Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) may disrupt mammary gland development and function; thereby inhibiting milk supply and breastfeeding duration. However, conclusions on the potential effects of PFAS and breastfeeding duration are limited by prior epidemiologic studies that inconsistently adjusted for past cumulative breastfeeding duration and by a lack of examination of the joint effects of PFAS mixtures.
Methods:
In Project Viva, a longitudinal cohort that enrolled pregnant participants from 1999–2002 in the greater Boston, MA area, we studied 1079 women who ever attempted to lactate. We investigated associations of plasma concentrations of select PFAS in early pregnancy (mean: 10.1 weeks gestation) with breastfeeding termination by 9 months, after which women typically cite self-weaning as the reason for terminating breastfeeding. We used Cox regression for single-PFAS models and quantile g-computation for mixture models, adjusting for sociodemographics, prior breastfeeding duration, and weeks of gestation at the time of blood draw.
Results:
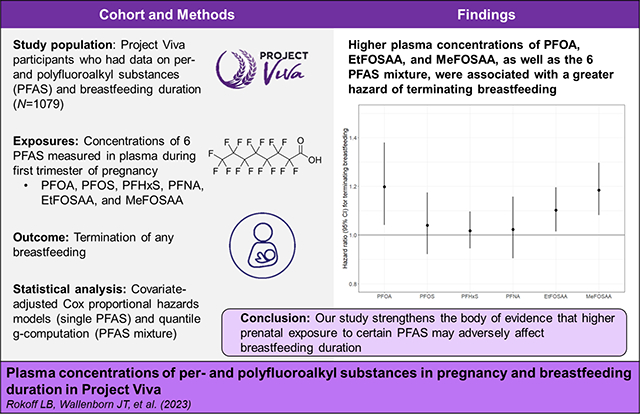
We detected 6 PFAS [perfluorooctane sulfonate; perfluorooctanoate (PFOA); perfluorohexane sulfonate; perfluorononanoate; 2-(N-ethyl-perfluorooctane sulfonamido) acetate (EtFOSAA); 2-(N-methyl-perfluorooctane sulfonamide) acetate (MeFOSAA)] in >98% of samples. Sixty percent of lactating women terminated breastfeeding by 9 months postpartum. Women with higher plasma concentrations of PFOA, EtFOSAA, and MeFOSAA had a greater hazard of terminating breastfeeding in the first 9 months postpartum [HR (95% CI) per doubling concentration: 1.20 (1.04, 1.38) for PFOA; 1.10 (1.01, 1.20) for EtFOSAA; 1.18 (1.08, 1.30) for MeFOSAA]. In the quantile g-computation model, simultaneously increasing all PFAS in the mixture by one quartile was associated with 1.17 (95% CI: 1.05, 1.31) greater hazard of terminating breastfeeding in the first 9 months.
Conclusion:
Our findings suggest that exposure to PFAS may be associated with reduced breastfeeding duration and draw further attention to environmental chemicals that may dysregulate human lactation.
Keywords: Breastfeeding, lactation, per- and polyfluoroalkyl substances
Graphical abstract
1. Introduction
Given the nutritional, immunological, and anti-inflammatory benefits of breastfeeding for infants, along with long-term benefits for maternal health (e.g., decreased risk of type 2 diabetes mellitus, breast cancer, ovarian cancer), the American Academy of Pediatrics (AAP) recommends continuing breastfeeding for 2 years or longer.1 However, only about 55% of United States (U.S.) women breastfeed beyond 6 months, and only 36% are still breastfeeding at 12 months.2 Duration of breastfeeding is influenced by a number of variables, including individual choice; pre- and postnatal care; and societal, sociocultural, psychological, and biological factors.3–5 Women who report terminating breastfeeding early often cite difficulties with the process of lactation, such as trouble with milk flow to start and insufficient milk supply.5 However, the reasons why some women face challenges with successful lactation are not well understood.
Lactation is regulated by a complex combination of hormones (e.g., prolactin, insulin, oxytocin),6 and endocrine-disrupting chemicals may affect mammary gland development, differentiation, and function and ultimately inhibit milk supply.7 Per- and polyfluoroalkyl substances (PFAS) are a class of synthetic endocrine disrupting chemicals, used in a broad range of industrial and consumer products, including textiles, non-stick cookware, and food packaging. PFAS are both widely used and persistent in the environment; resulting in nearly ubiquitous human exposure via contaminated drinking water, food, air, and dust.8,9 In fact, almost all individuals in the U.S. have detectable concentrations of PFAS in their blood.10
Prior epidemiologic studies examining associations of plasma or serum PFAS concentrations during pregnancy with time to breastfeeding termination have generally shown that higher PFAS concentrations are associated with shorter breastfeeding duration.11 However, not all prior studies could appropriately adjust for historical cumulative breastfeeding duration, a key confounder as (1) previous breastfeeding duration predicts the duration in a subsequent pregnancy and (2) lactation is a PFAS excretion pathway.12 The strength of the evidence is further hampered by minimal investigation of the joint effects of exposure to multiple PFAS (i.e., mixtures).
Our study leveraged data from a longitudinal cohort of U.S. women to assess the extent to which pregnancy plasma PFAS concentrations, evaluated as individual components and a mixture, were associated with hazard of terminating breastfeeding, after adjusting for prior breastfeeding duration.
2. Methods
2.1. Study population and design
Participants in this study were enrolled in Project Viva, a longitudinal pre-birth cohort based in the greater Boston, Massachusetts area. Pregnant participants were recruited from 1999–2002 during their first prenatal visit at the multi-specialty group practice Atrius Harvard Vanguard Medical Associates. During the enrollment period, these women resided in areas with no known community sources of PFAS exposure (e.g., contaminated drinking water). Eligibility criteria were singleton gestation, less than 22 weeks gestation, ability to answer questions in English, and no plans to move outside of the area before delivery.13 Of the 2128 live births in Project Viva, 1645 mother-child pairs had data available on plasma PFAS concentrations. We restricted our analysis to the 1094 women who put the baby to breast and/or fed the baby their own expressed human milk (i.e., successfully initiated breastfeeding) and then also reported their breastfeeding duration of the index child. We excluded 15 second pregnancies (i.e., multiple enrollments in Project Viva), resulting in a final analytic cohort of 1079 women.
Project Viva participants provided written informed consent, and the Institutional Review Boards at Harvard Pilgrim Health Care and other participating institutions approved study protocols.
2.2. Plasma PFAS concentrations during pregnancy
We measured PFAS in plasma collected at the initial prenatal study visit (mean ± SD: 10.1 ± 2.3 weeks gestation). As previously described,14 we stored all samples at −80°C prior to overnight shipping to the Division of Laboratory Sciences at the Centers for Disease Control and Prevention (CDC) (Atlanta, GA), where staff quantified concentrations of eight PFAS [perfluorohexane sulfonate (PFHxS); perfluorooctane sulfonate (PFOS); perfluorooctanoate (PFOA); perfluorononanoate (PFNA); perfluorodecanoate (PFDA); 2-(N-ethyl-perfluorooctane sulfonamido) acetate (EtFOSAA); 2-(N-methyl-perfluorooctane sulfonamide) acetate (MeFOSAA); perfluorooctane sulfonamide] using solid-phase extraction coupled with isotope dilution high performance liquid chromatography-tandem mass spectrometry.15 The limits of detection (LOD) were 0.2 ng/mL for PFOS and 0.1 ng/mL for the other PFAS.15 We replaced concentrations below the LOD with the . We a priori included PFAS detected in >60% of participants’ samples (PFOA, PFOS, PFHxS, PFNA, EtFOSAA, and MeFOSAA) in the statistical analyses. The analysis of the de-identified samples at the CDC laboratory did not constitute engagement in human subjects research.
