Abstract
RNase III Dicer produces small RNAs guiding sequence‐specific regulations, with important biological roles in eukaryotes. Major Dicer‐dependent mechanisms are RNA interference (RNAi) and microRNA (miRNA) pathways, which employ distinct types of small RNAs. Small interfering RNAs (siRNAs) for RNAi are produced by Dicer from long double‐stranded RNA (dsRNA) as a pool of different small RNAs. In contrast, miRNAs have specific sequences because they are precisely cleaved out from small hairpin precursors. Some Dicer homologs efficiently generate both, siRNAs and miRNAs, while others are adapted for biogenesis of one small RNA type. Here, we review the wealth of recent structural analyses of animal and plant Dicers, which have revealed how different domains and their adaptations contribute to substrate recognition and cleavage in different organisms and pathways. These data imply that siRNA generation was Dicer's ancestral role and that miRNA biogenesis relies on derived features. While the key element of functional divergence is a RIG‐I‐like helicase domain, Dicer‐mediated small RNA biogenesis also documents the impressive functional versatility of the dsRNA‐binding domain.
Keywords: Dicer, dsRBD, helicase, miRNA, siRNA
Subject Categories: RNA Biology, Structural Biology
This review discusses recent data on animal and plant Dicer substrate recognition and cleavage in small RNA pathways and highlights the helicase and dsRNA‐binding domains as key elements of functional divergence.

Introduction
Small RNAs (20–30 nucleotides [nt] long) loaded on Argonaute proteins function as sequence‐specific guides in numerous RNA silencing pathways (reviewed in Ketting, 2011). Small RNAs in many RNA silencing pathways are generated by RNase III Dicer from substrates with double‐stranded RNA structures (reviewed in Jaskiewicz & Filipowicz, 2008). Dicers and numbers of their paralogs in each species vary. Some organisms use a single Dicer for small RNA production, while others have two or more Dicers with distinct features. For example, mammals and Caenorhabditis elegans utilize a single Dicer, Drosophila two, and Arabidopsis has four (Fig 1A). This review summarizes recent remarkable progress on understanding the structural and functional variability of Dicer proteins in different model systems and discusses the main principles of Dicer protein function and evolution.
Figure 1. Dicer architecture.
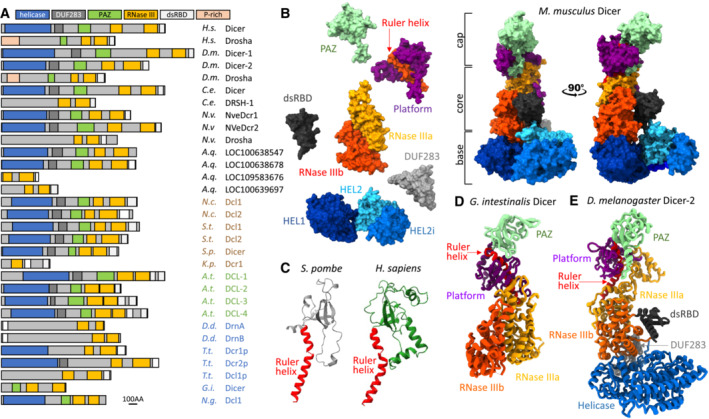
(A) Domain organization of selected Dicer and Dicer‐like proteins across eukaryotic kingdoms. Animal proteins are in a black font, fungi in brown, plants in green, and Protista in blue. Abbreviations of species names: H.s.—Homo sapiens, D.m.—Drosophila melanogaster, C.e.—Caenorhabditis elegans, N.v.—Nematostella vectensis (sea anemone), A.q.—Amphimedon queenslandica (sponge), N.c.—Neurospora crassa (red bread mold), S.t.—Sporotrichum thermophile (thermophilic fungus), S.p.—Schizosaccharomyces pombe (fission yeast), K.p.—Kluyveromyces polysporus, A.t.—Arabidopsis thaliana, D.d.—Dictyostelium discoideum (slime mold), T.t.—Tetrahymena thermophila, G.i.—Giardia intestinalis, and N.g.—Naegleria gruberi. Schemes were built based on domain annotations in Genbank, conserved domain search, sequence alignments, and inspection of AlphaFold predictions (Varadi et al, 2022). (B) Exploded and normal views of mammalian Dicer [PDB ID: 7YZ4] architecture depicting the key structural modules (Zapletal et al, 2022). (C) Comparison of the folds of the PAZ domain of S. pombe (gray fold) and H. sapiens (green fold). The red connector helix was retained in the fold to indicate orientation of the fold relative to the rest of Dicer. S. pombe fold is based on AlphaFold (Varadi et al, 2022), for the human Dicer a published structure [PDB ID: 5ZAM] was used (Liu et al, 2018). (D) A ribbon model of Dicer of Giardia intestinalis based on its crystal structure [PDB ID: 2FFL] (MacRae et al, 2006). This Dicer represents a simpler variant lacking the base and the C‐terminal dsRBD. (E) A ribbon model of the full architecture of Dicer‐2 of Drosophila melanogaster [PDB ID: 7W0B] (Su et al, 2022).
Dicer Architecture
Dicer is a general name for RNase III endoribonucleases producing small RNAs for RNA silencing pathways. There is a common order of specific modules present in Dicer across eukaryotic kingdoms, which reflects the common order of protein domains in the primary protein sequence. This protein organization constitutes the canonical Dicer architecture, despite some eukaryotic Dicers differing considerably from it (Fig 1A and B). The three‐dimensional architecture of canonical Dicer consists of three major structural regions: the cap (head), the core (body), and the base (Lau et al, 2009; Wang et al, 2009; Taylor et al, 2013; Liu et al, 2018; Fig 1B).
The core—an internal dimer of the RNase III domains
The rigid core of Dicer‐related enzymes (Fig 1B) is formed by an intramolecular dimer of two RNase III domains, which resembles a bacterial RNase III dimer (Zhang et al, 2004; MacRae et al, 2006). Each RNase III domain of Dicer cleaves one strand of the substrate (also known as “dicing”). The same intramolecular RNase III dimer organization is common for proteins called Dicer or Dicer‐like, even if they otherwise deviate from the canonical architecture. The RNase III core module is also present in Drosha, a Dicer‐related enzyme (Fig 1A), which likely evolved from a Dicer duplication in a Metazoan ancestor (Maxwell et al, 2012; Brate et al, 2018). Drosha supports animal miRNA biogenesis as the catalytic core of a nuclear Microprocessor complex that cleaves primary microRNA transcripts (pri‐miRNA) into precursor miRNA (pre‐miRNA) substrates for Dicer (reviewed in Bartel, 2018).
The cap—the PAZ domain and platform with a connector helix
The cap module is composed of the Piwi/Argonaute/Zwille (PAZ) domain, the platform, and the connector helix (Fig 1B). The PAZ domain is an RNA‐binding domain found in Dicer and Argonaute proteins (Lingel et al, 2004; Ma et al, 2004). In Dicer, PAZ is a dsRNA‐binding element (Ma et al, 2004; MacRae et al, 2006) that anchors one end of the substrate. The PAZ domain is present in animal and plant Dicers as well as in Dicer of Giardia intestinalis (MacRae et al, 2006), a flagellated unicellular parasite. The low sequence conservation of the PAZ domain impedes its recognition by sequence homology analysis with Dicers from the fungi Schizosaccharomyces pombe and Neurospora crassa (Colmenares et al, 2007; Vetukuri et al, 2011; Paturi & Deshmukh, 2021). However, despite the lack of sequence conservation, a PAZ‐like fold appears in the structural prediction for Dicer from S. pombe (Fig 1C), suggesting that the canonical Dicer architecture evolved in a common ancestor of the main eukaryotic kingdoms.
The PAZ/platform domains in different Dicers may contribute to discriminating Dicer substrates. While the PAZ has specificity for the two nucleotide 3′ overhang (3′ pocket; Lingel et al, 2004; Ma et al, 2004), the adjacent platform domain may carry a phosphate‐binding pocket, enabling combined binding of both strands of a substrate RNA duplex with the 3′ two nucleotide overhang and assuring efficient and accurate substrate processing (Park et al, 2011; Tian et al, 2014). The 5′ binding pocket is present in Drosophila Dicers and human Dicer but not in Giardia or S. pombe Dicer. Functional studies suggested that simultaneous anchoring of the 3′ and 5′ ends of the substrate terminus is a feature important for fidelity of small RNA biogenesis (Park et al, 2011; Fukunaga et al, 2014; Kandasamy & Fukunaga, 2016). A unique phosphate‐binding pocket exists in Drosophila Dicer‐2, which is required to bind the 5′ monophosphate of short dsRNA substrates but not long dsRNAs (Cenik et al, 2011; Fukunaga et al, 2014). Inorganic phosphate binding by the pocket suppresses miRNA processing in vivo and alters substrate specificity in favor of long dsRNAs (Fukunaga et al, 2014). At the same time, the phosphate‐binding pocket of Dicer‐2 supports length fidelity of siRNA production from long dsRNA (Kandasamy & Fukunaga, 2016). In monocot plants, divergence of the PAZ domain in DCL3 and DCL5 paralogs determines distinct substrate specificity in the biogenesis of distinct classes of 24‐nt siRNAs (Chen et al, 2022).
The PAZ domain is key to the biogenesis of small RNAs of defined length by different Dicers, as the length of the product is defined by the distance between the end of the substrate anchored in the PAZ domain and the catalytic center (MacRae et al, 2006, 2007). This distance is defined by an α helix (connector helix or ruler helix; Fig 1B, D and E), a structural component first reported in the Dicer structure from Giardia (MacRae et al, 2006), which directly connects the PAZ domain with the RNase III domains (Fig 1D).
