Abstract
As three-dimensional “organ-like” aggregates, human cortical organoids have emerged as powerful models for studying human brain evolution and brain disorders with unique advantages of human-specificity, fidelity and manipulation. Human cortical organoids derived from human pluripotent stem cells can elaborately replicate many of the key properties of human cortical development at the molecular, cellular, structural, and functional levels, including the anatomy, functional neural network, and interaction among different brain regions, thus facilitating the discovery of brain development and evolution. In addition to studying the neuro-electrophysiological features of brain cortex development, human cortical organoids have been widely used to mimic the pathophysiological features of cortical-related disease, especially in mimicking malformations of cortical development, thus revealing pathological mechanism and identifying effective drugs. In this review, we provide an overview of the generation of human cortical organoids and the properties of recapitulated cortical development and further outline their applications in modeling malformations of cortical development including pathological phenotype, underlying mechanisms and rescue strategies.
Key Words: cortical development, disease models, human cortical organoids, human cortical spheroids, human pluripotent stem cells, malformations of cortical development, telencephalon organoids, whole brain organoids
Introduction
During the evolution of the human brain, the cerebral cortex is a key brain region that plays a key role in the enhanced behavioral, cognitive and higher intellectual capacity of human as compared to non-human species (Bystron et al., 2008; Geschwind and Rakic, 2013). The development of the cerebral cortex involves a series of elaborately coordinated developmental events; the disruption of these events can directly cause malformations of cortical development or other brain disorders (Barkovich et al., 2012). Different types of in vivo and in vitro non-human models have been used to study the properties of human cortical development and disease pathology, thus helping us to construct our existing knowledge framework of the cerebral cortex. However, these models are associated with certain limitations, including species differences and insufficient fidelity. Over recent years, human cortical organoids (hCOs) have emerged as a novel research model to study human cortical development under normal or abnormal conditions (Taverna et al., 2014; Di Lullo and Kriegstein, 2017).
Human brain organoids are three-dimensional “organ-like” tissues that self-organize from human pluripotent stem cells (hPSCs), such as human embryonic stem cells and human induced pluripotent stem cells (Qian et al., 2019). Human brain organoids can form elaborately organized regions of the cerebral cortex within tissue and are known as hCOs; these can effectively recapitulate the key properties of cortical development, such as diverse cell types, organized architecture, neural activities, functionality and interaction between different regions (Agboola et al., 2021; Fernandes et al., 2021). Based on these key features, hCOs predominantly include whole brain organoids (WBOs), telencephalon organoids, and human cortical spheroids (hCSs) (Kadoshima et al., 2013; Lancaster et al., 2013; Pasca et al., 2015). Of these, WBOs can spontaneously form forebrain, midbrain and hindbrain regions and more refined sub-regions including the cortex, hippocampus, ventral forebrain, choroid plexus and immature retina. Telencephalic organoids contain regions with a ventral identity similar to the lateral ganglionic eminences as well as a dorsal identity that is specific to the cerebral cortex. hCSs almost exclusively generate the cortical region, in addition to expressing a few choroid plexus cells. Many researchers have used hCOs to unravel the roles of genes, transcription factors, secreted proteins, receptors, and hormones in brain development in vivo to shed light on the mechanisms underlying brain development (Kyrousi et al., 2021; Kelava et al., 2022). With the introduction of reprogramming and genomic engineering technologies, hCOs have been increasingly used to mimic multifarious brain disease-specific models which can facilitate the study of human-specific disease pathogenesis and the pharmacological assessment of drugs that can restore or alleviate different disease phenotypes (Lancaster and Huch, 2019; Sidhaye and Knoblich, 2021). Moreover, hCOs are powerful tools for studying embryology, evolution, toxicology, and regenerative medicine (Tang et al., 2022). Our laboratory has established a research platform using WBOs and performed a series of studies in the field of brain injury, including the feasibility of WBOs for rescuing traumatic brain injury and ischemic stroke as a form of transplantation regenerative therapy and to generate an ischemic stroke model for discovering potential anti-stroke drugs (Wang et al., 2017, 2020b, c, 2022). In this narrative review, we provide a comprehensive overview of different types of hCOs in terms of their generation, cortical developmental properties, and their application in malformations of cortical development.
Retrieval Strategy
We searched the existing literature using the PubMed database with the following keywords to select articles for evaluation focusing on brain organoids, cerebral organoids, forebrain organoids, telencephalon organoids, and cortical organoids. Most of the selected studies (85% of all references) were published between 2013 and 2022.
The Generation of Human Cortical Organoids
Three classical methodologies for the generation of hCOs
The generation of hCOs is a multi-step process that can be divided into three stages: (1) embryoid bodies and ectoderm formation, (2) neural induction, and (3) expansion and long-term maturation, as shown in Figure 1 (Kadoshima et al., 2013; Lancaster et al., 2013; Pasca et al., 2015). The main roles of chemical supplements and physical culture conditions at different developmental stages are summarized in Table 1. First, dissociated hPSCs or intact hPSCs clones self-organize into three-dimensional aggregates referred to as embryoid bodies in ultra-low-binding plates; this is followed by ectoderm differentiation. This step represents the initiation of neural specification and factors that may contribute to non-neural fates (such as mesoderm and endoderm differentiation) are often eliminated or inhibited, such as serum, transforming growth factor-β (TGFβ) and WNT signaling factors (Ying et al., 2003; Di-Gregorio et al., 2007). Furthermore, a selective Rho-associated kinase inhibitor, Y27632, needs to be provided as a supplement for the first several days in order to reduce the apoptosis of dissociated hPSCs (Watanabe et al., 2007). Then, the ectodermal layer undergoes neural induction; this is a critical period for determining the fates of neural cells in different brain regions. Finally, various supplements and physical culture conditions that mimic the in vivo human environment are employed to promote the expansion and maturation of hCOs.
Figure 1.

Schematic diagram depicting the generation of hCOs.
The generation of hCOs includes three stages of embryoid bodies (EBs) and ectoderm formation, neural induction, expansion, and long-term maturation, regardless of the different development timeline. The whole brain organoids (WBOs) culture method relies on intrinsic signaling factors to spontaneously self-organize into muti-regional organoids, whereas the methods used to culture telencephalon organoids and human cortical spheroids (hCSs) are directed to a relatively single telencephalon or cortex region by introducing exogenous transforming growth factor-β (TGFβ) inhibitors and WNT inhibitors. Finally, distinct chemical or physical culture conditions are employed to facilitate the expansion and maturation of hCOs. CDLC: Chemically defined lipid concentrate; EGF: epidermal growth factor; FBS: fetal bovine serum; FGF: fibroblast growth factor; hCOs: human cortical organoids; hPSCs: human pluripotent stem cells.
Table 1.
The role of chemical supplements and physical culture conditions in the generation of human cortical organoids
| Developmental stage | Supplements or physical culture conditions | Roles | References |
|---|---|---|---|
| EBs and ectoderm formation | Y27632 | Reduce the apoptosis of dissociated hPSCs | Watanabe et al., 2007 |
| Fetal bovine serum | Stabilize ectoderm | Lupo et al., 2014 | |
| TGFβ inhibitor, WNT inhibitor | Reduce non-ectodermal fates and enhance telencephalon specification | Eiraku et al., 2008 | |
| Neural induction | WNT agonist, SHH antagonist | Induce dorsal telencephalon identity | Wang et al., 2021 |
| SHH agonist | Induce ventral telencephalon identity | Qian et al., 2016; Bagley et al., 2017; Birey et al., 2017 | |
| Expansion and long-term maturation | Matrigel | Promote the expansion of neuroepithelium | Sato et al., 2009 |
| BDNF, GDNF, TGFβ, and cAMP | Promote differentiation | Mariani et al., 2012; Li et al., 2017a; Miura et al., 2020; Xiang et al., 2021 | |
| Leukemia inhibitory factor | Increase the expression of outer radial glia cells and astrocytes | Watanabe et al., 2017 | |
| Platelet-derived growth factor AA and insulin-like growth factor 1 | Induce oligodendrocyte progenitor cells and promote oligodendrocyte differentiation | Madhavan et al., 2018; Marton et al., 2019 | |
| Spinning bioreactors, hyperoxia, periodically bisected, and slicing | Improve the supplement of oxygen and nutrients | Kadoshima et al., 2013; Lancaster et al., 2013; Qian et al., 2020 |
BDNF: Brain-derived neurotrophic factor; cAMP: cyclic adenosine monophosphate; EBs: embryoid bodies; GDNF: glial cell-derived neurotrophic factor; hPSCs: human pluripotent stem cells; SHH: sonic hedgehog; TGFβ: transforming growth factor-β.
