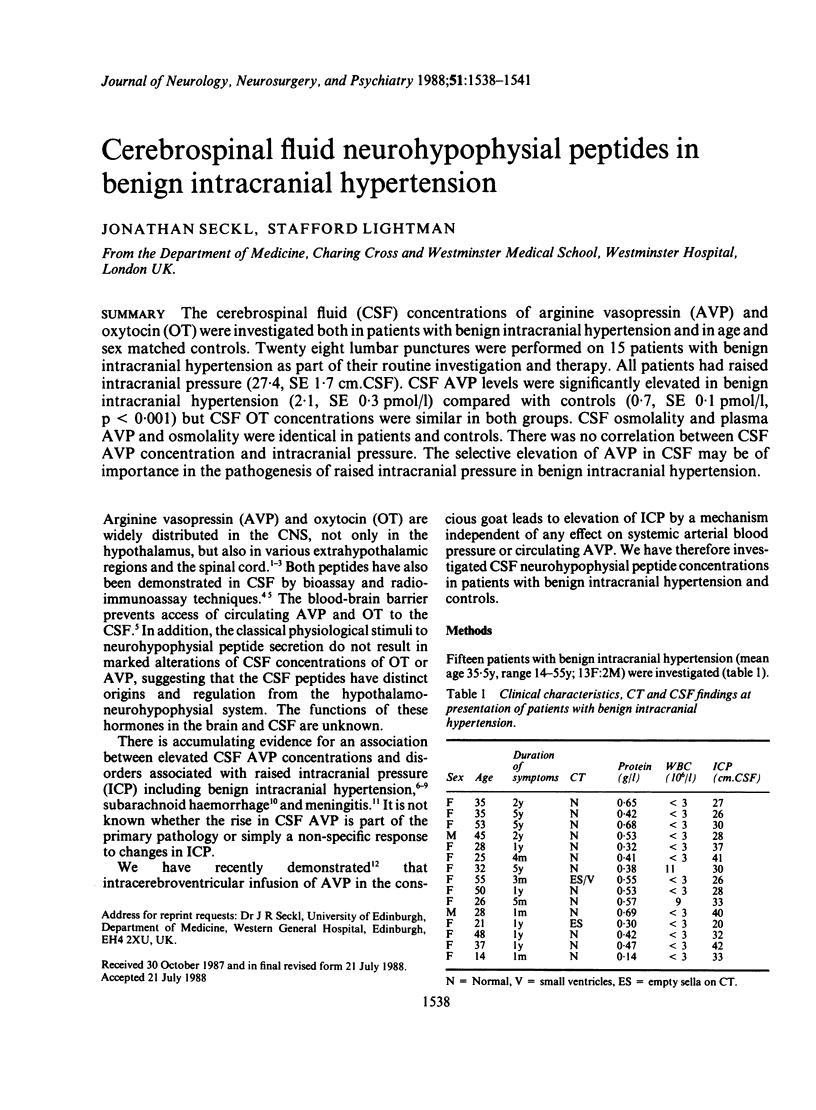


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artru A. A. Effects of halothane and fentanyl anesthesia on resistance to reabsorption of CSF. J Neurosurg. 1984 Feb;60(2):252–256. doi: 10.3171/jns.1984.60.2.0252. [DOI] [PubMed] [Google Scholar]
- Buijs R. M., Swaab D. F., Dogterom J., van Leeuwen F. W. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Cell Tissue Res. 1978 Jan 31;186(3):423–433. doi: 10.1007/BF00224932. [DOI] [PubMed] [Google Scholar]
- Corrêa F. M., Magro I. A., Peres-Polon V. L., Antunes-Rodrigues J. Mechanism of the CNS-mediated pressor response to intracerebroventricular injection of noradrenaline in unanaesthetized rats. Neuropharmacology. 1985 Sep;24(9):831–837. doi: 10.1016/0028-3908(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Davson H., Segal M. B. The effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. J Physiol. 1970 Jul;209(1):131–153. doi: 10.1113/jphysiol.1970.sp009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. O. Pathogenesis of pseudotumor cerebri syndromes. Neurology. 1981 Jul;31(7):877–880. doi: 10.1212/wnl.31.7.877. [DOI] [PubMed] [Google Scholar]
- Dóczi T., László F. A., Szerdahelyi P., Joó F. Involvement of vasopressin in brain edema formation: further evidence obtained from the Brattleboro diabetes insipidus rat with experimental subarachnoid hemorrhage. Neurosurgery. 1984 Apr;14(4):436–441. doi: 10.1227/00006123-198404000-00008. [DOI] [PubMed] [Google Scholar]
- Dóczi T., Szerdahelyi P., Gulya K., Kiss J. Brain water accumulation after the central administration of vasopressin. Neurosurgery. 1982 Sep;11(3):402–407. doi: 10.1227/00006123-198209000-00011. [DOI] [PubMed] [Google Scholar]
- Garcia H., Kaplan S. L., Feigin R. D. Cerebrospinal fluid concentration of arginine vasopressin in children with bacterial meningitis. J Pediatr. 1981 Jan;98(1):67–70. doi: 10.1016/s0022-3476(81)80537-9. [DOI] [PubMed] [Google Scholar]
- Hakim S., Venegas J. G., Burton J. D. The physics of the cranial cavity, hydrocephalus and normal pressure hydrocephalus: mechanical interpretation and mathematical model. Surg Neurol. 1976 Mar;5(3):187–210. [PubMed] [Google Scholar]
- Janny P., Chazal J., Colnet G., Irthum B., Georget A. M. Benign intracranial hypertension and disorders of CSF absorption. Surg Neurol. 1981 Mar;15(3):168–174. doi: 10.1016/0090-3019(81)90131-2. [DOI] [PubMed] [Google Scholar]
- Jenkins J. S., Mather H. M., Ang V. Vasopressin in human cerebrospinal fluid. J Clin Endocrinol Metab. 1980 Feb;50(2):364–367. doi: 10.1210/jcem-50-2-364. [DOI] [PubMed] [Google Scholar]
- Johnston I. Reduced C.S.F. absorption syndrome. Reappraisal of benign intracranial hypertension and related conditions. Lancet. 1973 Aug 25;2(7826):418–421. doi: 10.1016/s0140-6736(73)92277-0. [DOI] [PubMed] [Google Scholar]