2.3. Breastfeeding duration of the index child
At two time points (during an in-person or telephone interview at approximately 6 months postpartum and on a mailed questionnaire at 12 months postpartum), we asked participants if they had ever breastfed (i.e., initiated feeding by putting the baby to the breast and/or giving the baby their own human milk); if the participant was still breastfeeding at all; and if not, when they had stopped. We defined breastfeeding duration as the time up to 12 months (minimum: 0.3 months) that a child received any human milk (e.g., exclusive or combination feeding). At 6 months postpartum, we also asked about timing of introduction of solid food and other liquids, which enabled us to determine the duration of exclusive breastfeeding as the time in months that an infant had intake of human milk but no solid foods or other liquids (except water). Additionally, at 6 and 12 months postpartum, we asked women who terminated breastfeeding to indicate why they terminated; participants checked all reasons that applied, with one option being: “I wasn’t producing enough breastmilk to satisfy my child”. Other possible responses to this questionnaire included, but were not limited to: “I felt my child had the full benefits of breastfeeding”; “I returned to work or school”; and “Breastfeeding took too much time away from my other children”. We considered women to have perceived insufficient milk supply if they indicated that at least one of the reasons they terminated breastfeeding was not producing enough breastmilk.
2.4. Covariate assessment
We collected participant sociodemographic characteristics at enrollment, including age, race and ethnicity, education, marital status, parity, pre-pregnancy height and weight, smoking habits, and annual household income. At a research visit in midlife from 2017–2021 (~18 years post-pregnancy), participants reported information on duration of breastfeeding for every lifetime pregnancy, enabling us to calculate cumulative breastfeeding duration prior to the Project Viva index child. Among our analytic cohort of 1079 women, 76% (N=819) either provided cumulative lifetime breastfeeding data on the midlife questionnaire or were nulliparous at the index pregnancy.
2.5. Statistical analysis
We used Cox proportional hazards regression models to examine associations of single PFAS with breastfeeding termination in the first 9 months postpartum. In our primary analyses, we chose to “administratively censor” individuals at 9 months postpartum because up to 9 months, most women who stop breastfeeding cite concerns about milk supply as their reason for terminating, whereas after 9 months, most women cite baby self-weaning.16 We used Cox models rather than binomial modeling methods because we wanted to utilize an approach that was consistent across single PFAS and mixture analyses and was comparable with prior studies. To aid in interpretability, we log2-transformed plasma PFAS concentrations and calculated the hazard ratio (HR) of terminating breastfeeding in the first 9 months per doubling of plasma PFAS.
We used information from prior research11,14 to select the following possible confounders for inclusion in our models: age at enrollment (continuous, years), education (college graduate vs. not), marital status (married or cohabitating vs. not), race and ethnicity (as a proxy for the unmeasured consequences of racism; non-Hispanic White, non-Hispanic Black, Hispanic, Asian, other persons), smoking status (during pregnancy, former smoker, never smoked), parity (nulliparous vs. parous), prior breastfeeding duration (continuous, months), and pre-pregnancy body mass index (BMI; continuous, kg/m2). We also included gestational age at time of PFAS blood draw (continuous, weeks) as a covariate in our models to account for variability in PFAS concentrations due to hemodynamic changes that occur during pregnancy.17 We examined but did not include annual household income, as the inclusion versus exclusion of adjustment for this covariate did not materially change effect estimates. We did not control for gestational weight gain, as gestational weight gain has the potential to be on the causal pathway for the PFAS-breastfeeding duration relationship.18 In covariate-adjusted models, we assessed the proportional hazards assumption using Schoenfeld residuals as a diagnostic. While the residuals for all 6 PFAS did not suggest violation of this assumption, for some covariates (maternal education, parity, and prior breastfeeding duration) the proportional hazards was not supported; to address this, we relaxed this assumption by fitting a stratified Cox model which allows a different baseline hazard by levels of these covariates.
We used multiple imputation to account for missing data for maternal education, race and ethnicity, and pre-pregnancy BMI (all <1% missing), as well as cumulative breastfeeding duration in prior pregnancies (N=260, 24% missing). Using imputation by chained equations, we generated 50 imputed data sets, and combined estimates using Rubin’s rules19 for the single-PFAS models. We used data on the PFAS concentrations, outcomes, and additional covariates from 2100 individual participants in Project Viva (excluding 28 duplicate pregnancies) to generate the imputed data sets. For the analyses, we restricted to the 1079 participants who had initiated feeding human milk to their baby, had data on PFAS concentrations, and had data on the breastfeeding duration of the index child. We ran models that were unadjusted, adjusted for all covariates except prior breastfeeding duration (to examine the magnitude of confounding), and fully covariate-adjusted models.
We utilized quantile based g-computation to account for multiple PFAS and to estimate the association with the mixture, thereby more accurately reflecting human exposure scenarios.20 Quantile g-computation, an approach implemented via the ‘qgcomp’ package in R, provides 1) an estimate of the joint effect of the exposure mixture on an outcome, and 2) individual weights for each component, which represent the relative contribution to either the positive or negative scaled effect size. We applied quantile g-computation under a Cox proportional model 20 to estimate the adjusted association of a simultaneous quartile increase of all PFAS concentrations and the hazard of terminating breastfeeding in the first 9 months, using the same set of covariates as single-PFAS models and within one of 50 randomly selected imputed datasets. Analogous to single-PFAS models, we implemented quantile g-computation under a stratified Cox model which allowed a different baseline hazard by levels of maternal education, parity, and prior cumulative breastfeeding duration. Quantile g-computation allows for reporting of adjusted associations either conditional on the assumed confounders or marginalized with respect to confounders. Our estimates of conditional and marginal associations were similar, so we only report the conditional hazard ratio.
In sensitivity analyses, we ran single PFAS models among the 810 participants with all covariates measured (i.e., complete case analyses with no imputed data). To improve comparability with some prior studies,21–25 we also ran sensitivity analyses in which we restricted to nulliparous participants (N=560); included N=187 participants who never attempted to lactate (i.e., setting their breastfeeding duration to 0.01 months; N=1266 overall); adjusted for parity as a categorical variable (0, 1, 2, 3, or ≥4 prior births); and censored breastfeeding duration at 6 months. To assess the robustness of our results across analytic approaches, we used modified Poisson regression with robust error variance26 to estimate risk ratios between plasma concentrations of each PFAS and a binary outcome of terminating breastfeeding before versus after 9 months.
Additionally, we examined associations of plasma concentrations of each PFAS with exclusive breastfeeding termination in the first 3 months (N=1022 with data on exclusive breastfeeding, defined as no complimentary liquids or solids); during the years in which Project Viva women were enrolled into the study and began breastfeeding (1999–2002), the AAP recommended introducing solids foods as early as 4 months of age.27 Finally, we used modified Poisson regression models to examine associations of plasma concentrations of each PFAS with perceived insufficient milk supply (N=1077 with data on reason for terminating breastfeeding). We used R version 3.6.2 (R Core Team) to conduct all analyses.