The base—the N‐terminal helicase domain
The base of the enzyme consists of an N‐terminal helicase domain of the RIG‐I‐like receptor subgroup, which forms a clamp‐like structure near RNase III (Lau et al, 2009, 2012; Taylor et al, 2013; Liu et al, 2018). This domain is composed of three globular subdomains: an N‐terminal DExD/H domain (HEL1), an insertion domain (HEL2i), and a helicase superfamily C‐terminal domain (HEL2). As we will discuss later, the N‐terminal helicase is another structural element involved in substrate recognition and functional differentiation of Dicer homologs as shown by structural and functional analyses (Welker et al, 2011; Liu et al, 2012; Kidwell et al, 2014; Sinha et al, 2015; Wang et al, 2021; Wei et al, 2021; Jouravleva et al, 2022; Su et al, 2022; Yamaguchi et al, 2022; Zapletal et al, 2022; Aderounmu et al, 2023). It also mediates interactions with regulatory proteins (reviewed in Hansen et al, 2019).
dsRBD and DUF283 domains
Two additional domains are part of the common Dicer architecture: the C‐terminal dsRNA‐binding domain (dsRBD), which localizes near the catalytic core, and the DUF283 domain localized next to the helicase domain at the boundary between the core and the base (Fig 1). DUF283 has a dsRBD fold (Dlakic, 2006) and, like dsRBD, appears to interact with the substrate during dicing (Wang et al, 2021; Wei et al, 2021; Jouravleva et al, 2022; Su et al, 2022; Yamaguchi et al, 2022; Zapletal et al, 2022). The C‐terminal dsRBD has also been implicated in affecting the nucleocytoplasmic localization of Dicer (Vagin et al, 2009; Doyle et al, 2013).
While the above‐described canonical Dicer architecture (Fig 1B) exists across eukaryotic kingdoms, there are Dicer variants deviating from the cap‐core‐base architecture. The abovementioned Dicer from Giardia lacks the base as well as the C‐terminal dsRBD, representing a “minimal Dicer” composed of the core and head parts. There are other Dicers, whose architecture deviates even more. For example, DrnA/B in Dictyostelium retains the tandem RNase III core module, the dsRBD is placed at the N terminus and the central part of the protein does not show sequence homology with canonically built Dicers (Hinas et al, 2007; Kruse et al, 2016; Liao et al, 2018; Fig 1A). How DrnA/B determine the length of the product remains unclear. Similarly, abovementioned Drosha proteins may be considered highly derived Dicer variants retaining the core module with the dsRBD (Kwon et al, 2016). Remarkably, noncanonical Dicer Dcr1 in Kluyveromyces polysporus carrying just a single RNase III domain and a tandem dsRBD (Fig 1A) is also able to generate small RNAs of specific lengths (Weinberg et al, 2011). Structural analyses of Dcr1 suggested that the siRNA length of ~ 23 nt is generated upon cooperative binding of long dsRNA by an array of Dicers, where dicing by two adjacent Dicers yields a sized siRNA duplex.
Main Dicer Binding Partners
Dicer interacts with many proteins but two binding partner families stand out: (I) Aproteins from the Argonaute family, which bind small RNAs generated by Dicer and (II) dsRNA‐binding proteins with tandemly arrayed dsRBD, which facilitate substrate recognition, dicing fidelity, and Argonaute loading.
Argonaute proteins form the core of effector complexes and provide another layer for divergence of RNA silencing pathways (reviewed in Meister, 2013). Argonautes exist in both, prokaryotes and eukaryotes suggesting they emerged before the canonical Dicer architecture was formed. Eukaryotic Argonautes have a stereotypical bilobed architecture consisting of four domains: N‐terminal, PAZ, MID and PIWI. The Argonaute PAZ domain binds the 3′ end of the loaded small RNA (Lingel et al, 2003; Song et al, 2003; Yan et al, 2003). The 5′ end of the loaded small RNA is recognized by the MID domain (Boland et al, 2010; Frank et al, 2010). The PIWI domain has an RNaseH fold and provides nuclease activity for Argonaute homologs able to cleave RNAs complementary to the loaded small RNA (Liu et al, 2004; Meister et al, 2004). Interaction of human Dicer with AGO Argonaute subfamily was proposed through a region near the RNase IIIa domain (Sasaki & Shimizu, 2007).
The second type of common Dicer interaction partners is dsRNA‐binding proteins with three dsRBDs, such RDE‐4 in C. elegans (Tabara et al, 1999, 2002). Loquacious (LOQS) and R2D2 in Drosophila (Liu et al, 2003; Forstemann et al, 2005; Jiang et al, 2005; Hartig et al, 2009; Zhou et al, 2009), TARBP2 and PACT in mammals (Chendrimada et al, 2005; Haase et al, 2005; Lee et al, 2006) or double‐stranded RNA‐binding (DRB) family proteins in plants (Clavel et al, 2016). These proteins harbor a C‐terminal Type B dsRBD, which exhibits no interaction with RNA, but mediates protein–protein interaction. Interaction with Dicer can be exemplified by mammalian TARBP2 whose C‐terminal type B dsRBD (so‐called MEDIPAL domain) is degenerated and interacts with Dicer's HEL2i region (Laraki et al, 2008; Daniels et al, 2009; Wilson et al, 2015). However, despite their similar architectures, Dicer‐binding dsRBD proteins adapted different roles in substrate recognition and processing in RNA silencing in different species (Liu et al, 2003; Chendrimada et al, 2005; Haase et al, 2005; Parker et al, 2006, 2008; Marques et al, 2010; Hartig & Forstemann, 2011).
Dicer Substrates
Dicer substrates can be classified in different ways. Here, we divide substrates into three groups according to their structure and predictability of small RNA sequence (Fig 2A).
Figure 2. Dicer substrates and principles of their dicing.
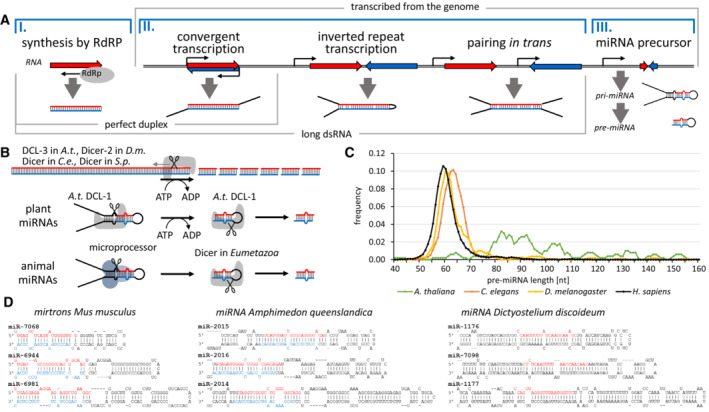
(A) Schematic representation of origins of different dsRNA substrates and their key features. Genomic transcripts producing dsRNA can originate from repetitive transcripts in pathways protecting genome integrity, or from genic sequences in pathways regulating gene expression. (B) Two modes of substrate processing. In the processive mode, Dicer is dicing long dsRNA molecules in a set of consecutive dicing where ATP‐dependent activity of the helicase domain “feeds” the substrate into Dicer. In the distributive mode, Dicer performs a single cleavage and then binds a new substrate. (C) Variability of lengths of pre‐miRNAs in human, C. elegans, D. melanogaster and A. thaliana. Pre‐miRNA lengths were estimated for miRNAs having both mature miRNA strands annotated in the miRBase (Kozomara et al, 2019). The curve was smoothed by calculating average length for five adjacent miRNAs, points in the graph correspond to the central size of the five. (D) Several examples of murine mirtrons and miRNA stem‐loop miRNA precursors from the sponge Amphimedon and slime mold Dictyostelium. Stem‐loop structures were obtained from miRBase (Kozomara et al, 2019). Annotated mature miRNAs are highlighted in red font when on the ascending strand (5p miRNA) and blue font when on descending strand (3p miRNA).
Perfectly complementary long dsRNAs with a blunt end
These are typically produced by an RNA‐dependent RNA polymerase (RdRP) that synthesizes the antisense strand from the end of a template. It may be a hallmark of replicating viral RNA and its processing by Dicer one of the mechanisms of innate immunity (reviewed in Hur, 2019). RdRPs are also an intrinsic element in many RNA silencing mechanisms (e.g., in transcriptional silencing in plants and S. pombe; Mourrain et al, 2000; Colmenares et al, 2007) where RdRPs convert specific RNAs into Dicer substrates. A blunt‐end dsRNA can be recognized through the PAZ domain (Zhang et al, 2002; MacRae et al, 2007). However, the point‐of‐entry for long dsRNA into Dicer may also be the RIG‐I‐like N‐terminal helicase domain (Welker et al, 2011; Sinha et al, 2018). In the processive mode of dicing (Fig 2B), the ATPase activity of the helicase domain facilitates the enzyme's movement along the substrate, which is processed by a single Dicer molecule in an ATP‐dependent series of consecutive dicing (Zamore et al, 2000; Bernstein et al, 2001; Ketting et al, 2001; Nykanen et al, 2001; Cenik et al, 2011; Naganuma et al, 2021).