- Martins A. N. Resistance to drainage of cerebrospinal fluid: clinical measurement and significance. J Neurol Neurosurg Psychiatry. 1973 Apr;36(2):313–318. doi: 10.1136/jnnp.36.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather H. M., Ang V., Jenkins J. S. Vasopressin in plasma and CSF of patients with subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1981 Mar;44(3):216–219. doi: 10.1136/jnnp.44.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman Q. J., Lawrence D., McLean L. Central effects of arginine vasopressin on blood pressure in rats. Endocrinology. 1982 Mar;110(3):1058–1060. doi: 10.1210/endo-110-3-1058. [DOI] [PubMed] [Google Scholar]
- Raichle M. E., Grubb R. L., Jr Regulation of brain water permeability by centrally-released vasopressin. Brain Res. 1978 Mar 17;143(1):191–194. doi: 10.1016/0006-8993(78)90766-7. [DOI] [PubMed] [Google Scholar]
- Reid A. C., Morton J. J. Arginine vasopressin levels in cerebrospinal fluid in neurological disease. J Neurol Sci. 1982 May;54(2):295–301. doi: 10.1016/0022-510x(82)90190-3. [DOI] [PubMed] [Google Scholar]
- Schultz W. J., Brownfield M. S., Kozlowski G. P. The hypothalamo-choridal tract. II. Ultrastructural response of the choroid plexus to vasopressin. Cell Tissue Res. 1977 Mar 1;178(1):129–141. doi: 10.1007/BF00232830. [DOI] [PubMed] [Google Scholar]
- Seckl J. R., Lightman S. L. Intracerebroventricular arginine vasopressin causes intracranial pressure to rise in conscious goats. Brain Res. 1987 Oct 13;423(1-2):279–285. doi: 10.1016/0006-8993(87)90850-x. [DOI] [PubMed] [Google Scholar]
- Seckl J. R., Williams T. D., Lightman S. L. Oral hypertonic saline causes transient fall of vasopressin in humans. Am J Physiol. 1986 Aug;251(2 Pt 2):R214–R217. doi: 10.1152/ajpregu.1986.251.2.R214. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V. Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. J Histochem Cytochem. 1980 May;28(5):475–478. doi: 10.1177/28.5.7381192. [DOI] [PubMed] [Google Scholar]
- Sørensen P. S., Gjerris A., Hammer M. Cerebrospinal fluid vasopressin in neurological and psychiatric disorders. J Neurol Neurosurg Psychiatry. 1985 Jan;48(1):50–57. doi: 10.1136/jnnp.48.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen P. S., Gjerris F., Hammer M. Cerebrospinal fluid vasopressin and increased intracranial pressure. Ann Neurol. 1984 May;15(5):435–440. doi: 10.1002/ana.410150506. [DOI] [PubMed] [Google Scholar]
- Vallejo M., Carter D. A., Lightman S. L. Haemodynamic effects of arginine-vasopressin microinjections into the nucleus tractus solitarius: a comparative study of vasopressin, a selective vasopressin receptor agonist and antagonist, and oxytocin. Neurosci Lett. 1984 Dec 21;52(3):247–252. doi: 10.1016/0304-3940(84)90169-1. [DOI] [PubMed] [Google Scholar]
- Wang B. C., Share L., Crofton J. T. Central infusion of vasopressin decreased plasma vasopressin concentration in dogs. Am J Physiol. 1982 Nov;243(5):E365–E369. doi: 10.1152/ajpendo.1982.243.5.E365. [DOI] [PubMed] [Google Scholar]


