Abstract
This review evaluates physical activity as a candidate for an adjunct treatment, in conjunction with antiretroviral therapy (ART), for people living with HIV (PLWH). Evidence is summarized that chronic, non-resolving inflammation (a principal feature of immune system dysfunction) and a dysfunctional state of the gut environment are key factors in HIV infection that persist despite treatment with ART. In addition, evidence is summarized that regular physical activity may restore normal function of both the immune system and the gut environment and may thereby ameliorate symptoms and non-resolving inflammation-associated comorbidities that burden PLWH. Physicians who care for PLWH could thus consider incorporating physical activity into treatment plans to complement ART. It is also discussed that different types of physical activity can have different effects on the gut environment and immune function, and that future research should establish more specific criteria for the design of exercise regimens tailored to PLWH.
Keywords: immunodeficiency, exercise intensity, microbiome, gut barrier, pro-inflammatory, recovery
“Incorporating physical activity into treatment plans may ameliorate lingering effects of HIV”
Introduction
Current treatment of infection with human immunodeficiency virus (HIV) focuses on countering the destruction of human immune cells and associated weakening of immune defenses. 1 Such intervention aims to prevent progression to stage 3 HIV, or acquired immunodeficiency syndrome (AIDS).2-4 At the same time, people living with HIV (PLWH) exhibit systemic, low-grade immune system activation, a hallmark of immune system dysfunction, that persists even when viral replication is effectively suppressed 5 by antiretroviral therapy (ART). This prolonged immune system activation is commonly referred to as chronic inflammation, but the term non-resolving inflammation has recently been introduced to emphasize that this persistent immune response primarily constitutes a failure to terminate pro-inflammatory signaling.6,7 Such non-resolving inflammation is a root cause of multiple diseases and disorders that can occur as comorbidities in PLWH.8-13
This review presents an overview of HIV effects that are successfully addressed by ART, as well as effects that persist in ART-treated PLWH. These lingering effects are aspects of immune system dysfunction, and can be linked to a persistent disruption of the gut environment. Since ART-treated PLWH experience such dysfunction, it is necessary and relevant in modern HIV treatment to understand how to restore a functional immune system and gut environment. Because dysfunction of these systems is implicated in chronic illnesses that disproportionately burden PLWH, ameliorating non-resolving inflammation should be a priority for physicians aiming to improve the quality of life of their patients with HIV.
Here, we review the effect of physical activity on immune system function and the gut environment. Attention is given to various types of physical activity and their apparent different impacts on the gut environment. Based on review of the available evidence, a proposal is made that certain types of physical activity may be candidates for adjunct treatment, complementary to ART, that may restitute the gut environment and restore normal immune system function (eliminate non-resolving inflammation) in PLWH. We propose that regular, voluntary (interest-based) physical activity with adequate periods of recovery is most likely to have a restorative effect on the gut environment and immune system function and is therefore an attractive candidate for an additional treatment complementary to ART in PLWH. Incorporating physical activity into treatment plans may ameliorate lingering effects of HIV that are not addressed by ART and enhance the quality of life of PLWH.
HIV Effects and Antiretroviral Therapy
Viruses like HIV interact with the immune system in complex ways; they use components of the immune system for their own replication, and at the same time precipitate a dysfunctional state of the immune system as a whole. The retrovirus HIV enters immune cells (especially CD4+ T cells) and employs their molecular vehicles for its own replication. 14 To do this, HIV activates gene regulators that trigger replication, such as the transcription factor NF-kB.15,16 The latter is a key regulator of the immune response and orchestrates inflammatory signaling.17,18 Constitutive, low-grade activation of NF-kB is associated with non-resolving inflammation in HIV-infected individuals.15,19 Furthermore, the take-over of CD4+ T cells by HIV eventually results in destruction of immune cells via programmed cell death—mainly as triggered by the human host’s immune system in an attempt to eliminate the virus. 5 Doitsh and Greene state that, during HIV infection, “most cells are not dying because of a toxic action of products encoded by HIV. Rather, death occurs as a consequence of a powerful defensive innate immune response launched by the host against the virus leading to a cellular form of suicide rather than virological murder.” 5 This attack by the immune system reverberates system-wide as non-resolving inflammation. The attack on immune cells involves reactive oxygen species (ROS) that function in the programmed cell death of CD4+ T cells 20 and also serve as signals that trigger a system-wide, snow-balling, and continuous mobilization of the immune system, 21 that is, non-resolving inflammation. This non-resolving inflammation involves systemic disruption of redox homeostasis (balance between oxidants and antioxidants) and the gut environment (see next section), and plays a key role in cardiovascular disease, obesity, GI distress, cancer, mental illness and many other conditions.22-27 The recognition of the role of non-resolving inflammation as a root cause for disease has been called “one of the most important scientific discoveries in health research in recent years.”28,29 Notably, PLWH exhibit a higher incidence of many of these and other comorbidities, which emphasizes the critical importance of addressing non-resolving inflammation in this population.
Another key component of the human immune system that interacts with HIV is the interferon system that detects pathogens and triggers defenses. The interferon system exhibits low activity in CD4+ cells 30 and is further suppressed by HIV infection. 31 Such impaired interferon activation in CD4+ cells allows sustained viral replication in these cells. Subsequent activation of interferon in other cells—once HIV infection is established—fails to eradicate the virus and may even promote non-resolving inflammation and further deterioration of host health. 30 As stated above, all aspects of immune-system activation involve ROS production that has the potential to snow-ball into non-resolving inflammation under exacerbating conditions, such as a dysfunctional gut environment (see section below). However, additional research is needed to elucidate the complex interactions between HIV and the interferon system. 32
Current HIV treatment based on ART arrests the replication cycle of HIV and has been shown to increase immune cell numbers in most PLWH.33-35 However, some PLWH are “immunological nonresponders” who do respond to ART with cessation of viral replication, but fail to exhibit restoration of CD4+ cell counts.36-38 More commonly yet, PLWH who receive regular ART treatment that halts viral replication typically show varying levels of persistent non-resolving inflammation.8,39-41 Addressing immune-system dysfunction in the context of HIV infection is thus critical even in the era of ART.
HIV and the Gut-Immune Link
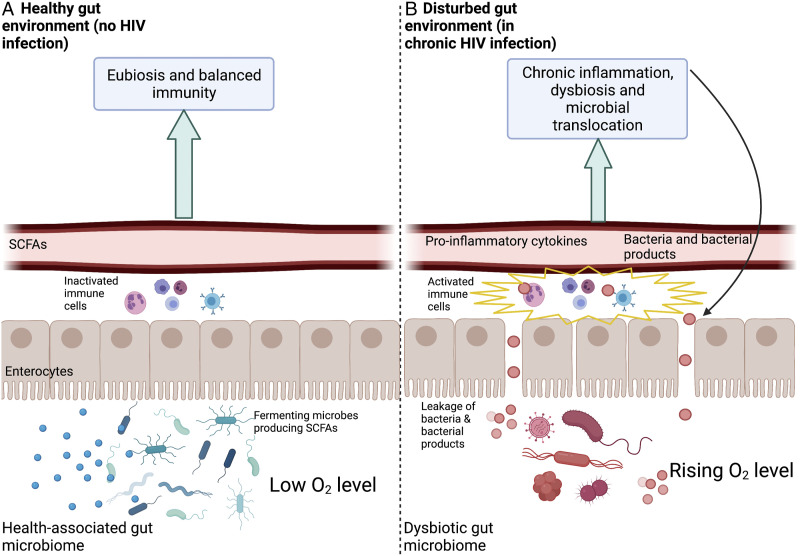
Additional mechanistic insight into the repercussions of non-resolving inflammation induced by HIV focuses on the gastrointestinal tract and the gut microbiome.42-46Figure 1 depicts the gut environment in its functional state without HIV infection (Figure 1A) as well as in its dysfunctional state during chronic HIV infection (Figure 1B).
Figure 1.
Schematic depiction of a healthy gut environment (A) and a gut environment (B) that is disturbed as the result of chronic HIV infection. Depicted are the gut content (bottom; fermenting microbes producing short-chain saturated fatty acids, SCFAs, especially in (A)), the cells forming the gut lining (enterocytes), immune cells (between enterocytes and blood vessels) that are either inactivated or activated, and a blood vessel that receives either SCFAs or pro-inflammatory cytokines, bacteria and bacterial products from the gut. Created with BioRender.com.
