Abstract
The regenerative capacity of the central nervous system is very limited and few effective treatments are currently available for spinal cord injury. It is therefore a priority to develop new drugs that can promote structural and functional recovery after spinal cord injury. Previous studies have shown that peptides can promote substantial repair and regeneration of injured tissue. While amphibians have a pronounced ability to regenerate the spinal cord, few studies have investigated the effect of amphibian spinal cord-derived peptides on spinal cord injury. Here we report for the first time the successful identification and isolation of a new polypeptide, VD11 (amino acid sequence: VDELWPPWLPC), from the spinal cord of an endemic Chinese amphibian (Odorrana schmackeri). In vitro experiments showed that VD11 promoted the secretion of nerve growth factor and brain-derived neurotrophic factor in BV2 cells stimulated with lipopolysaccharide, as well as the proliferation and synaptic elongation of PC12 cells subjected to hypoxia. In vivo experiments showed that intravertebral injection of VD11 markedly promoted recovery of motor function in rats with spinal cord injury, alleviated pathological damage, and promoted axonal regeneration. Furthermore, RNA sequencing and western blotting showed that VD11 may affect spinal cord injury through activation of the AMPK and AKT signaling pathways. In summary, we discovered a novel amphibian-derived peptide that promotes structural and functional recovery after spinal cord injury.
Key Words: Akt signaling pathway, amphibian-derived bioactive peptide, AMPK signaling pathway, axonal regeneration, brain-derived neurotrophic factor, ischemia/reperfusion injury, motor function, nerve growth factor, neuroprotective effect, spinal cord injury
Introduction
Spinal cord injury (SCI) caused by spinal trauma is associated with high morbidity and mortality (Devivo, 2012). SCI usually leads to partial or complete paraplegia and bowel and bladder dysfunction, which not only cause considerable physical and psychological harm but also impose a serious economic burden on patients, families, and society (Kumar et al., 2018). Current drugs and therapies for SCI treatment are mostly unsatisfactory or show poor clinical efficacy. At present, the only drug approved by the US Food and Drug Administration for the clinical treatment of SCI is the corticosteroid methylprednisolone (MPED) (Tsutsumi et al., 2006), which can cause serious complications, such as osteoporosis, hyperglycemia, gastrointestinal ulcers, electrolyte disorders, respiratory system complications, and infections (Gorio et al., 2005). In addition, while novel technologies such as gene therapy and stem cell transplantation show promise in the treatment of SCI (Uchida et al., 2014; Curtis et al., 2018; Islamov et al., 2021; Fadeev et al., 2021; Forbes and Andrews, 2021), many technical challenges need to be resolved before these therapies can be used in the clinic (Yousefifard et al., 2016). Therefore, exploring safe, effective, and convenient drugs for the treatment of SCI is a research priority.
Due to their excellent stability, biological activity, specificity, and safety, small molecule peptide drugs are becoming an increasingly important focus in drug research and development. Small molecule peptides derived from amphibians, which are an excellent natural source of such peptides, display remarkable repair and regeneration activity in damaged tissues (Yang et al., 2012, 2016; Cao et al., 2018, 2019; Zhang et al., 2021). In addition, some amphibians exhibit robust regeneration of the spinal cord; for example, quadrupedal salamanders can regenerate their spinal cords throughout their lifespan, while toads and frogs exhibit spinal cord regeneration before metamorphosis (Freitas et al., 2019). Amphibians may therefore contain certain substances that promote nervous system regeneration (Yang et al., 2019). In the current study, we identified a new peptide (VD11; amino acid sequence: VDELWPPWLPC) derived from the spinal cord of the odorous frog Odorrana schmackeri (O. schmackeri) and investigated whether it promotes structural and functional recuperation post-SCI.
Methods
Complementary DNA synthesis
All experimental animals were handled in strict accordance with Chinese regulations for experimental animal use, and animal care and handling were carried out in accordance with the regulations and requirements of the Kunming Medical University Ethical Staff Association (approval No. KMMU2020378, approval date September 24, 2020).
Adult odorous frogs (O. schmackeri) were collected from the field and maintained in the animal housing facility for 3 days. The water was changed every day, and food (bread worms) was provided every other day. After 3 days, the frogs were anesthetized with tricaine methanesulfonate, washed with deionized water, and then sacrificed. The spinal cord was removed, and immediately ground in liquid nitrogen. RNAiso (TaKaRa Bio Incorporation, Kusatsu, Japan) was used to extract the total spinal cord RNA, and mRNA was purified using an mRNA Purification Kit (Stratagene, Palo Alto, CA, USA) according to the manufacturers’ protocols. First- and second-strand cDNA synthesis was carried out using the Switching Mechanism at 5′ end of the RNA Transcript (SMART) technique, according to a previous study (Zhang et al., 2021) and primers specific for cDNAs encoding frog spinal cord bioactive precursors (5′-primer (5′-CCA AA(G/C) ATG TTC ACC (T/A)TG AAG AAA-3′) and 3′-primer (5′-ATT CTA GAG GCC GAG GCG GCC GAC ATG-3′)). A DNA Gel Extraction Kit (Sigma, St Louis, MO, USA) was used to recover the polymerase chain reaction products, which were subsequently sent out for commercial Illumina MiSeq sequencing (Exxon Technology Co. Ltd., Beijing, China). In brief, the PCR products (50–100 ng) were amplified for three cycles with KAPA HiFi Hot Start Ready Mix (Kapa Biosystems, Beijing, China) using infusion primer F (AAT GAT ACG GCG ACC ACC GAG ATC TAC ACT CTT TCC CTA CAC GAC GCT CTT CCG ATC T-specific 5′-primer) and infusion primer R (CAA GCA GAA GAC GGC ATA CGA GAT CTC AGA GTG ACT GGA GTT CAG ACG TGT GCT CTT CCG ATC T-specific 3′-primer). Amplicons were purified using Agencourt AMPure XP beads (Beckman Coulter Diagnostics, Inc., Pasadena, CA, USA) and quantified with a Qubit 2.0 Fluorometer (Life Invitrogen, Inc., Carlsbad, CA, USA). Purified amplicons were pooled in equimolar concentrations and subjected to paired-end sequencing (2300) on an Illumina MiSeq platform using standard protocols. After removing the adaptor sequences from the raw files, Fast Length Adjustment of Short reads (v1.2.11; The Center for Computational Biology, Baltimore, MD, USA) was used to assemble paired-end reads with the following criteria: overlap between 10 and 300 bp and a maximum mismatch rate of 0.1. Fastq files were obtained and converted into fasta files, from which repeating sequences were removed using the Fastx-toolkit software (v0.0.6) (Gaston Day School, Gastonia, NC, USA) (Song et al., 2019).
Peptide synthesis
The mature VD11 peptide (amino acid sequence: VDELWPPWLPC; molecular weight: 1357 Da) was synthesized by Wuhan Bioyeargene Biotechnology Co., Ltd. (Wuhan, China).
Lipopolysaccharide-induced inflammation model
BV2 cells (1 × 106 per well, American Type Culture Collection, Manassas, VA, USA, Cat# CRL-2467, RRID: CVCL_XD67) were seeded in 6-well plates and allowed to adhere before testing. Lipopolysaccharide (LPS, Sigma) at 1 mg/mL was applied to the BV2 cells for 6 hours. The medium was then removed and replaced with serum-free Dulbecco’s modified Eagle medium/Nutrient Mixture F-12 (DMEM-F12, GIBCO, Thermo Fisher Scientific, Waltham, MA, USA) medium or serum-free DMEM-F12 medium containing VD11 (at concentrations of 1, 10, and 100 nM). Cells were collected 12 hours later for further experiments.
Oxygen-glucose deprivation/reoxygenation model
PC12 cells (American Type Culture Collection, Cat# CRL-1721, RRID: CVCL_0481), which differentiate into neuron-like cells when induced with nerve growth factor (NGF), were cultured in DMEM-F12 with 10% fetal bovine serum at 37°C with 5% CO2. The medium was replaced daily, and cells were passaged until 70–80% confluency was reached. The oxygen-glucose deprivation/reoxygenation (OGD/R) model was established as described in a previous study (Deng et al., 2020). Briefly, the PC12 cells (1 × 106 per well in 6-well plates) were incubated in serum-free and glucose-free medium in a three-gas incubator (Heal Force, Shanghai, China) at 37°C with 90% N2, 5% O2, and 5% CO2 for 4 hours. Then the medium was removed and replaced with DMEM-F12 with or without VD11, and the cells were placed in a regular incubator (5% CO2, 95% air) for further culture for 24 hours. The cells were divided into eight groups: control group (without treatment), three VD11 groups (treated with 1, 10, or 100 nM VD11), model group (OGD/R model), and three model + VD11 groups (subjected to OGD/R and treated with 1, 10, or 100 nM VD11). Three replicates were included for each group.
