Abstract
Rho GTPases are essential regulators of the actin cytoskeleton. They are involved in various physiological and biochemical processes such as the regulation of cytoskeleton dynamics, development, proliferation, survival, and regeneration. During the development of cochlear hair cells, Rho GTPases are activated by various extracellular signals through membrane receptors to further stimulate multiple downstream effectors. Specifically, RhoA, Cdc42, and Rac1, members of the classical subfamily of the Rho GTPase family, regulate the development and maintenance of cilia by inducing the polymerization of actin monomers and stabilizing actin filaments. In addition, they also regulate the normal morphology orientation of ciliary bundles in auditory hair cells, which is an important element of cell polarity regulation. Moreover, the actin-related pathways mediated by RhoA, Cdc42, and Rac1 also play a role in the motility of outer hair cells, indicating that the function of Rho GTPases is crucial in the highly polar auditory sensory system. In this review, we focus on the expression of RhoA, Cdc42, and Rac1 in cochlear hair cells and how these small molecules participate in ciliary bundle morphogenesis and cochlear hair cell movement. We also discuss the progress of current research investigating the use of these small molecules as drug targets for deafness treatment.
Key Words: actin assembly, auditory sensory neurons, cell polarity, cell proliferation, electromotility, hair cell, hearing loss, morphogenesis, Rho GTPases, stereocilia
Introduction
The Rho family of GTPases is a family of small G proteins that is highly conserved in almost all eukaryotes, from yeast to humans. They participate in various cellular processes such as actin cytoskeleton assembly, gene expression regulation, vesicle trafficking, cell cycle progression, cell morphogenesis, cell polarity, and cell migration (Etienne-Manneville and Hall, 2002; Mosaddeghzadeh and Ahmadian, 2021). The human Rho GTPase family comprises 20 proteins that are divided into eight subfamilies: Cdc42, Rac, Rho, RhoU/RhoV, RhoBTB, RhoF/RhoD, RhoH, and Rnd. Moreover, they are further classified as classical or atypical Rho GTPases, according to their regulatory patterns (Figure 1A). The Rac, Rho, Cdc42, and RhoF/RhoD subfamilies are considered classical, and they act as molecular switches that cycle between the active GTP-bound form and inactive GDP-bound form, transducing upstream signaling stimuli to downstream effector molecules. During this process, the GTP/GDP ratio is co-regulated by guanine-nucleotide exchange factors, GTPase-activating proteins, and guanine nucleotide dissociation inhibitors (Crosas-Molist et al., 2022; Figure 1B). Rnd, RhoH, RhoU/RhoV, and RhoBTB belong to atypical subfamilies that mainly stay in GDP-binding forms and do not hydrolyze GTP (Lawson and Ridley, 2018). In addition, Rho GTPases are regulated by transcription and posttranslational modifications (PTMs), regardless of whether they are a classical or atypical subfamily (Hodge and Ridley, 2016).
Figure 1.

Rho GTPase family member classification and activation of classical subfamily members.
(A) Rootless phylogenetic tree of 20 Rho GTPase proteins based on amino acid sequences. The tree shows the relationships between different family members. Green represents members of the classical Rho GTPase subfamily, and gray represents members of the atypical Rho GTPase subfamily. The three core members of RhoA, Cdc42, and Rac1 reviewed in this article are labeled with red stars. (B) A cyclic process of atypical Rho GTPases between active GTP form and inactive GDP form. After Rho-GDP receives upstream signal stimuli, guanine-nucleotide exchange factors (GEFs) promote GDP and GTP exchange, GTPase-activating proteins (GAPs) enhance GTP hydrolytic activity, and guanine nucleotide dissociation inhibitors (GDIs) inhibit dissociation of the inactive form, thereby helping Rho GTPases to return to the active state. Thus, they jointly regulate the active and inactive cycles.
Rho GTPases are localized in both the cell membrane and cytoplasm in activated or inactivated states (Hurst et al., 2022). Various extramembrane signals activate the Rho GTPase-related signaling pathway to further regulate downstream cellular activities. For example, cadherin-mediated cell contact excites Rho GTPase to participate in the Wnt signaling pathway by regulating the expression of β-catenin to regulate tyrosine kinase signal transduction (Schlessinger et al., 2009). Through affinity chromatography, protein purification, and yeast two-hybrid screening, > 100 targets of Rho GTPases have been reported, including approximately 30 kinases and a large number of scaffold proteins such as Rho-related protein kinase (ROCK) (Wei et al., 2021), protein kinase C-related kinase (PKC) of the Rho subfamily, p21-activated kinase (PAK) (Mohammad et al., 2019; Dufies et al., 2021), mixed lineage kinase of the Rac subfamily (Prudnikova et al., 2015), tyrosine kinase, the formin family of scaffold protein mDia (Becker et al., 2022), and the Wiskott-Aldrich syndrome protein WASP (Mughees et al., 2021). Thus, the vast diversity of upstream regulators and downstream effectors enables the Rho GTPase complex signaling pathway to be involved in a wide range of physiological functions.
Rho GTPases such as RhoA, Rac1, and Cdc42 are critical regulators of actin cytoskeleton assembly (Kalpachidou et al., 2019). For example, RhoA regulates the assembly of microfilament bundles that are higher-order cytoskeletal structures composed of cross-linked actin filament bundles and myosin motor proteins (Landino et al., 2021). Cdc42 is responsible for the formation of filopodia and assembly of actin stress fibers, while Rac1 induces membrane folding and lamellipodia formation (Landino et al., 2021; Salameh et al., 2021; Tyckaert et al., 2022). The Rho GTPase family plays a critical role in actin cytoskeleton dynamics, and they are involved in many actin-dependent cellular processes such as neuronal guidance, cell proliferation/division, vesicle trafficking, cell motility/migration, cell polarity establishment, and cell-cell interactions (Bosco et al., 2009; Chircop, 2014; Ueyama, 2019). Interestingly, they are also crucial for the function of highly polarized sensory cells (organs), including cochlear hair cells (HCs) (Zhu et al., 2018; Kakiuchi et al., 2019). The stereocilia bundles of HCs own mechanosensitive channels that convert sound signals into electrical signals (Qiu and Müller, 2022). Then, the electrical signals are further transmitted through electromotility of outer hair cells (OHCs) mediated by actin polymerization (Ge et al., 2021). In addition, Rho GTPases are also involved in gene transcription regulation, apoptosis, cell cycle progression, reactive oxygen species (ROS) production, membrane transport, and other processes (Ngo et al., 2021; Crosas-Molist et al., 2022). Previous studies have shown that dysregulation of Rho GTPase activity is found in a variety of diseases such as neurological diseases, neurodegenerative diseases, inflammation, sensorineural deafness, and cancer (Nadif Kasri and Van Aelst, 2008; Chen et al., 2012; DeGeer and Lamarche-Vane, 2013; Han et al., 2015). Currently, RhoA, Cdc42, and Rac1 have attracted increasing attention as molecular switches that regulate the actin cytoskeleton and as promising targets for the improvement of sensorineural deafness. In this review, we focus primarily on the importance of the Rho GTPase family (RhoA, Cdc42, and Rac1) in the development of cochlear HCs and hearing maintenance.
Search Strategy
Literatures cited in this review were searched from the PubMed database. Relevant literature published from September 2000 to November 2022 were screened. A combination of the following text words (MeSH terms) was used to maximize search specificity and sensitivity: “neurosensory deafness,” “hearing loss,” “Rho GTPase,” “stereocilia,” “hair cell,” “cilia assembly,” “cell polarity,” “electromotility,” and “fasudil.” The results were further screened by the title and abstract. Studies exploring the relationship between Rho GTPase and cochlear HC were included.