3. Results
3.1. Population characteristics
Among all Project Viva participants (N=2100), those included in this analysis (imputed to N=1079) were more likely to be college graduates, non-Hispanic White persons, nulliparous, and to have never smoked as compared to those excluded (Table S1). Participants in the analytic cohort were on average 32.3 years old at enrollment. Participants were primarily non-Hispanic White persons (72%), married or cohabitating (92%), and college graduates (73%). On average, participants breastfed the index pregnancy 6.6 [standard deviation (SD): 4.2] months and exclusively breastfed the index pregnancy for 2.5 (SD: 2.0) months. Sixty percent terminated breastfeeding by 9 months postpartum. The 48% (N=519) participants who were parous had an average of 1.4 (SD: 0.7) prior births and spent an average of 11.3 (SD: 15.4) months total time breastfeeding across all prior pregnancies (Table 1).
Table 1.
Characteristics of study participants in the analytic cohort, overall and by quartiles of plasma perfluorooctanoate (PFOA) concentration during pregnancy, 1999–2002
| Overall | Quartile of perfluorooctanoate (PFOA) a | ||||
|---|---|---|---|---|---|
| N=1079 | Q1 N=272 |
Q2 N=276 |
Q3 N=270 |
Q4 N=261 |
|
| Participant characteristics | Mean ± Standard deviation or N (%) | ||||
| Age at enrollment (years) | 32.3 ± 4.9 | 33.5 ± 4.7 | 32.6 ± 4.7 | 31.5 ± 5.1 | 31.7 ± 4.9 |
| College degree b | |||||
| Yes | 786 (73) | 214 (79) | 204 (74) | 191 (71) | 177 (68) |
| No | 293 (27) | 58 (21) | 72 (26) | 79 (29) | 84 (32) |
| Married or cohabitating b | |||||
| Yes | 998 (92) | 253 (93) | 247 (89) | 251 (93) | 247 (95) |
| No | 81 (8) | 19 (7) | 29 (11) | 19 (7) | 14 (5) |
| Race/ethnicity b | |||||
| Non-Hispanic White | 777 (72) | 185 (68) | 196 (71) | 202 (75) | 195 (75) |
| Non-Hispanic Black | 143 (13) | 43 (16) | 39 (14) | 27 (10) | 33 (13) |
| Hispanic | 67 (6) | 14 (5) | 17 (6) | 19 (7) | 17 (7) |
| Asian | 50 (5) | 17 (6) | 16 (6) | 11 (4) | 6 (2) |
| Other c | 42 (4) | 13 (5) | 8 (3) | 11 (4) | 10 (4) |
| Smoking status b | |||||
| During pregnancy | 112 (10) | 15 (6) | 27 (10) | 35 (13) | 34 (13) |
| Former smoker | 211 (19) | 48 (18) | 49 (18) | 51 (19) | 63 (24) |
| Never smoked | 757 (70) | 209 (77) | 200 (72) | 184 (68) | 164 (63) |
| Nulliparous | |||||
| Yes | 560 (52) | 63 (23) | 127 (46) | 186 (69) | 184 (70) |
| No | 519 (48) | 209 (77) | 149 (54) | 84 (31) | 77 (30) |
| Prior breastfeeding duration (months) d, e | 5.4 ± 12.1 | 11.1 ± 15.8 | 5.0 ± 11.5 | 3.2 ± 9.1 | 2.0 ± 6.5 |
| Pre-pregnancy BMI (kg/m2) | 24.7 ± 5.4 | 24.3 ± 5.2 | 24.8 ± 5.6 | 24.7 ± 5.2 | 25.1 ± 5.4 |
| Weeks of gestation at time of PFAS blood draw | 10.1 ± 2.3 | 10.4 ± 2.3 | 10.1 ± 2.5 | 10.0 ± 2.3 | 9.7 ± 1.9 |
| Index pregnancy breastfeeding characteristics | |||||
| Breastfeeding duration (months) | 6.6 ± 4.2 | 8.1 ± 4.1 | 6.3 ± 4.3 | 6.6 ± 4.0 | 5.6 ± 4.2 |
| Terminated by 9 months postpartum | 651 (60) | 124 (46) | 171 (62) | 170 (63) | 186 (71) |
| Exclusive breastfeeding duration (months) f | 2.5 ± 2.0 | 3.1 ± 2.1 | 2.4 ± 2.0 | 2.4 ± 1.9 | 2.2 ± 1.9 |
| Perceived insufficient milk supply g | 345 (32) | 68 (25) | 96 (35) | 96 (36) | 85 (33) |
Abbreviations: Q, quartile; kg, kilograms
PFOA concentrations (ng/mL) for each quartile are Q1: 0.3 – 4.0; Q2: 4.1 – 5.7; Q3: 5.8 – 7.8; Q4: 7.9 – 36.7
Calculated from all imputations and Ns are rounded to nearest integer; values may not sum to 1079
Other race/ethnicity includes those who identified as American Indian or Alaskan Native, other, or more than one race
Cumulative breastfeeding duration of any pregnancies prior to the index pregnancy; data retrospectively collected at the midlife research visit and imputed in 255 participants with missing data
Among parous women (N=519; 48%), the overall mean ± SD of prior breastfeeding duration was 11.3 ± 15.4 months and for number of prior births was 1.4 ± 0.7
N =1022; exclusive breastfeeding duration not imputed
N = 1077; perceived insufficient milk supply not imputed
The 6 PFAS included in our analyses were detected in over 98% of samples. The highest concentrations were for PFOS [median (range): 25.0 (<LOD of 0.2 – 117) ng/mL]. Plasma PFAS concentrations were positively and moderately correlated [Spearman coefficients (rs): 0.20–0.74], with the strongest correlation between PFOS and PFOA concentrations (rs: 0.66, Table 2). Participants with higher plasma concentrations of PFOA in pregnancy were more likely to be non-Hispanic white persons, current or former smokers, nulliparous, and to have reported fewer months of prior breastfeeding (Table 1).
Table 2.
Distributions and Spearman correlation coefficients for the plasma PFAS concentrations (N=1079), 1999–2002
| Percentile | ||||||
|---|---|---|---|---|---|---|
| PFAS (ng/mL) | % detection a | Minimum | 25th | Median | 75th | Maximum |
| PFOA | 100 | 0.3 | 4.0 | 5.7 | 7.8 | 36.7 |
| PFOS | 99.8 | <LOD | 18.4 | 25.0 | 34.0 | 117 |
| PFHxS | 99.0 | <LOD | 1.6 | 2.4 | 3.7 | 46.4 |
| PFNA | 98.9 | <LOD | 0.5 | 0.7 | 0.9 | 6.0 |
| EtFOSAA | 99.8 | <LOD | 0.7 | 1.1 | 1.8 | 21.2 |
| MeFOSAA | 99.9 | <LOD | 1.2 | 1.9 | 3.0 | 29.7 |
| Spearman correlation coefficients | ||||||
| PFOA | PFOS | PFHxS | PFNA | EtFOSAA | MeFOSAA | |
| PFOA | - | - | - | - | - | - |
| PFOS | 0.74 | - | - | - | - | - |
| PFHxS | 0.55 | 0.53 | - | - | - | - |
| PFNA | 0.56 | 0.63 | 0.45 | - | - | - |
| EtFOSAA | 0.39 | 0.52 | 0.20 | 0.21 | - | - |
| MeFOSAA | 0.36 | 0.42 | 0.26 | 0.25 | 0.42 | - |
Abbreviations: PFAS, per- and polyfluoroalkyl substances; PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamido) acetate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamide) acetate
Limits of detection were 0.2 ng/mL for PFOS and 0.1 ng/mL for all other PFAS.
