Abstract
In the treatment of cancer, understanding the disease status, or accurate staging, is extremely important, and various imaging techniques are used. Computed tomography (CT), magnetic resonance imaging, and scintigrams are commonly used for solid tumors, and advances in these technologies have improved the accuracy of diagnosis. In the clinical practice of prostate cancer, CT and bone scans have been considered especially important for detecting metastases. Nowadays, CT and bone scans are called conventional methods because positron emission tomography (PET), especially prostate-specific membrane antigen (PSMA)/PET, is extremely sensitive in detecting metastases. Advances in functional imaging, such as PET, are advancing the diagnosis of cancer by allowing information to be added to the morphological diagnosis. Furthermore, PSMA is known to be upregulated depending on the malignancy of the prostate cancer grade and resistance to therapy. Therefore, it is often highly expressed in castration-resistant prostate cancer (CRPC) with poor prognosis, and its therapeutic application has been attempted for around two decades. PSMA theranostics refers to a type of cancer treatment that combines both diagnosis and therapy using a PSMA. The theranostic approach uses a radioactive substance attached to a molecule that targets PSMA protein on cancer cells. This molecule is injected into the patient’s bloodstream and can be used for both imaging the cancer cells with a PET scan (PSMA PET imaging) and delivering radiation directly to the cancer cells (PSMA-targeted radioligand therapy), with the aim of minimizing damage to healthy tissue. Recently, in an international phase III trial, the impact of 177Lu-PSMA-617 therapy was studied in patients with advanced PSMA-positive metastatic CRPC who had previously been treated with specific inhibitors and regimens. The trial revealed that 177Lu-PSMA-617 significantly extended both progression-free survival and overall survival compared to standard care alone. Although there was a higher incidence of grade 3 or above adverse events with 177Lu-PSMA-617, it did not negatively impact the patients’ quality of life. PSMA theranostics is currently being studied and used primarily for the treatment of prostate cancer, but it has the potential to be applied to other types of cancers as well.
Keywords: diagnosis, prostate cancer, PSMA, radiotherapy, theranostics
Introduction
Prostate cancer is the most frequently diagnosed malignant disease in Japan and the sixth leading cause of cancer death in men in 2019. 1 According to Japanese cancer statistics, it is estimated that 94,748 new cases of prostate cancer and 12,759 prostate cancer-specific deaths occur each year. 1
For patients with localized disease, robot-assisted radical prostatectomy has become the mainstay of surgical treatment in recent years in Japanese practice. Radiation therapies for curative purposes, such as intensity-modulated radiation therapy and particle therapy, including proton beam therapy and heavy ion therapy, are approved and covered by medical insurance in Japan.
However, 10–15% of patients have advanced disease at the time of initial diagnosis, and for these patients such curative therapies are not indicated. 2 Metastases may also occur in patients who have received prior curative treatment and subsequently relapse. These metastatic patients are initially treated with androgen deprivation therapy, but invariably develop advanced disease, a condition known as castration-resistant prostate cancer (CRPC).
Docetaxel is the only drug that has first shown efficacy and prolonged survival in CRPC patients. 3 Recently, the novel androgen receptor-targeting agents, abiraterone acetate and enzalutamide, and the novel taxane-based chemotherapeutic agent, cabazitaxel, have been effective for such patients with CRPC and were approved in 2014.4–8 In addition, the alpha-ray emitter radium-223 dichloride was also approved in 2016 for patients with bone metastatic CRPC. 9 These agents are rapidly being adopted in clinical practice in Japan for the treatment of CRPC because of their promising antitumor efficacy and manageable safety profiles, as demonstrated in their respective global phase III clinical trials.4–9
Metastatic CRPC remains fatal despite recent advances. Prostate-specific membrane antigen (PSMA) has been the subject of extensive investigation in the past two decades as a promising molecular target for prostate cancer. 10 Also known as folate hydrolase I or glutamate carboxypeptidase II, PSMA is a type II, 750 amino acid transmembrane protein. In benign prostatic cells, it is localized to the cytoplasmic and apical side of the prostate epithelium. As malignant transformation occurs, PSMA is transferred from the cytoplasm to the luminal surface of the prostatic ducts, where it presents a large extracellular domain to ligands. The biological function of PSMA remains unclear, but it is hypothesized to act as a transporter because PSMA ligands are internalized through endocytosis. PSMA ligand internalization theoretically enables specificity of synthetic PSMA radioligands for malignant prostatic tissue. 11 Furthermore, research suggests a 100- to 1000-fold increase in PSMA expression in prostatic adenocarcinoma compared to benign prostatic tissue.12,13 Although there is a growing number of studies indicating that PSMA expression is subject to inter- and intra-patient heterogeneity, PSMA expression generally increases with tumor dedifferentiation and later-stage metastatic CRPC (mCRPC). 14 Due to these properties, PSMA has become an appealing target for novel diagnostic and therapeutic – thus ‘theranostic’ – approaches to prostate cancer. Substantial evidence supports the high sensitivity of PSMA-targeted imaging for prostate cancer lesions, and there is growing evidence for the therapeutic efficacy of PSMA radioligand therapy for mCRPC. In this review, we present a broad overview of the current status of PSMA theranostics, including current evidence, potential clinical impact, and active areas of research.
Clinical significance and research implications
Prostate cancer diagnosis
With the advent of prostate-specific antigen (PSA) screening, a significant proportion of patients are diagnosed at an early stage that is confined to the organ. However, reflecting the heterogeneous nature of the disease, some patients present or progress to high-risk, progressive, or metastatic disease. 15
When prostate cancer is suspected, tissue biopsy remains the standard of care for diagnosis. However, improved risk stratification, advances in magnetic resonance and functional imaging, and the advent of biomarkers have made identification and characterization of the disease increasingly accurate.
Multiple management options
Currently, multiple management options exist for men diagnosed with prostate cancer. Active surveillance (the serial monitoring of disease progression) is considered safe and is the preferred approach for men with less aggressive prostate cancer, particularly those with PSA levels below 10 ng/mL and Gleason score 3 + 3 tumors. 16 Surgery and radiation remain curative treatments for localized disease but have serious side effects, including dysuria and sexual dysfunction, that may adversely affect quality of life. For metastatic disease, chemotherapy with androgen deprivation therapy as initial treatment appears to prolong survival compared to androgen deprivation therapy alone. 17 New hormonal therapies and bone-directed agents have proven effective in men with metastatic prostate cancer who have developed resistance to traditional hormonal therapy.
Accurate diagnosis and staging
Accurate diagnosis and staging combined with effective therapeutic options are essential for treating these patients. Patients who develop PSA recurrence after curative treatment benefit from the early initiation of salvage therapy. In addition, identifying and locating disease sites in patients with low-volume or oligometastatic disease can indicate alternative avenues of treatment. 18 Thus, there is a profound need to develop sensitive techniques for disease detection and monitoring. Conventional imaging modalities, such as computed tomography (CT) and bone scans, are usually performed to determine the extent of the lesion, but these have significant limitations, especially when PSA values are low. For this reason, positron emission tomography (PET) with choline or fluorodeoxyglucose-based tracers has gained attention for staging advanced disease. However, these tracers also have limitations in the setting of early metastatic disease and biochemical recurrence.
