Abstract
Introduction
The clinical manifestations of the worldwide pandemic, which began in mainland China in December 2019, were very similar to viral pneumonia and defined as Coronavirus disease 2019 (COVID-19). Complications such as acute respiratory distress syndrome (ARDS), acute cardiac tissue damage, secondary infections, isolated coagulopathy and pulmonary embolism have been reported with COVID-19 disease.
Clinical Findings
A 79-year-old woman admitted to the emergency room (ER) had complaints of fever and cough. The patient was admitted to the ER with the suspicion of COVID-19. Samples were collected with a nasopharyngeal swab and confirmed as COVID-19. In addition, a chest CT examination was performed. In the first evaluation after admittance, the D-dimer value was measured as 450 μg/L. In the follow-up of the patient, on the 18th day, increased respiratory distress and high D-dimer level (7893 μg/L) were detected in the laboratory findings.
Outcomes
A chest CT scan had ground-glass opacities compatible with COVID-19 pneumonia. A giant cavitary lesion was detected following the development of pulmonary embolism after COVID-19 disease.
Conclusions
In rare cases of COVID-19 cavitation development may occur after pulmonary infarction. In addition, it should be remembered that emphysema, giant bulla and pneumothorax may develop in COVID-19 pneumonia cases undergoing HFNC oxygen therapy. We present a case of a giant cavitary lesion that developed following a COVID-19-related pulmonary embolism.
Keywords: pulmonary embolism; lung diseases; COVID-19; coronavirus infections/complications; SARS-Cov-2; cavitation, d-dimer
Introduction
In December 2019, several unexplained cases of pneumonia were reported in Wuhan, Hubei Province, China. Clinical findings were very similar to viral pneumonia. A new coronavirus was identified as a pathogen by examining the samples taken from the respiratory tract of patients and the disease was named Coronavirus Disease 2019 (COVID-19). The patients shared common symptoms such as fever, cough, muscle pain and fatigue. Thoracic CT scans of the patients demonstrated findings consistent with viral pneumonia. The most common CT features of the viral pneumonia were pure ground-glass opacities (GGO), GGO with consolidation, rounded opacities, linear opacities, bronchiolar wall thickening and interlobular septal thickening. Complications such as acute respiratory distress syndrome (ARDS), acute cardiac tissue damage, secondary infections, isolated coagulopathy and pulmonary embolism have been reported with COVID-19 disease.1–3 Acute pulmonary embolism (PE) causes pulmonary infarction in only 10% of cases due to the dual blood supply to the lungs.4 Cavitation can occur in 4–7% of cases of pulmonary infarction.5 The development of a pulmonary infarction larger than 4 cm is a strong risk factor for aseptic necrosis, which may lead to the development of cavitation.6 Some patients with cavitation may develop an abscess. These patients classically present complaints such as fever, leukocytosis and sputum production. We present a case of a giant cavitary lesion that developed following a COVID-19-related pulmonary embolism.
Case Presentation
A 79-year-old woman was admitted to the emergency room with fever and dry cough complaints, which she had for three days. A nasopharyngeal swab was used to collect a sample for a Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) to detect COVID-19. When the patient developed dyspnea accompanied by hypoxemia, a thoracic CT scan was performed. In addition, due to faster results during the COVID-19 pandemic, patients with dyspnea were given chest CT examinations. Her exam revealed ground-glass opacities compatible with COVID-19 pneumonia, consolidation in the left lower lobe, and a pleural effusion on the left lobe. The patient’s RT-PCR test result returned positive, confirming the suspected diagnosis of COVID-19. In the first evaluation after acceptance, the D-dimer value was measured as 450 μg/L.
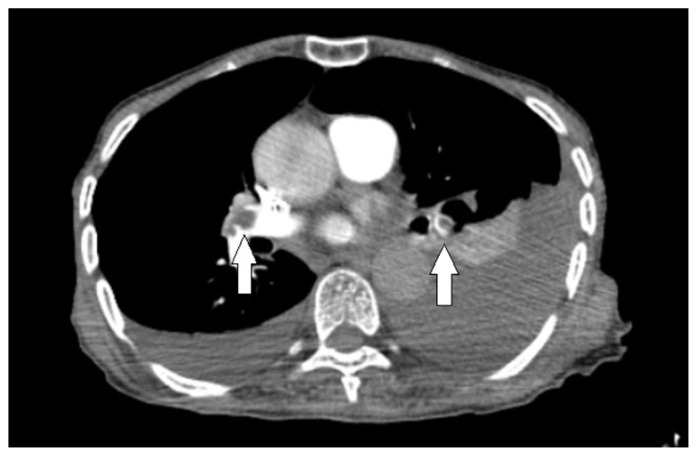
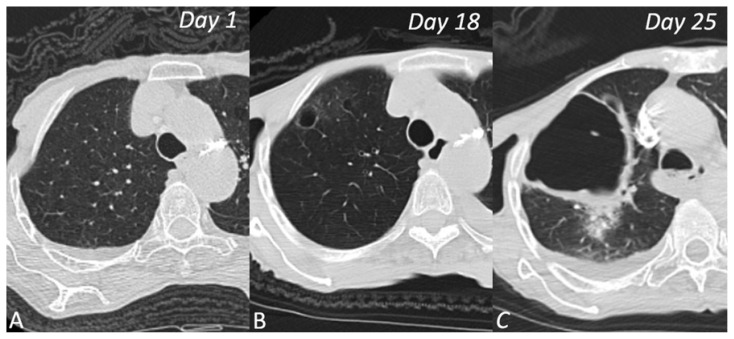
Patchy ground-glass infiltrates were observed in the right upper lobe on the patient’s first non-contrast thoracic CT on day 1. During the follow-up with the patient on day 18, a significantly elevated d-dimer level of 7893 μg/L was detected with worsening respiratory distress. In the PCTA examination performed on the 18th day, a 15-mm diameter, thin-walled air cyst in the anterior right lobe, a pulmonary embolus and ground-glass opacities were present. Also, PCTA demonstrated an acute thromboembolism was extending from both main pulmonary arteries to the lobar arteries. (Figure 1) In addition to COVID-19 treatment the patient was started on anticoagulant therapy for pulmonary embolism. During this period, high-flow nasal cannula (HFNC) oxygen therapy was initiated due to respiratory distress. In the follow-up scan performed on day 25, a giant cavitary lesion with an axial diameter exceeding 80 mm and thick walls surrounded by consolidation and ground-glass opacities were found in the right upper lobe anterior segment. (Figure 2) In addition, the new scan showed an increase in the left-sided pleural effusion and a newly developed pleural effusion on the right side. The atelectasis observed in the left lower lobe increased. It was determined that the general condition of the patient was not suitable for advanced treatment planning. Her condition rapidly deteriorated and she was taken to the intensive care unit for tracheal intubation and ventilator support but died a short time later.
