Abstract
Context
While a great deal of interest has been accorded to the cognitive effects of n-3 long-chain polyunsaturated fatty acids (LC PUFAs), there is a need for systematic review data that assess this outcome across the lifespan, accounting for population differences and highlighting methodological limitations of extant studies.
Objective
This systematic review addresses the effects of n-3s on human cognition and provides an overview on the current state of research and recommendations for future efforts.
Data Sources
Based on a thorough review of highly powered articles from PubMed (MEDLINE), Web of Science, and ProQuest Central, the authors evaluated articles published between 2000 and 2020 assessing LC PUFA status on cognition as a primary outcome measure. Using the PRISMA guidelines, the researchers’ primary aim was to provide a comprehensive overview of the articles.
Conclusions
The results indicate inconsistent effects of intervention, with benefits for specific groups on specific outcomes. Although results were rarely definitive across cognitive domains, and the majority of studies indicated the presence of a possible threshold effect in which LC PUFA needs were already being met, and supplementation did not have an additional effect, there is evidence for trends towards benefit in cognitive functions, in those experiencing early cognitive decline.
Keywords: Omega-3, cognitive impairment, dementia, supplement, Alzheimer’s
“Westernized diets and increased consumption of processed foods have meant that the majority of people consume significantly less n-3s than in previous years.”
Introduction
The cognitive effect of long-chain polyunsaturated fatty acids (LC PUFAs)—including docosahexaenoic acid, (DHA; 22:6, n-3), eicosapentaenoic acid (EPA; 20:5, n-3), alpha-linolenic acid (ALA; 18:3, n-3), and arachidonic acid (AA/ARA; 20:4, n-6)—has been addressed in a significant number of studies with various populations of interest. Given the global increase in the aging population, much of this research has focused on its neuroprotective effects among adults and the elderly. With lipids making up 50–60% of the brain’s dry weight and having effects on membrane fluidity and neurotransmission, it is particularly important to evaluate LC PUFA intake and supplementation’s potential effects on cognitive function and neuroprotection. 1
Unable to be synthesized de novo in human cells, dietarily derived LC PUFAs—and their consumption in the proper ratios—is a key area of interest in the nutrition field. Indeed, Westernized diets and increased consumption of processed foods have meant that the majority of people consume significantly less n-3s than in previous years. Estimates have suggested that there has been a significant shift from traditional diets with 1:1 ratios of n-6 to n-3 PUFAs to ratios closer to 20:1. 2 While it is evident that LC PUFA intake is necessary for cognitive function and their dietary lack is detrimental, empirical evidence on their efficacy for neuroprotective function has been mixed.
Because n-3 LC PUFAs function as cell membrane components, they affect both tissue formation and neuroprotection. 3 Therefore, extant studies on the subject can be divided into two categories: neurodevelopment and neuroprotection. The purpose of this systematic review is to evaluate the findings of highly powered studies designed to investigate the neuroprotective effects of LC PUFA supplementation, primarily including populations with Alzheimer’s Disease (AD), dementia, and mild cognitive decline (MCI). For comparative purposes, this review also includes studies conducted with populations of healthy adults, as well as those with depression, bipolar disorder, schizophrenia, and hypertension/cardiac comorbidities. Such studies also provide important insights concerning the cognitive mechanisms by which LC PUFAs affect cognition, the potential threshold effect of their consumption, and the specific cognitive outcome areas affected.
Methodology
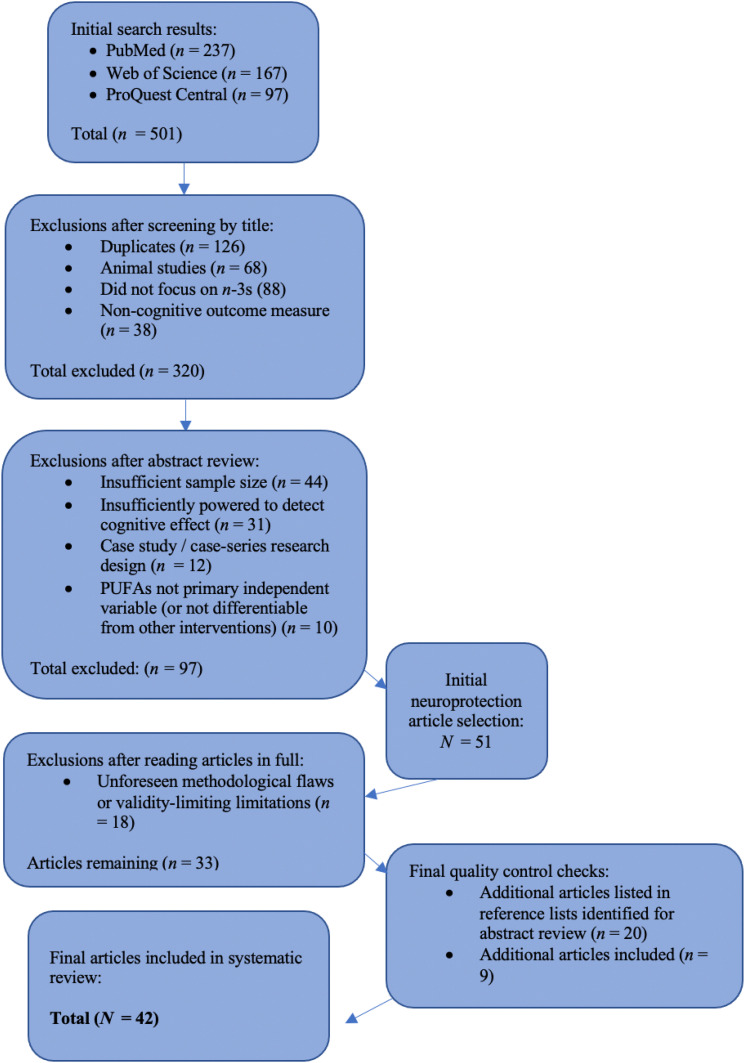
For the purposes of this systematic review, we searched PubMed (MEDLINE), Web of Science, and ProQuest Central, using filters for peer-reviewed articles published between 2000 and 2020. Search terms included: omega-3s; docosahexaenoic acid (DHA); cognitive function; macronutrients; healthy fats; polyunsaturated fatty acids; brain health; developmental outcomes; n-3 polyunsaturated fats; LC PUFAs; dietary fats; and brain function. In PubMed, results were additionally filtered to include only clinical trials conducted with humans. This initial search yielded 501 articles, which we reviewed first on the basis of title and metadata for the inclusion criteria of relevant, peer-reviewed articles published between 2000 and 2020 and written in English. After eliminating duplicates and narrowing the field to 181 articles, we then reviewed all abstracts to assess for the additional inclusion criteria of randomized controlled trials (RCTs), secondary analyses of RCTs, or sufficiently powered observational studies (case-control, prospective, or retrospective) with large sample sizes, conducted with human subjects. Exclusion criteria were case studies and case series study designs; underpowered studies; and those in which PUFAs were not a primary (or differentiable) independent variable (see Figure 1 for the literature search process and Table 1 for the PICOS inclusion criteria).
Figure 1.
Literature Search Flow Chart.
Table 1.
Salient PICOS Inclusion Criteria.
| Population | Intervention | Comparator | Outcome | Setting |
|---|---|---|---|---|
| Healthy adults and elderly, as well as those with MCI, AD, and other cognitive concerns | Supplementation of n-3s (either in food frequency questionnaire or supplement form) | Control groups of non-supplemented individuals | Cognitive function/level of cognitive decline | U.S. and international populations of adults (at times assessed from a wider cohort study) |
At this stage, it became evident that the extant literature could and should be subcategorized into that pertaining to the developing brain—studies that addressed the neurodevelopmental importance of n-3s from prenatal development to young adulthood—and that which addressed adulthood, focusing more on the neuroprotective effects of Omega-3s. As such, this article constitutes the second installment of a two-part series: neuroprotective effects of n-3 LC PUFA during adulthood and among the elderly. After abstract review, we selected 51 articles pertaining to neurodevelopment to read in full and narrowed these to a final set of 33 that met the inclusion criteria. To confirm the comprehensiveness of the literature included, we also consulted the reference lists of all articles published in the last 5 years, as well as previous literature review and meta-analysis articles, and used the “cited by” function in a university library database-wide search; we reviewed an additional 20 abstracts and included an additional 9 articles that met the inclusion criteria as a result of this process.
