Abstract
Nausea and vomiting (CINV) are distressful and widespread side effects of chemotherapy, and additional efficient regimens to alleviate CINV are urgently needed. In the present study, colorectal cancer (CRC) mice model induced by Azoxymethane (AOM)/Dextran Sodium Sulfate (DSS) was employed to evaluate the cancer suppression and CINV amelioration effect of the combination of thalidomide (THD) and Clostridium butyricum. Our results suggested that the combination of THD and C. butyricum abundantly enhanced the anticancer effect of cisplatin via activating the caspase-3 apoptosis pathway, and also ameliorated CINV via inhibiting the neurotransmitter (e.g., 5-HT and tachykinin 1) and its receptor (e.g., 5-HT3R and NK-1R) in brain and colon. Additionally, the combination of THD and C. butyricum reversed the gut dysbacteriosis in CRC mice by increasing the abundance of Clostridium, Lactobacillus, Bifidobacterium, and Ruminococcus at the genus level, and also led to increased expression of occludin and Trek1 in the colon, while decreased expression of TLR4, MyD88, NF-κB, and HDAC1, as well as the mRNA level of IL-6, IL-1β, and TNF-α. In all, these results suggest that the combination of THD and C. butyricum had good efficacy in enhancing cancer treatments and ameliorating CINV, which thus provides a more effective strategy for the treatment of CRC.
Keywords: CINV, thalidomide, intestinal microecology, Clostridium butyricum, gut- brain axis
1. Introduction
Colorectal cancer (CRC) is a cancer occurring in the colon or rectum, with over 1.9 million new cases and 935,000 deaths estimated in 2020, ranking the third in incidence and the second in mortality (1, 2). For patients’ treatment, systemic chemotherapy is the mainstay treatment besides surgery and local radiotherapy and has been wildly used in advanced CRC (3). Currently, platinum-based chemotherapy drugs which inhibits nuclear DNA transcription and replication and initiates programmed cell death are dominating (4). It is proposed that cyclooxygenase-2 (COX-2) inhibition can hasten Caspase-3 activation and poly (ADP-ribose) polymerase (PARP) cleavage, as well as suppress the expressions of anti-apoptotic proteins such as surviving, thus activating tumor apoptosis (5).
Despite the anticancer effects, chemotherapy drugs lead to severe side effect include gastrointestinal toxicities, myelosuppression, immunosuppression and neurotoxicity due to poor targeting (6, 7). Chemotherapy-induced nausea and vomiting (CINV) is a common adverse effects of chemotherapy that affects patient’s life and treatment effectiveness (8, 9). It has been reported that the expression of 5-hydroxytryptamine (5-HT) in intestinal mucosa was triggered by cytotoxic chemotherapy drugs, then stimulating the 5-HT receptors on adjacent vagal afferent nerves (VAN) (10). After nerves depolarization, vomiting center in brainstem was stimulated to induce a vomiting reflex (11). Consequently, there is a pressing need for alternative treatments that are both highly effective and have minimal side effects.
The most commonly used antiemetic agents belong to neurokinin-1 receptor antagonists (NK1-RAs), 5-hydroxytryptamine-3 receptor antagonists (5HT3-RAs) and dexamethasone but the therapeutic effect was still not satisfactory (12, 13). Thalidomide (THD), a derivative of glutamate, was originally used to treat nausea in pregnancy, later was banned because of its teratogenicity (14, 15). However, THD, as approved drugs, has been used in the treatment of a variety of solid tumors, with good efficacy and a certain degree of safety (16). At present, the effectiveness of THD in controlling CINV has become the focus of research. The randomized control trials have clinically confirmed the effectiveness of THD by calculating and analyzing the delayed and overall complete response rates to vomiting in cancer patients (17). Subsequently, several studies have demonstrated that THD’s potent immunomodulatory activity affects the expression and activity of various cytokines to influence anti-angiogenic effects and tumor defense (18, 19). However, numerous studies have shown that patients with CRC have an altered intestinal microbiota which lost a significant amount of butyrate-producing bacteria, such as Clostridium, Roseburia, and Eubacterium spp. (20). Chemotherapeutic drugs disrupt intestinal microecology, damage intestinal mucosal barrier, and trigger gut inflammation (21). Furthermore, the use of THD will lead to gastrointestinal side effects including constipation due to the certain neurotoxicity of THD (22).
At present, the gut microbiota has been widely evidenced to be related with the occurrence, development and treatment of cancers (23). Probiotics are beneficial microorganisms exerting probiotic role by regulating the gut microbiota (24, 25). Clostridium butyricum generates short-chain fatty acids (SCFAs), especially butyrate and acetate, and affects various physiological processes contributing to host health (26, 27). C. butyricum CBM 588 has been implicated in anti-inflammation, gut epithelial barrier protection and the increased abundance of Lactobacillus, Bifidobacterium in the gut microbiota (28). A study conducted by Danfeng Chen et al. revealed that C. butyricum can inhibit the development of CRC by the stimulation of apoptosis and the reconstruction of gut microbiota (29). Moreover, C. butyricum administration was found to significantly reduce cognitive dysfunction and histopathological changes, as well as neuronal apoptosis probably via the involvement in gut-brain axis modulation (30). Therefore, this suggests a possible therapeutic role for C. butyricum in the gastrointestinal toxic side effects of chemotherapy.
In clinic, we found that the combination of C. butyricum and THD almost eliminate CINV (data unshown). Therefore, to elucidate the efficacy and mechanisms of C. butyricum+THD on treatment of CINV, we investigated the antitumor function, the preventing of nausea and vomiting, the altering of microbiota composition and the enhancement of the intestinal epithelial barrier in CRC mouse model with chemotherapy. This study presents new ideas for the prevention and treatment of CINV with C. butyricum+THD.
2. Materials and methods
2.1. Animals and experimental design
Seventy-two male C57BL/6 mice were obtained from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, Hunan, China) and housed in an animal facility with a standard 12 h light–dark cycle. Figure 1A depicts the animal treatment schedule. After 1 week acclimation, the mice were randomly assigned to six different groups including: (i) C group, a control group with saline intraperitoneal injection; (ii) M group, a model group with injected Azoxymethane (AOM) (MP Biomedicals LLC, Santa Ana, America, 183971) (10 mg/kg) twice at the 1st and 19th day and fed 1% Dextran Sodium Sulfate (DSS) (Meilunbio, Dalian, China, MB5535) three times for 6 days each time at the 7th,19th and 29st day; (iii) MC group, treated with AOM/DSS, then received 2.5 mg/kg cisplatin (Macklin, Shanghai, China, D807330) after 1 month via intraperitoneal injection for 3 consecutive days; (iv) MCS group, after AOM/DSS and cisplatin treatment, treated with 25 mg/kg THD (Ark Pharm, Chicago, America, AK-91024) via intraperitoneal injection for 14 consecutive days; (v) MCL group, after AOM/DSS and cisplatin treatment, treated by gavage with 107 CFU C. butyricum for 21 consecutive days; (vi) MCSL group, after AOM/DSS and cisplatin treatment, treated both 107 CFU C. butyricum for 21 consecutive days and 25 mg/kg THD for 14 consecutive days.
