Abstract
Aims
The impact of long-term endurance sport participation (on top of a healthy lifestyle) on coronary atherosclerosis and acute cardiac events remains controversial.
Methods and results
The Master@Heart study is a well-balanced prospective observational cohort study. Overall, 191 lifelong master endurance athletes, 191 late-onset athletes (endurance sports initiation after 30 years of age), and 176 healthy non-athletes, all male with a low cardiovascular risk profile, were included. Peak oxygen uptake quantified fitness. The primary endpoint was the prevalence of coronary plaques (calcified, mixed, and non-calcified) on computed tomography coronary angiography. Analyses were corrected for multiple cardiovascular risk factors. The median age was 55 (50–60) years in all groups. Lifelong and late-onset athletes had higher peak oxygen uptake than non-athletes [159 (143–177) vs. 155 (138–169) vs. 122 (108–138) % predicted]. Lifelong endurance sports was associated with having ≥1 coronary plaque [odds ratio (OR) 1.86, 95% confidence interval (CI) 1.17–2.94], ≥ 1 proximal plaque (OR 1.96, 95% CI 1.24–3.11), ≥ 1 calcified plaques (OR 1.58, 95% CI 1.01–2.49), ≥ 1 calcified proximal plaque (OR 2.07, 95% CI 1.28–3.35), ≥ 1 non-calcified plaque (OR 1.95, 95% CI 1.12–3.40), ≥ 1 non-calcified proximal plaque (OR 2.80, 95% CI 1.39–5.65), and ≥1 mixed plaque (OR 1.78, 95% CI 1.06–2.99) as compared to a healthy non-athletic lifestyle.
Conclusion
Lifelong endurance sport participation is not associated with a more favourable coronary plaque composition compared to a healthy lifestyle. Lifelong endurance athletes had more coronary plaques, including more non-calcified plaques in proximal segments, than fit and healthy individuals with a similarly low cardiovascular risk profile. Longitudinal research is needed to reconcile these findings with the risk of cardiovascular events at the higher end of the endurance exercise spectrum.
Keywords: Athlete’s heart, Exercise, Coronary artery disease, Computed tomography coronary angiography
Structured Graphical Abstract
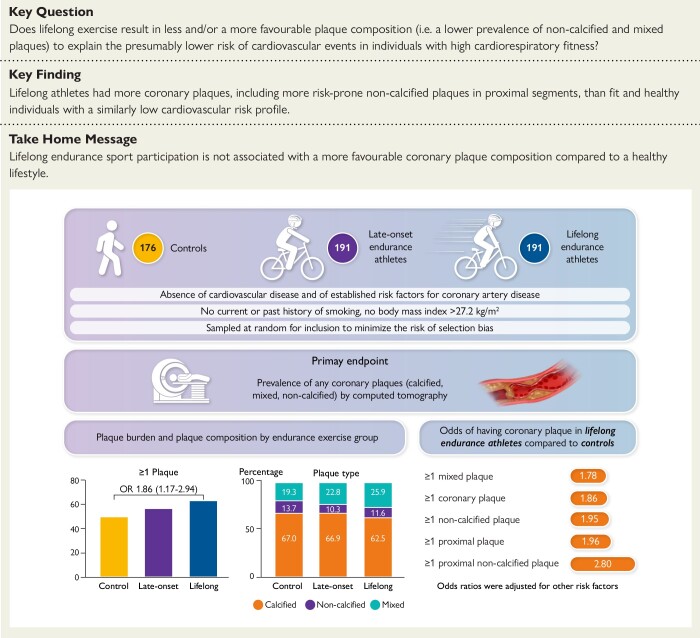
Structured Graphical Abstract.
The Master@Heart study compared the prevalence of calcified, mixed, and non-calcified coronary plaques by computed tomography between 176 controls, 191 late-onset endurance athletes, and 191 lifelong endurance athletes, in the absence of established risk factors for coronary artery disease. Lifelong endurance athletes had the highest coronary plaque burden regardless of plaque types.
See the editorial comment for this article ‘Fit to a fault? The paradox of coronary artery disease in veteran athletes’, by S. Fyyaz and M. Papadakis, https://doi.org/10.1093/eurheartj/ehad271.
Introduction
The dose–response relationship between long-term intensive endurance exercise and coronary heart disease has intrigued investigators for decades. Early studies indicated that regular endurance sport participation provides relative immunity from ischaemic heart disease.1 Yet, more recent studies reported an increased prevalence of coronary atherosclerotic plaques amongst highly trained athletes in comparison to healthy non-athletes.2–4 These findings spurred debate about a potential selection bias in these series, age-related differences and prior presence of cardiovascular risk factors such as smoking.3–5 Furthermore, these studies emphasized a relative difference in plaque composition with a higher likelihood of more stable calcified plaques in athletes as compared to non-athletes in whom plaque type was most frequently mixed. However, data on the absolute prevalence of calcified and non-calcified coronary plaques in athletes as compared to non-athletes are lacking. Hence, it is possible that endurance athletes may have a similar or even greater amount of non-calcified and mixed plaques just because the overall number of plaques is higher.
A better understanding of the upper range of the dose–response relationship between exercise and coronary artery disease is desirable given the popularity of intensive endurance sports in modern society. Regular exercise improves blood pressure control and lipid profiles, reduces the incidence of diabetes and myocardial infarction, and increases life expectancy.6–9 Increased cardiorespiratory fitness is associated with a progressive reduction of cardiovascular events, which would seem to be at odds with the aforementioned link between lifelong exercise load and increased coronary atherosclerosis.
The Master@Heart study aimed to investigate the apparent paradox of increased coronary atherosclerosis amongst highly trained endurance athletes despite having fewer cardiovascular events. We hypothesized that lifelong endurance exercise, and to a lesser degree late-onset training, would be associated with a lower prevalence of non-calcified and mixed plaques as compared to an active, healthy lifestyle without regular endurance training.
Methods
The rationale, design, and methodology of the Master@Heart study have been described previously and can be summarized as follows.10
Study design
The Master@Heart (Master Athlete’s Heart) study was a multicentre (University Hospitals Leuven, University Hospital Antwerp, and Jessa Hospital Hasselt) prospective cohort study, funded by the Fund for Scientific Research, Flanders (T003717N). ClinicalTrials.gov Identifier NCT03711539. The primary endpoint of the current manuscript was investigated in a cross-sectional design.
Study population
A dedicated web portal (www.masteratheart.be) informed potential study participants about the study and provided an online screening questionnaire to assess the eligibility of study candidates. The web portal was advertised to the wide community through media advertisements (e.g. traditional broadcasting, social media, and popular athletic magazines). The inclusion criteria for all participants were male sex and age between 45 and 70 years. Athletes were defined as follows: questionnaire reported engagement in cycling ≥8 h or running ≥ 6 h per week, or triathlon (combination of swimming, cycling, and running) ≥ 8 h per week for at least 6 months prior to baseline. Lifelong and late-onset athletes started regular endurance exercise training at <30 years and >30 years of age, respectively. Non-athletes engaged ≤ 3 h per week in physical activity without prior exposure to regular endurance exercise. The metabolic equivalent of task (MET) minutes per week was calculated as a measure of exercise volumes obtained by multiplying the MET score for the specific sport by the reported exercise volume (minutes/week).11
Exclusion criteria were a medical history of cardiovascular disease, history of established risk factors for coronary artery disease (diabetes mellitus, hypercholesterolaemia, or arterial hypertension), current or past history of smoking, a body mass index >27.2 kg/m², and a known allergy to iodine contrast agents.