The role of PET imaging in staging prostate cancer
EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer 19 discuss the use of imaging techniques for detecting lymph node (LN) invasion in patients with prostate cancer. Abdominal CT and magnetic resonance imaging (MRI) are commonly used but have low sensitivity. 20 Choline PET/CT has a sensitivity of 62% for pelvic LN metastases. 21 PSMA PET/CT has a higher sensitivity of 75% and a specificity of 99% for nodal staging on a per-node analysis. 22 In a study of newly diagnosed prostate cancer patients with negative bone scans, PSMA PET/CT had a per-patient sensitivity of 41.5% and specificity of 91%, resulting in a treatment change for 12.6% of patients. 23 Compared to mpMRI, PSMA PET/CT has a higher sensitivity of 0.65 and a comparable specificity of 0.94 for preoperative nodal staging in intermediate- and high-risk prostate cancer. 24
Prostate cancer bone metastases are most commonly evaluated using bone scintigraphy, which has a combined sensitivity and specificity of 79% and 82%, respectively. 25 However, diffusion-weighted whole-body and axial MRI are more sensitive in detecting bone metastases. 26 Whole-body MRI is also more sensitive and specific than combined bone scan, targeted radiography, and abdominopelvic CT. 27 Ga-PSMA PET has high sensitivity (33–92%) and good specificity (82–100%) for detecting metastases, with increased detection rates compared to conventional imaging. 28 This study recruited men with biopsy-proven prostate cancer and high-risk features at 10 hospitals in Australia. The patients were randomly assigned to either conventional imaging or PSMA PET-CT. The primary outcome was the accuracy of the first-line imaging for identifying pelvic nodal or distant metastatic disease. The results showed that PSMA PET-CT had a 27% greater accuracy than conventional imaging. PSMA PET-CT also had a higher sensitivity and specificity. First-line conventional imaging conferred management change less frequently and had more equivocal findings than PSMA PET-CT. Radiation exposure was higher for conventional imaging than for PSMA PET-CT. The study concluded that PSMA PET-CT is a suitable replacement for conventional imaging, providing superior accuracy in identifying prostate cancer metastases. However, the clinical benefit of detecting metastases at an earlier time point and the ideal management of patients diagnosed as metastatic by these more sensitive tests remain unclear. Results from randomized controlled trials evaluating the management and outcome of patients with (and without) metastases detected by more sensitive imaging are needed to make evidence-based decisions.
Multiparametric MRI
With recent improvements in accuracy, MRI is currently regarded as the most objective and reliable imaging test for the local staging of prostate cancer. In the United States, MRI has been used to localize prostate cancer since the late 1980s. Yet, its accuracy was limited because the marginal region of the normal prostate shows high signal on T2-weighted images, whereas prostate cancer shows a slightly lower signal.
In the late 1990s, diffusion-weighted MRI was used for the head, but in the 2000s, it became clinically applicable to the pelvic region as well. In prostate MRI, in addition to conventional morphological information based on T2-weighted images, diffusion-weighted images provide functional information on the diffusion of water molecules, which is the basis for the current standard prostate MRI imaging method, multiparametric (mp) MRI, which combines dynamic MRI with T2-weighted images. Today, mpMRI is highly favored over other conventional methods due to its superior ability for detecting and locally staging cancer (especially in the presence or absence of extracapsular invasion and seminal vesicle invasion), identifying LN metastasis, and enabling accurate grading. 29 Recently, MRI-transrectal ultrasound fusion image-guided prostate biopsy has also been performed in some centers.
Although mpMRI is highly recommended today, it has limitations in terms of cancer localization, and clinical staging [clinical tumor, node, and metastasis (TNM)] and pathological TNM do not always coincide.24,30
Preoperative diagnosis of cancer localization and clinical staging greatly influences the indication for surgery, the extent of surgical resection, the preservation of nerves involved in urinary symptoms and erection, and the extent of LN dissection. In addition, more accurate imaging is desired because it is closely related to cancer control, such as positive margins and recurrence, and to the patient’s quality of life, such as urinary incontinence and erectile dysfunction.
PET/MRI for initial staging
Gallium-68 (68Ga) and fluorine-18 (18F) PSMA PET/CT has emerged as a promising diagnostic and staging tool for advanced primary and recurrent prostate cancer. Integrated PET/MRI proved to have greater diagnostic value in locating prostate cancer than mpMRI or PET imaging alone. 31
A meta-analysis of the efficacy of PET/MRI for local diagnosis of prostate cancer was conducted using 23 articles, with pooled data on 2104 patients. Initial staging was the primary indication for PET/MRI. The most commonly used tracer was a radiolabeled PSMA. For primary lesions, the pooled sensitivity in the patient-based analysis was 94.9%. At restaging, the pooled detection rate was 80.9% and was 80.9% for radiolabeled PSMA over choline (81.8% and 77.3%, respectively). 32
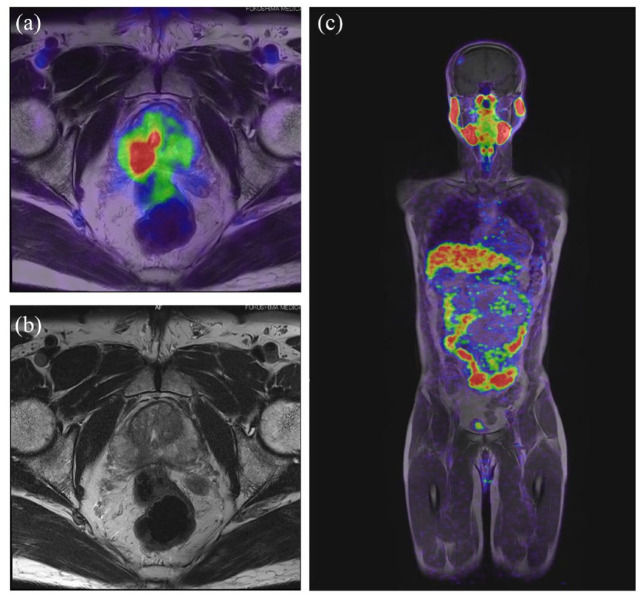
There are few prospective studies comparing mpMRI and 68Ga-PSMA-11 PET/MRI in prostate cancer diagnosis. Fukushima Medical University is conducting a specified clinical trial to diagnose local progression by PET/MRI using PSMA ligand (Prospective intra-individual comparison of multiparametric (mp) MRI versus68Gallium (Ga) PSMA−11 PET/MRI in a blinded read to evaluate diagnostic accuracy in patients with prostate cancer – RESTORATION study, jRCTs022220021 33 ; Figure 1).
Figure 1.
MRI of the prostate: T1-weighted imaging with PET fusion (a), T2-weighted imaging (b), and T1-weighted imaging with PET fusion on whole body (c).
Local recurrence after local therapy
When the cancer is confined to the prostate alone, robotic prostatectomy and radiation therapy may be used as local therapy. When surgery is performed as local therapy for localized prostate cancer, the tumor marker PSA drops below sensitivity. However, with follow-up, PSA recurrence, a gradual increase in PSA, may occur. CT and bone scan are performed to search for metastases, but if no metastases are found, local recurrence is considered present and salvage radiation therapy is administered to the prostatic bed. In retrospective studies, 68Ga-PSMA-11 PET imaging improves detection of biochemically recurrent prostate cancer compared with conventional imaging. 34 A total of 635 men were enrolled in the study, with a median age of 69 years (range, 44–95 years).
68Ga-PSMA-11 PET detected localized prostate cancer recurrence in 475 of 635 patients (75%), 38% for <0.5 ng/mL (n = 136), 57% for 0.5 to <1.0 ng/mL (n = 79), 84% for 1.0 to <2.0 ng/mL (n = 89), 86% for 2.0 to <5.0 ng/mL (n = 158), and 97% for ⩾5.0 ng/mL (n = 173, p < 0.001). Inter-reader reproducibility was fairly high (Fleiss κ, 0.65–0.78). In this situation, PSMA/PET is widely considered to be the best imaging option.