3.2. Single PFAS models
In single PFAS models that were unadjusted or adjusted for all covariates except prior breastfeeding duration, women with higher plasma concentrations of all six PFAS had a greater hazard of terminating breastfeeding in the first 9 months postpartum, although CIs for PFHxS included the null (Table S2). In fully adjusted models (i.e., after also accounting for prior cumulative breastfeeding duration), PFOA, EtFOSAA, and MeFOSAA remained significantly associated with terminating breastfeeding in the first 9 months. The hazard of terminating in the first 9 months was 20% [95% confidence interval (CI): 1.04, 1.38] higher per doubling of PFOA, 18% (95% CI: 1.08, 1.30) higher per doubling of MeFOSAA, and 10% (95% CI: 1.01, 1.20) higher per doubling of EtFOSAA. In fully adjusted models, women with higher PFOS, PFHxS, and PFNA concentrations also had greater hazard of terminating breastfeeding, but associations were weak, and the 95% CIs included the null (Figure 1, Table S3).
Figure 1.
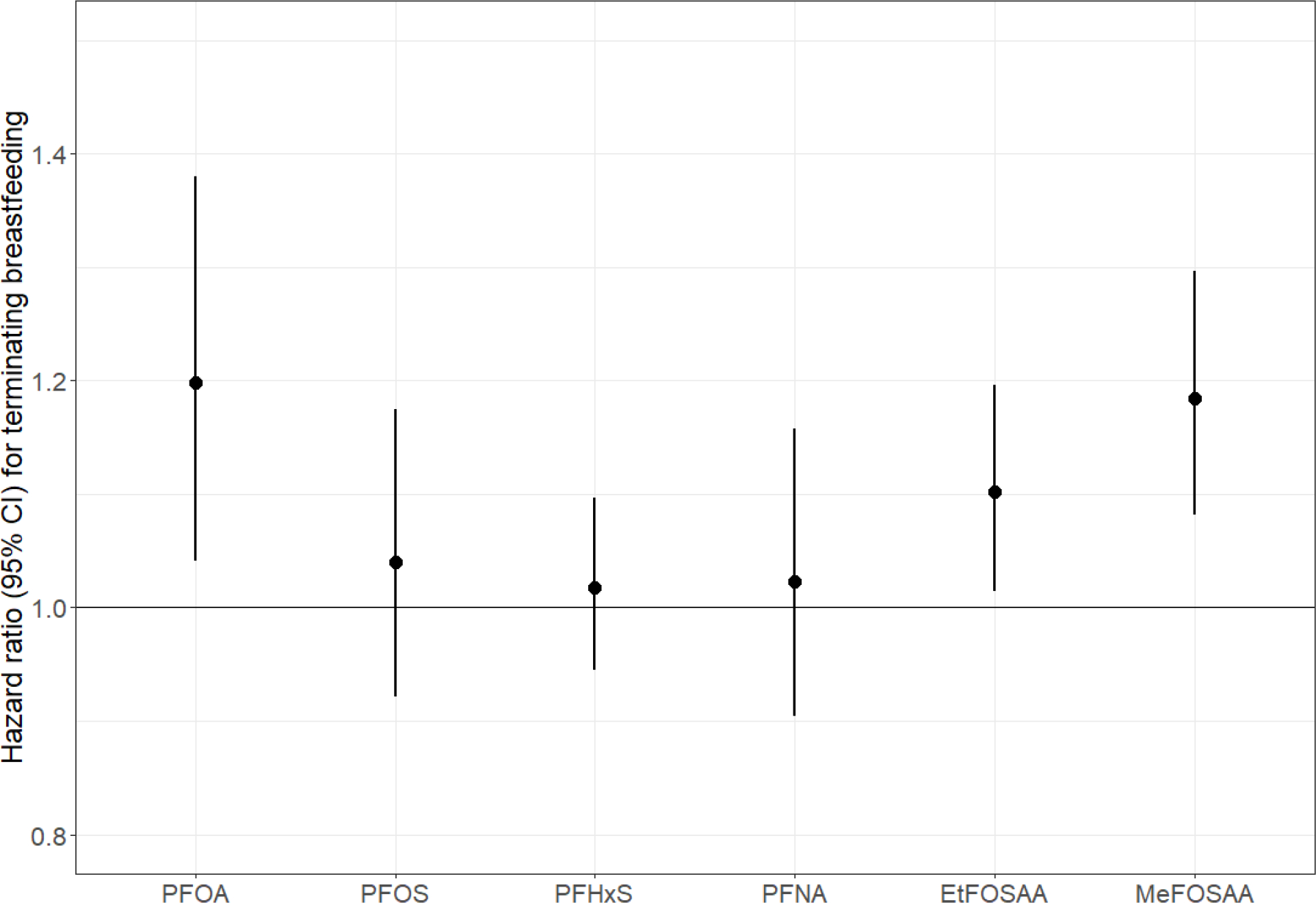
Adjusted hazard ratios (95% confidence intervals) for terminating breastfeeding in the first 9 months per doubling of plasma PFAS concentrations. Abbreviations: CI, confidence interval; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamido) acetate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamide) acetate. Notes: Adjusted for maternal age, race/ethnicity, pre-pregnancy body mass index, marital status, smoking during pregnancy, and weeks of gestation at time of PFAS blood draw. Cox model stratified by maternal education, parity, and prior breastfeeding duration. See Table S3 for corresponding numerical values.
3.3. PFAS mixture model
In the covariate-adjusted quantile g-computation model, we estimated that women had a 17% (95% CI: 1.05, 1.31) greater hazard of terminating breastfeeding per simultaneous quartile increase of each PFAS component in the mixture. Consistent with our single PFAS models, the positive association was driven by PFOA, EtFOSAA, and MeFOSAA (Figure 2).
Figure 2.