From all eligible subjects, 605 were sampled at random for inclusion to minimize the risk of selection bias. Sampling was however stratified by current age (45–53, 54–62, and 63–70 years). As the prevalence of high-level endurance athletes decreases with age, a participant distribution of 3/7 aged 45–53 years, 3/7 aged 54–62 years, and 1/7 aged 63–70 years was applied.
In addition, sampling was stratified by the age at which endurance training was started. Lifelong athletes were subdivided into <20 and 20–30 years of age at which endurance training was started. Late-onset athletes were subdivided into 31–40 and >40 years.
Dual-energy X-ray absorptiometry
A dual-energy X-ray absorptiometry scan (Discovery W, Hologic, Bedford, Massachusetts, USA – GE Lunar Prodigy Advance GE Healthcare, Horton Norway) was performed to measure body fat percentage.
Cardiopulmonary exercise testing
As a measure of aerobic capacity, peak oxygen uptake (VO2peak) was determined using a continuous bicycle stress test. The respiratory gas exchange was analysed using a breath-by-breath open-circuit spirometry system. Peak oxygen uptake was be determined as the highest 30 s of oxygen uptake.
Computed tomography coronary angiography
Computed tomography coronary angiography (CTCA) was acquired using a 128-slice dual-source CT scanner (Siemens Somatom Force - Siemens Healthineers, Forchheim, Germany), a 256-slice CT scanner (GE Revolution - GE Healthcare, Milwaukee, Wisconsin), or a 320-slice CT scanner (Aquilion ONE ViSION - Canon Medical Systems, Otawara, Japan). We administered esmolol intravenously when necessary to achieve target heart rates of <65 bpm. All subjects received 0.4 mg sublingual nitroglycerine 2 min before scanning. First, a non-enhanced ECG-synchronized scan was taken to quantify coronary calcium rendering the Agatston coronary artery calcium (CAC) score. Secondly, an ECG-triggered CTCA was acquired after the intravenous injection of iodinated contrast medium in an antecubital vein followed by a saline chaser.
All coronary atherosclerotic lesions were analysed using Syngo.Via (Siemens Healthineers, Forchheim, Germany) or GE Advanced Workstation (GE Healthcare, Milwaukee, Wisconsin) software. Computed tomography coronary angiography interpreters were blinded to the athletic background of the study participants and to the other study examinations. All coronary lesions were interpreted following the 2016 SCCT guidelines for their location, stenosis grade, composition (calcified, mixed, and non-calcified) as well as their vulnerability (positive remodeling, low-attenuation plaque, spotty calcification, and the napkin-ring sign).12,13 A density of >130 HU defined calcified areas. Calcified plaques were entirely composed of calcified areas. Mixed plaques were composed of both calcified and non-calcified areas. Non-calcified plaques were entirely composed of non-calcified areas. A proximal plaque was defined as a plaque in coronary segments 1 (right coronary artery), 5 (left main), 6 (left anterior descending), and 11 (circumflex). In addition, we calculated the segment stenosis score (SSS) and segment-involvement score (SIS) to assess the overall plaque burden. The SSS was calculated as the sum of the luminal stenosis score (0–3; 0%, < 50%, 50%–70%, > 70%) of each of the 15 coronary segments (minimum 0; maximum 45). The SIS was calculated as the sum of coronary segments with plaques irrespective of the degree of luminal stenosis (minimum = 0; maximum = 15).14 The SSS and SIS have demonstrated clinical validity in detecting major adverse cardiac events in multiple populations.15–19 Vulnerable plaques were defined by at least two high-risk plaque features: low attenuation, spotty calcification, positive remodelling, or the napkin sign.
Endpoints and hypothesis
The primary endpoint was the prevalence of coronary plaques (calcified, mixed, and non-calcified plaques) on CTCA assessed cross-sectionally at baseline. We hypothesized that more than late-onset training, lifelong endurance exercise would be associated with a lower prevalence of non-calcified plaques than non-athletes.
Statistical analysis
Assuming and incidence of mixed plaques of 35% in subjects with an exercise age of 0% and 22% in subjects with an exercise age of 25 years, a sample size of 486 subject offers a statistical power of 80% with a 5% probability of Type 1 error.3,4,10 The sample size calculation was based on the likelihood ratio χ2 test. Exercise age was assumed to be uniformly distributed between 0 and 40 years. A univariate logistic regression assessed the association between exercise age and the probability of mixed plaques. It was assumed that exercise can be assessed in the logistic regression as a linear, untransformed variable, i.e. log  (p/(1−p)) = α+β×exercise age. Statistical testing is two-sided and assessed at a significance level of 5%. PROC POWER in SAS V.9.4 (SAS/STAT V.14.1) was used to calculate the sample size for a univariate logistic regression with exercise age as the only covariate. A total sample size of at least 600 subjects (200 per group) gave a margin of up to 20% for exclusions due to errors in the online questions or uninterpretable data.
(p/(1−p)) = α+β×exercise age. Statistical testing is two-sided and assessed at a significance level of 5%. PROC POWER in SAS V.9.4 (SAS/STAT V.14.1) was used to calculate the sample size for a univariate logistic regression with exercise age as the only covariate. A total sample size of at least 600 subjects (200 per group) gave a margin of up to 20% for exclusions due to errors in the online questions or uninterpretable data.
Data were analysed using SPSS Statistics version 28 (IBM Corporation, Amonk, NY, USA). Normality was tested using the Shapiro–Wilk test. Continuous variables are presented as means (± standard deviation) or as medians (interquartile range) and categorical variables as percentages. For baseline characteristics, the between-group differences for continuous variables were assessed using a one-way analysis of variance or a Kruskal–Wallis test as appropriate with a Bonferroni post hoc correction for multiple comparisons. The χ2 test was used to assess categorical variables. For CTCA findings, multiple linear and logistic regression analysis that adjusted for age, body mass index (BMI), systolic and diastolic blood pressure, total cholesterol, low-density lipoprotein (LDL) cholesterol, glycated haemoglobin (HbA1c), and family history of coronary artery disease (defined as a first degree relative with myocardial infarction at the age of <50 years in males and/or <60 years in female relatives) was performed. We calculated the between-group difference and odds ratios (OR) with 95% confidence interval (CI) for continuous and categorical variables respectively. The widths of CIs have not been adjusted for multiplicity, and therefore the intervals should not be used to infer definitive exercise effects on primary outcomes. There were no missing data from population characteristics, exercise testing, and CTCA in any group. A P-value of <0.05 was considered statistically significant.
