Abstract
Background:
Differences in arsenic metabolism capacity may influence risk for type 2 diabetes, but the mechanistic drivers are unclear. We evaluated the associations between arsenic metabolism with overall diabetes prevalence and with static and dynamic measures of insulin resistance among Mexican Americans living in Starr County, Texas.
Methods:
We utilized data from cross-sectional studies conducted in Starr County, Texas, from 2010-2014. A Mendelian randomization approach was utilized to evaluate the associations between arsenic metabolism and type 2 diabetes prevalence using the intronic variant in the arsenic methylating gene, rs9527, as the instrumental variable for arsenic metabolism. To further assess mechanisms for diabetes pathogenesis, proportions of the urinary arsenic metabolites were employed to assess the association between arsenic metabolism and insulin resistance among participants without diabetes. Urinary biomarkers of arsenic metabolites were modeled as individual proportions of the total. Arsenic metabolism was evaluated both with a static outcome of insulin resistance, homeostatic measure of assessment (HOMA-IR), and a dynamic measure of insulin sensitivity, Matsuda Index.
Results:
Among 475 Mexican American participants from Starr County, higher metabolism capacity for arsenic is associated with higher diabetes prevalence driven by worse insulin resistance. Presence of the minor T allele of rs9527 is independently associated with an increase in the proportion of monomethylated arsenic (MMA%) and is associated with an odds ratio of 0.50 (95% CI: 0.24, 0.90) for type 2 diabetes. This association was conserved after potential covariate adjustment. Furthermore, among participants without type 2 diabetes, the highest quartile of MMA% was associated with 22% (95% CI: −33.5%, −9.07%) lower HOMA-IR and 56% (95% CI: 28.3%, 91.3%) higher Matsuda Index for insulin sensitivity.
Conclusions:
Arsenic metabolism capacity, indicated by a lower proportion of monomethylated arsenic, is associated with increased diabetes prevalence driven by an insulin resistant phenotype among Mexican Americans living in Starr County, Texas.
Keywords: Arsenic, Methylation, Type 2 diabetes, Insulin resistance, Insulin sensitivity, Endocrine disruptor
Introduction
Arsenic is a global health threat that contaminates drinking water, food, soil, and air for millions of people worldwide. There is significant evidence of the negative health consequences of arsenic exposure, including linkage to cancer, skin lesions, and cardiovascular disease (Kuo et al., 2017). There is also research that associates arsenic exposure with type 2 diabetes (T2D) risk (Sung et al., 2015); however, the mechanisms of arsenic’s metabolic toxicity are incompletely understood. Following exposure to inorganic arsenic (iAs), the metalloid is metabolized by multiple enzymes including arsenite methyltransferase (AS3MT) in the liver where it is methylated to increase the efficiency of excretion. Inorganic, monomethyl- (MMA), and dimethyl-arsenic (DMA) all differ with respect to both toxicity and clearance. Arsenic is commonly evaluated using urinaiy biomarkers where the proportion of the arsenic species can indicate the efficiency of methylation. Specifically, a higher proportion of MMA in urine indicates a lower methylation efficiency whereas a higher proportion of DMA indicates a higher methylation efficiency (Del Razo et al., 1997). How methylation efficiency influences arsenic’s toxicity has been studied extensively for cancer and cardiovascular disease outcomes with consistent evidence indicating MMA as the more pathogenic arsenic species; however, the evidence for metabolic disease and diabetes is conflicting. Additionally, research evaluating associations between arsenic methylation efficiency and diabetes among minority populations, whose exposure is elevated but not at or above the World Health Organization limit, is sparse.
Hispanics/Latinos are the largest and growing minority population in the United States, and they have both higher rates of chronic disease and a higher likelihood of toxic environmental exposures (Schulz and Sargis, 2021). Mexican Americans make up over 60% of the Hispanic/Latino population living in the United States, and many living in Southern Texas, at the Texas-Mexico border, have markedly elevated rates of chronic disease, including T2D (Hanis et al., 1983, U.S. Census Bureau, 2020). With the exception of a few regions, the majority of the United States has low to moderate levels of arsenic in groundwater (<50 ug/L) and community water systems (<10 ug/L). Texas ranks 10th for populations dependent upon domestic well water with over 1.3 million residents using domestic wells; located on the national border, Starr County specifically has a moderate concentration of private wells per square kilometer (Johnson and Belitz, 2017). Starr County groundwater has been noted to have arsenic levels above the EPA standard (10 ug/L) in the United States, and the county has markedly elevated rates of T2D (Hanis et al., 1983).
