Abstract
Background
There is a paucity of epidemiological data on the association between long-term variability of blood pressure (BP) and incident atrial fibrillation (AF).
Objectives
The purpose of this study was to evaluate the association of BP variability with incident AF in a large sample of adults with type 2 diabetes.
Methods
We included participants who had ≥5 BP measurements in the first 24 months of action to control cardiovascular risk in diabetes. The visit-to-visit variability of systolic blood pressure (SBP) and diastolic blood pressure (DBP) was estimated using the coefficient of variation, SD, and variability independent of the mean. Incident AF was recorded using follow-up electrocardiograms. Modified Poisson regression was used to generate risk ratios (RRs) and 95% CI for AF.
Results
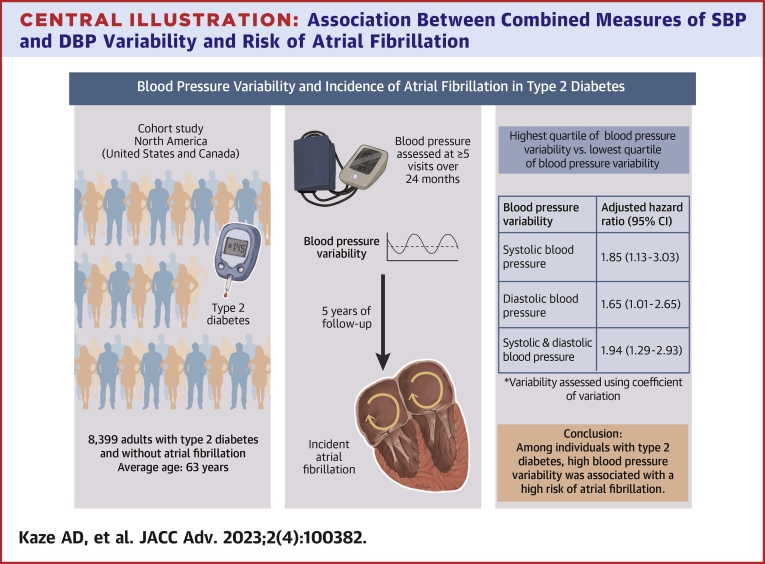
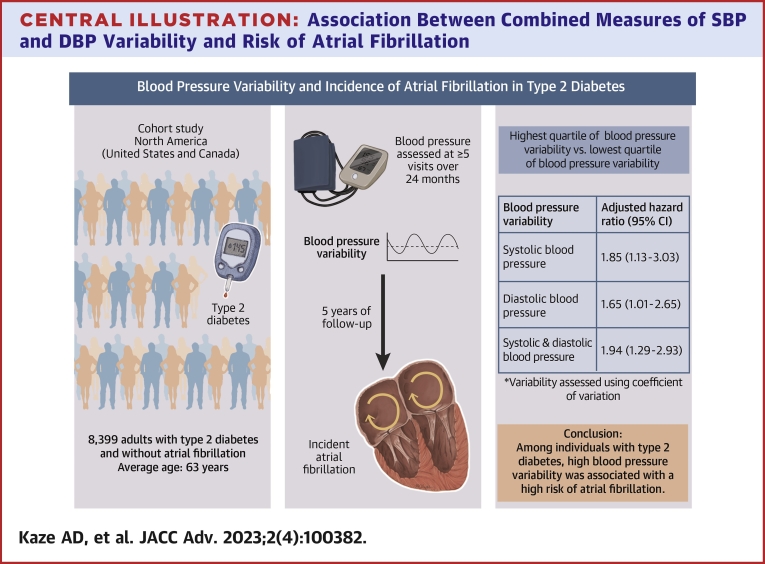
A total of 8,399 participants were included (average age 62.6 ± 6.5 years, 38.8% women, 63.2% White). Over a median follow-up of 5 years, 155 developed AF. Compared to the lowest quartile, the highest quartile of BP variability was associated with an increased risk of AF (RR: 1.85 [95% CI: 1.13-3.03] and 1.63 [95% CI: 1.01-2.65] for coefficient of variation of SBP and DBP, respectively). Participants in the highest quartile of both SBP and DBP had a 2-fold higher risk of AF compared to those in the lowest 3 quartiles of both SBP and DBP (RR: 1.94; 95% CI: 1.29-2.93).
Conclusions
In a large cohort of adults with type 2 diabetes, higher variability in SBP and DBP was independently associated with an increased risk of AF.
