Abstract
Background:
Antibiotic resistance is often spread through bacterial populations via conjugative plasmids. However, plasmid transfer is not well recognized in clinical settings because of technical limitations and healthcare- associated infections are usually caused by clonal transmission of a single pathogen. In 2015, multiple species of carbapenem-resistant Enterobacteriaceae (CRE), all producing a rare carbapenemase, were identified among patients in an intensive care unit (ICU). This observation suggested a large, previously unrecognized plasmid transmission chain and prompted our investigation.
Methods:
Electronic medical record review, infection control observations, and environmental sampling completed the epidemiologic outbreak investigation. Laboratory analysis, conducted on patient and environmental isolates, included long-read whole genome sequencing to fully elucidate plasmid DNA structures. Bioinformatics analyses were applied to infer plasmid transmission chains and results were subsequently confirmed using plasmid conjugation experiments.
Results:
Fourteen VIM-producing CRE were identified in twelve patients, and one additional isolate was obtained from a patient room sink drain. Whole genome sequencing identified horizontal transfer of blaVIM-1, a rare carbapenem resistance mechanism in the United States, via a promiscuous IncA/C2 plasmid which spread among five bacterial species isolated from patients and the environment.
Conclusions:
This investigation represents the largest known outbreak of VIM-producing CRE in the United States to date and comprises numerous bacterial species and strains. We present evidence of in-hospital plasmid transmission, as well as environmental contamination. Our findings demonstrate the potential for two types of hospital-acquired infection outbreaks: those due to clonal expansion and those due to spread of conjugative plasmids encoding antibiotic resistance across species.
Keywords: Hospital-acquired infection, Plasmid, Carbapenemase, Enterobacteriaceae
Summary:
We describe an outbreak investigation of VIM-producing CRE among twelve patients in an ICU. Horizontal spread of a rare carbapenem resistance mechanism occurred via a promiscuous plasmid that spread among five CRE species isolated from patients and the hospital environment.
Introduction:
Each year, approximately 2.8 million illnesses and 35,000 deaths in the United States (US) are attributable to antibiotic-resistant infections [1]. Due to decreasing susceptibility to other broad-spectrum antimicrobials, carbapenems have become the treatment of choice for infections caused by highly antibiotic-resistant bacteria [2]. The expanded use of carbapenem antimicrobials has coincided with a rise of carbapenem-resistant phenotypes, especially among the Enterobacteriaceae [3].
Enterobacteriaceae develop carbapenem resistance through several pathways, including expression of a carbapenemase or overproduction of certain beta-lactamases in combination with porin mutations and/or augmented efflux pump activity [4]. The rapid dissemination of carbapenem resistance is also driven by horizontal spread of resistance genes on conjugative plasmids, including among carbapenem-resistant Enterobacteriaceae (CRE) strains that have become successful in clinical settings [5]. Therefore, outbreak investigations and surveillance studies are increasingly focusing on plasmids that facilitate inter and intra-species transfer of antimicrobial resistance (AMR) [6,7].
While Klebsiella pneumoniae carbapenemase (KPC) is the most common carbapenemase in the US [4], metallo-ß- lactamases (MBL) are increasing. These MBL enzymes include New Delhi metallo-β-lactamase (NDM) [8], recently associated with duodenoscope exposure in the US [9], and Verona integron-encoded metallo-ß-lactamase (VIM) [10], among others. The VIM enzymes form one of the largest groups of MBLs comprising 51 reported variants (https://www.ncbi.nlm.nih.gov/bioproject/305729). VIM and its associated class-1 integrons are associated with numerous broad-range plasmids, including IncN, IncHI2, and IncA/C2, further facilitating their spread [11–13]. Production of VIM in Enterobacteriaceae has been relatively rare, in the US, with only 57 reported instances as of December 2017. Like other CRE, VIM-producers have been associated with more complicated patient courses, including higher relapse rates and a need for prolonged duration of antimicrobial therapy [14], and are an emerging threat to public health. The first VIM-producing CRE identified in the US occurred in an adult patient in 2006 [15], and a second travel-associated VIM-isolate was reported in 2010 [16].
From August 2015 through May 2016, 12 patients colonized with five different species of VIM-producing CRE were identified in an intensive care unit (ICU) at a tertiary care hospital in Kentucky [17]. Colonization with a VIM- producing CRE had been identified only once previously at this hospital, in an adult patient in 2013, as reported to the Kentucky Department for Public Health. The combination of a rare carbapenemase in CRE presenting in a single facility among a diverse set of pathogens prompted the use of next generation sequencing to characterize these VIM-producing CRE to better understand the driving forces behind this cluster.
Methods:
Case Definition, Finding, and Series
A case was defined as a PCR-confirmed blaVIM-containing CRE isolate, isolated from a clinical or surveillance culture from a patient who had a negative admission screening culture and was admitted to the affected hospital on or after August 1, 2015.
Suspected cases were initially identified through perirectal screening that included admission and weekly multidrug-resistant organism (MDRO) surveillance cultures. CRE were tested for blaVIM using Verigene nucleic acid test (Nanosphere, Chicago, IL) and VIM-positive isolates were submitted to CDC for PCR confirmation and whole genome sequence analysis, as described below. A review of infection control records over the prior year at the hospital was completed to identify historical cases.
Common upstream and downstream referral facilities from Hospital A were identified using Centers for Medicare & Medicaid Services (CMS) claims data from fee-for-service beneficiaries. Point prevalence perirectal surveillance of high-risk patients (extended length of stay, devices in place, or on ventilator) was conducted at four facilities (including tertiary care hospitals and long-term acute care hospitals) identified as most connected in the referral network of the hospital, including both upstream and downstream patient transfers. The activities involved in this investigation underwent human subjects review at the CDC and were determined to constitute an urgent public health response and therefore institutional review board exempt.
Environmental Assessment and Antimicrobial Resistance Testing
Environmental samples were collected from sinks, drains, environmental services carts, and surfaces in rooms that housed affected patients and common areas of affected units. Samples were collected using Sponge-Sticks (3M, St. Paul, Minnesota) and EnviroMax Plus foam paddle swabs (Puritan Medical, Guilford, Maine), then processed as described previously [18]. Dilutions were plated onto CHROMagar KPC (DRG International, Springfield, NJ), Trypticase™ Soy Agar with 5% Sheep Blood (TSA II™, BD, Sparks, Maryland) and MacConkey II (BD, Sparks, Maryland), and cultured overnight at 35°C.