Figure 1.
Axial CT angiography scan of the chest shows bilateral filling defects (white arrows) in both pulmonary arteries, representing thrombi.
Figure 2.
CT scans of 79-year-old woman who presented with fever and cough. A (Day 1) normal lung parenchyma, B (Day 18) small air cyst with ground-glass opacities and C (Day 25) giant cavitary lesion in the right upper lobe.
Discussion
Previous studies show that COVID-19 disease may have a poor prognosis with thromboembolic complications in patients with low platelet levels, increased D-dimer levels, increased prothrombin levels and significant comorbidity.7 D-dimer levels higher than 1000 μg/L in COVID-19 patients have been identified as an important risk factor for mortality.8 In our patient who was confirmed to have COVID-19 disease, an increase in D-dimer levels, acute PE development and acute giant cavitary lesion development in the lung parenchyma are observed. Although the cavitary lesion had thick walls and areas of consolidation in the surrounding parenchyma, there was no extensive consolidation/atelectasis compatible with the infarction in the surrounding parenchyma. Pulmonary infarction, which can develop after a pulmonary embolism, can cause cavitation development. In addition, factors such as old age, heart failure and chronic lung disease are reported as risk factors for the development of cavitation.5 Infected infarction leads to faster cavitation than infarcts with aseptic necrosis. In our case, the secondary infection was not considered due to thick-walled cavitary lesion and limited consolidation around it, as there was no significant fluid accumulation in it. However, aspiration and culture were not possible due to the rapid deterioration in the patient’s condition.
Sun et al. reported the development of a giant bulla in a patient with COVID-19 pneumonia.9 It is noted that, like our patient, COVID-19 patients who received HFNC oxygen therapy could develop giant bulla and pneumothorax with alveolar rupture. In our case, the origin of the giant cavitary lesion is not known exactly, but in the second scan, it was thought that it should be evaluated as a giant bulla due to the development of a thin-walled cyst and subsequent giant cystic cavitary lesion. However, pulmonary infarction and cavitary lesion development may also occur after pulmonary embolism. High mortality rates are reported for cavitations associated with pulmonary infarction, whether infected or not.10 Clinicians fighting the COVID-19 outbreak should consider the increased risk of thromboembolic complications. PE development in COVID-19 is common, especially in cases with pneumonia.11 In rare cases of COVID-19, cavitation development may occur after pulmonary infarction. In addition, it should be remembered that emphysema, giant bulla and pneumothorax may develop in COVID-19 pneumonia patients undergoing HFNC oxygen therapy.
Footnotes
Conflicts of Interest
The authors declare they have no conflicts of interest.
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/nejmoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie Y, Wang X, Yang P, Zhang S. COVID-19 Complicated by Acute Pulmonary Embolism. Radiol Cardiothorac Imaging. 2020 Apr 1;2(2):e200067. doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rajagopala S, Devaraj U, D’Souza G. Infected cavitating pulmonary infarction. Respir Care. 2011;56(5):707–709. doi: 10.4187/respcare.00828. [DOI] [PubMed] [Google Scholar]
- 5. Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985;64(5):342–348. doi: 10.1097/00005792-198509000-00006. [DOI] [PubMed] [Google Scholar]
- 6. Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol. 1986;37(4):327–333. doi: 10.1016/s0009-9260(86)80263-x. [DOI] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun R, Liu H, Wang X. Mediastinal Emphysema, Giant Bulla, and Pneumothorax Developed during the Course of COVID-19 Pneumonia. Korean J Radiol. 2020;21(5):541–544. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg. 1997;63(3):849–850. doi: 10.1016/s0003-4975(96)01253-2. [DOI] [PubMed] [Google Scholar]
- 11. Griffin DO, Jensen A, Khan M, et al. Pulmonary Embolism and Increased Levels of d-Dimer in Patients with Coronavirus Disease. Emerg Infect Dis. 2020;26(8):1941–1943. doi: 10.3201/eid2608.201477. [DOI] [PMC free article] [PubMed] [Google Scholar]