Key words: atrial fibrillation, blood pressure, diabetes type 2, epidemiology
Central Illustration
Atrial fibrillation (AF) is the most frequent sustained cardiac arrhythmia worldwide, and its prevalence is rapidly increasing.1,2 AF is associated with significant morbidity, mortality, and healthcare expenses.2, 3, 4 Hypertension and type 2 diabetes are 2 major comorbid illnesses in individuals with AF.5,6 Indeed, both diabetes and hypertension promote maladaptive profibrillatory structural and electrical remodeling in the left atrium, increasing the propensity to develop AF.7,8 Among patients with established AF, ∼17% have diabetes,5 and ∼60% to 80% have hypertension.6 Furthermore, blood pressure (BP) variability was increased among individuals with AF in a cohort of patients treated for hypertension.9 Although BP reduction is widely considered a priority among patients with hypertension and diabetes,10 long-term variability of BP has been linked to increased risks of adverse events including heart failure (HF) and stroke, independently of average BP.11, 12, 13, 14 The mechanisms through which high BP variability increases stroke risk remain uncertain. Given the positive relation between AF and stroke,2 AF may account for the association between BP variability and stroke. The relation of BP variability with AF risk has not been well studied, especially among patients with diabetes who inherently display higher BP variability,15 due in part to arterial stiffness as well as their propensity to develop autonomic nervous system dysregulation.15,16
Using data from the ACCORD (Action to Control Cardiovascular Risk in Diabetes study, we evaluated the associations of long-term variability of BP with incident AF in adults with type 2 diabetes. We hypothesized that greater BP variability would be associated with an increased risk of AF.
Methods
The datasets used for these analyses are publicly available through the BioLINCC (Biologic Specimen and Data Repository Information Coordinating Center) of the NHLBI (National Heart, Lung, and Blood Institute).
Study design
We conducted a secondary analysis of the ACCORD study, a double 2-by-2 randomized factorial trial that enrolled 10,251 participants from January 2001 to October 2005 from 77 centers in the United States and Canada. Participants were aged 40 to 79 years (with a history of cardiovascular disease [CVD]) or 55 to 79 years (with significant albuminuria, atherosclerosis, left ventricular hypertrophy, or a minimum of 2 CVD risk factors). They were randomly allocated to receive either an intensive glucose-lowering intervention with a glycated hemoglobin (HbA1C) goal <6% or standard glycemic treatment aiming for an HbA1C of 7.0% to 7.9%. Additionally, 5,518 participants were randomly assigned to either simvastatin plus fenofibrate or simvastatin plus placebo (ACCORD lipid trial), and the remaining 4,733 were randomized to either an intensive BP arm with a systolic BP (SBP) goal of <120 mm Hg or a standard BP arm with a SBP goal of <140 mm Hg (ACCORD BP trial). The full details about the rationale and design of ACCORD have been published elsewhere.17
We excluded participants with a history of AF/missing AF status at baseline (n = 171), missing AF status at follow-up (n = 769), or <5 BP measurements (n = 912) during the initial 24 months (BP variability assessment period). We included participants with ≥5 BP visits due to evidence showing the visit-to-visit variability of BP increases with the number of visits used for its calculation.18
The research protocol was approved by the institutional review board at all the participating centers, and each participant gave an informed consent.17
Assessment of long-term variability of blood pressure
At each study visit and location, BP was measured 3 times from the right arm with participants in the seated position using an automated device (OMRON HEM-907). The first reading was obtained after the participant had rested quietly for 5 minutes; the subsequent readings were recorded after an interval of 60 seconds between measurements. The average of the 3 readings was used as the visit BP. The schedule of BP visits in ACCORD has been reported previously.17 Briefly, BP measurements were recorded at baseline and 4-month intervals in the standard BP arm and at 2-month intervals in the intensive BP arm. To calculate visit-to-visit variability of SBP and diastolic BP (DBP), we used data from follow-up visits that occurred at 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 months after randomization (Supplemental Figure 1). We chose to begin the BP variability assessment period at the 4-month visit to avoid confounding by the initial reduction in BP that occurred at the beginning of the trial due to early medication titration.
The long-term variability of SBP and DBP variability was evaluated using 3 metrics: 1) the intraindividual SD across visits; 2) the coefficient of variation (CV) calculated as 100·SD/mean; and 3) the variability independent of the mean (VIM) calculated as 100·SD/meanα, where α is the regression coefficient based on the natural logarithm of SD as a function of the natural logarithm of the respective mean BP measure. We included multiple metrics to attempt to capture the entire spectrum of BP variability.
Ascertainment of atrial fibrillation
We included incident cases of AF that occurred after the BP variability assessment period from 4 months to 24 months (Supplemental Figure 1).
For each participant, a resting 12-lead electrocardiogram (ECG) was performed at enrollment and follow-up visits.17
Incident AF was ascertained from ECGs obtained at the biennial and exit visits and defined by any Minnesota code 8.3.19 All ECGs were digitally acquired by trained staff using a standardized protocol with a GE MAC 1200 electrocardiograph (GE) at a calibration of 10 mm/mV and a speed of 25 mm/s. The ECGs were transmitted via telephone line to the central core laboratory at the Epidemiological Cardiology Research Center, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA. ECGs were inspected visually for technical errors and inadequate quality and automatically processed using GE 12-SL Marquette Version 2001 (GE).