PSMA PET/CT at PSA failure after radical prostatectomy
Concerning the loco-regional recurrence, it should be stressed that 68Ga-PSMA PET has some limitations for the detection of pelvic recurrence due to the nonspecific tracer accumulation in bladder. Some reported that a study performed to evaluate the impact of forced diuresis and late-phase imaging on the accuracy and reader confidence in restaging prostate cancer using [68Ga]Ga-PSMA-11 PET/CT. 35 In all, 100 patients were included, and PET readers with varying levels of experience rated the images according to E-PSMA guidelines. 36 The study found that forced diuresis and late-phase imaging increased reader confidence and interobserver agreement for nodal restaging but only significantly improved diagnostic accuracy for certain scenarios. SUVmax kinetics were identified as a potential independent predictor of prostate cancer recurrence. The study does not support the systematic use of forced diuresis and late-phase imaging but suggests it may be beneficial in specific situations.
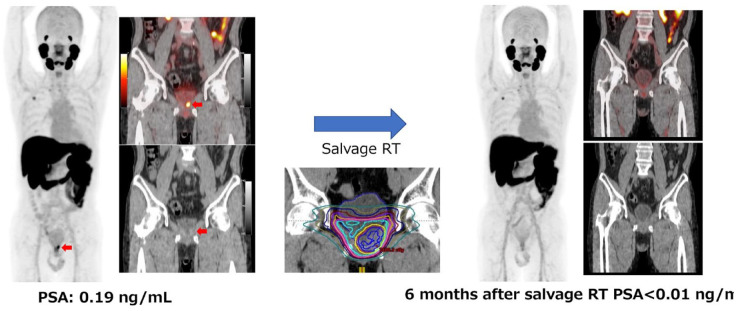
Figure 2 shows a published case study from a clinical trial at Osaka University Hospital (UMIN Clinical Trials Registry UMIN000037697 37 ). 18F-labeled PSMA ligand, [18F]PSMA-1007, has the benefit of a higher synthetic yield and minimal excretion in the urine. We manufactured the 18F-PSMA-1007 diagnostic on campus and were conducting a physician-initiated clinical study to administer it to prostate cancer patients. 38 The patient had PSA recurrence after radical prostatectomy and a PSA of 0.19 at the time of PET. The recurrence could not be detected by CT on the prostatic bed. However, PSMA PET was able to identify the region containing the tumor, and salvage radiation therapy was administered to that area. Six months after irradiation, PSA had decreased below the sensitivity of the assay, and the PSMA signal by PSMA PET had disappeared. 39 Without PET, more accurate irradiation could not have been performed. Until now, predicting whether the disease will remain localized or become metastatic is based on the PSA value and its doubling time. If no metastases are evident by CT and bone scans, salvage radiation therapy is again performed with the aim of curing the disease. Although this approach is often successful, in many cases, PSA may be elevated even after salvage radiation therapy, suggesting that the original rise in PSA was caused not by a local recurrence but a metastasis that could not be diagnosed by conventional imaging. With PSMA PET imaging, metastases and local recurrences can now be more clearly delineated. These advances obviate the need to explain to patients that they are assumed to have a local recurrence because there are no metastases or local recurrence images. Abnormal uptake was detected in 92.9% (26/28) of the patients with biochemical recurrence (BCR). The detection rates were 66.7% for 0.1 to <0.5 ng/mL, 85.7% or 0.5 to <1.0 ng/mL, and 100% for more than 1.0 ng/mL. High detection rate was observed in [18F]PSMA-1007.
Figure 2.
Case study from a clinical trial at Osaka University Hospital (UMIN Clinical Trials Registry UMIN000037697).
PSMA PET/CT for biochemical failure after radiation therapy
PMSA PET/CT is an effective response for PSA failure after radiation therapy for localized prostate cancer. Another case study illustrates PSA failure after 125I seed treatment. For this patient, no recurrent lesions were found upon CT, MR, and bone scan. However, PSMA PET revealed local recurrence with high uptake. Biopsy revealed recurrence, and salvage radiation therapy was performed. After radiation therapy, PSA decreased dramatically. In this instance, PSMA PET was essential for finding the appropriate treatment for this patient (data not shown). The efficacy of salvage radiation therapy has been confirmed for local recurrence after non-I-seed treatment for localized prostate cancer.
Metastatic prostate cancer and treatment
Prostate cancer may have metastases at the time of diagnosis, or the cancer may recur and metastasize after local therapy. In this situation, systemic therapy is applied. Androgen deprivation therapy with or without androgen receptor pathway inhibitors and anticancer drugs are used. Androgen deprivation therapy for prostate cancer is known to be highly effective, but it can lead to disease progression. This condition is called CRPC and has a very poor prognosis. Recently, a number of novel agents have been developed for CRPC patients, but they have not yet demonstrated sufficient improvement in prognosis, and targeted PSMA therapy is expected to be a novel treatment for CRPC patients.
CRPC and treatment
With androgen deprivation therapy, the development of CRPC is only a matter of time. CRPC is thought to progress through two overlapping mechanisms: an androgen receptor (AR)-independent mechanism and an AR-dependent mechanism. Docetaxel has been the only drug to show efficacy and prolonged survival in patients with CRPC. 3 Several new options for systemic therapy for CRPC patients include the androgen receptor-axis targeted (ARAT) compounds, abiraterone acetate and enzalutamide,4–6,8 and the novel taxane-based chemotherapeutic agent cabazitaxel. 7
In addition, the alpha-ray emitting agent radium-223 dichloride was approved in 2016 for patients with bone metastatic CRPC. 9 These agents are rapidly being adopted as CRPC treatment in clinical practice in Japan because they show promising antitumor efficacy and manageable safety profiles, as demonstrated in their respective global phase III clinical trials. Treatment options for CRPC have expanded. Currently, there is still debate regarding the optimal timing and sequence of administering ARAT agents and docetaxel, and currently there is no clear-cut consensus on the best approach.
Theranostics-targeting PSMA
Two new therapies using radionuclides, 223Ra-dichloride and radioligand therapy (RLT)-targeting PSMA, have been approved in the last decade for CRPC. The combined use of diagnosis and therapy in a unique approach is called theranostics for response prediction and assessment in patients receiving these therapies. 40 The theranostic approach involves using a radioactive substance that is linked to a PSMA-targeting molecule, which can then be injected into the patient’s bloodstream. The molecule travels through the body and attaches to the PSMA protein on the cancer cells, allowing them to be imaged using a PET scan. This diagnostic aspect is referred to as PSMA PET imaging.
In addition to providing information about the location and extent of the cancer, the radioactive substance that is attached to the PSMA molecule can also deliver radiation directly to the cancer cells. This is referred to as PSMA-targeted RLT, which aims to kill the cancer cells while minimizing damage to healthy tissue. In this way, PSMA allows clinicians to evaluate the likely therapeutic effect from diagnostic imaging. This is the concept of theranostics. PSMA theranostics is currently being studied and used primarily for the treatment of prostate cancer, but it has the potential to be applied to other types of cancers as well.
How do radiolabeled ligands bind PSMA on prostate cancer cells and serve as imaging and therapeutic tools?
PSMA is a transmembrane glycoprotein with three domains: intracellular, transmembrane, and extracellular domain. There is a ligand binding site in the extracellular domain where ligands bind PSMA on prostate cancer cells. PSMA ligands can be labeled with radionuclides for imaging and /or therapy. PSMA inhibitors are a class of drugs that target PSMA, which is a cell surface protein that is overexpressed in prostate cancer cells. PSMA inhibitors work by binding to the extracellular domain of PSMA, which is the portion of the protein that is located on the outside of the cell membrane. The extracellular domain of PSMA contains several binding sites that are targeted by PSMA inhibitors. One of the most important binding sites is the active site, which is where PSMA cleaves the amino acid glutamate from small peptides. PSMA inhibitors bind to this active site and block the enzymatic activity of PSMA, which can help to slow or stop the growth of prostate cancer cells.