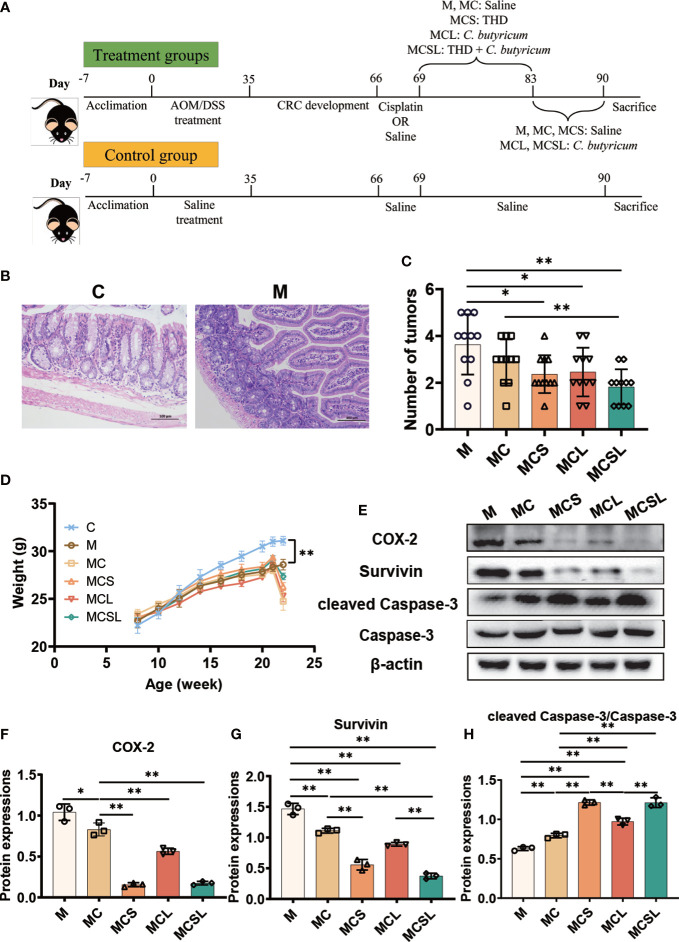
Figure 1.
The combination of THD and C. butyricum promoted tumor apoptosis in CRC mice. (A) The schematic diagram of CRC modeling and treatment process in C57BL/6 mice. (B) H&E staining of colon tissue. (C) The number of tumors in colon tissue (n=11). (D) Weight of mice in different treatment groups (n=11). (E) Western blotting of COX-2, Survivin, cleaved Caspase-3 and Caspase-3 (n=3). (F–H) Relative expressions of COX-2, Survivin and cleaved Caspase-3/Caspase-3 (n=3). Significance determined using one-way ANOVA with Tukey’s multiple comparison test and expressed as mean ± SD, *P < 0.05, **P < 0.01.
2.2. Behavioral experiments
Kaolin intake measurement was performed for 3 consecutive days during chemotherapy. Kaolin powder was prepared by mixing kaolin (Macklin, Shanghai, China, K812212) and Arabic gum (Meilunbio, Dalian, China, MB1728) at a 99:1 ratio and provided to mice. After drug intervention, the amount and residual of chow intake and kaolin intake were measured after drying every 24 h, and the ratio of kaolin intake to total food intake was calculated.
2.3. Sample collection
After stopping administration, fecal samples were collected and the mice were euthanized with isoflurane gas anesthesia. Colon, tumor, and brain tissues were immediately collected, fixed, or stored at -80 °C.
2.4. Histological staining analysis
The fixed colon and brain tissue samples were embedded in paraffin, cut into 2-μm-thick sections, and possessed with H&E staining to evaluate microscopically. And immunohistochemistry staining was possessed by primary antibody incubation ( Supplementary Material Table S1 ), washing, and corresponding secondary antibody incubation.
2.5. ELISA
Brain and colon tissues were homogenized and then the supernatant was collected for ELISA. According to the instructions of the ELISA kit (mlbio, Shanghai, China, ml001891), the protein expression of 5-HT was measured and determined at 450 nm with the microplate reader.
2.6. Quantitative real-time PCR
Total RNA was prepared from brain and colon tissues, reverse transcribed to cDNA and conducted forty cycles as follows: 95°C for 30 s, 60°C for 30 s, followed by 60 s at 95°C for polymerase activation using corresponding primers ( Supplementary Material Table S2 ).
2.7. Immunoblotting
Tumor and colon tissues were homogenized and the supernatant was collected for western blotting. Total proteins were fractionated with SDS–PAGE and transferred to the PVDF membrane. After blocking, staining with primary antibodies ( Supplementary Material Table S1 ), and incubating with HRP-conjugated secondary antibodies, the membranes were visualized by an ECL system.
2.8. High-throughput sequencing analysis
Total genome DNA from mouse feces was extracted, then the V4 hypervariable regions of 16S rDNA were amplified by PCR. After sequencing the PCR amplification products and quality filtering, the reads were clustered as OTU using VSEARCH clustering (v2.13.4_linux_x86_64) sequence. Based on the OTU profiles, observed species, alpha diversity (Shannon), and beta diversity (PCoA) were calculated. Venn Diagram was used to determine the relationships between communities among different treatment groups.
2.9. Statistical analysis
All data are presented as mean ± standard deviation. Statistical evaluation was performed using one-way ANOVA followed by Tukey’s multiple comparison test as post hoc tests via GraphPad Prism 9.0 software at P value of < 0.05 (*) or < 0.01 (**).
3. Results
3.1. The combination of THD and C. butyricum promoted tumor apoptosis in CRC mice
To verify the construction of the CRC mouse model, the colon of mice was collected for H&E staining after the AOM/DSS treatment. Compared to the C group, M group showed an increasing number of submucosal glands having dysplasia into the lumen and detachment of intestinal mucosal villi in colon, the enlarged and deeply stained cell nuclei, and mitotic figures, which confirmed the successful construction of the CRC mouse model ( Figure 1B ).
Then we treated the CRC mice with different therapies. In our study, cisplatin treatment reduced the colon tumor number of mice than M group (M vs. MC = 3.64 vs. 2.91, Figure 1C ), while the ingestion of THD (2.36, p < 0.05) and C. butyricum (2.45, p < 0.05) further promoted the anti-tumor effect. Notably, the combination of THD and C. butyricum-treated mice in MCSL group had markedly fewer colon tumor numbers than MC group (MC vs. MCSL =2.91 vs. 1.82, Figure 1C , p < 0.01). In addition, the body weight of CRC mice was significantly lower than that in C group (C vs. M = 31.10 vs. 28.60, Figure 1D , p < 0.01), which was aggravated after cisplatin treatment in MC group (24.76, p < 0.01) and was alleviated in MCS group (26.30, p < 0.01), MCL group (25.31, p < 0.01) and MCSL group (27.36, p < 0.01), indicating that the survival state of CRC mice was improved.
Furthermore, western blotting of tumor certified the decreased activity of COX-2 (p < 0.05) and Survivin (p < 0.01), and the increased activity of cleaved Caspase-3 (p < 0.01) in MC group than M group, which were remarkedly reversed in MCSL group (p < 0.01) ( Figures 1E–H ). What’s more, the expression of cleaved PARP (p < 0.01) was also increased after the combination of THD and C. butyricum ( Figure S1 ). Therefore, results indicated that combination therapy of THD and C. butyricum optimally enhanced the activation of Caspase-3 apoptotic pathway by cisplatin.
3.2. The combination of THD and C. butyricum reduced nausea and vomiting after chemotherapy in CRC mice
Since chemotherapeutic agents are closely connected with the occurrence of nausea and vomiting (31), we sought to explore whether C. butyricum and THD treatment could improve nausea and vomiting after chemotherapy. During the kaolin intake measurement, the ingestion of kaolin in each mice group is shown in Figure 2A , which is seen as a phenomenon similar to nausea and vomiting in humans.