Results
Study population and baseline characteristics
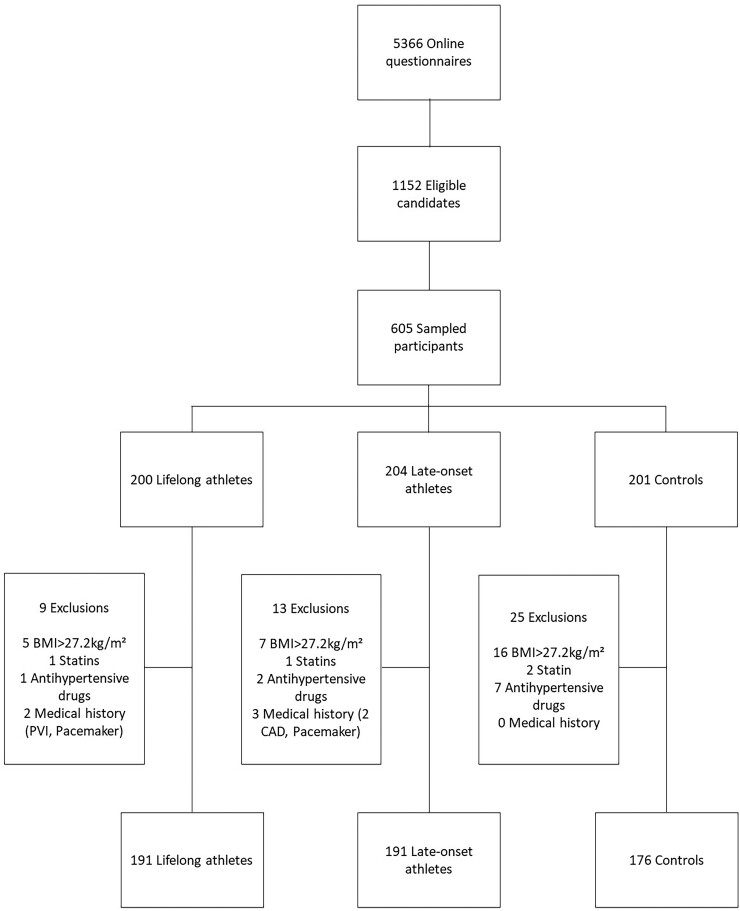
Out of 1152 eligible candidates, 605 individuals were sampled for further testing. On the day of testing, 47 participants were excluded. Twenty-eight individuals had a BMI >27.2 kg/m², and 10 were on treatment with antihypertensive drugs, four were on treatment with a statin, and five had a medical history of cardiovascular disease. Subsequent analysis was performed on 558 participants comprising 191 lifelong athletes, 191 late-onset athletes, and 176 non-athletic controls. Figure 1 shows the study inclusion and exclusion flow chart.
Figure 1.
Inclusion and exclusion flow chart of the Master@Heart study: the inclusion criteria for all participants were male sex and age between 45 and 70 years. Athletes were defined as follows: questionnaire reported engagement in cycling ≥8 h or running ≥ 6 h per week, or triathlon (combination of swimming, cycling, and running) ≥ 8 h per week for at least 6 months prior to baseline. Non-athletes were defined by engagement in ≤ 3 h per week of physical activity without prior exposure to regular endurance exercise. Exclusion criteria were a medical history of cardiovascular disease, current or past history of smoking, use of any antidiabetic drugs, statins or antihypertensive drugs, a BMI >27.2 kg/m², and a known allergy for iodine contrast agents.
Table 1 represents the baseline population characteristics. The mean age of all participants was 56 ± 6 years and was similar amongst all groups. All three groups did not differ in height, but controls had a higher body weight leading to a higher BMI than lifelong and late-onset athletes (24.0 vs. 23.2 vs. 23.5 kg/m²). Controls had a higher body fat percentage than lifelong and late-onset athletes (19.4% vs. 13.9% vs. 15%). Systolic blood pressure, diastolic blood pressure, total cholesterol, LDL cholesterol, and HbA1c were similar in all groups. The family incidence of coronary artery disease was 6.3% in lifelong athletes, 6.8% in late-onset athletes, and 6.3% in controls. Lifelong and late-onset athletes were mostly cyclists with smaller groups engaged in running or a combination of running, cycling, and swimming. Seventy-seven percent of controls performed exercise ≤3 h/week, mostly running and non-endurance sports. The remaining 23% of controls did not perform any type of regular exercise. There was no difference in the hours per week of endurance exercise between lifelong and late-onset athletes, but both athlete groups performed more hours than controls [11 h/week (10–14) vs. 10 h/week (8–12) vs. 1 h/week (0–3)]. Lifelong and late-onset had a similar VO2peak which was significantly higher than in controls [VO2peak 48 mL/min/kg (43–53) vs. 46 mL/min/kg (41–51) vs. 37 mL/min/kg (32–42); % predicted VO2peak 159% (143–177) vs. 155% (138–169) vs. 122% (108–138)].
Table 1.
Baseline characteristics: the baseline demographic and exercise characteristics of lifelong athletes, late-onset athletes, and non-athletic controls of the Master@Heart study
| Lifelong (n = 191) | Late-onset (n = 191) | Controls (n = 176) | P-value | |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) | 56 (51–61) | 55 (51–60) | 55 (50–61) | 0.636 |
| Weight (kg) | 75 ± 8 | 75 ± 8 | 77 ± 8 | 0.018 |
| Height (cm) | 179 (174–184) | 179 (174–183) | 179 (175–184) | 0.708 |
| BMI (kg/m²) | 23.2 (22.0–24.5) | 23.5 (22.0–24.9) | 24.0 (22.6–25.6) | 0.001 |
| BSA (m²) | 1.93 ± 0.13 | 1.93 ± 0.12 | 1.96 ± 0.13 | 0.092 |
| Bodyfat% | 13.9 (10.2–17.2) | 15 (10.6–18.9) | 19.4 (14.9–23.4) | <0.001 |
| Heart rate (bpm) | 54 (49–59) | 56 (50–62) | 60 (54–70) | <0.001 |
| Systolic BP (mmHg) | 122 (114–131) | 122 (115–130) | 123 (115–131) | 0.651 |
| Diastolic BP (mmHg) | 75 (70–80) | 76 (70–80) | 76 (70–80) | 0.469 |
| Total cholesterol (mg/dL) | 193 (172–212) | 196 (177–215) | 195 (171–217) | 0.733 |
| LDL cholesterol (mg/dL) | 121 (105–139) | 124 (105–143) | 131 (108–150) | 0.056 |
| HDL cholesterol (mg/dL) | 64 (55–74) | 64 (56–76) | 58 (49–66) | <0.001 |
| HbA1c (%) | 5.4 (5.3–5.6) | 5.4 (5.3–5.6) | 5.4 (5.2–5.6) | 0.215 |
| Family history of CAD – n(%) | 12 (6.3) | 13 (6.8) | 11 (6.3) | 0.970 |
| Exercise characteristics | ||||
| Sport – n (%) | ||||
| Cycling | 99 (51.8) | 113 (59.2) | 15 (8.5) | <0.001 |
| Running | 17 (8.9) | 35 (18.3) | 84 (47.7) | |
| Cycling and running | 48 (25.1) | 26 (13.6) | 5 (2.8) | |
| Duathlon | 5 (2.7) | 0 (0) | 0 (0) | |
| Triathlon | 22 (11.5) | 17 (8.9) | 0 (0) | |
| Non-endurance | 0 (0) | 0 (0) | 32 (18.2) | |
| None | 0 (0) | 0 (0) | 40 (22.8) | |
| Hours exercise per week | 11 (10–14) | 10 (8–12) | 1 (0–3) | <0.001 |
| Years of endurance exercise | 36 (29–44) | 14 (9–20) | 5 (0–26) | <0.001 |
| MET-min/week | 5070 (4500–6240) | 4884 (3720–5790) | 888 (420–1274) | <0.001 |
| VO2peak (mL/min/kg) | 48 (43–53)* | 46 (41–51) | 37 (32–42) | <0.001 |
| Percentage predicted VO2peak (%) | 159 (143–177) | 155 (138–169) | 122 (108–138) | <0.001 |
| VE/VCO2 slope | 30 (28–33) | 30 (27–33) | 31 (27–35) | 0.164 |
BMI, body mass index; BSA, body surface area; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CAD, coronary artery disease; VO2peak, oxygen uptake at peak exercise.