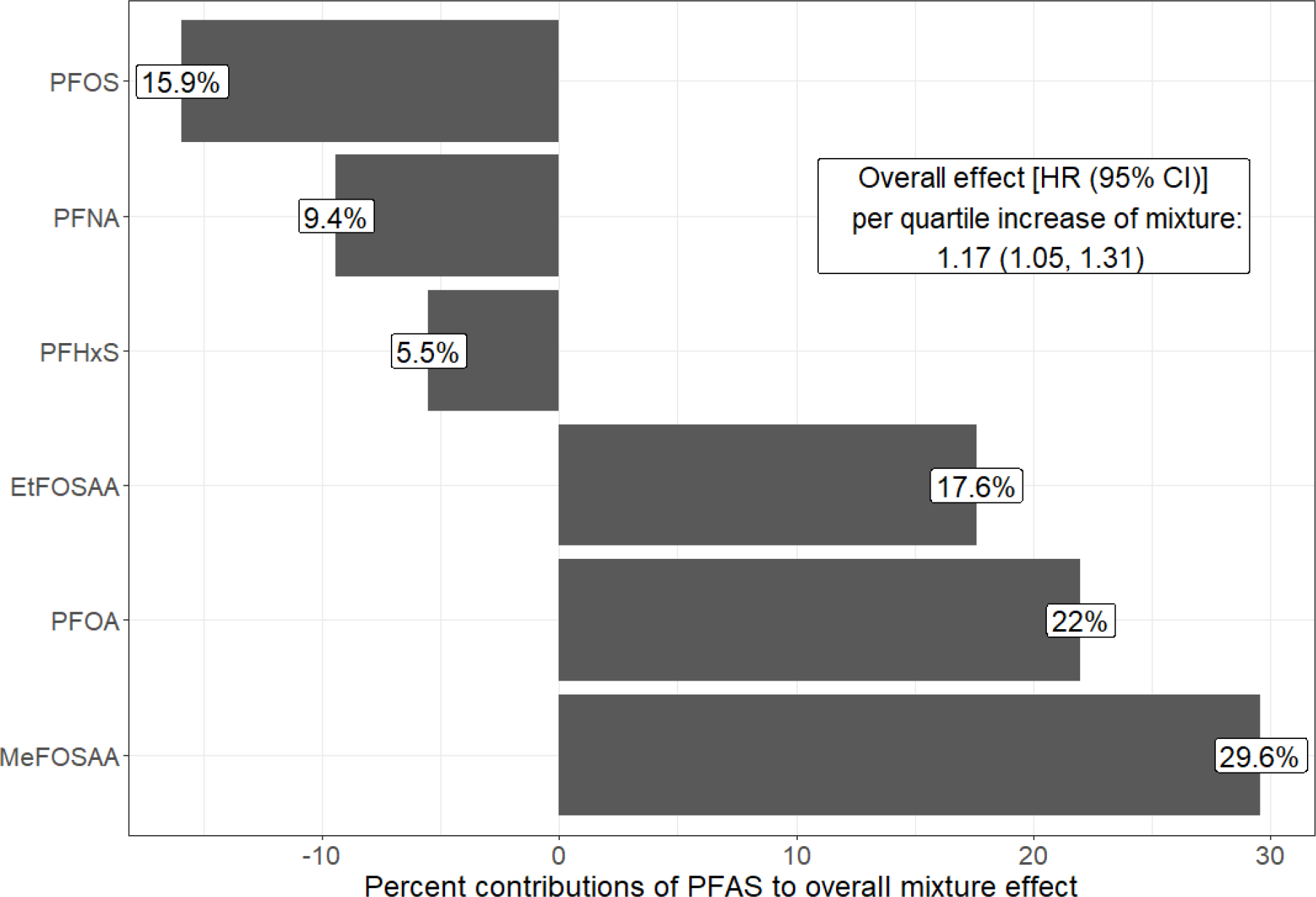
Adjusted quantile g-computation hazard ratio (95% confidence interval) for terminating breastfeeding in the first 9 months per quartile increment of all plasma PFAS concentrations in the mixture, and the percent contribution of each PFAS to the overall mixture effect (calculated using quantile g-computation output and taking into account the scaled effect size in each direction). Abbreviations: CI, confidence interval; HR, hazard ratio; PFAS, per- and polyfluoroalkyl substances; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; EtFOSAA, 2-(N-ethyl-perfluorooctane sulfonamido) acetate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamide) acetate. Notes: Adjusted for maternal age, race/ethnicity, pre-pregnancy body mass index, marital status, smoking during pregnancy, and weeks of gestation at time of PFAS blood draw. Cox model stratified by maternal education, parity, and prior breastfeeding duration.
3.4. Sensitivity analyses
When we examined only individuals with no missing covariate data, results were similar in direction and magnitude as compared to the cohort with imputed covariate data (Table S3). When we restricted to nulliparous participants (52% of overall analytic cohort), the direction and magnitude of effect estimates were generally similar, although CIs were wider. The association between a plasma PFOA concentration and hazard of terminating breastfeeding was somewhat attenuated among nulliparous participants compared to the overall analytic cohort [HR (95% CI): 1.14 (0.95, 1.37) versus 1.20 (1.04, 1.38) per doubling PFOA] (Table S4). When we included participants who never attempted to lactate (i.e., by setting their breastfeeding duration to 0.01 months) or when we adjusted for parity as a categorical rather than a binary variable, effect estimates were similar to those in our primary analyses (data not shown). When we examined associations of PFAS with breastfeeding termination within the first 6 months postpartum, direction and magnitude of effect estimates were similar except for slightly attenuated associations for PFOA [HR: 1.16 (95% CI: 0.98, 1.37) versus 1.20 (95% CI: 1.04, 1.38) per doubling PFOA when censored at 9 months postpartum] (Table S3). Results were robust across analytic approaches, with effect estimates for PFOA, EtFOSAA, and MeFOSAA being the strongest in magnitude and statistically significant at α=0.05 level whether using Cox models or modified Poisson with robust error variance (Table S5).
Women with higher concentrations of EtFOSAA and MeFOSAA during pregnancy had greater hazard of terminating exclusive breastfeeding in the first 3 months postpartum, but associations were weaker as compared to our primary analysis and CIs included the null [e.g., HR (95% CI) of 1.09 (0.99, 1.20) per doubling plasma MeFOSAA concentration] (Table S6). PFAS concentrations during pregnancy were generally not associated with perceived insufficient milk supply, although women with higher PFOS concentrations were less likely to report perceived insufficient milk supply as a reason for terminating breastfeeding (Table S7).
4. Discussion
Among Project Viva participants, higher plasma concentrations of PFOA, EtFOSAA, and MeFOSAA, as well as of the overall 6 PFAS mixture, were prospectively associated with a greater hazard of terminating breastfeeding. Our findings were consistent with results from the Danish National Birth Cohort,21 Health Outcomes and Measures of the Environment (HOME) Study,22 Children’s Health and the Environment in the Faroes (CHEF) cohort,23 Odense Child Cohort,24 and Ronneby cohort.28 Across prior studies, PFOA has been consistently associated with shorter breastfeeding duration,21–24 even after accounting for prior cumulative breastfeeding duration.22–24 Although analyses within some European cohorts have also shown higher concentrations of PFOS or PFNA to be associated with shorter breastfeeding duration, we observed no associations with PFOS21,23–25 or PFNA23,24 in Project Viva, similar to the U.S.-based HOME Study cohort.22 Also consistent with our findings, PFHxS has not been associated with shorter breastfeeding duration in prior studies.22–25 Our study is the first to evaluate EtFOSAA and MeFOSAA in relation to breastfeeding duration, and we found strong associations of both PFAS, particularly MeFOSAA, with shorter breastfeeding duration. Serum concentrations of EtFOSAA and MeFOSAA in the general population have declined over the past >20 years (i.e., since the time when Project Viva women were pregnant), with EtFOSAA largely no longer detectable. MeFOSAA, which is still detectable at relatively low concentrations in ~50% of the U.S. population,10 is an oxidation product of N-methyl perfluorooctanesulfonamidoethanol, used primarily in surface treatment applications of carpets and textiles;29,30 if our finding is replicated, it could have implications for exposure risk reduction in indoor environments among pregnant women.
In Project Viva, we collected plasma around the time of peak PFAS production in the U.S. (1999–2002).31 While PFAS concentrations in our cohort were similar to those observed in the 1999–2000 U.S. National Health and Nutrition Examination Survey (NHANES)10 and the Danish National Birth Cohort (1996–2002),21,32 concentrations were generally higher among our cohort than among other published studies evaluating PFAS and breastfeeding duration that used serum or plasma collected at a later time. For example, the median PFOS concentration in our study was 25 ng/mL, higher than 19 ng/mL in the CHEF cohort (1997–2000, 2007–2009),23 14 ng/mL in the HOME Study (2003–2006),22 13 ng/mL in MoBa (1999–2008),25 and 7.6 ng/mL in the Odense Child Cohort (2010–2012).24 In recent years, multiple communities have been identified with high levels of PFAS contamination28,33–37 (e.g., from historical use of aqueous film-forming foam mainly containing PFOS and other long-chain PFAS38–40). Median serum concentrations of PFOA, PFOS, and PFNA in some of these U.S. communities33–37 are in line with plasma concentrations in our study. Additionally, many women in our study had PFAS concentrations during pregnancy that meet the 2022 National Academies of Science clinical reference (20 ng/mL for the additive sum of PFHxS, PFOS, PFOA, PFNA, PFDA, MeFOSAA, and perfluoroundecanoic acid concentrations) for people who may face a higher risk of adverse health effects due to PFAS exposure.41 Thus, as compared to prior published studies, our findings may be somewhat more generalizable to individuals currently residing in communities with relatively high PFAS exposure.