Expression Profiles of RhoA, Cdc42, and Rac1 in the Cochlea
Many experimental data show that RhoA, Cdc42, and Rac1 are all expressed in the cochlea (Kirjavainen et al., 2015; Anttonen et al., 2017). In situ hybridization experiments showed that RhoA is ubiquitously expressed in the mouse cochlea (Anttonen et al., 2017). It has also been reported that Cdc42 is highly expressed in the stereocilia of mouse HCs and slightly expressed in the apical supporting cells of inner ear HCs (Kirjavainen et al., 2015). Likewise, immunostaining with anti-Rac1 antibody showed that Rac1 staining is particularly prominent in nascent bundles of mouse HCs (Grimsley-Myers et al., 2009). The expression pattern of RhoA, Rac1, and Cdc42 was also investigated in the guinea pig organ of Corti by immunostaining. The results showed that positive signals for these proteins are more intense in OHCs than in other Corti cell populations with a clear colocalization with the actin cytoskeleton (Kalinec et al., 2000). Taken together, these results show that RhoA, Cdc42, and Rac1 are all expressed in the cochlear HC and may be involved in the development of cochlear HCs and auditory conduction by actin regulation.
The Role of Rho GTPase in Cochlear Hair Cell Development
Cochlear HCs are highly polarized sensory epithelial cells, with one row of inner hair cells (IHCs) and three rows of OHCs in the cochlea. IHCs are responsible for detecting sound and transmitting information to the auditory cortex, while OHCs amplify sound waves in the cochlea by rapidly changing the cell length (McPherson, 2018). Each HC has specialized actin structures such as stereocilia, apical junction complexes, and cuticular plates (Du et al., 2019). HCs sense sound waves through bundles of stereocilia on their epidermal plates and then transform the mechanical signals into electrical signals to the central nervous system through mechanoelectrical transduction channels (Michalski and Petit, 2015; Liu et al., 2021; Qiu and Müller, 2022). In RhoA-, Cdc42-, and Rac1-mutant mice, the morphology and function of cochlear HCs or ciliary bundles undergo a varying degree of damage, which severely disrupts HC development and regeneration, resulting in hearing impairment.
In mouse cochlea with RhoA-specific inactivated, most OHCs have an enlarged apical surface area, with the OHCs breaking away from the surrounding supporting cells and diffusing into the endolymphatic fluid in the middle cochlea (Anttonen et al., 2017). Apical junction complexes composed of tight junctions and adherent junctions regulate epithelial homeostasis, paracellular permeability, and barrier properties (Otani and Furuse, 2020). It has been reported that the association of apical junction complexes with the actin-myosin cytoskeleton is strictly controlled by Rho GTPase (Matsuda et al., 2022). In HCs, one of the direct targets of RhoA is myosin II (NM II), which acts as a structural protein to promote the formation of F-actin fibers to stabilize intercellular connections (Ivanov et al., 2005; Quiros and Nusrat, 2014). Normally, NM II should be evenly distributed at the junction of OHCs. However, in the cochlea of RhoA-deficient mice, NM II diffuses away from the junction as OHCs are squeezed out (Anttonen et al., 2017). Thus, RhoA is essential for maintaining proper localization of NM II at the junction of OHCs and stability of cochlear OHCs.
The extracellular matrix (ECM) is the living cell microenvironment, and the mechanical signals generated by the ECM induce accumulation of RhoA that leads to proliferation and differentiation of inner ear progenitor cells (Ivanov et al., 2005). RhoA promotes the polymerization of the actin cytoskeleton, which further results in an increase in Yes-associated protein (YAP) and upregulation of the canonical Wnt signaling pathway (Ivanov et al., 2005). Therefore, this RhoA-YAP-β-catenin signaling axis transduces external mechanical signals to inner ear progenitor cells, connecting extracellular mechanical signaling with the proliferation and differentiation of cochlear progenitor cells.
Cdc42 is essential for early mammalian development and mediates phosphatidylinositol 4,5-bisphosphate (PIP2)-induced actin assembly (de Souza et al., 2021). After specifically knocking out Cdc42 in cochlear HCs by the Atoh1 promoter, the HCs of mice developed normally during the development stage but gradually degenerated after maturity, leading to progressive sensorineural hearing loss (Ueyama et al., 2014). Moreover, the stereocilia of IHC also show various abnormalities in Cdc42-knockout mouse, including scattered ciliary bundles and variable lengths of cilia in the same row, accompanied by a thinning circumferential actin belt at apical junction complexes. These changes are closely related to actin interactions, which indicates that Cdc42 likely plays a role in the maintenance of stable actin structure and cochlear HC function by regulating actin levels. Furthermore, Kirjavainen et al. (2015) inactivated Cdc42 in the late embryonic organ of Corti by crossing-breeding Cdc42loxP/loxP homozygous mice with Fgfr3-Cre-ERT2 mice. Cdc42 defects in mice lead to defective planar polarities with disordered hair bundle orientation and microtubule networks on the cell surface. This study further showed the essential roles of Cdc42 in HC planar cell polarity (PCP) and cellular patterning. In addition, there are significant stereocilia defects in Cdc42-KO cochlear HCs at the P8 stage. Meanwhile, treatment with ML141, a Cdc42 inhibitor, also results in similar defective phenotypes, including increased stereocilia height differences, decreased stereocilia width, and impaired PCP in neonatal mouse HCs (Du et al., 2021). In conclusion, these studies are sufficient to demonstrate that Cdc42 is a vital factor for the development of cochlear HCs and hearing maintenance.
Similar to RhoA and Cdc42, Rac1-KO mice also display severe defects in the morphology of the cochlear epithelium. With significant shortening of the cochlea in Rac1 mutant mice, HCs are also markedly reduced and become longer and flatter (Grimsley-Myers et al., 2009). Moreover, the planar polarity of HCs and the orientation of ciliary bundles are also impaired because of the deficiency of Rac1, as seen by the breakage, deformation, dislocation, and incorrect arrangements of ciliary bundles. Furthermore, the double knockout of Rac1 and Rac3 significantly decreases cell proliferation, thereby supporting cell differentiation and cell adhesion in the mouse cochlea, except for a severe defect in the HC bundle (Grimsley-Myers et al., 2012). These studies indicate the key functions of Rac1 in the morphogenesis of the otocyst and cochlear HC and ciliary bundles.
Rho GTPase Regulates Hair-Bundle Morphogenesis and Arrangement
The stereocilia of cochlear HCs are composed of hundreds of actin monomers (McGrath et al., 2021). Several studies have shown that loss of RhoA, Cdc42, and Rac1 causes severe morphological and functional defects in ciliary bundles (Du et al., 2021; Grimsley-Myers et al., 2009), which suggest that they are critical molecular switches for the regulation of actin assembly and maintenance. In addition, they are also involved in the noncanonical Wnt/PCP signaling pathway to regulate the polarity of cochlear HCs through the polarizing reorganization of the cytoskeleton (Anttonen et al., 2017; Du et al., 2021). The normal operation of these two aspects together are responsible for the complex morphology and function of cochlear HCs.
Rho GTPase participates in cilia assembly
Stereocilia development begins with a nucleation event in which three actin monomers (G-actin) interact to form a trimer called the actin core (Miyoshi et al., 2022). Then, the fiber rapidly elongates from the active core, with G-actin reversibly polymerizing on the two sides of F-actin to extend the actin core (Shin et al., 2013). In mature hair bundles, G-actin systematically binds to the actin filaments near the stereocilia tip, while depolymerization of F-actin at the base of the stereocilia occurs to maintain a fixed length of stereocilia (Pacentine et al., 2020). Rho GTPase-regulated actin dynamics are critical for stereocilia growth. Specifically, RhoA, Cdc42, and Rac1 regulate the assembly and maintenance of cilia by participating in G-actin polymerization and F-actin stabilization.