The associations between arsenic exposure and both T2D prevalence and incidence have been extensively studied in the United States through both cross-sectional and prospective investigations (Navas-Acien et al., 2008, Grau-Perez et al., 2017). It is postulated that the metabolism of inorganic arsenic may contribute to the metalloid’s toxicity, but limitations to biomarker evaluation and potential residual confounding introduce debate into the field. In contrast to the risk for cardiovascular disease and cancer, several studies have found an association between lower proportion of urinary MMA and increased prevalence of diabetes and obesity (Del Razo et al., 2011, Gribble et al., 2013, Rangel-Moreno et al., 2022). Additionally, analyses conducted using the Strong Heart Study cohort also reported associations between a lower proportion of urinary MMA and both diabetes incidence and insulin resistance (Grau-Perez et al., 2017, Kuo et al., 2015). On the other hand, a recent study conducted in New York City found associations between increased proportion of MMA with increased diabetes prevalence and increased hemoglobin A1c (Wu et al., 2021).
The distribution of organic arsenic species in urine has been shown to have genetic determinants, with single nucleotide polymorphisms (SNPs) in the gene encoding for AS3MT established as independent predictors of arsenic metabolite proportions (Pierce et al., 2012). As a result, these SNPs have been utilized as instrumental variables to assess associations between arsenic metabolism and health outcomes resistant to residual confounding and exposure misclassification (DiGiovanni et al., 2020). This method is referred to as Mendelian randomization (MR), which can be conceptualized as a type of instrumental variable study design, if the instrumental SNPs are associated with arsenic methylation and with type 2 diabetes, but that the association with diabetes is exclusively mediated through arsenic methylation (Burgess et al., 2013).
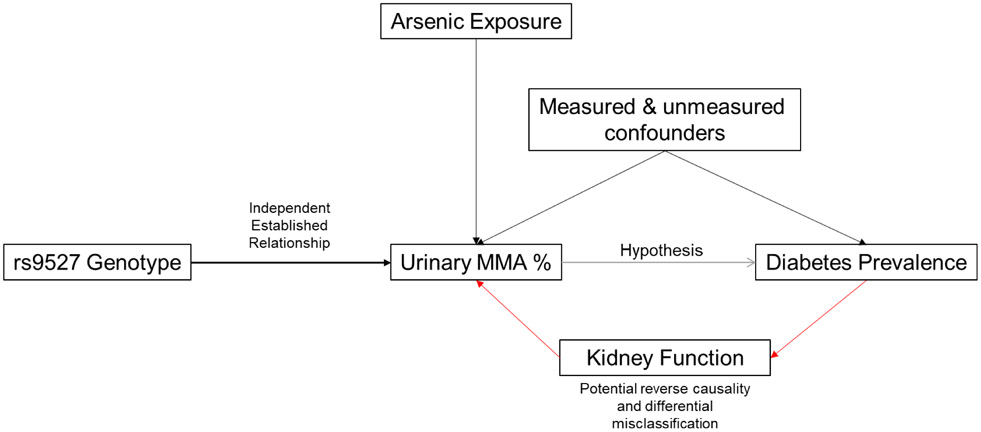
Utilizing a well-phenotyped cohort of Mexican American adults living in Starr County, Texas, this study used MR to evaluate the association between inorganic arsenic metabolism and diabetes prevalence in a minority population in the United States between the years 2010-2014. The SNP rs9527, an intronic variant in the AS3MT gene, has been established as a strong independent predictor of arsenic methylation efficiency and was used as an instrumental variable for arsenic metabolism in this study (Fig. 1) (Pierce et al., 2012, DiGiovanni et al., 2020, Das et al., 2016, Gao et al., 2019). To further elucidate mechanisms of toxicity, we investigated the associations of inorganic arsenic metabolism with continuous measures of insulin resistance among individuals without diabetes in the same cohort using both steady-state and dynamic measures.
Fig. 1.
Causal diagram for the relationship between rs9527 genotype, arsenic methylation, and diabetes prevalence. This relationship between the genetic variant with arsenic methylation is indicative of the use of this variant as a instrumental variable for mendelian randomization analysis.