Our study further extends prior literature by quantifying the association of a PFAS mixture with breastfeeding duration, while accounting for correlations between PFAS, and thus estimating a more ‘real world’ scenario of exposure to multiple PFAS. While the MoBa study used elastic net regression to account for multiple PFAS,25 our study is the first to evaluate the joint effect estimate of a PFAS mixture on breastfeeding duration. We found that women in Project Viva had a 17% greater hazard of terminating breastfeeding per quartile increase in each component of the PFAS mixture; similar to the observed 20% greater hazard of terminating per doubling ∑PFAS (i.e., summing concentrations of PFOA, PFOS, PFHxS, PFNA, and PFDA as a single exposure biomarker) in the Odense Child Cohort.24 Additionally, we carefully considered the potential for confounding by prior cumulative breastfeeding duration – as breastfeeding is both a major PFAS excretion pathway12 and a predictor of future (i.e., index pregnancy) breastfeeding duration.42,43 Prior studies have aimed to control for confounding by prior breastfeeding duration either via adjusting for prior cumulative breastfeeding duration as a covariate and/or restricting to nulliparous participants.21–25 We employed both approaches and found similar results, although observed that adjustment only for parity (without also adjusting for prior breastfeeding duration) was not sufficient to control confounding.
Consistent with prior cohorts,22–24 we found associations of PFAS concentrations with duration of exclusive breastfeeding to be weaker than with duration of any breastfeeding. Report of exclusive breastfeeding duration may be more susceptible to social desirability bias,44 and over-reporting exclusive breastfeeding duration could bias estimates toward the null. Additionally, a woman’s decision to stop exclusively breastfeeding (versus any breastfeeding) may be more largely impacted by external psychosocial (e.g., inconvenience, wanting someone else to feed the baby) and/or lifestyle (e.g., return to work, dietary restrictions while breastfeeding) factors compared to the decision to stop any breastfeeding. We found no association between plasma PFAS concentrations and self-reported insufficient milk supply. However, perceived insufficient milk supply does not consistently correlate with actual milk supply,45 and instead may be influenced by breastfeeding self-efficacy and infant behaviors (i.e., crying, sucking).46
PFAS act through several biological pathways that may disrupt mammary gland development and the lactation process.7 Specifically, in mice, higher exposure to PFOA impairs mammary gland development47,48 and differentiation49,50; activates the peroxisome proliferator activated receptor-α,51,52 which may impede mammary lobuloalveolar development53; alters expression of genes related to milk protein50; and inhibits the placental prolactin-growth hormone family,54 an important set of hormones for mammary gland development, differentiation, and milk production.55 In humans, these effects may impact a lactating person’s milk supply, leading to earlier termination of breastfeeding or supplementation with formula or complementary foods which has clinical implications for mother and infant. Longer breastfeeding duration lowers maternal risk of type 2 diabetes mellitus, breast cancer, and ovarian cancer.1 Notably, human milk is an excretion pathway for PFAS, and among infants, the consumption of human milk can be a main source of PFOS and PFOA.56–58 Even so, with limited information about PFAS in formula (often mixed with drinking water) and first foods;59 the nutritional, immunological, and anti-inflammatory benefits of breastfeeding for infants; and long-term benefits for maternal health, the CDC and AAP advise breastfeeding despite the possible presence of endocrine disrupting environmental chemicals like PFAS.60
We are not aware of any cohorts with direct measures of milk production, and our study is similarly limited by our use of breastfeeding duration as a proxy for human milk production. Social desirability or recall bias may also introduce outcome measurement error. However, recall of breastfeeding duration has high reliability and accuracy, particularly among women with a college degree.61,62 Furthermore, we expect any social desirability or recall bias to lead to non-differential error and thus bias our results towards the null; meaning there would be an underestimation of effect estimates. Generalizability may be limited, as Project Viva participants resided in the greater Boston, Massachusetts area and were of higher socioeconomic status. Finally, we used Cox modeling approach, which is advantageous in that it provides a convenient summary measure of estimated associations of exposure with a time-varying event process,63 but also limits interpretability of results due to the estimation of hazard ratios rather than risk ratios or risk differences.64,65 However, the robustness of our observed associations across methods (Cox models and modified Poisson regression with robust error variance) suggests that our findings are not simply an artifact of the modeling approach. Our study also has several strengths. As compared to most existing studies of PFAS and breastfeeding duration, we investigated a wider number of PFAS. We also examined the joint effects of a PFAS mixture, and carefully controlled for confounding by prior breastfeeding duration as well as other sociodemographic characteristics.
Our study strengthens existing evidence that higher prenatal exposure to certain PFAS, evaluated during pregnancy, individually and as a mixture, may adversely affect breastfeeding duration. As breastfeeding plays a beneficial role in the subsequent health of women and their children,60 our findings draw attention to endocrine disrupting chemicals, such as PFAS, that may disrupt human lactation.
Supplementary Material
Highlights.
Higher plasma PFAS in pregnancy were associated with shorter breastfeeding duration
PFOA, EtFOSAA, and MeFOSAA associations were strongest across modeling approaches
Study extends prior research by examining the joint effects of a 6 PFAS mixture
Acknowledgements:
We thank the participants and staff of Project Viva. We also thank Dr. Kayoko Kato, Ayesha Patel, Tao Jia, and the late Xiaoyun (Sherry) Ye for their help with PFAS measurements, and Hannah Chidekel for assistance with the literature review.
Funding:
This work was supported by the National Institutes of Health (R01ES021447, R01HD034568, R01ES030101, and UH3OD023286). Dr. M.E. Romano was supported in part by P20 GM104416 from the National Institute of General Medical Sciences. Dr. J.T. Wallenborn was funded by the Swiss National Science Foundation, grant No. 4626882.
Footnotes
Declaration of competing interest: The authors declare they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer:
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or National Institutes of Health. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the United States (U.S.) Department of Health and Human Services.