In addition to the active site, there are other binding sites on the extracellular domain of PSMA that are targeted by different PSMA inhibitors. Some inhibitors bind to the folate binding site, which is involved in the uptake of folate by cells. Other inhibitors bind to the zinc binding site, which is important for the stability of PSMA and its enzymatic activity.
Overall, the mechanisms by which PSMA inhibitors bind to the extracellular domain of PSMA vary depending on the specific inhibitor and the binding site targeted. However, by blocking these binding sites, PSMA inhibitors can help to slow or stop the growth of prostate cancer cells, making them a promising class of drugs for the treatment of prostate cancer.
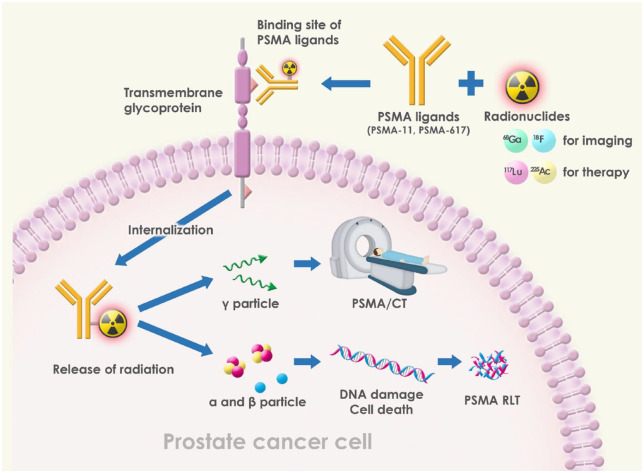
After radiolabeled PSMA ligands bind the extracellular ligand binding site, they are internalized into prostate cancer cells, releasing different particles. Gamma particles can be detected using PET scans for use in PET/CT imaging. Alpha and beta particles can cause DNA damage leading to cancer cell death and thus may be used in PSMA RLT (Figure 3). Beta particles have a lower mass and charge compared to alpha particles and can travel farther in tissue. This allows them to penetrate deeper into the body and target tumors located at a greater distance from the radiation source. However, beta particles have a lower linear energy transfer (LET), meaning they deposit less energy per unit length of tissue. As a result, beta radiation is less effective at killing cancer cells that are tightly packed together, or that are not actively dividing. Beta-emitting radionuclides are often used in systemic radionuclide therapy, where the radionuclide is administered intravenously and distributed throughout the body.
Figure 3.
Mechanism of action of PSMA theranostics.
In contrast, alpha particles have a higher mass and charge, and a shorter range in tissue. This makes them less suitable for treating tumors located deep within the body, but highly effective for treating small, localized tumors. Alpha radiation has a higher LET, allowing it to deposit more energy per unit length of tissue, resulting in more damage to cancer cells. Alpha-emitting radionuclides are often used in targeted alpha therapy (TAT), where the radionuclide is bound to a molecule that specifically targets cancer cells. This allows for highly targeted delivery of the radiation, minimizing damage to surrounding healthy tissue. 41
The applications of dual tracer PET/CT with 18F-FDG and PSMA ligands for patients’ selection before RLT
68Ga-PSMA PET/CT is commonly used for imaging in prostate cancers, but there have been few studies comparing its diagnostic efficiency to 18F-FDG PET/CT and evaluating whether a heterogeneous metabolic phenotype exists in patients with CRPC.42,43 In a retrospective analysis of 56 CRPC patients who underwent both 68Ga-PSMA and 18F-FDG PET/CT, it was found that although 68Ga-PSMA PET/CT had a higher detection rate and number of positive lesions, there were still patients with 68Ga-PSMA-negative and 18F-FDG-positive lesions. Patients with high Gleason scores and PSA levels were more likely to have these lesions, and they may benefit from additional 18F-FDG PET/CT. 44
Consensus for PSMA imaging
Evidence regarding the value of PSMA PET/CT in terms of long-term outcomes and effects on clinical decision-making is not robust. During the panel discussion on the use of PSMA PET/CT, it was confirmed that such a novel approach should only be used if a change in clinical management is expected from the results, as already emphasized in the EAU guidelines.45,46 For most statements, use of the words ‘in the majority of patients’ rather than ‘every’ or ‘any’ was preferred simply because it is very unlikely that any statement could apply to all patients affected by prostate cancer. This was the reason for the rephrasing of many statements. Significant concerns were raised regarding the management of patients with positive PSMA PET/CT and negative conventional imaging results, especially at initial staging, as it remains unclear if use of results from a more sensitive imaging tool to modify treatment has a demonstrable impact on meaningful outcomes, including survival. European Society for Medical Oncology guidelines suggest that patients with localized prostate cancer according to conventional imaging should not be denied radical local treatment solely because metastatic lesions are identified via novel imaging techniques. Nonetheless, there was a clear agreement on the use of PSMA PET/CT in staging all high-risk patients and selected patients with unfavorable intermediate-risk disease. As already stated in the EAU guidelines, the panel strongly endorsed the use of PSMA PET/CT in patients with BCR. Their results showed a consensus regarding the uncertainty of using PSMA PET/CT in patients with nonmetastatic CRPC (nmCRPC), which may be because of several factors, including patient heterogeneity, lack of long-term data regarding the benefit of metastasis directed therapy in CRPC (as a result of detecting distant lesions via PSMA PET/CT), and a lack of data on appropriate sequencing of treatment. The APCCC 2022 panel 46 discussed how to manage patients with metastatic hormone-sensitive prostate cancer (mHSPC) who have low volume on conventional imaging but high volume on next-generation imaging as PSMA PET becomes more commonly used for staging and re-staging. However, it should be noted that none of the trials for mHSPC have utilized next-generation imaging, and the current evidence is based on the number and presence of metastases seen on conventional imaging. Finally, there was a consensus against the systematic use of PSMA PET/CT to evaluate disease progression in patients with confirmed metastatic CRPC (mCRPC) based on a lack of data, possible lack of cost-effectiveness, and limited PSMA PET/CT availability in some countries.
177Lu-PSMA ligand therapy
Several recently published prospective and retrospective studies have shown that 177Lu-PSMA-617 and 177Lu-PSMA-I&T therapy significantly reduced PSA and prolonged radiologic progression-free survival (rPFS) and overall survival (OS). In a phase II trial (LuPSMA study 47 ), patients with metastatic CRPC whose disease had progressed after standard therapy, including taxane-based chemotherapy and second-generation antiandrogens, were recruited. Patients underwent screening for PSMA and FDG-PET/CT to confirm high PSMA expression. Eligible patients were required to have progressive disease defined by the presence of new pain in lesions evident on imaging or radiographs. Eligible patients received intravenous [177-Lu]-PSMA-617 for up to four cycles at 6-week intervals. Primary end points were PSA response (⩾50% PSA reduction from baseline) and Common Terminology Criteria for Adverse Events toxicity. Other primary end points were imaging response (measured by bone scan, CT, PSMA, and FDG PET/CT) and quality of life. Fifty-seven percent of patients achieved a PSA reduction of 50% or greater. There were no treatment-related deaths. The most common toxic effects associated with 177Lu-PSMA-617 were grade 1 dry mouth (87% of patients), grade 1 and 2 transient nausea (50%), and G1-2 fatigue (50%). Grade 3 or 4 thrombocytopenia, possibly attributable to [177Lu]-PSMA-617, occurred in 13% patients. Objective responses in nodal or visceral disease were reported in 82% with measurable disease. Clinically meaningful improvements in pain severity and interference scores were documented at all time points. 37% experienced an improvement of 10 or more points in their global health score by the second cycle of treatment.