Covariates
The covariates were selected based on their relationship with BP variability and/or AF. These included the following variables collected at baseline: age, sex, race/ethnicity, treatment arm, cigarette smoking, alcohol intake, duration of diabetes, use of antihypertensive medications, use of antiarrhythmics (beta-blockers, digoxin, calcium-channel blockers, and other antiarrhythmics), use of thiazolidinediones, history of atherosclerotic cardiovascular disease (ASCVD) (including prior myocardial infarction, coronary revascularization, carotid or peripheral revascularization, angina, or stroke) or HF.17 Additionally, the following covariates were collected during the BP variability assessment: average SBP, average body mass index (BMI: average of BMI values from baseline, 12-month and 24-month visits), average hemoglobin A1C (HbA1C: calculated from visits that occurred at 4, 8, 12, 16, 20, and 24 months), average ratio of total/high-density lipoprotein (HDL) cholesterol (mean of 4-, 8-, 12-, and 24-month visits), average estimated glomerular filtration rate (eGFR: calculated from 4-, 8-, 12-, 16-, 20-, and 24-month follow-up visits).
Statistical analyses
For each variability metric, the variability of SBP and DBP was evaluated as both quartiles and continuous variables (expressed per 1 SD). To explore the effect of the combined variability of SBP and DBP, we divided participants into 4 groups: those in the lowest 75th percentile of both SBP and DBP variability (Q1-Q3); those in the highest quartile of DBP variability but below the 75th percentile of SBP variability (DBP Q4 only); those in the highest quartile of SBP variability, but below the 75th percentile of DBP variability (SBP Q4 only); and participants in the highest quartiles of both SBP and DBP (both Q4). We compared the characteristics of participants according to quartiles of SBP and DBP variability using the analysis of variance, or Kruskal-Wallis test, for continuous variables and the chi-square test for categorical variables. We used multivariable Poisson regression with robust variance estimation to compute risk ratios (RRs) and associated 95% CI for AF. We built regression models in a sequential manner. Model 1 adjusted for age, sex, race, and treatment arm; Model 2 included variables in Model 1 plus cigarette smoking, alcohol drinking, mean BMI, mean total/HDL cholesterol ratio, mean eGFR, mean hemoglobin A1C, diabetes duration, antihypertensive medication use, thiazolidinedione use, use of antiarrhythmics (beta-blockers, digoxin, calcium-channel blockers, and other antiarrhythmics), prevalent ASCVD, and HF. Model 3 included model 2 with further adjustment for mean SBP (when assessing SBP variability), mean DBP (when evaluating DBP variability), or both when evaluating combined measures of SBP and DBP variability.
We tested for statistical interaction by age, sex, treatment arm (intensity of glycemic lowering), race, use of thiazolidinediones, and use of antiarrhythmics by adding an interaction term with variability quartiles to the fully adjusted model. In sensitivity analyses, we performed additional adjustments for the number of BP measurements. We also adjusted for the use of angiotensin-converting enzyme inhibitor (ACEI)/angiotensin-II receptor blockers (ARB), as these medications have been associated with a lower risk of AF.20,21 Finally, we restricted the analytical sample to individuals not on antiarrhythmic medications at baseline.
All statistical analyses were conducted using STATA 14.2 (Stata, Inc). A 2-sided P value <0.05 was deemed statistically significant.
Results
Characteristics of study participants