CRediT author statement
Lisa B. Rokoff: Methodology, Formal analysis, Data Curation, Writing – Original draft, Writing – Review & Editing, Visualization
Jordyn T. Wallenborn: Conceptualization, Methodology, Formal analysis, Data Curation, Writing – Original draft, Writing – Review & Editing
Maria H. Harris: Conceptualization, Methodology, Writing – Reviewing & Editing, Supervision
Sheryl L. Rifas-Shiman: Methodology, Validation, Data Curation, Writing – Reviewing & Editing
Rachel Criswell: Writing – Reviewing & Editing
Megan E. Romano: Writing – Reviewing & Editing
Jessica G. Young: Methodology, Writing – Reviewing & Editing
Antonia M. Calafat: Methodology, Resources, Writing – Reviewing & Editing
Emily Oken: Methodology, Writing – Reviewing & Editing, Project administration, Funding acquisition
Sharon Sagiv: Conceptualization, Methodology, Writing – Reviewing & Editing, Supervision, Project administration, Funding acquisition
Abby F. Fleisch: Methodology, Writing – Reviewing & Editing, Supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meek JY, Noble L. Technical Report: Breastfeeding and the Use of Human Milk. Pediatrics. Jul 1 2022;150(1)doi: 10.1542/peds.2022-057989 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, Division of Nutrition, Physical Activity, and Obesity. Breastfeeding Report Card, United States, 2022. Updated August 31, 2022. Accessed December 5, 2022. https://www.cdc.gov/breastfeeding/data/reportcard.htm
- 3.Thulier D, Mercer J . Variables associated with breastfeeding duration. J Obstet Gynecol Neonatal Nurs. May-Jun 2009;38(3):259–68. doi: 10.1111/j.1552-6909.2009.01021.x [DOI] [PubMed] [Google Scholar]
- 4.Sriraman NK, Kellams A . Breastfeeding: What are the Barriers? Why Women Struggle to Achieve Their Goals. J Womens Health (Larchmt). Jul 2016;25(7):714–22. doi: 10.1089/jwh.2014.5059 [DOI] [PubMed] [Google Scholar]
- 5.Odom EC, Li R, Scanlon KS, Perrine CG, Grummer-Strawn L. Reasons for earlier than desired cessation of breastfeeding. Pediatrics. Mar 2013;131(3):e726–32. doi: 10.1542/peds.2012-1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S, Kelleher SL. Biological underpinnings of breastfeeding challenges: the role of genetics, diet, and environment on lactation physiology. Am J Physiol Endocrinol Metab. Aug 1 2016;311(2):E405–22. doi: 10.1152/ajpendo.00495.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Criswell R, Crawford KA, Bucinca H, Romano ME. Endocrine-disrupting chemicals and breastfeeding duration: a review. Curr Opin Endocrinol Diabetes Obes. Dec 2020;27(6):388–395. doi: 10.1097/med.0000000000000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol. Mar 2019;29(2):131–147. doi: 10.1038/s41370-018-0094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. Oct 1 2011;45(19):7954–61. doi: 10.1021/es2011622 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention, National Center for Environmental Health, Division of Laboratory Sciences. Fourth National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Accessed February 7 2023, https://www.cdc.gov/exposurereport/ [Google Scholar]
- 11.Timmermann A, Avenbuan ON, Romano ME, et al. Per- and Polyfluoroalkyl Substances and Breastfeeding as a Vulnerable Function: A Systematic Review of Epidemiological Studies. Toxics. 2023;11(4):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mondal D, Weldon RH, Armstrong BG, et al. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environmental health perspectives. Feb 2014;122(2):187–92. doi: 10.1289/ehp.1306613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: Project Viva. International journal of epidemiology. Feb 2015;44(1):37–48. doi: 10.1093/ije/dyu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagiv SK, Rifas-Shiman SL, Webster TF, et al. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ Sci Technol. Oct 6 2015;49(19):11849–58. doi: 10.1021/acs.est.5b02489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A. Apr 15 2011;1218(15):2133–7. doi: 10.1016/j.chroma.2010.10.051 [DOI] [PubMed] [Google Scholar]
- 16.Li R, Fein SB, Chen J, Grummer-Strawn LM. Why mothers stop breastfeeding: mothers’ self-reported reasons for stopping during the first year. Pediatrics. Oct 2008;122 Suppl 2:S69–76. doi: 10.1542/peds.2008-1315i [DOI] [PubMed] [Google Scholar]
- 17.Sagiv SK, Rifas-Shiman SL, Fleisch AF, et al. Early-Pregnancy Plasma Concentrations of Perfluoroalkyl Substances and Birth Outcomes in Project Viva: Confounded by Pregnancy Hemodynamics? Am J Epidemiol. Apr 1 2018;187(4):793–802. doi: 10.1093/aje/kwx332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitro SD, Sagiv SK, Rifas-Shiman SL, et al. Per- and Polyfluoroalkyl Substance Exposure, Gestational Weight Gain, and Postpartum Weight Changes in Project Viva. Obesity (Silver Spring, Md). Oct 2020;28(10):1984–1992. doi: 10.1002/oby.22933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. Feb 20 2011;30(4):377–99. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 20.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environmental health perspectives. Apr 2020;128(4):47004. doi: 10.1289/ehp5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scand J Work Environ Health. Sep 2010;36(5):413–21. doi: 10.5271/sjweh.2908 [DOI] [PubMed] [Google Scholar]
- 22.Romano ME, Xu Y, Calafat AM, et al. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environmental research. Aug 2016;149:239–246. doi: 10.1016/j.envres.2016.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmermann CAG, Budtz-Jørgensen E, Petersen MS, et al. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reprod Toxicol. Mar 2017;68:164–170. doi: 10.1016/j.reprotox.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timmermann CAG, Andersen MS, Budtz-Jørgensen E, et al. Pregnancy Exposure to Perfluoroalkyl Substances and Associations With Prolactin Concentrations and Breastfeeding in the Odense Child Cohort. The Journal of Clinical Endocrinology & Metabolism. Jan 18 2022;107(2):e631–e642. doi: 10.1210/clinem/dgab638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen EM, Brantsæter AL, Carroll R, et al. Maternal Plasma Concentrations of Per- and polyfluoroalkyl Substances and Breastfeeding Duration in the Norwegian Mother and Child Cohort. Environ Epidemiol. Sep 2018;2(3):e027. doi: 10.1097/ee9.0000000000000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. Apr 1 2004;159(7):702–6. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 27.Kleinman RE. American Academy of Pediatrics recommendations for complementary feeding. Pediatrics. Nov 2000;106(5):1274. [PubMed] [Google Scholar]
- 28.Nielsen C, Li Y, Lewandowski M, Fletcher T, Jakobsson K. Breastfeeding initiation and duration after high exposure to perfluoroalkyl substances through contaminated drinking water: A cohort study from Ronneby, Sweden. Environmental research. May 1 2022;207:112206. doi: 10.1016/j.envres.2021.112206 [DOI] [PubMed] [Google Scholar]
- 29.Olsen GW, Church TR, Miller JP, et al. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environmental health perspectives. Dec 2003;111(16):1892–901. doi: 10.1289/ehp.6316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu XC, Dassuncao C, Zhang X, et al. Can profiles of poly- and Perfluoroalkyl substances (PFASs) in human serum provide information on major exposure sources? Environmental health : a global access science source. Feb 1 2018;17(1):11. doi: 10.1186/s12940-018-0355-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol. Oct 1 2011;45(19):8037–45. doi: 10.1021/es1043613 [DOI] [PubMed] [Google Scholar]
- 32.Fei C, McLaughlin JK, Tarone RE, Olsen J. Fetal growth indicators and perfluorinated chemicals: a study in the Danish National Birth Cohort. Am J Epidemiol. Jul 1 2008;168(1):66–72. doi: 10.1093/aje/kwn095 [DOI] [PubMed] [Google Scholar]
- 33.Agency for Toxic Substances and Disease Registry. PFAS Exposure Assessments Final Report: Findings Across Ten Exposure Assessment Sites. 2022. https://www.atsdr.cdc.gov/pfas/docs/PFAS-EA-Final-Report-508.pdf