RhoA/Cdc42/Rac1 are closely involved in the initiation of stereocilia growth
Among the downstream effectors of Rho GTPase, the formin protein family is closely related to actin monomer polymerization, as an effective nucleator of linear actin filaments (F-actin) (Le et al., 2020). Formins contain an N-terminal GTPase binding domain (GBD); formin homology domains (FH1, FH2, FH3); a dimerization domain; a coiled-coil domain; and a C-terminal autoregulatory domain. The FH2 domain directly binds to G- and F-actin to participate in actin nucleation and actin filament elongation, while the FH1 domain interacts with the actin regulator–profilin–to assist the FH2 domain in recruiting G-actin molecules for accelerating the rate of actin nucleation (Pinto-Costa and Sousa, 2020). These functions make the formins particularly important in the initial stage of stereocilia development. For example, in humans, a mutation in the formin protein DIA1 causes severe, hereditary nonsyndromic hearing loss and deafness (DFNA1 disease) which is characterized by progressive deafness starting in childhood. In addition, DIA1 mutant mice also experience progressive deafness and HC loss with various morphological malformations in the stereocilia, such as short, fused, elongated, and sparse cilia (Ueyama et al., 2016). Importantly, the FH3 domain recognizes the C-terminus to generate an intramolecular autoinhibitory complex in the resting state. However, when active RhoA-GTP binds to the GBD domain of formin, it relieves the autoinhibitory state to induce cilia assembly (Pinto-Costa and Sousa, 2020; Figure 2A). Therefore, the combination of active RhoA and formin proteins becomes a critical link in the stereocilia development of cochlear HCs.
Figure 2.
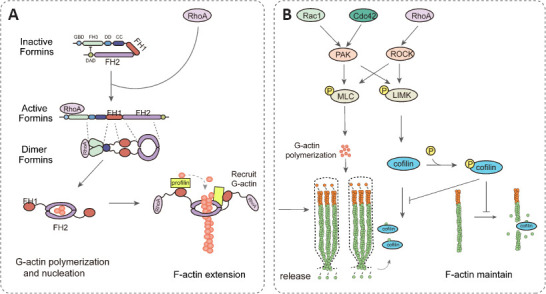
RhoA, Cdc42, and Rac1 are involved in the morphogenesis of stereocilia.
(A) At the initiation of stereocilia development, RhoA activates formins. The formin homology domains-2 (FH2) binds to G- and F-actin to participate in actin nucleation. The formin homology domains-1 (FH1) interact with profilin to assist the FH2 domain in recruiting G-actin, hence accelerating the rate of actin nucleation. (B) During stereocilia bundle elongation, RhoA, Rac1, and Cdc42 polymerize G-actin and inhibit F-actin depolymerization, promoting stereocilia extension and maintenance. MLC: Myosin light chain; PAK: p21-activated kinase; ROCK: Rho-related protein kinase.
Rho GTPases activate an important actin nucleation factor–the Arp2/3 complex–that localizes at the intersection site of actin filaments in mammalian cells to help actin networks develop branches. (Mughees et al., 2021). The effect of Cdc42 on this process is achieved by the N-WASP protein, an actin nucleation factor that promotes the polymerization of actin filaments (Katanov et al., 2020). N-WASP is in an autoinhibitory state when its N-terminal CRIB domain interacts with the C-terminal VCA domain. However, Cdc42 binds to the CRIB domain of WASP to ensure reversal of autoinhibition and subsequent activation (Watson et al., 2016). Then, the VCA domain binds to the Arp2/3 complex to stimulate its ability to polymerize actin. Similarly, Rac1 also activates WASP’s homolog, the WAVE protein, to regulate the Arp2/3 complex (Zhou et al., 2016). The WASP and Arp2/3 complexes constitute a core mechanism that directly connects upstream Rho-GTPase-mediated signaling and downstream actin assembly (Figure 2A). This is the first discovery of a signaling pathway for actin filament nucleation (Ridley, 2006). Interestingly, FCHSD2, which couples the actin cytoskeleton with plasma membranes, modulates F-actin assembly or maintenance in HC stereocilia by binding to WASP (Cao et al., 2013). These studies indicate that Cdc42 and Rac1 may be essential in the “nucleation event” at the beginning of stereocilia development by stimulating actin polymerization in a WASP-Arp2/3-dependent manner.
RhoA/Cdc42/Rac1 are closely involved in the extension of stereocilia
Cofilin, a member of the actin-depolymerizing factor protein family, is a ROCK substrate with actin-depolymerizing activity. The Rho-ROCK-LIMK pathway regulates cofilin phosphorylation and inhibits the depolymerization activity of actin filaments to stabilize actin filament structures (Lee et al., 2015; Figure 2B). It has been reported that LIMK2 is expressed in guinea OHCs, including hair bundles. Similar to LIMK2, cofilin and phosphorylated cofilin (p-cofilin) are also found in the hair bundle, the cuticular plate, and the actin-rich infracuticular structure (Matsumoto et al., 2010). Compared to unexposed cells, exposure of isolated OHCs to LPA (hemolytic phosphatidic acid, RhoA activator) for 30 min significantly increases the ratio of p-cofilin/cofilin; this increase is suppressed by C3-transferase (RhoA inhibitor) and Y-27632 (ROCK inhibitor) treatment (Matsumoto et al., 2010). Because p-cofilin is vital for maintenance of F-actin, we speculate that RhoA manipulates the phosphorylation of cofilin via the Rho-ROCK-LIMK pathway to inhibit the depolymerization of F-actin in stereocilia.
The substrates of ROCK also include actin-binding subunits of myosin light-chain phosphatase (MBS) and myosin light chain (MLC). ROCK inhibits the activity of myosin phosphatase by phosphorylating MBS, which further enhances the phosphorylation of the myosin light chain, reduces actin contraction, and facilitates the assembly of myosin filaments (Lawson and Ridley, 2018). In addition, PAK, a family of serine/threonine protein kinases that is activated by Rac1 and Cdc42, plays the same role as ROCK. It also participates in the MLC and LIMK pathways to regulate the polymerization and depolymerization of G-actins (Cheng et al., 2021; Figure 2B). Therefore, these results indicate that Rho GTPases are directly involved in the regulation of actin assembly to stereocilia through downstream targets such as ROCK, and that the underlying mechanism behind their interaction is complex and inseparable.
Activation of Rho GTPase by Wnt/Frizzled signaling is required for PCP
At the tissue level, hair bundles of HCs are evenly arranged along the medial-lateral axis of the cochlea and point toward the lateral edge of the cochlear duct, which is defined as PCP (Figure 3A and B). At the cell-intrinsic level, rows of stereocilia are arranged in a V-shaped ladder pattern, and the kinocilia situated at the vertex of the V-shaped stereocilia bundle edg (Figure 3B). The orientation of these cilia is called the HC-intrinsic polarity (Tarchini and Lu, 2019). Recently, it has been proven that the morphological defects of the HC in Cdc42 and Rac1 mutant mice are manifest not only during the loss and shortening of stereocilia but also in the disruption of planar polarity (Gerondopoulos et al., 2019; Nakayama et al., 2021).
Figure 3.
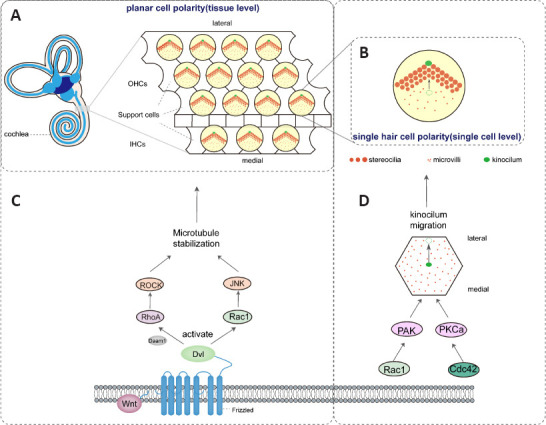
Rho GTPases participate in the polarity of mammalian cochlear hair cells.