Results
Of the 42 articles that met the selection criteria for this systematic review, 14 addressed a population of healthy individuals, with participants primarily below the age of 70.4–7 Six studies were conducted with healthy populations with the majority of participants over the age of 70. The majority focused on cognitive decline based on criteria for MCI (n = 8), while few studies addressed those with AD diagnoses (n = 3). Other population characteristics studied included bipolar disorder (n = 1), depression (n = 4), hypertension or other cardiac concerns (n = 3), psychosis (n = 1), and schizophrenia (n = 1).8–12 The sample sizes of the included articles ranged from 31 to 3,362, while the intervention lengths ranged from 4 weeks to 36 months. The majority of the studies included were randomized controlled trials (n = 31), with several secondary analysis (n = 7), highly powered observational (n = 3), and follow up (n = 1) studies. While the majority of studies included here were funded by governmental or institutional entities (n = 23), 7 studies were unspecified, and 12 were funded by industry sources. Table 2 provides the presents the study, intervention, and population characteristics of the studies included in this review, while Table 3 provides information on their instrumentation, results, limitations, and funding sources.
Table 2.
Study Types and Participant Characteristics.
| Study Type | Type/Dosage of PUFAs | N | Length of Intervention | Participant Age Range | Population Characteristics | |
|---|---|---|---|---|---|---|
| Andrieu et al. (2017) | RCT | 800 mg DHA and 225 mg EPA per day (alone or in combination with physical activity, cognitive training, and nutrition) | 1525 | 36 months | >70 | Memory complaints, but w/o dementia |
| Antypa et al. (2009) | RCT | 2.3 g of n-3 PUFA (1.74 g EPA, .25 g DHA) | 54 | 4 weeks | 18–27 | Healthy university students |
| Assmann et al. (2018) | 13-year follow up to previous intervention study (Supplementation with Antioxidant Vitamins and Minerals study) | PUFAs assessed with dietary questionnaire; antioxidant supplementation ([ascorbic acid (120 mg), vitamin E (30 mg, i.e., 45 IU), β-carotene (6 mg), selenium (100 μg), and zinc (20 mg) | 3362 | 8 years | Women aged 35–60 y and men aged 45–60 y | n/s |
| Benton et al. (2013) | RCT | 400 mg DHA/day | 285 | 50 days | Mean = 21.8 | Healthy young adults |
| Bo et al. (2017) | RCT | 480 mg of DHA and 720 mg of EPA daily | 86 | 6 months | >60 | Mild cognitive impairments |
| Bountziouka et al. (2009) | Observational | Non-intervention; food frequency questionnaire | 1190 | Non-intervention; study period 2005–2007 | >65 years | Older adults living in Cyprus and Greek islands |
| Ciappolino et al. (2020) | RCT | 1250 mg DHA/day | 31 | 12 weeks | 36 ± 12 years; 50.4 ± 11.3 years; 33.1 ± 12.4 years; 39.4 ± 13.9 years | Euthymic bipolar disorder patients and healthy controls |
| Dangour et al. (2010) | RCT | 200 mg EPA and 500 mg DHA per day | 876 | 24 months | 70–79 | Healthy |
| Dretsch et al. (2014) | RCT | 2.5 g per day of EPA and DHA in rates of 47% EPA, 38% DHA, 4% docosapentaenoic acid, and other FAs making up less than 2%) | 106 | 60 days | 20–54 | Soldiers |
| Duffy et al. (2015) | RCT (substudy of the Beyond Aging Project) | 1200 mg EPA and 800 mg DHA | 51 | 66–82 | 66–82 | At risk for major depression |
| Fontani et al. (2005) | RCT | Fish oil: 1.60 g of EPA, .80 g of DHA, and .40 g of alpha linolenic, stearidonic, eicosatetraenoic and docosapentaenoic acid | 33 | 35 days | 22–51 | Healthy |
| Freund–Levi et al. (2006) | RCT | 1.7 g of DHA and 0.6 g EPA per day | 174 | 6 months | 74±9 years | MMSE scores of 15 points or more |
| de Groot et al. (2007) | Non-intervention—blood fatty acid analyses | Blood analyses assessed levels of arachidonic acid, adrenic acid, Osbond acid, eicosapentaenoic acid, and docosahexaenoic acid | 54 | Non-intervention (22 weeks from baseline testing to study conclusion) | 20–40 | Healthy, non-pregnant women |
| Hashimoto et al. (2016) | RCT | 860 mg of DHA and 203 mg of EPA | 75 | 12 months | >75 years | Those living in elderly care facilities and nursing homes |
| Heude et al. (2003) | Longitudinal observational | Non-intervention; erythrocyte membrane fatty acid composition | 246 | 4 years between baseline and follow up measurement | 63–74 | Born between 1922 and 1932 |
| Hooper et al. (2017) | Secondary exploratory analysis of results from the Multidomain Alzheimer Preventive Trial (MAPT) | 800 mg of DHA and 225 mg of EPA per day for 3 years | 183 | 3 years | >70 | Subjective memory complaints |
| Howe et al. (2018) | RCT | 400 mg of DHA and 100 mg of EPA per day | 38 | 20 weeks | 40–85 | Mildly hypertensive |
| Jackson, Deary, et al. (2012) | RCT | DHA-rich fish oil (450 mg DHA; 90 mg EPA) or EPA-rich fish oil (300 mg EPA; 200 mg DHA) | 140 | 12 weeks | 18–35 | Healthy |
| Jackson, Reay, et al. (2012) | RCT | 1g FO or 2 g FO (DHA 5:1 EPA) | 65 | 12 weeks | 18–29 | Healthy |
| Jackson et al. (2016) | RCT | DHA (both alone and in a multi-nutrient supplement also containing Gingko biloba, phosphatidylserine and vitamins B9 and B12) | 86 | 6 months | 50–70 | Healthy |
| Jackson et al. (2018) | RCT | At least 840 mg n–3 PUFAs as ethyl esters | 320 | 2–5 days pre-op to hospital discharge or post-op day 10 | ≥18 | Post-cardiac surgery patients |
| Konagai et al. (2013) | RCT | .25 g krill oil (in which n-3 PUFAs are incorporated in phosphatidylcholine) or sardine oil (in which n-3 PUFAs are incorporated in triglycerides) | 45 | 12 weeks | 61–72 | Healthy |
| Mazereeuw et al. (2016) | RCT (substudy with a population of patients with coronary artery disease from the CAD Randomized Omega-3 Trial in Depression) | 1.2 g EPA and 0.6 g DHA | 92 | 12 weeks | 45–80 | Coronary artery disease |
| McGorry et al. (2017) | RCT | 1.4 g n-3PUFAs (840 mg of eicosapentaenoic acid and 560 mg of docosahexaenoic acid) | 304 | 6 months | 13–40 (mean age 19.1) | Patients at ultrahigh risk for psychosis |
| McNamara et al. (2018) | RCT | Fish oil containing 1.6 g EPA and 0.8 g DHA per day, blueberry powder, or both | 92 | 24 weeks | 62–80 | Subjective cognitive impairment |
| Moon et al. (2018) | Cross-sectional analysis of results from data from the Multidomain Alzheimer Preventive Trial | Non-intervention; measured n-3 PUFA levels in RBCs | 234 | 3 year follow up | ≥70 | Subjective memory complaints |
| Nilsson et al. (2012) | RCT | EPA 1500 mg, DHA 1050 mg | 40 | 5 weeks (separated by 5 week washout period) | 51–72 | Healthy |
| Nooyens et al. (2018) | Analysis of data from the Doetinchem Cohort Study | Non-intervention; measured fish consumption and fat intake via a self-administered semi-quantitative food frequency questionnaire | 2612 | 5-year follow up | 20–59 | n/s; stroke patients excluded |
| Otsuka et al. (2014) | Analysis of data from the National Institute of Longevity Services – Longitudinal Study of Aging | Non-intervention; blood sampling and serum FA analysis | 430 | Non-intervention; 10-year follow up | 60–79 | Elderly Japanese individuals, as that population tend to be exposed to more fish than others |
| Pawełczyk et al. (2018) | Secondary outcome analysis of the OFFER randomized controlled study | 2.2 g EPA and DHA per day, in a 3:2 ratio | Seventy-one patients were randomized, and in the final analysis, 18 participants in the intervention group and 11 in the placebo group were analysed. | 26 weeks | Mean 22–23 | First-episode schizophrenia |
| Phillips et al. (2015) | RCT | 600 mg EPA and 625 mg DHA per day | 76 | 4 months | Mean 71.1 | 57 patients with cognitive impairment no dementia (CIND) and 19 with early-stage Alzheimer’s disease |
| Pottala et al. (2016) | Analysis of data from the Women’s Health Initiative Memory Study | Non-intervention; measured DHA and EPA in red blood cells | 1111 | Non-intervention; 8-year follow up | 65–80 | Post-menopausal; free from dementia |
| Quinn et al. (2020) | RCT | 2g DHA/day | 295 | 18 months | n/s | Mild to moderate AD |
| Rizzo et al. (2012) | RCT | 2.5 g per day with a 2:1 EPA to DHA ratio | 46 | 8 weeks | 66–95 | Depressed and non-depressed elderly women |
| Rogers et al. (2008) | RCT | 630 mg EPA and 850 mg DHA per day | 218 individuals randomized; 190 completed | 12 weeks | 18–70 | Mild to moderately depressed |
| Schuchardt et al. (2016) | Observational | Non-intervention; LC PUFAs assessed using serum lipid levels | 111 | Non-intervention | 50–80 | Mild cognitive impairments |
| Shinto et al. (2014) | RCT | ω-3 (fish oil concentrate containing a daily dose of 675 mg DHA and 975 mg EPA; or ω-3 (fish oil concentrate containing a daily dose of 675 mg DHA and 975 mg EPA) plus LA (600 mg/day) | 39 | 12 months | ≥55 | Diagnosis of probable AD |
| Stonehouse et al. (2014) | RCT | 1.16 g DHA | 176 | 6 months | 18–45 | Healthy |
| Thesing et al. (2020) | Secondary analysis of 2 RCTs | Non-intervention; blood analysis | 474 | Non-intervention; 8-year follow up to original studies | 18–65 | Patients with Major Depressive Disorder |
| Wang et al. (2015) | Secondary analysis of data from the OmegAD study | 1.7 g DHA and 0.6 g EPA | 15 | Non-intervention; 6 months in original study | Mean 72.5 for intervention group; 70.4 for placebo | AD patients |
| Witte et al. (2014) | RCT | 1320 mg EPA and 880 mg DHA | 65 | 26 weeks | 50–75 | Healthy |
| Zhang et al. (2018) | RCT | 2g of algal-derived DHA per day | 240 | 24 months | ≥60 | Mild cognitive impairment |
Table 3.