The culture methodologies used for WBOs, telencephalon organoids, and hCSs, can be divided into three stages, as mentioned previously. Although there are many similarities in terms of culture systems, different types of hCOs are associated with differences in the culture timeline, medium composition, and physical culture conditions; these factors are important for the formation of individual cellular diversity and brain region composition (Kadoshima et al., 2013; Lancaster et al., 2013; Pasca et al., 2015). The culture system used for WBOs does not introduce exogenous TGFβ inhibitors and WNT inhibitors in the initial regulation of germ layer differentiation. In contrast, these inhibitors are supplemented in telencephalon organoids and hCSs culture methods to enhance neuronal conversion and telencephalon fate (Eiraku et al., 2008; Kadoshima et al., 2013; Lancaster and Knoblich, 2014; Pasca et al., 2015). TGFβ inhibitors, also known as SMAD inhibitors, commonly include bone morphogenetic proteins inhibitors (such as Noggin, Dorsomorphin, LDN193189, and BMPRIA-Fc), and Activin/Nodal inhibitors (such as Lefty and SB431542) (Zhou et al., 2010; Surmacz et al., 2012). Common WNT inhibitors include DKK1, IWR1, and IWP2, while WNT activators include WNT3a and CHIR99021 (Eiraku et al., 2008; Bagley et al., 2017). In contrast, the initial medium used for the culture of WBOs also contains fetal bovine serum and basic fibroblast growth factor, in which fibroblast growth factor may stabilize the ectoderm. Moreover, fetal bovine serum may be a key factor for the generation of mesoderm-derived cells in WBOs, as a previous study demonstrated that hPSCs were converted into cells derived from the mesoderm, endoderm and ectoderm in the presence of serum (Watanabe et al., 2005; Dang and Tropepe, 2006; Lupo et al., 2014). Following neural induction, the methods used to culture WBOs and telencephalon organoids are supplemented with Matrigel by bedding organoids or directly adding to the medium to provide a scaffold for neuroepithelial expansion, thus mimicking the basement membrane in vivo (Sato et al., 2009). Finally, the WBOs culture method adopts spinning bioreactors or alternative orbital shakers to promote the exchange of nutrients and oxygen, thus enabling long-term culture for up to 10 months. Telencephalon organoids need to be periodically cut into half size under hyperoxia culture conditions to facilitate the uptake of oxygen and nutrients to enable long-term culture over 13 weeks (Kadoshima et al., 2013). In contrast, the hCSs culture method, established by Pasca’s group, simplified the generation process without the need for extracellular matrix bedding, hyperoxia and rotational culture, thus achieving long-term culture for more than 24 months (Sloan et al., 2018; Yoon et al., 2019).
Modifications and optimizations of the method used to generate hCOs
Currently, there is no standardized method for the generation of hCOs. However, the classical methods have been modified and optimized to overcome certain limitations and adapt to different research purposes.
Studies have shown that only the superficial areas within a depth of 200 µm are fully permeable to oxygen and nutrients and that a lack of oxygen and nutrients inside the organoids can limit the late differentiation and maturation of hCOs (Pham et al., 2018; Qian et al., 2020). Therefore, developmental methods employed certain strategies during expansion and long-term maturation to improve the culture environment and promote differentiation or maturation. The most common optimization strategy is to supplement neurotrophic factors brain-derived neurotrophic factor and glial cell-derived neurotrophic factor, TGFβ and cyclic adenosine monophosphate, as well as dissolved Matrigel into the medium to promote the differentiation and maturation of hCOs (Mariani et al., 2012; Li et al., 2017a; Miura et al., 2020; Xiang et al., 2021). Another small molecule, CHIR99021, plays a slightly different role in different culture stages and can promote neuroepithelial expansion in the long-term culture stage; this factor also effectively reduces cell death when adding during the Matrigel embedding stage (Lancaster et al., 2017; Qian et al., 2018). Moreover, the addition of leukemia inhibitory factor activates STAT3 signaling, increases the generation of outer radial glia cells and astrocytes, and improves the formation of basement membrane (Watanabe et al., 2017). Some studies replaced basic DMEM/F12 and neurobasal medium with BrainPhys medium which supports neuronal functions such as action potential firing and synaptic activity (Kim et al., 2019; Fair et al., 2020; Dalgin et al., 2021). Orbital agitation or spinning bioreactors can also be replaced by hyperoxia culture and physical shearing in the telencephalon organoids culture method; this strategy extends long-term culture to at least 6 months (Velasco et al., 2019). Furthermore, periodic slicing the cultured hCOs into disk shapes in orbital shakers can ensure the effective diffusion of oxygen and nutrients (Qian et al., 2020).
Another modification strategy is intended to directionally induce specific cell fates and regional specification of brain. The WBOs culture method can also incorporate TGFβ inhibitors and optional WNT inhibitors to enhance neural fate (Albanese et al., 2020). A previous study demonstrated that the addition of dual SMAD inhibitors and WNT inhibitors during the early induction stage can effectively inhibit non-neural fate and enhance cortical identity (Rosebrock et al., 2022). Notably, WNT signaling factors direct cell fates towards dorsal telencephalon specification during the neural induction stage in a manner that is distinct from their role in the ectodermal formation stage (Wang et al., 2021). Typically, the WNT activator or sonic hedgehog (SHH) antagonist cyclopamine A is supplemented during the neural induction stage to enhance dorsal telencephalon identity, while the WNT inhibitor and SHH activator are added to the medium during the same stage to induce ventral forebrain identity (Qian et al., 2016; Bagley et al., 2017; Birey et al., 2017). In addition, reducing the concentration of heparin in the neural induction medium used for the culture of WBOs can enhance the expression of mesoderm-derived microglia (Ormel et al., 2018). In addition, several studies have complemented oligodendrocyte lineage cells by adding platelet-derived growth factor AA and insulin-like growth factor 1 to expand the formation of oligodendrocyte progenitor cells and promote oligodendrocyte differentiation and myelination (Madhavan et al., 2018; Marton et al., 2019).
Recapitulation of Cortical Developmental Properties in Human Cortical Organoids
Generally, one specific culture method is used to generate hCOs in an individual study, rather than generating different types of hCOs by the application of various culture methods. However, the cortical properties of different types of hCOs can differ. Thus, understanding the cortical developmental properties of different types of hCOs is vital when choosing an appropriate culture method to address the specific scientific question at hand. Overall, hCOs can mimic early human brain development spanning 8–24 weeks post-conception (Kelava and Lancaster, 2016). The three types of hCOs can exhibit similar cortical cell types and organized cortical architecture but with different neural activities and interactions within different brain regions.
Cell composition and organized structure
All types of hCOs preserve basic cortical cell types and organized structures similar to those of the human embryonic cortex, as shown in Figure 2 (Arlotta and Pasca, 2019). The cortex in hCOs is composed of diverse layer-specific radial glia cells, intermediate progenitor cells, outer radial glia cells, excitatory neurons and Cajal-Retzius cells, which are stereotypically organized into cortical laminar structures of the ventricular zone, subventricular zone, cortical plate, and marginal zone from the apical to the basal direction (Kadoshima et al., 2013; Quadrato et al., 2017; Yoon et al., 2019). In addition, telencephalon organoids recapitulate a cell-sparse intermediate zone and a Calretinin-marked subplate structure, whereas WBOs and hCSs do not generate these two zones. The major neural progenitor population of radial glia cells exhibits interkinetic nuclear migration behavior and undergoes symmetrical divisions to generate daughter neural progenitors and asymmetrical divisions to generate daughter neural progenitors and neurons (Kadoshima et al., 2013; Lancaster et al., 2013; Pasca et al., 2015), which are consistent with that observed in in vivo studies (Hartfuss et al., 2001). Astrocytes are an essential non-neuronal cellular component of the cortex which support the formation of neurons and synapses, and participate in neural circuits and the blood-brain barrier in vivo (Olsen et al., 2015; Appelt-Menzel et al., 2017). All hCOs can express markers of astrocytes. In particular, the astrocytes in hCSs exhibit a higher level of maturity and key functional properties of synaptic phagocytosis after long-term culture beyond 20 months (Sloan et al., 2017). Another important cortical cell composition, which is essentially absent in hCOs, is oligodendrocytes. Telencephalon organoids lack the cell cluster of oligodendrocyte precursors (Velasco et al., 2019). Although WBOs rarely express oligodendrocyte progenitor cells, there is no expression of oligodendrocytes (Quadrato et al., 2017; Ziffra et al., 2021). hCSs induced by specific growth factors and hormones are capable of expressing oligodendrocyte progenitor cells and oligodendrocytes, and can form compact myelin in axons (Madhavan et al., 2018; Marton et al., 2019). Overall, non-directed WBOs exhibit more diverse cellular types, such as mesodermal cells and mesenchymal cells (Lancaster et al., 2013; Camp et al., 2015; Quadrato et al., 2017). It is worth mentioning that the cellular diversity of WBOs can sacrifice inter-organoid reproducibility, at least to some extent (Quadrato et al., 2017). When compared with WBOs, individual telencephalon organoids and hCSs are relatively homogeneous and can reproducibly generate cortical cells (Velasco et al., 2019; Yoon et al., 2019).
Figure 2.
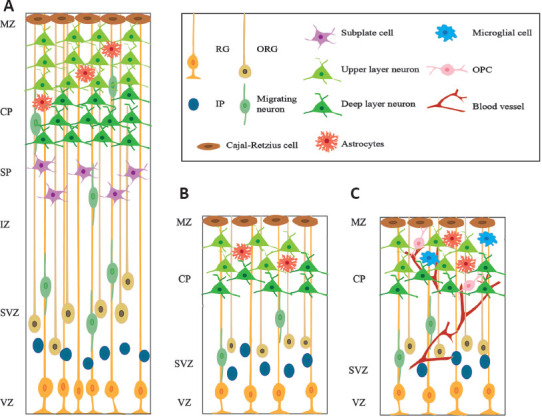
The cortical lamination structure of human embryonic cortex and hCOs.