(A) RhoA and Rac1 are activated by the Wnt/PCP pathway and are involved in regulating microtubule stability, thus controlling hair cell plane polarity. (B) Active Rac1 and Cdc42, with the assistance of p21-activated kinase (PAK) and protein kinase C-related kinase a (PKCa), control the migration of kinocilium, thus affecting the polarity of single hair cells. (C) In the cross-section of the cochlear catheter, the planar cell polarity at the tissue level is characterized by a V-shaped arrangement of stereocilia bundles, and the kinocilium points laterally along the medial side. (D) Single-cell polarity manifests as the migration of the kinocilium from the center of the apical plane of the hair cell to the lateral side and next to the highest static cilia. Dvl: Dishevelled; IHC: inner hair cell; OHC: outer hair cell; PAK: p21-activated kinase; PKC: protein kinase C-related kinase; ROCK: Rho-related protein kinase.
In the inner ear, core PCP proteins including Frizzled (Fzd3 and 6), Dishevelled (Dvl1-3), Van Gogh (Vangl1 and 2), and Flamingo (Celsr1-3) are distributed at the junctions of inner ear HCs and synergistically control the correct orientation of hair bundles (Duncan et al., 2017; Dong et al., 2018; Stoller et al., 2018). The formation of PCP is inseparable from the noncanonical Wnt/PCP pathway in which Rho GTPase participates (Navajas Acedo et al., 2019). Wnt proteins such as Wnt5a bind to the Frizzled transmembrane receptor on the cell surface and then activate RhoA/Rac1 via Dsh, subsequently assisting in the stabilization of microtubules and completion of the actin cytoskeleton (Feng et al., 2021). The RhoA activation process requires Daam1 involvement, and RhoA simultaneously activates ROCK (Montcouquiol and Kelley, 2020); Figure 3C). In Xenopus laevis, Wnt/Fz signaling coactivates RhoA and Rac1 to control cell polarity and motility during vertebrate gastrulation (Habas et al., 2003). Moreover, the reduction in RhoA activity effectively suppresses Fzd and Dvl overexpression in Drosophila, indicating that RhoA acts downstream of these two proteins in PCP signaling (Schlessinger et al., 2009). The phenomenon of misaligned hair bundles also occurs in the cochlea of RhoA and Cdc42 mutant mice. Therefore, it is speculated that Rho GTPase is involved in the polarization of cochlear HCs through the nonclassical Wnt/PCP pathways (Anttonen et al., 2017; Du et al., 2021). Rac1 and its effector Pak were the first identified regulators of HC intrinsic polarity. Rac-Pak signaling activity is enriched at the junctions of microtubules and HC surfaces to control the movement of kinocilia and the morphology of stereociliary bundles. (Grimsley-Myers et al., 2009; Figure 3D). Moreover, the scaffolding protein Par3, a functionally conserved polarity regulator, stimulates Rac1 signaling in HCs and SCs through interactions with guanine-nucleotide exchange factors. It has been experimentally verified that the expression of constitutively active Rac1 in cochlear HCs rescues the planar polarity defect in Par3-cKO mice, revealing the vital function of Rac1 and Par3 signaling in the regulation of HC-intrinsic polarity (Landin Malt et al., 2019). Therefore, data related to the mutant phenotypes of Rac1 and Cdc42 are sufficient to demonstrate that both Rac1 and Cdc42 are essential for the intrinsic polarity of cochlear HCs. Interestingly, planar polarization of individual HCs does not require PCP signaling. In mice, mutation of the core PCP protein leads to disruption of the hair bundle arrangement in the inner ear sensory epithelium (Goodrich and Strutt, 2011). Furthermore, the cochlear duct becomes short, with the HCs at the top of the cochlea that are arranged in multiple rows. However, the self-polarization structure of individual hair bundles does not appear to be affected. Moreover, the intrinsic polarity of the ciliary bundles of HCs is severely impaired in Cdc42 mutants, characterized by abnormal kinocilium localization and misdirected stereocilia bundles, with the disruption of the microtubule organization at the HC surface. However, the asymmetric localization of the core PCP protein remains unaffected (Kirjavainen et al., 2015). These results indicate that the reason for the inherent polarity impairment of the cochlear HCs is complex.
In conclusion, RhoA, Cdc42, and Rac1 are the best candidates for studying cochlear HC hair bundle morphogenesis due to their high expression in the inner ear, their specific effects on actin polymerization and their versatility as cell polarity signaling sensors. Regarding their regulatory network during cochlear HC development and maintenance, we propose the following model: at the initial development stage of stereocilia, RhoA binds to the GBD domain of formin to relieve its autoinhibitory state. Then, the FH2 domain of formin participates in actin nucleation, with the FH1 domain interacting with profilin to assist the recruitment of G-actin molecules. Meanwhile, Cdc42 and Rac1 activate the Arp2/3 complex through N-WASP to stimulate actin polymerization and form an actin core in the apical surface of HC. Furthermore, the FH1 domain activates profilins to polymerize actin, accelerating the rate of actin nucleation and facilitating the formation of actin filaments. During the extension phase of cilia, RhoA, Cdc42, and Rac1 are jointly involved in the phosphorylation of MLC/LIMK to regulate actin polymerization and depolymerization (Figure 2). In addition, Cdc42 and Rac1 promote the correct orientation of ciliary bundles to establish polarity via PCP-associated proteins (Figure 3).
Rho GTPases Regulate the Electromotility of Cochlear Outer Hair Cells
The low-intensity sound signal is mechanically amplified by the electromotility of the OHCs. Although prestin has been proved to be a major component for the process of electromotility (Ge et al., 2021), several studies regarding the role of Rho GTPases in this process have also been reported in recent years (Matsumoto et al., 2010; Park and Kalinec, 2015). Acetylcholine reportedly induces OHC shortening and a simultaneous increase in the amplitude of fast motility by activating Rac1 and Cdc42, which are essential for maintaining homeostasis and normal hearing in the cochlear amplifier (Kalinec et al., 2000). However, the signaling pathways mediated by these enzymes are yet to be elucidated. Subsequently, researchers found that this process relies, at least in part, on the control of the depolymerization rate of actin filaments by LIMK/cofilin-mediated signals (Matsumoto et al., 2010). For example, LIMK promotes cofilin phosphorylation to reduce the rate of actin filament depolymerization, which further causes an increase in the elongation of OHCs by stabilizing the cortical cytoskeleton after stimulation by RhoA activators. Conversely, a reduction in cofilin phosphorylation restrains the electromotive amplitude and length of OHCs (Matsumoto et al., 2010; Figure 4). In addition, the activation of RhoA/ROCK/PKCa signaling also induces the phosphorylation of adducin, which is a well-known promoter of actin-spectrin dissociation, to inhibit F-actin binding with spectrin (Park and Kalinec, 2015). The cortical cytoskeleton of OHCs is based on the actin-spectrin protein to maintain cell stability (Park and Kalinec, 2015). This process is responsible for the rearrangement of microdomains in the plasma membrane of OHCs (Kitani et al., 2013), which will, in turn, regulate the electromotility of these OHCs (Figure 4). Taken together, the current findings support the notion that the Rho GTPase pathway mediates changes in the cortical actin cytoskeleton, which remains one of the most attractive candidate mechanisms for the regulation of OHC electromotility.
Figure 4.
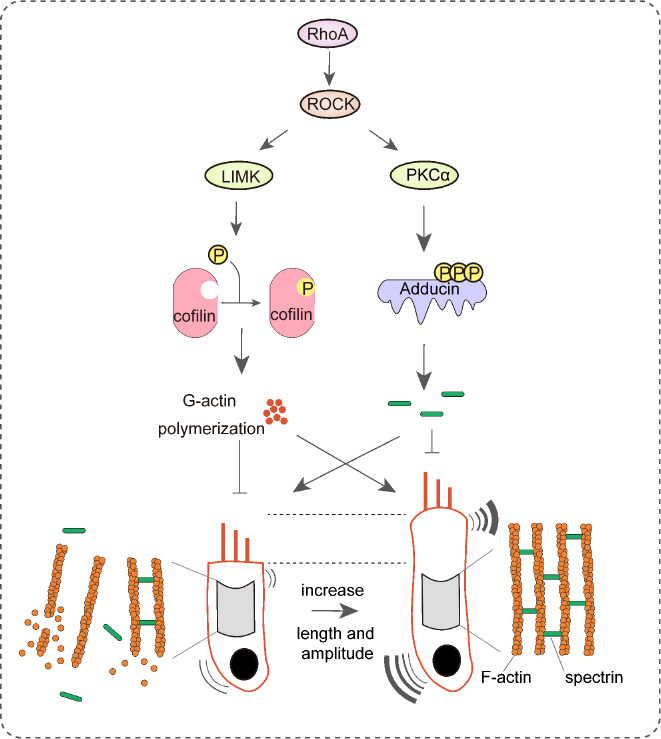
RhoA regulates outer hair cells electromotility.