Coronary artery computed tomography
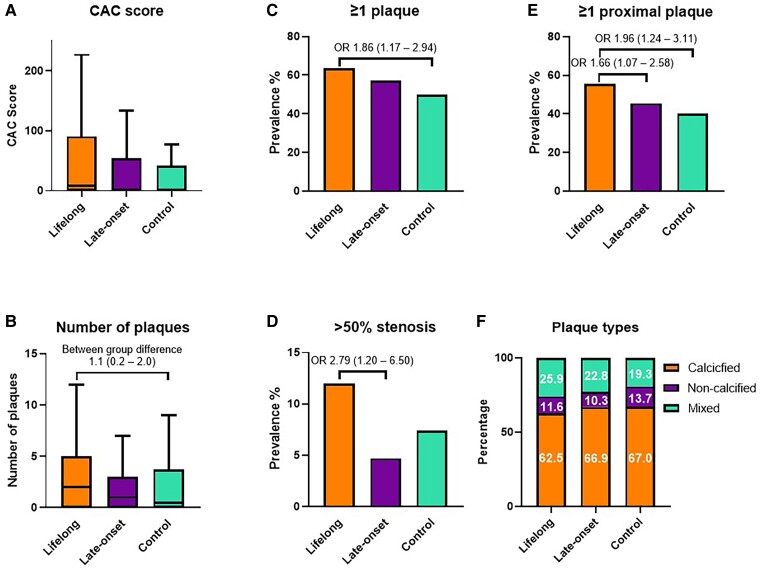
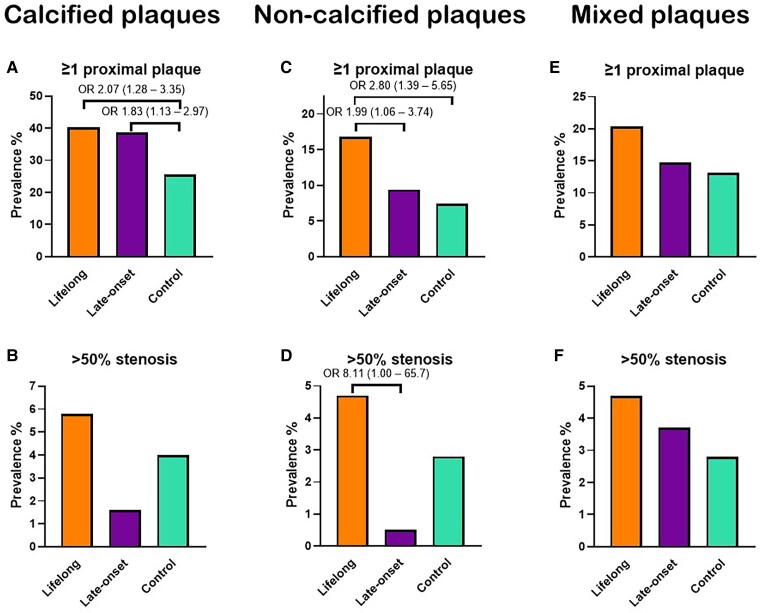
The results from CTCA are shown in Table 2. All individuals were in sinus rhythm at the time of the examination. The median number of plaques per individual [lifelong 2 (0–5) vs. late-onset 1 (0–3) vs. control 0 (0–4)] and the average CAC scores [8.5 (0–90.9) vs. 1.3 (0–54) vs. 0 (0–41.6)] (Figure 2) was similar between groups but lifelong athletes had a higher CAC percentile than controls (between-group difference 9.6 and 95% CI 2.4–16.8). The overall coronary plaque burden assessed by SSS and SSI was higher in lifelong athletes than controls (between-group difference 0.86 and 95% CI 0.22–1.51 and between-group difference 0.65 and 95% CI 0.20–1.10, respectively) (Table 2). When considering plaque types, the distribution of calcified (62.3% vs. 68.4% vs. 67%), non-calcified (11.8% vs. 9.8% vs. 13.7%), and mixed (25.9% vs. 21.8% vs. 19.3%) plaques was similar. In comparison to controls, lifelong endurance sport participation was associated with having ≥1 coronary plaque (OR 1.86, 95% CI 1.17–2.94), ≥ 1 proximal plaque (OR 1.96, 95% CI 1.24–3.11) ≥ 1 calcified plaques (OR 1.58, 95% CI 1.01–2.49), ≥ 1 calcified proximal plaque (OR 2.07, 95% CI 1.28–3.35), ≥ 1 non-calcified plaque (OR 1.95, 95% CI 1.12–3.40), ≥ 1 non-calcified proximal plaque (OR 2.80, 95% CI 1.39–5.65), and ≥1 mixed plaque (OR 1.78, 95% CI 1.06–2.99). In comparison to late-onset athletes, a ≥ 50% stenosis in any coronary segment (OR 2.79, 95% CI 1.20–6.50) and ≥50% stenosis in a proximal segment (OR 5.92, 95% CI 1.22–28.80) were more prevalent in lifelong athletes (Figure 2, Figure 3, and Table 2). Vulnerable plaques as defined by the presence of ≥2 high risk features were uncommon in all groups but a lifelong athletic lifestyle was associated with a lower prevalence (OR 0.11, 95% CI 0.01–0.98) (Table 2).
Table 2.