(A) The human embryonic cortex is composed of a ventricular zone (VZ), subventricular zone (SVZ), intermediate zone (IZ), subplate zone (SP), cortical plate (CP) and marginal zone (MZ) with specific cortical cells at around 20 weeks of gestation. (B) HCOs spontaneously organize into similar cortical layers defined as the VZ, SVZ, CP, and MZ. (C) Missing non-ectoderm derived cell types in hCOs are being complemented in diverse ways. hCOs: Human cortical organoids; IP: intermediate progenitor cell; RG: radial glial cell; ORG: outer radial glial cell; OPC: oligodendrocyte progenitor cell.
The in vivo generation of diverse cell types follows a temporal sequence, and newborn excitatory glutamatergic neurons can migrate radially to their destined positions in an “inside-out” pattern (Dehay and Kennedy, 2007; Silbereis et al., 2016). WBOs can reproduce the temporal sequence of generation for a diverse range of cortical cells. For example, Cajal-Retzius cells in WBOs are first generated and then migrate into the most superficial layer; subsequently, progenitors sequentially generate deep-layer neurons that express CTIP2 and a superficial layer of neurons that express SATB2; finally, the expression of astrocytes is enhanced after approximately 100 days of culture (Molyneaux et al., 2007; Renner et al., 2017). Neurogenesis in hCOs can also reproduce the stereotypic “inside-out” pattern in which late-born cortical neurons migrate to the more superficial side with early-born neurons located inside (Kadoshima et al., 2013; Lancaster et al., 2013).
Neural activity and function
Neural activity and functional neural networks underlie brain functionality and are indispensable for modeling brain disorders. There are action potentials in hCOs that can be blocked by the voltage-gated sodium-channel antagonist tetrodotoxin. Variations in neural network activity can be assessed by electrophysiological means via calcium imaging, patch clamping and multi-electrode arrays; this activity is directly related to the dynamic development and maturation of multiple neurons and glial cells (Lancaster et al., 2013; Pasca et al., 2015; Watanabe et al., 2017; Fair et al., 2020). When cultured for 76 to 104 days, telencephalon organoids exhibit spontaneous excitatory postsynaptic currents and spontaneous calcium dynamics, but with little synchronized activity in asynchronous calcium transients, thus implying relatively immature neural network functionality (Li et al., 2017a; Sakaguchi et al., 2019). WBOs not only exhibit calcium surges, they also possess a spontaneous active neuronal network in eight-month-old organoids (Quadrato et al., 2017). The electrophysiological properties of WBOs is dynamically mature, with weak spiking activities appeared after 1 month of culture, and increased synchronized burst firings from 120 to 161 days (Fair et al., 2020). WBOs also exhibit theta frequency oscillations that are synchronized with neuronal population bursts (Sharf et al., 2022). Synchronized burst firing and oscillatory activity with distinct frequencies are typical features of functional neural networks. hCSs also exhibit functional neural network activities that accompany developmental programming (Pasca et al., 2015; Trujillo et al., 2019). Research has also shown that 130-day-old hCSs exhibit spontaneous calcium spikes and that 180-day-old hCSs exhibit abundant synaptogenesis with spontaneous synaptic activity that can be completely blocked by pharmacological exposure to glutamate receptor antagonists (Pasca et al., 2015). hCSs exhibit spontaneous network events with developmental oscillations that transform from periodic and regular nested oscillations to more complex and variable oscillations, and the synchronous network events are similar to some features that have been observed in the electroencephalography of human preterm infants (Trujillo et al., 2019). Furthermore, fused organoids composed of hCOs and ganglionic eminence organoids exhibit neural oscillations with multiple frequencies that do not occur in unfused hCOs, thus indicating the important role of inhibitory interneurons in neural network activity (Samarasinghe et al., 2021).
Interaction between the cerebral cortex and other brain regions
Neural interactions between distinct brain regions, such as interneuron migration and axon tract extension, are very important for brain development and functionality (Bagley et al., 2017). Cortical interneurons primarily originate from the ventral telencephalon and integrate into the cortical neural network after long distance migration (Wonders and Anderson, 2006). The dorsal region of WBOs express positive immunostaining for interneurons, thus indicating the potential for interneuron migration (Lancaster et al., 2013). However, considering the variability and random generation of the ventral forebrain, WBOs are not an ideal model for detailed analysis of the interactions between different brain regions (Lancaster et al., 2013). Furthermore, the classic method for hCS culture only produces excitatory glutamatergic neurons, which also restricts the study of interneuron migration (Pasca et al., 2015). A novel modular strategy applied a fusion paradigm that fuses hCSs with ventral human forebrain spheroids or more refined human medial ganglionic eminence organoids, thus recapitulating the saltatory directional migration of GABAergic interneurons from the ventral forebrain to the dorsal forebrain and the formation of an integrated neural network (Bagley et al., 2017; Birey et al., 2017; Xiang et al., 2017).
Another important structural basis for interaction between the cerebral cortex and other brain regions is the axon tract which is responsible for connecting different brain regions and transmitting information (Swanson et al., 2017). Glutamatergic neurons in the cerebral cortex project to other brain regions and form functional circuits, such as the cortico-striatal circuits that are important for the regulation of motivated behaviors and movements, and the corticothalamic pathways that are important in transmitting sensorimotor information (Lopez-Bendito and Molnar, 2003; Stiles and Jernigan, 2010). Cortico-striatal assembloids can recapitulate YFP+ projections oriented from cortical neurons in the hCSs to neurons in human striatal spheroids connected by functional synapses (Miura et al., 2020). Research has shown that mCherry-labeled thalamus-like organoids and green fluorescent protein-labeled hCO assembloids can recapitulate reciprocal axon projections in the presence of specific cell feeders (Xiang et al., 2019). In addition, cortical glutamatergic neurons connect with the hindbrain and spinal cord through long-distance axons, thus forming functional cortico-motor circuits that activate muscles and produce movement (Shim et al., 2012; Kiehn, 2016). Recently, more complex three-component assembloids, containing hCSs, human spinal spheroids and human skeletal muscle spheroids, have been constructed by Pasca’s group, which can recapitulate the activation of muscle contractions by cortical neurons under glutamate uncaging photo-stimulation or optogenetic stimulation (Andersen et al., 2020). In addition to these interactions among brain organoids, one recent study has reported functional interactions between post-transplanted hCOs and the somatosensory cortex of rats (Revah et al., 2022). These post-transplanted hCOs can receive thalamocortical and corticocortical projections that could be activated by surrounding stimuli, and also extend axons to the brain that could drive reward-seeking behavior. Overall, hCOs not only provide an ideal model for investigating neural crosstalk and neural connections during brain development; they also promote the exploration and validation of specific actions or other manifestations controlled by specific regions of the brain cortex.
Application of Human Cortical Organoids in Modeling Malformations of Cortical Development
Abnormal cortical development caused by genetic, infectious, vascular, and metabolic factors, will lead to malformations of cortical development, a class of heterogeneous brain disorders that manifest as developmental delay, cerebral palsy or seizures (Severino et al., 2020). Specifically, malformations of cortical development mainly exhibit three abnormal phenotypes, including abnormal neuronal and glial proliferation or apoptosis, abnormal neuronal migration, and abnormal post-migration development (Barkovich et al., 2012). Since three-dimensional hCOs can efficiently reproduce the key developmental properties of the cortex, including progenitor proliferation, neuronal migration and organized structure, patient-derived or gene-edited hCOs have been used to investigate pathological phenotypes, underlying pathogenic mechanisms, and rescue strategies, for different types of malformations of cortical development, as shown in Table 2.
Table 2.