The Gut-Immune Link in Health
Figure 1A depicts a functional, health-promoting (eubiotic) gut microbiome characterized by prominent presence of anaerobic, fermenting bacteria that thrive in low-oxygen environments. 47 These microbes produce short-chain fatty acids (SCFAs) that serve as an energy source for the cells (enterocytes) lining the gut48-50 (for details, see below) and can also diffuse into the bloodstream.51,52 Locally, these SCFAs have essential roles in supporting gut-barrier integrity.53,54 For example, they act as gene activators for the production of proteins that maintain gut-barrier integrity.54-56 An example of a key SCFA is butyrate (produced by fermenting gut microbes) that increases expression of the tight-junction protein Claudin-1 involved in supporting gut-barrier integrity. 57 In addition, butyrate also represses a protein (Claudin-2) 58 that has been suggested to increase barrier permeability. 59 Gut-barrier integrity supports beneficial gut microbes and these gut microbes, in turn, maintain gut-barrier integrity. 47
A healthy gut is characterized by a steep oxygen gradient between the (hypoxic) gut lumen and the distal zone of the intestinal epithelium.60,61 The enterocytes of the intestinal epithelium require oxygen for their oxidative metabolism that supports their roles in barrier formation, 55 nutrient absorption 62 and immunity.61,63,64 Enterocytes use butyrate as the fuel they burn with oxygen to produce ATP for these various functions. 61 Therefore, butyrate produced by fermenting gut microbes stimulates oxygen uptake by enterocytes and helps maintain a low oxygen environment within the gut.55,65 By requiring oxygen for their own metabolism, enterocytes thus contribute to maintaining hypoxic concentrations within the gut lumen, 61 which supports anaerobic, SCFA-producing fermenting microbes.60,66 In addition to directly supporting gut-barrier integrity, SCFAs play a role in dampening the activity of NF-kB and the inflammatory response.67-71 In the state where gut microbial eubiosis and gut-barrier integrity are maintained, the immune cells associated with the gut lining are not activated to induce systemic effects72,73 (Figure 1A). Any condition that interferes with enterocyte function (eg, as seen during HIV infection) triggers production of messengers that initiate a pro-inflammatory cascade involving pro-inflammatory cytokines.74,75 Such adverse conditions include an insufficient supply of butyrate (as fuel) for enterocytes or of oxygen (to burn butyrate) as can be the case during exhaustive exercise (see Figure 2B in the next major section below).
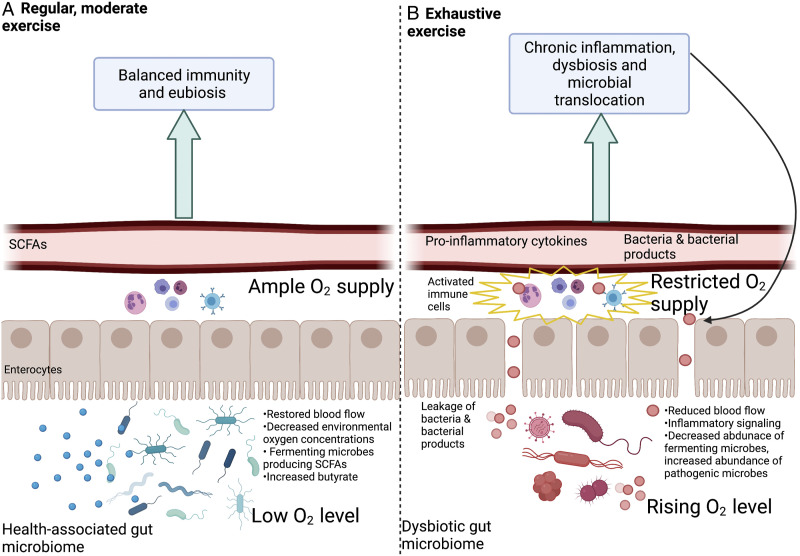
Figure 2.
The effects of a regimen of regular moderate physical activity (A) compared with the effects of exhaustive exercise (B). Acclimation to regular moderate physical activity (A) restores (splanchnic) blood flow to the gut during physical activity, which supports maintenance of low oxygen concentrations within the gut lumen and the activity of fermenting, SCFA-producing microbes, strengthens the gut barrier and decreases inflammation. Continuous exhaustive exercise (B) continuously reduces blood flow to the gut, leading to enterocyte dysfunction, gut microbiome dysbiosis, gut-barrier impairment, microbial translocation out of the gut, and non-resolving inflammation. Created with BioRender.com.
The Gut-Immune Link as Affected by HIV
Figure 1B illustrates a feed-forward loop of loss of gut-barrier integrity and non-resolving inflammatory responses in PLWH. Early HIV infection is characterized by widespread destruction of CD4+ T cells present in gut mucosa tissue. While ART causes some recovery of these cells, this recovery is not always complete and mucosa impairment may persist after individuals receive treatment.76-78 Disruption of T cells in the gut mucosa results in impaired gut-barrier integrity, which allows leakage of gut microbes and microbial products into the bloodstream (Figure 1B). Specifically, lipopolysaccharide (LPS), a cell surface component of gram-negative bacteria, is often identified as a marker of such microbial translocation and increased levels of LPS are observed during chronic HIV infection.79,80 Translocation of LPS activates the immune cells associated with the gut lining (Figure 2B) in a process mediated, for example, by NF-kB.81,82 Continuous activation of NF-kB contributes to a cascade of non-resolving immune system activation and may explain the persistent inflammatory state observed in PLWH.83-85
Furthermore, the disrupted gut barrier in chronic HIV infection (Figure 1B) results in increased oxygen concentrations within the gut, which shifts the gut microbiome to a disrupted state (dysbiosis) with specific losses in anaerobic fermenters and SCFA production. Increased concentrations of oxygen in the gut as a result of gut-barrier impairment also favor increases in gut bacteria that are facultative anaerobes capable of switching to aerobic (oxygen-dependent) cellular respiration and fast growth.86-88 These fast-growing facultative anaerobes can become pathogenic when undergoing such rapid growth.26,88,89 In addition to accelerating the cycle of leaky gut and non-resolving inflammation in PLWH shown in Figure 1B, this shift in microbiome composition is associated with GI distress and other symptoms.86,90
Since non-resolving inflammation and a dysfunctional gut microbiome are implicated in several conditions that disproportionately burden both untreated and treated PLWH, including cardiovascular disease, chronic kidney disease, cancer, and depression, restoration of the gut environment and normal immune function must be a priority for improving the quality of life of PLWH. The next section examines the potential of regular, moderate physical activity, used as a therapy complementary to ART, to reduce non-resolving inflammation and restore gut barrier integrity.
Physical Activity and the Gut-Immune Link
Figure 2 summarizes effects of physical activity on the gut microbiome, gut-barrier integrity, and inflammation that have emerged from recent studies in various human populations (Figures 2A and 2B).
Intensity, duration, and frequency of exercise, as well as age and training status of individual participants, can all influence the effect of exercise on health.91-95 More research is needed to elucidate the threshold of the transition between beneficial and detrimental exercise. A key consideration may be the effect of physical activity on blood flow to the gut, and the fact that acclimation (habituation) to a regular exercise regimen can restore this blood supply.
Effect of Regular Physical Activity With Replete Blood Flow to the Gut
It is well established that regular physical activity can reduce inflammation in various populations.96-98 In principle, physical activity has the potential to divert blood flow from the gut to the working muscles. 99 However, during the establishment of a regular regimen of physical activity, a process of acclimation (habituation) takes place that restores blood supply to the gut during physical activity. 75 Such regular physical activity is associated with fully functional enterocytes and a high abundance of fermenting, butyrate-producing microbes75,100-102 (Figure 2A). While some gut microbes produce butyrate from sugars derived from fiber or complex sugars,103,104 other microbes convert lactate produced by working muscles to SCFAs like butyrate.105,106 The butyrate produced serves to strengthen the gut barrier through the above-described support for enterocytes and, for example, stimulated expression of proteins involved in barrier tightening and downregulation of proteins involved in barrier loosening. A strengthened barrier and associated microbiome eubiosis decrease leakage of microbial products into the bloodstream, and decrease activation of immune system regulators such as NF-kB and the resulting non-resolving inflammation107-113 (Figure 2A). Suppression of NF-kB by physical activity was associated with reduced muscle loss in a mouse model 114 and this effect should also be assessed in humans. Exercise-associated decreases in muscle loss could offer important relief for PLWH since PLWH are often burdened by loss of muscle mass and function. 115
Effect of Physical Activity Associated With Continuous Low Blood Flow to the Gut
The type of exercise that increases the risk for non-resolving inflammation may be identified by its effect on the gut—as exercise that continuously exceeds the capacity for acclimatory restoration of blood flow to the gut at a level sufficient to prevent hypoxia in enterocytes. As far as impacts on the gut environment are concerned, a regimen of continuously exhaustive exercise has similar effects to those seen during the initial transition phase from inactivity to an established regimen of regular, moderate physical activity.74,75 Both activities draw blood away from the gut—either continuously or only during a transition phase of acclimation. Whereas acclimation to regular physical activity can restore blood flow to GI cells, 75 this may not be the case for a regimen of continuously exhaustive exercise. 116 Insufficient oxygen supply to the enterocytes is as detrimental as hypoxia within the gut lumen is beneficial. The ability to maintain sufficient blood flow to the gut during exercise is critical to supporting gut barrier integrity and gut microbiome eubiosis74,75,117,118 (Figure 2A). As detailed above, insufficient blood flow to enterocytes interferes with their functions and triggers production of messengers such as pro-inflammatory cytokines74,75 (Figure 2B).