Computed tomography coronary angiography: the findings on computed tomography coronary angiography in lifelong athletes, late-onset athletes, and non-athletic controls of the Master@Heart study
| Lifelong (n = 191) | Late-onset (n = 191) | Control (n = 176) | Adjusted difference lifelong vs. control (95% CI)a | Adjusted difference late-onset vs. control (95% CI)a | Adjusted difference lifelong vs. late-onset (95% CI)a | |
|---|---|---|---|---|---|---|
| CAC score | 8.5 (0–90.9) | 1.3 (0–54) | 0 (0–41.6) | 32.1 (−14.22–78.4)b | 1.5 (−44.8–47.9) b | 30.5 (−14.2–75.3) b |
| CAC score percentile | 44 (0–71) | 23 (0–63) | 0 (0–62) | 9.6 (2.4–16.8) b | 2.8 (−4.4–10.0)b | 6.8 (−0.15–13.7)b |
| CAC > 0 – n(%) | 109 (57.1) | 100 (52.4) | 80 (45.5) | 1.67 (1.06–2.63) | 1.31 (0.84–2.63) | 1.27 (0.82–1.97) |
| CAC > 10 – n(%) | 94 (49.2) | 72 (37.7) | 59 (33.5) | 2.11 (1.32–3.37) | 1.20 (0.75–1.93) | 1.75 (1.12–2.73) |
| CAC > 100 – n(%) | 44 (23) | 31 (16.2) | 26 (14.8) | 1.83 (1.02–3.27) | 1.11 (0.61–2.04) | 1.64 (0.95–2.83) |
| CAC > 400 – n(%) | 10 (5.2) | 9 (4.7) | 7 (4) | 1.16 (0.40–3.34) | 1.09 (0.37–3.19) | 1.07 (0.40–2.83) |
| Number of plaques | 2 (0–5) | 1 (0–3) | 0 (0–4) | 1.1 (0.2–2.0) b | 0.4 (−0.4–1.3)b | 0.7 (−0.2 1.5)b |
| ≥ 1 plaque – n(%) | 121 (63.4) | 109 (57.1) | 88 (50) | 1.86 (1.17–2.94) | 1.34 (0.85–2.10) | 1.39 (0.89–2.17) |
| ≥ 1 proximal plaque – n(%) | 106 (55.5) | 87 (45.5) | 71 (40.3) | 1.96 (1.24–3.11) | 1.18 (0.75–1.87) | 1.66 (1.07–2.58) |
| ≥50% luminal stenosis – n(%) | 23 (12) | 9 (4.7) | 13 (7.4) | 1.69 (0.78–3.68) | 0.61 (0.24–1.54) | 2.79 (1.20–6.50) |
| ≥50% luminal stenosis in proximal coronary artery – n(%) | 11 (5.8) | 2 (1.0) | 9 (5.1) | 0.88 (0.32–2.41) | 0.15 (0.03–0.77) | 5.92 (1.22–28.80) |
| ≥ 1 calcified plaque – n(%) | 95 (49.7) | 88 (46.1) | 68 (38.6) | 1.58 (1.01–2.49) | 1.31 (0.83–2.06) | 1.21 (0.79–1.86) |
| ≥ 1 calcified proximal plaque – n(%) | 77 (40.3) | 74 (38.7) | 45 (25.6) | 2.07 (1.28–3.35) | 1.83 (1.13–2.97) | 1.13 (0.73–1.76) |
| ≥ 1 non-calcified plaque – n(%) | 45 (23.6) | 33 (16.8) | 27 (15.3) | 1.95 (1.12–3.40) | 1.31 (0.73–2.33) | 1.49 (0.88–2.51) |
| ≥ 1 non-calcified proximal plaque – n(%) | 32 (16.8) | 18 (9.4) | 13 (7.4) | 2.80 (1.39–5.65) | 1.41 (0.66–3.00) | 1.99 (1.06–3.74) |
| ≥ 1 mixed plaque – n(%) | 57 (29.8) | 50 (26.2) | 39 (22.2) | 1.78 (1.06–2.99) | 1.44 (0.86–2.44) | 1.23 (0.76–1.99) |
| ≥ 1 mixed proximal plaque – n(%) | 37 (20.4) | 28 (14.7) | 23 (13.1) | 1.81 (0.98–3.35) | 1.31 (0.69–2.47) | 1.39 (0.78–2.46) |
| ≥50% luminal stenosis by a calcified plaque – n(%) | 11 (5.8) | 3 (1.6) | 7 (4.0) | 1.11 (0.37–3.29) | 0.29 (0.07–1.27) | 3.80 (0.95–15.25) |
| ≥50% luminal stenosis by a non-calcified plaque– n(%) | 9 (4.7) | 1 (0.5) | 5 (2.8) | 1.62 (0.50–5.21) | 0.20 (0.02–1.78) | 8.11 (1.00–65.7) |
| ≥50% luminal stenosis by a mixed plaque – n(%) | 9 (4.7) | 7 (3.7) | 5 (2.8) | 1.70 (0.53–5.51) | 1.45 (0.42–4.98) | 1.18 (0.41–3.39) |
| ≥50% luminal stenosis in proximal coronary artery by a calcified plaque – n(%) | 7 (3.7) | 2 (1.0) | 6 (3.4) | 0.82 (0.23–2.85) | 0.26 (0.05–1.45) | 3.17 (0.58–17.24) |
| ≥50% luminal stenosis in proximal coronary artery by a non-calcified plaque – n(%) | 5 (2.6) | 0 (0) | 0 (0) | 2.7 (0.7–4.6) † | 0.4 (−1.6–2.3) † | 2.3 (0.4–4.2) † |
| ≥50% luminal stenosis in proximal coronary artery by mixed plaque – n(%) | 4 (2.1) | 1 (0.5) | 3 (1.7) | 0.87 (0.17–4.44) | 0.19 (0.02–2.05) | 4.70 (0.48–45.89) |
| ≥1 high-risk plaque features – n (%) | 23 (12.0) | 13 (6.8) | 21 (11.9) | 1.31 (0.67–2.58) | 0.69 (0.32–1.48) | 1.90 (0.90–3.99) |
| Vulnerable plaques– n (%) | 1 (0.5) | 2 (1.0) | 6 (3.4) | 0.11 (0.01–0.98) | 0.20 (0.04–1.11) | 0.55 (0.05–6.38) |
Vulnerable plaques are defined as ≥2 high-risk plaque features.
Bold denotes a statistically significant difference, P < 0.05.
Odds ratios and between-group differences are adjusted for age, BMI, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HbA1c, and family history of coronary artery disease.
CAC, Agatston coronary artery calcium score; CI, confidence interval.
Denotes odds ratio and 95% confidence interval unless otherwise specified.
Between-group difference and 95% confidence interval.
Figure 2.
Overall coronary atherosclerotic burden: the overall coronary atherosclerotic burden between lifelong athletes, late-onset athletes, and non-athletic controls presented by (A) the Agatston coronary artery calcium score, (B) the number of plaques per individual, the prevalence of participants with (C) ≥ 1 coronary plaque, (D) ≥ 50% stenosis, (E) ≥ 1 proximal coronary plaque, and (F) the distribution of plaque types. Odds ratios and between-group differences are adjusted for age, body mass index, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HbA1c, and family history of coronary artery disease.
Figure 3.
Coronary atherosclerotic burden by plaque type. The coronary atherosclerotic burden by ≥1 proximal (A) calcified, (C) non-calcified, and (E) mixed plaques as well as by ≥50% stenosis by (B) calcified, (D) non-calcified, and (F) mixed plaques. Odds ratios are adjusted for age, body mass index, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HbA1c, and family history of coronary artery disease.
Discussion
The Master@Heart study is the largest and most comprehensive study to assess the dose–response relationship between intensive endurance exercise and coronary atherosclerosis. The training load and cardiorespiratory fitness of athletic and non-athletic participants was higher than that of other similar studies. The three groups were free of any classical risk factors for atherosclerosis and carefully stratified for age and exercise exposure. Nevertheless, lifelong endurance sports did not show to offer additional protection against coronary atherosclerosis compared to an active, healthy lifestyle. On the contrary, lifelong middle-aged athletes had more coronary plaques, including more unstable non-calcified plaques in proximal segments (Structured Graphical Abstract). The findings do not support the hypothesis that highly trained endurance athletes have a more benign plaque composition to explain their lower risk of cardiovascular events compared to non-athletes. As studies on the impact of physical activity in the upper range are lacking, our data open the question on whether coronary events are indeed less prevalent in this high-end exercise cohort, and if that is the case, on what explains the paradox. More and longitudinal research at the higher end of the endurance exercise spectrum is definitely needed.