Instrumentation, Results, and Limitations.
| Outcome Measures | Results | Conclusion | Confounding Variables/Limitations | Funding Source | ||
|---|---|---|---|---|---|---|
| Primary/Cognitive | Secondary/Other Measures | |||||
| Andrieu et al. (2017) | Composite Z score combining four cognitive tests: free and total recall of the Free and Cued Selective Reminding Test; 10 MMSE orientation items; Digit Symbol Substitution Test score from the Wechsler Adult Intelligence Scale—Revised; the Category Naming Test | MMSE score; Trail Making Test AA and B; Controlled Oral Word Association Test; visual analogue scales measuring memory functioning and consequences in everyday life; Short Physical Performance Battery; Alzheimer’s disease Cooperative Study—Activities of Daily Living Prevention Instrument; Clinical Dementia Rating; Fried’s frailty criteria; Geriatric Depression Scale | No significant difference b/w control and placebo | While no significant effect w/o the lifestyle intervention, future research should test this in an n-3 deficient population. | Not blinded regarding multidomain intervention; PET scans performed after baseline; no food frequency questionnaire | French Ministry of Health, Pierre Fabre Research Institute, Gerontopole, Exhonit Therapeutics, Avid Radiopharmaceuticals |
| Antypa et al. (2009) | Affective Go/No-Go task; An Attentional Go/No-Go task; 15 words test; Decision-making (gambling) task | Mini International Neuropsychiatric Interview; Beck Depression Inventory – II; Profile of Mood States; Behavioral Inhibition/Behavioral Activation Scales; Leiden Index of Depression Sensitivity – Revised | Limited effects of supplementation on mood states; supplemented group made fewer risk-averse decisions on the decision-making test and scored higher on the control/perfectionism aspect of the cognitive reactivity scale | Further research on depression-vulnerable samples should be conducted. | Only powered to detect moderately large effects; decision-making test only administered at post-test | N.W.O.-VICI grant |
| Assmann et al. (2018) | Neuropsychological test battery | Sex, age, physical activity, diabetes/hypertension information | Relationship between total MUFAs, total PUFAs, total n-6 PUFAs, LA, and ALA and improved cognitive functioning, but only in the group that had also received antioxidant supplementation | EPA, DHA, and DPA are more vulnerable to peroxidation than ALA, meaning that they are dependent on the presence of a sufficient amount of antioxidants. The results also demonstrated a generally positive cognitive effect of LA, as compared to a generally negative effect of AA | Sample tended to have higher education levels and better diets than the general population; no cognitive functioning assessment at baseline | n/s |
| Benton et al. (2013) | Cognitive test battery (recall of word list, recall of capitals, reaction times, rapid information processing task, mood assessment) | n/a | On the word list recall test, participants supplemented with DHA tended to forget more at the end of the study, although this result did not reach statistical significance. Interactions on other outcome measures also did not reach statistical significance, or were absent altogether. | The authors noted that the increase in forgetting with DHA supplementation requires further investigation, but that the results do not seem to indicate any beneficial cognitive effect of DHA supplementation among this population. They also noted that the lack of effect on mood was surprising, and that their null results may have been due to the lack of EPA supplementation and/or the relatively low DHA dosage provided | No baseline fatty acid status measure; | Novartis Nutrition Research A.G |
| Bo et al. (2017) | Basic Cognitive Aptitude Tests (BCAT) | n/a | Both groups’ cognitive scores improved, but the intervention group’s scores on perceptual speed, space imagery efficiency, and working memory metrics showed significant improvements | These findings contradict those of studies that did not find omega-3 supplementation to benefit individuals with mild to moderate AD, and suggested that small sample sizes may have been the cause of such findings | Small sample size; high dropout rate | n/s |
| Bountziouka et al. (2009) | Geriatric Depression Scale (GDS) | Education; activity levels; reported fish consumption | Higher levels of fish consumption correlated with lower scores on the GDS | Along with more education and higher activity levels, the highest levels of fish consumption were reported by those who also reported the lowest incidence of depressive symptoms | Cross-sectional study; potential recall bias | n/s |
| Ciappolino et al. (2020) | Brief Assessment of Cognition in Affective Disorder (BAC-A) | n/a | No significant between group differences, except on the emotion inhibition test, and only among healthy controls | That bipolar disorder patients often have unhealthy diets may explain the lack of supplementation effect on this population due to masking effects | Low sample size; older mean age of participants in bipolar disorder placebo group; fairly short intervention period | Italian Ministry of Health |
| Dangour et al. (2010) | Memory test from the California Verbal Learning Test (CVLT) | Other memory tests: Immediate and delayed recalls of a short story from the Wechsler Memory Scale; spatial memory; processing speed tests included a letter search; executive-function tests; prospective memory and digit span forward tests | No change in cognitive function, in either group, after the 24-month intervention | Study participants may already have been consuming sufficient amounts of n-3 LC PUFAs, as both the intervention and control groups exhibited a relatively high concentration after the intervention | Potential threshold | UK Food Standards Agency |
| Dretsch et al. (2014) | Central Nervous System Vital Signs battery | Other mood, sleep, and symptom questionnaires | No effect on neurocognitive functioning or psychological health measures, while increased DHA and EPA levels was correlated with reported reductions in daytime sleepiness | Authors studied this novel population due to the potential protective effects of n-3 PUFA supplementation on depression, but the results did not show this | Few participants reported depression, so room for improvement may have been limited | U.S. Army Aeromedical Research Laboratory |
| Duffy et al. (2015) | Neuropsychological assessment (Visuomotor speed: This was assessed using the Trail Making Test, Part A; Verbal learning and memory: The Rey Auditory Verbal Learning Test (RAVLT); Executive functions: Set-shifting was examined using the Trail Making Test, Part B) | Depressive symptoms were assessed using the PHQ-9; Proton magnetic resonance spectroscopy to measure oxidative stress via glutathione (GSH). | The GSH levels among the placebo group increased significantly after the intervention, which were moderately associated with increased depressive symptoms. They found that, rather than increasing GSH among the intervention group, the intervention attenuated that effect among the placebo group, suggesting the protective, rather than proactive, effects of omega-3 supplementation | The authors suggested that their results indicated that supplementation with omega-3 fatty acids may protect against worsening of depressive symptoms among vulnerable groups | Did not collect dietary data; baseline blood samples were destroyed | Bupa Health Foundation |
| Fontani et al. (2005) | Alert, Go/No-Go, Choice and Sustained Attention tests; event-related potentials measured by electroencephalogram (EEG) and electromyography (EMG) | Profile of Mood States | Omega-3 supplementation correlated with improved mood, reduced anger, and reduced anxiety and depression states; reaction time in Go/No-Go and Sustained Attention tests decreased after supplementation | The results indicate that n-3 supplementation can improve central nervous system functioning | Small sample size; no gender comparison component | n/s |
| Freund–Levi et al. (2006) | MMSE and the cognitive portion of the Alzheimer Disease Assessment Scale | n/a | No significant difference between groups on primary outcome measures at either 6 or 12 months; however, based on the suggestion that n-3 PUFA supplementation may primarily benefit those with mild AD, the researchers performed subgroup analysis according to participants’ MMSE scores. In the group with the mildest AD, the decline rate appeared to be slower during the first months in the treatment group than the placebo group | n-3 PUFA supplementation may have a protective effect for individuals with mild AD symptomology | Second 6 months of the study not placebo-controlled | Pronova Biocare A/S; Capio; Gamla Tjanarinnor; Swedish Alzheimer Foundation; Odd Fellow; Swedish Society of Physicians; Lion’s Sweden |
| de Groot et al. (2007) | Visual verbal words learning tasks; concept shifting test; Stroop color–word interference test; Letter digit substitution test (LDST) | Level of education | Fatty acids did not significantly correlate with cognitive test battery results; however, higher DHA levels were found to correlate with slower learning curve on the Stroop task, and AA levels correlated with a faster learning curve | The authors noted that, particularly as their result associating higher DHA levels with lower cognitive performance was unexpected, further research should be conducted | Exploratory design; intervention studies should be conducted with this population; sample only included women | n/s |
| Hashimoto et al. (2016) | Hasegawa’s Dementia Scale-Revised (HDS-R) and Mini-Mental State Examination (MMSE) | Apathy (using the Zung Self-Rating Depression Sscale [SDS]), caregiver burden | The MMSE subitem 3 score, “registration,” was found to be higher in the active than the placebo group, indicating a positive effect on cognitive function; From baseline to the conclusion of the study, both SDS and apathy scores were lower among the active than the placebo group. | Higher levels of DHA consumption can protect age-related cognitive decline. Further, two measures on the caregiver burden scale used (Zarit Caregiver Burden Interview [J-ZBI_8]), were lower in the active group than the placebo group, suggesting that individuals’ reduction in apathy and improved cognitive function can reduce their caregivers’ burden | Dosage and length of intervention should be considered in future studies | Ministry of Education Culture, Sports, Science and Technology of Japan |
| Heude et al. (2003) | Mini-Mental State Examination (MMSE) | Sex; age; education; lifestyle factors; history of coronary artery disease | Higher total n-6 polyunsaturated fatty acids and stearic acid levels were associated with increased cognitive decline | The study indicates the role of FAs in cognitive decline, and more research on the subject is warranted. | No dietary intake data collected | EISAI Laboratory |