In summary, exercise that continuously exceeds the capacity to maintain sufficient blood flow to the gut results in enterocyte hypoxia, which impairs gut-barrier integrity, 119 increases oxygen concentrations in the gut lumen, and decreases the abundance of anaerobic fermenting microbes120,121 (Figure 2B). Such microbiome dysbiosis and impaired barrier parallel changes observed during chronic HIV infection and promote microbial translocation, chronic activation of gut mucosa-associated immune cells, upward-spiraling immune system activation and further impairment of the gut barrier (Figure 2B).
Additional Effects of Physical Activity on the Gut and Immune System
Additional mechanisms may also contribute to the effects of physical activity on the immune system. For example, adipose tissue around the waist (visceral fat) is metabolically active and releases inflammatory hormones. 122 Excess visceral fat thereby leads to chronic NF-kB activation and non-resolving inflammation.123,124 Exercise can contribute to preventing excess adipose tissue as one avenue to reduce non-resolving inflammation.96,125-127
Moreover, regular physical activity may also restore immune system balance by supporting redox homeostasis. 128 All exercise generates oxidants (reactive oxygen species, ROS) in working muscles which, in turn, stimulate production of endogenous antioxidant enzymes that help keep ROS in check.129,130 Moderate amounts of ROS are essential to induce these important antioxidant enzymes. 131 Redox homeostasis supports immune function, that is, stimulates immunity against infection without inducing excessive self-attack or non-resolving inflammation. 132 Regular non-exhaustive physical activity presumably stimulates ROS and antioxidant production in a balanced ratio that maintains redox homeostasis. Antioxidants are needed to keep ROS from triggering excessive NF-kB activation and programmed cell death.133,134 Continuously exhaustive exercise may produce ROS at a level that exceeds the capacity for antioxidant enzyme production and results in chronic redox imbalance. It should also be noted that most of the antioxidant enzymes induced by physical activity require dietary mineral cofactors, such as zinc, selenium, copper, and manganese, and also cooperate with dietary antioxidant vitamins, such as vitamins C and E. 135
The Transition Between Beneficial and Detrimental Exercise
Intensity and Habituation
There is interest in identifying a quantifiable transition point between beneficial and detrimental physical activity. The authors 136 of a recent review of the relevant evidence proposed that “vigorous endurance training with ≥60 min at ≥70% of VO 2max increases the intestinal permeability, with an enhanced effect observed in hot environments, at high altitude, and under dehydration.” Exercise at 70-80% of VO2max (maximal aerobic capacity, or maximal oxygen consumption) was reported to be the point at which blood flow to the gut decreased by other authors.137,138 It should be noted that this recommendation ties the threshold to individual work capacity as a feature of individual fitness level that is, in turn, presumably associated with acclimation/habituation to a particular exercise regimen and varies with individual differences in training status as well as other personal factors.
Evidence for Effects of Physical Activity on Inflammatory Markers in PLWH
There is some evidence that exercise may influence immune markers in PLWH (Table 1). Table 1 shows that exercise can either lower inflammation markers, have no effect, or increase inflammation markers. The intensity at which training programs are completed may affect the response, with moderate and intense exercise reported as having contrasting effects on inflammatory markers in PLWH. 139 However, some of the studies finding decreases in pro-inflammatory markers in PLWH also involved what can be considered rather intense exercise.
Table 1.
Summary of Selected Studies Published Over the Past Ten Years on the Effects of Interventional Physical Activity Programs on Inflammatory Markers and/or CD4+ Cell Counts in PWLH. Significant Effects (P<.05) Are Noted in Relation to the Duration and Type of the Physical Activity Completed. Longitudinal Study Assesses Variables Over an Extended Period of Time.
| Author(s) | Type of study (original or review) | Duration and type of physical activity | Effects on inflammatory markers | Effects on CD4+ T-cell count |
|---|---|---|---|---|
| Anti-inflammatory effects | ||||
| Bonato et al, 179 2017 | Original study | 12 weeks of a) combined aerobic (walking) and strength training or b) aerobic training (walking) alone | Both groups: decrease in pro-inflammatory hsCRP and IL-18; aerobic-only group: decrease in pro-inflammatory IL-6 | No longitudinal measure |
| Bonato et al, 143 2020 | Review | Longitudinal, interventional training program | Decrease in pro-inflammatory CRP, IL-8 and IL-6 | No longitudinal measure |
| Dudgeon et al, 144 2012 | Original study | 6 weeks of moderate intensity combined aerobic and strength training | Decrease in salivary cortisol as a stress marker at wake | No longitudinal measure |
| Ghayomzadeh et al, 140 2021 | Original study | 6-month combined aerobic and resistance training; aerobic exercise completed at 65–80% maximal heart rate | Decrease in pro-inflammatory IL-6 and TNF-α | No longitudinal measure |
| Pedro et al, 180 2017 | Original study | 16-week heart rate-guided aerobic program | Decrease in pro-inflammatory IL-8 | No longitudinal measure |
| Zanetti et al, 141 2016a | Original study | 12-week resistance training program | Decrease in pro-inflammatory IL-1β, IL-6, IL-8 and TNF-α | Increase in CD4+ count |
| Zanetti et al, 181 2016b | Original study | 12-week resistance training program | Decrease in pro-inflammatory CRP | No longitudinal measure |
| Zanetti et al, 142 2017 | Original study | 12-week resistance training program | Decrease in pro-inflammatory IL-1β, IL-6, IL-8, and TNF-α | No longitudinal measure |
| Zanetti et al, 182 2020 | Original study | 12-week resistance training program; mild to moderate intensity | Decrease in pro-inflammatory IL-1β and CRP; increase in anti-inflammatory IL-10 | No longitudinal measure |
| No significant effects | ||||
| Cutrono et al, 183 2016 | Original study | 12 weeks of combined aerobic and resistance training; aerobic sessions completed at 60-80% of age-determined maximum heart rate | No significant changes in inflammatory markers | No longitudinal measure |
| Ibeneme et al, 145 2019a | Review | Aerobic training alone, resistance training alone, and combination of aerobic and resistance training | No significant changes in inflammatory markers | No longitudinal measure |
| Vingren et al, 184 2018 | Original study | 6-week resistance training program | No significant changes in inflammatory markers | No longitudinal measure |
| Zanetti et al, 151 2021 | Review | 6–24 weeks of resistance training programs | No significant changes in inflammatory markers | Increase in CD4+ cell count |
| Pro-inflammatory effects | ||||
| Erlandson et al, 139 2020 | Original study | 24 weeks of combined aerobic and strength training; first 2 weeks at low intensity; next 10 weeks at moderate intensity (40–50% baseline VO 2max ); last 12 weeks at moderate or high (60–70% of week 12 VO 2max ) intensity | Increase in pro-inflammatory IL-6 in high intensity group | No longitudinal measure |
| Zanetti et al, 142 2017 | Original study | 12-week resistance training program | Decrease in anti-inflammatory IL-10 | No longitudinal measure |
| No inflammatory markers measured | ||||
| Asogwa et al, 150 2020 | Original study | 6 weeks of moderate intensity aerobic training | NA | Increase in CD4+ count |
| de Brito-Neto et al, 149 2019 | Original study | 12-week resistance training program | NA | Increase in CD4+ cell count |
| Dianatinasab et al, 185 2018 | Original study | 12-week combined aerobic and resistance training | NA | Decrease in CD4+ cell count |
| Ezema et al, 147 2014 | Original study | 8 weeks of moderate intensity aerobic training completed at 60–79% of maximum heart rate | NA | Increase in CD4+ cell count |
| Ibeneme et al, 154 2019b | Review | Aerobic and/or resistance training | NA | No significant changes in CD4+ count |
| Maduagwu et al, 148 2017 | Original study | 12-week moderate intensity aerobic training program completed at 50–75% of heart rate reserve | NA | Increase in CD4+ count |
| O’Brien et al, 153 2016 | Review | Aerobic training alone or a combination of aerobic and resistance training | NA | No significant changes in CD4+ count |
| Tiozzo, 152 2011 | Original study | 12-week moderate intensity combined aerobic and resistance training program | NA | No significant changes in CD4+ count |
Abbreviations: CRP, C-reactive protein; hsCRP, high-sensitivity C-reactive protein; IL, interleukin; NA, not applicable (no inflammatory marker measured) TNF, tumor necrosis factor.
A recent clinical trial consisting of combined aerobic and resistance training over six months, with aerobic exercise performed at 65–80% maximal heart rate, resulted in statistically significant decreases in percent body fat and decreased levels of the pro-inflammatory cytokines IL-6 and TNF-α. 140 A 12-week resistance training program also significant decreased subcutaneous body fat, IL-6 and TNF-α in sedentary PLWH 141 as well as in PLWH with metabolic syndrome. 142 A recent review 143 concluded that physical activity is associated with positive changes in the inflammatory environment of PLWH. However, other trials found a mix of decreases in some inflammatory markers and no change in others (eg, Dudgeon et al, 2012). 144 A meta-analysis 145 of a limited number of studies using diverse training regimens concluded that physical activity does not significantly reduce inflammation in PLWH.