Methods
Study population
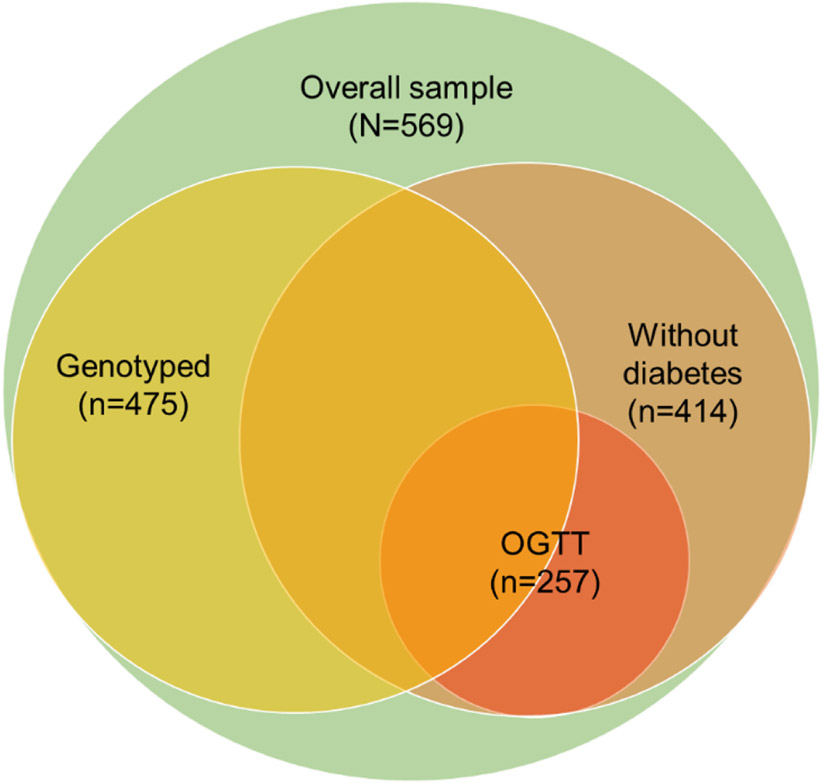
The study sample for this analysis included Mexican American individuals residing in Starr County, Texas, who had participated in a previous study of novel diabetes risk factors (Hanis et al., 2016) and individuals participating in an ongoing study of the gut microbiome and progression of dysglycemia (Jun et al., 2020). Briefly, a systematic survey, largely representative of the age and sex distribution of the Starr County population, was conducted in 3,085 households within 309 blocks in Starr County from 2002-2006 to determine the frequency of diabetes. Predominantly from those who answered the survey, 1200 individuals from independent households (selected to include approximately half with diabetes) returned for a follow-up examination of novel diabetes risk factors in 2010-2014 (Hanis et al., 2016). Of these participants, 412 individuals were randomly selected for urinary metal analyses. The sample size was then supplemented with an additional 157 individuals (identified from the original survey) without known diabetes participating in an ongoing study examining the impact of the gut microbiome on the progression of dysglycemia. In total, 569 individuals were selected for urinary metal analyses, of which 475 participants aged 31 to 80 years had undergone genetic testing. To evaluate the association between arsenic metabolism and insulin resistance, 414 individuals without diabetes were included. Complete oral glucose tolerance tests were completed on a subgroup of 257 of the 414 individuals. The overall analytical subsample selection is depicted in Fig. 2.
Fig. 2.
Diagram depicting relationship between study subsamples.
Assessment of outcomes
Hemoglobin A1c (HbA1c) (Siemens DCA Vantage Analyzer point of care device, Malvern, PA), fasting plasma glucose (FPG), post-load glucose, and fasting plasma insulin (Roche Cobas Analyzer, Chicago, IL) concentrations were analyzed from blood samples collected at enrollment. Seventy-five-gram oral glucose tolerance tests (OGTTs) were conducted with blood drawn at 0, 15, 30, 60, 90, and 120 minutes. Glucose was measured using a YSI 2300 STAT Plus Glucose and Lactate Analyzer (YSI Life Sciences, Yellow Springs, Ohio). Diabetes status was determined using the American Diabetes Association diagnostic criteria defined as meeting any of the following: 1) hemoglobin A1c greater than or equal to 6.5%, 2) fasting plasma glucose greater than or equal to 126 mg/dl, or 3) 2-hour postload glucose greater than or equal to 200 mg/dl. Additionally, two independent insulin resistance measures were calculated, the homeostatic model assessment of insulin resistance (HOMA-IR), an estimate of steady-state insulin resistance, and the Matsuda Index for insulin sensitivity, a dynamic measure of insulin-glucose dynamics. HOMA-IR was calculating using (Matthews et al., 1985), where represents fasting insulin, and represents fasting glucose. The Matsuda Index is a measurement of insulin sensitivity using the 6 time points of an oral glucose tolerance test with both glucose and insulin measurements in the following equation (Matsuda and DeFronzo, 1999). These outcomes were further supplemented with the evaluation of the homeostatic model assessment of β-cell function (HOMA-β), which was calculated using , and HbA1c.