Perfectly and nearly perfectly complementary long dsRNAs with longer single‐stranded overhangs
Such dsRNAs typically arise from genomic transcription followed by intra or intermolecular base pairing of cellular transcripts (reviewed in Chen & Hur, 2022). Such structures usually have one or two long single‐stranded overhangs (Fig 2A), as such transcripts are unlikely to match precisely to generate a blunt end. This precludes direct access of Dicer to ends of the double‐stranded region. However, Dicer is able to dice such substrates by a poorly understood endonucleolytic activity (Zhang et al, 2002), which yields fragments with two nucleotide overhangs, which are then efficiently recognized and processed by Dicer.
Small hairpin substrates
Small hairpin substrates are common substrates for the biogenesis of miRNA‐like molecules. Annotated miRNAs in animals, plants, and other taxonomic groups (Kozomara et al, 2019) show that miRNAs are produced from a heterogeneous assortment of substrates that are not processed in a uniform way. At the same time, a common feature of miRNAs is that their biogenesis results in a small RNA with a defined sequence.
In plants, miRNAs are generated by a single Dicer, DCL‐1, that cuts a miRNA precursor twice. Many plant miRNAs evolved from eroded larger inverted repeats originally producing long dsRNA (reviewed in Svoboda & Di Cara, 2006) and often suppress complementary sequences by RNAi‐like endonucleolytic cleavage. Consequently, many plant miRNAs retain longer stems (Fig 2C) and are diced by DCL1 in two consecutive cleavages in an ATP‐dependent manner reminiscent of siRNA biogenesis (Fig 2B).
Canonical animal miRNAs are a distinct small RNA class whose biogenesis involves a two‐step processing by two RNase III family proteins—Drosha and Dicer (Fig 2B). Nuclear Drosha generates pre‐miRNA, a small precursor hairpin, from a long pri‐miRNA transcript. Subsequently, cytoplasmic Dicer cleaves the pre‐miRNA and releases a ~ 22 nt miRNA, which is loaded onto Argonaute. Canonical miRNAs in Eumetazoan animals typically emerge de novo (Chen & Rajewsky, 2007; Meunier et al, 2013), presumably from random small hairpin structures entering the miRNA biogenesis machinery. Given the distinct modes of biogenesis and independent adaptation of animal Dicers for miRNA biogenesis, miRNAs in Eumetazoan animals have shorter stems and smaller loops than plant miRNAs while showing slight differences in precursor length distribution (Fig 2C). There are also noncanonical animal miRNAs, such as mirtrons, which are Drosha‐independent, and are Dicer hairpin substrates derived from specific small spliced‐out introns (Fig 2D). How the biogenesis of annotated miRNAs from species from other taxonomic groups (e.g., Dictyostelium or Phytophthora) occurs is unclear. But the precursors of these miRNAs seem to have longer stems and loops, such as plant precursors do (Fig 2D).
Taken together, Dicer substrates have different structural features, which facilitate their recognition and cleavage. Three types of endonucleolytic cleavages by Dicer can be recognized: ATP‐dependent processive cleavage of dsRNA from its terminus, poorly understood internal endonucleolytic cleavage of long dsRNA with inaccessible ends, and loop removal from small hairpin miRNA precursors.
Dicer Function at the Molecular Level
While Dicer was identified as an enzyme producing small RNAs more than two decades ago (Bernstein et al, 2001), only recent structural analyses of Dicers in plants (Wang et al, 2021; Wei et al, 2021) Drosophila (Jouravleva et al, 2022; Su et al, 2022; Yamaguchi et al, 2022) and mammals (Liu et al, 2018; Zapletal et al, 2022; Lee et al, 2023b) have provided detailed structural insights into miRNA and siRNA biogenesis by Dicer. Several Dicer homologs were captured in apo form and in multiple RNA‐bound states (Table 1), revealing details about substrate selection, catalysis, and product release, and how Dicer cooperates with dsRBP cofactors during RNA processing. These structural data from multiple organisms allow us to take a closer look at common and derived features of Dicer in substrate recognition and processing.
Table 1.
Available structures of full‐length Dicers.
| PDB ID | Protein/complex designation | Species | Note | References |
|---|---|---|---|---|
| 2FFL | Dicer | Giardia | Apo form | MacRae et al (2006) |
| 2QVW | Dicer | Giardia | Apo form | MacRae & Doudna (2007) |
| 7ELD | DCL1–pri‐miRNA complex | Arabidopsis | No Mg/ATP, pri‐miRNA dicing state | Wei et al (2021) |
| 7ELE | DCL1–pre‐miRNA complex | Arabidopsis | No Mg/ATP, pre‐miRNA dicing state | Wei et al (2021) |
| 7VG3 | DCL‐3–30 bp RNA complex | Arabidopsis | Dicing state | Wang et al (2021) |
| 7VG2 | DCL‐3–40 bp RNA complex | Arabidopsis | Dicing state | Wang et al (2021) |
| 6BUA | Dcr‐2 cap‐core | Drosophila | Apo form | Sinha et al (2018) |
| 7V6B | Dcr‐2:R2D2 | Drosophila | Apo form | Yamaguchi et al (2022) |
| 7V6C | Dcr‐2:R2D2:siRNA | Drosophila | Composite recognition/strand select | Yamaguchi et al (2022) |
| 7W0A | Dcr‐2:LOQS‐PD | Drosophila | Dimer | Su et al (2022) |
| 7W0B | Dcr‐2:LOQS‐PD:50 bp dsRNA | Drosophila | −ATP, apo state | Su et al (2022) |
| 7W0C | Dcr‐2:LOQS‐PD:50 bp dsRNA | Drosophila | +ATP, Mg2+, early‐translocation | Su et al (2022) |
| 7W0D | Dcr‐2:LOQS‐PD:50 bp dsRNA | Drosophila | +ATP, Mg2+, mid‐translocation | Su et al (2022) |
| 7W0E | Dcr‐2:LOQS‐PD:50 bp dsRNA | Drosophila | +ATP, Mg2+, dicing | Su et al (2022) |
| 7W0F | Dcr‐2:LOQS‐PD:50 bp dsRNA | Drosophila | +ATP, Mg2+, post‐dicing | Su et al (2022) |
| 8DGI | Dcr‐1:LOQS‐PB | Drosophila | Apo form, closed conformation (Ia) | Jouravleva et al (2022) |
| 8DGJ | Dcr‐1:LOQS‐PB | Drosophila | Apo form, closed conformation (Ib) | Jouravleva et al (2022) |
| 8DFV | Dcr‐1:LOQS‐PB:pre‐let‐7 | Drosophila | Ca2+, dicing‐competent state (IIa) | Jouravleva et al (2022) |
| 8DG5 | Dcr‐1:LOQS‐PB:pre‐let‐7 | Drosophila | Mg2+, dicing‐competent state (IIb) | Jouravleva et al (2022) |
| 8DG7 | Dcr‐1:LOQS‐PB:pre‐let‐7 | Drosophila | Mg2+, 5′ arm dicing state (III) | Jouravleva et al (2022) |
| 8DGA | Dcr‐1:LOQS‐PB:pre‐let‐7 | Drosophila | Mg2+, 3′ arm dicing state (IV) | Jouravleva et al (2022) |
| 7YZ4 | Dicer | Mouse | Apo form | Zapletal et al (2022) |
| 7YYM | Dicer:pre‐miR‐15 | Mouse | Pre‐dicing state | Zapletal et al (2022) |
| 7YYN | DicerO:pre‐miR‐15 | Mouse | Dicing state | Zapletal et al (2022) |
| 7ZPK | Dicer:pre‐miR‐15:TARBP2 | Mouse | Pre‐dicing state | Zapletal et al (2022) |
| 7ZPI | Dicer:pre‐miR‐15:TARBP2 | Mouse | Dicing state | Zapletal et al (2022) |
| 5ZAK | Dicer:TARBP2 | Human | Apo form | Liu et al (2018) |
| 5ZAL | Dicer:TRBP:pre‐let‐7 | Human | Pre‐dicing (class I) | Liu et al (2018) |
| 5ZAM | Dicer:TRBP:pre‐let‐7 | Human | Pre‐dicing (classII) | Liu et al (2018) |
| 7XW2 | Dicer‐pre‐miRNA | Human | Dicing state | Lee et al (2023b) |
| 7XW3 | Dicer | Human | Apo form | Lee et al (2023b) |
Dicer helicases determine how the enzyme selects and loads its substrate
The determined structures of Dicer from invertebrates (Drosophila Dicer‐1 and Dicer‐2) and Dicer‐1 from mammals all adopt very similar closed “L”‐shaped conformations in the apo state (Liu et al, 2018; Jouravleva et al, 2022; Su et al, 2022; Yamaguchi et al, 2022; Zapletal et al, 2022; Lee et al, 2023b). In this autoinhibited conformation reflecting in vitro data (Ma et al, 2008), the helicase domain is associated with RNase IIIb and limits the loading of RNA into the catalytic site. The helicase domains of these Dicers have diverged during evolution. For example, Drosophila Dicer‐1 has a degenerate helicase domain and is an ATP‐independent enzyme (Tsutsumi et al, 2011), Drosophila Dicer‐2 has a conserved helicase domain that hydrolyzes ATP (Zamore et al, 2000; Nykanen et al, 2001; Cenik et al, 2011; Sinha et al, 2015), and mammalian Dicer‐1, despite conservation of its helicase domain, does not hydrolyze ATP (Zhang et al, 2002). Accordingly, Dicers employ distinct mechanisms of substrate recognition and loading. This view is in line with the recent attempt to reconstruct ancestral helicase sequences of animal Dicers and analyze their properties in vitro (Aderounmu et al, 2023).