- 34.Kotlarz N, McCord J, Collier D, et al. Measurement of Novel, Drinking Water-Associated PFAS in Blood from Adults and Children in Wilmington, North Carolina. Environmental health perspectives. Jul 2020;128(7):77005. doi: 10.1289/ehp6837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daly ER, Chan BP, Talbot EA, et al. Per- and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. Int J Hyg Environ Health. Apr 2018;221(3):569–577. doi: 10.1016/j.ijheh.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 36.Nair AS, Ma ZQ, Watkins SM, Wood SS. Demographic and exposure characteristics as predictors of serum per- and polyfluoroalkyl substances (PFASs) levels - A community-level biomonitoring project in Pennsylvania. Int J Hyg Environ Health. Jan 2021;231:113631. doi: 10.1016/j.ijheh.2020.113631 [DOI] [PubMed] [Google Scholar]
- 37.McDonough CA, Choyke S, Barton KE, et al. Unsaturated PFOS and Other PFASs in Human Serum and Drinking Water from an AFFF-Impacted Community. Environ Sci Technol. Jun 15 2021;55(12):8139–8148. doi: 10.1021/acs.est.1c00522 [DOI] [PubMed] [Google Scholar]
- 38.Barzen-Hanson KA, Roberts SC, Choyke S, et al. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ Sci Technol. Feb 21 2017;51(4):2047–2057. doi: 10.1021/acs.est.6b05843 [DOI] [PubMed] [Google Scholar]
- 39.Annunziato KM, Doherty J, Lee J, et al. Chemical Characterization of a Legacy Aqueous Film-Forming Foam Sample and Developmental Toxicity in Zebrafish (Danio rerio). Environmental health perspectives. Sep 2020;128(9):97006. doi: 10.1289/ehp6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leeson A, Thompson T, Stroo HF, et al. Identifying and Managing Aqueous Film-Forming Foam-Derived Per- and Polyfluoroalkyl Substances in the Environment. Environ Toxicol Chem. Jan 2021;40(1):24–36. doi: 10.1002/etc.4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Academies of Sciences, Engineering, and Medicine, Health, and, Medicine, Division, et al. The National Academies Collection: Reports funded by National Institutes of Health. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. National Academies Press (US); 2022. [PubMed] [Google Scholar]
- 42.Nagy E, Orvos H, Pál A, Kovács L, Loveland K. Breastfeeding duration and previous breastfeeding experience. Acta Paediatr. Jan 2001;90(1):51–6. doi: 10.1080/080352501750064879 [DOI] [PubMed] [Google Scholar]
- 43.Bai DL, Fong DY, Tarrant M . Previous breastfeeding experience and duration of any and exclusive breastfeeding among multiparous mothers. Birth. Mar 2015;42(1):70–7. doi: 10.1111/birt.12152 [DOI] [PubMed] [Google Scholar]
- 44.Greiner T Exclusive breastfeeding: measurement and indicators. Int Breastfeed J. 2014;9:18. doi: 10.1186/1746-4358-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galipeau R, Dumas L, Lepage M. Perception of Not Having Enough Milk and Actual Milk Production of First-Time Breastfeeding Mothers: Is There a Difference? Breastfeed Med. May 2017;12:210–217. doi: 10.1089/bfm.2016.0183 [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Liu Y, Yu XY, Zeng TY. The rates and factors of perceived insufficient milk supply: A systematic review. Matern Child Nutr. Jan 2022;18(1):e13255. doi: 10.1111/mcn.13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E, Fenton SE. The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod Toxicol. Jul 2015;54:26–36. doi: 10.1016/j.reprotox.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang C, Tan YS, Harkema JR, Haslam SZ. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reprod Toxicol. Jun 2009;27(3–4):299–306. doi: 10.1016/j.reprotox.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environmental health perspectives. Aug 2011;119(8):1070–6. doi: 10.1289/ehp.1002741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White SS, Calafat AM, Kuklenyik Z, et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicological Sciences. Mar 2007;96(1):133–44. doi: 10.1093/toxsci/kfl177 [DOI] [PubMed] [Google Scholar]
- 51.Abbott BD, Wolf CJ, Schmid JE, et al. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicological Sciences. Aug 2007;98(2):571–81. doi: 10.1093/toxsci/kfm110 [DOI] [PubMed] [Google Scholar]
- 52.Schlezinger JJ, Puckett H, Oliver J, Nielsen G, Heiger-Bernays W, Webster TF. Perfluorooctanoic acid activates multiple nuclear receptor pathways and skews expression of genes regulating cholesterol homeostasis in liver of humanized PPARα mice fed an American diet. Toxicol Appl Pharmacol. Oct 15 2020;405:115204. doi: 10.1016/j.taap.2020.115204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Q, Kurotani R, Yamada A, Kimura S, Gonzalez FJ. Peroxisome proliferator-activated receptor alpha activation during pregnancy severely impairs mammary lobuloalveolar development in mice. Endocrinology. Oct 2006;147(10):4772–80. doi: 10.1210/en.2006-0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suh CH, Cho NK, Lee CK, et al. Perfluorooctanoic acid-induced inhibition of placental prolactin-family hormone and fetal growth retardation in mice. Mol Cell Endocrinol. Apr 30 2011;337(1–2):7–15. doi: 10.1016/j.mce.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 55.Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front Physiol. 2018;9:1091. doi: 10.3389/fphys.2018.01091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haug LS, Huber S, Becher G, Thomsen C. Characterisation of human exposure pathways to perfluorinated compounds--comparing exposure estimates with biomarkers of exposure. Environment international. May 2011;37(4):687–93. doi: 10.1016/j.envint.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 57.Criswell RL, Wang Y, Christensen B, et al. Concentrations of Per- and Polyfluoroalkyl Substances in Paired Maternal Plasma and Human Milk in the New Hampshire Birth Cohort. Environ Sci Technol. Jan 10 2023;57(1):463–472. doi: 10.1021/acs.est.2c05555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blomberg AJ, Haug LS, Lindh C, et al. Changes in perfluoroalkyl substances (PFAS) concentrations in human milk over the course of lactation: A study in Ronneby mother-child cohort. Environmental research. Feb 15 2023;219:115096. doi: 10.1016/j.envres.2022.115096 [DOI] [PubMed] [Google Scholar]
- 59.LaKind JS, Naiman J, Verner MA, Lévêque L, Fenton S. Per- and polyfluoroalkyl substances (PFAS) in breast milk and infant formula: A global issue. Environmental research. Feb 15 2023;219:115042. doi: 10.1016/j.envres.2022.115042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.American Academy of Pediatrics, Council on Environmental Health. Pediatric Environmental Health, 4th Edition. Breast Milk. American Academy of Pediatrics; 2019:238. [Google Scholar]
- 61.Li R, Ingol TT, Smith K, Oza-Frank R, Keim SA. Reliability of Maternal Recall of Feeding at the Breast and Breast Milk Expression 6 Years After Delivery. Breastfeed Med. Apr 2020;15(4):224–236. doi: 10.1089/bfm.2019.0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amissah EA, Kancherla V, Ko YA, Li R. Validation Study of Maternal Recall on Breastfeeding Duration 6 Years After Childbirth. J Hum Lact. May 2017;33(2):390–400. doi: 10.1177/0890334417691506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young JG, Stensrud MJ, Tchetgen Tchetgen EJ, Hernán MA. A causal framework for classical statistical estimands in failure-time settings with competing events. Stat Med. Apr 15 2020;39(8):1199–1236. doi: 10.1002/sim.8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinussen T, Vansteelandt S, Andersen PK. Subtleties in the interpretation of hazard contrasts. Lifetime Data Anal. Oct 2020;26(4):833–855. doi: 10.1007/s10985-020-09501-5 [DOI] [PubMed] [Google Scholar]
- 65.Hernán MA. The hazards of hazard ratios. Epidemiology. Jan 2010;21(1):13–5. doi: 10.1097/EDE.0b013e3181c1ea43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