Table 1 also includes a summary of recent studies on the effect of exercise on CD4+ T cell counts in PLWH. Non-resolving inflammation and dysbiosis are linked to diminished CD4+ cell counts 146 and low CD4+ counts persist in immunological non-responders (see above). Restoring CD4+ cells is a priority of HIV treatment, and elimination of non-resolving inflammation may facilitate recovery of CD4+ T cell counts in PLWH. Exercise increased CD4+ cell counts in some trials on exercise in PLWH141,147-151 but not in others.152-154 The potential of physical activity to increase CD4+ counts thus warrants further study as a potential avenue to strengthen immunity in PLWH.
Resolving Inflammation and Recovery Time
It should be noted that classification of inflammation regulators into pro- vs anti-inflammatory molecules does not capture the context-dependent roles of these molecules, which applies to both cytokine hormones 155 and another key class of inflammation regulators, the fatty-acid-based eicosanoids.156,157 Moreover, increased acute inflammation following physical activity is not problematic and instead plays a critical role in triggering the synthesis of antioxidant enzymes 131 and thus maintaining immune balance.129,132,158 In fact, when oxidative signals are eliminated by high-dose antioxidant vitamins taken during athletic training, synthesis of endogenous antioxidant enzymes as well as muscle building is prevented. 131 Similarly, anti-inflammatory treatments (eg, NSAIDS) targeting exercise-associated injury can actually prevent healing by blocking the pro-resolution “stop signals” involved in the inflammatory response. 159 Physical activity can thereby acutely increase inflammatory markers while also strengthening pro-resolution pathways. 160 Taken together, these results suggest that short-term increases in markers of inflammation following physical activity may boost, rather than weaken, the anti-inflammatory resolution of inflammation.
The recovery time available for the resolution of acute inflammation may play a role. In other words, exercise performed at an excessive duration, 161 without sufficient recovery time between sessions, 162 may be what is most detrimental. 163 For example, what was described as “vigorous” exercise by the authors164,165 increased the levels of the stress hormone cortisol, whereas “moderate” exercise had no such effect. Likewise, “forced” treadmill running in mice promoted significantly greater increases in cortisol than “voluntary” wheel running. 166 For such exhaustive exercise, constitutive activation of NF-kB and subsequent tissue degeneration, oxidative stress, gut barrier breakdown, gut microbiome dysbiosis and non-resolving inflammation have been demonstrated.167-171 At this point, it can thus not be excluded that detrimental exercise modalities described as “high-intensity,” “vigorous,” or “forced,” may also be lacking in adequate time for recovery and resolution of inflammation between sessions. A notable study by Schlabe et al (2017) 172 suggests that even marathon training, performed as “moderate endurance training” over a period of 12 months, can be safe for PLWH.
Future research is needed that includes assessment of time allowed for recovery and resolution of inflammation, and uses consistent standards to quantify work capacity (such as VO2max), what percent of this capacity was reached and for how long during the physical activity, as well as blood flow to the gut across a range of different physical activities. Furthermore, individualized physical activity regimens likely need to be customized for different needs. As stated above, acclimation to a regimen of physical activity can restore blood supply to the gut during exercise. Such acclimation presumably raises the ceiling of work capacity. However, there likely is an upper limit of this acclimation effect that may be exceeded by certain types of exhaustive exercise. The current understanding thus suggests that physical activity recommendations should be expanded to comprehensively emphasize movement that is interest-based, voluntary, completed at an enjoyable intensity, performed regularly, and allows adequate time for recovery and resolution of inflammation.75,173-178
Proposed Benefits of Physical Activity for PLWH
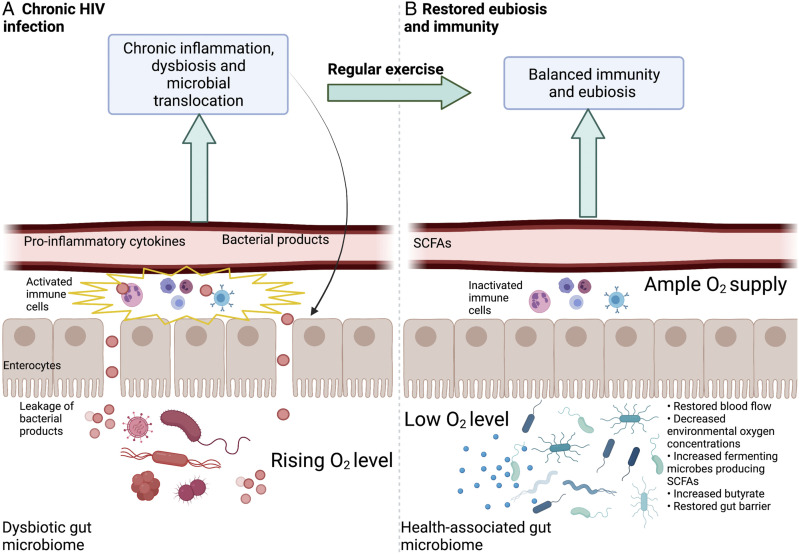
The evidence reviewed above supports a proposal that regular physical activity—allowing sufficient acclimation and recovery that restore blood flow to the gut—may facilitate the resolution of inflammation by ameliorating the leaky gut present in many treated and untreated PLWH (Figure 3). As stated above (Figure 1B), many PLWH experience a dysbiotic gut microbiome, characterized by gut-barrier disruption and microbial translocation that persist despite ART treatment (Figures 1B and 3A). This microbial translocation activates the immune system and leads to release of pro-inflammatory cytokines, microbes, and microbial products into the bloodstream, further triggering widespread immune activation and gut barrier breakdown (see above; Figures 1B and 3A). The non-resolving inflammation experienced by PLWH (Figure 3B) may thus be reduced by adoption of a regimen of regular physical activity that allows sufficient blood flow to the GI tract, increases abundance of fermenting microbes and SCFAs, and strengthens the gut barrier integrity. This type of physical activity may protect against various comorbidities in both treated and untreated PLWH and serve as a complement to traditional ART (Figure 3B).
Figure 3.
Schematic depiction of proposed changes in the gut of PLWH induced by regular, moderate intensity, interest-driven physical activity. (A) Dysbiosis and impaired barrier present during chronic HIV infection (B) Restored blood flow, eubiosis and reduction of inflammation due to regular physical activity. Created with BioRender.com.