Upon RNA binding, the horseshoe‐shaped structure of the helicase domain of Drosophila Dicer‐2 associates with the DUF283 domain to form a ring (similar to RIG‐I‐like receptor) that threads the RNA substrate into the enzyme (Fig 3A; Sinha et al, 2018; Su et al, 2022). Using this mechanism, Dicer‐2 can process not only dsRNA with a blunt end (Sinha et al, 2018) but also those with an overhang at the 3′‐end, as suggested by quantitative biochemical evidence (Cenik et al, 2011), single‐molecule fluorescent microscopy (Naganuma et al, 2021), and a recent structural study (Su et al, 2022). While it is still controversial whether or not the initial binding of RNA requires ATP, the subsequent translocation of the RNA substrate through the helicase‐DUF283 ring is ATP‐dependent (Sinha et al, 2018; Naganuma et al, 2021; Singh et al, 2021; Su et al, 2022).
Figure 3. Substrate recognition and dicing by Dicer.
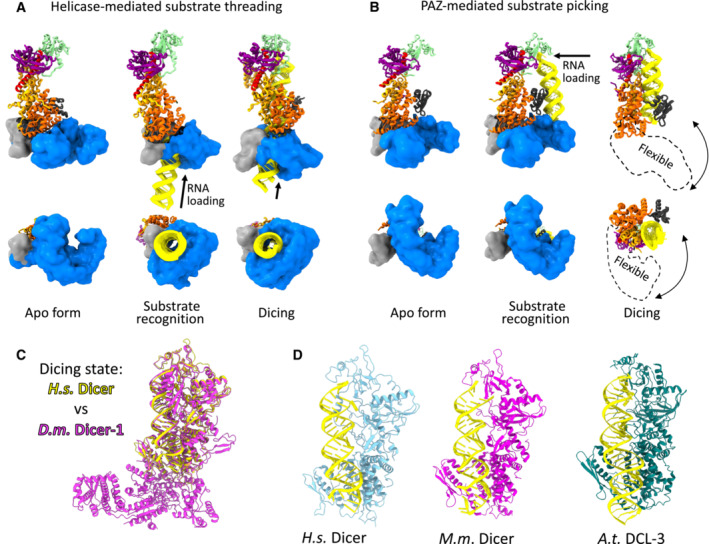
(A) Substrate recognition and threading of dsRNA substrate through the helicase domain exemplified on Drosophila Dicer‐2 (PDB IDs: 7W0B, 7W0A [monomer], and 7W0E) (Su et al, 2022). (B) Substrate recognition involving PAZ domain exemplified by miRNA recognition by mouse Dicer‐1 (PDB ID: 7YZ4, 7YYM, and 7YYN) (Zapletal et al, 2022). In the dicing state, the helicase becomes flexible and its precise position cannot be determined. (C) Overlay of the dicing state of human Dicer‐1 (in yellow) and Drosophila Dicer–1 (in magenta; PDB IDs: 7XW2 and 8DG7) (Jouravleva et al, 2022; Lee et al, 2023b). (D) Dicing states of human, murine and Arabidopsis Dicers (PDB IDs: 7XW2, 7YYN, and 7VG2; Wang et al, 2021; Zapletal et al, 2022; Lee et al, 2023b). Position of the helicase domain in these dicing states was not determined.
By contrast, the helicase domain of mammalian Dicer‐1 is not rearranged and remains in the closed “L”‐shaped conformation and encircles the pre‐miRNA substrate during initial RNA binding. This mechanism discriminates between authentic miRNA precursors and other RNA hairpins by anchoring three elements: the RNA ends, the central region, and the terminal loop (Fig 3B). Upon binding of the substrate (and TARBP2), Dicer switches into an active open state that allows repositioning of the substrate into the RNA processing center. This substrate repositioning requires disengagement of the helicase domain and DUF283 from the Dicer core, and the unlocked helicase becomes highly flexible in the dicing state (invisible in cryo‐EM), and the same has been reported for Dicer in mice (Zapletal et al, 2022) and humans (Lee et al, 2023b). Interestingly, the helicase domain of Dicer‐1 in flies remains fully bound to the Dicer core and undergoes only a small conformational change toward an open conformation that is sufficient to accommodate authentic miRNA precursors (Jouravleva et al, 2022).
Structural studies in plants showed that DCL‐3, an siRNA‐producing enzyme, adopts the same active conformation as mammalian miRNA‐producing Dicer‐1, whose helicase domain and DUF283 are disengaged from the Dicer core and are highly flexible (Wang et al, 2021). In contrast, the miRNA‐producing DCL‐1 in plants, which combines the activities of both Drosha and Dicer in mammals, uses a helicase‐mediated threading mechanism typical of the siRNA‐producing Dicers in animals. The DCL‐1 structures showed that the helicase‐DUF238 threading ring transfers the RNA substrate in an ATP‐dependent manner, while the enzyme performs two cuts, first pri‐miRNA to form pre‐miRNA and then pre‐miRNA to miRNA (Wei et al, 2021).
Dicing mechanism
The set of recent structures showed that the adaptation of Dicer to specific roles in different organisms and pathways (Box 1) is encoded in the substrate selection and in the loading mechanism, while the actual mechanism of catalysis remains invariant. The superposition of Dicer–RNA structures, regardless of organism or pathway, shows virtually identical conformations of the core elements involved in the catalysis (Fig 3C and D; Wang et al, 2021, Jouravleva et al, 2022 #744; Zapletal et al, 2022; Lee et al, 2023b). This includes the insertion of 3′ and 5′ ends of pre‐miRNA into a basic pocket of the PAZ‐Platform cassette, which aligns the substrate and determines the dicing site. The pre‐miRNA is further aligned in the positively charged groove formed by the RNase IIIa/b domains. The internal dsRBD of Dicer clamps the RNA in the catalytic sites of Dicer. A recent study proposed that the dsRBD of human Dicer recognizes the GYM motif (G guanine; Y, paired C/U; M, mismatched nucleotide) and stabilizes the interaction between the RNA and Dicer for a more efficient dicing reaction (Lee et al, 2023b). This molecular interaction could explain the evolutionary conservation of the GYM motif in a subset of miRNAs (Lee et al, 2023a).
Box 1. Characteristics of Dicer structures.
Drosophila Dicer‐2
Inactive form: L‐shaped.
Active form: the horseshoe‐shaped structure of the helicase domain associates with the DUF283 domain to form a ring that threads the RNA substrate into the enzyme (yet side‐loading (ATP‐independent; distributive) can occur for the 3′overhang substrate).
Drosophila Dicer‐1
Inactive form: L‐shaped.
Active form: small opening of the helicase domain during the miRNA substrate loading, the helicase stays associated with the core.
Vertebrate Dicer‐1
Inactive form: L‐shaped.
Active form: the helicase domain and DUF283 entirely disengage from the Dicer core.
Plant DCL‐3
Inactive form: not published.
Active form: the helicase domain and DUF283 entirely disengage from the Dicer core.
Plant DCL‐1
Inactive form: not published.
Active form: the horseshoe‐shaped structure of the helicase domain associates with the DUF283 domain to form a ring that threads the RNA substrate into the enzyme.
Internal and external dsRBDs modulate Dicer's activities
Recent structures of mammalian Dicer‐1 unexpectedly revealed that its internal C‐terminal dsRBD has two distinct RNA‐binding sites. The first RNA‐binding site, situated on the β‐sheet surface of the domain, is responsible for binding the central double‐helical region of the pre‐miRNA in the pre‐dicing state. In contrast, the second RNA‐binding site is located on the α‐helical surface that is at the opposite side of the domain and is used to clamp RNA in the catalytic sites of Dicer in the dicing state (Fig 4B and C; Zapletal et al, 2022; Lee et al, 2023b). The fact that the dsRBD switches between its two RNA‐binding sites during the multistep catalysis process of Dicer demonstrates the remarkable plasticity of this important domain. The structural studies of D.m. Dicer‐2 showed that the dsRBD also helps to induce bending of the dsRNA substrate, which facilitates the proper alignment of the substrate with the core of Dicer and its translocation into the PAZ domain (Su et al, 2022).
Figure 4. Different modes of RNA binding by dsRBD in substrate selection, dicing, and substrate release by Dicer.
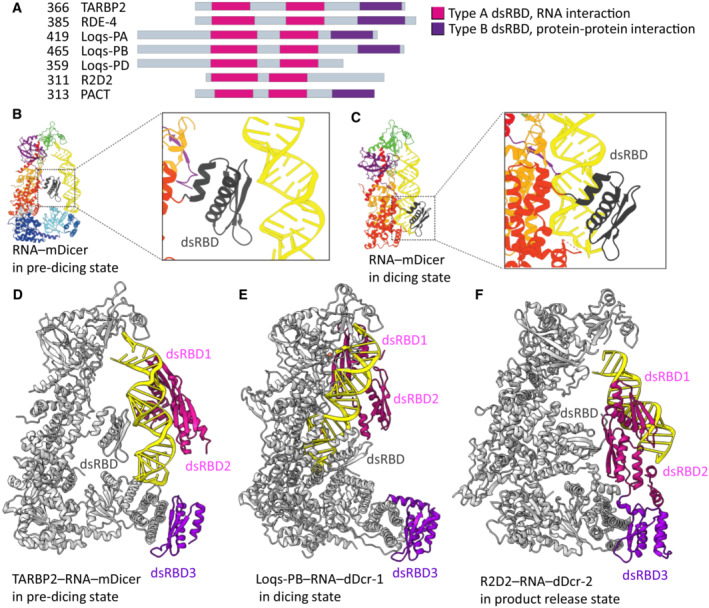
(A) Overview of Dicer cofactors that contain dsRBD. (B) In pre‐dicing state, the internal dsRBD of mouse Dicer unusually uses its β‐sheet surface to recognize RNA (Zapletal et al, 2022; PDB ID: 7YYM). (C) In dicing state, the internal dsRBD of mouse Dicer utilizes its α‐helical face for RNA recognition (Zapletal et al, 2022; PDB ID: 7YYN). (D) TABRB2 dsRBD1 and 2 bind the central region of pre‐miRNA (Zapletal et al, 2022; PDB ID: 7ZPK). (E) Dicer‐1 dsRBD and Loqs‐PB dsRBD encircle the RNA and lock it in the catalytic site (Jouravleva et al, 2022; PDB ID: 8DFV). (F) While Dicer–2 releases the substrate, R2D2 asymmetrically recognizes the end of the siRNA duplex with the higher base‐pairing stability (PDB ID: 7V6C). dDcr‐1, Drosophila Dicer–1; dDcr–2, Drosophila Dicer–2; mDicer, murine Dicer1.