Application of hCOs in modeling malformations of cortical development
| Disease | Organoids type | Modeling disease method | Disease phenotypes | Disease mechanism | Rescue strategy | Reference |
|---|---|---|---|---|---|---|
| Primary microcephaly | WBOs | CDK5RAP2 mutation | Smaller neural tissues, reduced neuroepithelial tissues, decreased RGs, and increased neurons | Loss CDK5RAP2 protein | Introducing CDK5RAP2 protein | Lancaster et al., 2013 |
| WBOs | ASPM mutation | Loss of neural progenitors, reduced calcium activity, and asynchronized neuronal activity | / | / | Li et al., 2017a | |
| Telencephalon organoids | NARS1 mutation | Smaller cortical organoids and reduced proliferation of RGs | / | / | Wang et al., 2020a | |
| Virus infected microcephaly | hCSs | ZIKV infection | Reduced organoids size, increased cell death, and reduced proliferation | / | / | Qian et al., 2016 |
| Telencephalon organoids | ZIKV infection | Reduced organoids size and progenitor apoptosis | / | Cholesterol 25-hydroxylase, R428, Duramycin, Ivermectin | Watanabe et al., 2017 | |
| WBOs | A clinical-like Cytomegalovirus exposure | Decreased proliferation cells, increased apoptotic cells, reduced calcium signaling, and neural network activity | PDGFRa and EGFR are involved in HCMV infection of the brain organoids | Neutralizing antibodies Nabs | Sun et al., 2020 | |
| WBOs | Cytomegalovirus infection | Impaired organized structure, reduced calcium level, and lost response to stimulation | / | Cytomegalovirus inhibitor maribavir | Sison et al., 2019 | |
| WBOs | Cytomegalovirus infection | Downregulated cortical neurodevelopmental and functional genes | / | / | O’Brien et al., 2022 | |
| Macrocephaly | WBOs | RAB39b mutation | Enlarged size and increased proliferation | Promoted PI3K-AKT-mTOR signaling | / | Zhang et al., 2020 |
| Pretzel syndrome | hCSs | STRADA mutation | Increased size, delayed neurogenesis, increased proliferation, disrupted primary cilia architecture, and subsequent decreased oRGs | / | / | Dang et al., 2021 |
| Tuberous sclerosis complex | hCSs | Homozygous deletion of TSC1 or TSC2 | Reduced or delayed neurons, increased glial cells, and morphological changes of neurons and glial cells | Hyperactivation of mTORC1 signaling | Rapamycin | Blair et al., 2018 |
| Periventricular heterotopia | WBOs | DCHS1 and FAT4 mutations | Disrupted morphology in neural progenitors and abnormal migration dynamics of a subset of neurons | Relatively high expression of GNG5 gene | / | Klaus et al., 2019; Ayo-Martin et al., 2020 |
| Miller Dieker syndrome | Telencephalon organoids | Patient derivation | Smaller organoids, increased apoptosis and decreased vertical divisions of neural progenitors, abnormal neuronal migration, and mitosis defect of oRGs | / | / | Bershteyn et al., 2017 |
| Forebrain organoids | Patient derivation | Reduced organoid size, altered organization of microtubule network and ventricular niche, and distribution adhesion molecules | Non-cell-autonomous disruption of N-cadherin/β-catenin/WNT signaling axis | WNT activator CHIR99021 | Iefremova et al., 2017 | |
| Rett syndrome | WBOs | MECP2 mutation | Increased ventricular area, decreased radial thickness, alterations in neurogenesis and neuronal differentiation, and migration defects | Upregulated miR-199 and miR-214 regulate ERK and AKT signalings | / | Mellios et al., 2018 |
| hCSs | MECP2 mutation | Premature differentiation of immature neurons, decreased progenitors, and interneurons migration defects | / | / | Gomes et al., 2020 | |
| Assembloids of hCOs and medial ganglionic eminence organoids | MECP2 mutation | Synapse formation defects, hyperexcitability and hypersynchrony, and epileptiform-appearing spikes and high frequency oscillations | / | Pifithrin-α | Samarasinghe et al., 2021 | |
| Angelman syndrome | WBOs | UBE3A mutation | Early silencing of UBE3A protein and higher calcium transient frequencies | / | Topoisomerase inhibitors topotecan or indotecan | Sen et al., 2020 |
| hCSs | UBE3A mutation | Augmented neuronal excitability and elevated after-hyperpolarization | Augmented big potassium channels | Big potassium antagonist paxilline | Sun et al., 2019 |
hCOs: Human cortical organoids; hCSs: human cortical spheroids; oRGs: outer radial glia cells; RGs: radial glia cells; WBOs: whole brain organoids.
Microcephaly
Primary microcephaly is a genetically heterogeneous disorder with several identified associated mutations of genetic loci, including MCPH1, WDR62, CDK5RAP2, and ASPM (Passemard et al., 2013). HCOs carrying specific genetic mutations of primary microcephaly exhibit a reduced size, a key phenotype of primary microcephaly. WBOs derived from patients with CDK5RAP2 mutations exhibit smaller neural tissues and reduced neuroepithelial tissues, with a reduced number of radial glia cells and an increased number of neurons; these manifestations are indicative of the premature neural differentiation caused by the loss of CDK5RAP2 protein (Lancaster et al., 2013). Another microcephalic model of WBOs derived from patients with ASPM mutation or ASPM-knockdown lines also exhibit a severe reduction in size, the absence of a cortical laminar structure, and defective progenitor proliferation with the loss of neural progenitors (Li et al., 2017a). In addition, functional dysregulation was observed and manifested by reduced calcium activity and asynchronized neuronal activity (Li et al., 2017a). Telencephalon organoids derived from patients with microcephaly and NARS1 mutations also had a reduced organoid size with reduced proliferation of radial glia cells (Wang et al., 2020a).
In addition to genetic mutations, viral infections can also cause microcephaly, including ZIKV virus, cytomegalovirus, herpes simplex virus, and rubella virus (Devakumar et al., 2018). HCOs exposed to ZIKV virus and cytomegalovirus also exhibit a microcephalic phenotype. ZIKV virus has been shown to invade hCSs and predominantly target neuron progenitors, thus resulting in reduced thickness of the ventricular zone and neuronal layer and an enlarged lumen, possibly associated with a reduction in the number of proliferating cells and increased cell death (Qian et al., 2016). Watanabe et al. (2017) also reported dramatically increased cell death and the reduced overall size of ZIKV-infected telencephalon organoids. They also proposed that innate immune responses may contribute to ZIKV-triggered programmed cell death and tested whether multiple drugs could effectively ameliorate the impaired phenotype. Cytomegalovirus-infected WBOs exhibited severe neurodevelopmental disorders and neural function defects, including a reduced number of proliferating cells, an increased number of apoptotic cells, impairment in structural organization, and reduced calcium signaling and neural network activity; these phenotypes were effectively prevented by Nabs, an antibody that specifically targets the cytomegalovirus pentamer complex (Sison et al., 2019; Sun et al., 2020). Transcriptional analysis also demonstrated that human cytomegalovirus can cause the downregulation of cortical development and functional genes in WBOs (O’Brien et al., 2022).
Macrocephaly
Macrocephaly refers to a general increase in head size with an occipital-frontal circumference that exceeds the mean for age and gender by at least two standard deviations (Severino et al., 2020). A previous study reported that RAB39b-mutated WBOs successfully reproduced the key phenotype of human macrocephaly of increasing head size and proliferation (Zhang et al., 2020). The underlying mechanism may be that RAB39b deletion promotes PI3K-AKT-mTOR signaling in neuronal progenitors; furthermore, the inhibition of AKT signaling was shown to rescue these disease phenotypes.
Pretzel syndrome
Pretzel syndrome, also known as polyhydramnios, megalocephaly, and symptomatic epilepsy syndrome, is caused by homozygous germline mutations in the STRADA gene (Puffenberger et al., 2007). HCSs derived from patients with STRADA mutation show an increased size and other phenotypes such as delayed neurogenesis, increased proliferation, disrupted primary cilia architecture, along with a reduced number of outer radial glia cells (Dang et al., 2021).
Tuberous sclerosis complex
Tuberous sclerosis complex is a multisystem disorder with the hallmark pathological feature of cortical tubers and is caused by germline heterozygous mutations in the TSC1 or TSC2 genes that regulate hyperactivation of the mTOR pathway (Blair and Bateup, 2020). hCSs possessing the homozygous deletion of TSC1 or TSC2 exhibit the reduced expression of neuronal markers or delayed neurogenesis, an increased expression of glial cell markers, and morphological changes (Blair et al., 2018). Rapamycin can reverse these phenotypes by blocking mTORC1 signaling at key developmental stages.
Periventricular heterotopia
Periventricular heterotopia is characterized by the dysregulated migration of newborn neurons located in the lateral ventricles (Romero et al., 2018). Several gene mutations are implicated in this pathology, including FLNA, ARFGEF2, DCHS1, and FAT4 (Stouffer et al., 2016). WBOs derived from patients with DCHS1 and FAT4 mutations or from isogenic knockout lines can successfully recapitulate periventricular heterotopia-related phenotypes of disrupted morphology in neural progenitors and the abnormal migration dynamics in a subset of neurons (Klaus et al., 2019). Relatively high expression levels of the GNG5 gene may be an underlying factor that contributes to the abnormal phenotypes of periventricular heterotopia (Klaus et al., 2019; Ayo-Martin et al., 2020).
Miller Dieker syndrome
Miller Dieker syndrome is a severe form of classical lissencephaly that is caused by microdeletion within chromosome 17p13.3 and exhibits symptoms of intellectual disability, seizures, and craniofacial dysmorphisms (Blazejewski et al., 2018). Three-dimensional telencephalon organoids derived from patients with Miller Dieker syndrome have helped to investigate disease-related phenotype defects, including increased apoptosis and reduced vertical cell divisions of neural progenitors, abnormal neuronal migration, as well as the prolonged mitotic cycle of outer radial glia cells (Bershteyn et al., 2017). Iefremova et al. (2017) reproduced consistent Miller Dieker syndrome-related phenotypes in a similar organoid system, including reduced cortical expansion, altered organization in the ventricular niche and adhesion molecules, as well as non-cell-autonomous disruption in the N-cadherin/β-catenin/WNT signaling axis. Furthermore, administration of the WNT activator CHIR99021 was shown to rescue the reduced expansion phenotype (Iefremova et al., 2017).