RhoA activates the Rho-related protein kinase (ROCK)/LIM kinases (LIMK) pathway to phosphorylate cofilin and the Rho-related protein kinase (ROCK)/protein kinase C-related kinase a (PKCa) pathway to phosphorylate adducin, thereby disrupting the cortical actin cytoskeleton, and altering the length of F-actin and the outer hair cytoskeleton. Both pathways antagonize each other, thereby regulating the electromotility of outer hair cells.
Role of Rho GTPase in Hearing Disease
An increasing number of studies have shown that the Rho GTPase signaling pathway is inextricably linked with the pathogenic mechanism of neurosensory deafness (Chen et al., 2012; Han et al., 2015; Zhu et al., 2018; Ueyama, 2019). Noise exposure, auditory nerve synapses, ototoxic drug use, and genetic defects are common deafness factors. Interestingly, transient ATP depletion with active RhoA levels is significantly reduced during hearing loss caused by noise treatment, which further leads to the depolymerization of actin filaments and their instability (Chen et al., 2012). However, treatment with lysophosphatidic acid, an activator of the Rho pathway, results in a significant reversal of attenuated OHC death caused by noise exposure, along with F/G-actin ratio rescue in the cochleae of adult mice (Han et al., 2015). Moreover, the cochlear synaptopathy caused by the disorder of auditory nerve synapses has been clarified in recent years, and it is recognized as a major reason for sensorineural hearing loss (Liberman and Kujawa, 2017). Interestingly, ROCK inhibitor Y-27632 that has been reported to have neuroprotective and regenerative effects on synaptic pathways could promote regeneration of the cochlear nerve axons and synapses between the auditory nerve and the inner hair cell after excitotoxic injury to the cochlea (Koizumi et al., 2020). In addition, Y-27632 treatment could also significantly increase the amplitude of wave in the auditory brainstem response and the number of synapses in mice with cochlear synaptopathy caused by laser-induced shock wave (Koizumi et al., 2021). These results clearly demonstrate the promising clinical applications of using the Rho pathway activator or inhibitor to cure hearing loss.
The use of aminoglycoside antibiotics such as streptomycin, kanamycin, and gentamicin, could also lead to permanent hearing loss. They induce actin depolymerization and increase oxygen free radicals, which leads to the accumulation of iron ions and ROS to trigger the death pathway of intracochlear cells, thereby reducing auditory function (Hammill and Campbell, 2018; Rybak et al., 2021). It has been reported that kanamycin injection leads to the abnormal expression of Rac1 and RhoA, as well as the accumulation of ROS in mice cochleae, which impairs the original actin pathway and ultimately leads to aminoglycoside-induced HC loss (Jiang et al., 2006). Fasudil, a Rho kinase inhibitor, has been widely used for the clinical treatment of cerebral vasospasm after subarachnoid hemorrhage and for clinical evaluation in angina pectoris (Vicari et al., 2005). Interestingly, current studies have also shown that fasudil could significantly inhibit the Rho signaling pathway in both whole-organ cultured cochleae and auditory HEI-OC1 (house ear institute-organ of corti 1) cells after neomycin exposure, which further reduces the levels of ROS accumulation to decrease the apoptosis of HCs and HEI-OC1 cells (Zhang et al., 2019). Taken together, these results suggest that Rho signal pathway might be a potential therapeutic target for the prevention of aminoglycoside-induced HC damage.
These studies sufficiently demonstrate the critical role of Rho GTPase and its kinases in normal hearing maintenance by targeting the cytoskeleton. In addition, other signaling cascade members of Rho GTPase also have actin-regulating properties, and whether they may also be important targets for treating noise or aminoglycoside-induced hearing loss requires further investigation.
Limitations
While this review aimed to be comprehensive, it has some limitations. First, only the PubMed database was used to search for literature; thus, incomplete retrieval of identified research was inevitable. Second, articles that were published in languages other than English or those unavailable online were not selected, which could have caused a reporting bias in our review. Third, owing to inadequate research, the current understanding of interactions between members of Rho GTPase is limited, and it is challenging to use them as therapeutic targets for hearing diseases.
Conclusion and Future Perspectives
The function of the Rho GTPase family has been increasingly focused on and researched in the past nearly five decades. They are the fundamental molecules of multiple signal transduction pathways in cells and play a bridging role in intracellular signal transduction, where they serve as molecular switches that participate in the regulation of regular cell proliferation, differentiation, and apoptosis. The signaling pathway mediated by RhoA, Cdc42, and Rac1 plays a critical role in auditory HC development and auditory conduction. Their activity and interaction directly determine the morphological development, electromotility process, and polar state of cochlear HCs (Table 1). Moreover, in hearing impairment caused by aminoglycosides, the activity and expression levels of RhoA and Rac1 are abnormal. However, the mechanisms that drive these physiological or disease processes remain poorly understood and warrant further investigation.
Table 1.
The mechanisms of action of Rho GTPase family
| Rho GTPase | Mechanism of action | Reasons for hearing loss | Studies |
|---|---|---|---|
| RhoA | 1. Relieve the autoinhibitory state of Formins to recruit G-actin nucleation. 2. Regulate cofilin phosphorylation and inhibit the depolymerization of actin filaments. 3. Participate in the MLC and LIMK pathways to regulate the polymerization of G-actins. 4. Reduce cofilin phosphorylation to restrain the electromotive of OHCs. 5. Promote F-actin binding with spectrin to maintain cell stability. | 1. Stereocilia defects, inability to convert sound into electrical signals. 2. The polarity of planar cells is impaired, and the hair bundles are in the wrong direction. 3. The electromotility of OHCs is disturbed to preventing the sound amplification. | Matsumoto et al., 2010; Lee et al., 2015; Park and Kalinec, 2015; Gerondopoulos et al., 2019; Pinto-Costa and Sousa, 2020; Cheng et al., 2021 |
| CDC42 | 1. Regulate cofilin phosphorylation and inhibit the depolymerization of actin. 2. Participate in the MLC and LIMK pathways to regulate the polymerization of G-actins. 3. Control the movement of kinocilia and the morphology of cilia bundles. | 1. Stereocilia defects, inability to convert sound into electrical signals. 2. The polarity of single cells is damaged and the kinocilia are mispositioned. | Grimsley-Myers et al., 2009; Lee et al., 2015; Cheng et al., 2021 |
| RAC1 | 1. Regulate cofilin phosphorylation and inhibit the depolymerization of actin filaments. 2. Participate in the MLC and LIMK pathways to regulate the polymerization of G-actins. 3. Assisting the stabilization of microtubules and the completeness of the actin cytoskeleton. 4. Control the movement of kinocilia and the morphology of cilia bundles by Rac-Pak signaling pathway. | 1. Stereocilia defects, inability to convert sound into electrical signals. 2. The polarity of planar cells is impaired, and the hair bundles are in the wrong direction to fail to transmit sound signals. | Lee et al., 2015; Gerondopoulos et al., 2019; Landin Malt et al., 2019; Montcouquiol and Kelley, 2020; Cheng et al., 2021 |
| ROCK | 1. Participate in the MLC and LIMK pathways to regulate the polymerization of G-actins. 2. Assisting the stabilization of microtubules and the completeness of the actin cytoskeleton. 3. Reduce cofilin phosphorylation to restrain the electromotive of OHCs. 4. Induces the phosphorylation of adducin and promote F-actin binding with spectrin to maintain cell stability. | 1. Stereocilia defects, inability to convert sound into electrical signals. 2. The electromotility of OHCs is disturbed to preventing the sound amplification. | Kalinec et al., 2000; Matsumoto et al., 2010; Kitani et al., 2013; Park and Kalinec, 2015; Montcouquiol and Kelley, 2020; Cheng et al., 2021 |
MLC: Myosin light chain; OHCs: outer hair cells; ROCK: Rho-related protein kinase.