Supplemental Table 1 compares the baseline characteristics of included participants to those of excluded participants (exclusion criteria listed in Supplemental Figure 1). The characteristics of study participants by variability of SBP, DBP, and combined variability of both are displayed in Table 1 and Supplemental Tables 2 and 3. Compared to those in lower quartiles, participants in the highest quartile (Q4) of BP variability were older and more frequently Black or women. They also had a longer duration of diabetes, lower eGFR, higher averages of BMI, HbA1C, SBP, and DBP, as well as higher rates of CVD and use of insulin and ACEI/ARB at baseline.
Table 1.
Characteristics of Participants by SBP Variability
| Total (N = 8,399) |
Quartiles of SBP CV, % |
P Value | ||||
|---|---|---|---|---|---|---|
| <5.66 (n = 2,100) |
5.66-7.70 (n = 2,101) |
7.71-10.13 (n = 2,099) |
>10.13 (n = 2,099) |
|||
| At baseline | ||||||
| Age, y | 62.6 ± 6.5 | 61.6 ± 6.5 | 62.4 ± 6.4 | 63.0 ± 6.5 | 63.2 ± 6.6 | <0.001 |
| Women, % | 38.8 | 33.4 | 36.7 | 40.7 | 44.6 | <0.001 |
| Race/ethnicity, % | <0.001 | |||||
| White | 63.2 | 62.2 | 64.8 | 64.5 | 61.1 | |
| Black | 18.3 | 13.6 | 17.9 | 19.3 | 22.5 | |
| Hispanic | 6.9 | 6.7 | 6.0 | 7.2 | 7.7 | |
| Other | 11.6 | 17.43 | 11.2 | 9.0 | 8.7 | |
| Treatment arm, % | 0.576 | |||||
| Intensive glycemic lowering | 49.6 | 49.3 | 50.0 | 48.5 | 50.5 | |
| Standard glycemic lowering | 50.4 | 50.7 | 50.0 | 51.5 | 49.5 | |
| Current smoking, % | 13.8 | 12.6 | 13.7 | 13.1 | 15.8 | 0.015 |
| Alcohol drinking, % | 24.3 | 25.4 | 26.9 | 22.6 | 22.3 | 0.001 |
| Use of BP-lowering drug, % | 83.7 | 79.0 | 82.9 | 84.5 | 88.4 | <0.001 |
| Use of ACEI/ARB, % | 70.1 | 64.8 | 69.2 | 72.0 | 74.2 | <0.001 |
| Use of antiarrhythmics, % | 43.1 | 36.5 | 39.2 | 43.8 | 52.9 | <0.001 |
| Use of thiazolidinediones, % | 22.8 | 21.7 | 25.2 | 22.0 | 22.3 | 0.023 |
| Use of insulin, % | 35.1 | 29.6 | 34.6 | 37.0 | 39.2 | <0.001 |
| Duration of diabetes, y | 10.0 (5.0-15.0) | 9.0 (5.0-14.0) | 9.0 (5.0-15.0) | 10.0 (5.0-15.0) | 10.0 (5.0-16.0) | <0.001 |
| Prevalent ASCVD, % | 34.3 | 31.4 | 32.1 | 33.6 | 40.2 | <0.001 |
| Prevalent HF, % | 4.1 | 2.9 | 3.0 | 4.1 | 6.4 | <0.001 |
| Over BPV assessment period | ||||||
| Number of BP measurements | <0.001 | |||||
| 5 | 9.1 | 9.3 | 8.0 | 9.2 | 9.7 | |
| 6 | 63.8 | 68.9 | 64.3 | 60.8 | 61.3 | |
| ≥7 | 27.1 | 21.9 | 27.7 | 29.9 | 29.0 | |
| Mean BMI | 32.7 ± 5.5 | 32.1 ± 5.5 | 32.6 ± 5.4 | 32.8 ± 5.6 | 33.1 ± 5.7 | <0.001 |
| Mean total/HDL cholesterol ratio | 4.4 ± 2.0 | 4.3 ± 1.7 | 4.4 ± 2.3 | 4.4 ± 2.0 | 4.4 ± 1.9 | 0.147 |
| Mean eGFR, mL/min/1.73 m2 | 81.4 ± 21.1 | 84.1 ± 20.5 | 83.0 ± 21.7 | 80.5 ± 20.6 | 77.8 ± 21.1 | <0.001 |
| Mean hemoglobin A1C, % | 7.2 ± 0.9 | 7.1 ± 0.9 | 7.1 ± 0.9 | 7.2 ± 0.8 | 7.2 ± 0.9 | <0.001 |
| Mean SBP, mm Hg | 128.1 ± 12.5 | 127.0 ± 11.4 | 127.3 ± 11.9 | 128.6 ± 12.8 | 129.7 ± 13.6 | <0.001 |
| Mean DBP, mm Hg | 69.9 ± 8.5 | 70.8 ± 7.9 | 69.8 ± 8.3 | 69.8 ± 8.5 | 69.4 ± 9.0 | <0.001 |
Values are mean ± SD, %, or median (IQR).
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; BP = blood pressure; BPV = blood pressure variability; CV = coefficient of variation; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; HF = heart failure; SBP = systolic blood pressure.
Variability of systolic blood pressure
Over a median follow-up period of 5 years, 155 participants developed incident AF. The adjusted HRs for AF by SBP variability are shown in Table 2. After multivariable adjustment, the RRs for incident AF per each SD in intraindividual CV, SD, and VIM of SBP were 1.26 (95% CI: 1.09-1.44), 1.24 (95% CI: 1.11-1.37), and 1.25 (95% CI: 1.09-1.44), respectively (Central Illustration). Participants in the highest quartile of SBP CV had a 1.9-fold higher risk of incident AF compared to those in the lowest quartile (RR: 1.85, 95% CI: 1.13-3.03). The corresponding HRs relating AF to the SD and VIM of SBP were 1.89 (95% CI: 1.13-3.18) and 1.75 (95% CI: 1.08-2.85), respectively.
Table 2.