Environmental isolates were screened for antimicrobial resistance using 10 µg meropenem disks placed between the first and second quadrant of growth. Suspect colonies were selected within a 21 mm zone, then screened for Metallo-ß-lactamase (MBL) activity by comparing imipenem broth microdilution MICs in the presence (32 to 0.25 mg/ml) and absence (64 to 0.5 mg/ml) of metal chelators (0.2 mM EDTA and 0.02 mM phenanthroline). Resistance determinants were identified from cultured isolates by multiplex PCR for detection of blaOXA-48, blaVIM, blaNDM, and blaKPC [19].
Isolate Preparation and Genomic Analysis
A detailed description of molecular methods is provided in the Supplemental Appendix. In brief; nineteen isolates, including 14 from 12 case-patients, two environmental isolates, and three historical specimens, underwent short- read sequencing using a MiSeq (Illumina, San Diego, CA). A subset of six isolates was selected for long-read sequencing on a RSII instrument (Pacific Biosciences, Menlo Park, CA). Clean short-reads were assembled de novo into contigs using SPAdes 3.9.0 [20]. Acquired antimicrobial resistance (AR) genes were detected using SSTAR [21]and the ResFinder database [22].
PacBio reads were assembled using HGAP and subsequently polished with Quiver [23]. Known resistance mechanisms were determined as described for Illumina contigs. Clean Illumina reads from all IncA/C2 plasmid replicon-containing isolates were mapped to the core plasmid genome using lyve-SET1.1.4f [24]. Raw sequencing reads, PacBio assemblies, and drug susceptibility data were placed under NCBI BioProject PRJNA302185.
Results:
Case Finding and Case Series
From August 2015 through May 2016, 14 CRE carrying blaVIM-1 were identified in 12 patients from perirectal swabs. No patients had symptomatic infection, suggesting that all positive cultures represented colonization. The epidemic curve by hospital location is shown in Figure 1A. In the first five weeks of the outbreak, VIM-producing isolates represented four CRE species, including two different strains of Klebsiella pneumoniae, but subsequent isolates were exclusively Enterobacter hormaechei (Figure 1B). In total, the most prevalent species was E. hormaechei (n=10; 71%) followed by K. pneumoniae (n=2; 12%), Escherichia coli (n=1; 7%), and Raoultella ornithinolytica (n=1; 7%).
Figure 1.
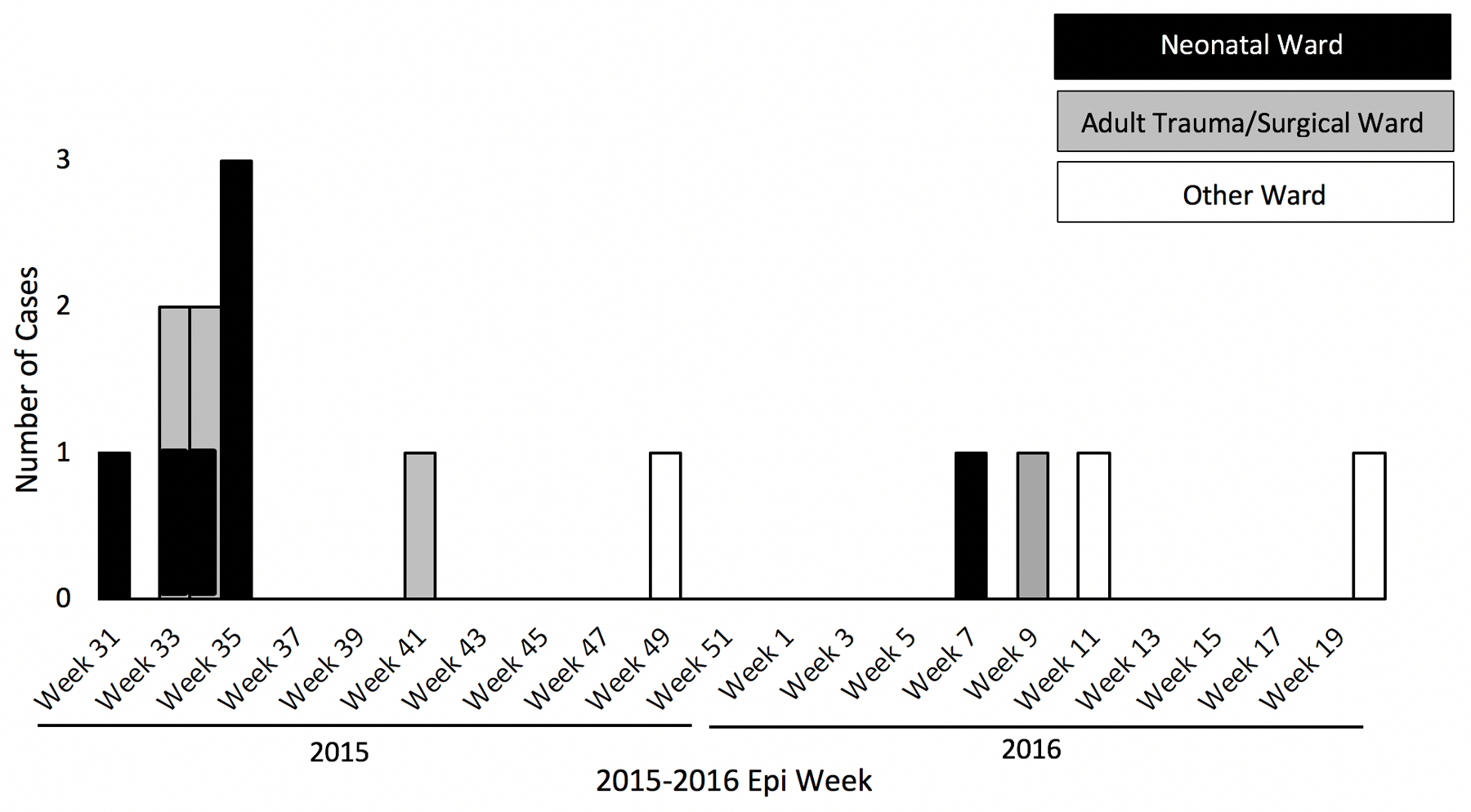
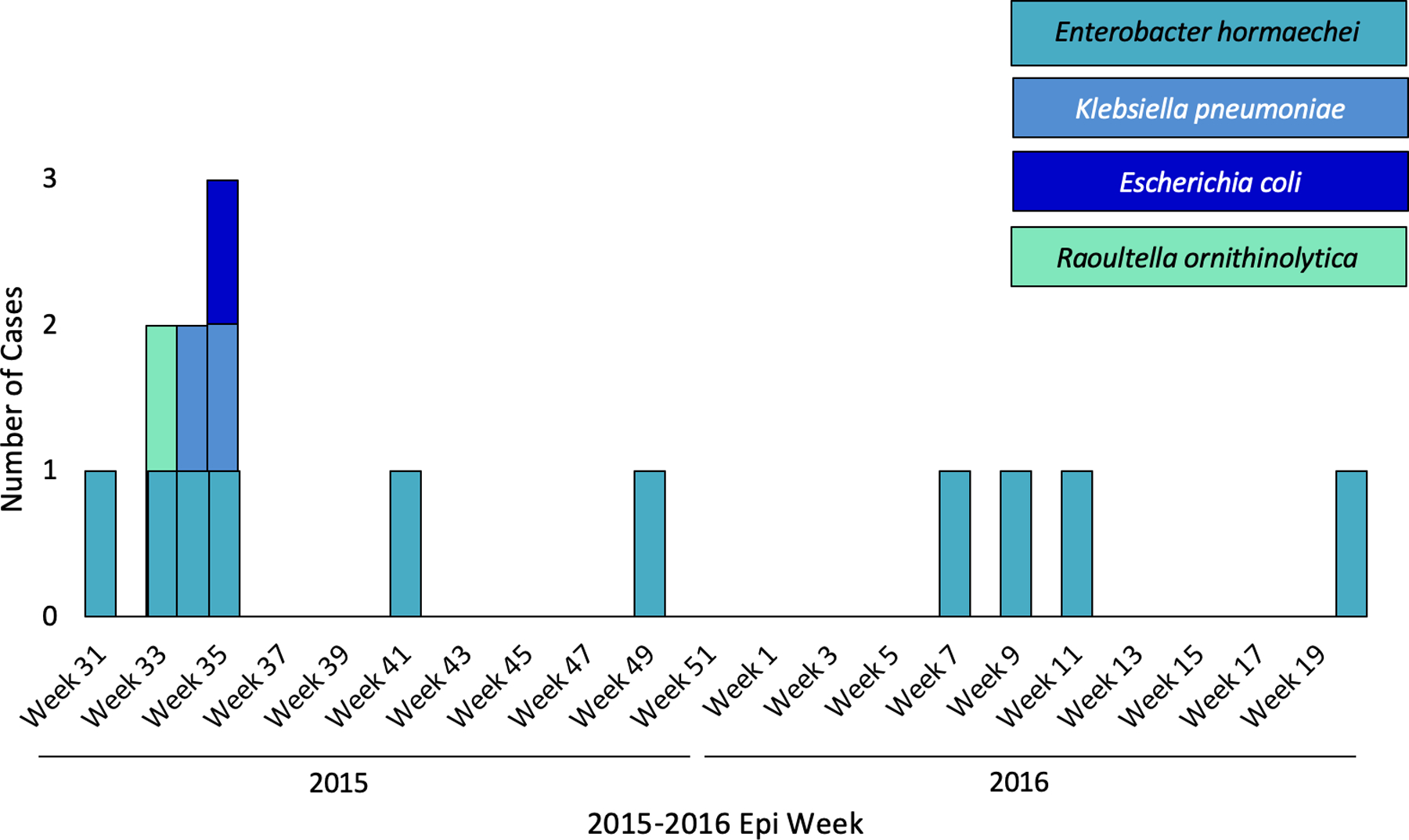
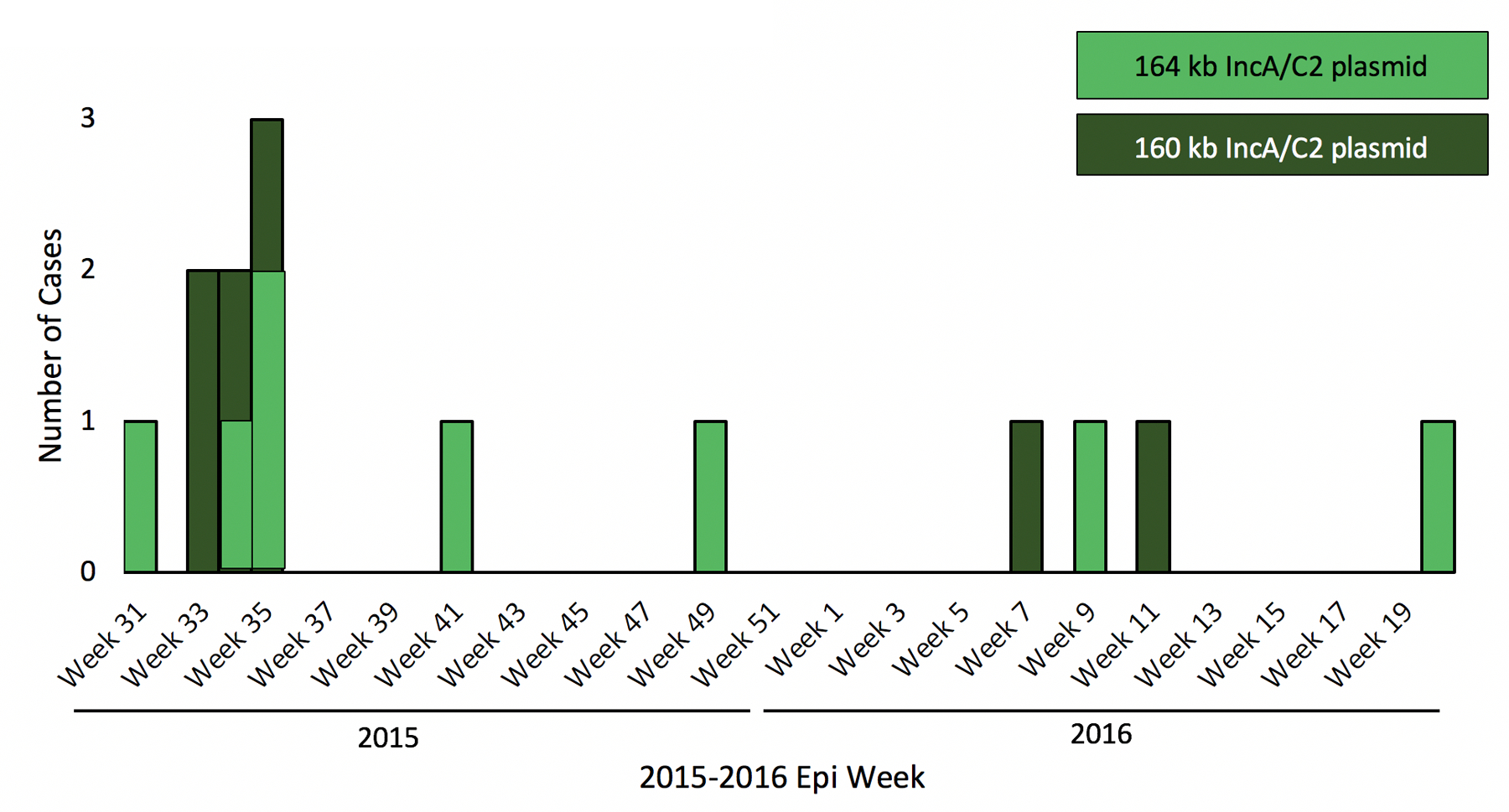
A-C. Epidemic curve of VIM-producing CRE by hospital location, organism species, and plasmid variant.