Assessment of exposure
Spot urine samples were collected from each participant and stored at −70°C until analyses were performed. The urine samples were analyzed for iAs, MMA, DMA, and arsenobetaine (AsB) at the Trace Element Analysis Core at Dartmouth College. Briefly, samples were oxidized with 10% H2O2, this converts all inorganic arsenic to arsenate, which simplifies and enhances the chromatography but does not otherwise affect the organic arsenic species (Scheer et al., 2012). The urine samples were analyzed by anion chromatography, Hamilton PRPX100 column with carbonate eluant, coupled to ICP-MS using an Aglient 1260 HPLC and 8900 ICP-MS (Agilent, Wilmington DE) (Jackson, 2015). Quality control included duplicate and spiked species samples and continuing analysis of reference urines NIST 2669 level 1 and 2 every ten samples. Within this study population, 0.2% of samples were below the limit of detection (LOD) for DMA and MMA, 35% for iAs, and 3.8% for arsenobetaine (AsB). Samples below the limit of detection were assigned calibration values from the instrument.
As the primary objective of this study is to evaluate the impact of inorganic arsenic metabolism on diabetes risk and insulin resistance independent of seafood consumption, the metabolites were adjusted for other organic sources of arsenic exposure. AsB is a lipid form of arsenic commonly found in fish and other marine animals that is metabolized into MMA and DMA (Jones et al., 2016). As a result, the measured urinary arsenic from participants was adjusted for AsB. The residual method was utilized to determine the estimated arsenic species concentration independent of AsB by regressing log transformed AsB on the log transformed arsenic species and extracting the model residuals using the following equation: , where represents the estimated arsenic species concentration independent of AsB. These estimated concentrations were utilized to calculate the relative proportion of each species that were utilized in the subsequent analysis.
The arsenic methylation measures were calculated from the estimated measured concentrations of iAs, MMA, and DMA. Total arsenic concentration was calculated as a sum of each estimated arsenic species, namely MMA, DMA, and iAs. The relative proportion of each species (MMA%, DMA%, and iAs%) was standardized by the total arsenic concentration.
Genotyping
DNA was isolated and extracted from whole blood samples. Genotyping was conducted using the Affymetrix Genome-Wide SNP Array 6.0 (Affymetrix, Santa Clara, CA, USA) and custom genome-wide array of for insulin sensitivity, a dynamic measure of insulin-glucose dynamics the Broad Institute and imputed with 1000 Genomes phase 3 reference panel using the Michigan Imputation Server (Michigan Imputation Server). Of the 475 participants with rs9527 genotype data, the minor allele frequency (T) was observed to be 0.15. Genotype was coded as a binary variable with 1 representing the presence of at least one minor T allele.
Assessment of covariates
Self-reported participant characteristics, including age (years), sex (male, female), years of education, employment status (full time, part time, unemployed, retired, or disabled), smoking status (current, former, never), pack-years of smoking, height (m), weight (kg), and alcohol consumption (yes, no) were available from baseline questionnaires administered to participants. Participant’s height in meters and weight in kilograms were used to calculate body mass index (kg/m2). Urinary creatinine was quantified from the spot urine samples using DetectX Urinary Creatinine Kit (Arbor Assays, Ann Arbor, Michigan). Models were adjusted for covariates noted to be associated with either arsenic exposure or diabetes risk from prior literature (Rahman et al., 2006, Lindberg et al., 2010, Jain, 2015).
Statistical analyses
Demographic characteristics were summarized for the 475 individuals with genetic data, the 414 diabetes-free participants with HOMA data, and the 257 diabetes-free participants with glucose tolerance testing data. Creatinine standardized total arsenic concentration was estimated by dividing the urinary arsenic concentration (μg/L) by urinary creatinine (mg/dL) and multiplying by a factor of 100 for a standardized arsenic concentration of μg/g of creatinine. The mean and standard deviation (SD) for creatinine-standardized total arsenic and each proportion of arsenic species are presented for all participants.
To demonstrate the relationship between the instrumental variable and exposure of interest, linear regression was utilized to estimate the association between the minor T rs9527 allele with MMA%. Logistic regression was used to estimate both the association between MMA% and diabetes status and rs9527 T allele with diabetes status. Each model underwent a stepwise, a priori adjustment approach where the crude; adjusted for age and sex; and adjusted for age, sex, smoking status, and body mass index are presented for each model. A cross product term between genotype and creatinine-adjusted arsenic exposure, dichotomized at the median concentration, was included, and both stratum-specific associations for genotype and P-values for interaction are presented for each model.