The multicenter, phase II trial compared the efficacy and safety of 177Lu-PSMA-617, a radiolabeled small molecule targeting PSMA (TheraP), with cabazitaxel in treating mCRPC. The study took place in 11 Australian centers and involved men with mCRPC who were considered suitable for cabazitaxel. 48 Participants underwent PET scans to confirm PSMA-positive disease and were then randomly assigned to receive either 177Lu-PSMA-617 or cabazitaxel. The primary end point was a reduction in PSA levels by at least 50%. Out of 291 screened men, 200 were eligible, and almost all of them received the study treatment. PSA responses were more common in the 177Lu-PSMA-617 group than in the cabazitaxel group, with 65 versus 37 responses (66% versus 37% by intention to treat). In addition, grade 3–4 adverse events were less common in the 177Lu-PSMA-617 group (33% versus 53%). The study concluded that 177Lu-PSMA-617 led to a higher PSA response and fewer severe adverse events than cabazitaxel, marking it as a new effective class of therapy and a potential alternative for mCRPC treatment.
In Japan, a newer open-label, multicenter, single-arm phase II clinical trial (NCT05114746) has begun enrollment in January 2022. The study will evaluate the efficacy, tolerability, safety, pharmacokinetics, and dosing of 177Lu-PSMA-617. 49
The FDA-granted breakthrough therapy designation of 177Lu-PSMA-617 for mCRPC is detailed in the recent results of the Phase III VISION trial (NCT03511664). 50 The VISION trial prospectively randomized 831 mCRPC patients with advanced disease to receive either 177Lu-PSMA-617 with protocol-accepted best standard of care (BSOC) or BSOC alone as defined by the investigators.
The study reported a nearly 40% reduction in risk of death when 177Lu-PSMA-617 was added to standard therapy compared to standard therapy alone. Median OS was improved by 4 months compared to standard therapy alone (15.3 versus 11.3 months). RLT also improved median rPFS by 5.3 months (8.7 versus 3.4 months) and reduced the risk of progression or death by 60% (HR, 0.40). Additional end points included safety, patient-reported health-related quality of life (HRQoL), and pain. 51 Analyses were conducted to assess the time to the first occurrence of HRQoL/pain worsening, disease progression, or death. The analysis of HRQoL included 581 out of the 831 randomized patients, with 385 in the 177Lu-PSMA-617 arm and 196 in the control arm. The HRQoL and pain time-to-worsening analyses showed better outcomes in the 177Lu-PSMA-617 arm, despite a higher incidence of severe adverse events compared to standard care alone. No new or unexpected safety concerns were observed. The addition of 177Lu-PSMA-617 to standard care was generally well tolerated and delayed the worsening of HRQoL and pain compared to standard care alone in patients with advanced mCRPC. These results led the FDA to approve lutetium PSMA for the treatment of adult patients with PSMA-positive mCRPC who have been treated with AR pathway inhibitors and taxanes.
Further studies utilizing lutetium are underway, including trials for PSMA-I&T, which uses a different ligand than PSMA-617. In addition, an open-label randomized phase III clinical trial (NCT05204927) 52 comparing the safety and efficacy of 177Lu-PSMA-I&T versus hormonal therapy (abiraterone plus prednisone or enzalutamide) in mCRPC patients began enrollment in February 2022. The primary outcome measure to be investigated is rPFS.
225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy
PSMA-based radioligand therapy (RLT) with the therapeutic radionuclide 177Lu has been used in European countries since 2015 for compassionate use in patients with mCRPC.53,54 Since then, several studies have reported positive results with 177Lu-PSMA-RLT.55,56 However, up to 30–40% of patients were found to be refractory to 177Lu-PSMA-RLT during clinical trials, with hemotoxicity limiting dose expansion. 47 In PSMA-based RLT, alpha-particle emitters with high energy transfer rates and short pathlengths have received significant attention as alternatives to beta-ray emitters. 57
225Ac has become the first-line alpha-particle emitter in recent experimental PSMA-based RLT for the management of mCRPC patients.58–66 However, given the limited availability of 225Ac and the unsystematized clinical setting of these exploratory studies, there is no strong evidence to guide physicians in the management of mCRPC patients using alpha-particle emitting in experimental PSMA-based RLT for the management of mCRPC patients.58–66 A current phase II study (company-initiated clinical trial) is ongoing in the United States. 67
Emerging data on switching non-responders from 177Lu to 225Ac-PSMA have shown surprising and excellent results even in cases refractory to beta therapy. 68 They suggest that the combination of 225Ac and 177Lu-labeled PSMA ligands in TANDEM therapy is feasible, safe, and effective and may even be synergistic.
One report detailed the results of a retrospective study of Actinium-225-PSMA-617 in advanced metastatic CRPC after failure of Lutetium-177-PSMA as follows.
Ac-225-PSMA-617 was administered for 61 cycles (median number of cycles 2, median activity 9 MBq) for 26 mCRPC cases that had progressed after 177Lu-PSMA administration. A 50% reduction in PSA was achieved in 17/26 patients. Median PSA-PFS, cPFS, and OS periods were 3.5, 4.1, and 7.7 months, respectively. Liver metastases were associated with PSA-PFS (median 1.9 versus 4.0 months, p = 0.02), cPFS (median 1.8 versus 5.2 months, p = 0.001), and OS (median 4.3 versus 10.4 months, p = 0.01). 38
Hematologic grade 3/4 toxicities were anemia (35%), leukopenia (27%), and thrombocytopenia (19%).
Grade 1/2 xerostomia was also present in all patients. Two and six patients discontinued treatment due to hematologic toxicity and xerostomia, respectively. At the conclusion of the study, 225Ac-PSMA-617 showed measurable antitumor effects after 177Lu-PSMA was discontinued. Grade 3/4 hematologic side effects were observed in up to one-third of patients, and a significant number of patients had xerostomia leading to treatment interruption. 58
PSMA treatment with astatine is also being developed for alpha therapy in prostate cancer
Astatine 211 has physical properties that make it a good candidate as a source for alpha-particle radionuclide therapy (targeted alpha therapy: TAT). 211At is a 100% alpha emitter with only one alpha particle emitted per decay, which prevents unpredictable dose localization due to detachment of the radioactive daughter from the carrier vector. This is comparable with other alpha-emitting nuclides such as 227Th, 223Ra, 212Pb, 212Bi, and 225Ac, but these molecules have longer decay series and recoil problems. With a half-life (t½) of 7.2 h, the residual radioactivity of 211At after 2 days is less than 1%, potentially reducing normal tissue exposure, while the length of transport is another advantage.69–72 To date, RLT using astatine has been studied in a variety of carcinomas and with a variety of probes. Studies on glioblastoma, 73 ovarian cancer,74–77 and cancer of the tongue 78 have already been completed and published. Studies on multiple myeloma,79,80 leukemia,81,82 thyroid cancer,83,84 and malignant pheochromocytoma 85 are currently underway around the world, and trials for thyroid cancer and malignant pheochromocytoma have been initiated in Japan. 83
Fukushima Medical University also developed a novel labeling method based on the substitution reaction of 211At for dihydroxyboryl groups.66,67 Furthermore, Osaka University Hospital developed a newly designed precursor based on the structure of [18F]-PSMA-1007, 39 which is considered suitable for 211At labeling. In this study, we evaluated the properties of the new 211At-labeled PSMA compound ([211At]PSMA5) and its therapeutic efficacy in a mouse xenograft model of prostate cancer and compared it with closely related new derivatives, [211At]PSMA1 and [211At]PSMA6. 86
PSMA-directed imaging and radioguided surgery with single-photon emission CT
PSMA is a valuable target for prostate cancer diagnosis and therapy. PSMA inhibitors labeled with radionuclides emitting positrons or gamma-photons can be used for imaging with PET/CT or single photon emission computed tomography (SPECT). 87 There are different approaches for obtaining PSMA ligands labeled with gamma-emitting nuclides and discussions for the applications of PSMA SPECT imaging in various clinical settings, as well as its potential use in radioguided surgery (RGS). Intraoperative PSMA-targeted radioguidance has been proven valuable for detecting prostate cancer lesions during open surgery, and the rapid growth of robot-assisted, minimally invasive surgery has led to an increased need for robot-compliant PSMA-RGS. Some study evaluated the feasibility of using a miniaturized gamma probe called DROP-IN to carry out robot-assisted PSMA-RGS in men with recurrent prostate cancer. 88 The study found that using the DROP-IN probe, 90% of PSMA-avid lesions could be resected robotically, with a sensitivity of 86% and a specificity of 100%. The procedure was technically feasible and effective for detecting nodal or local PSMA-avid prostate cancer recurrences.