| Hooper et al. (2017) | Battery of cognitive tests | Fatty acid assessment in RBCs | Subjects in the n-3 intervention group improved in performance on the Controlled Oral Word Association Test (COWAT), while those in the placebo group exhibited decreased performance. On all other cognitive tests, the intervention group demonstrated decreased levels of cognitive decline, but these were insignificant | Because the COWAT is a test of executive functioning, the authors concluded that n-3 supplementation may have a neuroprotective effect in this area. | As a secondary analysis, this study was not powered to detect a significant between group difference | Gérontopole of Toulouse; the French Ministry of Health; Pierre Fabre Research Institute (manufacturer of the omega-3 supplement); Exhonit Therapeutics SA; Avid Radiopharmaceuticals Inc; the French National Agency for Research |
| Howe et al. (2018) | Cerebrovascular responsiveness (CVR) to hypercapnia; cerebrovascular function (transcranial Doppler ultrasound of blood flow in the middle cerebral artery at rest and whilst performing a battery of cognitive tasks) | Cognitive test battery/mood questionnaires | Cerebrovascular responsiveness (CVR) to hypercapnia, increased 26% in women; there was no change in men; neurovascular coupling increased significantly in men only; the latter correlated with an increase of EPA in erythrocytes. There was no associated improvement of mood or cognition in either men or women. | LCn-3PUFA supplementation has the potential to enhance blood flow in the brain in response to both hypercapnic and cognitive stimuli | Transcranial Doppler ultrasound technique was able to identify vasodilator responses, but not localization of those responses | Westfund Limited Australia |
| Jackson, Deary, et al. (2012) | Cognition (Computerized Mental Performance Assessment System and a cognitive demand battery) | Mood (with Bond-Lader visual analogue scales and the Depression, Anxiety and Stress Scales) | For the cognitive performance battery, only two measures (reaction time on the Stroop Task and number of correctly matched items on the Names-to-Faces) showed a significant treatment effect. For the cognitive demand battery, the only significant effect was lower reported mental fatigue scores among the EPA-rich fish oil group. The treatment had no effect on either mood measure. | The authors noted that Jensen et al. has established an n-3 PUFA level in a rat study, and suggested that a similar threshold could exist in humans, which should be investigated. | A longer supplementation period may have been needed | Ginsana SA, Switzerland |
| Jackson, Reay, et al. (2012) | Corsi blocks; numeric working memory; simple reaction time; 4-choice reaction time; choice reaction time; Stroop task; rapid visual information processing task; serial 7s | Hemoglobin analysis | Omega-3 supplementation resulted in significant increases to cerebral region blood flow during task performance, with a dose-response effect (across all tasks with 2g FO, and only Stoop and rapid visual information processing with 1g FO) | More research should evaluate this topic to produce more nuanced results on task performance effects | No baseline measures of cognitive performance used | Ginsana SA, Switzerland |
| Jackson et al. (2016) | Hemoglobin and deoxygenated hemoglobin concentrations during cognitive tasks (from the Cognitive Demand Battery) using Near Infared Spectroscopy | n/a | No significant differences in cognitive test performance were identified between groups, nor in cerebral blood parameters | The authors noted that these null results may have been due to an already sufficient omega-3 level among participants, or the focus on DHA rather than provision of larger amounts of EPA. | Cognitive tests may not have been sufficiently sensitive | Efamol Ltd. |
| Jackson et al. (2018) | Repeatable Battery for Assessment of Neuropsychological Status (RBANS) | n/a | Neither group showed persistent perioperative cognitive decline 30 days after surgery, and the researchers did not identify any significant differences between groups on cognitive functioning measures | The authors noted that not only did their results not indicate the benefit of n-3 PUFA supplementation in post-operative cardiac supplements, but also did not align with previous findings on the presence of cognitive decline among non-supplemented post-surgery patients | ||
| Konagai et al. (2013) | Changes in oxyhemoglobin concentration during working memory and calculation tasks | n/a | Both the sardine oil group and the krill oil group showed significantly higher changes in oxyhemoglobin concentrations at week 12 in response to the working memory test | The authors suggested that these results indicate that supplementation with sardine or krill oil serves to activate the dorsolateral prefrontal cortex, which promotes working memory and prevents cognitive deterioration among healthy elderly individuals | Small sample size; male subjects only | Nippon Suisan Kaisha Ltd |
| Mazereeuw et al. (2016) | Vascular test battery for cognitive impairment | Hamilton Depression Rating Scale and the Beck Depression Inventory II | There were no significant differences between scores on either depression scale between the intervention and placebo groups after 12 weeks of the intervention, or any significant effects on the cognitive measures. | The authors noted that, as mild cognitive effects are common among those with coronary artery disease, this study complemented those that have found n-3 PUFAs to have a beneficial effect on such cognitive issues. | Small sample size in subgroups | Ontario Mental Health Foundation |
| McGorry et al. (2017) | Transition to psychosis status at 6 months | Brief Psychiatric Rating Scale; Scale for the Assessment of Negative Symptoms; Montgomery-Asberg Depression Rating Scale; Young Mania Rating Scale; Social and Occupational Functioning Assessment Scale; Global Functioning: Social and Role scale | No significant difference between control and intervention groups in rates of transition to psychosis status | Since both groups showed symptomatic improvements, there may have been a threshold effect with other treatments patients were receiving | Population under investigation cannot be tested without the other treatments, so effect of n-3 supplementation in isolation cannot be assessed | Stanley Medical Research Institute; NHMRC Australia Program; Colonial Foundation |
| McNamara et al. (2018) | Dysexecutive Questionnaire (DEX) | Trail Making Test, Controlled Oral Word Production procedures, and the Hopkins Verbal Learning Test | Compared to the placebo group, the fish oil group demonstrated reduced cognitive symptoms in everyday activities. The blueberry group similarly demonstrated reduced cognitive symptoms, but neither group demonstrated improvements in the other cognitive domains | The authors noted that the improvements in the fish oil group may have been due to improved brain glucose uptake, as opposed to the blueberry intervention, which may have produced improved circulation and neural signalling. | A period of abstinence from both berries and fish oil before the study may have been useful; cognitive measures may not have been sufficiently sensitive | National Institute of Health |
| Moon et al. (2018) | White matter hyperintensities (which have been shown to have associations with cognitive impairment) and n-3 PUFA levels in red blood cells, since both factors have been associated with cognition | n/a | The results did not indicate any association between n-3 PUFA levels and white matter hyperintensities | That participants were a fairly homogenous and dementia-free group may have impacted the results; a longer longitudinal study should be conducted with this population | Small sample size may have affected statistical power | Gérontopole of Toulouse; the French Ministry of Health; Pierre Fabre Research Institute (manufacturer of the omega-3 supplement); Exhonit Therapeutics SA; Avid Radiopharmaceuticals Inc; the French National Agency for Research |
| Nilsson et al. (2012) | Working memory and selective attention using the test by developed by Radeborg et al for working memory and a computerized, image-based test for selective attention | n/a | The results of the working memory test improved after PUFA supplementation, with the improvements being more significant for the most demanding parts of the test. For word retrieval and figure retrieval components of the working memory test, the results improved after intervention. While performance after supplementation on the selective attention task did tend to improve, these results were not determined to be significant | This study was also focused on cardiometabolic risk factors of cognitive function, and found systolic blood pressure to be inversely related to cognitive test performance | Issues with placebo/intervention group blinding/did not assess blood PUFA concentrations | VINNOVA excellence center Anti Diabetic Food Center (AFC), Lund, Sweden |
| Nooyens et al. (2018) | Neuropsychological testing including 15 Words Verbal Learning Test, the Stroop Color–Word Test, the Word Fluency test, and the Letter Digit Substitution Test | n/a | While the results did not indicate associations between fish or fatty fish consumption and cognitive function, those with a low level of fatty fish intake, compared to those with none, exhibited slower decline in memory function. Higher cholesterol was associated with faster decline in global cognitive function, higher n-3 PUFA intake was associated with slower memory decline and higher ALA intake was associated with slower decline in global cognitive function and memory | The researchers also found that EPA and DHA intake was only beneficial for individuals with the apolipoprotein genotype ε4/ε4. | Loss to follow up | Internationale Stichting Alzheimer Onderzoek |
| Otsuka et al. (2014) | Cognitive function measured with the MMSE | n/a | After adjusting for age, sex, and education, the results indicated that higher serum DHA levels were significantly associated with lower levels of cognitive decline; EPA was not found to have the same correlation | The authors noted that, significantly, their results suggest that—rather than high DHA levels having a neuroprotective effect—low DHA levels formed over time may increase the risk of cognitive decline | Only used a general cognitive test; | Japanese Ministry for Education, Culture, Sports, Science and Technology; Research Funding for Longevity Sciences from the National Center for Geriatrics and Gerontology, Japan |