Two patients were colonized with two different bacterial species from the same culture site. Demographics of the 12 case-patients are shown in Table 1. No patients reported healthcare exposure outside the United States, and chart review did not reveal any known risk factors for acquisition such as prior major healthcare exposure or long term care. Among colonized infants, all five spent time on the neonatal ICU/intermediate care unit (neonatal ward) during their hospitalizations. The neonatal ward is arranged into pods of between two and six neonate beds; all five case-infants spent time in pods that other case-infants once occupied, and two case-infants overlapped temporally in the same pod before positive culture.
Table 1.
Demographic and clinical characteristics of affected patients
| Demographic and Clinical Characteristics | Case Patients (n=12) |
|---|---|
| Male, No. (%) | 7(58%) |
| Infants less than 1 year, No. (%) | 5 (42%) |
| Adults over 18 years, No. (%) | 7 (58%) |
| Age at First Positive Culture | |
| Mean age of infants, days (range) | 29 (9–54) |
| Mean age of adults, years (range) | 52 (41–68) |
| Length of Stay | |
| Median length of stay to first positive culture, days (range) | 16.5 (8–54) |
| Hospital Location | |
| Neonatal ICU or intermediate care ward, No. (%) | 5 (42%) |
| Adult Trauma/Surgical ICU or intermediate care ward, No. (%) | 4 (33%) |
| Other, No. (%) | 3 (25%) |
Four (57%) of seven adult patients spent time on the adult trauma/surgical ICU/intermediate care unit (adult trauma/surgical ward) during their hospitalizations; two spent time in the same adult trauma/surgical ward room, separated by four months. The remaining three adult patients (43%) were never admitted to the adult trauma/surgical ward.
Four referral network facilities in the region were identified as highly connected to the affected hospital and agreed to colonization screening at their facility. Ten high-risk patients in each facility underwent perirectal colonization testing, for a total of 40 patients tested; none were positive for VIM-producing CRE.
Infection Control Observations and Environmental Assessment
Multiple lapses in infection control were observed, including adherence to hand hygiene and use of personal protective equipment (PPE). Lapses were corrected when observed, and systematic changes based on these observations were recommended. Recommendations implemented by the facility included healthcare personnel education, hand hygiene monitoring, increased surveillance, cohorting of affected patients, and increased attention on environmental services practices.
Initial environmental sampling yielded two VIM-producing carbapenem-resistant isolates in the adult trauma/surgical ward. A VIM-1-producing Citrobacter amalonaticus was isolated from a case-patient room sink drain and a likely unrelated VIM-2 (10% amino acid dissimilarity with VIM-1 [25]) producing Pseudomonas putida was isolated from the surface of an environmental services cart on the same ward. No additional VIM-2-producing bacteria were identified during the study.
Characterization of VIM CRE
blaVIM-1 presence was confirmed in 18 isolates, including 14 outbreak samples from 12 patients, three historical patient isolates from 2013, and one environmental specimen. Several early findings in the investigation prompted further analysis with long-read sequencing to retrieve fully closed high-quality plasmid sequences and evaluate alternative mechanisms for the spread of blaVIM-1. These findings included the presence of blaVIM-1 across multiple species and strains, 100% concordance between the presence and absence of blaVIM-1 and the IncA/C2 plasmid replicon marker gene, and a lack of concordance between blaVIM-1 presence and all other replicons (Table S1, Data S1). We observed extensive genetic diversity among all VIM producing species, including 8 and 3 different strain-types of E. hormaechei and K. pneumoniae, respectively (Table S1).
Among the 18 VIM-producing isolates, numerous other resistance mechanisms were detected, including genes conferring resistance to aminoglycosides, sulfonamides, fluoroquinolones, and trimethoprim (Data S1). A fraction of VIM producers harbored a complete CRISPR defense mechanism (n=7; 39%) which could provide immunity against plasmid introduction (Data S1).
For six VIM-positive isolates, we assembled full-length, circular plasmid structures using PacBio SMRT long-read sequencing technology. Computational screening of these plasmid sequences confirmed that blaVIM-1 was located on a plasmid of incompatibility group A/C2. In addition, each IncA/C2 plasmid carried blaVIM-1 on a previously described and widely distributed 4482 bp class 1 In1209 integron that shared 100% sequence similarity with all other In1209 integrons in this data set [26]. The In1209 integron also carried additional resistance genes, aacA7, aadA1 and dfrA1, conferring resistance to aminoglycosides and trimethoprim (Figure 2). Additionally, all six isolates that underwent PacBio sequencing contained numerous plasmids of various incompatibility groups harboring a vast array of antimicrobial resistance genes (Data S2). All further discussion of plasmid characteristics will be limited to these six IncA/C2 plasmids harboring blaVIM-1, unless otherwise stated.
Figure 2.