Rho GTPase proteins are involved in different signaling pathways, and we are still in the preliminary phase of understanding the interaction between Rho GTPase proteins and related signaling mechanisms. eExcept RhoA, Cdc42, and Rac1, the functions of other members of the Rho GTPase family in auditory sensory neurons are lack of knowledge. In addition, most Rho GTPases-related proteins or signaling pathways are studied in isolation during HC development and auditory conduction, and it is not clear how distinct signaling mechanisms cooperate with each other and/or how they are triggered in response to diverse physiological processes. Therefore, further research is warranted to elucidate the physiological and molecular aspects of these small GTPase proteins in the auditory system. The function and interactions of Rho GTPase proteins with other members in the development of auditory HCs also needs to be further researched to evaluate potential prospects for the treatment of hearing disorders. In the future, more detailed studies on the Rho GTPase family are likely to reveal more complex regulatory networks.
Further, GTPase proteins have been tested as drug targets for certain vascular and neurological diseases (Vicari et al., 2005; Labandeira-Garcia et al., 2015), and the corresponding drugs also have potential for curing auditory organ injury and hearing loss. However, a main challenge in targeting Rho GTPase and its kinases for the treatment of hearing diseases is that different Rho GTPase subtypes have non-overlapping functions, which needs further detailed research. For example, existing inhibitors cannot distinguish between ROCK1 and ROCK2. Moreover, in addition to the auditory organs, Rho GTPases are expressed in a variety of tissues and affects crucial cellular functions such as cell proliferation and cell motility (Riento & Ridley, 2003). Therefore, interfering with these basic cellular functions might have undesirable effects and can also be an important issue that needs to be addressed in pharmaceutics. In summary, the development of GTPases-targeted therapeutics has the potential to be a new target for auditory disease.
Footnotes
Author contributions: Review conception and design: YBD, JG; data collection, analysis and interpretation: XG, JG; manuscript writing: YBD, DL, JG; project administration: DL, JG. All authors approved the final version of the manuscript.
Conflicts of interest: No potential conflict of interest relevant to this article is declared.
Data availability statement: No additional data are available.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Anttonen T, Belevich I, Laos M, Herranen A, Jokitalo E, Brakebusch C, Pirvola U. Cytoskeletal stability in the auditory organ in vivo: rhoa is dispensable for wound healing but essential for hair cell development. eNeuro. 2017;4:ENEURO.0149–17.2017. doi: 10.1523/ENEURO.0149-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker KN, Pettee KM, Sugrue A, Reinard KA, Schroeder JL, Eisenmann KM. The cytoskeleton effectors Rho-kinase (ROCK) and mammalian diaphanous-related (mDia) formin have dynamic roles in tumor microtube formation in invasive glioblastoma cells. Cells. 2022;11:1559. doi: 10.3390/cells11091559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: a “Rac”of all trades. Cell Mol Life Sci. 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao H, Yin X, Cao Y, Jin Y, Wang S, Kong Y, Chen Y, Gao J, Heller S, Xu Z. FCHSD1 and FCHSD2 are expressed in hair cell stereocilia and cuticular plate and regulate actin polymerization in vitro. PLoS One. 2013;8:e56516. doi: 10.1371/journal.pone.0056516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen FQ, Zheng HW, Hill K, Sha SH. Traumatic noise activates Rho-family GTPases through transient cellular energy depletion. J Neurosci. 2012;32:12421–12430. doi: 10.1523/JNEUROSCI.6381-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng C, Hou Y, Zhang Z, Wang Y, Lu L, Zhang L, Jiang P, Gao S, Fang Q, Zhu C, Gao J, Liu X, Xie W, Jia Z, Xu Z, Gao X, Chai R. Disruption of the autism-related gene Pak1 causes stereocilia disorganization, hair cell loss, and deafness in mice. J Genet Genomics. 2021;48:324–332. doi: 10.1016/j.jgg.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Chircop M. Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells. Small GTPases. 2014;5:e29770. doi: 10.4161/sgtp.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosas-Molist E, Samain R, Kohlhammer L, Orgaz JL, George SL, Maiques O, Barcelo J, Sanz-Moreno V. Rho GTPase signaling in cancer progression and dissemination. Physiol Rev. 2022;102:455–510. doi: 10.1152/physrev.00045.2020. [DOI] [PubMed] [Google Scholar]
- 9.de Souza LB, Ong HL, Liu X, Ambudkar IS. PIP2 and septin control STIM1/Orai1 assembly by regulating cytoskeletal remodeling via a CDC42-WASP/WAVE-ARP2/3 protein complex. Cell Calcium. 2021;99:102475. doi: 10.1016/j.ceca.2021.102475. [DOI] [PubMed] [Google Scholar]
- 10.DeGeer J, Lamarche-Vane N. Rho GTPases in neurodegeneration diseases. Exp Cell Res. 2013;319:2384–2394. doi: 10.1016/j.yexcr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Dong B, Vold S, Olvera-Jaramillo C, Chang H. Functional redundancy of frizzled 3 and frizzled 6 in planar cell polarity control of mouse hair follicles. Development. 2018;145:dev168468. doi: 10.1242/dev.168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du H, Zhou H, Sun Y, Zhai X, Chen Z, Wang Y, Xu Z. The Rho GTPase cell division cycle 42 regulates stereocilia development in cochlear hair cells. Front Cell Dev Biol. 2021;9:765559. doi: 10.3389/fcell.2021.765559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du TT, Dewey JB, Wagner EL, Cui R, Heo J, Park JJ, Francis SP, Perez-Reyes E, Guillot SJ, Sherman NE, Xu W, Oghalai JS, Kachar B, Shin JB. LMO7 deficiency reveals the significance of the cuticular plate for hearing function. Nat Commun. 2019;10:1117. doi: 10.1038/s41467-019-09074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufies O, Doye A, Courjon J, Torre C, Michel G, Loubatier C, Jacquel A, Chaintreuil P, Majoor A, Guinamard RR, Gallerand A, Saavedra PHV, Verhoeyen E, Rey A, Marchetti S, Ruimy R, Czerucka D, Lamkanfi M, Py BF, Munro P, et al. Escherichia coli Rho GTPase-activating toxin CNF1 mediates NLRP3 inflammasome activation via p21-activated kinases-1/2 during bacteraemia in mice. Nat Microbiol. 2021;6:401–412. doi: 10.1038/s41564-020-00832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan JS, Stoller ML, Francl AF, Tissir F, Devenport D, Deans MR. Celsr1 coordinates the planar polarity of vestibular hair cells during inner ear development. Dev Biol. 2017;423:126–137. doi: 10.1016/j.ydbio.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 17.Feng D, Wang J, Yang W, Li J, Lin X, Zha F, Wang X, Ma L, Choi NT, Mii Y, Takada S, Huen MSY, Guo Y, Zhang L, Gao B. Regulation of Wnt/PCP signaling through p97/VCP-KBTBD7-mediated Vangl ubiquitination and endoplasmic reticulum-associated degradation. Sci Adv. 2021;7:eabg2099. doi: 10.1126/sciadv.abg2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge J, Elferich J, Dehghani-Ghahnaviyeh S, Zhao Z, Meadows M, von Gersdorff H, Tajkhorshid E, Gouaux E. Molecular mechanism of prestin electromotive signal amplification. Cell. 2021;184:4669–4679.e13. doi: 10.1016/j.cell.2021.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerondopoulos A, Strutt H, Stevenson NL, Sobajima T, Levine TP, Stephens DJ, Strutt D, Barr FA. Planar Cell Polarity Effector Proteins Inturned and Fuzzy Form a Rab23 GEF Complex. Curr Biol. 2019;29:3323–3330.e8. doi: 10.1016/j.cub.2019.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimsley-Myers CM, Sipe CW, Géléoc GS, Lu X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J Neurosci. 2009;29:15859–15869. doi: 10.1523/JNEUROSCI.3998-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimsley-Myers CM, Sipe CW, Wu DK, Lu X. Redundant functions of Rac GTPases in inner ear morphogenesis. Dev Biol. 2012;362:172–186. doi: 10.1016/j.ydbio.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammill TL, Campbell KC. Protection for medication-induced hearing loss: the state of the science. Int J Audiol. 2018;57:S67–S75. doi: 10.1080/14992027.2018.1455114. [DOI] [PubMed] [Google Scholar]