Previous studies have investigated the dose–response relationship between exercise and coronary artery disease and reported a higher CAC burden in healthy marathon runners compared to risk-factor matched non-athletes.2 In 2017, two other studies added a relevant nuance that athletes presenting with coronary plaques were more likely to have calcified plaques, whereas non-athletes with plaques more frequently had mixed plaques.3,4 Since calcified plaques are more stable and less prone to rupture than mixed and non-calcified plaques, these findings were interpreted as benign and non-alarming.20 Importantly, these prior studies only looked at the relative distribution of plaque type in individuals with evidence of plaque, but differences in the absolute prevalence of calcified, mixed, and non-calcified plaques were not reported.
Contrary to these previous studies, the Master@Heart study did investigate the absolute prevalence of different coronary plaque types. As a primary finding, our data did not reveal a more benign plaque composition in endurance athletes, neither in lifelong nor in late-onset athletes, than in non-athletic controls. There even seems to be a dose–response relationship, with late-onset athletes fitting in between lifelong athletes and non-athletes. The most prevalent plaque type in both athletes and non-athletes was calcified plaques, followed by mixed and non-calcified plaques. Moreover, a greater proportion of lifelong athletes had proximal plaques and lesions with significant stenosis, as well as plaques of non-calcified and mixed morphology. The non-calcified/mixed characteristics, a stenosis grade of ≥50%, and the proximal location of coronary plaques are all established risk factors for ischaemic heart disease.14,21 Finally, the overall coronary plaque burden, which is associated with higher mortality risk, was greater in lifelong athletes.14 As such, our results do not support the hypothesis that the observed lower risk of cardiovascular events amongst individuals with high cardiorespiratory fitness can be explained by a more benign (i.e. stable) plaque composition.22
Importantly, current evidence cannot relate an increased risk of ischaemic heart disease events in endurance athletes.23–26 Some studies have shown that endurance exercise reduces the risk for ischaemic events regardless of CAC score, and this reduction is greatest at the higher CAC scores.24,26 A first potential explanation for this apparent paradox can be found in our study as vulnerable plaques, which carry the highest risk for events, were rare and not associated with an athletic lifestyle. Secondly, whilst we did observe an increased plaque burden in lifelong athletes (i.e. SSS >0–5 and SIS >0–3), this did not extend beyond an SSS >10 or SIS >6 meaning that the overall plaque burden remains low in athletes. Thirdly, it is known that endurance athletes have larger coronary arteries and a greater vasodilatory potential, meaning that the plaque-to-vessel ratio in athletes could be lower and thus lead to less significant stenosis.27,28 Finally, despite levels of cholesterol and HbA1c generally falling within normal ranges, dietary habits such as high caloric intake and high protein diets may have contributed to coronary atherosclerosis in endurance athletes. Our study protocol planned to follow up the study subjects for 2 years to observe clinical events; with the given findings, we will extend that follow-up longer.
In contrast to previous studies, non-athletes in the Master@Heart study did not have a predominance of mixed plaques but rather a propensity towards calcified plaques and smaller proportions of mixed and non-calcified plaques. Several explanations may contribute to this. Firstly, studies differed in cardiovascular risk profiles in both athletes and controls. Smoking history was a non-negligible confounder in the papers by Molhenkamp et al. and Aengevaeren et al.2,4 Similarly, some athletes and controls were on medical treatment in previous studies, whereas the Master@Heart study excluded all individuals with a history of arterial hypertension, smoking, dyslipidaemia, or diabetes mellitus. As a result, none of the study participants was taking antihypertensive drugs or statins.2,4
Another key area of discrepancy with previous studies is the participants’ training load and physical fitness. Lifelong and late-onset athletes from the Master@Heart study performed more training hours per week and had a higher METmin/week with a higher VO2peak than in other studies.3,4 Interestingly, also the non-athletes of Master@Heart had a higher aerobic capacity than controls in previous studies. Seventy-seven percent of non-athletes performed leisure-time physical activity. Forty-eight percent were recreational runners (<3 h/week), 9% were cyclists, and 18% were participating in non-endurance sports (e.g. football, tennis). Hence, our non-athletes exhibited a higher level of fitness with a higher METmin/week than the controls by Aengevaeren et al. (888 vs. 669 METmin/week) and a greater average percentage predicted VO2peak of 122% compared to 96% reported by Merghani et al.3,4
Also, a difference in the type of sports between trials can be noted. In the paper by Merghani et al. and Molhenkamp et al., athletes were mostly engaged in running whilst the athletic population in the trial by Aengevaeren et al. was more diverse with 29% cyclists and 25% runners.2–4 There is evidence to suggest that runners have a higher prevalence of coronary plaques than cyclists.29 In the Master@Heart study, cyclists represented the largest group within the athlete population, whereas active controls were mostly engaged in running. The observation that lifelong athletes exhibited more coronary plaques, despite the potentially more favourable effects of cycling, strengthens the association between high endurance exercise exposure and coronary atherosclerosis.
Considering that a higher VO2peak is associated with a lower lipid volume, higher fibrous volume, and thicker fibrous cap in coronary plaques, we speculate that the dose–response relationship between endurance exercise and coronary atherosclerosis might be reverse J-shaped rather than a descending logarithmic function.30 A sedentary and unhealthy lifestyle carries the highest risk of ischaemic heart disease with a significant burden of high-risk plaques. Conversely, a lower prevalence of cardiac risk factors with low physical activity levels is associated with reduced coronary atherosclerosis but unfavourable plaque characteristics.3,4 Our data demonstrate that a healthy lifestyle and above-average cardiorespiratory training and fitness prevents coronary atherosclerosis with a more favourable pattern of more calcified and less mixed or non-calcified plaques. Additional increments of endurance sports training load and associated increases in fitness do not affect the distribution of plaque types. However, lifetime intensive endurance training increases the overall coronary atherosclerotic burden with more plaques, including non-calcified and mixed plaques, plaques in proximal segments, and those with a significant degree of stenosis. In support of this premise, a recent study by Rubies et al. in a rodent model demonstrated that long-term intensive but not moderate training promoted adverse changes in the structural and functional properties of elastic and muscular arteries through a process mediated by the renin-angiotensin-aldosterone system, miR-212/132 and miR-146b, and matrix metalloproteinase 9.31 These findings may in part explain why moderate and vigorous physical activity beyond a specific amount of time does not offer additional reduction in mortality risk, thereby exhibiting a nadir in the dose–response relationship.32,33
One of the key strengths of our study was the comprehensive nature of the participant recruitment, which was carefully designed to minimize the risk of selection bias. As detailed in Figure 1, more than 5000 individuals registered through the web portal after responding to study advertisements in the broad community. As a result, 1152 individuals were eligible for inclusion in the study, which was twice the size of the target study population. This stringent process enabled us to include study participants at random, thereby diminishing the possibility of including individuals who registered because of personal health concerns. Importantly, the final sample size of the Master@Heart study remains twice as large as that of previous studies.2–4
Limitations
The Master@Heart study was limited to male individuals and did not provide insights on the exercise dose–response relationship in women. For statistical power, we only included men as the risk of coronary artery disease is significantly lower in women. All participants of the Master@Heart study were of White ethnicity, so our results may not be extrapolated to other ethnicities. All athletes denied using illicit performance-enhancing drugs that might induce or accelerate coronary atherosclerosis. However, no laboratory tests were performed to exclude this possibility. The primary endpoint from the Master@Heart study was investigated in a cross-sectional design. Therefore, a definitive causal relationship between endurance exercise and coronary artery disease cannot be made. We applied stringent inclusion criteria in order to minimize the influence of traditional cardiovascular risk factors. However, we did not perform blood sampling or blood pressure monitoring prior to study inclusion. Nevertheless, we do not believe that the higher prevalence of coronary plaques in athlete was influenced by occult arterial hypertension or dyslipidaemia as blood pressure was similar between groups and athletes had even more favourable lipid profiles than controls. Multiple comparisons were performed between lifelong athletes, late-onset athletes, and controls which may increase the risk of Type 1 errors. Finally, lifetime training load was assessed using questionnaires rather than continuous tracking of training logs over time. We do assess training load as part of the prospective follow-up of all study participants, such that we will be able to disentangle exercise intensity and duration and their relationship with coronary atherosclerosis in the future. We cannot exclude that some athletes may have temporarily interrupted exercise training during their lifetime. Nevertheless, superior aerobic capacity and lowest body fat percentage provide indirect evidence of superior training levels in the athletic population.