Quantitative polymerase chain reaction assay
A total RNA extraction kit (Tiangen Biotech, Beijing, China) was used to extract total RNA from BV2 cells pretreated with VD11 for 12 hours. The RNA purity and concentration were measured using an ultra-microspectrophotometer (Thermo Fisher Scientific). The cDNA was reverse transcribed using a SureScript kit (GeneCopoeia, Guangzhou, China) in a total volume of 20 μL at 25°C for 5 minutes, 42°C for 15 minutes, and 85°C for 5 minutes, and then held at 4°C. A BlazeTaq kit was used for polymerase chain reaction (PCR) amplification (GeneCopoeia, Guangzhou, China). β-Actin (5′-TCA TCA CTA TTG GCA ACG AGC-3′, 5′-AAC AGT CCG CCT AGA AGC AC-3′), NGF (5′-TCT ATA CTG GCC GCA GTG AG-3′, 5′-GGA CAT TGC TAT CTG TGT ACG G-3′), and BDNF (5′-CTC CTG GGT TCC TGA GCA TC-3′, 5′-TTC ACT CCC TGA GTC ACA GC-3′) primers were purchased from GeneCopoeia. A Life Technologies thermocycler (Thermo, Waltham, MA, USA) was used to perform the qPCR reaction. The 2–ΔΔCt method was used to calculate the mRNA expression of NGF and BDNF, which was normalized to β-actin (Yin et al., 2021).
Enzyme-linked immunosorbent assay
After trypsin digestion, the BV2 cells were centrifuged at 1000 × g, 25°C for 20 minutes, and the supernatant was removed for testing. A commercial enzyme-linked immunosorbent assay kit (Neobio Science, Shanghai, China) was used to detect NGF and BDNF concentrations in the supernatant following the manufacturer’s instructions.
Cell viability assays
PC12 cells were inoculated in 96-well plates and cultured overnight in culture medium (100 μL/well). The OGD/R model was then established. After adding the peptide (VD11 group) and incubating for 24 hours, the cells were cultured with 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (20 μL/well; Promega, Beijing, China) in an incubator (37°C, 5% CO2) for 4 hours. Absorbance was read using a microplate reader (Thermo Fisher Scientific) at 490 nm and normalized to the control group. Cell viability data are expressed as percentages.
Animals and surgery
Specific pathogen-free-grade 4-week-old female Sprague-Dawley rats (n = 60), weighing 200–230 g, were provided by the Laboratory Animal Center of Kunming Medical University (license No. SYXK (Dian) K2020-0006). Three rats per cage were housed in ventilated cages, and after SCI each rat was housed singly. The temperature was maintained at 20–24°C, the humidity was maintained at 40–70%, there was a 12/12-hour light/dark cycle, and ad libitum access to food diet and water was provided. All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020).
Rats were randomly separated into three groups (i.e., vehicle, MPED, and VD11), and anesthetized by intraperitoneal injection with pentobarbital sodium (10 mg/kg, Mread, Beijing, China). The spinal cord was surgically exposed, a T10 laminectomy was performed, and the spinal cord was entirely severed with ophthalmic scissors. Then, a subcutaneous catheter (Jiangxi Fenglin Medical Appliances Co., Fenglin, Jiangxi, China) was inserted beneath the spinal dura mater to allow further intraspinal injection. In detail, the skin was cut 3 to 4 cm above the injury site, and the catheter was inserted, passed subcutaneously to the injury site, and fixed when suturing the muscle layer so that the open end of the catheter terminates under the muscle layer (Kwiecien et al., 2019). Stratified sutures were then used to close the skin.
Before awakening from anesthesia, all rats were subcutaneously injected with 0.4 mL of ketoprofen (10 mg/mL, PKU HealthCare Corp., Ltd., Beijing, China) for analgesia and placed on a heating mat (Kwiecien et al., 2019). Penicillin (200,000 units/100 g, intramuscular injection twice a day) was given for 7 days after surgery to fight infection. Manually assisted urination was performed three times a day. Mortality was recorded on days 0, 3, 7, 14, 21, and 28.
Drug treatment
Rats in the vehicle, MPED, and VD11 groups were given an intraspinal injection of 0.9% normal saline (0.2 mL), MPED (30 mg/kg, Pfizer Inc, New York City, NY, USA), and peptide VD11 (0.1 mg/kg), respectively, twice a day for 7 days.
Evaluation of lower limb function
The Basso-Beattie-Bresnahan (BBB) motor score system (Vipin et al., 2016) was used to assess hind limb motor function in rats before and after injury. The rats were placed alone on an open flat surface, and their behaviors were recorded using a video camera. A score of 21 indicated the same motor function as normal uninjured rats, while a score of 0 indicated no hind limb movement. Assessment of behavioral scores was performed on days 0, 3, 7, 14, 21, and 28. All assessments were made by three independent, blinded observers.
Tissue preparation and histochemical analysis
Thirty days post-operation, rats were anesthetized with pentobarbital sodium (10 mg/kg) and subjected to cardiac perfusion with 0.9% normal saline, and their spines were removed and fixed in 4% paraformaldehyde overnight. The spinal cord was photographed, and the length of the scar was measured with a ruler before the cord was dehydrated in 10%, 20%, and 30% sucrose, successively. After embedding in frozen section embedding agent (Sakura, Tokyo, Japan), the spinal cords were cut into 8-μm-thick tissue sections with a cryostat (Leica, Witzler, Hesse, Germany) for hematoxylin and eosin (H&E), Nissl, and immunofluorescence staining. ImageJ (v1.8.0, National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012) was used to analyze tissue cavity area and neuron survival. For H&E staining, the spinal cord tissue sections were stained with hematoxylin (Biosharp Life Science, Hefei, Anhui, China), acidified with 1% hydrochloric acid, and stained with eosin (Biosharp Life Science). For Nissl staining, the tissue sections were stained with methyl violet solution and differentiated with differentiation solution (Biosharp Life Science). Finally, neutral balata fixation was performed. The tissue cavity was outlined and its area was calculated by ImageJ, with the cavity area in the vehicle group set to a value of 1. For immunofluorescence staining and labeling, the PC12 cells or spinal cord tissue sections were treated with a blocking buffer containing 5% goat serum and 0.3% Triton-X-100-phosphate-buffered saline at room temperature for 1 hour. The tissue sections were then incubated overnight at 4°C with a rabbit anti-Tau primary antibody (1:200, Genetex, Irvine, CA, USA, Cat# GTX130462, RRID: AB_2886280) to label neuronal axons and a mouse anti-glial fibrillary acidic protein (GFAP) primary antibody (1:200, Cell Signaling Technology, Boston, MA, USA, Cat#56522, RRID: AB_2314536) to label astrocytes. Then, the sections were incubated for 1 hour at room temperature away from light with secondary antibodies, including Cy3-goat anti-rabbit (1:200, Proteintech, Wuhan, Hubei, China, Cat# SA00009-2, RRID: AB_2890957) and fluorescein isothiocyanate (FITC)-goat anti-rat (1:200, Proteintech, Cat# SA00003-11, RRID: AB_2890989). The nuclei were stained and sealed with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Xiao et al., 2018). Then, sections were observed and photographed with a fluorescence microscope (Leica). The cavity area in the lesion site center was outlined, and its area was calculated by ImageJ, with the cavity area in the vehicle group set to 100%. PC12 cells were also labeled for Tau fluorescence. The Tau-positive area represented the axon growth, and the value in the control group was set to 100%. Three replicates were performed for each group, with each value representing mean cell number across three random visual fields.