Preliminary results of PSMA-targeted RGS are promising, but larger studies are needed to validate this surgical approach. PSMA-targeted SPECT/CT has potential as a cost-effective alternative to PSMA PET/CT, and future research will investigate novel SPECT technologies or algorithms for this purpose.
Consensus for PSMA ligand therapy
The EANM FOCUS1 meeting in 2019 89 which was held in Valencia, Spain, stated that among the therapeutic radiopharmaceuticals available for patients with metastatic CRPC, radium-223 is preferred. Furthermore, patients with metastatic CRPC should only be considered for therapy with PSMA radiopharmaceuticals within appropriate clinical trials. Recently, the EAU and EANM collaborated to produce consensus statements as interim guidance on PSMA PET/CT imaging in patients suitable for [177Lu]Lu-PSMA therapy in August 2021. 90 The panel members reached a consensus on the usefulness of dosimetric evaluation performed via SPECT/CT, although it is not strictly required for patient selection before or during therapy. They agreed against performing PSMA PET/CT after every course of therapy and [18F]FDG PET/CT after the completion of treatment. There was uncertainty about the role of additional PSMA PET/CT at the end of planned therapy. There was a minor discrepancy between statements 33 and 7 regarding the use of PSMA PET/CT to evaluate the response to [177Lu]Lu-PSMA therapy. There was a strong agreement regarding the need to follow procedural guidelines for [177Lu]Lu-PSMA therapy.
Another consensus panel, APCCC 2022, 46 was held, and a panel of experts voted with a strong consensus (92%) to recommend performing a baseline PSMA PET even if it is not required for the selection of 177Lu-PSMA therapy. However, 74% of the same panel recommended that the threshold of uptake for selecting treatment with 177Lu-PSMA therapy be based on VISION criteria, while 24% recommended using TheraP criteria, and 2% said that PSMA PET is not necessary for treatment selection. There was no consensus for any of these options, with 17 abstentions.
The present and future direction
In the early days of drug development for CRPC, docetaxel was the only drug available to prolong prognosis, so ARAT was designed to compare post-docetaxel outcomes with those of placebo. Subsequently, trials were conducted to prove efficacy in CRPC prior to chemotherapy, resulting in the worldwide use of ARAT agents with and without chemotherapy. In addition, several ARATs have been approved for use before CRPC, that is, in hormone-sensitive prostate cancer.91–94
In PSMA RLT, different PSMA ligands and different radioligands are being developed. Although it is still being developed as a treatment for CRPC, which has a poor prognosis, now that chemotherapeutic and ARAT agents have been established, RLT is being tested to prove its usefulness in many post-treatment CRPC patients.
In addition, as with ARAT drug development, trials are underway to confirm the utility of PSMA RLT for CRPC prior to the use of chemotherapeutic agents and ARAT agents. 95 This is also being tested for usefulness in hormone-sensitive prostate cancer as well, 96 and a future is anticipated in which it will be necessary to discuss when PSMA RLT should be used in what is still a complex sequential treatment.97–100
Conclusion
In the treatment of prostate cancer, accurate diagnosis is essential for superior treatment. In this regard, theranostics is an excellent drug delivery system to deliver drugs to patients who express them, and could be a very important diagnostic and therapeutic tool in the treatment of metastatic prostate cancer and CRPC, which have a poor prognosis. The issue with PSMA theranostics is that it is currently not widely available and accessible to all patients due to various reasons such as limited production of the radiotracers, high cost, and regulatory barriers. This has resulted in unequal access to this promising treatment option for patients with prostate cancer. With the advent of ARATs, the treating physician already has many options, but the right drug for the right patient has not yet been established, and the availability of RLTs may further complicate the treatment regimen, but we believe we have a revolutionary tool in our hands.
Acknowledgments
Not applicable.
Footnotes
ORCID iD: Motohide Uemura  https://orcid.org/0000-0001-7263-7965
https://orcid.org/0000-0001-7263-7965
Contributor Information
Motohide Uemura, Department of Urology, Fukushima Medical University School of Medicine, 1, Hikarigaoka, Fukushima 960-1295, Japan; Department of Urology, Iwase General Hospital, Sukagawa, Fukushima, Japan; Department of Urology, Osaka University Graduate School of Medicine, Suita, Osaka, Japan.
Tadashi Watabe, Department of Nuclear Medicine and Tracer Kinetics, Osaka University Graduate School of Medicine, Suita, Osaka, Japan.
Seiji Hoshi, Department of Urology, Fukushima Medical University School of Medicine, Fukushima, Japan.
Ryo Tanji, Department of Urology, Fukushima Medical University School of Medicine, Fukushima, Japan.
Kei Yaginuma, Department of Urology, Fukushima Medical University School of Medicine, Fukushima, Japan.
Yoshiyuki Kojima, Department of Urology, Fukushima Medical University School of Medicine, Fukushima, Japan.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Motohide Uemura: Conceptualization; Investigation; Project administration; Writing – original draft; Writing – review & editing.
Tadashi Watabe: Investigation; Project administration.
Seiji Hoshi: Investigation; Writing – original draft.
Ryo Tanji: Conceptualization; Visualization.
Kei Yaginuma: Investigation.
Yoshiyuki Kojima: Investigation; Project administration.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
MP and J-YS declare no relationships/activities/interests.
Availability of data and materials: Not applicable.