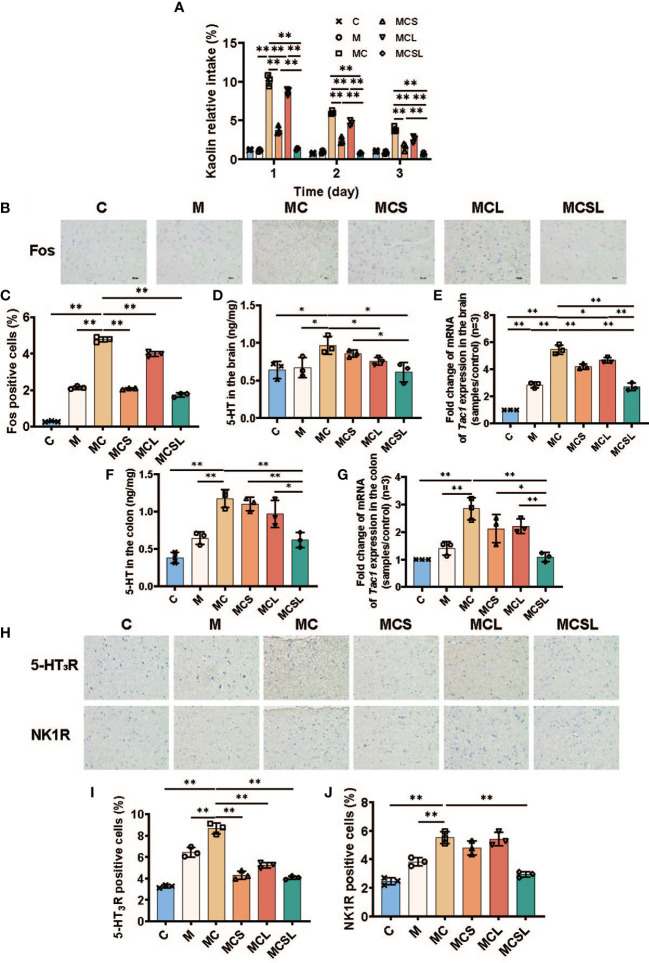
Figure 2.
The combination of THD and C. butyricum reduced CINV in CRC mice. (A) The kaolin consumption of mice in different treatment groups (n=3). (B) Fos protein in brain by IHC staining. (C) Fos positive cells were semiquantitatively assessed (n=3). (D) 5-HT of brain in different treatment groups (n=3). (E) the mRNA levels of Tac1 in brain (n=3). (F) 5-HT of colon in different treatment groups (n=3). (G) the mRNA levels of Tac1 in colon (n=3). (H) 5-HT3R and NK1R protein in brain by IHC staining. (I, J) 5-HT3R (I) and NK1R (J) positive cells were semiquantitatively assessed (n=3). Significance determined using one-way ANOVA with Tukey’s multiple comparison test and expressed as mean ± SD, *P < 0.05, **P < 0.01.
During the assay, the kaolin intake of MC group on the 1st day was significantly increased than M group (M vs. MC = 1.10% vs. 10.17%, Figure 2A , p < 0.01). Moreover, compared with MC group, nausea and vomiting was markedly alleviated in MCL group (8.72%, p < 0.01), MCS group (3.77%, p < 0.01), and MCSL group (1.35%, p < 0.01), respectively. Over the next two days, the combination of THD and C. butyricum still showed a best therapeutic effect on nausea and vomiting after chemotherapy than MCS group (p < 0.01) and MCL group (p < 0.01).
To further elucidate the mechanisms in regulation of CINV, we detected the expression of Fos in area postrema (AP) using immunohistochemical staining. In our research, compared to the C group, the expression of Fos in AP of MC group was increased (C vs. MC = 0.28% vs. 4.78%, Figure 2C , p < 0.01), while both THD (2.08%, p < 0.01), C. butyricum (3.98%, p < 0.01) and the combination treatment (1.74%, p < 0.01) could reduce the expression of Fos ( Figures 2B, C ). Notably, the combination of THD and C. butyricum treatment completely eliminated Fos expression in mouse brain of MCSL group.
5-HT and tachykinin 1 (Tac1), neurochemical mediators in the brainstem, are associated with emesis caused by cisplatin (31). Therefore, we detected the expression of 5-HT and Tac1 mRNA in mice brains by ELISA and q-PCR, respectively. The expression of 5-HT (M vs. MC = 0.67 ng/mg vs. 0.97 ng/mg, Figure 2D , p < 0.05) in mice brains was significantly increased after cisplatin treatment, which was reduced after the combined therapy of THD and C. butyricum (MC vs. MCSL = 0.97 ng/mg vs. 0.61 ng/mg, p < 0.05). Consistently, Tac1 mRNA in mice brains was markedly upregulated in MC group (M vs. MC = 2.84 vs. 5.45, Figure 2E , p < 0.01) and this increase could be reversed in MCS group (4.20, p < 0.01), MCL group (4.65, p < 0.05) and MCSL group (2.70, p < 0.01).
Furthermore, cisplatin has been shown to evoke the release of 5-HT and Tac1, which lead to the activation of a vomiting reflex (11). The content of 5-HT was quantified by ELISA of colon tissue to found that it in MC group was significantly raised than M group (M vs. MC = 0.65 ng/mg vs. 1.17 ng/mg, Figure 2F , p < 0.01), which was substantially decreased by combined administration in MCSL group (0.62 ng/mg, p < 0.01). In addition, Tac1 mRNA in mice colon was increased after cisplatin injection (M vs. MC = 1.41 vs. 2.84, Figure 2G , p < 0.01), and the combination of THD and C. butyricum could notably downregulate the relative expression of Tac1 mRNA in CRC mouse with cisplatin chemotherapy (1.09, p < 0.01).
5-HT and Tac1 stimulate its receptors (5-HT3R and NK-1R) on VAN to stimulate emesis (32). Hence, we further investigated the amount of 5-HT3R and NK-1R in AP using immunohistochemical staining. Compared to M group, cisplatin chemotherapy led to abnormal aggregation of 5-HT3R (M vs. MC = 6.46% vs. 8.66%, Figure 2I , p < 0.01) and NK-1R (M vs. MC = 3.83% vs. 5.51%, Figure 2J , p < 0.01), however, it was significantly reduced in MCSL group (4.07%, Figure 2I , p < 0.01; 2.93%, Figure 2J , p < 0.01) ( Figures 2H–J ).
3.3. The combination of THD and C. butyricum reversed dysbacteriosis in CRC mice
Since disturbed intestinal microbiota is associated with CRC and cisplatin chemotherapy (33), we further analyzed intestinal microbial composition. The Shannon index was markedly reduced in MC group (p < 0.05) than C group, and this reduction was ameliorated after C. butyricum treatment (MCL group) (p < 0.01) ( Figure 3A ). PCoA plots showed that the samples of M group clustered separately from those of C group. Compared to M group, the dysbacteriosis was further exacerbated after cisplatin chemotherapy (MC group), however, it was reversed by the combined treatment of THD and C. butyricum ( Figure 3B ). Next, according to the Venn results, there are 304 core OTUs in all of these fix groups, and 1201, 994, 638, 690, 1115 and 871 unique OTUs discovered in C, M, MC, MCS, MCL and MCSL group, separately ( Figure 3C ).