Conclusion
Lifelong endurance sport participation on top of a healthy lifestyle is not associated with a more favourable coronary plaque composition. Lifelong middle-aged athletes had more coronary plaques, including more non-calcified and mixed plaques and plaques in proximal segments with significant luminal stenosis, than fit and healthy individuals with a similarly low cardiovascular risk profile. The dose–response relationship between endurance training and coronary atherosclerosis may be reverse J-shaped. Longitudinal research is needed to reconcile these findings with the risk of cardiovascular events at the higher end of the endurance exercise spectrum.
Author contributions
All authors read, gave final approval, and agreed to be accountable for all aspects of the work, ensuring integrity.
Supplementary Material
Acknowledgements
The authors would like to thank the many staff members at the three sites for helping conduct this study. We would particularly like to thank the clinical research assistants Sofie van Soest, Dorien Vermeulen, and Daisy Thijs for their dedication and devoted efforts to include, test, and follow up participants. We would like to thank Ann Belmans for calculating the sample size and statistical advice. We would also like to thank Kris Van der Mieren (Sport- en keuringsartsen), Tom Teuglinckx (Sport- en keuringsartsen), Reynout Van Schuylenbergh (Triatlon Vlaanderen), Frank Glorieux (Cycling Vlaanderen), Paul Rowe (Sport Vlaanderen), Gert Van Goolen (Golazo), and Sven Nys (Golazo and godfather of Master@Heart) as members of the advisory committee for their insightful input on the design, the launch, and approach of the Master@Heart study.
Contributor Information
Ruben De Bosscher, Department of Cardiovascular Sciences, KU Leuven, Herestraat 49, 3000 Leuven, Belgium; Division of Cardiology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Christophe Dausin, Department of Movement Sciences, KU Leuven, Tervuursevest 101, 3001 Leuven, Belgium.
Piet Claus, Department of Cardiovascular Sciences, KU Leuven, Herestraat 49, 3000 Leuven, Belgium.
Jan Bogaert, Division of Radiology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Steven Dymarkowski, Division of Radiology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Kaatje Goetschalckx, Division of Cardiology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Olivier Ghekiere, Division of Radiology, Jessa Ziekenhuis, Stadsomvaat 11, 3500 Hasselt, Belgium; Department of Medicine and Life Sciences, University of Hasselt, Stadsomvaart 11, 3500 Hasselt, Belgium.
Caroline M Van De Heyning, Division of Cardiology, University Hospital Antwerp, Drie Eikenstraat 655, 2650 Edegem, Belgium; Cardiovascular Research, University of Antwerp, Drie Eikenstraat 655, 2650 Edegem, Belgium.
Paul Van Herck, Division of Cardiology, University Hospital Antwerp, Drie Eikenstraat 655, 2650 Edegem, Belgium; Cardiovascular Research, University of Antwerp, Drie Eikenstraat 655, 2650 Edegem, Belgium.
Bernard Paelinck, Division of Cardiology, University Hospital Antwerp, Drie Eikenstraat 655, 2650 Edegem, Belgium; Cardiovascular Research, University of Antwerp, Drie Eikenstraat 655, 2650 Edegem, Belgium.
Haroun El Addouli, Division of Cardiology, University Hospital Antwerp, Drie Eikenstraat 655, 2650 Edegem, Belgium; Cardiovascular Research, University of Antwerp, Drie Eikenstraat 655, 2650 Edegem, Belgium.
André La Gerche, Department of Cardiology, Baker Heart and Diabetes Institute, 75 Commercial Road, Melbourne, Victoria 3004, Australia.
Lieven Herbots, Department of Medicine and Life Sciences, University of Hasselt, Stadsomvaart 11, 3500 Hasselt, Belgium; Division of Cardiology, Hartcentrum, Jessa Ziekenhuis, Stadsomvaart 11, 3500 Hasselt, Belgium.
Rik Willems, Department of Cardiovascular Sciences, KU Leuven, Herestraat 49, 3000 Leuven, Belgium; Division of Cardiology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Hein Heidbuchel, Division of Cardiology, University Hospital Antwerp, Drie Eikenstraat 655, 2650 Edegem, Belgium; Cardiovascular Research, University of Antwerp, Drie Eikenstraat 655, 2650 Edegem, Belgium.
Guido Claessen, Department of Cardiovascular Sciences, KU Leuven, Herestraat 49, 3000 Leuven, Belgium; Department of Medicine and Life Sciences, University of Hasselt, Stadsomvaart 11, 3500 Hasselt, Belgium; Department of Cardiology, Baker Heart and Diabetes Institute, 75 Commercial Road, Melbourne, Victoria 3004, Australia; Division of Cardiology, Hartcentrum, Jessa Ziekenhuis, Stadsomvaart 11, 3500 Hasselt, Belgium.
Supplementary data
Supplementary data is available at European Heart Journal online.
Data availability
The data that support the findings of this study are available upon reasonable request.
Funding
This study is supported by a research grant (T003717N) and R.W. is supported as a post-doctoral clinical researcher by the Fund for Scientific Research Flanders (FWO Vlaanderen), Brussels, Belgium.