At this time, there is some evidence that exercise can reduce markers of inflammation and improve body composition in PLWH (see above; Table 1).141,143,179 However, more research is needed to establish this because findings are presently not consistent. 145 It is clear, however, that physical activity interacts with the same molecular players that are affected by HIV, such as NF-kB. Whereas an acute bout of exercise was associated with acute activation of NF-kB, acclimation to regular exercise suppressed NF-kB in mice models and prevented non-resolving inflammation. 114
Physical activity thus offers great promise as a potential lifestyle modification for reducing non-resolving inflammation and inflammation-associated comorbidities in PLWH. Physicians and other healthcare providers should be aware of the specific benefits physical activity may offer to PLWH and consider incorporating physical activity into treatment plans as a complement to ART. Additional research is needed to design individualized training plans customized to match individual fitness levels and interest.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Elizabeth Enichen https://orcid.org/0000-0002-0752-5390
Robert B. Adams https://orcid.org/0000-0002-7950-5188
References
- 1.Phanuphak N, Gulick RM. HIV treatment and prevention 2019: current standards of care. Curr Opin HIV AIDS. 2020;15(1):4-12. doi: 10.1097/COH.0000000000000588 [DOI] [PubMed] [Google Scholar]
- 2.Sterne JA, Hernán MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366(9483):378-384. doi: 10.1016/S0140-6736(05)67022-5 [DOI] [PubMed] [Google Scholar]
- 3.Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res. 2010;85(1):1-18. doi: 10.1016/j.antiviral.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC . About HIV/AIDS. https://www.cdc.gov/hiv/basics/whatishiv.html. Published June 1, 2021. Accessed September 25, 2021. [Google Scholar]
- 5.Doitsh G, Greene WC. Dissecting how CD4 T cells are lost during HIV infection. Cell Host Microbe. 2016;19(3):280-291. doi: 10.1016/j.chom.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schett G, Neurath MF. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun. 2018;9(1):3261. doi: 10.1038/s41467-018-05800-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley CD, Gilroy DW, Serhan CN. Pro-Resolving lipid mediators and Mechanisms in the resolution of acute inflammation. Immunity. 2014;40(3):315-327. doi: 10.1016/j.immuni.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39(4):633-645. doi: 10.1016/j.immuni.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maniar A, Ellis C, Asmuth D, Pollard R, Rutledge J. HIV infection and atherosclerosis: evaluating the drivers of inflammation. Eur J Prev Cardiol. 2013;20(5):720-728. doi: 10.1177/2047487312447843 [DOI] [PubMed] [Google Scholar]
- 10.Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS. 2016;30(10):1495-1509. doi: 10.1097/QAD.0000000000001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zicari S, Sessa L, Cotugno N, et al. Immune activation, inflammation, and Non-AIDS Co-Morbidities in HIV-infected patients under long-term ART. Viruses. 2019;11(3):200. doi: 10.3390/v11030200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloch M, John M, Smith D, Rasmussen T, Wright E. Managing HIV-associated inflammation and ageing in the era of modern ART. HIV Med. 2020;21(S3):2-16. doi: 10.1111/hiv.12952 [DOI] [PubMed] [Google Scholar]
- 13.Cribbs SK, Crothers K, Morris A. Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol Rev. 2020;100(2):603-632. doi: 10.1152/physrev.00039.2018 [DOI] [PubMed] [Google Scholar]
- 14.Freed EO. HIV-1 and the host cell: an intimate association. Trends Microbiol. 2004;12(4):170-177. doi: 10.1016/j.tim.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Roulston A, Lin R, Beauparlant P, Wainberg MA, Hiscott J. Regulation of human immunodeficiency virus type 1 and cytokine gene expression in myeloid cells by NF-kappa B/Rel transcription factors. Microbiol Rev. 1995;59(3):481-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bren GD, Trushin SA, Whitman J, Shepard B, Badley AD. HIV gp120 induces, NF-κB Dependent, HIV replication that requires Procaspase 8. PLoS One. 2009;4(3):e4875. doi: 10.1371/journal.pone.0004875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldwin AS. THE NF-κB AND IκB PROTEINS: new discoveries and insights. Annu Rev Immunol. 1996;14(1):649-681. doi: 10.1146/annurev.immunol.14.1.649 [DOI] [PubMed] [Google Scholar]
- 18.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayden MS, West AP, Ghosh S. NF- κ B and the immune response. Oncogene. 2006;25(51):6758-6780. doi: 10.1038/sj.onc.1209943 [DOI] [PubMed] [Google Scholar]
- 20.Pajusto M, Toivonen TH, Tarkkanen J, Jokitalo E, Mattila PS. Reactive oxygen species induce signals that lead to apoptotic DNA degradation in primary CD4+ T cells. Apoptosis. 2005;10(6):1433-1443. doi: 10.1007/s10495-005-2050-5 [DOI] [PubMed] [Google Scholar]
- 21.Chelombitko MA. Role of reactive oxygen species in inflammation: a minireview. Mosc Univ Biol Sci Bull. 2018;73(4):199-202. doi: 10.3103/S009639251804003X [DOI] [Google Scholar]
- 22.O’Malley D, Quigley EMM, Dinan TG, Cryan JF. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav Immun. 2011;25(7):1333-1341. doi: 10.1016/j.bbi.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 23.Myers JL, Allen JC. Nutrition and inflammation: insights on dietary pattern, obesity, and Asthma. Am J Lifestyle Med. 2012;6(1):14-17. doi: 10.1177/1559827611424259 [DOI] [Google Scholar]
- 24.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126-1167. doi: 10.1089/ars.2012.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Candales A, Hernández Burgos PM, Hernandez-Suarez DF, Harris D. Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J Nat Sci. 2017;3(4):e341. [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10(1):18-26. doi: 10.1038/mi.2016.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci. 2019;1437(1):57-67. doi: 10.1111/nyas.13712 [DOI] [PubMed] [Google Scholar]
- 28.Slavich GM. Understanding inflammation, its regulation, and relevance for health: a top scientific and public priority. Brain Behav Immun. 2015;45:13-14. doi: 10.1016/j.bbi.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822-1832. doi: 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg RK, Rahbek SH, Kofod-Olsen E, et al. T cells detect intracellular DNA but fail to induce type I IFN responses: implications for restriction of HIV Replication. PLoS One. 2014;9(1):e84513. doi: 10.1371/journal.pone.0084513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467(7312):214-217. doi: 10.1038/nature09337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Utay NS, Douek DC. Interferons and HIV infection: the good, the bad, and the ugly. Pathog Immun. 2016;1(1):107-116. doi: 10.20411/pai.v1i1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV Disease. Science. 1997;277(5322):112-116. doi: 10.1126/science.277.5322.112 [DOI] [PubMed] [Google Scholar]
- 34.Ledergerber B, Egger M, Opravil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Lancet. 1999;353(9156):863-868. doi: 10.1016/S0140-6736(99)01122-8 [DOI] [PubMed] [Google Scholar]
- 35.Silveira MPT, Silveira CPT, Guttier MC, et al. Long-term immune and virological response in HIV-infected patients receiving antiretroviral therapy. J Clin Pharm Ther. 2016;41(6):689-694. doi: 10.1111/jcpt.12450 [DOI] [PubMed] [Google Scholar]
- 36.Gazzola L, Tincati C, Bellistrì GM, et al. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;48(3):328-337. doi: 10.1086/595851 [DOI] [PubMed] [Google Scholar]
- 37.Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;48(3):350-361. doi: 10.1086/595888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massanella M, Gómez-Mora E, Carrillo J, et al. Increased ex vivo cell death of central memory CD4 T cells in treated HIV infected individuals with unsatisfactory immune recovery. J Transl Med. 2015;13:230. doi: 10.1186/s12967-015-0601-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lichtfuss GF, Hoy J, Rajasuriar R, et al. Biomarkers of immune dysfunction following combination antiretroviral therapy for HIV infection. Biomark Med. 2011;5(2):171-186. doi: 10.2217/bmm.11.15 [DOI] [PubMed] [Google Scholar]
- 40.Lichtfuss GF, Cheng WJ, Farsakoglu Y, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol Baltim Md 1950. 2012;189(3):1491-1499. doi: 10.4049/jimmunol.1200458 [DOI] [PubMed] [Google Scholar]
- 41.Rhoades N, Mendoza N, Jankeel A, et al. Altered immunity and microbial dysbiosis in aged individuals with long-term controlled HIV infection. Front Immunol. 2019;10:463. doi: 10.3389/fimmu.2019.00463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brenchley J, Douek D. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1(1):23-30. doi: 10.1038/mi.2007.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinh DM, Volpe GE, Duffalo C, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV Infection. J Infect Dis. 2015;211(1):19-27. doi: 10.1093/infdis/jiu409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zevin AS, McKinnon L, Burgener A, et al. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS. 2016;11(2):182-190. doi: 10.1097/COH.0000000000000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alzahrani J, Hussain T, Simar D, et al. Inflammatory and immunometabolic consequences of gut dysfunction in HIV: parallels with IBD and implications for reservoir persistence and non-AIDS comorbidities. EBioMedicine. 2019;46:522-531. doi: 10.1016/j.ebiom.2019.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vujkovic-Cvijin I, Sortino O, Verheij E, et al. HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat Commun. 2020;11(1):2448. doi: 10.1038/s41467-020-16222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iebba V, Totino V, Gagliardi A, et al. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 2016;39(1):1-12. [PubMed] [Google Scholar]