Risk Ratios for Incident Atrial Fibrillation by SBP Variability
| Measure of Variability | Quartiles of SBP Variability | P Value for T trend | Per 1-SD Increment | |||
|---|---|---|---|---|---|---|
| SBP CV, % | <5.66 | 5.66-7.70 | 7.71-10.13 | >10.13 | ||
| No events/no at risk | 28/2,100 | 29/2,101 | 38/2,099 | 60/2,099 | 155/8,399 | |
| Model 1 | 1.00 (reference) | 0.97 (0.58-1.63) | 1.22 (0.75-2.00) | 2.09 (1.32-3.29)b | 0.001 | 1.33 (1.19-1.49)c |
| Model 2 | 1.00 (reference) | 1.06 (0.62-1.81) | 1.22 (0.72-2.06) | 1.86 (1.14-3.04)a | 0.007 | 1.26 (1.09-1.45)b |
| Model 3 | 1.00 (reference) | 1.06 (0.62-1.81) | 1.22 (0.72-2.06) | 1.85 (1.13-3.03)a | 0.008 | 1.26 (1.09-1.44)b |
| SBP SD, mm Hg | <7.07 | 7.07-9.77 | 9.78-13.13 | >13.13 | ||
| No events/no at risk | 26/2,101 | 40/2,099 | 32/2,101 | 57/2,098 | 155/8,399 | |
| Model 1 | 1.00 (reference) | 1.41 (0.86-2.31) | 1.14 (0.67-1.92) | 2.10 (1.31-3.36)b | 0.005 | 1.24 (1.14-1.35)c |
| Model 2 | 1.00 (reference) | 1.55 (0.92-2.61) | 1.11 (0.63-1.93) | 1.88 (1.13-3.12)a | 0.044 | 1.23 (1.11-1.35)c |
| Model 3 | 1.00 (reference) | 1.55 (0.92-2.61) | 1.11 (0.63-1.95) | 1.89 (1.13-3.18)a | 0.049 | 1.24 (1.11-1.37)c |
| SBP VIM | <1.69 | 1.69-2.29 | 2.30-3.03 | >3.03 | ||
| No events/no at risk | 29/2,100 | 25/2,100 | 43/2,100 | 58/2,099 | 155/8,399 | |
| Model 1 | 1.00 (reference) | 0.82 (0.48-1.39) | 1.34 (0.83-2.15) | 1.94 (1.23-3.07)b | 0.001 | 1.34 (1.18-1.51)c |
| Model 2 | 1.00 (reference) | 0.89 (0.51-1.54) | 1.32 (0.79-2.19) | 1.75 (1.07-2.84)a | 0.007 | 1.25 (1.08-1.45)b |
| Model 3 | 1.00 (reference) | 0.89 (0.51-1.55) | 1.32 (0.79-2.19) | 1.75 (1.08-2.85)a | 0.007 | 1.25 (1.09-1.44)b |
Values are RR (95% CI) unless otherwise indicated. Model 1 adjusted for age, sex, race, and treatment arm; Model 2 includes model 1 plus cigarette smoking, alcohol drinking, mean BMI, mean total/HDL cholesterol ratio, mean eGFR, mean hemoglobin A1C, diabetes duration, antihypertensive medication use, thiazolidinedione use, use of antiarrhythmics (beta-blockers, digoxin, calcium-channel blockers, and other antiarrhythmics), prevalent ASCVD and HF. Model 3 includes model 2 plus mean SBP.
ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; CV = coefficient of variation; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; HF = heart failure; SBP = systolic blood pressure; SD = standard deviation; VIM = variability independent of the mean.
P < 0.05.
P < 0.01.
P < 0.001.
Central Illustration.
Association Between Combined Measures of SBP and DBPVariability and Risk of Atrial Fibrillation
Variability of diastolic blood pressure
As displayed in Table 3, the RRs for incident AF per each SD increment in CV, SD, and VIM of DBP were 1.30 (95% CI: 1.13-1.50), 1.30 (95% CI: 1.14-1.48), and 1.30 (95% CI: 1.13-1.49), respectively. The RRs for the top compared to the bottom quartiles were 1.63 (95% CI: 1.01-2.65), 2.02 (95% CI: 1.24-3.30), and 1.87 (95% CI: 1.15-3.04) for CV, SD, and VIM of DBP, respectively.
Table 3.
Risk Ratios for Incident Atrial Fibrillation by DBP Variability
| Measure of Variability | Quartiles of DBP Variability | P Value for Trend | Per 1-SD Increment | |||
|---|---|---|---|---|---|---|
| DBP CV, % | <6.37 | 6.37-8.46 | 8.46-10.92 | >10.92 | ||
| No events/no at risk | 26/2,100 | 31/2,100 | 40/2,100 | 58/2,099 | 155/8,399 | |
| Model 1 | 1.00 (reference) | 1.11 (0.66-1.88) | 1.40 (0.85-2.30) | 1.95 (1.22-3.13)b | 0.002 | 1.34 (1.19-1.51)c |
| Model 2 | 1.00 (reference) | 1.00 (0.58-1.70) | 1.25 (0.76-2.08) | 1.59 (0.98-2.57) | 0.029 | 1.29 (1.11-1.48)b |
| Model 3 | 1.00 (reference) | 1.01 (0.59-1.72) | 1.28 (0.77-2.13) | 1.63 (1.01-2.65)a | 0.021 | 1.30 (1.13-1.50)c |
| DBP SD, mm Hg | <4.41 | 4.41-5.82 | 5.83-7.62 | >7.62 | ||
| No events/no at risk | 25/2,104 | 39/2,097 | 33/2,102 | 58/2,096 | 155/8,399 | |
| Model 1 | 1.00 (reference) | 1.58 (0.96-2.61) | 1.34 (0.79-2.26) | 2.56 (1.60-4.10)c | <0.001 | 1.33 (1.19-1.48)c |
| Model 2 | 1.00 (reference) | 1.42 (0.86-2.36) | 1.17 (0.69-1.98) | 2.06 (1.27-3.36)b | 0.008 | 1.31 (1.15-1.48)c |
| Model 3 | 1.00 (reference) | 1.42 (0.85-2.36) | 1.17 (0.69-1.97) | 2.02 (1.24-3.30)b | 0.011 | 1.30 (1.14-1.48)c |
| DBP VIM | <63.70 | 63.71-84.02 | 84.03-108.80 | >108.81 | ||
| No events/no at risk | 25/2,100 | 34/2,100 | 38/2,100 | 58/2,099 | 155/8,399 | |
| Model 1 | 1.00 (reference) | 1.32 (0.79-2.21) | 1.46 (0.88-2.43) | 2.31 (1.44-3.70)c | <0.001 | 1.34 (1.20-1.50)c |
| Model 2 | 1.00 (reference) | 1.20 (0.71-2.02) | 1.29 (0.77-2.16) | 1.89 (1.16-3.06)a | 0.008 | 1.30 (1.14-1.49)c |
| Model 3 | 1.00 (reference) | 1.20 (0.71-2.03) | 1.29 (0.77-2.16) | 1.87 (1.15-3.04)a | 0.010 | 1.30 (1.13-1.49)c |
Values are RR (95% CI) unless otherwise indicated. Model 1 adjusted for age, sex, race, and treatment arm; Model 2 includes model 1 plus cigarette smoking, alcohol drinking, mean BMI, mean total/HDL cholesterol ratio, mean eGFR, mean hemoglobin A1C, diabetes duration, antihypertensive medication use, thiazolidinedione use, use of antiarrhythmics (beta-blockers, digoxin, calcium-channel blockers, and other antiarrhythmics), prevalent ASCVD and HF. Model 3 includes model 2 plus mean DBP.
ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; CV = coefficient of variation; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; HF = heart failure; SD = standard deviation; VIM = variability independent of the mean.
P < 0.05.
P < 0.01.
P < 0.001.
Combined measures of SBP and DBP variability
Compared to participants in the lowest 75th percentile of both SBP and DBP variability, participants in the highest quartile of both SBP and DBP variability had an increased risk of AF, with RRs being 1.94 (95% CI: 1.29-2.93), 1.99 (95% CI: 1.31-3.00), 2.02 (95% CI: 1.35-3.03) for CV, SD, and VIM of DBP, respectively (Central Illustration, Supplemental Table 4).
Supplementary analyses
We did not observe any effect modification by age, sex, race, glycemia treatment arm, use of thiazolidinediones, or use of antiarrhythmics (all P interaction >0.05).
Additionally, we tested the robustness of our findings by performing additional adjustments for the number of BP measurements and use of ACEI/ARB. Consistent with our main results, higher variability of SBP, DBP, and both measures combined remained significantly associated with an increased risk of AF (Supplemental Table 5).
Discussion
We conducted a comprehensive assessment of the relations between long-term variability of BP and incident AF in a large cohort of adults with type 2 diabetes. We found that a greater variability of SBP and DBP in isolation and combined was independently associated with an increased risk of incident AF after accounting for other AF risk factors. Our findings were consistent across variability measures. Our results underscore the importance of stable and consistent BP control over time for AF risk reduction among patients with type 2 diabetes.
Our study is one of only a few to investigate the effect of long-term variability of BP on incident AF as the outcome exclusively in patients with type 2 diabetes. Long-term variability of BP has received significant attention as an independent predictor of adverse cardiovascular outcomes, including stroke.11, 12, 13, 14 However, the mechanisms for the increased risk of stroke among people with high BP variability have remained unclear. Our findings of a positive association between high visit-to-visit variability of BP and an increased risk of AF corroborate a recent study of Korean individuals, although the prior study had a number of limitations, including the lack of racial diversity, the assessment of BP variability using <5 BP visits (two-thirds of the study sample had only 3 BP visits), and the fact that it was not exclusive to people with diabetes.22
The exact pathways linking BP variability to a higher risk of AF are unknown, but a few hypotheses may be formulated. First, one potential mechanism relates to the effects of long-term BP variability on cardiac remodeling. Indeed, in a large sample of US adults, a greater variability of SBP and DBP was found to be associated with impaired left ventricular (LV) relaxation and increased LV filling pressures,23 abnormalities that may precede left atrial remodeling, and pulmonary vein dilation; this eventually led to a reduction in the atrial effective refractory period, which increases the vulnerability to AF.24, 25, 26 Second, the link with endothelial dysfunction may provide another pathway, as high BP variability may impair endothelial function by inhibiting nitric oxide formation, which has been associated with AF in animal models.27 Third, BP variability has been shown to be associated with arterial stiffness and arterial remodeling,16 findings which were found to predict incident AF in the Framingham Heart Study.28 Other mechanisms include the neurohormonal changes—including the link with cardiac autonomic neuropathy,15,29 and the renin-angiotensin system30—inflammatory changes, myocardial fibrosis, cardiac hypertrophy, and LV systolic dysfunction.31 Furthermore, behavioral and lifestyle factors such as the lack of adherence to BP medication and the excessive use of alcohol could also contribute to increased BP variability.