Additionally, Dicers associate with accessory dsRBD‐containing proteins such as TARBP2, ADAR1, PKR, PACT in mammals, and Loquacious‐PA/PB/PD and R2D2, in invertebrates (Fig 4A; Hansen et al, 2019). These external regulatory dsRBD‐containing proteins associate with the Dicer helicase domain via their C‐terminal Type B dsRBD (protein–protein interacting domain; Doyle & Jantsch, 2002; Hansen et al, 2019) as in TARPB2, R2D2, and Loquacious‐PA/PB (Fig 4D–F) or via unstructured regions as in Loquacious‐PD (Su et al, 2022). In general, these binding partners facilitate miRNA or siRNA precursor interaction with Dicers. Recent structural work on D.m. Dicer‐2 showed that the C‐terminal tail of Loquacious‐PD binds to the helicase domain of Dicer‐2 and its two dsRBDs support substrate binding (Su et al, 2022). However, the dsRBDs of Loquacious‐PD were not observed in the density map of the initial binding state, suggesting that the dsRBDs have only an assisting role in the initial binding of dsRNA to the Dicer‐2 helicase domain. In mammals, TARBP2 stimulates the transition from the pre‐dicing to dicing‐competent state (Zapletal et al, 2022). In the pre‐dicing state, the dsRBD3 of TARBP2 stably associates with the Dicer‐1 helicase (Fig 4D; Liu et al, 2018; Zapletal et al, 2022), while the first two dsRBDs bind dsRNA in a flexible manner, similarly as observed for Loquacious‐PD (Fig 4D; Zapletal et al, 2022). Akin to TARBP2, the C‐terminal dsRBD3 of Loquacious‐PB interacts with the helicase domain of D.m. Dicer‐1. By contrast, the first two dsRBDs of Loquacious‐PB are stably bound to the miRNA precursor and assist D.m. Dicer‐1 in the positioning of the pre‐miRNA into the RNA processing center (Fig 4E; Jouravleva et al, 2022).
Recent structures of the Dicer‐2‐R2D2‐siRNA complexes provided insight into substrate release and strand‐selection state mediated by R2D2 in the siRNA‐loading process. In this state, the internal dsRBD of Dicer is dissociated from the siRNA and R2D2 stably and asymmetrically recognizes the end of the siRNA duplex with the higher base‐pairing stability, while the other end (the guide strand for target silencing) is accessible for loading onto AGO2 (Fig 4F; Yamaguchi et al, 2022).
In previous structural studies, the isolated dsRBDs were shown to bind dsRNA with little or no specificity (Green & Mathews, 1992; St Johnston et al, 1992; Bass et al, 1994; Bycroft et al, 1995; Kharrat et al, 1995; Nanduri et al, 1998; Ryter & Schultz, 1998; Lehmann & Bass, 1999; Fierro‐Monti & Mathews, 2000; Ramos et al, 2000; Blaszczyk et al, 2004; Gan et al, 2006; Stefl et al, 2006; Masliah et al, 2018), but several studies have reported that they can bind preferentially when irregularities (e.g., mismatches, internal loops, and stem‐loop junctions) are present in the RNA double helix (Stefl et al, 2005, 2010; Wang et al, 2011; Jayachandran et al, 2016; Lazzaretti et al, 2018; Yadav et al, 2020). It was shown that the irregularities widen the minor groove of the RNA double helix, thereby facilitating the positioning of the α1 helix of the dsRBD on dsRNA. Recent Dicer–RNA structures with dsRBD‐containing regulatory proteins show that the mode of dsRBD binding is governed by the dynamic structural context of multistep Dicer catalysis. This versatility of dsRBDs ranges from nonspecific interactions with multiple dsRNA registers during initial substrate recruitment to highly specific interactions (mainly dsRBDs α1 helix in the minor dsRNA groove) that directly modulate Dicer catalysis and control substrate release to properly feed AGO2 with a guide strand for target silencing (Liu et al, 2018; Jouravleva et al, 2022; Su et al, 2022; Yamaguchi et al, 2022; Zapletal et al, 2022; Lee et al, 2023b).
Ancestral and Derived Dicer Functions
For a more detailed overview of the evolution of Dicer and its paralogs, we refer readers to the works of Mukherjee et al (2013) and Jia et al (2017). Here, we will discuss only selected issues, which have emerged from the recent structural analyses. As mentioned earlier, the complex canonical Dicer architecture found in animals, plants, fungi, and Protista implies that the canonical Dicer architecture with a functional ATPase/helicase had evolved already in the common ancestor of all three multicellular kingdoms and at least some extant Protista. The RIG‐I‐like helicases serve as cytoplasmic dsRNA sensors for antiviral immunity across animals (Yoneyama & Fujita, 2007; Kowalinski et al, 2011; Guo et al, 2013). RIG‐I recognizes blunt‐end dsRNA with 5′ triphosphate (Kowalinski et al, 2011), which is a hallmark of viral replication. The primary function of the canonical Dicer architecture was thus most likely linked to dsRNA processing in antiviral defense in early eukaryotes living in aquatic environments which hosted virioplankton. While it is unknown how dense and diverse virioplankton was in primeval oceans, the exposure of early eukaryotes to viruses was likely intense. This notion is consistent with high throughput analyses of seawater nowadays, which reveal unexpected density and diversity of extant virioplankton, including RNA viruses (Vlok et al, 2019; Wolf et al, 2020). The antiviral role is highly plausible for explaining the emergence and success of the canonical Dicer architecture. Given the major regulatory potential of sequence‐specific targeting by small RNA, it is not surprising that other RNA silencing pathways emerged, such as the gene expression‐regulating miRNA pathway or transcriptional silencing mechanisms.
The major requirement for the miRNA pathway is biogenesis of precisely defined small RNAs, which enables their specific engagement in gene regulation. In plants and animals, miRNA biogenesis involves distinct mechanisms (reviewed in Rogers & Chen, 2013; Bartel, 2018). In plants, two cleavages by DCL1 release a miRNA from its precursor. In animals, the mechanism involves first nuclear cleavage by Drosha and then cytoplasmic cleavage by Dicer. In this context, Drosha appears as a highly specialized animal Dicer variant emerging from Dicer duplication early in Metazoan evolution. Animal Dicer‐1 shows distinct adaptations to miRNA biogenesis in different animal lineages further supporting the notion that animal miRNA biogenesis by Dicer is a derived character. The main adaptation concerned the helicase domain, whose evolution in animal Dicers took diverse paths. In C. elegans DCR‐1, the helicase is fully functional as the enzyme is efficiently processing both types of substrates—long dsRNA and pre‐miRNAs. In Drosophila, the helicase of miRNA‐producing Dicer‐1 lost its original function and degenerated, while siRNA‐producing Dicer‐2 carries a functional RIG‐I‐like helicase hydrolyzing ATP and feeding dsRNA substrate into the enzyme. A recent attempt to reconstruct ancestral helicase sequences of animal Dicers suggests that decline of the functionality of the helicase started relatively early in the lineage leading to Deuterostomes and vertebrates (Aderounmu et al, 2023). Notably, Dicer in the earliest branching animal groups (ctenophores, sponges, and cnidarians; Schultz et al, 2023) uses a binding partner, which resembles HYL1 from plants rather than Loquacious/TARBP2 (Moran et al, 2013) suggesting that some miRNA‐like mechanism preceded the existence of miRNA pathways in animals.
Mammalian Dicer‐1, which primarily generates miRNAs, represents a remarkable case where the helicase lost its helicase/ATPase function, yet amino acid residues critical for ATPase function are highly conserved across mammals. Structural and functional analyses of the helicase showed that it acquired an ATP‐independent unique structural role in miRNA biogenesis (Zapletal et al, 2022). The previously observed dicing‐incompetent “pre‐dicing state” where a pre‐miRNA interacts with PAZ, dsRBD, and helicase domains (Liu et al, 2018) appears to be a specific adaptation of mammalian Dicer for recognition of bona fide pre‐miRNAs. In Drosophila, the single‐stranded region of the pre‐miRNA also interacts with the helicase but the arrangement is different from that in mammals (Jouravleva et al, 2022).