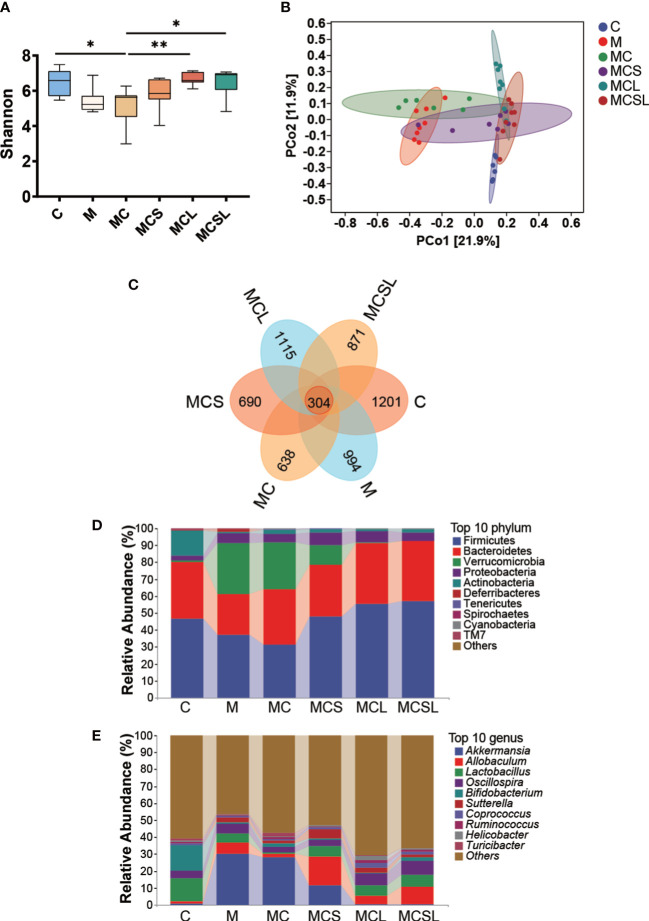
Figure 3.
The combination of THD and C. butyricum reversed dysbacteriosis in CRC mice. (A) The Shannon indexes. (B) Principal coordinate analysis (PCoA). (C) Venn representation. (D) The relative abundance of the bacteria at phylum level. (E) The relative abundance of the bacteria at genus level. *P < 0.05, **P < 0.01.
At the phylum level, a lower abundance of Firmicutes, Bacteroidetes, and Actinobacteria, and an increasing amount of Verrucomicobiota were detected in M and MC group compared to C group. After being treated with C. butyricum, the relative abundance of the aforementioned phyla significantly recovered ( Figure 3D ). At the genus level, we found that Akkermansia was abnormally increased and Bifidobacterium and Lactobacillus were reduced in feces of M group and MC group than C group. After C. butyricum treatment, dysbiosis of these genera was substantially restored than MC group ( Figure 3E ).
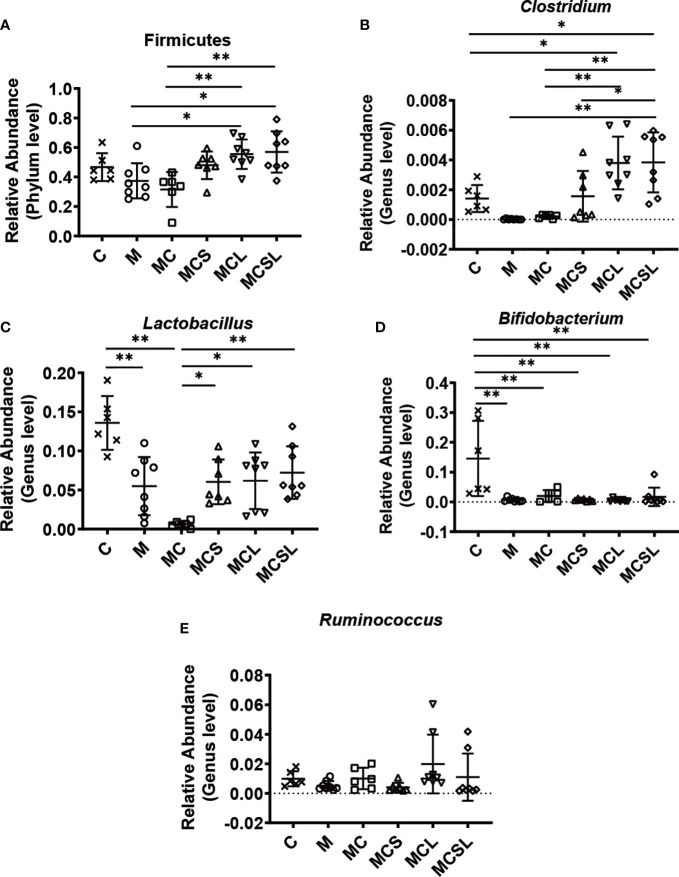
Furthermore, we selected some representative probiotics closely related to CRC and cisplatin-induced vomiting for analysis. Compared with MC group, the relative abundance of the phylum Firmicutes and the genera Clostridium was increased in MCL group (p < 0.01) and MCSL group (p < 0.01) ( Figures 4A, B ). After cisplatin chemotherapy, the relative abundance of the genera Lactobacillus was significantly reduced in MC group (p < 0.01), while it was markedly increased in MCS group (p < 0.05), MCL group (p < 0.05), and MCSL group (p < 0.01) ( Figure 4C ). Then, we found that the relative abundance of the genera Bifidobacterium was greatly reduced after all treatments by comparing with control group (p < 0.01), whereas it was slightly elevated in MCSL group ( Figure 4D ). Besides, compared with MCS group, the relative abundance of the genera Ruminococcus were up-regulated in MCL group and MCSL group ( Figure 4E ). These results suggested that the composition of gut microbiota could be altered after incidence of CRC and cisplatin treatment, but the use of C. butyricum restored the imbalance of the microbiome to a standard one.
Figure 4.
The combination of THD and C. butyricum regulated anti-cancer related probiotics in CRC mice. (A) The relative abundance of Fimicutes (n=6). (B) The relative abundance of Clotridium (n=6). (C) The relative abundance of Lactobacillus (n=6). (D) The relative abundance of Bifidobacterium (n=6). (E) The relative abundance of Ruminococcus (n=6). Significance determined using one-way ANOVA with Tukey’s multiple comparison test and expressed as mean ± SD, *P < 0.05, **P < 0.01.