- 25.Han Y, Wang X, Chen J, Sha SH. Noise-induced cochlear F-actin depolymerization is mediated via ROCK2/p-ERM signaling. J Neurochem. 2015;133:617–628. doi: 10.1111/jnc.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17:496–510. doi: 10.1038/nrm.2016.67. [DOI] [PubMed] [Google Scholar]
- 27.Hurst M, McGarry DJ, Olson MF. Rho GTPases: Non-canonical regulation by cysteine oxidation. Bioessays. 2022;44:e2100152. doi: 10.1002/bies.202100152. [DOI] [PubMed] [Google Scholar]
- 28.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H, Sha SH, Schacht J. Rac/Rho pathway regulates actin depolymerization induced by aminoglycoside antibiotics. J Neurosci Res. 2006;83:1544–1551. doi: 10.1002/jnr.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakiuchi A, Kohno T, Kakuki T, Kaneko Y, Konno T, Hosaka Y, Hata T, Kikuchi S, Ninomiya T, Himi T, Takano K, Kojima T. Rho-kinase and PKCαinhibition induces primary cilia elongation and alters the behavior of undifferentiated and differentiated temperature-sensitive mouse cochlear cells. J Histochem Cytochem. 2019;67:523–535. doi: 10.1369/0022155419841013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalinec F, Zhang M, Urrutia R, Kalinec G. Rho GTPases mediate the regulation of cochlear outer hair cell motility by acetylcholine. J Biol Chem. 2000;275:28000–28005. doi: 10.1074/jbc.M004917200. [DOI] [PubMed] [Google Scholar]
- 32.Kalpachidou T, Spiecker L, Kress M, Quarta S. Rho GTPases in the physiology and pathophysiology of peripheral sensory neurons. Cells. 2019;8:591. doi: 10.3390/cells8060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katanov C, Novak N, Vainshtein A, Golani O, Dupree JL, Peles E. N-wasp regulates oligodendrocyte myelination. J Neurosci. 2020;40:6103–6111. doi: 10.1523/JNEUROSCI.0912-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirjavainen A, Laos M, Anttonen T, Pirvola U. The Rho GTPase Cdc42 regulates hair cell planar polarity and cellular patterning in the developing cochlea. Biol Open. 2015;4:516–526. doi: 10.1242/bio.20149753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitani R, Park C, Kalinec F. Microdomains shift and rotate in the lateral wall of cochlear outer hair cells. Biophys J. 2013;104:8–18. doi: 10.1016/j.bpj.2012.11.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koizumi Y, Ito T, Mizutari K, Kakehata S. Regenerative effect of a ROCK inhibitor, Y-27632, on excitotoxic trauma in an organotypic culture of the cochlea. Front Cell Neurosci. 2020;14:572434. doi: 10.3389/fncel.2020.572434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koizumi Y, Mizutari K, Kawauchi S, Sato S, Shiotani A, Kakehata S. Y-27632, a ROCK inhibitor, improved laser-induced shock wave (LISW)-induced cochlear synaptopathy in mice. Mol Brain. 2021;14:105. doi: 10.1186/s13041-021-00819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labandeira-Garcia JL, Rodríguez-Perez AI, Villar-Cheda B, Borrajo A, Dominguez-Meijide A, Guerra MJ. Rho kinase and dopaminergic degeneration: a promising therapeutic target for Parkinson's disease. Neuroscientist. 2015;21:616–629. doi: 10.1177/1073858414554954. [DOI] [PubMed] [Google Scholar]
- 39.Landin Malt A, Dailey Z, Holbrook-Rasmussen J, Zheng Y, Hogan A, Du Q, Lu X. Par3 is essential for the establishment of planar cell polarity of inner ear hair cells. Proc Natl Acad Sci U S A. 2019;116:4999–5008. doi: 10.1073/pnas.1816333116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landino J, Leda M, Michaud A, Swider ZT, Prom M, Field CM, Bement WM, Vecchiarelli AG, Goryachev AB, Miller AL. Rho and F-actin self-organize within an artificial cell cortex. Curr Biol. 2021;31:5613–5621.e5. doi: 10.1016/j.cub.2021.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawson CD, Ridley AJ. Rho GTPase signaling complexes in cell migration and invasion. J Cell Biol. 2018;217:447–457. doi: 10.1083/jcb.201612069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le S, Yu M, Bershadsky A, Yan J. Mechanical regulation of formin-dependent actin polymerization. Semin Cell Dev Biol. 2020;102:73–80. doi: 10.1016/j.semcdb.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Lee SR, Xu YN, Jo YJ, Namgoong S, Kim NH. The Rho-GTPase effector ROCK regulates meiotic maturation of the bovine oocyte via myosin light chain phosphorylation and cofilin phosphorylation. Mol Reprod Dev. 2015;82:849–858. doi: 10.1002/mrd.22524. [DOI] [PubMed] [Google Scholar]
- 44.Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear Res. 2017;349:138–147. doi: 10.1016/j.heares.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Wang S, Zou L, Xiong W. Mechanisms in cochlear hair cell mechano-electrical transduction for acquisition of sound frequency and intensity. Cell Mol Life Sci. 2021;78:5083–5094. doi: 10.1007/s00018-021-03840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuda M, Chu CW, Sokol SY. Lmo7 recruits myosin II heavy chain to regulate actomyosin contractility and apical domain size in Xenopus ectoderm. Development. 2022;149:dev200236. doi: 10.1242/dev.200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto N, Kitani R, Maricle A, Mueller M, Kalinec F. Pivotal role of actin depolymerization in the regulation of cochlear outer hair cell motility. Biophys J. 2010;99:2067–2076. doi: 10.1016/j.bpj.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGrath J, Tung CY, Liao X, Belyantseva IA, Roy P, Chakraborty O, Li J, Berbari NF, Faaborg-Andersen CC, Barzik M, Bird JE, Zhao B, Balakrishnan L, Friedman TB, Perrin BJ. Actin at stereocilia tips is regulated by mechanotransduction and ADF/cofilin. Curr Biol. 2021;31:1141–1153.e7. doi: 10.1016/j.cub.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McPherson DR. Sensory hair cells: an introduction to structure and physiology. Integr Comp Biol. 2018;58:282–300. doi: 10.1093/icb/icy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michalski N, Petit C. Genetics of auditory mechano-electrical transduction. Pflugers Arch. 2015;467:49–72. doi: 10.1007/s00424-014-1552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyoshi T, Belyantseva IA, Kitajiri SI, Miyajima H, Nishio SY, Usami SI, Kim BJ, Choi BY, Omori K, Shroff H, Friedman TB. Human deafness-associated variants alter the dynamics of key molecules in hair cell stereocilia F-actin cores. Hum Genet. 2022;141(3-4):363–382. doi: 10.1007/s00439-021-02304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohammad RM, Li Y, Muqbil I, et al. Targeting Rho GTPase effector p21 activated kinase 4 (PAK4) suppresses p-Bad-microRNA drug resistance axis leading to inhibition of pancreatic ductal adenocarcinoma proliferation. Small GTPases. 2019;10:367–377. doi: 10.1080/21541248.2017.1329694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montcouquiol M, Kelley MW. Development and patterning of the cochlea: from convergent extension to planar polarity. Cold Spring Harb Perspect Med. 2020;10:a033266. doi: 10.1101/cshperspect.a033266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosaddeghzadeh N, Ahmadian MR. The RHO family GTPases: mechanisms of regulation and signaling. Cells. 2021;10:1831. doi: 10.3390/cells10071831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mughees M, Bano F, Wajid S. Mechanism of WASP and WAVE family proteins in the progression of prostate cancer. Protoplasma. 2021;258:683–693. doi: 10.1007/s00709-021-01608-2. [DOI] [PubMed] [Google Scholar]