| Pawełczyk et al. (2018) | Cortical thickness via structural MRI | n/a | The results indicate decreased loss of cortical thickness in the patients who received the intervention, specifically in the left parieto-occipital cortex | The authors noted that their results indicate that n-3 PUFA supplementation can have neuroprotective effects for healthy individuals, as well as those with the early stages of neurodegenerative disorders like schizophrenia, MCI, and AD | No objective adherence measure; small sample with useable MRI scans; intervention period may have been too short | Polish National Science Center |
| Phillips et al. (2015) | Mini–mental state examination MMSE, the Hopkins Verbal Learning Test—Revised (HVLT-R), and the Brief Assessment Schedule Depression Cards (BASDEC) | n/a | While the placebo concentrations of DHA and EPA increased among members of the intervention group, the researchers did not identify any positive effect of increasing these levels on mood or cognition among those with early-stage AD or mild cognitive impairment | However, the authors noted that the constant cognitive decline that characterizes AD/this phase of life means that any improvements as a result of this type of intervention may be countered by the progression of the disease | Small sample size in AD group; measurements may not have been sufficiently sensitive | Sir Halley Stewart Trust |
| Pottala et al. (2016) | MRI brain volumes 8 years after the original study | n/a | Compared to omega-3 index as a whole, the researchers identified a marginal correlation between DHA (p = .063) and total brain volume, as well as a smaller correlation between EPA (p = .11) and total brain volume | These results indicate that omega-3 index (RBC levels of EPA and DHA) are directly related to normal brain and hippocampal volumes, which suggests that supplementation can have a neuroprotective effect on the effects of and diseases related to brain atrophy | Women only; non-intervention | National Heart, Lung, and Blood Institute |
| Quinn et al. (2020) | Alzheimer’s Disease Assessment Scale (ADAS-cog)16 and the Clinical Dementia Rating (CDR) | MMSE, the ADCS’s activities of daily living (ADCS-ADL) scale, the Neuropsychiatric Inventory (NPI), and the Quality of Life Alzheimer’s Disease scale | Among 44 participants who participated in a voluntary draw of cerebrospinal fluid, the DHA subgroup had undergone a significant 38% increase in cerebrospinal fluid DHA | The primary and secondary outcome measures did not indicate a significant treatment effect of DHA supplementation; the authors noted that this could have been due to not starting the intervention earlier | Earlier DHA intervention may have had an effect | National Institute on Aging |
| Rizzo et al. (2012) | MMSE, a neurologic and psychiatric evaluation, and the Geriatric Depression Scale (GDS) | n/a | The results indicate that, at baseline, the AA/EPA ratio of depressed individuals was higher than that of the healthy individuals and that, after supplementation, the ratio decreased significantly. The researchers noted that the ratios of supplementation used were able to restore the EPA concentration in red blood cell membrane to normal values, but was not able to increase the saturation of EPA and DHA in the red blood cells to healthy levels | The authors suggested that their results indicate that n-3 PUFA supplementation can be effective in treating depression among the elderly, and that whole blood AA/EPA ration should be used to assess n-3 status | Women only | Regione Lombardia; Italian Space Agency |
| Rogers et al. (2008) | Depression, Anxiety and Stress Scales (DASS) | Beck Depression Inventory (BDI); battery of cognitive tests | After the 12-week intervention, EPA and DHA concentration in plasma had doubled in the intervention group, but not the placebo group, but this increase did not correlate with any improvements to any mood or mental health measures | Excepting the insignificant possibility that the impulsivity task performance may have been improved with supplementation, the DHA and EPA intervention did not affect any measures of cognitive performance | Unclear results on lowest dose (2g) evaluated | Food Standards Agency, UK government and by the University of Bristol, UK |
| Schuchardt et al. (2016) | Associations between FADS gene clusters (FADS1, FADS2, and FADS3) and PUFA levels | Associations between FADS genotypes and single LC PUFAs with serum lipid levels and depressive symptoms | Their results indicate a significant relationship between several FADS genotypes (rs174546, rs174548 (FADS1), rs3834458, rs1535, rs174574, rs174575, rs174576, and rs174578 (FADS2)) for higher LA and ALA levels and lower AA levels in erythrocyte membranes in minor allele carriers. Minor allele carriers of the FADS genotypes investigated in this study have lower EPA and DHA levels erythrocyte membranes than major allele carriers | Because lower DHA status and omega-3 index was associated with depressive symptoms in this study, the researchers noted that future research should expand on this subject as well as how LC PUFA levels and FADS genotypes affect cognitive decline | No healthy control group evaluated | Deutsche Forschungsgemeinschaft; Bundesministerium fur Bildung und Forschung |
| Shinto et al. (2014) | Urine F2-isoprostane level | Alzheimer Disease Assessment Scale-cognitive sub-scale, MMSE, and Activities of Daily Living/Instrumental Activities of Daily Living | The results, adjusted for age and education, suggested that, while n-3 supplementation alone did not significantly affect the cognitive outcome measures, n-3 plus LA did have a protective effect against cognitive decline according to the MMSE score, compared to the placebo group. The urinary F2 isoprostane levels were not different between placebo and intervention groups when assessed at 6 or 12 months. Both intervention groups demonstrated reduced functional impairment after 12 months, compared to the placebo group | The results suggest that the intervention slowed decline, but that a larger study is necessary | ||
| Stonehouse et al. (2014) | Computerized Mental Performance Assessment System | n/a | For episodic memory, no effects were seen among men, but the measure improved significantly among women in the DHA intervention group, and the reaction time for episodic memory improved regardless of gender. The intervention did not affect working memory scores, but did improve working memory reaction time (this occurred in both women and men, but was only significant for men). No significant effects occurred regarding attention or processing speed | The authors noted that this study was unique in its assessment of sex interactions, and that further research with larger sample sizes must be conducted to confirm or supplement its results | Insufficient statistical power to evaluate apolipoprotein E genotype (APOE) according to sex | Massey University Research Fund, Neuro- logical Foundation of New Zealand, and Oakley Mental Health Research Fund |
| Thesing et al. (2020) | Time until Major Depressive Disorder recurrence | n/a | No association between PUFAs and time to recurrence of Major Depressive Disorder | Correcting deficits and improper ratios of PUFAs is not a promising prevention technique for Major Depressive Disorder recurrence | Possibility of memory bias as recurrence assessed retrospectively; some PUFA data missing from original studies | BBMRI-NL; Dutch Brain Foundation |
| Wang et al. (2015) | MMSE | n/a | The results indicate that the supplementation had a supportive effect on SPM production by peripheral blood mononuclear cells. The placebo group results—a decline in SPM secretion after 6 months—suggests that the ability of these cells to produce SPM decreases over time, which allows AD to progress more quickly | The authors suggested that their results did not yet allow identification of the mechanism by which this beneficial effect occurs, but suggested that it may relate to increased availability of SPM precursors. They therefore suggested that using SPMs instead of their precursors could be a beneficial AD treatment and that n-3 PUFA supplementation may help prevent a reduction in levels of SPM release | Limited sample size | Pronova Biocare A/S; Swedish Research Council; Chinese Scholarship Council; Stockholm County Council; Swedish Alzheimer Foundation; Gun och Bertil Stohnes Stiftelse; Stiftelsen for Gamla tjanarinnor |
| Witte et al. (2014) | White matter integrity, gray matter volume, CIMT, and peripheral parameters (omega-3 index, glucose/insulin metabolism, inflammatory markers, and neurotrophins) to determine mechanisms, and assessed cognitive function on the basis of composite scores of memory and executive function | n/a | The results indicate that LC-PUFA supplementation had a significant effect on executive functions, which improved by 26% in the intervention group compared to the placebo group. There was no significant effect on the composite score for memory, but memory consolidations after intervention did show improvements, compared to the placebo. The researchers also found that LC-PUFA supplementation improved white matter microstructural integrity, gray matter volume, and vascular parameters | The authors noted that their results indicate potential neuroprotective effects of PUFA supplementation | Small sample size; longer intervention period may have yielded more significant results | Deutsche For- schungsgemeinschaft; Else-Kroner fresenius Stiftung; Bundesministerium fur Bildung und Forschung |
| Zhang et al. (2018) | Chinese version of the Wechsler Adult Intelligence Scale-Revised (WAIS-RC) | n/a | The full-scale IQ (FSIQ), verbal IQ (VIQ), information and digit span test measures were found to be significantly higher in the intervention group than the control group. The results indicate DHA to have protective properties on brain health, in part by decreasing the production and accumulation of the Aβ peptide, which has been identified as a major cause of AD | The population in this study were experiencing MCI, which has been shown to develop into AD (10%–15% per year), so the authors suggested longer-term studies be conducted to assess for neuroprotective benefits of DHA supplementation in more detail over a longer period of time | Optimal DHA dosage unknown; quality seafood in the region in which the study was conducted may have biased the results | 2012 Chinese Nutrition society Nutrition Research Foundation—DsM Research Fund; National Natural Science Foundation of China |
Key Populations/Demographic Interactions
Mild Cognitive Impairment
Many studies have addressed populations with mild, rather than severe, levels of cognitive impairment, and have yielded mixed results. Bo et al. 13 identified cognitive improvements in individuals with mild-moderate AD, which contradict the results of previous studies—the authors suggested that this is due to the large sample size in their work. Freund–Levi et al. 14 found, via subgroup analysis, that decline rate was slower in the treatment group, but only among those with the mildest AD symptoms. Moon et al. 15 studied a similar population, but did not identify any positive effect of omega-3 supplementation.