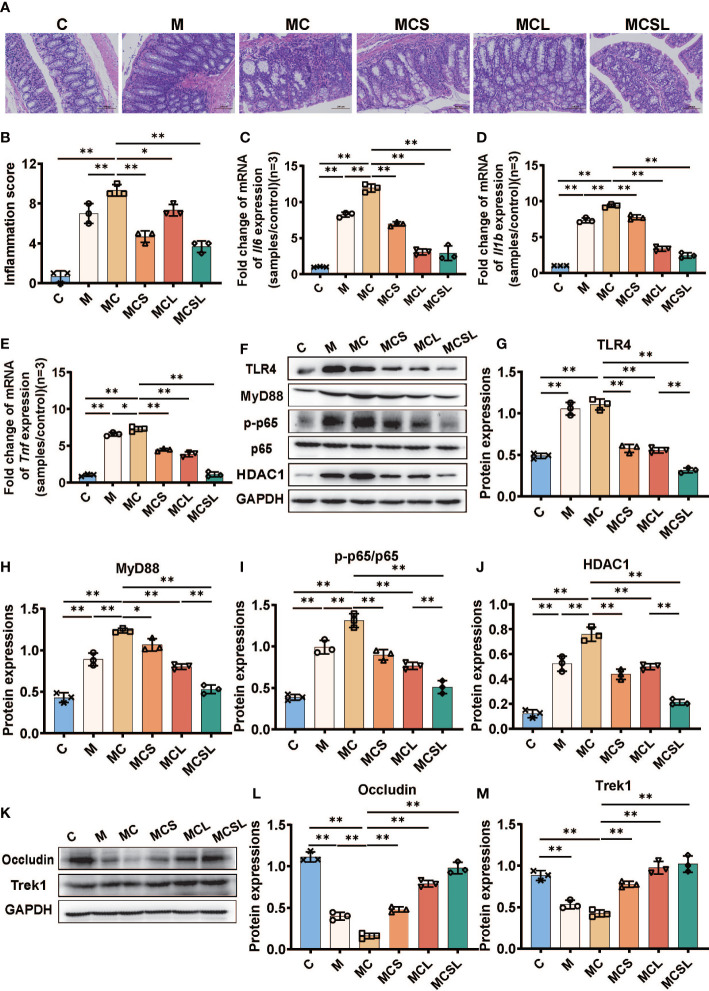
3.4. The combination of THD and C. butyricum inhibited colon inflammation and enhanced intestinal barrier
Cisplatin will lead to gastrointestinal side effects including colon inflammation and intestinal barrier destruction (34). Hence, we detected colonic inflammation at the end of experiment and the pathological changes of colon showed that the colonic crypts disappearance and inflammatory cell infiltration in M and MC group was significantly alleviated after the combination treatment of THD and C. butyricum in MCSL group ( Figures 5A, B ). After that, we performed the qRT-PCR of the intestinal tissue to access the level of inflammatory cytokines. Compared with C group, the mRNA expression of Il6 (C vs. M = 1.00 vs. 8.24, Figure 5C , p < 0.01), Il1b (C vs. M = 1.00 vs. 7.33, Figure 5D , p < 0.01) and Tnf (C vs. M = 1.01 vs. 6.57, Figure 5E , p < 0.01) in CRC mice were significantly elevated. Cisplatin chemotherapy in MC group would further upregulate the relative expression of Il6 (11.92, p < 0.01), Il1b (9.36, p < 0.01) and Tnf (7.28, p < 0.05) than M group, whereas THD (p < 0.01), C. butyricum (p < 0.01), and the combination of THD and C. butyricum (p < 0.01) inhibited the up-regulated levels of these inflammatory cytokines in CRC mice with cisplatin chemotherapy.
Figure 5.
The combination of THD and C. butyricum inhibited colon inflammation and improved intestinal barrier in AOM/DSS-induced CRC mice. (A) H&E staining of colon tissue. (B) Histological scores of inflammations (H&E staining) (n=3). (C–E) The mRNA levels of Il6, Il1b, and Tnf (n=3). (F) Western blotting of TLR4, MyD88, p-p65, p65, HDAC1 (n=3). (G–J) The relative abundance of TLR4, MyD88, p-p65/p65, HDAC1 (n=3). (K) Western blotting of occludin and Trek1 (n=3). (L, M) The relative abundance of occludin and Trek1 (n=3). Significance determined using one-way ANOVA with Tukey’s multiple comparison test and expressed as mean ± SD, *P < 0.05, **P < 0.01.
Then, we further determined HDAC1 protein, key proteins of TLR4/NF-κB inflammatory pathway, and intestinal tight-junction associated proteins in colon by Western blotting. In contrast to normal mice, we detected a marked up-expression of TLR4 (p < 0.01), MyD88 (p < 0.01), p-p65 (p < 0.01), and HDAC1 (p < 0.01) in CRC mice. What’s more, cisplatin treatment further aggravated the quantity of MyD88 (p < 0.01), p-p65 (p < 0.01), and HDAC1 (p < 0.01) than M group. On the contrary, the colon inflammation could be suppressed after THD and C. butyricum administration (p < 0.01) ( Figures 5F–J ).
Meanwhile, Western blotting also revealed the decreased expression of occludin (p < 0.01) and Trek1 (p < 0.01) in M group than C group, while these reductions were markedly restored in MCS group (p < 0.01), MCL group (p < 0.01), and MCSL group (p < 0.01) ( Figures 5K–M ).
4. Discussion
CRC is the third most common cancer worldwide, with high morbidity and mortality rates (35). Currently, chemotherapy, the primary treatment for advanced CRC, causes severe side effects, especially nausea and vomiting (31). CINV which threatens patient’s life and health (8, 9), leads to an urgent need to create alternative therapies that are highly effective and have minimal side effects.
Clinically, we observed that the combination of C. butyricum and THD almost eliminate CINV (data not shown), but the mechanism for the prevention of CINV remains unclear. Herein, we used AOM/DSS-induced CRC mouse models that received cisplatin chemotherapy to investigate the primary role of the combination of C. butyricum and THD in addition to its possible therapeutic mechanism. Our results indicated that AOM/DSS caused the occurrence of CRC in mice, but the mice receiving cisplatin with the combination of THD and C. butyricum showed a markedly decreasing tumor number. Our findings suggested that COX-2 and survivin in tumor are highly expressed, and cleaved Caspase-3 and cleaved PARP are lowly expressed in M group. Additionally, COX-2, which is elevated expressed in CRC, contributes to tumorigenesis by excited angiogenesis, suppressed apoptosis, and enhanced cell invasiveness (36). What’s more, survivin is an inhibitor of programmed cell death that is negatively correlated with apoptosis and directly suppresses caspase-3 activity to prevent apoptosis (37, 38). After activation of caspase-3, the DNA repair enzyme PARP was cleaved leading to loss of the ability of DNA repair resulting in apoptosis (39). Herein, we found that the combination of THD and C. butyricum can inhibit the expression of COX-2 and survivin to activate Caspase-3 and cleave PARP to induce tumor apoptosis ( Figure 1 ).
Cisplatin, a chemotherapeutic agent frequently used in clinical practice, is associated with emesis (40). Hence, we used kaolin intake measurement, an index of nausea and emesis in animal studies (41), to evaluate nausea and vomiting of mice with chemotherapy. Mice ingested cisplatin severely vomited than M group, but the combination therapy of THD and C. butyricum significantly relieved emesis. Fos in AP, a marker of neuronal activity whose expression correlates with brain stimulation resulting from the stimulation of neurotransmitter receptors on adjacent vagal afferents, was increased after cisplatin chemotherapy (42, 43). Our findings suggested that the combination of THD and C. butyricum could abundantly decrease the fos expression in the AP, confirming reduced brain neuron activation. Furthermore, it was currently reported that cisplatin-induced release of the 5-HT and Tac1 interacted with receptors (i.e., 5-HT3R and NK-1R) of the vagus nerve in brain and gastrointestinal tract to cause the above neuronal activation (7, 44). We found that a combination of THD and C. butyricum abundantly reduces the 5-HT expression and the relative Tac1 mRNA level in brain and colon compared with MC group. Additionally, we found that the combination therapy inhibited aggregation of 5-HT3R and NK-1R in brain of CRC mice with cisplatin chemotherapy. In summary, these results suggested that the combined administration of THD and C. butyricum reversed the up-release of neurotransmitters and activation of receptors in cisplatin-treated CRC mice model, to relieve the cisplatin-induced nausea and vomiting ( Figure 2 ).