Rett syndrome
Rett syndrome, a rare neurodevelopmental disorder, is primarily caused by X-linked gene mutations in the MECP2 gene with the characteristic symptoms of loss of acquired speech and motor skills, repetitive hand movements and seizures (Kyle et al., 2018). Three-dimensional WBOs carrying MECP2 mutations exhibited an increased ventricular area, decreased radial thickness, altered neurogenesis and neuronal differentiation, and migration defects; these effects can be affected by the upregulation of miR-199 and miR-214 to regulate ERK and AKT signaling (Mellios et al., 2018). hCSs and ventral forebrain organoids and their assembloids derived from female patients with Rett syndrome with MECP2 mutations exhibited a reduced number of neural progenitors, the premature differentiation of immature neurons, and defects in interneuronal migration (Gomes et al., 2020). MECP2-mutant assembloids of hCOs and human medial ganglionic eminence organoids exhibited synapse formation defects, hyperexcitability and hypersynchrony, as well as epileptiform-like spikes and high frequency oscillations; these effects could be rescued by Pifithrin-α, a putative TP53 target inhibitor (Samarasinghe et al., 2021). MECP2-mutant interneurons have been shown to be the major cause of dysfunction in the neural network, as MECP2-mutant interneurons exhibited abnormalities in molecular and functional maturation in subsequent studies (Xiang et al., 2020; Samarasinghe et al., 2021).
Angelman syndrome
Angelman syndrome is a complex neurodevelopmental disorder that is characterized by delayed development, seizures, and intellectual disability; this condition is predominantly caused by the loss of ubiquitin ligase E3 UBE3A (Lopez et al., 2018). WBOs derived from maternal UBE3A-deleted Angelman syndrome patients replicated the early silencing of the UBE3A protein with higher calcium transient frequency; these effects could be partially rescued by topoisomerase inhibitors (Sen et al., 2020). Another form of UBE3A knockout hCSs exhibited augmented neuronal excitability and elevated after-hyperpolarization due to the augmentation of large potassium channels; the administration of the large potassium channel antagonist paxilline was shown to restore normal neuronal excitability and network activity (Sun et al., 2019).
In addition to malformations of cortical development modeled by hCOs as outlined in this review, hCOs have allowed the generation of a broad range of model systems for diseases such as psychiatric disorders, neurodegenerative diseases, and other brain disorders caused by trauma, infection, or cancer, thus providing a powerful platform for studying complex disease pathology as well as for drug development, as shown in Figure 3. For instance, hCOs exposed to severe acute respiratory syndrome coronavirus 2 have been applied to interrogate the infection mechanisms and neurotoxicity associated with the current global outbreak of corona virus disease 2019 (Ramani et al., 2020). Furthermore, hCOs are candidate sources for transplantation therapy in regenerative medicine with a sufficient and diverse range of cells and functional neural networks, thus contributing to the regeneration and reconstruction of functional neural networks in vivo.
Figure 3.
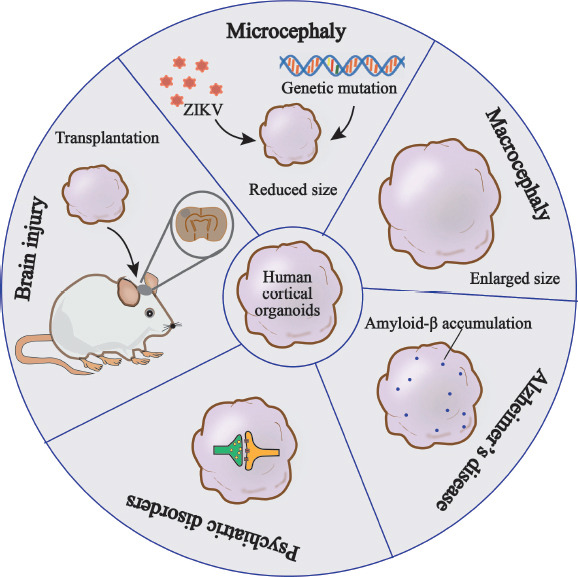
Overview of the application of hCOs in different disease models.
hCOs have been widely used in the study of neurodevelopmental diseases, neurodegenerative disease, neuropsychiatric disorders and other neurological diseases, such as brain injury. Disease models generated by hCOs can recapitulate key disease-specific phenotypes, such as the reduced head size associated with microcephaly, the enlarged head size associated with macrocephaly, the accumulation of amyloid-β in Alzheimer’s disease and the synaptic dysregulation in neuropsychiatric disorders. Furthermore, the transplantation of hCOs into brain injury models is a prominent field of research in regenerative medicine. hCOs: Human cortical organoids.
Limitations and Prospects
Although hCOs have been widely used to mimic human brain development and the modeling of different types of brain disorders, there are still unsolved limitations in with regards to the application of hCOs. For example, we do not yet have a full understanding of hCOs with regards to the fidelity of cell types, subtypes, and tissue structures.
The cell lineages of hCOs are largely derived from the ectoderm and rarely from the mesoderm; this means that hCOs are not expressed in endothelial cells and rarely expressed in microglia (Tanaka et al., 2020). Co-culture strategies involving the culture of hCOs with microglia or endothelial cells has been proposed to complement these missing cell types (Bejoy et al., 2019; Song et al., 2019). In addition, the in vivo transplantation of organoids into animals can provide a blood supply and nutritional support for organoids; this may represent a powerful means of constructing primary blood vessels within organoids as an in vivo organoid research model (Mansour et al., 2018; Pham et al., 2018; Shi et al., 2020). A previous study constructed vascularized hCOs with certain blood-brain barrier properties by employing vascular endothelial growth factor to induce vasculogenesis without impairing neurogenesis (Ham et al., 2020). The enhanced expression of endothelial signature proteoglycan clusters may generate precursor cells of endothelial lineage which may represent a key target for inducing angiogenesis (Tanaka et al., 2020).
Furthermore, the cortical structure of hCOs cannot convincingly replicate the subplate layer and subsequent canonical six layered structure of the cortical plate; neither can these structures preserve gyrencephalic folding and the complete spatial topography patterned by morphogen gradients. The subplate is a transient layer in the process of cortical development and plays crucial roles in cortical connections, microcircuitry, and synaptogenesis (Molnar et al., 2019). Organotypic culture of fetal human brain has contributed to investigations of subplate-related synaptic networks (McLeod et al., 2022). Transcription factors and growth factors have been demonstrated to play essential roles in regulating cortical expansion and folding (Rash et al., 2013; Lui et al., 2014). A genetically modified hCO system that deletes the PTEN gene and sequentially enhances the PTEN-AKT signaling pathway has been used to reproduce cortical expansion and continuous folding (Li et al., 2017b). A previous study also engineered a hCO system possessing a triggered SHH protein gradient to refine the patterning and topographical organization of the forebrain region (Cederquist et al., 2019).
In addition, heterogeneity among individual organoids, even in one culture batch, remains an obstacle that still needs to be overcome. Studies have shown that multiple factors such as hPSCs linage, signaling factors, extracellular matrix, and spinning bioreactors are responsible for the variability observed in hCOs, and that variations mostly occur in the neural induction stage (Yoon et al., 2019; Strano et al., 2020). In the future, developing new signaling factors and a matrix with definite components is of great significance for the development of hCOs. The transcriptomic trajectories of the human brain developing in vivo provide a path to explore key signaling factors to pattern hCOs more specifically. In addition, hyaluronic acid, a major extracellular matrix in the human brain, may be considered as an alternative extracellular matrix that may activate WNT signaling and exhibit a caudalizing effect when combined with heparin (Bejoy et al., 2018; Yi et al., 2021). The combination of organoids with biomaterials, nanotechnology, and bioengineering strategies such as three-dimensional (3D) printers and microfluidic devices, may ameliorate these limitations and drive the development and refinement of the organoids field.
Conclusions
As a novel versatile model with unique advantages, hCOs possess tremendous potential for uncovering unknown mechanisms related to brain development and even the trajectory of embryonic development, thus making it possible to investigate early brain developmental events and disease mechanisms in the human brain. In addition to the applications mentioned earlier, broader applications of organoids, including hCOs, are already being investigated. For example, culturing individual specific hCOs in combination with genome-editing technology or cell trans-differentiation may help to identify defects in fetal brain development by introducing whole genome sequencing and proteomics; these strategies might be important for reproductive optimization. There is no doubt that extensive study in the future will enable hCOs to better mimic human brain structure and function, thereby strengthening basic and clinical studies involving hCOs.
Footnotes
Author contributions: XPZ and XYW retrieved and analyzed concerned literatures. XPZ and SNW designed and wrote the manuscript. CYM designed and revised the manuscript. All the authors approved the final version of the manuscript.
Conflicts of interest: The authors declare no conflict of interest.
