Abstract
Introduction
Granulosa cell tumors (GCTs) are part of the sex cord-stromal tumors occurring with a rare incidence rate that only makes up about 2–5% of all ovarian malignancies.
Case Presentation
A 28-year-old woman, gravida 2, para 1, presented with a juvenile-type granulosa cell tumor at 31 weeks gestation, which appeared as a rapidly growing mass with rupture. She under-went an exploratory laparotomy with unilateral salpingo-oophorectomy, and consequently had a successful vaginal delivery. Post-operatively she was treated with paclitaxel and carboplatin chemotherapy regimen with no evidence of recurrence after one year.
Conclusion
Radical surgical management is recommended for these tumors due to the high recurrence rate, but more conservative surgical options may be considered based on the fertility goals of the patient.
Keywords: granulosa cell tumor, sex cord-gonadal stromal tumors, ovarian neoplasms, ovarian diseases, pregnancy, pregnancy complications
Introduction
Granulosa cell tumors (GCTs) are part of the sex cord-stromal tumors that occur with a rare incidence rate that makes up 2–5% of all ovarian malignancies.1,2 These tumors are classified into 2 distinct subtypes: adult GCT (AGCT) and juvenile GCT (JGCT), with the juvenile type constituting only 5% of GCTs and occurring predominantly in women under age 30.2 The incidence of ovarian tumors amongst pregnant women is less than 0.07 in 1000, with only 10% of GCTs occurring during pregnancy.3 This report describes a case of JGCT in a pregnant woman at 31 weeks gestation, with the rapid growth of the mass and rupture at presentation. We discuss her treatment course, surveillance plan, and include a literature review on managing these tumors during pregnancy.
Case Description
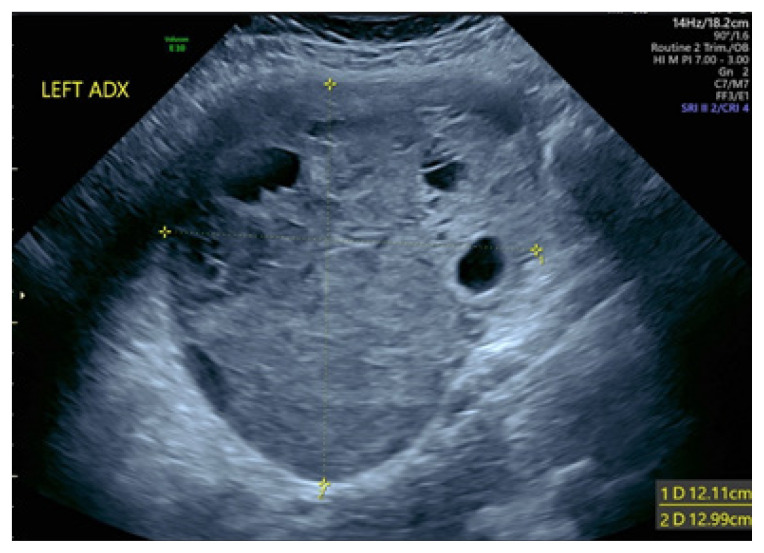
The patient was a 28-year-old woman, gravida 2, para 1 with an elevated body mass index (BMI >30) and an obstetrical history significant for a previous cesarean section. She had a normal 20-week anatomy ultrasound performed by Maternal-Fetal Medicine (MFM) and was referred back to MFM for management of gestational diabetes at 28 weeks. At this time, an incidental 12 x 12 cm heterogeneous left-sided cystic adnexal mass was noted on her ultrasound (Figure 1). An MRI was ordered for further evaluation, with results pending at the time of presentation.
Figure 1.
An ultrasound image of the left adnexa shows a large, complex, cystic, solid mass measuring 12.11 cm x 12.99 cm.
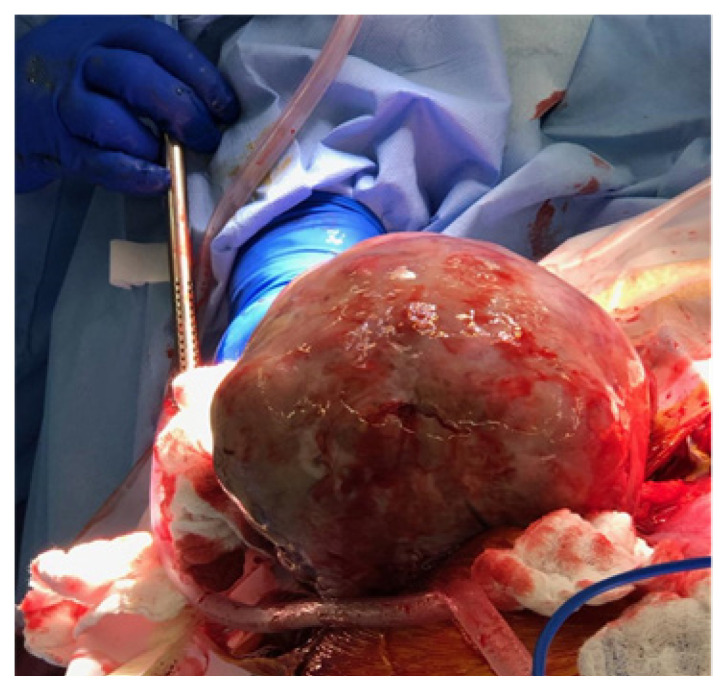
At 31 weeks gestation, the patient presented to obstetrics triage with acute onset abdominal pain. Upon physical examination, she was noted to have an acute abdomen with generalized abdominal pain, hypotension, and suspected hemoperitoneum on her ultrasound. An exploratory laparotomy revealed a gravid uterus measuring about 32 cm, a normal right ovary, a fallopian tube, and a left ovarian neoplasm measuring 20 x 25 cm at the broad ligament adjacent to the uterus. The ovarian tumor capsule was ruptured with pale yellow soft tissue densities protruding through the defect (Figure 2). The patient then underwent a left-salpingo-oophorectomy, which evacuated approximately 500 ml of clotted blood from the peritoneal cavity. All visible tumor was removed. As a result of the preoperative rupture of the tumor capsule, the patient met staging consistent with the International Federation of Gynecology and Obstetrics (FIGO) Stage 1C2.