Western blotting
Spinal cord tissues were harvested 30 days after SCI, and PC12 cells were collected after different treatments. Lysis solution radio-immunoprecipitation assay + phenyl methane sulfonyl fluoride (1000:1, Meilun Biotechnology, Dalian, China) was added to centrifuge tubes containing the tissue or cells, followed by centrifugation at 14,000 × g and 4°C for 15 minutes. The supernatant was removed, and protein quantification was performed using the bicinchoninic acid assay method (Olson and Markwell, 2007). Western blot analysis was used to determine protein concentration, as previously reported (Yin et al., 2021). The following antibodies were used: rabbit anti-phosphorylated 5′-monophosphate-activated protein kinase (P-AMPK, 1:2000, Cell Signaling Technology, Cat# 4186, RRID: AB_10897959), rabbit anti-5′-monophosphate-activated protein kinase (AMPK, 1:2000, Cell Signaling Technology, Cat# 4150, RRID: AB_1586882), rabbit anti-phosphorylated Akt (P-Akt, 1:2000, Cell Signaling Technology, Cat# 4060, RRID: AB_2225022), rabbit anti-AKT (1:2000, Proteintech, Cat#10176-2-AP, RRID: AB_2224574), rabbit anti-β-actin (1:2000, Proteintech, Cat# 81115-1-RR, RRID: AB_2750915), rabbit anti-BDNF (1:2000, Abcam, Cambridge, UK, Cat#ab108319, RRID: AB_2064312) rabbit anti-NGF (1:2000, Abcam, Cat# ab52918, RRID: AB_2493038), and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:2000, Proteintech, Cat#SA00001-2, RRID: AB_2722564). The membranes were incubated in primary antibody at 4°C overnight and in secondary antibody for 1 hour at 25°C. Bands were detected using an enhanced chemiluminescence kit (Biosharp Life Science) and a chemiluminescence imager (Bio-Rad Laboratories, Hercules, CA, USA), and quantified using ImageJ.
RNA sequencing
The spinal cords were weighed and incubated with RNA Later (1:100, Thermo Fisher Scientific) overnight at 4°C. Then, the spinal cords were pre-cooled at –80°C for 1–2 hours and sent out to Beijing Fruit Shell Biotechnology Co., Ltd. (China) on dry ice for RNA sequencing (RNA-seq) and analysis. After creating maps of the differentially expressed genes (DEGs), we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.genome.jp/kegg) pathway enrichment analysis of the DEGs using KOBAS (Bu et al., 2021).
Statistical analysis
An estimated sample size of nine rats per group would provide at least 80% power for detecting a 20% change in lesion volume at α = 0.05 (two-sided). A total of 60 rats were used in this study. Data are expressed as means ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA, www. graphpad.com). For behavioral tests, data was analyzed by two-way analysis of variance with the Bonferroni’s post hoc test. The remaining data were analyzed using one-way analysis of variance with the Bonferroni’s post hoc test. P < 0.05 was considered statistically significant.
Results
Discovery of VD11
We discovered a cDNA sequence encoding a peptide precursor in the spinal cord of O. schmackeri. This 58-amino acid residue precursor was encoded by a 221-bp mRNA, as illustrated in Figure 1A. An NCBI BLAST search found that the most similar sequence structure to this precursor was “Nigrocin-2N” (Figure 1B). The mature sequence of the precursor was VDELWPPWLPC. The peptide was named VD11 based on its amino acid sequence (VD: initial two amino acids, 11: peptide length). Unlike Nigrocin-2N, which exhibits a cyclic motif, VD11 featured a linear motif and showed no evident sequence similarity to any other peptide. Thus, VD11 was considered unique. We discovered that VD11 greatly enhanced the transcription of BDNF and NGF mRNA using qPCR screening (Figure 2A and B). As a result, we concluded that VD11 may have neuroprotective action following nerve damage, and we conducted additional experimental verification. There are currently no reports of Nigrocin-2N with pro-regenerative activity, demonstrating that VD11 is distinct from Nigrocin-2N.
Figure 1.
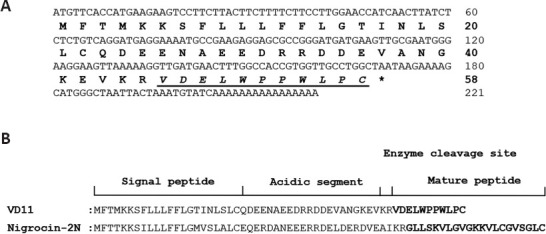
VD11 cDNA sequence.
(A) VD11 cDNA sequence. The VD11 precursor contains 58 amino acid residues encoded by a 221-bp mRNA, and the mature sequence is VDELWPPWLPC (underlined italics). (B) Sequence alignment of VD11 and Nigrocin-2N showed similar precursor sequences but no apparent similarity between the mature peptides. cDNA: Complementary DNA.
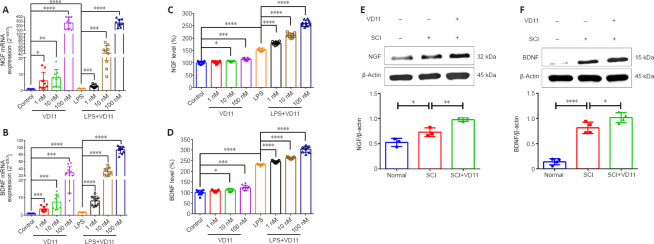
Figure 2.
VD11 increases NGF and BDNF secretion in vitro and in vivo.
(A, B) VD11 significantly increased NGF (A) and BDNF (B) mRNA expression in BV2 cells, as detected by qPCR. NGF and BDNF expression levels were normalized to β-actin. (C, D) VD11 promoted NGF and BDNF secretion in a dose-dependent manner, as determined by enzyme-linked immunosorbent assay. Data were normalized to the control group. (E, F) Western blot assay showed that VD11 promoted BDNF (n = 4) and NGF (n = 3) secretion after SCI in rats. Data are presented as mean ± SD from three independent experiments performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way analysis of variance with the Bonferroni’s post hoc test). BDNF: Brain-derived neurotrophic factor; NGF: nerve growth factor; SCI: spinal cord injury.
VD11 promotes NGF and BDNF secretion in vitro and in vivo
The immortalized BV2 cell line retains many of the morphological, phenotypic, and functional properties of microglia. NGF and BDNF are essential for neuronal survival, morphogenesis, and plasticity (Li et al., 2018). Therefore, qPCR was used to measure the amount of NGF and BDNF secreted by LPS-stimulated and peptide-treated BV2 cells.
VD11 induced a significant and dose-dependent increase in NGF and BDNF mRNA and protein expression in normal and LPS-stimulated BV2 cells. As shown in Figure 2A–D, these changes were most pronounced at a VD11 concentration of 100 nM, so this concentration was used for subsequent experiments. Treatment with 100 nM VD11 increased NGF and BDNF mRNA and protein expression in BV2 cells compared with untreated cells, and the LPS + VD11 group exhibited greater NGF and BDNF mRNA and protein expression than the LPS group. These findings indicate that NGF and BDNF secretion increase after cell injury.
To test the positive effect of VD11 on BDNF and NGF expression in vivo, spinal cord tissues were obtained from normal rats and SCI rats (rats after SCI for 30 days) and subjected to WB experiments. As shown in Figure 2E and F, compared with the normal group, NGF and BDNF expression were both increased in the SCI group. After VD11 administration, NGF and BDNF expression were even higher than SCI group. These results indicate that VD11 can significantly increase NGF and BDNF levels in vivo.
Taken together, these findings indicate that VD11 promotes NGF and BDNF secretion in vitro and in vivo.
VD11 promotes PC12 cell proliferation, axon outgrowth, and survival in the context of OGD/R
PC12 cells stimulated with physiological NGF are commonly employed in in vitro studies of neurological illnesses (Wiatrak et al., 2020). To investigate the effects of VD11 on PC12 cells subjected to OGD/R, PC12 cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay. Compared with the control group, cell viability was higher in the 1, 10, and 100 nM VD11 groups, respectively. Cell viability was lower in the OGD/R group compared with the control group, indicating that the model was successfully established. Compared with the OGD/R group, cell viability increased after treatment with 1, 10, and 100 nM VD11, respectively (Figure 3A). In addition, after OGD/R, PC12 cells showed a reduction in the number of cells and axon growth compared with the control group (Figure 3B), whereas treatment with VD11 rescued these effects.
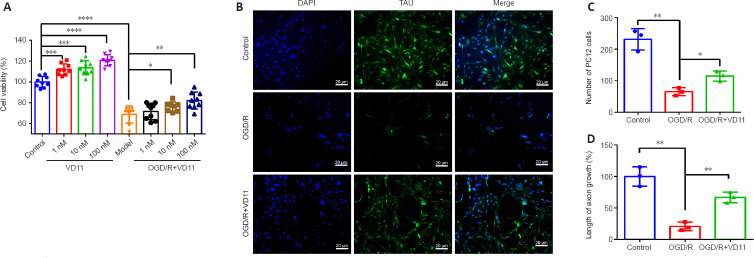
Figure 3.