References
- 1. Bassler TJ. More on immunity to atherosclerosis in marathon runners. N Engl J Med 1978;299:201. 10.1056/NEJM197807272990416 [DOI] [PubMed] [Google Scholar]
- 2. Mohlenkamp S, Lehmann N, Breuckmann F, Brocker-Preuss M, Nassenstein K, Halle M, et al. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J 2008;29:1903–1910. 10.1093/eurheartj/ehn163 [DOI] [PubMed] [Google Scholar]
- 3. Merghani A, Maestrini V, Rosmini S, Cox AT, Dhutia H, Bastiaenan R, et al. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation 2017;136:126–137. 10.1161/CIRCULATIONAHA.116.026964 [DOI] [PubMed] [Google Scholar]
- 4. Aengevaeren VL, Mosterd A, Braber TL, Prakken NHJ, Doevendans PA, Grobbee DE, et al. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation 2017;136:138–148. 10.1161/CIRCULATIONAHA.117.027834 [DOI] [PubMed] [Google Scholar]
- 5. Schmermund A. Letter by Schmermund regarding article, “Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes With a Low Atherosclerotic Risk Profile”. Circulation 2018;137:539–540. 10.1161/CIRCULATIONAHA.117.029490 [DOI] [PubMed] [Google Scholar]
- 6. Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 2005;46:667–675. 10.1161/01.HYP.0000184225.05629.51 [DOI] [PubMed] [Google Scholar]
- 7. Kelley GA, Kelley KS, Franklin B. Aerobic exercise and lipids and lipoproteins in patients with cardiovascular disease: a meta-analysis of randomized controlled trials. J Cardiopulm Rehabil 2006;26:131–139. quiz 40-1, discussion 42-4. 10.1097/00008483-200605000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev 2006;3:CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koolhaas CM, Dhana K, Golubic R, Schoufour JD, Hofman A, van Rooij FJ, et al. Physical activity types and coronary heart disease risk in middle-aged and elderly persons: the Rotterdam study. Am J Epidemiol 2016;183:729–738. 10.1093/aje/kwv244 [DOI] [PubMed] [Google Scholar]
- 10. De Bosscher R, Dausin C, Claus P, Bogaert J, Dymarkowski S, Goetschalckx K, et al. Endurance exercise and the risk of cardiovascular pathology in men: a comparison between lifelong and late-onset endurance training and a non-athletic lifestyle - rationale and design of the Master@Heart study, a prospective cohort trial. BMJ Open Sport Exerc Med 2021;7:e001048. 10.1136/bmjsem-2021-001048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 12. Cury RC, Abbara S, Achenbach S, Agatston A, Berman DS, Budoff MJ, et al. Coronary Artery Disease - Reporting and Data System (CAD-RADS): an expert consensus document of SCCT, ACR and NASCI: endorsed by the ACC. JACC Cardiovasc Imaging 2016;9:1099–1113. 10.1016/j.jcmg.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 13. Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr 2017;11:74–84. 10.1016/j.jcct.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 14. Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–1170. 10.1016/j.jacc.2007.03.067 [DOI] [PubMed] [Google Scholar]
- 15. Al-Mallah MH, Qureshi W, Lin FY, Achenbach S, Berman DS, Budoff MJ, et al. Does coronary CT angiography improve risk stratification over coronary calcium scoring in symptomatic patients with suspected coronary artery disease? Results from the prospective multicenter international CONFIRM registry. Eur Heart J Cardiovasc Imaging 2014;15:267–274. 10.1093/ehjci/jet148 [DOI] [PubMed] [Google Scholar]
- 16. Tesche C, Plank F, De Cecco CN, Duguay TM, Albrecht MH, Varga-Szemes A, et al. Prognostic implications of coronary CT angiography-derived quantitative markers for the prediction of major adverse cardiac events. J Cardiovasc Comput Tomogr 2016;10:458–465. 10.1016/j.jcct.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 17. Nadjiri J, Hausleiter J, Deseive S, Will A, Hendrich E, Martinoff S, et al. Prognostic value of coronary CT angiography in diabetic patients: a 5-year follow up study. Int J Cardiovasc Imaging 2016;32:483–491. 10.1007/s10554-015-0785-9 [DOI] [PubMed] [Google Scholar]
- 18. Motwani M, Dey D, Berman DS, Germano G, Achenbach S, Al-Mallah MH, et al. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J 2017;38:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van den Hoogen IJ, van Rosendael AR, Lin FY, Lu Y, Dimitriu-Leen AC, Smit JM, et al. Coronary atherosclerosis scoring with semiquantitative CCTA risk scores for prediction of major adverse cardiac events: propensity score-based analysis of diabetic and non-diabetic patients. J Cardiovasc Comput Tomogr. 2020;14:251–257. 10.1016/j.jcct.2019.11.015 [DOI] [PubMed] [Google Scholar]
- 20. Hou ZH, Lu B, Gao Y, Jiang SL, Wang Y, Li W, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging 2012;5:990–999. 10.1016/j.jcmg.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 21. Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684–692. 10.1016/j.jacc.2014.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation 2008;118:800–807. 10.1161/CIRCULATIONAHA.108.785626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao JW, Hao QY, Lu LY, Han JJ, Huang FF, Vuitton DA, et al. Associations of long-term physical activity trajectories with coronary artery calcium progression and cardiovascular disease events: results from the CARDIA study. Br J Sports Med 2022;56:854–861. 10.1136/bjsports-2021-105092 [DOI] [PubMed] [Google Scholar]
- 24. German CA, Fanning J, Singleton MJ, Shapiro MD, Brubaker PH, Bertoni AG, et al. Physical activity, coronary artery calcium, and cardiovascular outcomes in the Multi-Ethnic Study of Atherosclerosis (MESA). Med Sci Sports Exerc 2022;54:800–806. 10.1249/MSS.0000000000002856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeFina LF, Radford NB, Barlow CE, Willis BL, Leonard D, Haskell WL, et al. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol 2019;4:174–181. 10.1001/jamacardio.2018.4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radford NB, DeFina LF, Leonard D, Barlow CE, Willis BL, Gibbons LW, et al. Cardiorespiratory fitness, coronary artery calcium, and cardiovascular disease events in a cohort of generally healthy middle-age men: results from the Cooper Center Longitudinal Study. Circulation 2018;137:1888–1895. 10.1161/CIRCULATIONAHA.117.032708 [DOI] [PubMed] [Google Scholar]
- 27. Parker JL, Oltman CL, Muller JM, Myers PR, Adams HR, Laughlin MH. Effects of exercise training on regulation of tone in coronary arteries and arterioles. Med Sci Sports Exerc 1994;26:1252–1261. 10.1249/00005768-199410000-00012 [DOI] [PubMed] [Google Scholar]
- 28. Haskell WL, Sims C, Myll J, Bortz WM, St Goar FG, Alderman EL. Coronary artery size and dilating capacity in ultradistance runners. Circulation 1993;87:1076–1082. 10.1161/01.CIR.87.4.1076 [DOI] [PubMed] [Google Scholar]
- 29. Aengevaeren VL, Mosterd A, Sharma S, Braber TL, Thompson PD, Velthuis BK, et al. Coronary atherosclerosis in athletes: exploring the role of sporting discipline. JACC Cardiovasc Imaging 2019;12:1587–1589. 10.1016/j.jcmg.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 30. Yoshikawa D, Ishii H, Kurebayashi N, Sato B, Hayakawa S, Ando H, et al. Association of cardiorespiratory fitness with characteristics of coronary plaque: assessment using integrated backscatter intravascular ultrasound and optical coherence tomography. Int J Cardiol 2013;162:123–128. 10.1016/j.ijcard.2011.05.047 [DOI] [PubMed] [Google Scholar]
- 31. Rubies C, Batlle M, la Garza M S-d, Dantas AP, Jorba I, Fernandez G, et al. Long-term strenuous exercise promotes vascular injury by selectively damaging the Tunica Media: experimental evidence. JACC Basic Transl Sci 2022;7:681–693. 10.1016/j.jacbts.2022.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahmadi MN, Clare PJ, Katzmarzyk PT, Del Pozo Cruz B, Lee IM, Stamatakis E. Vigorous physical activity, incident heart disease, and cancer: how little is enough? Eur Heart J 2022;43:4801–4814. 10.1093/eurheartj/ehac572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee DH, Rezende LFM, Joh HK, Keum N, Ferrari G, Rey-Lopez JP, et al. Long-term leisure-time physical activity intensity and all-cause and cause-specific mortality: a prospective cohort of US adults. Circulation 2022;146:523–534. 10.1161/CIRCULATIONAHA.121.058162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.