Disordered gut microbiota is associated with weakened health (45) and is involved in gastrointestinal carcinogenesis, while cisplatin chemotherapy further destroyed its homeostasis (46, 47). In addition, Chen et al. discovered that C. butyricum is beneficial in the reconstruction of gut microbiota (48), and the reversed gut microbiota disorder further plays a role in treatment of intestinal diseases (49). Therefore, we explored whether C. butyricum could improve the intestinal microbiota disorder caused by CRC and chemotherapy via high-throughput sequencing. The results demonstrated the therapeutics of the combination treatment (MCSL group) in restoration of intestinal microbiota diversity ( Figure 3 ). Moreover, it was observed that certain genera of bacteria had reduced abundance in both M group and MC group, including Clostridium, Lactobacillus, Bifidobacterium and, Ruminococcus. Furthermore, such dysbiosis could be restored by combined treatment with THD and C. butyricum, which is consistent with the published reports about the microbiota regulatory effect of C. butyricum and ( Figures 4B–E ). Among them, the increased relative abundance of Clostridium may be related to the well intestinal colonization of C. butyricum (50). After its colonization, C. butyricum furthur regulates the richness and composition of intestinal microbiota, alleviates intestinal inflammation, further improve the efficacy of chemotherapy which may contribute to the remission of CINV (26, 51). Meng et al. discovered that L. plantarum orally administration significantly increased the abundance of Lactobacillus and Bifidobacterium in cecal content of CTX-treated mice, and markedly reduced the nausea and vomiting symptoms, suggesting that the high abundance of Lactobacillus and Bifidobacterium is positively correlated with the relief of nausea, vomiting (52). What’s more, the increased abundance of Lactobacillus limits intestinal tumor growth via gut microbiota reconstruction, tumor cell proliferation suppression and apoptosis activation (53, 54). Bifidobacterium, one of the butyrate-producing bacteria, has similarly been verified effective in decreasing pro-inflammatory cytokines and inhibiting cancer cells to suppress gastrointestinal cancer (55, 56). Notably, Ruminococcus, another strain can produce butyrate, showed a slight increase in abundance after C. butyricum administration. And there are published papers supporting that Ruminococcus could partially inhibit inflammation and protect the intestinal mucosa after cisplatin therapy in various cancer models (51, 57). At the phylum level, the findings of Burkhardt Flemer et al. (58) and our research both pointed to the decreased abundance of Firmicutes in CRC mice ( Figure 4A ). As suggested by another study, the diminished Firmicutes would be the possible mechanism responsible for cisplatin-associated side effects (47). In conclusion, the improved gut microbiota dysbiosis mediated by C. butyricum administration may be associated with anti-tumor, neurotransmitter secretion decrease and brain activation reduction, as well as anti-inflammation in colon.
In recent years, evidence has been mounting to suggest that chronic inflammation produces considerable inflammatory mediators (IL-6, IL-1β, TNF-α), activating NF-κB, thus leading to intestinal barrier function loss and colon carcinogenesis (59). Hence, we utilized H&E staining to find that the combination therapy (MCSL group) greatly suppresses colonic inflammation and attenuates histopathological changes in colon ( Figures 5A–B ). Additionally, our findings suggested that the combined treatment of THD and C. butyricum degraded the levels of IL-6, IL-1β, and TNF-α and downregulated the activity of HDAC1, TLR4, MyD88, and p-p65 ( Figures 5C–J ). Among them, histone deacetylase 1 (HDAC1) can be inhibited by butyrate to eliminate inflammation in colonic epithelial cells, subsequently suppressing colonic inflammation (60). The intestinal mucosal barrier functions are associated with intestinal tight-junction associated proteins, such as occludin (61). Besides, recent reports have suggested the importance of Trek1 in preserving the integrity of the intestinal epithelial barrier (62). We discovered that occludin and Trek1 activity in colon was significantly decreased in both M and MC groups. However, combination administration (MCSL group) substantially restored those reductions ( Figures 5K–M ). Collectively, these outcomes demonstrated that the combination of C. butyricum and THD has a notable impact on protecting intestinal barrier function and anti-inflammatory effect in CRC mice undergoing cisplatin chemotherapy.
5. Conclusions
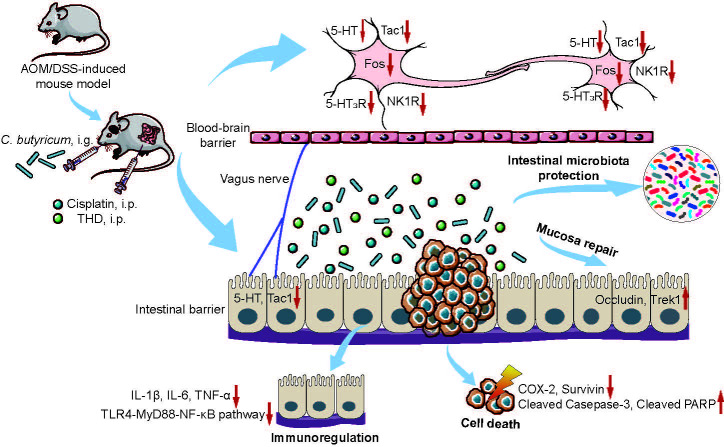
We conclude that the combination of THD and C. butyricum improved cancer suppression efficacy of cisplatin in CRC mice by activation of caspase-3 apoptotic pathway. Moreover, our study confirmed that the inhibition of neurotransmitters (e.g., 5-HT and Tac1) and its receptor (e.g., 5-HT3R and NK-1R), the regulation of intestinal microbiota, the inhibition of inflammation in colon, and the protection of intestinal mucosa barrier via the combination therapy are extremely important to alleviate CINV in CRC mice ( Figure 6 ). It should be noted that in this work, we only investigated the ability of C. butyricum combined with THD in the treatment of CRC and CINV via gut microbiota and vagus nerve activity modulation. However, it is important to evaluate the efficacy of additional probiotics for the development of chemotherapy adjuvant drugs. Moreover, we will fully consider the necessary of verifying the efficacy of the combination of C. butyricum and THD in CRC patients receiving chemotherapy and its chemotherapy associated side effects in the clinic.
Figure 6.
Schematic diagram of the underlying mechanisms of the combination of THD and C. butyricum in cancer treatments and CINV amelioration. The combination of THD and C. butyricum enhanced anti-tumor effects and ameliorated CINV via activating caspase-3 apoptosis pathway, inhibiting neurotransmitter (e.g., 5-HT and Tac1) and its receptors (e.g., 5-HT3R and NK-1R), and reversing intestinal dysbiosis in Azoxymethane/Dextran Sodium Sulfate induced colorectal cancer (CRC) mice. THD, thalidomide; C. butyricum, Clostridium butyricum; CINV, chemotherapy-induced nausea and vomiting; 5-HT, 5-hydroxytryptamine; Tac1, tachykinin 1; 5-HT3R, 5-hydroxytryptamine-3 receptor; NK1R, neurokinin-1 receptor.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA941024.
Ethics statement
This study and the animal experimental protocol were reviewed and approved by the Laboratory Animal Ethics Committee of Nanchang Leyou Biotechnology Co. Ltd. (RyE2021070902). All experiments were conducted in accordance with the guidelines.
Author contributions
XZ contributed with methodology, investigation, formal analysis, visualization and writing - original draft. HWu and RZ contributed with methodology, investigation and visualization. GS and JW contributed with investigation. HS and PT contributed with methodology. TC and HWe contributed with conceptualization, funding acquisition, supervision, writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding Statement
This study was supported by the National Natural Science Foundation of China (Grant No. 82060638 to TC) and Double Thousand Plan of Jiangxi Province to TC (High‐End Talents Project of Scientific and Technological Innovation).
Conflict of interest
Author GS was employed by Eastsea Pharma Co. LTD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1220165/full#supplementary-material
The combination of THD and C. butyricum promote PARP cleaved in CRC mice. (A) Western blotting of cleaved PARP (n=3). (B) Relative expressions of cleaved PARP (n=3). Significance determined using one-way ANOVA with Tukey’s multiple comparison test and expressed as mean ± SD, * P < 0.05, ** P < 0.01.