As mentioned above, a recent reconstruction of ancestral helicases of animal Dicers (Aderounmu et al, 2023) suggests that degeneration of the helicase activity in the lineage leading to mammals likely started already in early deuterostomes and the helicase was inactive in vertebrate ancestors already. This creates a remarkable paradox—removal of HEL1 from a mammalian Dicer increases its ability to dice long dsRNA (Ma et al, 2008; Flemr et al, 2013), which is the opposite of its original role to facilitate long dsRNA dicing. Furthermore, there is no evidence that mammalian Dicer lacking HEL1 is truly processive. Analyses of small RNA populations generated by the full‐length Dicer and the shorter isoform suggest that the shorter isoform might only be more active (Flemr et al, 2013; Zapletal et al, 2022).
Summary and Outlook
Structural analyses of Dicers with canonical Dicer architectures reveal features associated with miRNA and siRNA biogenesis and provide a good framework for interpreting future structures and/or their predictions (Box 2). It is likely that future studies of Dicer in nematodes, mollusks and other animals will identify additional unique adaptations of Dicer for miRNA biogenesis. At the same time, some organisms rely on Dicers, which retain only the core module and the mechanism by which these Dicers generate small RNAs of defined length has not yet been determined. AlphaFold‐based models (Varadi et al, 2022) offer interesting insights into predicted structural organization of these divergent Dicers and suggest that the future may hold additional mechanisms for small RNA biogenesis by Dicer.
Box 2. In need of answers.
How do noncanonical Dicers, such as DrnA and DrnB in Dictyostelium, recognize and cleave substrates to produce defined lengths of small RNAs? Are there other Dicer architectures in Protists?
A single Dicer in C. elegans produces both, miRNAs and siRNAs. Are long dsRNA and pre‐miRNA substrates recognized and cleaved in a similar way or differently? That is, is long dsRNA recognized and loaded into the enzyme through the helicase domain and do pre‐miRNAs interact with Dicer primarily through the PAZ domain? Cryo‐EM structures of C. elegans Dicer with both substrates would answer this question.
How does AGO bind Dicer and select 5p/3p miRNAs? It remains unclear how AGO interacts with Dicer and binds cleavage products in different model organisms. Once a cleavage product is released from Dicer, thermodynamic sensing promotes selection and loading of the main strand. However, structural insights into the organization of the RISC loading complex and the loading process are still needed.
What is the physiological relevance of PACT, a mammalian TARBP2 paralog? Is it having any specific role?
How do other Dicer‐binding partners regulate its activity/substrate specificity?
Author contributions
David Zapletal: Visualization; writing – review and editing. Karel Kubicek: Visualization; writing – review and editing. Richard Stefl: Conceptualization; funding acquisition; visualization; writing – original draft; writing – review and editing. Petr Svoboda: Conceptualization; funding acquisition; visualization; writing – original draft; writing – review and editing.
Disclosure and competing interests statement
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to thank Soung‐Hun Roh for providing human Dicer‐1 coordinates prior to their public release. The main funding was provided by the Czech Science Foundation EXPRO grant 20‐03950X to PS. Computational resources were provided by the e‐INFRA CZ project (ID: 90140), supported by the Ministry of Education, Youth and Sports of the Czech Republic.
EMBO reports (2023) 24: e57215
Contributor Information
Petr Svoboda, Email: svobodap@img.cas.cz.
Richard Stefl, Email: richard.stefl@ceitec.muni.cz.
References
- Aderounmu AM, Aruscavage PJ, Kolaczkowski B, Bass BL (2023) Ancestral protein reconstruction reveals evolutionary events governing variation in Dicer helicase function. Elife 12: e85120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2018) Metazoan microRNAs. Cell 173: 20–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BL, Hurst SR, Singer JD (1994) Binding properties of newly identified Xenopus proteins containing dsRNA‐binding motifs. Curr Biol 4: 301–314 [DOI] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409: 363–366 [DOI] [PubMed] [Google Scholar]
- Blaszczyk J, Gan J, Tropea JE, Court DL, Waugh DS, Ji X (2004) Noncatalytic assembly of ribonuclease III with double‐stranded RNA. Structure 12: 457–466 [DOI] [PubMed] [Google Scholar]
- Boland A, Tritschler F, Heimstadt S, Izaurralde E, Weichenrieder O (2010) Crystal structure and ligand binding of the MID domain of a eukaryotic Argonaute protein. EMBO Rep 11: 522–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brate J, Neumann RS, Fromm B, Haraldsen AAB, Tarver JE, Suga H, Donoghue PCJ, Peterson KJ, Ruiz‐Trillo I, Grini PE et al (2018) Unicellular origin of the animal microRNA machinery. Curr Biol 28: 3288–3295.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft M, Grunert S, Murzin AG, Proctor M, St Johnston D (1995) NMR solution structure of a dsRNA binding domain from Drosophila staufen protein reveals homology to the N‐terminal domain of ribosomal protein S5. EMBO J 14: 3563–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenik ES, Fukunaga R, Lu G, Dutcher R, Wang Y, Tanaka Hall TM, Zamore PD (2011) Phosphate and R2D2 restrict the substrate specificity of Dicer‐2, an ATP‐driven ribonuclease. Mol Cell 42: 172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Hur S (2022) Cellular origins of dsRNA, their recognition and consequences. Nat Rev Mol Cell Biol 23: 286–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Rajewsky N (2007) The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet 8: 93–103 [DOI] [PubMed] [Google Scholar]
- Chen S, Liu W, Naganuma M, Tomari Y, Iwakawa HO (2022) Functional specialization of monocot DCL3 and DCL5 proteins through the evolution of the PAZ domain. Nucleic Acids Res 50: 4669–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R (2005) TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436: 740–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel M, Pelissier T, Montavon T, Tschopp MA, Pouch‐Pelissier MN, Descombin J, Jean V, Dunoyer P, Bousquet‐Antonelli C, Deragon JM (2016) Evolutionary history of double‐stranded RNA binding proteins in plants: identification of new cofactors involved in easiRNA biogenesis. Plant Mol Biol 91: 131–147 [DOI] [PubMed] [Google Scholar]
- Colmenares SU, Buker SM, Buhler M, Dlakic M, Moazed D (2007) Coupling of double‐stranded RNA synthesis and siRNA generation in fission yeast RNAi. Mol Cell 27: 449–461 [DOI] [PubMed] [Google Scholar]
- Daniels SM, Melendez‐Pena CE, Scarborough RJ, Daher A, Christensen HS, El Far M, Purcell DF, Laine S, Gatignol A (2009) Characterization of the TRBP domain required for Dicer interaction and function in RNA interference. BMC Mol Biol 10: 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlakic M (2006) DUF283 domain of Dicer proteins has a double‐stranded RNA‐binding fold. Bioinformatics 22: 2711–2714 [DOI] [PubMed] [Google Scholar]
- Doyle M, Jantsch MF (2002) New and old roles of the double‐stranded RNA‐binding domain. J Struct Biol 140: 147–153 [DOI] [PubMed] [Google Scholar]
- Doyle M, Badertscher L, Jaskiewicz L, Guttinger S, Jurado S, Hugenschmidt T, Kutay U, Filipowicz W (2013) The double‐stranded RNA binding domain of human Dicer functions as a nuclear localization signal. RNA 19: 1238–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro‐Monti I, Mathews MB (2000) Proteins binding to duplexed RNA: one motif, multiple functions. Trends Biochem Sci 25: 241–246 [DOI] [PubMed] [Google Scholar]
- Flemr M, Malik R, Franke V, Nejepinska J, Sedlacek R, Vlahovicek K, Svoboda P (2013) A retrotransposon‐driven Dicer isoform directs endogenous small interfering RNA production in mouse oocytes. Cell 155: 807–816 [DOI] [PubMed] [Google Scholar]
- Forstemann K, Tomari Y, Du T, Vagin VV, Denli AM, Bratu DP, Klattenhoff C, Theurkauf WE, Zamore PD (2005) Normal microRNA maturation and germ‐line stem cell maintenance requires loquacious, a double‐stranded RNA‐binding domain protein. PLoS Biol 3: e236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank F, Sonenberg N, Nagar B (2010) Structural basis for 5′‐nucleotide base‐specific recognition of guide RNA by human AGO2. Nature 465: 818–822 [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Colpan C, Han BW, Zamore PD (2014) Inorganic phosphate blocks binding of pre‐miRNA to Dicer‐2 via its PAZ domain. EMBO J 33: 371–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan J, Tropea JE, Austin BP, Court DL, Waugh DS, Ji X (2006) Structural insight into the mechanism of double‐stranded RNA processing by ribonuclease III. Cell 124: 355–366 [DOI] [PubMed] [Google Scholar]
- Green SR, Mathews MB (1992) Two RNA‐binding motifs in the double‐stranded RNA‐activated protein kinase, DAI. Genes Dev 6: 2478–2490 [DOI] [PubMed] [Google Scholar]