VD11 promotes PC12 cell proliferation and axon extension.
(A) Effect of VD11 on PC12 cell viability, normalized to the control group (n = 9). (B) Representative immunofluorescence images of the effects on OGD/R and VD11 on the cell survival and axon growth (detected by Tau expression, green-fluorescein isothiocyanate) in PC12 cells. Axon length decreased after OGD/R and increased after VD11 treatment. DAPI indicates nuclei (blue). Scale bars: 20 μm. (C, D) VD11 (100 nM) alleviated the decrease in cell number (C) and increased axon length (D) in PC12 cells. Three replicates were performed for each group, with each value representing mean cell number in three random visual fields. Data are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way analysis of variance with the Bonferroni’s post hoc test). DAPI: 4′,6-Diamidino-2-phenylindole; OGD/R: oxygen glucose deprivation/reoxygenation.
The immunofluorescence staining results showed that, compared with normal cultured cells (the control group), both the number of cells and the axon length at 48 hours after OGD/R were markedly reduced (n = 3, P < 0.01 and n = 3, P < 0.01, respectively). However, compared with the OGD/R model group, the number of cells in the OGD/R + VD11 (100 nM) group was increased after 48 hours (n = 3, P < 0.05), and the axon length was increased as well (n = 3, P < 0.01). The increase in PC12 cell axon length after VD11 treatment may indicate that VD11 has a neuroprotective effect in the context of OGD/R (Figure 3B–D).
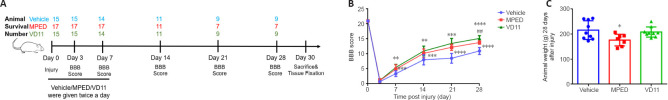
VD11 promotes hind limb functional recovery in rats with SCI
To study the effect of VD11 on hind limb functional recovery in rats after SCI, we performed T10 total spinal cord transection in rats (Figure 4A) and measured motor functional recovery for 4 weeks after surgery based using the BBB scale (Figure 4B). All rats had normal hind limb function before surgery (21.00 ± 0.00) and exhibited hind limb paralysis after surgery. The lowest hind limb function scores were recorded 3 days after operation. On day 28 after SCI, the score in the VD11 group increased to 15.15 ± 0.31 (n = 9, P < 0.0001), which was significantly higher than that in the vehicle (10.96 ± 0.39, n = 9, P < 0.001) and MPED groups (13.79 ± 0.22, n = 7, P < 0.01). These results indicate that VD11 significantly promoted hind limb functional recovery, and to a greater degree than MPED. In addition, 28-day mortality in the VD11 group (40.0%) was lower than that in the MPED group (64.7%), but similar to that in the vehicle group (40.0%) (Figure 4A). There was no significant difference in body weight between the VD11 and vehicle groups 28 days after injury (Figure 4C), whereas body weight was significantly decreased (P < 0.05) in the MPED group (n = 9), indicating that VD11 was safer and less toxic in rats compared with MPED.
Figure 4.
VD11 promotes hind limb functional recovery in rats after SCI, as determined by motor function assessment.
(A) Experimental design and timeline. (B) Changes in BBB locomotor score. Data represent means of scores from three independent observers. (C) Animal weight 28 days after injury. Data are presented as mean ± SD (n = 9). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, vs. vehicle group; ##P < 0.01, vs. MPEP group (two-way (B) or one-way (C) analysis of variance with the Bonferroni’s post hoc test). BBB: Basso-Beattie-Bresnahan; MPED: methylprednisolone; SCI: spinal cord injury.
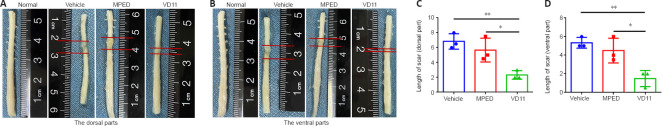
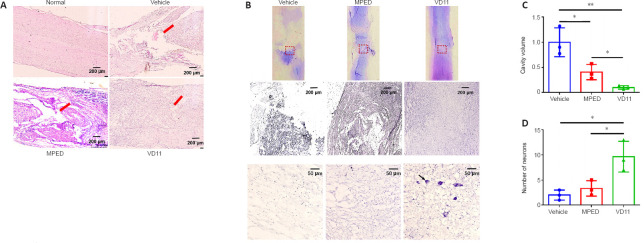
VD11 accelerates nervous tissue repair in rats with SCI
To study the effect of VD11 on tissue repair in rats, spinal cords were analyzed 30 days after SCI. The presence of both ventral and dorsal scars on the spinal cord indicated successful transection (Figure 5A and B). Compared with the vehicle and MPED groups, the ventral and dorsal scar lengths in the VD11 group were much shorter (n = 3, P < 0.01 and n = 3, P < 0.05, respectively; Figure 5C and D). Thus, rats treated with VD11 exhibited less scar formation and improved spinal cord healing compared with rats treated with normal saline or MPED. H&E staining and Nissl staining also showed that VD11 reduced the area of the tissue defect (Figure 6A and B). Based on analysis of H&E-stained spinal cord tissue, the tissue cavity area was markedly decreased in the VD11 group compared with the vehicle (n = 3, P < 0.01) and MPED groups (n = 3, P < 0.05; Figure 6C). Nissl staining showed that the number of surviving neurons was significantly higher in the VD11 group (P < 0.01 and P < 0.05, respectively; Figure 6D) than in the vehicle and MPED groups. Thus, VD11 exhibited a strong neuroprotective effect post-SCI.
Figure 5.
VD11 reduces scar lengths in the spinal cord of rats after SCI.
(A, B) Representative dorsal (A) and ventral (B) images of the spinal cord 30 days after SCI. Rats treated with MPED showed a reduction in spinal cord scarring compared with vehicle group, while rats treated with VD11 showed a more substantial reduction in scarring. Scar edge is denoted by red lines. (C, D) Spinal cord scar lengths (mm) in rats 30 days after surgery. Data are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01 (one-way analysis of variance with the Bonferroni’s post hoc test). MPED: Methylprednisolone; SCI: spinal cord injury.
Figure 6.
VD11 promotes tissue structure repair in SCI rats, as determined by H&E and Nissl staining.
(A, B) Representative images of H&E (A) and Nissl (B) staining of spinal cord tissue from rats 30 days after SCI. The images in the second row of (B) are magnifications of the red boxes in the images in the first row. The images in the third row are high-magnification representative images of neurons with a diameter of 5 mm at the injury center. Some residual neurons could be seen in the VD11 group, but almost no neurons could be seen in the control and MPED groups. Red arrows indicate tissue cavities, and black arrows indicate neurons. (C, D) Statistical analysis of the cavity area in the spinal cord tissue stained with H&E (C) and the number of neurons stained with Nissl (D) in rats 30 days after surgery. Three replicates were performed for each group, with each value representing mean neuron number in three random visual fields. Data are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01 (one-way analysis of variance with the Bonferroni’s post hoc test). H&E: Hematoxylin and eosin; MPED: methylprednisolone; SCI: spinal cord injury.
VD11 alleviates nerve injury and promotes nerve regeneration in rats with SCI
To assess nerve tissue damage and regeneration at the SCI sites in rats, GFAP and Tau were used for immunofluorescence labeling of astrocytes (Sullivan, 2014) and neuronal axons (Yu and Rasenick, 2006), respectively. As shown in Figure 7A, typical GFAP-positive astrocytes and Tau-positive neuronal axons were observed in the VD11 group within the lesion site 30 days after SCI, indicating alleviation of nerve injury and/or the occurrence of astrocyte regeneration and axon extension, and these effects were much more pronounced than in the vehicle and MPED groups. In particular, in the center of the lesion site there were large cavities, and almost no GFAP+ astrocytes and Tau+ axons were observed, in the vehicle and MPED groups. The GFAP+ content of the cavity area was significantly lower in the VD11 group (23.79 ± 8.90%) than in the vehicle (100.00 ± 12.82%, n = 3, P < 0.01) and MPED groups (47.16 ± 10.57, n = 3, P < 0.05). In addition, the Tau+ content of the cavity area was significantly lower in the VD11 group (11.38 ± 4.82%) than in the vehicle (100.00 ± 22.39%, n = 3, P < 0.01) and MPED groups (49.99 ± 10.25%, n = 3, P < 0.01) (Figure 7B and C). These results indicate that VD11 alleviated nerve injury and promoted nerve regeneration after SCI.
Figure 7.