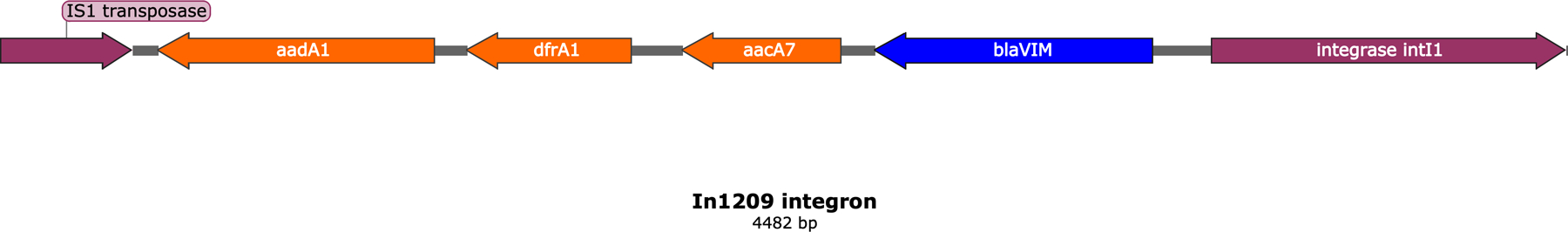
Molecular structure of the In1209 integron identified among all IncA/C2 outbreak plasmids. Resistance genes, except blaVIM, are in orange.
IncA/C2 plasmid classification
Assessment of the rhs gene and other features classified all six IncA/C2 plasmids as type 1 [27]; the most common IncA/C2 group, which often harbors other beta-lactamase genes, including blaCMY and blaNDM. Moreover, these plasmids were part of pMLST group ST1.1, the largest sub-group worldwide, and not previously described to harbor blaVIM (Supplementary results, Table S2). All six IncA/C2 plasmids from this outbreak were most closely related to historical IncA/C2 plasmid pRMH760 from Australia isolated in 1997 (KF976462.2), and plasmid pKPC_CAV1344 isolated in the US in 2010 (CP011622), which contained a blaKPC (Supplementary results, Figure S1).
Two highly related IncA/C2 sub-groups were identified during the outbreak (Figure 1C) through granular plasmid genome characterization (Figure 3). Three of the sequenced plasmids were approximately ~160 Kb and contained a 3366 bp class 1 In27 integron that carried aadA2 (aminoglycoside resistance) and dfrA12 (dihydrofolate reductase, trimethoprim resistance). The remaining three plasmids were slightly larger (~164 Kb) and lacked the 3366 bp In27 integron, but instead harbored a 2197 bp class 1 In7 integron structure carrying aadB (aminoglycoside resistance), which was absent in the first group of plasmids. Both integrons were conserved and shared 100% sequence similarity within their respective plasmid sub-groups. The 164 Kb plasmids also carried a blaTEM-1B beta-lactamase transported by a Tn3 element (5216 bp), which were absent from the 160 Kb plasmids. After aligning all six IncA/C2 plasmid sequences with each other, we were able to fully differentiate the two plasmid sub-groups by presence and absence of transposable elements and their associated antimicrobial resistance genes mentioned above (Figure 4).
Figure 3.
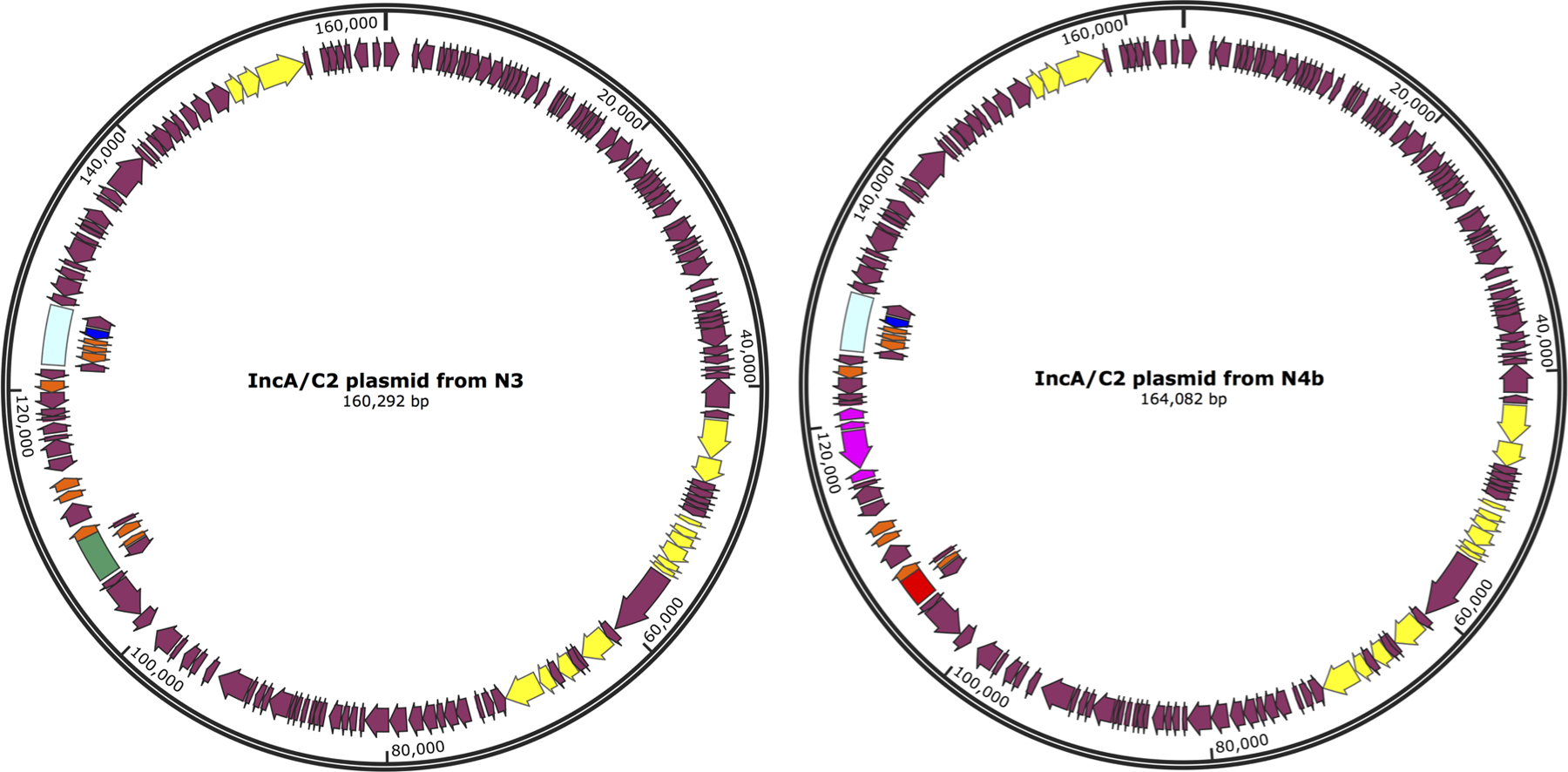
A representative for each sub-group of IncA/C2 plasmid. Genes are denoted as arrows. Conjugal transfer genes are depicted in yellow, the blaVIM gene is in dark blue, the associated In1209 integron is in light blue, the blaTEM-1B gene and associated mobile elements are purple, the In7 integron is in red, and the In27 integron in green.