Data availability statement: No additional data are available.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Agboola OS, Hu X, Shan Z, Wu Y, Lei L. Brain organoid: a 3D technology for investigating cellular composition and interactions in human neurological development and disease models in vitro. Stem Cell Res Ther. 2021;12:430. doi: 10.1186/s13287-021-02369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese A, Swaney JM, Yun DH, Evans NB, Antonucci JM, Velasco S, Sohn CH, Arlotta P, Gehrke L, Chung K. Multiscale 3D phenotyping of human cerebral organoids. Sci Rep. 2020;10:21487. doi: 10.1038/s41598-020-78130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen J, Revah O, Miura Y, Thom N, Amin ND, Kelley KW, Singh M, Chen X, Thete MV, Walczak EM, Vogel H, Fan HC, Pasca SP. Generation of functional human 3D cortico-motor assembloids. Cell. 2020;183:1913–1929.e1926. doi: 10.1016/j.cell.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelt-Menzel A, Cubukova A, Gunther K, Edenhofer F, Piontek J, Krause G, Stuber T, Walles H, Neuhaus W, Metzger M. Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri- and multipotent stem cells. Stem Cell Reports. 2017;8:894–906. doi: 10.1016/j.stemcr.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arlotta P, Pasca SP. Cell diversity in the human cerebral cortex: from the embryo to brain organoids. Curr Opin Neurobiol. 2019;56:194–198. doi: 10.1016/j.conb.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Ayo-Martin AC, Kyrousi C, Di Giaimo R, Cappello S. GNG5 controls the number of apical and basal progenitors and alters neuronal migration during cortical development. Front Mol Biosci. 2020;7:578137. doi: 10.3389/fmolb.2020.578137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagley JA, Reumann D, Bian S, Levi-Strauss J, Knoblich JA. Fused cerebral organoids model interactions between brain regions. Nat Methods. 2017;14:743–751. doi: 10.1038/nmeth.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bejoy J, Wang Z, Bijonowski B, Yang M, Ma T, Sang QX, Li Y. Differential effects of heparin and hyaluronic acid on neural patterning of human induced pluripotent stem cells. ACS Biomater Sci Eng. 2018;4:4354–4366. doi: 10.1021/acsbiomaterials.8b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bejoy J, Yuan X, Song L, Hua T, Jeske R, Sart S, Sang QA, Li Y. Genomics analysis of metabolic pathways of human stem cell-derived microglia-like cells and the integrated cortical spheroids. Stem Cells Int. 2019;2019:2382534. doi: 10.1155/2019/2382534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bershteyn M, Nowakowski TJ, Pollen AA, Di Lullo E, Nene A, Wynshaw-Boris A, Kriegstein AR. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell. 2017;20:435–449.e434. doi: 10.1016/j.stem.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, O'Rourke NA, Steinmetz LM, Bernstein JA, Hallmayer J, Huguenard JR, Pasca SP. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair JD, Hockemeyer D, Bateup HS. Genetically engineered human cortical spheroid models of tuberous sclerosis. Nat Med. 2018;24:1568–1578. doi: 10.1038/s41591-018-0139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair JD, Bateup HS. New frontiers in modeling tuberous sclerosis with human stem cell-derived neurons and brain organoids. Dev Dyn. 2020;249:46–55. doi: 10.1002/dvdy.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blazejewski SM, Bennison SA, Smith TH, Toyo-Oka K. Neurodevelopmental genetic diseases associated with microdeletions and microduplications of chromosome 17p13.3. Front Genet. 2018;9:80. doi: 10.3389/fgene.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 17.Camp JG, Badsha F, Florio M, Kanton S, Gerber T, Wilsch-Brauninger M, Lewitus E, Sykes A, Hevers W, Lancaster M, Knoblich JA, Lachmann R, Paabo S, Huttner WB, Treutlein B. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A. 2015;112:15672–15677. doi: 10.1073/pnas.1520760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cederquist GY, Asciolla JJ, Tchieu J, Walsh RM, Cornacchia D, Resh MD, Studer L. Specification of positional identity in forebrain organoids. Nat Biotechnol. 2019;37:436–444. doi: 10.1038/s41587-019-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalgin G, Tryba AK, Cohen AP, Park SY, Philipson LH, Greeley SAW, Garcia AJ., 3rd Developmental defects and impaired network excitability in a cerebral organoid model of KCNJ11 p V59M-related neonatal diabetes. Sci Rep. 2021;11:21590. doi: 10.1038/s41598-021-00939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang L, Tropepe V. Neural induction and neural stem cell development. Regen Med. 2006;1:635–652. doi: 10.2217/17460751.1.5.635. [DOI] [PubMed] [Google Scholar]
- 21.Dang LT, Vaid S, Lin G, Swaminathan P, Safran J, Loughman A, Lee M, Glenn T, Majolo F, Crino PB, Parent JM. STRADA-mutant human cortical organoids model megalencephaly and exhibit delayed neuronal differentiation. Dev Neurobiol. 2021;81:696–709. doi: 10.1002/dneu.22816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- 23.Devakumar D, Bamford A, Ferreira MU, Broad J, Rosch RE, Groce N, Breuer J, Cardoso MA, Copp AJ, Alexandre P, Rodrigues LC, Abubakar I. Infectious causes of microcephaly: epidemiology, pathogenesis, diagnosis, and management. Lancet Infect Dis. 2018;18:e1–13. doi: 10.1016/S1473-3099(17)30398-5. [DOI] [PubMed] [Google Scholar]
- 24.Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 2017;18:573–584. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di-Gregorio A, Sancho M, Stuckey DW, Crompton LA, Godwin J, Mishina Y, Rodriguez TA. BMP signalling inhibits premature neural differentiation in the mouse embryo. Development. 2007;134:3359–3369. doi: 10.1242/dev.005967. [DOI] [PubMed] [Google Scholar]
- 26.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Fair SR, Julian D, Hartlaub AM, Pusuluri ST, Malik G, Summerfied TL, Zhao G, Hester AB, Ackerman WE, 4th, Hollingsworth EW, Ali M, McElroy CA, Buhimschi IA, Imitola J, Maitre NL, Bedrosian TA, Hester ME. Electrophysiological maturation of cerebral organoids correlates with dynamic morphological and cellular development. Stem Cell Reports. 2020;15:855–868. doi: 10.1016/j.stemcr.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes S, Klein D, Marchetto MC. Unraveling human brain development and evolution using organoid models. Front Cell Dev Biol. 2021;9:737429. doi: 10.3389/fcell.2021.737429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes AR, Fernandes TG, Vaz SH, Silva TP, Bekman EP, Xapelli S, Duarte S, Ghazvini M, Gribnau J, Muotri AR, Trujillo CA, Sebastiao AM, Cabral JMS, Diogo MM. Modeling rett syndrome with human patient-specific forebrain organoids. Front Cell Dev Biol. 2020;8:610427. doi: 10.3389/fcell.2020.610427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ham O, Jin YB, Kim J, Lee MO. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem Biophys Res Commun. 2020;521:84–90. doi: 10.1016/j.bbrc.2019.10.079. [DOI] [PubMed] [Google Scholar]
- 32.Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- 33.Iefremova V, Manikakis G, Krefft O, Jabali A, Weynans K, Wilkens R, Marsoner F, Brandl B, Muller FJ, Koch P, Ladewig J. An organoid-based model of cortical development identifies non-cell-autonomous defects in Wnt signaling contributing to Miller-Dieker Syndrome. Cell Rep. 2017;19:50–59. doi: 10.1016/j.celrep.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 34.Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A. 2013;110:20284–20289. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelava I, Lancaster MA. Stem cell models of human brain development. Cell Stem Cell. 2016;18:736–748. doi: 10.1016/j.stem.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Kelava I, Chiaradia I, Pellegrini L, Kalinka AT, Lancaster MA. Androgens increase excitatory neurogenic potential in human brain organoids. Nature. 2022;602:112–116. doi: 10.1038/s41586-021-04330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci. 2016;17:224–238. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Xu R, Padmashri R, Dunaevsky A, Liu Y, Dreyfus CF, Jiang P. Pluripotent stem cell-derived cerebral organoids reveal human oligodendrogenesis with dorsal and ventral origins. Stem Cell Reports. 2019;12:890–905. doi: 10.1016/j.stemcr.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klaus J, Kanton S, Kyrousi C, Ayo-Martin AC, Di Giaimo R, Riesenberg S, O'Neill AC, Camp JG, Tocco C, Santel M, Rusha E, Drukker M, Schroeder M, Gotz M, Robertson SP, Treutlein B, Cappello S. Altered neuronal migratory trajectories in human cerebral organoids derived from individuals with neuronal heterotopia. Nat Med. 2019;25:561–568. doi: 10.1038/s41591-019-0371-0. [DOI] [PubMed] [Google Scholar]