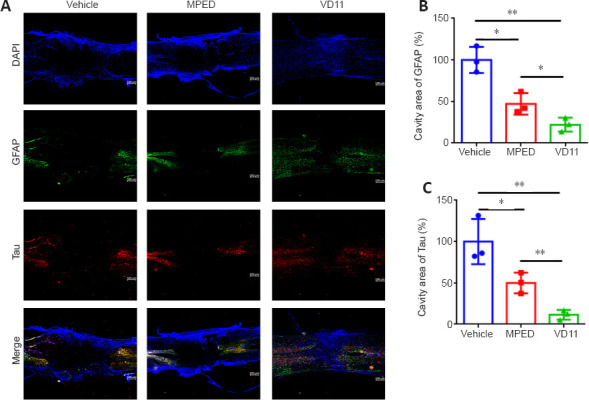
VD11 alleviates nerve injury and promotes nerve regeneration in SCI rats, as determined by immunofluorescence staining.
(A) Representative immunofluorescence staining images of reactive astrocytes (marked with GFAP, green-fluorescein isothiocyanate) and neuronal axons (marked with Tau, red-Cy3) in the spinal cord 30 days after SCI. DAPI indicates nuclei (blue). Scale bars: 500 µm. (B, C) Statistical analysis of the GFAP+ (B) and Tau+ (C) areas. The area in the vehicle group was defined as 100%. Data are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01 (one-way analysis of variance with the Bonferroni’s post hoc test). DAPI: 4′,6-Diamidino-2-phenylindole; GFAP: glial fibrillary acidic protein; MPED: methylprednisolone; SCI: spinal cord injury.
Mechanism underlying the neuroprotective effects of VD11
Total RNA from spinal cord tissues of rats in the normal, model, and model + VD11 groups was sequenced, followed by transcriptome analysis to examine the mechanism underlying the effects of VD11 on axon extension after SCI. Maps of DEGs were created for the three different groups, as illustrated in Figure 8A. We performed KEGG pathway enrichment analysis of the DEGs and sorted them in terms of importance (Figure 8B). DEGs were enriched in the AMPK and AKT signaling pathways and other nerve injury-related pathways. The effects of VD11 on the AMPK and AKT signaling pathways were confirmed by western blotting. As shown in Figure 8C and D, p-AMPK and p-AKT levels were considerably lower in the OGD/R group than the control group, whereas treatment with VD11 treatment increased the levels of these two phosphorylated proteins. Thus, the neuroprotective effects of VD11 may be mediated by activation of the AMPK and AKT signaling pathways.
Figure 8.
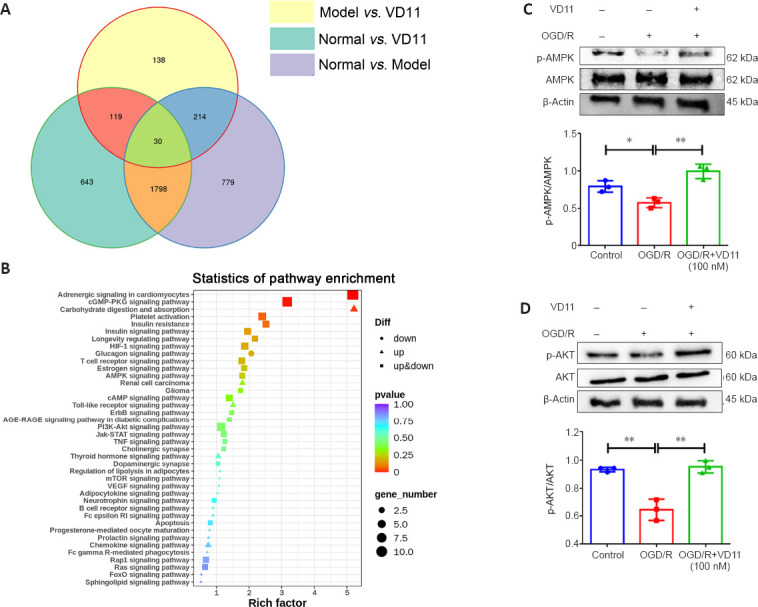
Mechanism underlying the effects of VD11 on neurological recovery.
(A) DEGs as determined by RNA-seq analysis. (B) KEGG pathways enriched in DEGs based on RNA-seq analysis, arranged from top to bottom in terms of significance and presented as a bubble graph. (C, D) VD11 administration resulted in decreased AMPK (C) and AKT (D) phosphorylation compared with the OGD/R group. The value for the control group was set to 100%. Data are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01 (one-way analysis of variance with the Bonferroni’s post hoc test). AMPK: 5′-Monophosphate-activated protein kinase; DEG: differentially expressed gene; KEGG: Kyoto Encyclopedia of Genes and Genomes; OGD/R: oxygen-glucose deprivation/reoxygenation; P-Akt: phosphorylated Akt; P-AMPK: phosphorylated 5′-monophosphate-activated protein kinase; RNA-seq: RNA sequencing.
Discussion
Viable treatments for SCI in humans remain scarce due to the relatively restricted spontaneous regeneration capacity of the central nervous system (CNS) (Tator, 2006). In this study, we identified and cloned a new peptide (VD11) expressed in the spinal cord of O. schmackeri, and showed that it promoted PC12 cell proliferation and axon extension, improved hind limb motor function in rats after SCI, and reduced tissue damage at the SCI site. These findings demonstrate that VD11 has a neuroprotective effect and may potentially play a role in axonal regeneration. We then went on to explore the mechanism underlying these effects.
Various amphibian-derived peptides show antioxidant, antimicrobial, and pro-repair properties (Liu et al., 2021; Zhang et al., 2021). Furthermore, peptide-loaded nanomaterials can substantially improve the tissue regeneration and repair activity of amphibian-derived peptides (Qin et al., 2021; Sun et al., 2021). However, although the amphibian spinal cord shows a pronounced capacity for regeneration, few studies have explored the effects of amphibian-derived peptides on SCI. Notably, VD11 is the first peptide obtained from an amphibian spinal cord reported to promote SCI repair. We previously showed that OM-LV20, a peptide derived from frog skin, can promote SCI healing and confers neuroprotective effects (Li et al., 2018; Yin et al., 2022; Zhao et al., 2022). Compared with OM-LV20 (1967.5 Da, 20 amino acid), however, VD11(1357 Da, 11 amino acid) has a lower molecular weight and a shorter sequence, and is thus easier and more affordable to synthesize. In addition, VD11 treatment demonstrated superior performance to OM-LV20 in terms of decreasing scar size (reducing glial scar area more than three-fold), surviving more neurons and increasing axon length and astrocyte numbers post-SCI. This indicates that VD11 has a more potent neuroprotective effect after SCI than OM-LV20.
To determine whether VD11 is neuroprotective, we first examined NGF and BDNF mRNA and protein expression. Both NGF and BDNF play key roles in axonal regeneration (Li et al., 2020). Using modern axon-tracking techniques, studies have shown that CNS axons can regrow to the distal end, although this regrowth depends on neurotrophic factors produced by distal cells (Hagg et al., 1991). A lack of NGF, BDNF, and other nerve growth factors may cause spontaneous axonal regeneration failure after CNS injury (Alto et al., 2009). In our study, we found that VD11 may play a neuroprotective role after SCI by stimulating BDNF and NGF secretion.
To verify the neuroprotective effect of VD11 in vitro, an OGD/R model was used to replicate the ischemia/reperfusion injury induced by SCI. SCI pathophysiology is categorized into primary and secondary injury (McDonald and Sadowsky, 2002). Acute primary damage immediately destroys neurons, axons, blood vessels, and glia (Choo et al., 2007). Secondary damage includes injury-related vascular injury, inflammation, hypoxia, and oxidative stress, which can lead to delayed and progressive neurodegeneration, axonal degeneration, demyelination, and cystic cavity formation at the lesion site (Yong et al., 2001; Moon et al., 2012). These factors can influence the repair process and extent of long-term neurological impairment (Norenberg et al., 2004). As a result, secondary damage is traditionally regarded as a significant target in SCI therapy for neuroprotection and functional preservation (Hilton et al., 2017; Wang et al., 2019). Our results showed that VD11 promoted PC12 cell proliferation and axon prolongation after hypoxic injury.