Figure 4.
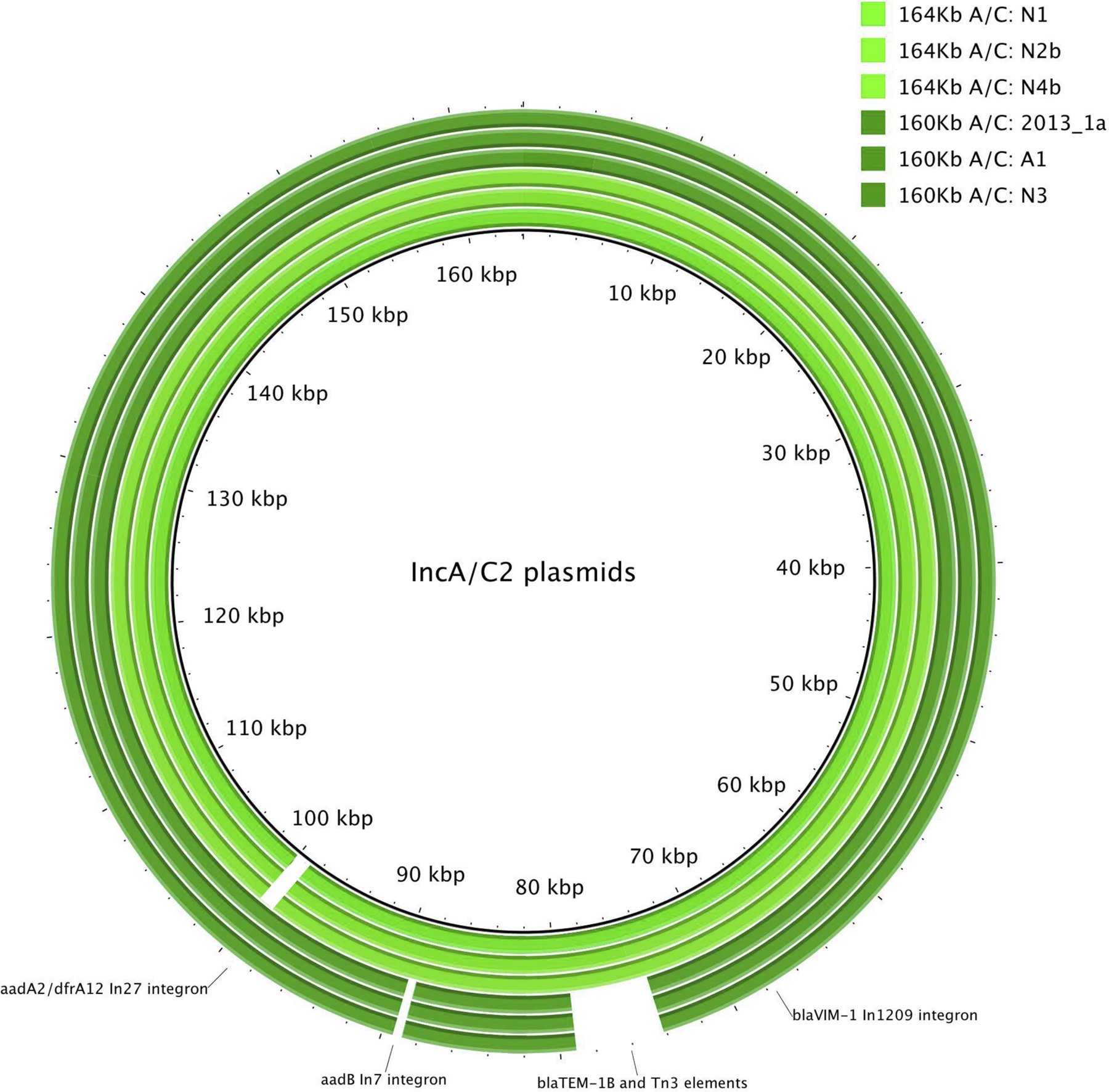
Circular BLAST alignment of all six outbreak IncA/C2 plasmids from PacBio long-read sequencing. White areas indicate absence of a certain genomic region.
Phylogenetic analysis and IncA/C2 conjugation
An estimated 157 Kb genomic region was shared among all six IncA/C2 plasmids (core plasmid genome). After mapping Illumina short reads from all 18 VIM and IncA/C2-positive isolates against this core, the genetic diversity of all plasmids ranged between 0 and 16 SNPs (data not shown). These SNPs grouped all 18 IncA/C2 plasmids in two distinct clades representing the 160Kb- and 164Kb-sized plasmids. No further transmission events were inferred from short-read data.
However, we tested conjugation capacity of both IncA/C2 plasmid variants for all five CRE species involved in this outbreak and observed conjugation efficiencies ranging from 1.3 x 10-2 to 2.1 x 10-5 conjugants/recipient colony forming units (Supplementary results, Table S3).
Discussion:
This investigation represents the largest known outbreak of VIM-producing CRE in the United States to date, comprising a diverse set of bacterial species, and was driven by horizontal plasmid transmission. In accordance with recent trends, we leveraged genomics data for investigation of horizontal plasmid transmission between bacterial species. During the course of the investigation we demonstrated that blaVIM-1, a rare carbapenemase gene among CRE in the US, inserted into a broad host range IncA/C2 plasmid and spread within a tertiary care hospital. Plasmids of incompatibility group A/C2 are strongly associated with resistance to clinically relevant third-generation cephalosporins and carbapenems, and are therefore of great concern to public health [28,29]. Reported healthcare-associated outbreaks are generally caused by a single, clonal strain, however in this case the range of genera and strains of the same species (e.g., 8 strains among 10 E. hormaechei isolates) carrying blaVIM-1 pointed to an alternate path of transmission, namely a promiscuous plasmid. In recent years there has been growing interest in conjugative plasmids due to their importance in the dissemination of antibiotic resistance in pathogenic bacteria [7,30,31].