Figure 2.
This image shows a gross, intra-operative mass measuring 20 x 25 cm. Mogor et al. (2023) 4:1. https://doi.org/10.36518/2689-0216.1461
Post-operatively, the patient had regular contractions progressing to active labor over the next 24 hours. She went on to have a spontaneous vaginal delivery of a vigorous baby girl at 31 weeks 2 days, weighing 1830 g and Apgar scores of 8 and 9 at 1 and 5 minutes. Both the mother and baby did well post-partum. The patient received 6 cycles of paclitaxel and carboplatin chemotherapy and showed no evidence of disease recurrence 1 year after her surgery. She also desired fertility preservation.
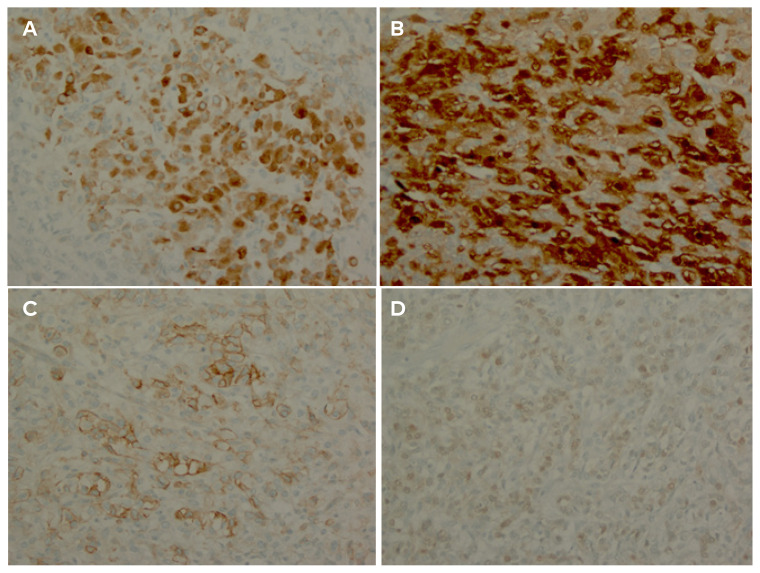
The tumor was 18 x 14 x 9 cm, weighing 1395 grams. Pathology returned a sex cord-stromal neoplasm with a nodular growth pattern and variably-sized follicles lined by epithelioid cells. The tumor had abundant vacuolated cytoplasm surrounding immature-appearing nuclei, a basophilic myxoid background, and aggregates of luteinized cells, a characteristic of JGCT. A tumor mitotic rate of 11 mitoses per 10 high-power fields was noted. Immunohistochemical analysis revealed that the tumor cells were positive for inhibin, calretinin, CD99, and S100. The cells were negative for SALL4 and AFP (Figure 3).
Figure 3.
The tumor cells are positive for inhibin (A), calretinin (B), CD99 (C), and S100 (D). The cells were negative for SALL4 and AFP.