A rat model of complete spinal cord transection was established, using MPED as a positive control, to determine the neuroprotective effect of VD11 in vivo. MPED has a long history of clinical usage in the treatment of acute SCI because it can improve the prognosis of patients with SCI by inhibiting the immune system, lowering inflammation, and decreasing the frequency of secondary infection (Zhang et al., 2013). However, systemic use of MPED in excessive dosages might create serious problems. Thus, the use of MPED is currently contentious, and several animal trials have raised doubt regarding its neuroprotective impact (Rabchevsky et al., 2002; Akhtar et al., 2009). Recent studies have shown that the use of topical MPED or MPED conveyed by specific carriers can greatly enhance motor function after SCI by reducing glucocorticoid side effects (Baltin et al., 2021; Suetsugu et al., 2021). This is also why MPED is still used as a positive control drug in some SCI experiments (Karabey-Akyurek et al., 2017). In our study we administered a low dose of topical MPED to the spinal canal in the positive control group, and the findings demonstrated that this treatment enhanced motor function in rats after SCI. At 28 days after SCI, the BBB exercise scores were significantly higher in the VD11 group than in the vehicle and MPED groups, and mortality and weight loss were lower in the VD11 group than in the MPED group. Furthermore, compared with MPED, VD11 had a greater positive effect on motor function recovery after SCI, with fewer toxic side effects. Subsequent histological examination also showed that the VD11 group had less scarring and cavity formation, as observed both visually and microscopically. Moreover, the MPED dose used was 300 times greater than that of VD11, indicating that VD11 possesses superior activity at low doses, and thus has higher efficiency. Furthermore, as compared to the other two groups, rats of MPED group showed more limited hip mobility and considerable lower limb muscle atrophy, which might be attributed to glucocorticoid-induced femoral head necrosis. We assumed that this is also why MPED is less effective in other animal experiments. In addition, female rats were used in this study instead of male rats, since most rat SCI models use female rats due to male rats having a longer and curved urethra that is more prone to urine retention, which leads to greater mortality (He et al., 2019).
To further determine whether VD11 is beneficial to nerve tissue survival or regeneration after injury, immunofluorescence analysis was performed on rat spinal cord tissues after SCI. The results showed that astrocyte and axon regeneration was significantly increased in the VD11 group compared with the control and MPED groups. Axonal regeneration is necessary for motor function recovery after SCI. However, when neurons mature, they lose their ability to generate axons (Goldberg et al., 2002), and axonal growth following injury is typically minimal (He and Jin, 2016). Damage to the central nervous system initiates reactive gliosis (Norton et al., 1992), which is characterized by the presence of hypertrophic astrocytes, also known as reactive astrocytes. Scarring astrocytes have long been thought to be the main cause of axonal regeneration failure in the CNS (Silver and Miller, 2004), but this view has since been questioned (Wu et al., 2015). For example, one study showed that, when reactive astrocytes were selectively removed after spinal cord crush injury in rats, both axonal degeneration and demyelination increased, and motor function recovery decreased. This suggests that the role of glial scarring is not to inhibit axonal growth, but rather to hide or sequester the damage caused by injury and to promote tissue repair (Faulkner et al., 2004). Another study showed that mature CNS axons regenerate along astrocytes after injury when stimulated with appropriate growth factors or in response to gene activation (Kawaja and Gage, 1991). Similarly, axons grow along astrocytes during development (Brosius Lutz and Barres, 2014). Spontaneous axon regeneration occurs along astroglial bridges formed by connective tissue growth factors (Mokalled et al., 2016) after SCI in lower vertebrates. Therefore, astrocyte scarring may help rather than hinder axonal regeneration (Anderson et al., 2016). In this study, immunofluorescence analysis showed a significant increase in astrocyte and axonal regeneration in the VD11 group compared with the control and MPED groups. This suggests that VD11 can alleviate nerve damage and promote axonal regeneration after SCI. Studies have shown that hypertrophic astrocytes bind to surviving neurons in active tissues and may affect axonal bud development by controlling the expression of peripheral and related neural factors (Burda and Sofroniew, 2014). Therefore, the ability of VD11 to promote axonal regeneration may be related to its ability to promote astrocyte regeneration. In addition, more cells and deeper immunofluorescence staining were observed at the tissue edges in the control and MPED groups, indicating thicker scars in the control and MPED groups compared with the VD11 group.
Finally, we explored the possible mechanism of VD11-mediated nerve regeneration. Activation of the AMPK and AKT signaling pathways, which are related to neurological recovery (Chen et al., 2017; Hu et al., 2021), was examined by RNA-seq. AMPK is a serine/threonine kinase that functions as an energy sensor in cells (Tian et al., 2019). The AMPK signaling pathway is linked to energy supply and oxidative stress (de Oliveira et al., 2021), both of which can significantly disrupt metabolic processes.
Ischemia/reperfusion injury following SCI can result in a significant increase in reactive oxygen species (Wang et al., 2020). AMPK phosphorylation plays an active part in axonal repair following spinal cord damage (Hu et al., 2021). Compared to the OGD/R group, p-AMPK was increased in the VD11 group. AKT plays a vital function in neuronal preservation (Chen et al., 2017). Here, we found that treatment with VD11 activated the AKT signaling pathway. Thus, the neuroprotective effects of VD11 may be mediated by activation of the AMPK and AKT pathways, which then protects neurons from OGD/R damage.
The experimental design of this study had some limitations. For example, the structure of VD11 was not optimized, and the cellular receptors that VD11 binds to were not investigated. In a future study, we plan to perform a detailed exploration of the mechanism of VD11 action.
In conclusion, we identified a new peptide (VD11; amino acid sequence: VDELWPPWLPC) derived from the spinal cord of the odorous frog O. schmackeri. Analysis showed that VD11 promoted NGF and BDNF secretion in microglia and increased PC12 cell proliferation and axon prolongation after hypoxic injury. In rats, intraspinal application of VD11 significantly improved hind limb motor function and reduced tissue damage following complete transection of the spinal cord. This demonstrates that VD11 has a neuroprotective effect and may potentially play a role in axonal regeneration. The underlying mechanism of VD11 activity was explored by RNA-seq, which revealed that the adenosine AMPK and AKT pathways were significantly enriched in various DEGs. Western blotting confirmed that VD11 activated these two signaling pathways. Thus, the neuroprotective effects of VD11 after SCI may be related to activation of the AMPK and Akt signaling pathways. This study identified a novel amphibian-derived peptide that accelerated structural and functional repair post-SCI, indicating that it may be a potential candidate for clinical SCI treatment. VD11 is the first peptide derived from the amphibian spinal cord that has been reported to promote SCI repair. Furthermore, this study is the first to investigate the link between amphibian-derived peptides and axonal regeneration, thereby laying the groundwork for future drug development.
Open peer reviewers: Andrew M Michael Hamilton, University of California Davis, USA; Seth Herr, Purdue University, USA.
Additional file: Open peer review reports 1 (111.6KB, pdf) and 2 (96.4KB, pdf) .
Footnotes
Author contributions: Study conceptualization: SSL, YW, XWY; main experiment implementation: SSL, SYW, LJW, YHS, JZ, SGY, ZQW; data curation: BYZ; data analysis: YZ; data validation: NXL, YLL; manuscript draft: SSL, BYZ; manuscript revision and project administration: JS, YW, XWY; funding acquisition: BYZ, JS, YW, XWY; supervision: YW, XWY. All authors have read and approved the final version of the manuscript.
Conflicts of interest: The authors declare there are no conflicts of interest.
Data availability statement: The data are available from the corresponding author on reasonable request.