References
- 1. Mao W, Fan Y, Cheng C, Yuan X, Lan T, Mao K, et al. Efficacy and safety of kanglaite injection combined with chemotherapy for colorectal cancer: a protocol for systematic review and meta-analysis. Med (Baltimore) (2020) 99:e22357. doi: 10.1097/MD.0000000000022357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ren C, Han H, Pan J, Chang Q, Wang W, Guo X, et al. DLGAP1 − AS2 promotes human colorectal cancer progression through trans − activation of Myc. Mamm Genome (2022) 33:672–83. doi: 10.1007/s00335-022-09963-y [DOI] [PubMed] [Google Scholar]
- 3. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin (2022) 72:409–36. doi: 10.3322/caac.21731 [DOI] [PubMed] [Google Scholar]
- 4. Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalt Trans (2018) 47:6645–53. doi: 10.1039/c8dt00838h [DOI] [PubMed] [Google Scholar]
- 5. Yang Y, Zhao Y, Liu L, Zhu W, Jia S, Li X, et al. The anti-apoptotic role of COX-2 during In vitro infection of human intestinal cell line by giardia duodenalis and the potential regulators. Infect Immun (2022) 90:1–11. doi: 10.1128/iai.00672-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) (2011) 3:1351–71. doi: 10.3390/cancers3011351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rapoport BL. Delayed chemotherapy-induced nausea and vomiting: pathogenesis, incidence, and current management. Front Pharmacol (2017) 8:19. doi: 10.3389/fphar.2017.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Zhang J, Teng Y, Zhang L, Yu P, Jin B, et al. Thalidomide improves prevention of chemotherapy-induced gastrointestinal side effects following a modified FOLFOX7 regimen: results of a prospective randomized crossover study. Tumori (2009) 95:691–6. doi: 10.1177/030089160909500609 [DOI] [PubMed] [Google Scholar]
- 9. Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ. Patient characteristics associated with medication adherence. Clin Med Res (2013) 11:54–65. doi: 10.3121/cmr.2013.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hornby PJ. Central neurocircuitry associated with emesis. Am J Med (Elsevier Inc.) (2001), 106–12. doi: 10.1016/s0002-9343(01)00849-x [DOI] [PubMed] [Google Scholar]
- 11. Minami M, Endo T, Hirafuji M, Hamaue N, Liu Y, Hiroshige T, et al. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther (2003) 99:149–65. doi: 10.1016/S0163-7258(03)00057-3 [DOI] [PubMed] [Google Scholar]
- 12. Rao KV, Faso A. Chemotherapy-induced nausea and vomiting: optimizing prevention and management. Am Heal Drug Benefits (2012) 5:232–40. [PMC free article] [PubMed] [Google Scholar]
- 13. Navari RM. Management of chemotherapy-induced nausea and vomiting in pediatric patients. Pediatr Drugs (2017) 19:213–22. doi: 10.1007/s40272-017-0228-2 [DOI] [PubMed] [Google Scholar]
- 14. J.D’Amato R, S.Loughnan M, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Med Sci (1994) 91:4082–5. doi: 10.1073/pnas.91.9.4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kenyon BM, Browne F, D’Amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res (1997) 64:971–8. doi: 10.1006/exer.1997.0292 [DOI] [PubMed] [Google Scholar]
- 16. Wang N, Xu P, Liu Y, Zhao P, Ruan J, Zheng Y, et al. Efficacy and safety of thalidomide for chemotherapy-induced nausea and vomiting. J Cancer (2020) 11:4560–70. doi: 10.7150/jca.45678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L, Qu X, Teng Y, Shi J, Yu P, Sun T, et al. Efficacy of thalidomide in preventing delayed nausea and vomiting induced by highly emetogenic chemotherapy: a randomized, multicenter, double-blind, placebo-controlled phase III trial (CLOG1302 study). J Clin Oncol (2017) 35:3558–65. doi: 10.1200/JCO.2017.72.2538 [DOI] [PubMed] [Google Scholar]
- 18. Wang X, Shen Y, Li S, Lv M, Zhang X, Yeng J, et al. Importance of the interaction between immune cells and tumor vasculature mediated by thalidomide in cancer treatment (Review). Int J Mol Med (2016) 38:1021–9. doi: 10.3892/ijmm.2016.2724 [DOI] [PubMed] [Google Scholar]
- 19. Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Rajen N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: Clinical application. Br J Haematol (2005) 128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x [DOI] [PubMed] [Google Scholar]
- 20. Chen HM, Yu YN, Wang JL, Lin YW, Kong X, Yang CQ, et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutr (2013) 97:1044–52. doi: 10.3945/ajcn.112.046607 [DOI] [PubMed] [Google Scholar]
- 21. Yixia Y, Sripetchwandee J, Chattipakorn N, Chattipakorn SC. The alterations of microbiota and pathological conditions in the gut of patients with colorectal cancer undergoing chemotherapy. Anaerobe (2021) 68:102361. doi: 10.1016/j.anaerobe.2021.102361 [DOI] [PubMed] [Google Scholar]
- 22. Cool RM, Herrington JD. Thalidomide for the treatment of relapsed and refractory multiple myeloma. Pharmacotherapy (2002) 22:1019–28. doi: 10.1592/phco.22.12.1019.33606 [DOI] [PubMed] [Google Scholar]
- 23. Meng C, Bai C, Brown TD, Hood LE, Tian Q. Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinforma (2018) 16:33–49. doi: 10.1016/j.gpb.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang N, Zhan Y, Wan J, Li Y, Hu X, Liu W. Effects of lacidophilin tablets, yogurt, and bifid triple viable capsules on the gut microbiota of mice with antibiotic-associated diarrhea. Can J Infect Dis Med Microbiol (2022) 2022:1–10. doi: 10.1155/2022/6521793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aragon G, Graham DB, Borum M, Doman DB. Probiotic therapy for irritable bowel syndrome. Gastroenterol Hepatol (2010) 6:39–44. [PMC free article] [PubMed] [Google Scholar]
- 26. Stoeva MK, Garcia-So J, Justice N, Myers J, Tyagi S, Nemchek M, et al. Butyrate-producing human gut symbiont, clostridium butyricum, and its role in health and disease. Gut Microbes (2021) 13:1–28. doi: 10.1080/19490976.2021.1907272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 28. Hagihara M, Kuroki Y, Ariyoshi T, Higashi S, Fukuda K, Yamashita R, et al. Clostridium butyricum modulates the microbiome to protect intestinal barrier function in mice with antibiotic-induced dysbiosis. iScience (2020) 23:100772. doi: 10.1016/j.isci.2019.100772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen D, Jin D, Huang S, Wu J, Xu M, Liu T, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating wnt signaling and gut microbiota. Cancer Lett (2020) 469:456–67. doi: 10.1016/j.canlet.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 30. Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, et al. Neuroprotective effects of clostridium butyricum against vascular dementia in mice via metabolic butyrate. BioMed Res Int (2015) 2015:1–12. doi: 10.1155/2015/412946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta K, Walton R, Kataria SP. Chemotherapy-induced nausea and vomiting: pathogenesis, recommendations, and new trends. Cancer Treat Res Commun (2021) 26:100278. doi: 10.1016/j.ctarc.2020.100278 [DOI] [PubMed] [Google Scholar]
- 32. Aapro M. CINV: still troubling patients after all these years. Support Care Cancer (2018) 26:5–9. doi: 10.1007/s00520-018-4131-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Li H. Gut microbiota modulation: a tool for the management of colorectal cancer. J Transl Med (2022) 20:1–14. doi: 10.1186/s12967-022-03378-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zou YT, Zhou J, Wu CY, Zhang W, Shen H, Xu JD, et al. Protective effects of poria cocos and its components against cisplatin-induced intestinal injury. J Ethnopharmacol (2021) 269:113722. doi: 10.1016/j.jep.2020.113722 [DOI] [PubMed] [Google Scholar]