Our findings have several implications, especially for individuals with type 2 diabetes. Contemporary guidelines recommend the optimization of BP in the management of patients with diabetes, but they focus only on average BP goals. The long-term variability of BP has been linked to greater risks of adverse cardiovascular outcomes including stroke. However, the exact mechanisms for the increased risk of stroke in relation to BP variability have remained unknown. Our data suggest that AF may potentially serve as the connecting link between BP variability and stroke. Additional research is needed to evaluate optimal methods to capture long-term BP variability in clinical settings and its potential role as a therapeutic target. The added value of accounting for BP variability in risk tools for predicting AF is a potential clinical application that should be explored. Such an endeavor would require external validation on a separate cohort. Additionally, studies of therapeutic strategies targeting both BP variability and AF risk are needed.
Our study has multiple strengths. First, this was a prospective study that included a large, multiracial/ethnic sample of adults with type 2 diabetes. Second, BP was recorded using a standardized protocol at multiple preset study visits. Third, ACCORD collected data on several confounders following a standardized protocol, and we conducted robust adjustments for these confounders. Finally, the adjudication of AF events was standardized using central ECG core analysis.
Study Limitations
Our findings should be interpreted in the context of a few limitations. First, incident AF cases were ascertained using short ECG recordings performed at 24-monthly and study exit visits; continuous electrocardiographic monitoring was not performed in ACCORD. As AF was not a prespecified outcome of the ACCORD trial and we did not have access to all medical and hospital records, it is possible that we did not detect certain AF events, such as paroxysmal AF occurring in-between visits. This might have resulted in a nondifferential misclassification of the outcome, leading to a bias towards the null. Moreover, we could not evaluate the differential effect of BP variability on AF subtypes such as paroxysmal, persistent, or permanent AF. Second, as cardiac imaging data were not collected in ACCORD, we could not assess the effect of the left atrial volume index on our results, as this may affect the risk of AF. Third, we did not have data on adherence to antihypertensive medications, which influence BP variability.32 We also did not have extensive longitudinal data on medications used for indications other than hypertension (eg, nonsteroidal anti-inflammatory drugs and decongestants), which can influence BP. Fourth, we used the average values of covariates over the BP variability assessment period in our models, which may be less efficient than fitting these as time-varying covariates. Finally, our study was observational; therefore, there is the possibility of residual confounding.
Conclusions
In a large sample of individuals with type 2 diabetes, greater long-term variability in BP was independently associated with a higher risk of incident AF, above and beyond mean BP. Our data highlight the relevance of BP variability in the prevention and prediction of AF and underscore the need to minimize BP fluctuations among patients with type 2 diabetes.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: In a large sample of adults with type 2 diabetes, a greater variability in each of systolic and diastolic blood pressure was independently associated with an increased risk of atrial fibrillation, independent of several known risk factors and independently of diabetes duration.
TRANSLATIONAL OUTLOOK: Our findings highlight the relevance of visit-to-visit variability of blood pressure in atrial fibrillation risk assessment and the necessity to maintain normal and consistent BP over time in people with type 2 diabetes. More mechanistic research is needed to elucidate the pathways from blood pressure variability to incident atrial fibrillation.
Funding support and author disclosures
ACCORD has been funded by federal funds from the National Heart Lung and Blood Institute (NHLBI). The data from the ACCORD study were supplied to the investigators by the NHLBI through the Central Repository BioLINCC. Dr Echouffo Tcheugui was supported by NIH/NHLBI grant K23 HL153774. Dr Fonarow has done consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Janssen, Medtronic, Merck, and Novartis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors wish to thank the staff and participants of the ACCORD study for their valuable contributions.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure and tables, please see the online version of this paper.
Supplementary data
References
- 1.Go A.S., Hylek E.M., Phillips K.A., et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 3.Kim M.H., Johnston S.S., Chu B.-C., Dalal M.R., Schulman K.L. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 4.Piccini J.P., Hammill B.G., Sinner M.F., et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Staa T.P., Setakis E., Di Tanna G.L., Lane D.A., Lip G.Y.H. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost. 2011;9(1):39–48. doi: 10.1111/j.1538-7836.2010.04085.x. [DOI] [PubMed] [Google Scholar]
- 6.Nabauer M., Gerth A., Limbourg T., et al. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11(4):423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S.-J., Choisy S.C.M., Barman P., et al. Atrial remodeling and the substrate for atrial fibrillation in rat hearts with elevated afterload. Circ Arrhythm Electrophysiol. 2011;4(5):761–769. doi: 10.1161/CIRCEP.111.964783. [DOI] [PubMed] [Google Scholar]