P-Reviewers: Hamilton AMH, Herr S; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Crow E, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Akhtar AZ, Pippin JJ, Sandusky CB. Animal studies in spinal cord injury: a systematic review of methylprednisolone. Altern Lab Anim. 2009;37:43–62. doi: 10.1177/026119290903700108. [DOI] [PubMed] [Google Scholar]
- 2.Alto LT, Havton LA, Conner JM, Hollis ER, 2nd, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltin ME, Sabirova DE, Kiseleva EI, Kamalov MI, Abdullin TI, Petrova NV, Ahmetov NF, Sachenkov OA, Baltina TV, Lavrov IA. Comparison of systemic and localized carrier-mediated delivery of methylprednisolone succinate for treatment of acute spinal cord injury. Exp Brain Res. 2021;239:627–638. doi: 10.1007/s00221-020-05974-w. [DOI] [PubMed] [Google Scholar]
- 5.Brosius Lutz A, Barres BA. Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev Cell. 2014;28:7–17. doi: 10.1016/j.devcel.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Bu D, Luo H, Huo P, Wang Z, Zhang S, He Z, Wu Y, Zhao L, Liu J, Guo J, Fang S, Cao W, Yi L, Zhao Y, Kong L. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49:W317–W325. doi: 10.1093/nar/gkab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao X, Tang J, Fu Z, Feng Z, Wang S, Yang M, Wu C, Wang Y, Yang X. Identification and characterization of a novel gene-encoded antioxidant peptide from odorous frog skin. Protein Pept Lett. 2019;26:160–169. doi: 10.2174/0929866525666181114153136. [DOI] [PubMed] [Google Scholar]
- 9.Cao X, Wang Y, Wu C, Li X, Fu Z, Yang M, Bian W, Wang S, Song Y, Tang J, Yang X. Cathelicidin-OA1, a novel antioxidant peptide identified from an amphibian, accelerates skin wound healing. Sci Rep. 2018;8:943. doi: 10.1038/s41598-018-19486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Wang Z, Zheng Z, Chen Y, Khor S, Shi K, He Z, Wang Q, Zhao Y, Zhang H, Li X, Li J, Yin J, Wang X, Xiao J. Neuron and microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent neuroinflammation to improve functional recovery after spinal cord injury. Cell Death Dis. 2017;8:e3090. doi: 10.1038/cddis.2017.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choo AM, Liu J, Lam CK, Dvorak M, Tetzlaff W, Oxland TR. Contusion, dislocation, and distraction: primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J Neurosurg Spine. 2007;6:255–266. doi: 10.3171/spi.2007.6.3.255. [DOI] [PubMed] [Google Scholar]
- 12.Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, Van Gorp S, Leerink M, Tadokoro T, Marsala S, Jamieson C, Marsala M, Ciacci JD. A first-in-human, phase i study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–950.e6. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira MR, de Souza ICC, Brasil FB. Promotion of mitochondrial protection by emodin in methylglyoxal-treated human neuroblastoma SH-SY5Y cells: involvement of the AMPK/Nrf2/HO-1 axis. Neurotox Res. 2021;39:292–304. doi: 10.1007/s12640-020-00287-w. [DOI] [PubMed] [Google Scholar]
- 14.Deng Z, Ou H, Ren F, Guan Y, Huan Y, Cai H, Sun B. LncRNA SNHG14 promotes OGD/R-induced neuron injury by inducing excessive mitophagy via miR-182-5p/BINP3 axis in HT22 mouse hippocampal neuronal cells. Biol Res. 2020;53:38. doi: 10.1186/s40659-020-00304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 16.Fadeev FO, Bashirov FV, Markosyan VA, Izmailov AA, Povysheva TV, Sokolov ME, Kuznetsov MS, Eremeev AA, Salafutdinov II, Rizvanov AA, Lee HJ, Islamov RR. Combination of epidural electrical stimulation with ex vivo triple gene therapy for spinal cord injury: a proof of principle study. Neural Regen Res. 2021;16:550–560. doi: 10.4103/1673-5374.293150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes LH, Andrews MR. Advances in human stem cell therapies: pre-clinical studies and the outlook for central nervous system regeneration. Neural Regen Res. 2021;16:614–617. doi: 10.4103/1673-5374.295287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freitas PD, Yandulskaya AS, Monaghan JR. Spinal cord regeneration in amphibians: a historical perspective. Dev Neurobiol. 2019;79:437–452. doi: 10.1002/dneu.22669. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 21.Gorio A, Madaschi L, Di Stefano B, Carelli S, Di Giulio AM, De Biasi S, Coleman T, Cerami A, Brines M. Methylprednisolone neutralizes the beneficial effects of erythropoietin in experimental spinal cord injury. Proc Natl Acad Sci U S A. 2005;102:16379–16384. doi: 10.1073/pnas.0508479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagg T, Gulati AK, Behzadian MA, Vahlsing HL, Varon S, Manthorpe M. Nerve growth factor promotes CNS cholinergic axonal regeneration into acellular peripheral nerve grafts. Exp Neurol. 1991;112:79–88. doi: 10.1016/0014-4886(91)90116-t. [DOI] [PubMed] [Google Scholar]
- 23.He Z, Jin Y. Intrinsic control of axon regeneration. Neuron. 2016;90:437–451. doi: 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 24.He Z, Zang H, Zhu L, Huang K, Yi T, Zhang S, Cheng S. An anti-inflammatory peptide and brain-derived neurotrophic factor-modified hyaluronan-methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury. Int J Nanomedicine. 2019;14:721–732. doi: 10.2147/IJN.S187854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilton BJ, Moulson AJ, Tetzlaff W. Neuroprotection and secondary damage following spinal cord injury: concepts and methods. Neurosci Lett. 2017;652:3–10. doi: 10.1016/j.neulet.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Xia N, Lin J, Li D, Zhang C, Ge M, Tian H, Mei X. Zinc regulates glucose metabolism of the spinal cord and neurons and promotes functional recovery after spinal cord injury through the AMPK signaling pathway. Oxid Med Cell Longev. 2021;2021:4331625. doi: 10.1155/2021/4331625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Islamov RR, Bashirov FV, Sokolov ME, Izmailov AA, Fadeev FO, Markosyan VA, Davleeva MA, Zubkova OV, Smarov MM, Logunov DY, Naroditskyi BS, Salafutdinov II, Rizvanov AA, Turaev RG. Gene-modified leucoconcentrate for personalized ex vivo gene therapy in a mini pig model of moderate spinal cord injury. Neural Regen Res. 2021;16:357–361. doi: 10.4103/1673-5374.290902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karabey-Akyurek Y, Gurcay AG, Gurcan O, Turkoglu OF, Yabanoglu-Ciftci S, Eroglu H, Sargon MF, Bilensoy E, Oner L. Localized delivery of methylprednisolone sodium succinate with polymeric nanoparticles in experimental injured spinal cord model. Pharm Dev Technol. 2017;22:972–981. doi: 10.3109/10837450.2016.1143002. [DOI] [PubMed] [Google Scholar]
- 29.Kawaja MD, Gage FH. Reactive astrocytes are substrates for the growth of adult CNS axons in the presence of elevated levels of nerve growth factor. Neuron. 1991;7:1019–1030. doi: 10.1016/0896-6273(91)90346-2. [DOI] [PubMed] [Google Scholar]
- 30.Kumar R, Lim J, Mekary RA, Rattani A, Dewan MC, Sharif SY, Osorio-Fonseca E, Park KB. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg. 2018;113:e345–363. doi: 10.1016/j.wneu.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 31.Kwiecien JM, Dabrowski W, Marzec-Kotarska B, Kwiecien-Delaney CJ, Yaron JR, Zhang L, Schutz L, Lucas AR. Myxoma virus derived immune modulating proteins, M-T7 and Serp-1, reduce early inflammation after spinal cord injury in the rat model. Folia Neuropathol. 2019;57:41–50. doi: 10.5114/fn.2019.83830. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Pu Q, Hintze M, Wang Y, Eckhardt M, Gieselmann V, Tiemann I, Qi X, Cai D, Wang J, Huang R. BDNF and NGF signals originating from sensory ganglia promote cranial motor axon growth. Exp Brain Res. 2020;238:111–119. doi: 10.1007/s00221-019-05694-w. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Wang Y, Zou Z, Yang M, Wu C, Su Y, Tang J, Yang X. OM-LV20, a novel peptide from odorous frog skin, accelerates wound healing in vitro and in vivo. Chem Biol Drug Des. 2018;91:126–136. doi: 10.1111/cbdd.13063. [DOI] [PubMed] [Google Scholar]
- 34.Liu N, Meng B, Bian W, Yang M, Shu L, Liu Y, Fu Z, Wang Y, Wang Y, Yang X. The beneficial roles of poisonous skin secretions in survival strategies of the odorous frog Odorrana andersonii. Naturwissenschaften. 2021;109:4. doi: 10.1007/s00114-021-01776-4. [DOI] [PubMed] [Google Scholar]