References
- 1.Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan, Ministry of Health, Labour and Welfare. Cancer Statistics, 2023. [Google Scholar]
- 2.Harris WP, Mostaghel EA, Nelson PS, et al. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol 2009; 6: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512. [DOI] [PubMed] [Google Scholar]
- 4.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13: 983–992. [DOI] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–160. [DOI] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 7.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 8.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223. [DOI] [PubMed] [Google Scholar]
- 10.O’Keefe DS, Bacich DJ, Huang SS, et al. A perspective on the evolving story of PSMA Biology, PSMA-Based imaging, and endoradiotherapeutic strategies. J Nucl Med 2018; 59: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen DP, Xiong PL, Liu H, et al. Induction of PSMA and internalization of an anti-PSMA mAb in the vascular compartment. Mol cancer res 2016; 14: 1045–1053. [DOI] [PubMed] [Google Scholar]
- 12.Sweat SD, Pacelli A, Murphy GP, et al. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998; 52: 637–640. [DOI] [PubMed] [Google Scholar]
- 13.Silver DA, Pellicer I, Fair WR. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997; 3: 81–85. [PubMed] [Google Scholar]
- 14.Paschalis A, Sheehan B, Riisnaes R, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol 2019; 76: 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartsch G, Horninger W, Klocker H, et al. Prostate cancer mortality after introduction of prostate-specific antigen mass screening in the Federal State of Tyrol, Austria. Urology 2001; 58: 417–424. [DOI] [PubMed] [Google Scholar]
- 16.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013; 63: 597–603. [DOI] [PubMed] [Google Scholar]
- 17.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015; 373: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tosoian JJ, Gorin MA, Ross AE, et al. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol 2017; 14: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2021; 79: 243–262. [DOI] [PubMed] [Google Scholar]
- 20.Hövels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 2008; 63: 387–395. [DOI] [PubMed] [Google Scholar]
- 21.von Eyben FE, Kairemo K. Meta-analysis of 11C-choline and 18F-choline PET/CT for management of patients with prostate cancer. Nucl Med Commun 2014; 35: 221–230. [DOI] [PubMed] [Google Scholar]
- 22.Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in Advanced Prostate Cancer—updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol 2020; 77: 403–417. [DOI] [PubMed] [Google Scholar]
- 23.van Kalmthout LWM, van Melick HHE, Lavalaye J, et al. Prospective validation of gallium-68 prostate specific membrane antigen-positron emission tomography/Computerized tomography for primary staging of prostate cancer. Urol J 2020; 203: 537–545. [DOI] [PubMed] [Google Scholar]
- 24.Woo S, Suh CH, Kim SY, et al. Diagnostic performance of prostate imaging reporting and data system version 2 for detection of Prostate Cancer: a systematic review and diagnostic meta-analysis. Eur Urol 2017; 72: 177–188. [DOI] [PubMed] [Google Scholar]
- 25.Shen G, Deng H, Hu S, et al. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol 2014; 43: 1503–1513. [DOI] [PubMed] [Google Scholar]
- 26.Lecouvet FE, El Mouedden J, Collette L, et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol 2012; 62: 68–75. [DOI] [PubMed] [Google Scholar]
- 27.Pasoglou V, Larbi A, Collette L, et al. One-step TNM staging of high-risk prostate cancer using magnetic resonance imaging (MRI): toward an upfront simplified “all-in-one” imaging approach? Prostate 2014; 74: 469–477. [DOI] [PubMed] [Google Scholar]
- 28.Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020; 395: 1208–1216. [DOI] [PubMed] [Google Scholar]
- 29.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618–629. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–822. [DOI] [PubMed] [Google Scholar]
- 31.Eapen RS, Nzenza TC, Murphy DG, et al. PSMA PET applications in the prostate cancer journey: from diagnosis to theranostics. World J Urol 2019; 37: 1255–1261. [DOI] [PubMed] [Google Scholar]
- 32.Evangelista L, Zattoni F, Cassarino G, et al. PET/MRI in prostate cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2021; 48: 859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.https://jrct.niph.go.jp/en-latest-detail/jRCTs022220021.
- 34.Fendler WP, Calais J, Eiber M. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol 2019; 5: 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauckneht M, Miceli A, Signori A, et al. Combined forced diuresis and late acquisition on [68Ga]Ga-PSMA-11 PET/CT for biochemical recurrent prostate cancer: a clinical practice-oriented study. Eur Radiol 2023; 33: 3343–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceci F, Oprea-Lager DE, Emmett L, et al. E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging 2021; 48: 1626–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000042995.
- 38.Naka S, Watabe T, Kurimoto K, et al. Automated [18F]PSMA-1007 production by a single use cassette-type synthesizer for clinical examination. EJNMMI Radiopharm Chem 2020; 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watabe T, Uemura M, Soeda F, et al. High detection rate in [18F]PSMA-1007 PET: interim results focusing on biochemical recurrence in prostate cancer patients. Ann Nucl Med 2021; 35: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filippi L, Urso L, Bianconi F, et al. Radiomics and theranostics with molecular and metabolic probes in prostate cancer: toward a personalized approach. Expert Rev Mol Diagn 2023; 23: 243–255. [DOI] [PubMed] [Google Scholar]
- 41.Filippi L, Chiaravalloti A, Schillaci O, et al. The potential of PSMA-targeted alpha therapy in the management of prostate cancer. Expert Rev Anticancer Ther 2020; 20: 823–829. [DOI] [PubMed] [Google Scholar]
- 42.Perez PM, Hope TA, Behr SC, et al. Intertumoral heterogeneity of 18F-FDG and 68Ga-PSMA uptake in prostate cancer pulmonary metastases. Clin Nucl Med 2019; 44: e28–e32. [DOI] [PubMed] [Google Scholar]
- 43.Parida GK, Tripathy S, Datta Gupta S, et al. Adenocarcinoma prostate with neuroendocrine differentiation: potential utility of 18F-FDG PET/CT and 68Ga-DOTANOC PET/CT over 68Ga-PSMA PET/CT. Clin Nucl Med 2018; 43: 248–249. [DOI] [PubMed] [Google Scholar]
- 44.Chen R, Wang Y, Zhu Y, et al. The added value of 18F-FDG PET/CT compared with 68Ga-PSMA PET/CT in patients with castration-resistant prostate cancer. J Nucl Med 2022; 63: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogl UM, Beer TM, Davis ID, et al. Lack of consensus identifies important areas for future clinical research: Advanced Prostate Cancer Consensus Conference (APCCC) 2019 findings. Eur J Cancer2022; 160: 24–60. [DOI] [PubMed] [Google Scholar]
- 46.Gillessen S, Bossi A, Davis ID, et al. Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer2023; 185: 178–215. [DOI] [PubMed] [Google Scholar]
- 47.Hofman MS, Violet J, Hicks RJ, et al. [177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol 2018; 19: 825–833. [DOI] [PubMed] [Google Scholar]
- 48.Hofman MS, Emmett L, Sandhu S. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 2021; 397: 797–804. [DOI] [PubMed] [Google Scholar]
- 49.https://clinicaltrials.gov/ct2/show/NCT05114746.
- 50.Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med 2021; 385: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fizazi K, Herrmann K, Krause BJ, et al. 576MO health-related quality of life (HRQoL), pain and safety outcomes in the phase III VISION study of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Ann Oncol 2021; 32: S627–S628. [Google Scholar]
- 52.ClinicalTrials.gov. 177Lu-PSMA-I&T for metastatic castration-resistant prostate cancer, https://www.clinicaltrials.gov/ct2/show/NCT05204927.
- 53.Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med 2016; 57: 1170–1176. [DOI] [PubMed] [Google Scholar]
- 54.Weineisen M, Schottelius M, Simecek J, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med 2015; 56: 1169–1176. [DOI] [PubMed] [Google Scholar]
- 55.Kim YJ, Kim YI. Therapeutic responses and survival effects of 177Lu-PSMA-617 radioligand therapy in metastatic castrate-resistant prostate cancer: a meta-analysis. Clin Nucl Med 2018; 43: 728–734. [DOI] [PubMed] [Google Scholar]
- 56.Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study Investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med 2017; 58: 85–90. [DOI] [PubMed] [Google Scholar]
- 57.Parker C, Lewington V, Shore N, et al. Targeted alpha therapy, an emerging class of cancer agents: a review. JAMA Oncol 2018; 4: 1765–1772. [DOI] [PubMed] [Google Scholar]
- 58.Feuerecker B, Tauber R, Knorr K, et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur Urol 2021; 79: 343–350. [DOI] [PubMed] [Google Scholar]
- 59.Rosar F, Krause J, Bartholomä M, et al. Efficacy and safety of [(225)Ac]Ac-PSMA-617 augmented [(177)Lu]Lu-PSMA-617 radioligand therapy in patients with highly advanced mCRPC with poor prognosis. Pharmaceutics 2021; 13: 722–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sen I, Thakral P, Tiwari P, et al. Therapeutic efficacy of 225Ac-PSMA-617 targeted alpha therapy in patients of metastatic castrate resistant prostate cancer after taxane-based chemotherapy. Ann Nucl Med 2021; 35: 794–810. [DOI] [PubMed] [Google Scholar]
- 61.Zacherl MJ, Gildehaus FJ, Mittlmeier L, et al. First clinical results for PSMA-targeted α-therapy using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J Nucl Med 2021; 62: 669–674. [DOI] [PubMed] [Google Scholar]
- 62.Khreish F, Ebert N, Ries M, et al. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging 2020; 47: 721–728. [DOI] [PubMed] [Google Scholar]
- 63.Satapathy S, Mittal BR, Sood A, et al. Health-related quality-of-life outcomes with actinium-225-prostate-specific membrane antigen-617 therapy in patients with heavily pretreated metastatic castration-resistant prostate cancer. Indian J Nucl Med 2020; 35: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sathekge M, Bruchertseifer F, Vorster M, et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving 225Ac-PSMA-617 radioligand therapy. J NuclMed 2020; 61: 62–69. [DOI] [PubMed] [Google Scholar]
- 65.Yadav MP, Ballal S, Sahoo RK, et al. Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant prostate cancer patients. Theranostics 2020; 10: 9364–9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted α-Therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med 2018; 59: 795–802. [DOI] [PubMed] [Google Scholar]
- 67.https://clinicaltrials.gov/ct2/show/NCT05219500.