- 56.Nadif Kasri N, Van Aelst L. Rho-linked genes and neurological disorders. Pflugers Arch. 2008;455:787–797. doi: 10.1007/s00424-007-0385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakayama S, Yano T, Namba T, Konishi S, Takagishi M, Herawati E, Nishida T, Imoto Y, Ishihara S, Takahashi M, Furuta K, Oiwa K, Tamura A, Tsukita S. Planar cell polarity induces local microtubule bundling for coordinated ciliary beating. J Cell Biol. 2021;220:e202010034. doi: 10.1083/jcb.202010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navajas Acedo J, Voas MG, Alexander R, Woolley T, Unruh JR, Li H, Moens C, Piotrowski T. PCP and Wnt pathway components act in parallel during zebrafish mechanosensory hair cell orientation. Nat Commun. 2019;10:3993. doi: 10.1038/s41467-019-12005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ngo A, Parra-Izquierdo I, Aslan JE, McCarty O. Rho GTPase regulation of reactive oxygen species generation and signalling in platelet function and disease. Small GTPases. 2021;12:440–457. doi: 10.1080/21541248.2021.1878001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otani T, Furuse M. Tight junction structure and function revisited. Trends Cell Biol. 2020;30:805–817. doi: 10.1016/j.tcb.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Pacentine I, Chatterjee P, Barr-Gillespie PG. Stereocilia rootlets: actin-based structures that are essential for structural stability of the hair bundle. Int J Mol Sci. 2020;21:324. doi: 10.3390/ijms21010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park C, Kalinec F. PKCα-mediated signals regulate the motile responses of cochlear outer hair cells. Biophys J. 2015;108:2171–2180. doi: 10.1016/j.bpj.2015.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinto-Costa R, Sousa MM. Profilin as a dual regulator of actin and microtubule dynamics. Cytoskeleton (Hoboken) 2020;77:76–83. doi: 10.1002/cm.21586. [DOI] [PubMed] [Google Scholar]
- 64.Prudnikova TY, Rawat SJ, Chernoff J. Molecular pathways: targeting the kinase effectors of RHO-family GTPases. Clin Cancer Res. 2015;21:24–29. doi: 10.1158/1078-0432.CCR-14-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu X, Müller U. Sensing sound: cellular specializations and molecular force sensors. Neuron. 2022;110:3667–3687. doi: 10.1016/j.neuron.2022.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quiros M, Nusrat A. RhoGTPases, actomyosin signaling and regulation of the epithelial apical junctional complex. Semin Cell Dev Biol. 2014;36:194–203. doi: 10.1016/j.semcdb.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 69.Rybak LP, Ramkumar V, Mukherjea D. Ototoxicity of non-aminoglycoside antibiotics. Front Neurol. 2021;12:652674. doi: 10.3389/fneur.2021.652674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salameh J, Cantaloube I, Benoit B, Poüs C, Baillet A. Cdc42 and its BORG2 and BORG3 effectors control the subcellular localization of septins between actin stress fibers and microtubules. Curr Biol. 2021;31:4088–4103.e5. doi: 10.1016/j.cub.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23:265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 72.Shin JB, Krey JF, Hassan A, et al. Molecular architecture of the chick vestibular hair bundle. Nat Neurosci. 2013;16:365–374. doi: 10.1038/nn.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stoller ML, Roman O, Jr, Deans MR. Domineering non-autonomy in Vangl1;Vangl2 double mutants demonstrates intercellular PCP signaling in the vertebrate inner ear. Dev Biol. 2018;437:17–26. doi: 10.1016/j.ydbio.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tarchini B, Lu X. New insights into regulation and function of planar polarity in the inner ear. Neurosci Lett. 2019;709:134373. doi: 10.1016/j.neulet.2019.134373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tyckaert F, Zanin N, Morsomme P, Renard HF. Rac1, the actin cytoskeleton and microtubules are key players in clathrin-independent endophilin-A3-mediated endocytosis. J Cell Sci. 2022;135:jcs259623. doi: 10.1242/jcs.259623. [DOI] [PubMed] [Google Scholar]
- 76.Ueyama T, Sakaguchi H, Nakamura T, Goto A, Morioka S, Shimizu A, Nakao K, Hishikawa Y, Ninoyu Y, Kassai H, Suetsugu S, Koji T, Fritzsch B, Yonemura S, Hisa Y, Matsuda M, Aiba A, Saito N. Maintenance of stereocilia and apical junctional complexes by Cdc42 in cochlear hair cells. J Cell Sci. 2014;127(Pt 9):2040–2052. doi: 10.1242/jcs.143602. [DOI] [PubMed] [Google Scholar]
- 77.Ueyama T, Ninoyu Y, Nishio SY, Miyoshi T, Torii H, Nishimura K, Sugahara K, Sakata H, Thumkeo D, Sakaguchi H, Watanabe N, Usami SI, Saito N, Kitajiri SI. Constitutive activation of DIA1 (DIAPH1) via C-terminal truncation causes human sensorineural hearing loss. EMBO Mol Med. 2016;8:1310–1324. doi: 10.15252/emmm.201606609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ueyama T. Rho-family small GTPases: from highly polarized sensory neurons to cancer cells. Cells. 2019;8:92. doi: 10.3390/cells8020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, Bittar N, Weiss RJ, Morales-Ballejo H, Thadani U Fasudil Study Group. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46:1803–1811. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 80.Watson JR, Fox HM, Nietlispach D, Gallop JL, Owen D, Mott HR. Investigation of the Interaction between Cdc42 and its effector TOCA1: Handover of Cdc42 to the actin regulator N-WASP is facilitated by differential binding affinities. J Biol Chem. 2016;291:13875–13890. doi: 10.1074/jbc.M116.724294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei X, Lou H, Zhou D, Jia Y, Li H, Huang Q, Ma J, Yang Z, Sun C, Meng Y, Xu S, Yang X, Li X, Ji T, Guo Z, Gao Q. TAGLN mediated stiffness-regulated ovarian cancer progression via RhoA/ROCK pathway. J Exp Clin Cancer Res. 2021;40:292. doi: 10.1186/s13046-021-02091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Li W, He Z, Wang Y, Shao B, Cheng C, Zhang S, Tang M, Qian X, Kong W, Wang H, Chai R, Gao X. Pre-treatment with fasudil prevents neomycin-induced hair cell damage by reducing the accumulation of reactive oxygen species. Front Mol Neurosci. 2019;12:264. doi: 10.3389/fnmol.2019.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou T, Wang CH, Yan H, Zhang R, Zhao JB, Qian CF, Xiao H, Liu HY. Inhibition of the Rac1-WAVE2-Arp2/3 signaling pathway promotes radiosensitivity via downregulation of cofilin-1 in U251 human glioma cells. Mol Med Rep. 2016;13:4414–4420. doi: 10.3892/mmr.2016.5088. [DOI] [PubMed] [Google Scholar]
- 84.Zhu C, Cheng C, Wang Y, Muhammad W, Liu S, Zhu W, Shao B, Zhang Z, Yan X, He Q, Xu Z, Yu C, Qian X, Lu L, Zhang S, Zhang Y, Xiong W, Gao X, Xu Z, Chai R. Loss of ARHGEF6 causes hair cell stereocilia deficits and hearing loss in mice. Front Mol Neurosci. 2018;11:362. doi: 10.3389/fnmol.2018.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]