This outbreak was characterized by two highly related plasmids that drove the spread of antibiotic resistance within a single facility. The IncA/C2 plasmids in this study contained numerous genes conferring antimicrobial resistance. Furthermore, the observed plasmid conjugation efficiencies demonstrated the mobile nature of these IncA/C2 plasmids, even when some recipient cells within this outbreak might contain active CRISPR systems known to ward off transfer of such mobile elements into bacterial cells [32,33]. This combination of multiple resistance mechanisms and the demonstrated mobile nature of this particular IncA/C2 plasmid within and across species barriers, likely resulted in its occurrence among multiple host species.
Our findings in this outbreak investigation provide evidence of in-hospital transmission. It is also possible that additional silent spread occurred, which was not captured in our screening approach. Interventions included instituting enhanced infection control and prevention measures as detailed in the 2015 CDC CRE Toolkit Update [34]. Given the promiscuity of this plasmid and the emerging nature of this resistance mechanism, the emphasis on early aggressive containment was imperative to preventing further spread.
These interventions appear to have halted transmission at the facility and, along with communication of the case-patients’ colonization status at transfer, appear to have limited further spread.
The results of this investigation also highlight the potential role of persistent environmental sources in the spread of CRE, since a VIM-producing Citrobacter carrying an outbreak-associated plasmid was identified from sinks of affected units. Persistent bacteria in biofilms in the premise plumbing could have served as reservoir for plasmid transmission. Although no point source was identified, a possible introduction was through a patient treated at the facility in 2013. Carbapenem-resistant organisms in sink drain biofilms have been noted during outbreaks in the past [35,36], and biofilms provide a permissive environment for plasmid transfer between organisms [37]. Although best methods for eradication of sink drain biofilms are not known, practices like daily cleaning of sinks and surrounding surfaces, and eliminating storage of equipment and medication preparation near sinks can likely decrease transmission risk.
There are several limitations of our investigation. This was not a planned study, but was conducted as part of a public health investigation. Due to this, multiple interventions and evaluations were implemented over an extended time frame until cessation of transmission was documented. In addition, there was no control group. Therefore, it is not possible to definitively determine the effect of our interventions. Point prevalence surveys done outside of the affected facility were limited in scope and the power to detect other VIM-producing CRE colonized or infected patients was small, especially considering that CMS data may not be well suited to describe the movement of neonates across healthcare facilities.
Only six of 19 isolates underwent long-read sequencing; based on those results, it is possible that we missed additional diversity among the IncA/C2 plasmids and that this introduced gaps in our inferred transmission network. For instance, investigation suggests that adults and neonatal patients all shared the same plasmid, but it seems implausible that much cross exposure occurred between these two groups. Additional sampling and long- read sequencing might have elucidated additional routes of transmission that were not identified using our approaches. We mapped short reads onto a long-read plasmid assembly, however this approach may yield misleading results when plasmid plasticity is high [38]. Several integron mobilization events among A/C2 plasmids were identified in a relatively short period of time, therefore we cannot assume that our long-read plasmid assemblies represent a stable structure; this further limits our transmission inference investigation.
Early recognition of this plasmid outbreak by long-read whole genome sequencing represented an opportunity to implement control measures to prevent VIM-producing CRE from further regional spread. Our investigation highlights horizontal spread of antibiotic resistance through mobile genetic elements and identifies a potential threat to efforts to contain antibiotic resistance. Improving our understanding of this mechanism of transmission will strengthen, accelerate, and likely alter public health responses and infection control practices.
Supplementary Material
Acknowledgements:
We thank Kentucky Department for Public Health’s Katie Myatt, Kimberly Daniels, Lynn Roser, Kimberly Porter, Robert Brawley, Douglas Thoroughman, Kraig Humbaugh, and Rachel Zinner. We also thank Centers for Disease Control and Prevention (CDC) staff, including Terri Forster, Hollis Houston, Kristen Allison Perry, Bette Jensen, James Matheson, Kaitlin Tagg, and Alicia Shams. The findings and conclusions are those of the authors and do not necessarily represent the official position of the CDC.
Funding:
This work was supported by, in part, CDC’s investments to combat antibiotic resistance and the Advanced Molecular Detection (AMD) program at CDC. Steven Hancock is supported by the Australian Postgraduate Award.
Footnotes
Potential conflicts of interest: Authors declare no conflicts of interest.
References:
- 1.CDC. Antibiotic resistance threats in the United States, 2019 www.cdc.gov 2019;
- 2.Paterson DL. Resistance in gram-negative bacteria: enterobacteriaceae. The American journal of medicine 2006; 119:S20–8-discussion S62–70. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PA. Sparing carbapenem usage. J Antimicrob Chemoth 2017; 72:2410–2417. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Cliniical Infectious Diseases 2011; 53:60–67. [DOI] [PubMed] [Google Scholar]
- 5.Mathers AJ, Peirano G, Pitout JD. The Role of Epidemic Resistance Plasmids and International High-Risk Clones in the Spread of Multidrug-Resistant Enterobacteriaceae. Clinical Microbiology Reviews 2015; 28:565–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammerum AM, Hansen F, Nielsen H, et al. Use of WGS data for investigation of a long-term NDM-1-producing Citrobacter freundii outbreak and secondary in vivo spread of blaNDM-1 to Escherichia coli, Klebsiella pneumoniae and Klebsiella oxytoca. J Antimicrob Chemoth 2016; 71:3117–3124. [DOI] [PubMed] [Google Scholar]