- 68.Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med 2016; 57: 1941–1944. [DOI] [PubMed] [Google Scholar]
- 69.Feng Y, Zalutsky MR. Production, purification and availability of (211)At: near term steps towards global access. Nucl Med Biol 2021; 100-101: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindegren S, Albertsson P, Bäck T, et al. Realizing clinical trials with astatine-211: the chemistry infrastructure. Cancer Biother Radiopharm 2020; 35: 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guérard F, Maingueneau C, Liu L, et al. Advances in the chemistry of astatine and implications for the development of radiopharmaceuticals. Acc Chem Res 2021; 54: 3264–3275. [DOI] [PubMed] [Google Scholar]
- 72.Yang H, Wilson JJ, Orvig C, et al. Harnessing α-emitting radionuclides for therapy: radiolabeling method review. J Nucl Med 2022; 63: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zalutsky MR, Reardon DA, Akabani G, et al. Clinical experience with alpha-particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med 2008; 49: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andersson H, Cederkrantz E, Bäck T, et al. Intraperitoneal α-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of 211At-MX35 F(ab′)2—a phase I study. J Nucl Med 2009; 50: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 75.Hallqvist A, Bergmark K, Bäck T, et al. Intraperitoneal α-Emitting radioimmunotherapy with (211)At in relapsed ovarian cancer: long-term follow-up with individual absorbed dose estimations. J Nucl Med 2019; 60: 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cederkrantz E, Andersson H, Bernhardt P, et al. Absorbed doses and risk estimates of (211)At-MX35 F(ab’)2 in intraperitoneal therapy of ovarian cancer patients. Int J Radiat Oncol Biol Phys 2015; 93: 569–576. [DOI] [PubMed] [Google Scholar]
- 77.Leidermark E, Hallqvist A, Jacobsson L. Estimating the risk for secondary cancer following targeted alpha therapy with astatine-211 intraperitoneal radioimmunotherapy. J Nucl Med 2022; 10: 2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doberenz I, Doberenz W, Wunderlich G, et al. Endoarterielle therapie eines zungenkarzinoms mit 211 at-markierten humanserumalbumin-mikrosphären—erste klinische erfahrungen. NucCompact 1990; 21: 124–127. [Google Scholar]
- 79.ClinicalTrials.gov. Radioimmunotherapy (211At-OKT10-B10) and chemotherapy (Melphalan) before stem cell transplantation for the treatment of multiple myeloma, https://clinicaltrials.gov/ct2/show/NCT04466475
- 80.ClinicalTrials.gov. ²¹¹At-OKT10-B10 and fludarabine alone or in combination with cyclophosphamide and low-dose TBI before donor stem cell transplant for the treatment of newly diagnosed, recurrent, or refractory high-risk multiple myeloma, https://clinicaltrials.gov/ct2/show/NCT04579523
- 81.ClinicalTrials.gov. 211At-BC8-B10 followed by donor stem cell transplant in treating patients with relapsed or refractory high-risk acute leukemia or myelodysplastic syndrome, https://clinicaltrials.gov/ct2/show/NCT03670966.
- 82.ClinicalTrials.gov. 211At-BC8-B10 before donor stem cell transplant in treating patients with high-risk acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndrome, or mixed-phenotype acute leukemia, https://clinicaltrials.gov/ct2/show/NCT03128034
- 83.ClinicalTrials.gov. Targeted alpha therapy using astatine (At-211) against differentiated thyroid cancer, https://clinicaltrials.gov/ct2/show/NCT05275946.
- 84.Watabe T, Kaneda-Nakashima K, Ooe K, et al. Extended single-dose toxicity study of [211At]NaAt in mice for the first-in-human clinical trial of targeted alpha therapy for differentiated thyroid cancer. Ann Nucl Med 2021; 35: 702–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ukon N, Higashi T, Hosono M, et al. Manual on the proper use of meta-[211At] astato-benzylguanidine ([211At] MABG) injections in clinical trials for targeted alpha therapy (1st edition). Ann Nucl Med 2022; 36: 695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watabe T, Kaneda-Nakashima K, Shirakami Y, et al. Targeted α-therapy using astatine (211At)-labeled PSMA1, 5, and 6: a preclinical evaluation as a novel compound. Eur J Nucl Med Mol Imaging 2023; 50: 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Filippi L, Palumbo B, Frantellizzi V, et al. Prostate-specific membrane antigen-directed imaging and radioguided surgery with single-photon emission computed tomography: state of the art and future outlook. Expert Rev Med Devices 2022; 19: 815–824. [DOI] [PubMed] [Google Scholar]
- 88.de Barros HA, van Oosterom MN, Donswijk ML. Robot-assisted prostate-specific membrane antigen-radioguided salvage surgery in recurrent prostate cancer using a DROP-IN gamma probe: the first prospective feasibility study. Eur Urol 2022; 82: 97. [DOI] [PubMed] [Google Scholar]
- 89.Fanti S, Minozzi S, Antoch G, et al. Consensus on molecular imaging and theranostics in prostate cancer. Lancet Oncol 2018; 19: e696–e708. [DOI] [PubMed] [Google Scholar]
- 90.Fanti S, Briganti A, Emmett L, et al. EAU-EANM consensus statements on the role of prostate-specific membrane antigen positron emission tomography/computed tomography in patients with prostate cancer and with respect to [177Lu]Lu-PSMA radioligand therapy. Eur Urol Oncol 2022; 5: 530–536. [DOI] [PubMed] [Google Scholar]
- 91.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017; 377: 352–360. [DOI] [PubMed] [Google Scholar]
- 92.Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. New Engl J Med 2019; 381: 13–24. [DOI] [PubMed] [Google Scholar]
- 93.Davis ID, Martin AJ, Stockler MR. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 2019; 381: 121–131. [DOI] [PubMed] [Google Scholar]
- 94.Smith MR, Hussain M, Saad F. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med 2022; 386: 1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sathekge M, Bruchertseifer F, Knoesen O. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging 2019; 46: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banda A, Privé BM, Allach Y, et al. PSMA-RLT in patients with Metastatic Hormone-sensitive prostate cancer: a retrospective study. OA Cancer 2022; 15: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sudo H, Tsuji AB, Sugyo A, et al. Preclinical evaluation of the acute radiotoxicity of the α-Emitting molecular-targeted therapeutic agent 211At-MABG for the treatment of malignant pheochromocytoma in normal mice. Transl Oncol 2019; 12: 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohshima Y, Sudo H, Watanabe S, et al. Antitumor effects of radionuclide treatment using α-emitting meta-211At-astato-benzylguanidine in a PC12 pheochromocytoma model. Eur J Nucl Med Mol Imaging 2018; 45: 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000042995.
- 100.Wu H, Xu T, Wang X. Diagnostic performance of (68)gallium labelled prostate-specific membrane antigen positron emission tomography/computed tomography and magnetic resonance imaging for staging the prostate cancer with intermediate or high risk prior to radical prostatectomy. World J Mens Health 2020; 38: 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]