- 40.Kyle SM, Vashi N, Justice MJ. Rett syndrome: a neurological disorder with metabolic components. Open Biol. 2018;8:170216. doi: 10.1098/rsob.170216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyrousi C, O'Neill AC, Brazovskaja A, He Z, Kielkowski P, Coquand L, Di Giaimo R, D'Andrea P, Belka A, Forero Echeverry A, Mei D, Lenge M, Cruceanu C, Buchsbaum IY, Khattak S, Fabien G, Binder E, Elmslie F, Guerrini R, Baffet AD, et al. Extracellular LGALS3BP regulates neural progenitor position and relates to human cortical complexity. Nat Commun. 2021;6298;12 doi: 10.1038/s41467-021-26447-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014;9:2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, Knoblich JA. Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol. 2017;35:659–666. doi: 10.1038/nbt.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lancaster MA, Huch M. Disease modelling in human organoids. Dis Model Mech. 2019;12:dmm039347. doi: 10.1242/dmm.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li R, Sun L, Fang A, Li P, Wu Q, Wang X. Recapitulating cortical development with organoid culture in vitro and modeling abnormal spindle-like (ASPM related primary) microcephaly disease. Protein Cell. 2017a;8:823–833. doi: 10.1007/s13238-017-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M, Gehrke L, Knoblich JA, Jaenisch R. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell. 2017b;20:385–396.e383. doi: 10.1016/j.stem.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez SJ, Segal DJ, LaSalle JM. UBE3A: An E3 ubiquitin ligase with genome-wide impact in neurodevelopmental disease. Front Mol Neurosci. 2018;11:476. doi: 10.3389/fnmol.2018.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Bendito G, Molnar Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- 50.Lui JH, Nowakowski TJ, Pollen AA, Javaherian A, Kriegstein AR, Oldham MC. Radial glia require PDGFD-PDGFRbeta signalling in human but not mouse neocortex. Nature. 2014;515:264–268. doi: 10.1038/nature13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lupo G, Bertacchi M, Carucci N, Augusti-Tocco G, Biagioni S, Cremisi F. From pluripotency to forebrain patterning: an in vitro journey astride embryonic stem cells. Cell Mol Life Sci. 2014;71:2917–2930. doi: 10.1007/s00018-014-1596-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, Karl M, Clayton BLL, Factor DC, Allan KC, Barbar L, Jain T, Douvaras P, Fossati V, Miller RH, Tesar PJ. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat Methods. 2018;15:700–706. doi: 10.1038/s41592-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mansour AA, Goncalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36:432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, Horvath TL, Vaccarino FM. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, Huguenard JR, Pasca SP. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. 2019;22:484–491. doi: 10.1038/s41593-018-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLeod F, Dimtsi A, Marshall AC, Lewis-Smith D, Thomas R, Clowry GJ, Trevelyan AJ. Altered synaptic connectivity in an in vitro human model of STXBP1 encephalopathy. Brain. 2022:awac396. doi: 10.1093/brain/awac396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mellios N, Feldman DA, Sheridan SD, Ip JPK, Kwok S, Amoah SK, Rosen B, Rodriguez BA, Crawford B, Swaminathan R, Chou S, Li Y, Ziats M, Ernst C, Jaenisch R, Haggarty SJ, Sur M. MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol Psychiatry. 2018;23:1051–1065. doi: 10.1038/mp.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miura Y, Li MY, Birey F, Ikeda K, Revah O, Thete MV, Park JY, Puno A, Lee SH, Porteus MH, Pasca SP. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat Biotechnol. 2020;38:1421–1430. doi: 10.1038/s41587-020-00763-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molnar Z, Clowry GJ, Sestan N, Alzu'bi A, Bakken T, Hevner RF, Hppi PS, Kostovic I, Rakic P, Anton ES, Edwards D, Garcez P, Hoerder-Suabedissen A, Kriegstein A. New insights into the development of the human cerebral cortex. J Anat. 2019;235:432–451. doi: 10.1111/joa.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 61.O'Brien BS, Mokry RL, Schumacher ML, Pulakanti K, Rao S, Terhune SS, Ebert AD. Downregulation of neurodevelopmental gene expression in iPSC-derived cerebral organoids upon infection by human cytomegalovirus. iScience. 2022;25:104098. doi: 10.1016/j.isci.2022.104098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, Rouach N. New insights on astrocyte ion channels: critical for homeostasis and neuron-glia signaling. J Neurosci. 2015;35:13827–13835. doi: 10.1523/JNEUROSCI.2603-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ormel PR, Vieira de SáR, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, Johansen LE, van Dijk RE, Scheefhals N, Berdenis van Berlekom A, Ribes Martínez E, Kling S, MacGillavry HD, van den Berg LH, Kahn RS, Hol EM, de Witte LD, Pasterkamp RJ. Microglia innately develop within cerebral organoids. Nat Commun. 2018;9:4167. doi: 10.1038/s41467-018-06684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O'Rourke NA, Nguyen KD, Smith SJ, Huguenard JR, Geschwind DH, Barres BA, Pasca SP. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Passemard S, Kaindl AM, Verloes A. Microcephaly. Handb Clin Neurol. 2013;111:129–141. doi: 10.1016/B978-0-444-52891-9.00013-0. [DOI] [PubMed] [Google Scholar]
- 66.Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, Waldau B. Generation of human vascularized brain organoids. Neuroreport. 2018;29:588–593. doi: 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puffenberger EG, Strauss KA, Ramsey KE, Craig DW, Stephan DA, Robinson DL, Hendrickson CL, Gottlieb S, Ramsay DA, Siu VM, Heuer GG, Crino PB, Morton DH. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain. 2007;130:1929–1941. doi: 10.1093/brain/awm100. [DOI] [PubMed] [Google Scholar]
- 68.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc. 2018;13:565–580. doi: 10.1038/nprot.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qian X, Song H, Ming GL. Brain organoids: advances, applications and challenges. Development. 2019;146:dev166074. doi: 10.1242/dev.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, Su K, Li S, Lu L, Jacob F, Nguyen PTT, Huh S, Hoke A, Swinford-Jackson SE, Wen Z, Gu X, Pierce RC, Wu H, Briand LA, Chen HI, et al. Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell. 2020;26:766–781.e9. doi: 10.1016/j.stem.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, Boyden ES, Lichtman JW, Williams ZM, McCarroll SA, Arlotta P. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramani A, Müller L, Ostermann PN, Gabriel E, Abida-Islam P, Müller-Schiffmann A, Mariappan A, Goureau O, Gruell H, Walker A, Andrée M, Hauka S, Houwaart T, Dilthey A, Wohlgemuth K, Omran H, Klein F, Wieczorek D, Adams O, Timm J, et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;39:e106230. doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rash BG, Tomasi S, Lim HD, Suh CY, Vaccarino FM. Cortical gyrification induced by fibroblast growth factor 2 in the mouse brain. J Neurosci. 2013;33:10802–10814. doi: 10.1523/JNEUROSCI.3621-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Renner M, Lancaster MA, Bian S, Choi H, Ku T, Peer A, Chung K, Knoblich JA. Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J. 2017;36:1316–1329. doi: 10.15252/embj.201694700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Revah O, Gore F, Kelley KW, Andersen J, Sakai N, Chen X, Li MY, Birey F, Yang X, Saw NL, Baker SW, Amin ND, Kulkarni S, Mudipalli R, Cui B, Nishino S, Grant GA, Knowles JK, Shamloo M, Huguenard JR, et al. Maturation and circuit integration of transplanted human cortical organoids. Nature. 2022;610:319–326. doi: 10.1038/s41586-022-05277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romero DM, Bahi-Buisson N, Francis F. Genetics and mechanisms leading to human cortical malformations. Semin Cell Dev Biol. 2018;76:33–75. doi: 10.1016/j.semcdb.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 78.Rosebrock D, Arora S, Mutukula N, Volkman R, Gralinska E, Balaskas A, Aragones Hernandez A, Buschow R, Brandl B, Muller FJ, Arndt PF, Vingron M, Elkabetz Y. Enhanced cortical neural stem cell identity through short SMAD and WNT inhibition in human cerebral organoids facilitates emergence of outer radial glial cells. Nat Cell Biol. 2022;24:981–995. doi: 10.1038/s41556-022-00929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakaguchi H, Ozaki Y, Ashida T, Matsubara T, Oishi N, Kihara S, Takahashi J. Self-organized synchronous calcium transients in a cultured human neural network derived from cerebral organoids. Stem Cell Reports. 2019;13:458–473. doi: 10.1016/j.stemcr.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samarasinghe RA, Miranda OA, Buth JE, Mitchell S, Ferando I, Watanabe M, Allison TF, Kurdian A, Fotion NN, Gandal MJ, Golshani P, Plath K, Lowry WE, Parent JM, Mody I, Novitch BG. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat Neurosci. 2021;24:1488–1500. doi: 10.1038/s41593-021-00906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 82.Sen D, Voulgaropoulos A, Drobna Z, Keung AJ. Human cerebral organoids reveal early spatiotemporal dynamics and pharmacological responses of UBE3A. Stem Cell Reports. 2020;15:845–854. doi: 10.1016/j.stemcr.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Severino M, Geraldo AF, Utz N, Tortora D, Pogledic I, Klonowski W, Triulzi F, Arrigoni F, Mankad K, Leventer RJ, Mancini GMS, Barkovich JA, Lequin MH, Rossi A. Definitions and classification of malformations of cortical development: practical guidelines. Brain. 2020;143:2874–2894. doi: 10.1093/brain/awaa174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharf T, van der Molen T, Glasauer SMK, Guzman E, Buccino AP, Luna G, Cheng Z, Audouard M, Ranasinghe KG, Kudo K, Nagarajan SS, Tovar KR, Petzold LR, Hierlemann A, Hansma PK, Kosik KS. Functional neuronal circuitry and oscillatory dynamics in human brain organoids. Nat Commun. 2022;13:4403. doi: 10.1038/s41467-022-32115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, Li P, Guo L, Fang A, Chen R, Ge WP, Wu Q, Wang X. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020;18:e3000705. doi: 10.1371/journal.pbio.3000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shim S, Kwan KY, Li M, Lefebvre V, Sestan N. Cis-regulatory control of corticospinal system development and evolution. Nature. 2012;486:74–79. doi: 10.1038/nature11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sidhaye J, Knoblich JA. Brain organoids: an ensemble of bioassays to investigate human neurodevelopment and disease. Cell Death Differ. 2021;28:52–67. doi: 10.1038/s41418-020-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The cellular and molecular landscapes of the developing human central nervous system. Neuron. 2016;89:248–268. doi: 10.1016/j.neuron.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sison SL, O'Brien BS, Johnson AJ, Seminary ER, Terhune SS, Ebert AD. Human cytomegalovirus disruption of calcium signaling in neural progenitor cells and organoids. J Virol. 2019;93:e00954–19. doi: 10.1128/JVI.00954-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, Reimer R, Quake SR, Barres BA, Pasca SP. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron. 2017;95:779–790. doi: 10.1016/j.neuron.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sloan SA, Andersen J, Pasca AM, Birey F, Pasca SP. Generation and assembly of human brain region-specific three-dimensional cultures. Nat Protoc. 2018;13:2062–2085. doi: 10.1038/s41596-018-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song L, Yuan X, Jones Z, Vied C, Miao Y, Marzano M, Hua T, Sang QA, Guan J, Ma T, Zhou Y, Li Y. Functionalization of brain region-specific spheroids with isogenic microglia-like cells. Sci Rep. 2019;9:11055. doi: 10.1038/s41598-019-47444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stouffer MA, Golden JA, Francis F. Neuronal migration disorders: Focus on the cytoskeleton and epilepsy. Neurobiol Dis. 2016;92:18–45. doi: 10.1016/j.nbd.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strano A, Tuck E, Stubbs VE, Livesey FJ. Variable outcomes in neural differentiation of human PSCs arise from intrinsic differences in developmental signaling pathways. Cell Rep. 2020;31:107732. doi: 10.1016/j.celrep.2020.107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun AX, Yuan Q, Fukuda M, Yu W, Yan H, Lim GGY, Nai MH, D'Agostino GA, Tran HD, Itahana Y, Wang D, Lokman H, Itahana K, Lim SWL, Tang J, Chang YY, Zhang M, Cook SA, Rackham OJL, Lim CT, et al. Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science. 2019;366:1486–1492. doi: 10.1126/science.aav5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun G, Chiuppesi F, Chen X, Wang C, Tian E, Nguyen J, Kha M, Trinh D, Zhang H, Marchetto MC, Song H, Ming GL, Gage FH, Diamond DJ, Wussow F, Shi Y. Modeling human cytomegalovirus-induced microcephaly in human iPSC-derived brain organoids. Cell Rep Med. 2020;1:100002. doi: 10.1016/j.xcrm.2020.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Surmacz B, Fox H, Gutteridge A, Fish P, Lubitz S, Whiting P. Directing differentiation of human embryonic stem cells toward anterior neural ectoderm using small molecules. Stem Cells. 2012;30:1875–1884. doi: 10.1002/stem.1166. [DOI] [PubMed] [Google Scholar]
- 99.Swanson LW, Hahn JD, Sporns O. Organizing principles for the cerebral cortex network of commissural and association connections. Proc Natl Acad Sci U S A. 2017;114:E9692–9701. doi: 10.1073/pnas.1712928114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tanaka Y, Cakir B, Xiang Y, Sullivan GJ, Park IH. Synthetic analyses of single-cell transcriptomes from multiple brain organoids and fetal brain. Cell Rep. 2020;30:1682–1689.e1683. doi: 10.1016/j.celrep.2020.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang XY, Wu S, Wang D, Chu C, Hong Y, Tao M, Hu H, Xu M, Guo X, Liu Y. Human organoids in basic research and clinical applications. Signal Transduct Target Ther. 2022;7:168. doi: 10.1038/s41392-022-01024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taverna E, Gotz M, Huttner WB. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- 103.Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, Wang A, Wu W, Haddad GG, Chaim IA, Domissy A, Vandenberghe M, Devor A, Yeo GW, Voytek B, Muotri AR. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell. 2019;25:558–569. doi: 10.1016/j.stem.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, Paulsen B, Nguyen L, Adiconis X, Regev A, Levin JZ, Arlotta P. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature. 2019;570:523–527. doi: 10.1038/s41586-019-1289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, Li Z, Sievert D, Smith DEC, Mendes MI, Chen DY, Stanley V, Ghosh S, Wang Y, Kara M, Aslanger AD, Rosti RO, Houlden H, Salomons GS, Gleeson JG. Loss of NARS1 impairs progenitor proliferation in cortical brain organoids and leads to microcephaly. Nat Commun. 2020a;11:4038. doi: 10.1038/s41467-020-17454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang SN, Wang Z, Xu TY, Cheng MH, Li WL, Miao CY. Cerebral organoids repair ischemic stroke brain injury. Transl Stroke Res. 2020b;11:983–1000. doi: 10.1007/s12975-019-00773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang SN, Wang Z, Wang XY, Zhang XP, Xu TY, Miao CY. Humanized cerebral organoids-based ischemic stroke model for discovering of potential anti-stroke agents. Acta Pharmacol Sin. 2022 doi: 10.1038/s41401-022-00986-4. doi:10.1038/s41401-022-00986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang YF, Liu C, Xu PF. Deciphering and reconstitution of positional information in the human brain development. Cell Regen. 2021;10:29. doi: 10.1186/s13619-021-00091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Z, Wang SN, Xu TY, Miao ZW, Su DF, Miao CY. Organoid technology for brain and therapeutics research. CNS Neurosci Ther. 2017;23:771–778. doi: 10.1111/cns.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Z, Wang SN, Xu TY, Hong C, Cheng MH, Zhu PX, Lin JS, Su DF, Miao CY. Cerebral organoids transplantation improves neurological motor function in rat brain injury. CNS Neurosci Ther. 2020c;26:682–697. doi: 10.1111/cns.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 112.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 113.Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, Khakh BS, Coppola G, Pearson CA, Yamauchi K, Gong D, Dai X, Damoiseaux R, Aliyari R, Liebscher S, Schenke-Layland K, Caneda C, Huang EJ, Zhang Y, Cheng G, Geschwind DH, et al. Self-organized cerebral organoids with human-specific features predict effective drugs to combat Zika virus infection. Cell Rep. 2017;21:517–532. doi: 10.1016/j.celrep.2017.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 115.Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N, Cakir B, Kim KY, Lombroso AP, Hwang SM, Zhong M, Stanley EG, Elefanty AG, Naegele JR, Lee SH, Weissman SM, Park IH. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell. 2017;21:383–398. doi: 10.1016/j.stem.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, Sun P, Kang YJ, Zhong M, Liu X, Patra P, Lee SH, Weissman SM, Park IH. hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell. 2019;24:487–497. doi: 10.1016/j.stem.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xiang Y, Tanaka Y, Patterson B, Hwang SM, Hysolli E, Cakir B, Kim KY, Wang W, Kang YJ, Clement EM, Zhong M, Lee SH, Cho YS, Patra P, Sullivan GJ, Weissman SM, Park IH. Dysregulation of BRD4 function underlies the functional abnormalities of MeCP2 mutant neurons. Mol Cell. 2020;79:84–98. doi: 10.1016/j.molcel.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xiang Y, Cakir B, Park IH. Deconstructing and reconstructing the human brain with regionally specified brain organoids. Semin Cell Dev Biol. 2021;111:40–51. doi: 10.1016/j.semcdb.2020.05.023. [DOI] [PubMed] [Google Scholar]
- 119.Yi SA, Zhang Y, Rathnam C, Pongkulapa T, Lee KB. Bioengineering approaches for the advanced organoid research. Adv Mater. 2021;33:e2007949. doi: 10.1002/adma.202007949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 121.Yoon SJ, Elahi LS, Pasca AM, Marton RM, Gordon A, Revah O, Miura Y, Walczak EM, Holdgate GM, Fan HC, Huguenard JR, Geschwind DH, Pasca SP. Reliability of human cortical organoid generation. Nat Methods. 2019;16:75–78. doi: 10.1038/s41592-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang W, Ma L, Yang M, Shao Q, Xu J, Lu Z, Zhao Z, Chen R, Chai Y, Chen JF. Cerebral organoid and mouse models reveal a RAB39b-PI3K-mTOR pathway-dependent dysregulation of cortical development leading to macrocephaly/autism phenotypes. Genes Dev. 2020;34:580–597. doi: 10.1101/gad.332494.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou J, Su P, Li D, Tsang S, Duan E, Wang F. High-efficiency induction of neural conversion in human ESCs and human induced pluripotent stem cells with a single chemical inhibitor of transforming growth factor beta superfamily receptors. Stem Cells. 2010;28:1741–1750. doi: 10.1002/stem.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ziffra RS, Kim CN, Ross JM, Wilfert A, Turner TN, Haeussler M, Casella AM, Przytycki PF, Keough KC, Shin D, Bogdanoff D, Kreimer A, Pollard KS, Ament SA, Eichler EE, Ahituv N, Nowakowski TJ. Single-cell epigenomics reveals mechanisms of human cortical development. Nature. 2021;598:205–213. doi: 10.1038/s41586-021-03209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