- Guo X, Zhang R, Wang J, Ding SW, Lu R (2013) Homologous RIG‐I‐like helicase proteins direct RNAi‐mediated antiviral immunity in C. elegans by distinct mechanisms. Proc Natl Acad Sci USA 110: 16085–16090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AD, Jaskiewicz L, Zhang H, Laine S, Sack R, Gatignol A, Filipowicz W (2005) TRBP, a regulator of cellular PKR and HIV‐1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep 6: 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SR, Aderounmu AM, Donelick HM, Bass BL (2019) Dicer's helicase domain: a meeting place for regulatory proteins. Cold Spring Harb Symp Quant Biol 84: 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig JV, Forstemann K (2011) Loqs‐PD and R2D2 define independent pathways for RISC generation in Drosophila . Nucleic Acids Res 39: 3836–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig JV, Esslinger S, Bottcher R, Saito K, Forstemann K (2009) Endo‐siRNAs depend on a new isoform of loquacious and target artificially introduced, high‐copy sequences. EMBO J 28: 2932–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinas A, Reimegard J, Wagner EG, Nellen W, Ambros VR, Soderbom F (2007) The small RNA repertoire of Dictyostelium discoideum and its regulation by components of the RNAi pathway. Nucleic Acids Res 35: 6714–6726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur S (2019) Double‐stranded RNA sensors and modulators in innate immunity. Annu Rev Immunol 37: 349–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskiewicz L, Filipowicz W (2008) Role of Dicer in posttranscriptional RNA silencing. Curr Top Microbiol Immunol 320: 77–97 [DOI] [PubMed] [Google Scholar]
- Jayachandran U, Grey H, Cook AG (2016) Nuclear factor 90 uses an ADAR2‐like binding mode to recognize specific bases in dsRNA. Nucleic Acids Res 44: 1924–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Kolaczkowski O, Rolland J, Kolaczkowski B (2017) Increased affinity for RNA targets evolved early in animal and plant Dicer lineages through different structural mechanisms. Mol Biol Evol 34: 3047–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Ye X, Liu X, Fincher L, McKearin D, Liu Q (2005) Dicer‐1 and R3D1‐L catalyze microRNA maturation in Drosophila . Genes Dev 19: 1674–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouravleva K, Golovenko D, Demo G, Dutcher RC, Hall TMT, Zamore PD, Korostelev AA (2022) Structural basis of microRNA biogenesis by Dicer‐1 and its partner protein Loqs‐PB. Mol Cell 82: 4049–4063.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy SK, Fukunaga R (2016) Phosphate‐binding pocket in Dicer‐2 PAZ domain for high‐fidelity siRNA production. Proc Natl Acad Sci USA 113: 14031–14036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketting RF (2011) The many faces of RNAi. Dev Cell 20: 148–161 [DOI] [PubMed] [Google Scholar]
- Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH (2001) Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans . Genes Dev 15: 2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharrat A, Macias MJ, Gibson TJ, Nilges M, Pastore A (1995) Structure of the dsRNA binding domain of E. coli RNase III. EMBO J 14: 3572–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell MA, Chan JM, Doudna JA (2014) Evolutionarily conserved roles of the Dicer helicase domain in regulating RNA interference processing. J Biol Chem 289: 28352–28362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, Grigorov B, Gerlier D, Cusack S (2011) Structural basis for the activation of innate immune pattern‐recognition receptor RIG‐I by viral RNA. Cell 147: 423–435 [DOI] [PubMed] [Google Scholar]
- Kozomara A, Birgaoanu M, Griffiths‐Jones S (2019) miRBase: from microRNA sequences to function. Nucleic Acids Res 47: D155–D162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J, Meier D, Zenk F, Rehders M, Nellen W, Hammann C (2016) The protein domains of the Dictyostelium microprocessor that are required for correct subcellular localization and for microRNA maturation. RNA Biol 13: 1000–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SC, Nguyen TA, Choi YG, Jo MH, Hohng S, Kim VN, Woo JS (2016) Structure of human DROSHA. Cell 164: 81–90 [DOI] [PubMed] [Google Scholar]
- Laraki G, Clerzius G, Daher A, Melendez‐Pena C, Daniels S, Gatignol A (2008) Interactions between the double‐stranded RNA‐binding proteins TRBP and PACT define the Medipal domain that mediates protein‐protein interactions. RNA Biol 5: 92–103 [DOI] [PubMed] [Google Scholar]
- Lau PW, Potter CS, Carragher B, MacRae IJ (2009) Structure of the human Dicer‐TRBP complex by electron microscopy. Structure 17: 1326–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PW, Guiley KZ, De N, Potter CS, Carragher B, MacRae IJ (2012) The molecular architecture of human Dicer. Nat Struct Mol Biol 19: 436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaretti D, Bandholz‐Cajamarca L, Emmerich C, Schaaf K, Basquin C, Irion U, Bono F (2018) The crystal structure of Staufen1 in complex with a physiological RNA sheds light on substrate selectivity. Life Sci Alliance 1: e201800187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN (2006) The role of PACT in the RNA silencing pathway. EMBO J 25: 522–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YY, Kim H, Kim VN (2023a) Sequence determinant of small RNA production by DICER. Nature 615: 323–330 [DOI] [PubMed] [Google Scholar]
- Lee YY, Lee H, Kim H, Kim VN, Roh SH (2023b) Structure of the human DICER‐pre‐miRNA complex in a dicing state. Nature 615: 331–338 [DOI] [PubMed] [Google Scholar]
- Lehmann KA, Bass BL (1999) The importance of internal loops within RNA substrates of ADAR1. J Mol Biol 291: 1–13 [DOI] [PubMed] [Google Scholar]
- Liao Z, Kjellin J, Hoeppner MP, Grabherr M, Soderbom F (2018) Global characterization of the dicer‐like protein DrnB roles in miRNA biogenesis in the social amoeba Dictyostelium discoideum . RNA Biol 15: 937–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingel A, Simon B, Izaurralde E, Sattler M (2003) Structure and nucleic‐acid binding of the Drosophila Argonaute 2 PAZ domain. Nature 426: 465–469 [DOI] [PubMed] [Google Scholar]
- Lingel A, Simon B, Izaurralde E, Sattler M (2004) Nucleic acid 3′‐end recognition by the Argonaute2 PAZ domain. Nat Struct Mol Biol 11: 576–577 [DOI] [PubMed] [Google Scholar]
- Liu Q, Rand TA, Kalidas S, Du F, Kim HE, Smith DP, Wang X (2003) R2D2, a bridge between the initiation and effector steps of the Drosophila RNAi pathway. Science 301: 1921–1925 [DOI] [PubMed] [Google Scholar]
- Liu JD, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua‐Tor L, Hannon GJ (2004) Argonaute2 is the catalytic engine of mammalian RNAi. Science 305: 1437–1441 [DOI] [PubMed] [Google Scholar]
- Liu C, Axtell MJ, Fedoroff NV (2012) The helicase and RNaseIIIa domains of Arabidopsis Dicer‐Like1 modulate catalytic parameters during microRNA biogenesis. Plant Physiol 159: 748–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang J, Cheng H, Ke X, Sun L, Zhang QC, Wang HW (2018) Cryo‐EM structure of human Dicer and its complexes with a pre‐miRNA substrate. Cell 173: 1191–1203.e12 [DOI] [PubMed] [Google Scholar]
- Ma JB, Ye K, Patel DJ (2004) Structural basis for overhang‐specific small interfering RNA recognition by the PAZ domain. Nature 429: 318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma E, MacRae IJ, Kirsch JF, Doudna JA (2008) Autoinhibition of human Dicer by its internal helicase domain. J Mol Biol 380: 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae IJ, Doudna JA (2007) Ribonuclease revisited: structural insights into ribonuclease III family enzymes. Curr Opin Struct Biol 17: 138–145 [DOI] [PubMed] [Google Scholar]
- MacRae IJ, Zhou K, Li F, Repic A, Brooks AN, Cande WZ, Adams PD, Doudna JA (2006) Structural basis for double‐stranded RNA processing by Dicer. Science 311: 195–198 [DOI] [PubMed] [Google Scholar]
- MacRae IJ, Zhou K, Doudna JA (2007) Structural determinants of RNA recognition and cleavage by Dicer. Nat Struct Mol Biol 14: 934–940 [DOI] [PubMed] [Google Scholar]
- Marques JT, Kim K, Wu PH, Alleyne TM, Jafari N, Carthew RW (2010) Loqs and R2D2 act sequentially in the siRNA pathway in Drosophila . Nat Struct Mol Biol 17: 24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah G, Maris C, Konig SL, Yulikov M, Aeschimann F, Malinowska AL, Mabille J, Weiler J, Holla A, Hunziker J et al (2018) Structural basis of siRNA recognition by TRBP double‐stranded RNA binding domains. EMBO J 37: e97089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell EK, Ryan JF, Schnitzler CE, Browne WE, Baxevanis AD (2012) MicroRNAs and essential components of the microRNA processing machinery are not encoded in the genome of the ctenophore Mnemiopsis leidyi . BMC Genomics 13: 714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G (2013) Argonaute proteins: functional insights and emerging roles. Nat Rev Genet 14: 447–459 [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T (2004) Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15: 185–197 [DOI] [PubMed] [Google Scholar]
- Meunier J, Lemoine F, Soumillon M, Liechti A, Weier M, Guschanski K, Hu H, Khaitovich P, Kaessmann H (2013) Birth and expression evolution of mammalian microRNA genes. Genome Res 23: 34–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran Y, Praher D, Fredman D, Technau U (2013) The evolution of microRNA pathway protein components in Cnidaria. Mol Biol Evol 30: 2541–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourrain P, Beclin C, Elmayan T, Feuerbach F, Godon C, Morel JB, Jouette D, Lacombe AM, Nikic S, Picault N et al (2000) Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 101: 533–542 [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Campos H, Kolaczkowski B (2013) Evolution of animal and plant Dicers: early parallel duplications and recurrent adaptation of antiviral RNA binding in plants. Mol Biol Evol 30: 627–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma M, Tadakuma H, Tomari Y (2021) Single‐molecule analysis of processive double‐stranded RNA cleavage by Drosophila Dicer‐2. Nat Commun 12: 4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri S, Carpick BW, Yang Y, Williams BR, Qin J (1998) Structure of the double‐stranded RNA‐binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA‐mediated activation. EMBO J 17: 5458–5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykanen A, Haley B, Zamore PD (2001) ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell 107: 309–321 [DOI] [PubMed] [Google Scholar]