- 7.Sheppard AE, Stoesser N, Wilson DJ, et al. Nested Russian Doll-Like Genetic Mobility Drives Rapid Dissemination of the Carbapenem Resistance Gene blaKPC. Antimicrobial Agents and Chemotherapy 2016; 60:3767–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrobial Agents and Chemotherapy 2009; 53:5046–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein L, Hunter JC, Arwady AM, et al. New Delhi Metallo-β-Lactamase–Producing Carbapenem-Resistant Escherichia coliAssociated With Exposure to Duodenoscopes. JAMA 2014; 312:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauretti L, Riccio M, Mazzariol A, et al. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrobial Agents and Chemotherapy 1999; 43:1584–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falgenhauer L, Ghosh H, Guerra B, et al. Comparative genome analysis of IncHI2 VIM-1 carbapenemase- encoding plasmids of Escherichia coli and Salmonella enterica isolated from a livestock farm in Germany. Veterinary microbiology 2017; 200:114–117. [DOI] [PubMed] [Google Scholar]
- 12.Miriagou V, Papagiannitsis C, Kotsakis S, et al. Sequence of pNL194, a 79.3-kilobase IncN plasmid carrying the blaVIM-1 metallo-beta-lactamase gene in Klebsiella pneumoniae. Antimicrobial Agents and Chemotherapy 2010; 54:4497–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papagiannitsis CC, Dolejska M, Izdebski R, et al. Characterisation of IncA/C2 plasmids carrying an In416-like integron with the blaVIM-19 gene from Klebsiella pneumoniae ST383 of Greek origin. International Journal of Antimicrobial Agents 2016; 47:158–162. [DOI] [PubMed] [Google Scholar]
- 14.Ruppe E, Armand-Lefevre L, Estellat C, et al. High Rate of Acquisition but Short Duration of Carriage of Multidrug-Resistant Enterobacteriaceae After Travel to the Tropics. Cliniical Infectious Diseases 2015; 61:593–600. [DOI] [PubMed] [Google Scholar]
- 15.Moland E, Hong S, Cleary T, Morris M, Hanson N, Thomson K. U.S. isolate of Klebsiella pneumoniae (KP) Producing VIM Metallo-β-lactamase (MBL) and SHV-5-like ESBL 46th Interscience Conference on Antimicrobial Agents and Chemotherapy 2006; [Google Scholar]
- 16.CDC. Update: detection of a verona integron-encoded metallo-beta-lactamase in Klebsiella pneumoniae --- United States, 2010. MMWR Morbidity and mortality weekly report 2010; 59:1212. [PubMed] [Google Scholar]
- 17.Yaffee AQ, Roser L, Daniels K, et al. Notes from the Field: Verona Integron-Encoded Metallo-Beta-Lactamase- Producing Carbapenem-Resistant Enterobacteriaceae in a Neonatal and Adult Intensive Care Unit--Kentucky, 2015. MMWR Morbidity and mortality weekly report 2016; 65:190. [DOI] [PubMed] [Google Scholar]
- 18.ams A, Rose LJ, Edwards JR, et al. Assessment of the Overall and Multidrug-Resistant Organism Bioburden on Environmental Surfaces in Healthcare Facilities. Infection control and hospital epidemiology 2016; 37:1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasheed KJ, Kitchel B, Zhu W, et al. New Delhi Metallo-β-Lactamase–producing Enterobacteriaceae, United States. Emerging Infectious Diseases 2013; 19:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single- cell sequencing. Journal of computational biology : a journal of computational molecular cell biology 2012; 19:455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Man TJ, Limbago BM. SSTAR, a Stand-Alone Easy-To-Use Antimicrobial Resistance Gene Predictor. mSphere 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zankari E, Hasman H, Cosentino S, et al. Identification of acquired antimicrobial resistance genes. Journal of Antimicrobial Chemotherapy 2012; 67:2640–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin C-S, Alexander DH, Marks P, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nature Methods 2013; 10:563–569. [DOI] [PubMed] [Google Scholar]
- 24.Katz LS, Griswold T, Williams-Newkirk AJ, et al. A Comparative Analysis of the Lyve-SET Phylogenomics Pipeline for Genomic Epidemiology of Foodborne Pathogens. Frontiers in Microbiology 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirel L, Naas T, Nicolas D, et al. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrobial Agents and Chemotherapy 2000; 44:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson GK, Snyder JW, McElheny CL, Thomson KS, Doi Y. Coproduction of KPC-18 and VIM-1 Carbapenemases by <span class="named-content genus-species" id="named-content- 1">Enterobacter cloacae</span>: Implications for Newer β-Lactam–β-Lactamase Inhibitor Combinations. J Clin Microbiol 2016; 54:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harmer CJ, Hall RM. The A to Z of A/C plasmids. Plasmid 2015; 80:63–82. [DOI] [PubMed] [Google Scholar]
- 28.Carattoli A, Villa L, Poirel L, Bonnin RA, Nordmann P. Evolution of IncA/C blaCMY-(2)-carrying plasmids by acquisition of the blaNDM-(1) carbapenemase gene. Antimicrobial Agents and Chemotherapy 2012; 56:783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carattoli A. Plasmids and the spread of resistance. International journal of medical microbiology : IJMM 2013; 303:298–304. [DOI] [PubMed] [Google Scholar]
- 30.Conlan S, Thomas PJ, Deming C, et al. Single-molecule sequencing to track plasmid diversity of hospital- associated carbapenemase-producing Enterobacteriaceae. Science Translational Medicine 2014; 6:254ra126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khong W, Marimuthu K, Teo J, et al. Tracking inter-institutional spread of NDM and identification of a novel NDM-positive plasmid, pSg1-NDM, using next-generation sequencing approaches. The Journal of antimicrobial chemotherapy 2016; 71:3081–3089. [DOI] [PubMed] [Google Scholar]
- 32.Stern A, Keren L, Wurtzel O, Amitai G, Sorek R. Self-targeting by CRISPR: gene regulation or autoimmunity? Trends in Genetics 2010; 26:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doron S, Melamed S, Ofir G, et al. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 2018; 359:eaar4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CDC. 2015 CRE Toolkit—Guidance for Control of Carbapenem-resistant Enterobacteriaceae (CRE) www.cdc.gov 2015;
- 35.Wendel AF, Kolbe-Busch S, Ressina S, et al. Detection and termination of an extended low-frequency hospital outbreak of GIM-1-producing Pseudomonas aeruginosa ST111 in Germany. AJIC: American Journal of Infection Control 2015; 43:635–639. [DOI] [PubMed] [Google Scholar]
- 36.Mathers AJ, Vegesana K, Mesner I, et al. Intensive Care Unit Wastewater Interventions to Prevent Transmission of Multi-species Klebsiella pneumoniae Carbapenemase (KPC) Producing Organisms. Clinical Infectious Diseases 2018; [DOI] [PubMed]
- 37.Cook LC, nny G. The influence of biofilms in the biology of plasmids. Microbiology spectrum 2:0012–0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orlek A, Stoesser N, Anjum MF, et al. Plasmid Classification in an Era of Whole-Genome Sequencing: Application in Studies of Antibiotic Resistance Epidemiology. Front Microbiol 2017; 8:182–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