AD/More Severe Cognitive Impairment
Overall, fewer studies have addressed populations with more severe levels of cognitive decline and AD. In part, this has been due to difficulties discerning the potential effects of n-3 supplementation in the presence of progressive cognitive decline. For example, Phillips et al. 16 suggested that cognitive disease progression may have caused their lack of significant findings of supplementation among a population with cognitive impairment or AD. Similarly, Quinn et al. 17 did not identify a significant treatment effect in relation to AD.
Sex-based Differences
Various studies have addressed sex-response differences in the cognitive efficacy of n-3 supplementation. Howe et al. 18 found that LC PUFA supplementation improved cerebrovascular responsiveness in women only and neurovascular coupling in men only, and suggested that future work should address gender differences in more detail. Similarly, Bo et al. 13 found that, while supplementation significantly improved perceptual speed, space imagery efficiency, working memory, and total BCAT scores in males, it only improved perceptual speed, space imagery efficiency, and total BCAT scores in females.
Limited Effect in a Younger, Healthy Population
Studies largely found limited effects of interventions conducted among members of a younger and healthy population. One study conducted with healthy university students found no effect of 4 weeks of n-3 PUFA supplementation on mood states or attention, memory, emotion recognition, or memory inhibition, though the results did indicate that members of the intervention group made fewer risk-averse decisions on a decision-making test and had higher scores on the control/perfectionism measure of the cognitive reactivity score. 19 A small study (N = 33) found n-3 supplementation for 35 days to improve mood states and decrease reaction time on attentional tasks. 20 The results of another study conducted on healthy young adults found that supplementation with 2 g of fish oil for 12 weeks resulted in increased cerebral region blood flow during cognitive task performance, but not with improved performance in those tasks. 21 In a study conducted with healthy, non-pregnant women, the authors found no effect of blood FA levels on cognitive performance, excepting an association between higher DHA levels and slower learning curve on the Stroop task. 22 Another study found no effect of fish oil supplementation on mood or cognition in a population aged 18–35. 23
Moon et al. 15 suggested that their null results may have been due to their population’s characteristics as relatively stable, dementia-free, younger than in other studies, and with less risk of vascular issues. Benton et al. 1 tested healthy young adults supplemented with DHA for 50 days and did not find any significant interactions between treatment and cognitive outcomes, but did find that patients treated with DHA tend to forget more on the word recall list test, which requires further investigation.
Mental Health Interactions
Emotional Effects and Depressive Symptoms
Dretsch et al. 24 studied soldiers regarding the potential protective effect of n-3 PUFAs on depression, due to the high prevalence of depression among that population, but the results did not support this finding. Duffy et al. 25 demonstrated a protective effect—that supplementation may protect against the worsening of depressive symptoms among members of vulnerable groups. Hashimoto et al. 26 found that supplementation improved patients’ apathy, and thereby reduced levels of caregiver burden.
An 8-year follow up of two previous large-scale RCTs found no effect of PUFA levels on the likelihood of recurrence of Major Depressive Disorder. 27 However, Rizzo et al. 28 found that PUFA supplementation may have a positive effect on depression in elderly women. Mazereeuw et al. 29 also measured depressive symptoms, but did not find any effect of n-3 supplementation. Phillips et al. 16 assessed mood, and did not find any particular effects of n-3 supplementation. Rogers et al. 30 did not find supplementation to affect cognitive function or depressive symptoms.
Schizophrenia
Pawełczyk et al. 31 noted that their results indicate that n-3 PUFA supplementation can have neuroprotective effects for healthy individuals, as well as those with the early stages of neurodegenerative disorders like schizophrenia, MCI, and AD.
Bipolar Disorder
A study conducted on a population of euthymic Bipolar Disorder patients and healthy controls found that supplementation with DHA only caused improvement among the healthy control group. 32 Further, improvement was only seen on the emotion inhibition test included in the neuropsychological test battery. The authors noted that the lack of effect on members of the Bipolar Disorder group may have been due to a masking effect of their tendency toward poorer diet and other unhealthy lifestyle characteristics.
PUFA Characteristics
Antioxidant Effects
Assman et al. found that long-term (8 years) supplementation with PUFAs produced improvements to cognitive functioning, but only if antioxidant supplementation is also provided. 33 McNamara et al. compared PUFAs with blueberry powder based on the antioxidant effects and found that both the fish oil and blueberry groups demonstrated a reduction in cognitive symptoms in everyday activities after the intervention. 34 Interestingly, they also noted the surprising finding that the combined fish oil and blueberry powder group did not demonstrate improvements, and suggested that this may have been due to a combined reaction of the two undermining potential benefits.
Which PUFAs/Their Source/Intervention Length
Otsuka et al. 35 found that higher serum DHA levels were significantly associated with lower levels of cognitive decline; EPA was not found to have the same correlation. This study was conducted among a sample of elderly Japanese individuals, as a population that tends to have a high level of fish consumption. Konagai et al. found that, while both krill and sardine oil promoted activity during the working memory task, only krill oil was shown to have activation effects during the calculation task; the difference between the two appears to be in their chemical incorporation form rather than differences in the amounts of EPA or DHA. 36 The researchers also studied event-related potential, and found that, compared to the MCT group, the group that received krill oil showed a significant decrease in latency, which reflects the rate of information processing. While both krill and sardine oil promoted activity during the working memory task, only krill oil was shown to have activation effects during the calculation task; the difference between the two appears to be in their chemical incorporation form rather than differences in the amounts of EPA or DHA.
Relatively few studies have assessed the effects of dietarily derived (rather than supplement-based) omega-3 consumption. A secondary analysis of a larger RCT used a food frequency questionnaire to assess consumption, and found that, compared to those who reported no fish consumption, those with a low level of fatty fish intake exhibited slower cognitive decline. 5
Key Types of Findings
The Threshold
Several studies suggested that their research should be repeated in populations deficient in n-3 PUFAs, as their population may already have been at the threshold for its benefits. Hooper et al. 37 conducted a secondary analysis of results with the MAPT study of those with the lowest omega-3 index scores, and found some effect of supplementation. More specifically, the results indicated that 3 years of n-3 supplementation may have a neuroprotective effect in the area of executive functioning, but only for those with low omega-3 index scores. Jackson et al. 38 also suggested that the null results of their 6-month DHA supplementation (alone and in combination with a multi-nutrient intervention) study may be explained by the fact that participants already had a significant omega-3 level. Dangour et al. 39 did not identify any effect of 24 months of n-3 supplementation on cognitive function, and suggested that this may have been attributable to the fact that study participants were already consuming a sufficient amount of LC PUFAs.