- 35.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 36.Mokalled MH, Patra C, Dickson AL, Endo T, Stainier DY, Poss KD. Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science. 2016;354:630–634. doi: 10.1126/science.aaf2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon YJ, Lee JY, Oh MS, Pak YK, Park KS, Oh TH, Yune TY. Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. J Neurosci Res. 2012;90:243–256. doi: 10.1002/jnr.22734. [DOI] [PubMed] [Google Scholar]
- 38.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 39.Norton WT, Aquino DA, Hozumi I, Chiu FC, Brosnan CF. Quantitative aspects of reactive gliosis: a review. Neurochem Res. 1992;17:877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- 40.Olson BJ, Markwell J. Assays for determination of protein concentration. Curr Protoc Pharmacol Appendix. 2007;3:3A. doi: 10.1002/0471141755.pha03as38. [DOI] [PubMed] [Google Scholar]
- 41.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin P, Meng Y, Yang Y, Gou X, Liu N, Yin S, Hu Y, Sun H, Fu Z, Wang Y, Li X, Tang J, Wang Y, Deng Z, Yang X. Mesoporous polydopamine nanoparticles carrying peptide RL-QN15 show potential for skin wound therapy. J Nanobiotechnology. 2021;19:309. doi: 10.1186/s12951-021-01051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabchevsky AG, Fugaccia I, Sullivan PG, Blades DA, Scheff SW. Efficacy of methylprednisolone therapy for the injured rat spinal cord. J Neurosci Res. 2002;68:7–18. doi: 10.1002/jnr.10187. [DOI] [PubMed] [Google Scholar]
- 44.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ:25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 46.Song Y, Wu C, Zhang X, Bian W, Liu N, Yin S, Yang M, Luo M, Tang J, Yang X. A short peptide potentially promotes the healing of skin wound. Biosci Rep. 2019;39:BSR20181734. doi: 10.1042/BSR20181734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suetsugu K, Muraki S, Fukumoto J, Matsukane R, Mori Y, Hirota T, Miyamoto T, Egashira N, Akashi K, Ieiri I. Effects of letermovir and/or methylprednisolone coadministration on voriconazole pharmacokinetics in hematopoietic stem cell transplantation: a population pharmacokinetic study. Drugs R D. 2021;21:419–429. doi: 10.1007/s40268-021-00365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan SM. GFAP variants in health and disease: stars of the brain and gut. J Neurochem. 2014;130:729–732. doi: 10.1111/jnc.12754. [DOI] [PubMed] [Google Scholar]
- 49.Sun H, Wang Y, He T, He D, Hu Y, Fu Z, Wang Y, Sun D, Wang J, Liu Y, Shu L, He L, Deng Z, Yang X. Hollow polydopamine nanoparticles loading with peptide RL-QN15: a new pro-regenerative therapeutic agent for skin wounds. J Nanobiotechnology. 2021;19:304. doi: 10.1186/s12951-021-01049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. discussion 982-987. [DOI] [PubMed] [Google Scholar]
- 51.Tian L, Cao W, Yue R, Yuan Y, Guo X, Qin D, Xing J, Wang X. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J Pharmacol Sci. 2019;139:352–360. doi: 10.1016/j.jphs.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Tsutsumi S, Ueta T, Shiba K, Yamamoto S, Takagishi K. Effects of the Second National Acute Spinal Cord Injury Study of high-dose methylprednisolone therapy on acute cervical spinal cord injury-results in spinal injuries center. Spine (Phila Pa 1976) 2006;31:2992–2996. doi: 10.1097/01.brs.0000250273.28483.5c. discussion 2997. [DOI] [PubMed] [Google Scholar]
- 53.Uchida K, Nakajima H, Guerrero AR, Johnson WE, Masri WE, Baba H. Gene therapy strategies for the treatment of spinal cord injury. Ther Deliv. 2014;5:591–607. doi: 10.4155/tde.14.20. [DOI] [PubMed] [Google Scholar]
- 54.Vipin A, Thow XY, Mir H, Kortelainen J, Manivannan J, Al-Nashash H, All AH. Natural progression of spinal cord transection injury and reorganization of neural pathways. J Neurotrauma. 2016;33:2191–2201. doi: 10.1089/neu.2015.4383. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Zheng Z, Han W, Yuan Y, Li Y, Zhou K, Wang Q, Xie L, Xu K, Zhang H, Xu H, Wu Y, Xiao J. Metformin promotes axon regeneration after spinal cord injury through inhibiting oxidative stress and stabilizing microtubule. Oxid Med Cell Longev. 2020;2020:9741369. doi: 10.1155/2020/9741369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Smith GM, Selzer ME, Li S. Emerging molecular therapeutic targets for spinal cord injury. Expert Opin Ther Targets. 2019;23:787–803. doi: 10.1080/14728222.2019.1661381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiatrak B, Kubis-Kubiak A, Piwowar A, Barg E. PC12 cell line: cell types, coating of culture vessels, differentiation and other culture conditions. Cells. 2020;9:958. doi: 10.3390/cells9040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu D, Klaw MC, Connors T, Kholodilov N, Burke RE, Tom VJ. Expressing constitutively active rheb in adult neurons after a complete spinal cord injury enhances axonal regeneration beyond a chondroitinase-treated glial scar. J Neurosci. 2015;35:11068–11080. doi: 10.1523/JNEUROSCI.0719-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao ZF, He JB, Su GY, Chen MH, Hou Y, Chen SD, Lin DK. Osteoporosis of the vertebra and osteochondral remodeling of the endplate causes intervertebral disc degeneration in ovariectomized mice. Arthritis Res Ther. 2018;20:207. doi: 10.1186/s13075-018-1701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang X, Lee WH, Zhang Y. Extremely abundant antimicrobial peptides existed in the skins of nine kinds of Chinese odorous frogs. J Proteome Res. 2012;11:306–319. doi: 10.1021/pr200782u. [DOI] [PubMed] [Google Scholar]
- 61.Yang X, Wang Y, Zhang Y, Lee WH, Zhang Y. Rich diversity and potency of skin antioxidant peptides revealed a novel molecular basis for high-altitude adaptation of amphibians. Sci Rep. 2016;6:19866. doi: 10.1038/srep19866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang X, Wang Y, Wu C, Ling EA. Animal venom peptides as a treasure trove for new therapeutics against neurodegenerative disorders. Curr Med Chem. 2019;26:4749–4774. doi: 10.2174/0929867325666181031122438. [DOI] [PubMed] [Google Scholar]
- 63.Yin S, Yang M, Li Y, Li S, Fu Z, Liu N, Wang Y, Hu Y, Xie C, Shu L, Pang A, Gu Y, Wang Y, Sun J, Yang X. Peptide OM-LV20 exerts neuroprotective effects against cerebral ischemia/reperfusion injury in rats. Biochem Biophys Res Commun. 2021;537:36–42. doi: 10.1016/j.bbrc.2020.12.053. [DOI] [PubMed] [Google Scholar]
- 64.Yin S, Pang A, Liu C, Li Y, Liu N, Li S, Li C, Sun H, Fu Z, Wang Y, Zhang Y, Yang M, Sun J, Wang Y, Yang X. Peptide OM-LV20 protects astrocytes against oxidative stress via the 'PAC1R/JNK/TPH1'axis. J Biol Chem. 2022;298:102429. doi: 10.1016/j.jbc.2022.102429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yong CS, Choi JS, Quan QZ, Rhee JD, Kim CK, Lim SJ, Kim KM, Oh PS, Choi HG. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int J Pharm. 2001;226:195–205. doi: 10.1016/s0378-5173(01)00809-2. [DOI] [PubMed] [Google Scholar]
- 66.Yousefifard M, Rahimi-Movaghar V, Nasirinezhad F, Baikpour M, Safari S, Saadat S, Moghadas Jafari A, Asady H, Razavi Tousi SM, Hosseini M. Neural stem/progenitor cell transplantation for spinal cord injury treatment;A systematic review and meta-analysis. Neuroscience. 2016;322:377–397. doi: 10.1016/j.neuroscience.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 67.Yu JZ, Rasenick MM. Tau associates with actin in differentiating PC12 cells. FASEB J. 2006;20:1452–1461. doi: 10.1096/fj.05-5206com. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, Feng C, Wang S, Wang Y, Fu Z, Zhang Y, Sun H, Xie C, Fu Y, Tao J, Luo M, Yang X. A novel amphibian-derived peptide alleviated ultraviolet B-induced photodamage in mice. Biomed Pharmacother. 2021;136:111258. doi: 10.1016/j.biopha.2021.111258. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Z, Li F, Sun T. An expert consensus on the evaluation and treatment of acute thoracolumbar spine and spinal cord injury in China. Neural Regen Res. 2013;8:3077–3086. doi: 10.3969/j.issn.1673-5374.2013.33.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao J, Pang A, Yin S, Yang M, Zhang X, Zhang R, Liu J, Gu Y, Li S, Hu Y, Zhang Y, Ba Y, Meng B, Yang X. Peptide OM-LV20 promotes structural and functional recovery of spinal cord injury in rats. Biochem Biophys Res Commun. 2022;598:124–130. doi: 10.1016/j.bbrc.2022.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.