- 35. Kim J, Lee HK. Potential role of the gut microbiome in colorectal cancer progression. Front Immunol (2022) 12:807648. doi: 10.3389/fimmu.2021.807648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Konturek PC, Rembiasz K, Burnat G, Konturek SJ, Tusinela M, Bielanski W, et al. Effects of cyclooxygenase-2 inhibition on serum and tumor gastrins and expression of apoptosis-related proteins in colorectal cancer. Dig Dis Sci (2006) 51:779–87. doi: 10.1007/s10620-006-3206-z [DOI] [PubMed] [Google Scholar]
- 37. Choi J, Chang HK. The expression of MAGE and SSX, and correlation of COX2, VEGF, and survivin in colorectal cancer. Anticancer Res (2012) 32:559–64. [PubMed] [Google Scholar]
- 38. Temraz S, Mukherji D, Shamseddine A. Potential targets for colorectal cancer prevention. Int J Mol Sci (2013) 14:17279–303. doi: 10.3390/ijms140917279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu F-H, Wei H-Z, Deng H-Y, Xiao G-H, Zhang Y-C. PARP in colorectal cancer: molecular mechanisms, immunity, clinical trials, and drug combinations. Neoplasma (2023) 70:1–14. doi: 10.4149/neo_2022_220724N745 [DOI] [PubMed] [Google Scholar]
- 40. Han ZX, Xu J, Wang HM, Ma J, Sun X, Du XP. Antiemetic role of thalidomide in a rat model of cisplatin-induced emesis. Cell Biochem Biophys (2014) 70:361–5. doi: 10.1007/s12013-014-9921-8 [DOI] [PubMed] [Google Scholar]
- 41. Goineau S. Comparison of three preclinical models for nausea and vomiting assessment. J Pharmacol Toxicol Methods (2016) 82:45–53. doi: 10.1016/J.VASCN.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 42. Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol (1990) 296:517–30. doi: 10.1002/cne.902960402 [DOI] [PubMed] [Google Scholar]
- 43. Li S, Lei Y, Chen JDZ. Chemotherapy-induced pica in rats reduced by electroacupuncture. Neuromodulation (2018) 21:254–60. doi: 10.1111/ner.12712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marx W, Ried K, McCarthy AL, Vitetta L, Sali A, McKavanagh D, et al. Ginger–mechanism of action in chemotherapy-induced nausea and vomiting: a review. Crit Rev Food Sci Nutr (2017) 57:141–6. doi: 10.1080/10408398.2013.865590 [DOI] [PubMed] [Google Scholar]
- 45. Li X, Wu J, Kang Y, Chen D, Chen G, Zeng X, et al. Yeast mannoproteins are expected to be a novel potential functional food for attenuation of obesity and modulation of gut microbiota. Front Nutr (2022) 9:1019344. doi: 10.3389/fnut.2022.1019344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene (2020) 39:4925–43. doi: 10.1038/s41388-020-1341-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gori S, Inno A, Belluomini L, Bocus P, Bisoffi Z, Russo A, et al. Gut microbiota and cancer: how gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit Rev Oncol Hematol (2019) 143:139–47. doi: 10.1016/j.critrevonc.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 48. Chen X, Yi H, Liu S, Zhang Y, Su Y, Liu X, et al. Probiotics improve eating disorders in mandarin fish (Siniperca chuatsi) induced by a pellet feed diet via stimulating immunity and regulating gut microbiota. Microorganisms (2021) 9:1288. doi: 10.3390/microorganisms9061288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang H, Chen G, Li X, Zheng F, Zeng X. Yeast β-glucan, a potential prebiotic, showed a similar probiotic activity to inulin. Food Funct (2020) 11:10386–96. doi: 10.1039/D0FO02224A [DOI] [PubMed] [Google Scholar]
- 50. Luo X, Kong Q, Wang Y, Duan X, Wang P, Li C, et al. Colonization of clostridium butyricum in rats and its effect on intestinal microbial composition. Microorganisms (2021) 9:1573. doi: 10.3390/microorganisms9081573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hsiao Y-P, Chen H-L, Tsai J-N, Lin M-Y, Liao J-W, et al. Administration of lactobacillus reuteri combined with clostridium butyricum attenuates cisplatin-induced renal damage by gut microbiota reconstitution, increasing butyric acid production, and suppressing renal inflammation. Nutrients (2021) 13:2792. doi: 10.3390/nu13082792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meng Y, Wang J, Wang Z, Zhang G, Liu L, Huo G, et al. Lactobacillus plantarum KLDS1.0318 ameliorates impaired intestinal immunity and metabolic disorders in cyclophosphamide-treated mice. Front Microbiol (2019) 10:731. doi: 10.3389/fmicb.2019.00731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sugimura N, Li Q, Chu ESH, Lau HCH, Fong W, Liu W, et al. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut (2022) 71:2011–21. doi: 10.1136/gutjnl-2020-323951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dong Y, Zhu J, Zhang M, Ge S, Zhao L. Probiotic lactobacillus salivarius ren prevent dimethylhydrazine-induced colorectal cancer through protein kinase b inhibition. Appl Microbiol Biotechnol (2020) 104:7377–89. doi: 10.1007/s00253-020-10775-w [DOI] [PubMed] [Google Scholar]
- 55. Zaharuddin L, Mokhtar NM, Najmi K, Nawawi M, Affendi R, Ali R. A randomized double-blind placebo- controlled trial of probiotics in post-surgical colorectal cancer. (2019) 19:131. doi: 10.1186/s12876-019-1047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bahmani S, Azarpira N, Moazamian E. Anti-colon cancer activity of bifidobacterium metabolites on colon cancer cell line SW742. Turkish J Gastroenterol (2019) 30:835–42. doi: 10.5152/tjg.2019.18451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perales-Puchalt A, Perez-Sanz J, Payne KK, Svoronos N, Allegrezza MJ, Chaurio RA, et al. Frontline science: microbiota reconstitution restores intestinal integrity after cisplatin therapy. J Leukoc Biol (2018) 103:799–805. doi: 10.1002/JLB.5HI1117-446RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Flemer B, Lynch DB, Brown JMR, Jeffery IB, Ryan FJ, Claesson MJ, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut (2017) 66:633–43. doi: 10.1136/gutjnl-2015-309595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol (2011) 11:9–20. doi: 10.1038/nri2891 [DOI] [PubMed] [Google Scholar]
- 60. Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, et al. Butyrate suppresses colonic inflammation through HDAC1-dependent fas upregulation and fas-mediated apoptosis of T cells. Am J Physiol - Gastrointest Liver Physiol (2012) 302:1405–15. doi: 10.1152/ajpgi.00543.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem (1998) 273:29745–53. doi: 10.1074/jbc.273.45.29745 [DOI] [PubMed] [Google Scholar]
- 62. Huang H, Liu JQ, Yu Y, Mo LH, Ge RT, Zhang HP, et al. Regulation of TWIK-related potassium channel-1 (Trek1) restitutes intestinal epithelial barrier function. Cell Mol Immunol (2016) 13:110–8. doi: 10.1038/cmi.2014.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The combination of THD and C. butyricum promote PARP cleaved in CRC mice. (A) Western blotting of cleaved PARP (n=3). (B) Relative expressions of cleaved PARP (n=3). Significance determined using one-way ANOVA with Tukey’s multiple comparison test and expressed as mean ± SD, * P < 0.05, ** P < 0.01.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA941024.