Discussion
GTCs comprise only 2–5% of all malignant ovarian tumors.1,2 These tumors arise from the granulosa cells of the ovaries. As a result, they typically present with manifestations of elevated levels of estrogen. Symptoms of hyperestrogenism can vary based on the menstruation status of the woman: precocious puberty occurring in prepubescent girls, irregular menstruation in premenopausal women, and abnormal uterine bleeding in postmenopausal women.4 However, the hormonal profile in pregnancy can fluctuate, resulting in subtle hormonal symptoms during pregnancy compared to non-pregnant women. These patients commonly present after an incidental finding of an enlarged tumor during routine imaging or, as in our case, with an acute abdomen due to the rupture of the rapidly growing tumor.5
The use of imaging modalities cannot adequately distinguish JGCT from other ovarian neoplasms due to the absence of any characteristic features. Therefore, evaluation using histology and immunochemical assays can help provide a confirmatory diagnosis.6 Histologically, the cells of the juvenile-type tumors are irregular in shape and size. They may present with immature nuclei, significant atypia, an increased nuclear-cytoplasmic ratio, and a high mitotic rate. In contrast, the more common adult-type granulosa cell presents with a low mitotic rate, round nuclei, and Call-Exner bodies.2 The FIGO staging system for epithelial ovarian cancer is also used in GCTs to determine the involvement of disease, risk of recurrence, and overall survival. Over 90% of JGCT cases are diagnosed at FIGO stage 1, which poses a favorable prognosis with a 5-year survival rate of over 90%.7
Surgical management is the mainstay for the definitive treatment of these tumors, as it is helpful for tissue diagnosis, staging, and debulking. GCTs are usually confined to 1 ovary. At initial diagnosis, only a few cases have been reported with lymph node involvement.8 Therefore, pelvic and para-aortic lymphadenectomy is routinely omitted from the surgical staging. In young patients who desire future fertility, ipsilateral salpingo-oophorectomy is adequate. A total hysterectomy can be performed for older patients or those who have completed childbearing.9 In patients with a higher disease stage or stage 1 with a ruptured tumor, outcomes are less favorable. Adjuvant chemotherapy is recommended for patients with stage 1C disease or higher or in cases with a high mitotic index greater than 20 per 10 high power fields.10
Adequate follow-up after initial treatment is crucial in these cases. Routine follow-up should be done at 2–3 month intervals for the first 2 years followed by 4–6 month intervals over the next 3 years. Then there should be annual follow-ups for another 5 years. Any evidence of recurrence should be followed up with imaging of the abdomen and pelvis where the physician should look for recurrent tumors.9
GCTs are rare but potentially malignant tumors that can recur years after initial treatment and resolution. There is a paucity of reported cases of juvenile-type tumors presenting in pregnancy and management of these rare tumors in this unique population. The hormone level variance of pregnancy can mask the typical presentation compared to non-pregnant patients. When feasible, treatment is guided by the childbearing goals of the patient, with fertility-preserving surgery being the most appropriate option for younger patients. Appropriate interval follow-up should be arranged with a multi-disciplinary team approach recommended between the gynecologic oncologist, obstetrician, and, in some cases, a psychologist to ensure adequate recovery and monitoring.
Conclusion
We report this interesting case of a rare tumor that can be especially dangerous in pregnancy since it often goes unnoticed. We also wanted to highlight the management of this patient and consequent follow-up for recurrence, leading to a successful outcome thus far.
Funding Statement
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
References
- 1. Stine JE, Pierce S, Soper JT. A comprehensive review of diagnostic and treatment options for granulosa cell tumors of the ovary. Obstet Gynecol Surv. 2014;69(1):29–38. doi: 10.1097/ogx.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 2. Young RH. Sex cord-stromal tumors of the ovary and testis: their similarities and differences with consideration of selected problems. Mod Pathol. 2005;18(2):S81. doi: 10.1038/modpathol.3800311. [DOI] [PubMed] [Google Scholar]
- 3. Hasiakos D, Papakonstantinou K, Goula K, Karvouni E, Fotiou S. Juvenile granulosa cell tumor associated with pregnancy: report of a case and review of the literature. Gynecol Oncol. 2006;100(2):426–429. doi: 10.1016/j.ygyno.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 4. Evans AT, III, Gaffey TA, Malkasian GD, Jr, Annegers JF. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. 1980;55(2):231–238. [PubMed] [Google Scholar]
- 5.Barakat RR, Markman M, Randall M, editors. Principles and Practice of Gynecologic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 6. Outwater EK, Wagner BJ, Mannion C, McLarney JK, Kim B. Sex cord-stromal and steroid cell tumors of the ovary. Radiographics. 1998;18(6):1523–1546. doi: 10.1148/radiographics.18.6.9821198. [DOI] [PubMed] [Google Scholar]
- 7. Calaminus G, Wessalowski R, Harms D, Göbel U. Juvenile granulosa cell tumors of the ovary in children and adolescents: results from 33 patients registered in a prospective cooperative study. Gynecol Oncol. 1997;65(3):47–452. doi: 10.1006/gyno.1997.4695. [DOI] [PubMed] [Google Scholar]
- 8. Pectasides D, Pectasides E, Psyrri A. Granulosa cell tumor of the ovary. Cancer Treat Rev. 2008;34(1):1–12. doi: 10.1016/j.ctrv.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 9. Colombo N, Parma G, Zanagnolo V, Insinga A. Management of ovarian stromal cell tumors. J Clin Oncol. 2007;25(20):2944–2951. doi: 10.1200/JCO.2007.11.1005. [DOI] [PubMed] [Google Scholar]
- 10. Schneider DT, Calaminus G, Harms D, Göbel U. German Maligne Keimzelltumoren Study Group. Ovarian sex cord-stromal tumors in children and adolescents. J Reprod Med. 2005;50(6):439–446. [PubMed] [Google Scholar]