- 48.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70(2):567-590. doi: 10.1152/physrev.1990.70.2.567 [DOI] [PubMed] [Google Scholar]
- 49.Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994;35(suppl 1):S35-S38. doi: 10.1136/gut.35.1_Suppl.S35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.den Besten G, van Eunen K, Groen AK, Venema K, Reinjngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325-2340. doi: 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B. 1987;86(3):439-472. doi: 10.1016/0305-0491(87)90433-0 [DOI] [PubMed] [Google Scholar]
- 52.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol Biochem. 2018;49(1):190-205. doi: 10.1159/000492853 [DOI] [PubMed] [Google Scholar]
- 54.Parada Venegas D, De la Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17(5):662-671. doi: 10.1016/j.chom.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan H, Ajuwon KM. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One. 2017;12(6):e0179586. doi: 10.1371/journal.pone.0179586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57(12):3126-3135. doi: 10.1007/s10620-012-2259-4 [DOI] [PubMed] [Google Scholar]
- 58.Krishnan M, Singh A, Smith J, et al. HDAC inhibitors regulate claudin-1 expression in colon cancer cells through modulation of mRNA stability. Oncogene. 2010;29(2):305-312. doi: 10.1038/onc.2009.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3(1-2):e977176. doi: 10.4161/21688370.2014.977176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albenberg L, Esipova T, Judge C, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota in humans and mice. Gastroenterology. 2014;147(5):1055-1063.e8. doi: 10.1053/j.gastro.2014.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. a review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309(6):C350-C360. doi: 10.1152/ajpcell.00191.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ward JBJ, Keely SJ, Keely SJ. Oxygen in the regulation of intestinal epithelial transport. J Physiol. 2014;592(Pt 12):2473-2489. doi: 10.1113/jphysiol.2013.270249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miron N, Cristea V. Enterocytes: active cells in tolerance to food and microbial antigens in the gut. Clin Exp Immunol. 2012;167(3):405-412. doi: 10.1111/j.1365-2249.2011.04523.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng L, Kelly CJ, Battista KD, et al. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of Claudin-2. J Immunol. 2017;199(8):2976-2984. doi: 10.4049/jimmunol.1700105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7(5):281-287. doi: 10.1038/nrgastro.2010.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29-41. doi: 10.1111/1462-2920.13589 [DOI] [PubMed] [Google Scholar]
- 67.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118(4):724-734. doi: 10.1016/S0016-5085(00)70142-9 [DOI] [PubMed] [Google Scholar]
- 68.Segain J, de la Bletiere DR, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn’s disease. Gut. 2000;47(3):397-403. doi: 10.1136/gut.47.3.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gassull MA. Review article: the intestinal lumen as a therapeutic target in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24(s3):90-95. doi: 10.1111/j.1365-2036.2006.03067.x [DOI] [PubMed] [Google Scholar]
- 70.Liu T, Li J, Liu Y, et al. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation. 2012;35(5):1676-1684. doi: 10.1007/s10753-012-9484-z [DOI] [PubMed] [Google Scholar]
- 71.Yang Q, Ouyang J, Sun F, Yang J. Short-chain fatty acids: a soldier fighting against inflammation and protecting from tumorigenesis in people with diabetes. Front Immunol. 2020;11:590685. doi: 10.3389/fimmu.2020.590685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macpherson AJ, Geuking MB, McCoy KD. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology. 2005;115(2):153-162. doi: 10.1111/j.1365-2567.2005.02159.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008;153(s1):3-6. doi: 10.1111/j.1365-2249.2008.03713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Royes LFF. Cross-talk between gut and brain elicited by physical exercise. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165877. doi: 10.1016/j.bbadis.2020.165877 [DOI] [PubMed] [Google Scholar]
- 75.Keirns BH, Koemel NA, Sciarrillo CM, Anderson KL, Emerson SR. Exercise and intestinal permeability: another form of exercise-induced hormesis? Am J Physiol Gastrointest Liver Physiol. 2020;319(4):G512-G518. doi: 10.1152/ajpgi.00232.2020 [DOI] [PubMed] [Google Scholar]
- 76.Mehandru S, Poles MA, Tenner-Racz K, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3(12):e484. doi: 10.1371/journal.pmed.0030484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Somsouk M, Estes JD, Deleage C, et al. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS Lond Engl. 2015;29(1):43-51. doi: 10.1097/QAD.0000000000000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ponte R, Mehraj V, Ghali P, Couëdel-Courteille A, Cheynier R, Routy JP. Reversing gut damage in HIV infection: using non-human primate models to instruct clinical research. EBioMedicine. 2016;4:40-49. doi: 10.1016/j.ebiom.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655-666. doi: 10.1038/nrmicro2848 [DOI] [PubMed] [Google Scholar]
- 80.Ramendra R, Isnard S, Mehraj V, et al. Circulating LPS and (1→3)-β-D-Glucan: A Folie à Deux Contributing to HIV-associated immune activation. Front Immunol. 2019;10:465. doi: 10.3389/fimmu.2019.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andreakos E, Sacre SM, Smith C, et al. Distinct pathways of LPS-induced NF-κB activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood. 2004;103(6):2229-2237. doi: 10.1182/blood-2003-04-1356 [DOI] [PubMed] [Google Scholar]
- 82.Candelli M, Franza L, Pignataro G, et al. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int J Mol Sci. 2021;22(12):6242. doi: 10.3390/ijms22126242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12(1):86. doi: 10.1186/1476-4598-12-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol. 2019;10:85. doi: 10.3389/fimmu.2019.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. ISME J. 2013;7(7):1256-1261. doi: 10.1038/ismej.2013.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stecher B. The roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. Microbiol Spectr. 2015;3(3): MBP-0008-2014. doi: 10.1128/microbiolspec.MBP-0008-2014 [DOI] [PubMed] [Google Scholar]
- 88.Rivera-Chavez F, Zhang L, Faber F. Depletion of butyrate-producing clostridia from the gut microbiome drives an aerobic luminal expansion of Salmonella. Cell Host Microbe. 2016;19(4):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winter SE, Bäumler AJ. Why related bacterial species bloom simultaneously in the gut: principles underlying the “Like will to like” concept. Cell Microbiol. 2014;16(2):179-184. doi: 10.1111/cmi.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655-662. doi: 10.1038/s41586-019-1237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shephard RJ. Absolute versus relative intensity of physical activity in a dose-response context. Med Sci Sports Exerc. 2001;33(6):S400. [DOI] [PubMed] [Google Scholar]
- 92.Woo JS, Derleth C, Stratton JR, Levy WC. The influence of age, gender, and training on exercise efficiency. J Am Coll Cardiol. 2006;47(5):1049-1057. doi: 10.1016/j.jacc.2005.09.066 [DOI] [PubMed] [Google Scholar]
- 93.Clark J. The impact of duration on effectiveness of exercise, the implication for periodization of training and goal setting for individuals who are overfat, a meta-analysis. Biol Sport. 2016;33(4):309-333. doi: 10.5604/20831862.1212974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seifi-skishahr F, Damirchi A, Farjaminezhad M, Babaei P. Physical training status determines oxidative stress and redox changes in response to an acute aerobic exercise. Biochem Res Int. 2016;2016:e3757623. doi: 10.1155/2016/3757623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raffin J, Barthélémy JC, Dupré C, et al. Exercise frequency determines heart rate variability gains in older people: a meta-analysis and meta-regression. Sports Med Auckl NZ. 2019;49(5):719-729. doi: 10.1007/s40279-019-01097-7 [DOI] [PubMed] [Google Scholar]
- 96.Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis. 2011;3(1):130-140. [PMC free article] [PubMed] [Google Scholar]
- 97.Simpson RJ, Kunz H, Agha N, Graff R. Chapter fifteen - exercise and the regulation of immune functions. In: Bouchard C, ed. Progress in Molecular Biology and Translational Science. Vol 135. Molecular and Cellular Regulation of Adaptation to Exercise. Academic Press; 2015:355-380. doi: 10.1016/bs.pmbts.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 98.Scheffer DdL, Latini A. Exercise-induced immune system response: anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165823. doi: 10.1016/j.bbadis.2020.165823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Otte JA, Oostveen E, Geelkerken RH, Groeneveld ABJ, Kolkman JJ. Exercise induces gastric ischemia in healthy volunteers: a tonometry study. J Appl Physiol. 2001;91(2):866-871. doi: 10.1152/jappl.2001.91.2.866 [DOI] [PubMed] [Google Scholar]
- 100.Evans CC, LePard KJ, Kwak JW, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 2014;9(3):e92193. doi: 10.1371/journal.pone.0092193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang SS, Jeraldo PR, Kurti A, et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014;9(1):36. doi: 10.1186/1750-1326-9-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017:1-8. doi: 10.1155/2017/3831972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perrin P, Pierre F, Patry Y, et al. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut. 2001;48(1):53-61. doi: 10.1136/gut.48.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng C, Wei H, Peng J. 370 Dietary soluble fiber increases the intestinal butyrate-producing bacteria, reduces intestinal permeability, and improves metabolic syndrome in sows during perinatal period. J Anim Sci. 2019;97(suppl 3):133. doi: 10.1093/jas/skz258.27130388227 [DOI] [Google Scholar]