- Park JE, Heo I, Tian Y, Simanshu DK, Chang H, Jee D, Patel DJ, Kim VN (2011) Dicer recognizes the 5′ end of RNA for efficient and accurate processing. Nature 475: 201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GS, Eckert DM, Bass BL (2006) RDE‐4 preferentially binds long dsRNA and its dimerization is necessary for cleavage of dsRNA to siRNA. RNA 12: 807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GS, Maity TS, Bass BL (2008) dsRNA binding properties of RDE‐4 and TRBP reflect their distinct roles in RNAi. J Mol Biol 384: 967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturi S, Deshmukh MV (2021) A glimpse of “Dicer biology” through the structural and functional perspective. Front Mol Biosci 8: 643657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Grunert S, Adams J, Micklem DR, Proctor MR, Freund S, Bycroft M, St Johnston D, Varani G (2000) RNA recognition by a Staufen double‐stranded RNA‐binding domain. EMBO J 19: 997–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K, Chen X (2013) Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell 25: 2383–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter JM, Schultz SC (1998) Molecular basis of double‐stranded RNA‐protein interactions: structure of a dsRNA‐binding domain complexed with dsRNA. EMBO J 17: 7505–7513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Shimizu N (2007) Evolutionary conservation of a unique amino acid sequence in human DICER protein essential for binding to Argonaute family proteins. Gene 396: 312–320 [DOI] [PubMed] [Google Scholar]
- Schultz DT, Haddock SHD, Bredeson JV, Green RE, Simakov O, Rokhsar DS (2023) Ancient gene linkages support ctenophores as sister to other animals. Nature 618: 110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Jonely M, Leslie E, Rejali NA, Noriega R, Bass BL (2021) Transient kinetic studies of the antiviral Drosophila Dicer‐2 reveal roles of ATP in self‐nonself discrimination. Elife 10: e65810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha NK, Trettin KD, Aruscavage PJ, Bass BL (2015) Drosophila Dicer‐2 cleavage is mediated by helicase‐ and dsRNA termini‐dependent states that are modulated by loquacious‐PD. Mol Cell 58: 406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha NK, Iwasa J, Shen PS, Bass BL (2018) Dicer uses distinct modules for recognizing dsRNA termini. Science 359: 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Liu J, Tolia NH, Schneiderman J, Smith SK, Martienssen RA, Hannon GJ, Joshua‐Tor L (2003) The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nat Struct Biol 10: 1026–1032 [DOI] [PubMed] [Google Scholar]
- St Johnston D, Brown NH, Gall JG, Jantsch M (1992) A conserved double‐stranded RNA‐binding domain. Proc Natl Acad Sci USA 89: 10979–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefl R, Skrisovska L, Allain FH (2005) RNA sequence‐ and shape‐dependent recognition by proteins in the ribonucleoprotein particle. EMBO Rep 6: 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefl R, Xu M, Skrisovska L, Emeson RB, Allain FH (2006) Structure and specific RNA binding of ADAR2 double‐stranded RNA binding motifs. Structure 14: 345–355 [DOI] [PubMed] [Google Scholar]
- Stefl R, Oberstrass FC, Hood JL, Jourdan M, Zimmermann M, Skrisovska L, Maris C, Peng L, Hofr C, Emeson RB et al (2010) The solution structure of the ADAR2 dsRBM‐RNA complex reveals a sequence‐specific readout of the minor groove. Cell 143: 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Wang J, Deng T, Yuan X, He J, Liu N, Li X, Huang Y, Wang HW, Ma J (2022) Structural insights into dsRNA processing by Drosophila Dicer‐2‐Loqs‐PD. Nature 607: 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P, Di Cara A (2006) Hairpin RNA: a secondary structure of primary importance. Cell Mol Life Sci 63: 901–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC (1999) The rde‐1 gene, RNA interference, and transposon silencing in C. elegans . Cell 99: 123–132 [DOI] [PubMed] [Google Scholar]
- Tabara H, Yigit E, Siomi H, Mello CC (2002) The dsRNA binding protein RDE‐4 interacts with RDE‐1, DCR‐1, and a DExH‐box helicase to direct RNAi in C. elegans . Cell 109: 861–871 [DOI] [PubMed] [Google Scholar]
- Taylor DW, Ma E, Shigematsu H, Cianfrocco MA, Noland CL, Nagayama K, Nogales E, Doudna JA, Wang HW (2013) Substrate‐specific structural rearrangements of human Dicer. Nat Struct Mol Biol 20: 662–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Simanshu DK, Ma JB, Park JE, Heo I, Kim VN, Patel DJ (2014) A phosphate‐binding pocket within the platform‐PAZ‐connector helix cassette of human Dicer. Mol Cell 53: 606–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi A, Kawamata T, Izumi N, Seitz H, Tomari Y (2011) Recognition of the pre‐miRNA structure by Drosophila Dicer‐1. Nat Struct Mol Biol 18: 1153–1158 [DOI] [PubMed] [Google Scholar]
- Vagin VV, Wohlschlegel J, Qu J, Jonsson Z, Huang XH, Chuma S, Girard A, Sachidanandam R, Hannon GJ, Aravin AA (2009) Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev 23: 1749–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A et al (2022) AlphaFold protein structure database: massively expanding the structural coverage of protein‐sequence space with high‐accuracy models. Nucleic Acids Res 50: D439–D444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetukuri RR, Avrova AO, Grenville‐Briggs LJ, Van West P, Soderbom F, Savenkov EI, Whisson SC, Dixelius C (2011) Evidence for involvement of dicer‐like, Argonaute and histone deacetylase proteins in gene silencing in Phytophthora infestans . Mol Plant Pathol 12: 772–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlok M, Lang AS, Suttle CA (2019) Marine RNA virus Quasispecies are distributed throughout the oceans. mSphere 4: e00157‐19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Noland C, Siridechadilok B, Taylor DW, Ma E, Felderer K, Doudna JA, Nogales E (2009) Structural insights into RNA processing by the human RISC‐loading complex. Nat Struct Mol Biol 16: 1148–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hartman E, Roy K, Chanfreau G, Feigon J (2011) Structure of a yeast RNase III dsRBD complex with a noncanonical RNA substrate provides new insights into binding specificity of dsRBDs. Structure 19: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Xue Y, Zhang L, Zhong Z, Feng S, Wang C, Xiao L, Yang Z, Harris CJ, Wu Z et al (2021) Mechanism of siRNA production by a plant Dicer‐RNA complex in dicing‐competent conformation. Science 374: 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Ke H, Wen A, Gao B, Shi J, Feng Y (2021) Structural basis of microRNA processing by Dicer‐like 1. Nat Plants 7: 1389–1396 [DOI] [PubMed] [Google Scholar]
- Weinberg DE, Nakanishi K, Patel DJ, Bartel DP (2011) The inside‐out mechanism of Dicers from budding yeasts. Cell 146: 262–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker NC, Maity TS, Ye X, Aruscavage PJ, Krauchuk AA, Liu Q, Bass BL (2011) Dicer's helicase domain discriminates dsRNA termini to promote an altered reaction mode. Mol Cell 41: 589–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Tambe A, Kidwell MA, Noland CL, Schneider CP, Doudna JA (2015) Dicer‐TRBP complex formation ensures accurate mammalian microRNA biogenesis. Mol Cell 57: 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf YI, Silas S, Wang Y, Wu S, Bocek M, Kazlauskas D, Krupovic M, Fire A, Dolja VV, Koonin EV (2020) Doubling of the known set of RNA viruses by metagenomic analysis of an aquatic virome. Nat Microbiol 5: 1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav DK, Zigackova D, Zlobina M, Klumpler T, Beaumont C, Kubickova M, Vanacova S, Lukavsky PJ (2020) Staufen1 reads out structure and sequence features in ARF1 dsRNA for target recognition. Nucleic Acids Res 48: 2091–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Naganuma M, Nishizawa T, Kusakizako T, Tomari Y, Nishimasu H, Nureki O (2022) Structure of the Dicer‐2‐R2D2 heterodimer bound to a small RNA duplex. Nature 607: 393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Yan S, Farooq A, Han A, Zeng L, Zhou MM (2003) Structure and conserved RNA binding of the PAZ domain. Nature 426: 468–474 [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T (2007) RIG‐I family RNA helicases: cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev 18: 545–551 [DOI] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP (2000) RNAi: double‐stranded RNA directs the ATP‐dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101: 25–33 [DOI] [PubMed] [Google Scholar]
- Zapletal D, Taborska E, Pasulka J, Malik R, Kubicek K, Zanova M, Much C, Sebesta M, Buccheri V, Horvat F et al (2022) Structural and functional basis of mammalian microRNA biogenesis by Dicer. Mol Cell 82: e4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kolb FA, Brondani V, Billy E, Filipowicz W (2002) Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J 21: 5875–5885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W (2004) Single processing center models for human Dicer and bacterial RNase III. Cell 118: 57–68 [DOI] [PubMed] [Google Scholar]
- Zhou R, Czech B, Brennecke J, Sachidanandam R, Wohlschlegel JA, Perrimon N, Hannon GJ (2009) Processing of Drosophila endo‐siRNAs depends on a specific loquacious isoform. RNA 15: 1886–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]