Neuroprotective Effect/Slowing Decline
Several studies have suggested that supplementation with omega-3s may slow decline rather than improving cognitive function.14,26,36 Several researchers identified the presence of reduced cognitive symptoms, or lower rates of cognitive decline, but no improvement as such.34,35 Phillips et al. 16 noted that their null findings may have been due to cognitive disease progression. Pottala et al. 40 indicated a potential neuroprotective effect of omega-3 levels; by measuring for cortical thickness, the researchers found that blood levels of EPA and DHA correlate with brain and hippocampal volumes, meaning that adequate omega-3 consumption may have a neuroprotective effect for diseases of brain atrophy.
Quinn et al. 17 addressed the fact that their null findings may have been due to not starting the intervention earlier. Heude et al. found that higher total n-6 polyunsaturated fatty acids and stearic acid levels were associated with increased cognitive decline a 4-year follow up study of a sample aged 63–74. 41 Jackson et al. did not find an effect of pre- and post-operative supplementation on cardiac surgery patients, inconsistent with the results of previous research. The authors noted that the study period may have been too brief, and that the mechanisms for improving cognitive function and preventing cognitive decline are different, which may explain the null results in this study. Andrieu et al. did not find a significant difference between placebo and control in a 3-year study with omega-3 supplementation and a multidomain intervention. Exploratory subgroup analysis suggested that n-3 supplementation in combination with the multidomain intervention may slow cognitive decline in certain groups, but the study design was not powered to detect this.
Specific Cognitive Outcomes
Executive Functioning
Based on the results of a Controlled Oral Word Association Test—a test of executive functioning—Hooper et al. 37 concluded that n-3 supplementation may have a neuroprotective effect in this area.
Working Memory
Konagi et al. found that supplementation improved working memory; Mazereeuw et al. found that supplementation did improve verbal memory performance significantly among a subgroup of participants who did not meet depressive episode criteria; Nilsson et al. identified improvements to working memory after 5 weeks of supplementation with EPA and DHA particularly in the more demanding parts of the test, and with word retrieval and figure retrieval.29,36,42,43
Mechanisms/Determinants of PUFAs’ Effects
Cerebral Mechanisms by which n-3s May Affect Cognition
Howe et al. 18 (2018) studied cerebrovascular responsiveness as a potential mechanism by which n-3s affect cognition. Similarly, Konagai et al. (2013) tested changes in oxyhemoglobin concentrations in the cerebral cortex during memory and calculation tests. McNamara et al. suggested that the positive cognitive effect they determined may have been due to brain glucose uptake. 34 Moon et al. 15 studied white matter hyperintensities (which have been shown to have associations with cognitive impairment). Pawełczyk et al. 31 noted reduced brain volume and cortical thickness in patients with schizophrenia, and that n-3 deficiencies have been linked to schizophrenia. Pottala et al. 40 addressed the connection between EPA/DHA and brain volume.
Cardiovascular Interactions
Jackson et al. 44 studied post-cardiac surgery population because of the reported cognitive decline after cardiac surgery, but did not find an effect of supplementation, but also did not produce results consistent with previous studies that had identified this cognitive decline. Mazereeuw et al. 29 studied a population with coronary artery disease, and did not identify any significant effect of pre- and post-operative n-3 supplementation on cognitive function. Nilsson et al. 42 found systolic blood pressure to be inversely related to cognitive test performance.
Discussion
Key findings of this systematic literature review are addressed briefly in this section, including the following:
• Equivocal results regarding cognitive improvements among individuals with MCI—observed in some studies but not others;
• Due to disease progression, LC PUFA supplementation may slow decline rather than yield improvements, but at times without clear results; and
• LC PUFA supplementation appeared to have a limited effect among younger and healthy populations, potentially indicating the presence of a threshold effect and/or that sample participants were not deficient n-3s before the intervention.
• Dosage of LC PUFA supplementation varies widely across studies, indicating the need for increased understanding of ideal EPA/DHA ratios and best practices in dosage amounts.
While public interest in and supplement availability of n-3s has increased in recent years, additional research is required to confirm their beneficial effects for certain populations. Several studies have failed to find supplementation to produce improvement in populations with MCI. 13 Compared to results with AD or more severe levels of cognitive impairment, this may indicate that LC PUFA supplementation is indicated more strongly for those with milder levels of cognitive decline. Among those with AD diagnoses, given the severity and progression of the illness, results tend to be still more equivocal. However, some studies have suggested that—while they did not facilitate improvement—intervention may have slowed the rate of cognitive decline. The relatively limited number of studies addressing AD or severe cognitive impairment, as well as the difficulty of measuring efficacy in terms of slower rates of decline, make the results difficult to parse and indicate that further research on this subject is required. Similarly, in populations with depression, bipolar disorder, and other mental health concerns, results have been shown to be unclear and effects may have taken the form of mitigating decline rather than yielding improvement.
Importantly, there remains uncertainty regarding the most effective dosages and ratios of LC PUFAs relative to their efficacy for an adult and aging population. In addition to a very limited number of studies assessing dietarily derived n-3s through food frequency questionnaires and serum/erythrocyte/RBC analyses, the majority of studies assessed the effects of supplementation with some combination of EPA and DHA. However, DHA dosages ranged from 560 mg to 1.7 g per day, and EPA dosages ranged still more widely from 90 mg to 1.7 g per day. While the majority of studies featured a combination, several studies assessed the effects of DHA in isolation. Further, there was a fairly even split of studies that supplemented with more EPA than DHA and those that included a higher ratio of DHA to EPA. These differences do not yet indicate a favorable pattern regarding which LC PUFAs/ratios are most effective, which should indicate that there is much more to be discerned regarding dosage before the results of such studies can be understood more definitively.
Another limitation of extant studies is—like those conducted with populations in periods of neurodevelopment—the presence of a threshold effect. Several researchers have suggested that their studies may not have yielded significant results due to the fact that research participants were already consuming an adequate amount of n-3s. Results also yielded several sex-differences, and unclear results regarding the cognitive mechanisms through which n-3s function, indicating the necessity of further research on the topic.
Conclusion
Several pervasive limitations consistently affected the results of the studies included in this systematic review, including lack of food frequency questionnaire or diet data; studies insufficiently powered to detect small effects and between group differences; pre-/post-test inconsistencies; sample populations with higher educational levels/better diets than the general population; short intervention periods/small sample sizes; the threshold effect/difficulty determining slowed decline as opposed to cognitive improvements; lack of clear gender comparison; inconsistencies in general and diverse domain-specific cognitive testing; and the lack of knowledge regarding the ideal dosage and ratios of LC PUFAs. Importantly, studies addressed in this systematic review included intervention in the form of a broad range of LC PUFA types and dosages, including DHA, EPA, but few included dietarily derived n-3s or food frequency questionnaires. As there may be an advantage in the bioavailability of dietarily derived over supplement-based n-3s over supplements, this may be a significant lacuna in the current research. 45
Though the results are inconsistent and not conclusive, several studies have indicated neuroprotective effects of n-3 LC PUFA supplementation on adult populations—including the elderly, those with varying degrees of cognitive decline, and various mental health concerns. Given the increasingly aging global population, prevalence of n-3 supplements in the market, the potential cognitive benefit of such supplementation, and paucity of comprehensive studies, future highly powered research is needed to conclusively identify the population that would most benefit the appropriate dosage, and method of delivery.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability: Data described in the manuscript, code book, and analytic code will be made available upon request pending approval of manuscript.