- 105.Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol. 2007;9(5):1101-1111. doi: 10.1111/j.1462-2920.2007.01281.x [DOI] [PubMed] [Google Scholar]
- 106.Scheiman J, Luber JM, Chavkin TA, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25(7):1104-1109. doi: 10.1038/s41591-019-0485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heuvelin E, Lebreton C, Grangette C, Pot B, Cerf-Bensussan N, Heyman M. Mechanisms involved in alleviation of intestinal inflammation by bifidobacterium breve soluble factors. PLoS One. 2009;4(4):e5184. doi: 10.1371/journal.pone.0005184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yan F, Polk DB. Disruption of NF-κB signalling by ancient microbial molecules: novel therapies of the future? Gut. 2010;59(4):421-426. doi: 10.1136/gut.2009.179614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ferreira CM, Vieira AT, Vinolo MAR, Oliveira FA, Curi R, Martins FdS. The central role of the gut microbiota in chronic inflammatory diseases. J Immunol Res. 2014;2014:e689492. doi: 10.1155/2014/689492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mu Q, Kirby J, Reilly CM, Luo XM. Leaky gut as a danger signal for autoimmune diseases. Front Immunol. 2017;8:598. doi: 10.3389/fimmu.2017.00598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lazar V, Ditu LM, Pircalabioru GG, et al. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol. 2018;9:1830. doi: 10.3389/fimmu.2018.01830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Divella R, Palma GD, Tufaro A, et al. Diet, probiotics and physical activity: the right allies for a healthy microbiota. Anticancer Res. 2021;41(6):2759-2772. doi: 10.21873/anticanres.15057 [DOI] [PubMed] [Google Scholar]
- 113.Singh R, Zogg H, Wei L, et al. Gut microbial dysbiosis in the pathogenesis of gastrointestinal dysmotility and metabolic disorders. J Neurogastroenterol Motil. 2021;27(1):19-34. doi: 10.5056/jnm20149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu HW, Chang SJ. Moderate exercise suppresses NF-κB signaling and activates the SIRT1-AMPK-PGC1α Axis to Attenuate Muscle Loss in Diabetic db/db Mice. Front Physiol. 2018;9:636. doi: 10.3389/fphys.2018.00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oliveira VHF, Borsari AL, Webel AR, Erlandson KM, Deminice R. Sarcopenia in people living with the human immunodeficiency Virus: a systematic review and meta-analysis. Eur J Clin Nutr. 2020;74(7):1009-1021. doi: 10.1038/s41430-020-0637-0 [DOI] [PubMed] [Google Scholar]
- 116.Clark A, Mach N. The Crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol. 2017;8:319. doi: 10.3389/fphys.2017.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Wijck K, Lenaerts K, van Loon LJC, Peters WHM, Buurman WA, Dejong CHC. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS One. 2011;6(7):e22366. doi: 10.1371/journal.pone.0022366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Codella R, Luzi L, Terruzzi I. Exercise has the guts: how physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis. 2018;50(4):331-341. doi: 10.1016/j.dld.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 119.Lian P, Braber S, Varasteh S, Wichers HJ, Folkerts G. Hypoxia and heat stress affect epithelial integrity in a Caco-2/HT-29 co-culture. Sci Rep. 2021;11(1):13186. doi: 10.1038/s41598-021-92574-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Litvak Y, Byndloss MX, Bäumler AJ. Colonocyte metabolism shapes the gut microbiota. Science. 2018;362(6418):eaat9076. doi: 10.1126/science.aat9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Konjar Š, Pavšič M, Veldhoen M. Regulation of oxygen homeostasis at the intestinal epithelial barrier site. Int J Mol Sci. 2021;22(17):9170. doi: 10.3390/ijms22179170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Adams MS, Adams RB, Wessman CA, Demmig-Adams B. Nutritional cues tie living organisms to their environment and its sustainability. Front Nutr. 2016;3:28. doi: 10.3389/fnut.2016.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010-1013. doi: 10.2337/db06-1656 [DOI] [PubMed] [Google Scholar]
- 124.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation and metabolic disease. Cell Metab. 2011;13(1):11-22. doi: 10.1016/j.cmet.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Flynn MG, McFarlin BK, Markofski MM. The anti-inflammatory actions of exercise training. Am J Lifestyle Med. 2007;1(3):220-235. doi: 10.1177/1559827607300283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mika A, Macaluso F, Barone R, Di Felice V, Sledzisnki T. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Front Physiol. 2019;10:26. doi: 10.3389/fphys.2019.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Niemiro GM, Rewane A, Algotar AM. Exercise and fitness effect on obesity. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov/books/NBK539893/. Accessed October 6, 2021. [PubMed] [Google Scholar]
- 128.Margaritelis NV, Paschalis V, Theodorou AA, Kyparos A, Nikolaidis MG. Redox basis of exercise physiology. Redox Biol. 2020;35:101499. doi: 10.1016/j.redox.2020.101499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44(2):153-159. doi: 10.1016/j.freeradbiomed.2007.01.029 [DOI] [PubMed] [Google Scholar]
- 130.Freiberg MS, Chang C-CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614-622. doi: 10.1001/jamainternmed.2013.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Adams R, Egbo K, Demmig-Adams B. High-dose vitamin C supplements diminish the benefits of exercise in athletic training and disease prevention. Nutr Food Sci. 2014;44:95-101. [Google Scholar]
- 132.Amir Aslani B, Ghobadi S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016;146:163-173. doi: 10.1016/j.lfs.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 133.Baruchel S, Wainberg MA. The role of oxidative stress in disease progression in individuals infected by the human immunodeficiency virus. J Leukoc Biol. 1992;52(1):111-114. doi: 10.1002/jlb.52.1.111 [DOI] [PubMed] [Google Scholar]
- 134.Malorni W, Rivabene R, Teresa Santini M, Donelli G. N-Acetylcysteine inhibits apoptosis and decreases viral particles in HIV-chronically infected U937 cells. FEBS Lett. 1993;327(1):75-78. doi: 10.1016/0014-5793(93)81043-Y [DOI] [PubMed] [Google Scholar]
- 135.Tran E, Demmig‐Adams B. Vitamins and minerals: powerful medicine or potent toxins? Nutr Food Sci. 2007;37(1):50-60. doi: 10.1108/00346650710726959 [DOI] [Google Scholar]
- 136.Ribeiro FM, Petriz B, Marques G, Kamilla LH, Franco OL. Is there an exercise-intensity threshold capable of avoiding the leaky gut? Front Nutr. 2021;8:75. doi: 10.3389/fnut.2021.627289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Casey E, Mistry DJ, MacKnight JM. Training room management of medical conditions: sports gastroenterology. Clin Sports Med. 2005;24(3):525-540, viii. doi: 10.1016/j.csm.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 138.van Wijck K, Lenaerts K, Grootjans J, et al. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol Gastrointest Liver Physiol. 2012;303(2):G155-G168. doi: 10.1152/ajpgi.00066.2012 [DOI] [PubMed] [Google Scholar]
- 139.Erlandson KM, Wilson MP, MaWhinney S, et al. The impact of moderate or high-intensity combined exercise on systemic inflammation among older persons with and without HIV. J Infect Dis. 2020;223(7):1161-1170. doi: 10.1093/infdis/jiaa494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ghayomzadeh M, Earnest CP, Hackett D, et al. Combination of resistance and aerobic exercise for six months improves bone mass and physical function in HIV infected individuals: a randomized controlled trial. Scand J Med Sci Sports. 2021;31(3):720-732. doi: 10.1111/sms.13871 [DOI] [PubMed] [Google Scholar]
- 141.Zanetti HR, da Cruz LG, Lourenço CLM, de F Neves F, Silva-Vergara ML, Mendes EL. Non-linear resistance training reduces inflammatory biomarkers in persons living with HIV: a randomized controlled trial. Eur J Sport Sci. 2016. a;16(8):1232-1239. doi: 10.1080/17461391.2016.1167962 [DOI] [PubMed] [Google Scholar]
- 142.Zanetti HR, da Cruz LG, Lourenço CL, Neves FF, Silva-Vergara ML, Mendes EL. Does nonlinear resistance training reduce metabolic syndrome in people living with HIV? A randomized clinical trial. J Sports Med Phys Fitness. 2017;57(5):678-684. doi: 10.23736/s0022-4707.16.06294-0 [DOI] [PubMed] [Google Scholar]
- 143.Bonato M, Turrini F, De Zan V, et al. A mobile application for exercise intervention in people living with HIV. Med Sci Sports Exerc. 2020;52(2):425-433. doi: 10.1249/MSS.0000000000002125 [DOI] [PubMed] [Google Scholar]
- 144.Dudgeon WD, Jaggers JR, Phillips KD, et al. Moderate-intensity exercise improves body composition and improves physiological markers of stress in HIV-infected men. ISRN AIDS. 2012;2012:e145127. doi: 10.5402/2012/145127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ibeneme SC, Omeje C, Myezwa H, et al. Effects of physical exercises on inflammatory biomarkers and cardiopulmonary function in patients living with HIV: a systematic review with meta-analysis. BMC Infect Dis. 2019. a;19(1):359. doi: 10.1186/s12879-019-3960-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lu D, Zhang JB, Wang YX, et al. Association between CD4+ T cell counts and gut microbiota and serum cytokines levels in HIV-infected immunological non-responders. BMC Infect Dis. 2021;21(1):742. doi: 10.1186/s12879-021-06491-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ezema CI, Onwunali AA, Lamina S, Ezugwu UA, Amaeze AA, Nwankwo MJ. Effect of aerobic exercise training on cardiovascular parameters and CD4 cell count of people living with human immunodeficiency virus/acquired immune deficiency syndrome: a randomized controlled trial. Niger J Clin Pract. 2014;17(5):543. doi: 10.4103/1119-3077.141414 [DOI] [PubMed] [Google Scholar]