- 8.Russo I., Frangogiannis N.G. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84–93. doi: 10.1016/j.yjmcc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehlum M.H., Liestøl K., Wyller T.B., Hua T.A., Rostrup M., Berge E. Blood pressure variability in hypertensive patients with atrial fibrillation in the VALUE trial. Blood Press. 2019;28(2):77–83. doi: 10.1080/08037051.2018.1524707. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111–S134. doi: 10.2337/dc20-S010. [DOI] [PubMed] [Google Scholar]
- 11.Gosmanova E.O., Mikkelsen M.K., Molnar M.Z., et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;68(13):1375–1386. doi: 10.1016/j.jacc.2016.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehlum M.H., Liestøl K., Kjeldsen S.E., et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J. 2018;39(24):2243–2251. doi: 10.1093/eurheartj/ehx760. [DOI] [PubMed] [Google Scholar]
- 13.Kaze A.D., Santhanam P., Erqou S., Bertoni A.G., Ahima R.S., Echouffo-Tcheugui J.B. Long-term variability of blood pressure and incidence of heart failure among individuals with type 2 diabetes. ESC Heart Fail. 2021;8(4):2959–2967. doi: 10.1002/ehf2.13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaze A.D., Santhanam P., Erqou S., et al. Long-term variability of blood pressure, cardiovascular outcomes, and mortality: the Look AHEAD study. Am J Hypertens. 2021;34(7):689–697. doi: 10.1093/ajh/hpaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spallone V. Blood pressure variability and autonomic dysfunction. Curr Diab Rep. 2018;18(12):137. doi: 10.1007/s11892-018-1108-z. [DOI] [PubMed] [Google Scholar]
- 16.Zhou T.L., Henry R.M.A., Stehouwer C.D.A., van Sloten T.T., Reesink K.D., Kroon A.A. Blood pressure variability, arterial stiffness, and arterial remodeling. Hypertension. 2018;72(4):1002–1010. doi: 10.1161/HYPERTENSIONAHA.118.11325. [DOI] [PubMed] [Google Scholar]
- 17.Buse J.B., Bigger J.T., Byington R.P., et al. Action to control cardiovascular risk in diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99(12A):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Levitan E.B., Kaciroti N., Oparil S., Julius S., Muntner P. Blood pressure measurement device, number and timing of visits, and intra-individual visit-to-visit variability of blood pressure. J Clin Hypertens (Greenwich) 2012;14(11):744–750. doi: 10.1111/jch.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prineas R.J., Crow R.S., Zhang Z.-M. Springer Science & Business Media; 2009. The Minnesota Code Manual of Electrocardiographic Findings. [Google Scholar]
- 20.Du H., Fan J., Ling Z., et al. Effect of nifedipine versus telmisartan on prevention of atrial fibrillation recurrence in hypertensive patients. Hypertension. 2013;61(4):786–792. doi: 10.1161/HYPERTENSIONAHA.111.202309. [DOI] [PubMed] [Google Scholar]
- 21.Schaer B.A., Schneider C., Jick S.S., Conen D., Osswald S., Meier C.R. Risk for incident atrial fibrillation in patients who receive antihypertensive drugs: a nested case-control study. Ann Intern Med. 2010;152(2):78–84. doi: 10.7326/0003-4819-152-2-201001190-00005. [DOI] [PubMed] [Google Scholar]
- 22.Lee S.-R., Choi Y.-J., Choi E.-K., et al. Blood pressure variability and incidence of new-onset atrial fibrillation: a nationwide population-based study. Hypertension. 2020;75(2):309–315. doi: 10.1161/HYPERTENSIONAHA.119.13708. [DOI] [PubMed] [Google Scholar]
- 23.Nwabuo C.C., Yano Y., Moreira H.T., et al. Association between visit-to-visit blood pressure variability in early adulthood and myocardial structure and function in later life. JAMA Cardiol. 2020;5(7):795–801. doi: 10.1001/jamacardio.2020.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsang T.S.M., Gersh B.J., Appleton C.P., et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40(9):1636–1644. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 25.Triposkiadis F., Tentolouris K., Androulakis A., et al. Left atrial mechanical function in the healthy elderly: new insights from a combined assessment of changes in atrial volume and transmitral flow velocity. J Am Soc Echocardiogr. 1995;8(6):801–809. doi: 10.1016/s0894-7317(05)80004-5. [DOI] [PubMed] [Google Scholar]
- 26.Ravelli F., Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96(5):1686–1695. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 27.Cai H., Li Z., Goette A., et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106(22):2854–2858. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 28.Shaikh A.Y., Wang N., Yin X., et al. Relations of arterial stiffness and brachial flow-mediated dilation with new-onset atrial fibrillation: the Framingham Heart study. Hypertension. 2016;68(3):590–596. doi: 10.1161/HYPERTENSIONAHA.116.07650. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal S.K., Norby F.L., Whitsel E.A., et al. Cardiac autonomic dysfunction and incidence of atrial fibrillation: results from 20 years follow-up. J Am Coll Cardiol. 2017;69(3):291–299. doi: 10.1016/j.jacc.2016.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goette A., Staack T., Röcken C., et al. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35(6):1669–1677. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- 31.Kudo H., Kai H., Kajimoto H., et al. Exaggerated blood pressure variability superimposed on hypertension aggravates cardiac remodeling in rats via angiotensin II system-mediated chronic inflammation. Hypertension. 2009;54(4):832–838. doi: 10.1161/HYPERTENSIONAHA.109.135905. [DOI] [PubMed] [Google Scholar]
- 32.Eguchi K. Effects of antihypertensive therapy on blood pressure variability. Curr Hypertens Rep. 2016;18(10):75. doi: 10.1007/s11906-016-0680-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.