ORCID iD
Ayesha Z. Sherzai https://orcid.org/0000-0003-0600-294X
References
- 1.Benton D, Donohoe RT, Clayton DE, Long SJ. Supplementation with DHA and the psychological functioning of young adults. Br J Nutr. 2013;109:155-161. [DOI] [PubMed] [Google Scholar]
- 2.Simopoulos A. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornstra G. Essential fatty acids in mothers and their neonates. Am J Clin Nutr. 2000;71:1262S-1269S. [DOI] [PubMed] [Google Scholar]
- 4.Bountziouka V, Polychronopoulos E, Zeimbekis A, et al. Long-term fish intake is associated with less severe depressive symptoms among elderly men and women: The MEDIS (MEDiterranean ISlands Elderly) Epidemiological Study. J Aging Health. 2009;21:864-880. [DOI] [PubMed] [Google Scholar]
- 5.Nooyens ACJ, van Gelder BM, Bueno-de-Mesquita HB, van Boxtel MPJ, Verschuren WMM. Fish consumption, intake of fats and cognitive decline at middle and older age: The Doetinchem Cohort Study. Eur J Nutr. 2018;57:1667-1675. [DOI] [PubMed] [Google Scholar]
- 6.Stonehouse W, Conlon CA, Podd J, et al. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am J Clin Nutr. 2013;97:1134-1143. [DOI] [PubMed] [Google Scholar]
- 7.Witte AV, Kerti L, Hermannstädter HM, et al. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cerebr Cortex. 2014;24:3059-3068. [DOI] [PubMed] [Google Scholar]
- 8.McGorry PD, Nelson B, Markulev C, et al. Effect of ω-3 Polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: The NEURAPRO Randomized Clinical Trial. JAMA Psychiatr. 2017;74:19-27. [DOI] [PubMed] [Google Scholar]
- 9.Schuchardt JP, Köbe T, Witte V, et al. Genetic variants of the FADS gene cluster are associated with erythrocyte membrane LC PUFA levels in patients with mild cognitive impairment. J Nutr Health Aging. 2016;20:611-620. [DOI] [PubMed] [Google Scholar]
- 10.Shinto L, Quinn J, Montine T, et al. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s Disease. J Alzheimers Dis. 2014;38(1):111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Hjorth E, Vedin I, et al. Effects of n-3 FA supplementation on the release of proresolving lipid mediators by blood mononuclear cells: the OmegAD study. J Lipid Res. 2015;56:674-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Lou Y, Hu J, Miao R, Ma F. DHA supplementation improves cognitive function via enhancing Aβ-mediated autophagy in Chinese elderly with mild cognitive impairment: A randomised placebo-controlled trial. J Neurol Neurosurg Psychiatr. 2018;89:382-388. [DOI] [PubMed] [Google Scholar]
- 13.Bo Y, Zhang X, Wang Y, et al. The n-3 polyunsaturated fatty acids supplementation improved the cognitive function in the chinese elderly with mild cognitive impairment: A double-blind randomized controlled trial. Nutrients. 2017;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, et al. ω-3 Fatty Acid treatment in 174 patients with mild to moderate Alzheimer Disease: OmegAD Study: A Randomized Double-blind Trial. Arch Neurol. 2006;63:1402-1408. [DOI] [PubMed] [Google Scholar]
- 15.Moon SY, de Souto Barreto P, Chupin M, et al. Association between red blood cells omega-3 polyunsaturated fatty acids and white matter Hyperintensities: The MAPT Study. J Nutr Health Aging. 2018;22:174-179. [DOI] [PubMed] [Google Scholar]
- 16.Phillips MA, Childs CE, Calder PC, Rogers PJ. No effect of omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable Alzheimer’s Disease: A randomised controlled trial. Int J Mol Sci. 2015;16:24600-24613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA. 2010;304:1903-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howe PRC, Evans HM, Kuszewski JC, Wong RHX. Correction: Howe et al. Effects of long chain omega-3 polyunsaturated fatty acids on brain function in mildly hypertensive older adults. Nutrients. 2019;11:1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antypa N, Van der Does A, Smelt A, Rogers R. Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J Psychopharmacol. 2009;23:831-840. [DOI] [PubMed] [Google Scholar]
- 20.Fontani G, Corradeschi F, Felici A, Alfatti F, Migliorini S, Lodi L. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur J Clin Invest. 2005;35:691-699. [DOI] [PubMed] [Google Scholar]
- 21.Jackson PA, Reay JL, Scholey AB, Kennedy DO. Docosahexaenoic acid-rich fish oil modulates the cerebral hemodynamic response to cognitive tasks in healthy young adults. Biol Psychol. 2012;89:183-190. [DOI] [PubMed] [Google Scholar]
- 22.de Groot RHM, Hornstra G, Jolles J. Exploratory study into the relation between plasma phospholipid fatty acid status and cognitive performance. Prostagl Leukot Essent Fat Acids. 2007;76:165-172. [DOI] [PubMed] [Google Scholar]
- 23.Jackson PA, Deary ME, Reay JL, Scholey AB, Kennedy DO. No effect of 12 weeks’ supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18–35 years. Br J Nutr. 2012;107:1-12. [DOI] [PubMed] [Google Scholar]
- 24.Dretsch MN, Johnston D, Bradley RS, MacRae H, Deuster PA, Harris WS. Effects of omega-3 fatty acid supplementation on neurocognitive functioning and mood in deployed U.S. soldiers: a pilot study. Mil Med. 2014;179:396-403. [DOI] [PubMed] [Google Scholar]
- 25.Duffy SL, Lagopoulos J, Cockayne N, et al. The effect of 12-wk ω-3 fatty acid supplementation on in vivo thalamus glutathione concentration in patients “at risk” for major depression. Nutrition. 2015;31:1247-1254. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto M, Kato S, Tanabe Y, et al. Beneficial effects of dietary docosahexaenoic acid intervention on cognitive function and mental health of the oldest elderly in Japanese care facilities and nursing homes. Geriatr Gerontol Int. 2017;17:330-337. [DOI] [PubMed] [Google Scholar]
- 27.Thesing CS, Lok A, Milaneschi Y, et al. Fatty acids and recurrence of major depressive disorder: Combined analysis of two Dutch clinical cohorts. Acta Psychiatr Scand. 2020;141:362-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzo AM, Corsetto PA, Montorfano G, et al. Comparison between the AA/EPA ratio in depressed and non depressed elderly females: omega-3 fatty acid supplementation correlates with improved symptoms but does not change immunological parameters. Nutr J. 2012;11:82-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazereeuw G, Herrmann N, Oh PI, et al. Omega-3 fatty acids, depressive symptoms, and cognitive performance in patients with coronary artery disease: Analyses from a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2016;36:436-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: A randomised controlled trial. Br J Nutr. 2008;99:421-431. [DOI] [PubMed] [Google Scholar]
- 31.Pawełczyk T, Piątkowska-Janko E, Bogorodzki P, et al. Omega-3 fatty acid supplementation may prevent loss of gray matter thickness in the left parieto-occipital cortex in first episode schizophrenia: A secondary outcome analysis of the OFFER randomized controlled study. Schizophr Res. 2018;195:168-175. [DOI] [PubMed] [Google Scholar]
- 32.Ciappolino V, DelVecchio G, Prunas C, et al. The Effect of DHA supplementation on cognition in patients with bipolar disorder: An exploratory randomized control trial. Nutrients. 2020;12:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assmann KE, Adjibade M, Hercberg S, Galan P, Kesse-Guyot E. Unsaturated fatty acid intakes during midlife are positively associated with later cognitive function in older adults with modulating effects of antioxidant supplementation. J Nutr. 2018;148:1938-1945. [DOI] [PubMed] [Google Scholar]
- 34.McNamara RK, Kalt W, Shidler MD, et al. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol Aging. 2018;64:147-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otsuka R, Tange C, Nishita Y, et al. Serum docosahexaenoic and eicosapentaenoic acid and risk of cognitive decline over 10 years among elderly Japanese. Eur J Clin Nutr. 2014;68:503-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koagai C, Yanagimoto K, Hayamizu K, Han L, Tsuji T, Koga Y. Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: A randomized controlled trial in healthy elderly volunteers. Clin Interv Aging. 2013;8:1247-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooper C, de Souto Barreto P, Coley N, et al. Cognitive changes with omega-3 polyunsaturated fatty acids in non-demented older adults with low omega-3 index. J Nutr Health Aging. 2017;21:988-993. [DOI] [PubMed] [Google Scholar]
- 38.Jackson PA, Forster JS, Bell JG, Dick JR, Younger I, Kennedy DO. DHA supplementation alone or in combination with other nutrients does not modulate cerebral Hemodynamics or cognitive function in healthy older adults. Nutrients. 2016;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dangour AD, Allen E, Elbourne D, et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: A randomized, double-blind, controlled trial. Am J Clin Nutr. 2010;91:1725-1732. [DOI] [PubMed] [Google Scholar]
- 40.Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI Study. Neurology. 2014;82:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heude B, Ducimetière P, Berr C, EVA Study . Cognitive decline and fatty acid composition of erythrocyte membranes--The EVA Study. Am J Clin Nutr. 2003;77:803-808. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson A, Radeborg K, Salo I, Bjorck I. Effects of supplementation with n-3 polyunsaturated fatty acids on cognitive performance and cardiometabolic risk markers in healthy 51 to 72 years old subjects: A randomized controlled cross-over study. Nutr J. 2012;11:99-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017;16:377-389. [DOI] [PubMed] [Google Scholar]
- 44.Jackson JC, Mozaffarian D, Graves AJ, et al. Fish oil supplementation does not affect cognitive outcomes in cardiac surgery patients in the omega-3 fatty acids for prevention of Post-Operative Atrial Fibrillation (OPERA) Trial. J Nutr. 2018;148:472-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Handeland K, Øyen J, Skotheim S, et al. Fatty fish intake and attention performance in 14-15 year old adolescents: FINS-TEENS - a randomized controlled trial. Nutr J. 2017;16:64-110. [DOI] [PMC free article] [PubMed] [Google Scholar]