- 148.Maduagwu S, Kaidal A, Gashau W, et al. Effect of aerobic exercise on CD4 cell count and lipid profile of HIV infected persons in North Eastern Nigeria. J AIDS Clin Res. 2015;06:508. doi: 10.4172/2155-6113.1000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.de Brito-Neto JG, de Andrade MF, de Almeida VD, et al. Strength training improves body composition, muscle strength and increases CD4+ T lymphocyte levels in people living with HIV/AIDS. Infect Dis Rep. 2019;11(1):7925. doi: 10.4081/idr.2019.7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Asogwa EI, Obeagu EI, Ekine RS, et al. Effects of 6-weeks moderate intensity aerobic exercise on CD4 count, bone mineral density and weight of people living with HIV/AIDS in Alex-Ekwueme federal university teaching hospital Ebonyi State. J Pharm Res Int. 2020;25:13-22. doi: 10.9734/jpri/2020/v32i2330784 [DOI] [Google Scholar]
- 151.Zanetti HR, Lopes LTP, Gonçalves A, et al. Effects of resistance training on muscle strength, body composition and immune-inflammatory markers in people living with HIV: a systematic review and Meta-analysis of randomized controlled trials. HIV Res Clin Pract. 2021;22(5):119-127. doi: 10.1080/25787489.2021.1975448 [DOI] [PubMed] [Google Scholar]
- 152.Tiozzo E. The Effect of Combined Moderate-Intensity Training on Immune Functioning, Metabolic Variables, and Quality of Life in HIV-infected Individuals Receiving Highly Active Antiretroviral Therapy. Univ Miami. Published Online 2011. Accessed December 15, 2021. https://www.semanticscholar.org/paper/The-Effect-of-Combined-Moderate-Intensity-Training-Tiozzo/7c556549e41891eeeeab54a7838bc53da93015ed [Google Scholar]
- 153.O’Brien KK, Tynan AM, Nixon SA, Glazier RH. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis. 2016;16:182. doi: 10.1186/s12879-016-1478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ibeneme SC, Irem FO, Iloanusi NI, et al. Impact of physical exercises on immune function, bone mineral density, and quality of life in people living with HIV/AIDS: a systematic review with meta-analysis. BMC Infect Dis. 2019;19(1):340. doi: 10.1186/s12879-019-3916-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta BBA - Mol Cell Res 2011;1813(5):878-888. doi: 10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 156.Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14(10):461-469. doi: 10.1016/j.molmed.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 157.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15(8):511-523. doi: 10.1038/nri3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Caillaud C, Py G, Eydoux N, Legros P, Prefaut C, Mercier J. Antioxidants and mitochondrial respiration in lung, diaphragm, and locomotor muscles: effect of exercise. Free Radic Biol Med. 1999;26(9):1292-1299. doi: 10.1016/S0891-5849(98)00342-6 [DOI] [PubMed] [Google Scholar]
- 159.Markworth JF, Maddipati KR, Cameron-Smith D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc Immunol Rev. 2016;22:110-134. [PubMed] [Google Scholar]
- 160.Vella L, Markworth JF, Farnfield MM, Maddipati KR, Russell AP, Cameron-Smith D. Intramuscular inflammatory and resolving lipid profile responses to an acute bout of resistance exercise in men. Physiol Rep. 2019;7(13):e14108. doi: 10.14814/phy2.14108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stranahan AM, Mattson MP. Exercise-induced hormesis. In: Mattson MP, Calabrese EJ, eds. Hormesis: A Revolution in Biology, Toxicology and Medicine. Totowa, NJ: Humana Press; 2010:109-122. doi: 10.1007/978-1-60761-495-1_6 [DOI] [Google Scholar]
- 162.Mattson MP, Calabrese EJ. Hormesis: what it is and why it matters. In: Hormesis: A Revolution in Biology, Toxicology and Medicine. Humana Press Inc; 2010:1-13. doi: 10.1007/978-1-60761-495-1_1 [DOI] [Google Scholar]
- 163.Degerstrøm J, Østerud B. Increased inflammatory response of blood cells to repeated bout of endurance exercise. Med Sci Sports Exerc. 2006;38(7):1297-1303. doi: 10.1249/01.mss.0000227315.93351.8d [DOI] [PubMed] [Google Scholar]
- 164.Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008;31(7):587-591. doi: 10.1007/BF03345606 [DOI] [PubMed] [Google Scholar]
- 165.Ponce P, Del Arco A, Loprinzi P. Physical activity versus psychological stress: effects on salivary cortisol and working memory performance. Med Kaunas Lith. 2019;55(5):E119. doi: 10.3390/medicina55050119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sasaki H, Hattori Y, Ikeda Y, et al. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Sci Rep. 2016;6(1):27607. doi: 10.1038/srep27607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rosa JC, Lira FS, Eguchi R, et al. Exhaustive exercise increases inflammatory response via Toll like receptor-4 and NF-κBp65 pathway in rat adipose tissue. J Cell Physiol. 2011;226(6):1604-1607. doi: 10.1002/jcp.22490 [DOI] [PubMed] [Google Scholar]
- 168.Clark A, Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. 2016;13:43. doi: 10.1186/s12970-016-0155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dokladny K, Zuhl MN, Moseley PL. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physiol. 2016;120(6):692-701. doi: 10.1152/japplphysiol.00536.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liao P, He Q, Zhou X, et al. Repetitive bouts of exhaustive exercise induces a systemic inflammatory response and multi-organ damage in rats. Front Physiol. 2020;11:685. doi: 10.3389/fphys.2020.00685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Suzuki K, Tominaga T, Ruhee RT, Ma S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants. 2020;9(5):401. doi: 10.3390/antiox9050401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schlabe S, Vogel M, Boesecke C, et al. Moderate endurance training (marathon-training) – effects on immunologic and metabolic parameters in HIV-infected patients: the 42 KM cologne project. BMC Infect Dis. 2017;17(1):550. doi: 10.1186/s12879-017-2651-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hillsdon M, Thorogood M, Anstiss T, Morris J. Randomised controlled trials of physical activity promotion in free living populations: a review. J Epidemiol Community Health. 1995;49(5):448-453. doi: 10.1136/jech.49.5.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fernandes JL, Serrano CV, Toledo F, et al. Acute and chronic effects of exercise on inflammatory markers and B-type natriuretic peptide in patients with coronary artery disease. Clin Res Cardiol. 2011;100(1):77-84. doi: 10.1007/s00392-010-0215-x [DOI] [PubMed] [Google Scholar]
- 175.Ke Z, Yip SP, Li L, Zheng XX, Tong KY. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain Ischemia Model. PLoS One. 2011;6(2):e16643. doi: 10.1371/journal.pone.0016643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cook MD, Martin SA, Williams C, et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of Colitis. Brain Behav Immun. 2013;33:46-56. doi: 10.1016/j.bbi.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chair SY, Cheng HY, Chew HSJ, Zang YL, Siow EKC, Cao X. Leisure-time physical activity and depressive symptoms among patients with coronary heart disease: the mediating role of physical activity self-efficacy. Worldviews Evid Based Nurs. 2020;17(2):144-150. doi: 10.1111/wvn.12425 [DOI] [PubMed] [Google Scholar]
- 178.Berry A, McCabe CS, Halls S, Muir S, Walsh N. Beliefs, motives and gains associated with physical activity in people with osteoarthritis. Musculoskeletal Care. 2021;19(1):52-58. doi: 10.1002/msc.1507 [DOI] [PubMed] [Google Scholar]
- 179.Bonato M, Galli L, Passeri L, et al. A pilot study of brisk walking in sedentary combination antiretroviral treatment (cART)- treated patients: benefit on soluble and cell inflammatory markers. BMC Infect Dis. 2017;17(1):61. doi: 10.1186/s12879-016-2095-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pedro RE, Candido N, Guariglia DA, et al. Exercise improves cytokine profile in HIV-infected people: a randomized clinical trial. Cytokine. 2017;99:18-23. doi: 10.1016/j.cyto.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 181.Zanetti HR, da Cruz LG, Lourenço CL, et al. Nonlinear resistance training enhances the lipid profile and reduces inflammation marker in people living with HIV: a randomized clinical trial. J Phys Act Health. 2016;13(7):765-770. doi: 10.1123/jpah.2015-0540 [DOI] [PubMed] [Google Scholar]
- 182.Zanetti HR, Gonçalves A, Teixeira Paranhos Lopes L, et al. Effects of exercise training and statin use in people living with human immunodeficiency virus with dyslipidemia. Med Sci Sports Exerc. 2020;52(1):16-24. doi: 10.1249/MSS.0000000000002120 [DOI] [PubMed] [Google Scholar]
- 183.Cutrono S, Lewis J, Perry A, Signorile J, Tiozzo E, Jacobs K. The effect of a community-based exercise program on inflammation, metabolic risk, and fitness levels among persons living with HIV/AIDS. AIDS Behav. 2016;20:1123-1131. doi: 10.1007/s10461-015-1245-1 [DOI] [PubMed] [Google Scholar]
- 184.Vingren JL, Curtis JH, Levitt DE, et al. Adding resistance training to the standard of care for inpatient substance abuse treatment in men with human immunodeficiency virus improves skeletal muscle health without altering cytokine concentrations. J Strength Cond Res. 2018;32(1):76-82. doi: 10.1519/JSC.0000000000002289 [DOI] [PubMed] [Google Scholar]
- 185.Dianatinasab M, Fararouei M, Padehban V, et al. The effect of a 12-week combinational exercise program on CD4 count and mental health among HIV infected women: a randomized control trial. J Exerc Sci Fit. 2018;16(1):21-25. doi: 10.1016/j.jesf.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]