To evaluate associations between the arsenic metabolite proportions and continuous glycemic traits, we constructed quartiles of metabolite percentages based on the distribution in the study sample to capture potential non-linear relationships. The first quartile, representing participants with the lowest metabolite proportion levels, was used as the reference group. We used multivariable linear regression to estimate the percent differences in HOMA-IR, Matsuda index, HOMA-β, and HbA1c as for each quartile compared to the referent. The P-value for trend was obtained by modeling the quartiles as a single ordinal variable. All 4 outcomes were natural log-transformed due to skewed distributions. Models were adjusted for a priori confounders, including sex (male, female), age (years), smoking status (current, former, never), pack-years of smoking, BMI (kg/m2), and alcohol consumption (yes, no) (Rahman et al., 2006, Lindberg et al., 2010, Jain, 2015).
Additionally, to account for potential confounding by initial dosage of total arsenic, creatinine-adjusted total arsenic was included as a model covariate (Willett et al., 1997). As a result, the association between an increase in one arsenic species proportion corresponds to a decrease in either of the other two species. As the second methylation step, which converts MMA to DMA, is the final methylation step for arsenic methylation, discerning the relationship between changes in these two species is vital for understanding methylation efficiency. To address this, the leave-one-out approach was utilized where two metabolite proportions are included in the model and the third is left out (Kuo et al., 2015). This method allows us to present the relative measures of association. Specifically, the association for each metabolite proportion will be presented twice, each measure relative to a decrease in the two counterparts individually.
All statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC), R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria), and Stata 17 (StataCorp, College Station, TX).
Results
Participant Characteristics and Arsenic Metabolite Distributions
Table 1 shows the characteristics of each study participant subsample. Most participants were female, had a BMI in the obese range, worked full-time, had a high school education, and did not smoke or drink alcohol. Table 2 shows the mean and standard deviation of the creatinine-standardized total arsenic and metabolite proportions for each sample. There are minimal differences between the two diabetes-free participant classes. As a result, the same confounders were conditioned on for the continuous glycemic outcome analysis and a stepwise adjustment approach was utilized for the Mendelian randomization analysis.
Table 1.
Selected participant characteristics.
| Characteristic | Genotyped Participants (N=475) |
Diabetes Free Participants (N=414) |
OGTT Participants (n=257) |
|---|---|---|---|
| Age, mean ± SD | 51 ± 10 | 47 ± 9 | 46 ± 10 |
| Gender, n (%) | |||
| Male | 138 (29) | 99 (24) | 60 (24) |
| Female | 337 (71) | 315 (76) | 197 (76) |
| BMI, mean ± SD | 32 ± 6 | 32 ± 7 | 31 ± 7 |
| Employment Status, n (%) | |||
| Full time | 258 (55) | 252 (61) | 169 (66) |
| Part time | 106 (22) | 98 (24) | 68 (27) |
| Not working | 54 (11) | 42 (10) | 6 (2) |
| Retired | 50 (11) | 18 (4) | 14 (6) |
| Disabled | 5 (1) | 4 (1) | 0 (0) |
| Education years, mean ± SD | 10 ± 4 | 10 ± 4 | 10 ± 4 |
| Pack years, mean ± SD | 4 ± 11 | 3 ± 9 | 3 ± 9 |
| Smoking status, n (%) | |||
| Current | 80 (17) | 67 (16) | 45 (17) |
| Former | 77 (16) | 52 (13) | 30 (12) |
| Never | 318 (67) | 295 (71) | 182 (71) |
| Alcohol drinking, n (%) | |||
| Yes | 140 (30) | 137 (33) | 77 (30) |
| No | 334 (30) | 277 (67) | 180 (70) |
Table 2.
Distribution of Arsenic Metabolite Proportions.
| Arsenic Measure Mean (SD) |
Genotyped Participants (N=475) |
Diabetes Free Participants (N=414) |
OGTT Participants (N=257) |
|---|---|---|---|
| Σ species, ug/g creatinine | 5.65 (3.41) | 5.46 (3.40) | 5.89 (3.60) |
| MMA, % | 0.12 (0.08) | 0.12 (0.06) | 0.11 (0.06) |
| DMA, % | 0.84 (0.40) | 0.82 (0.10) | 0.83 (0.09) |
| iAs, % | 0.06 (0.05) | 0.06 (0.04) | 0.05 (0.03) |
Mendelian Randomization
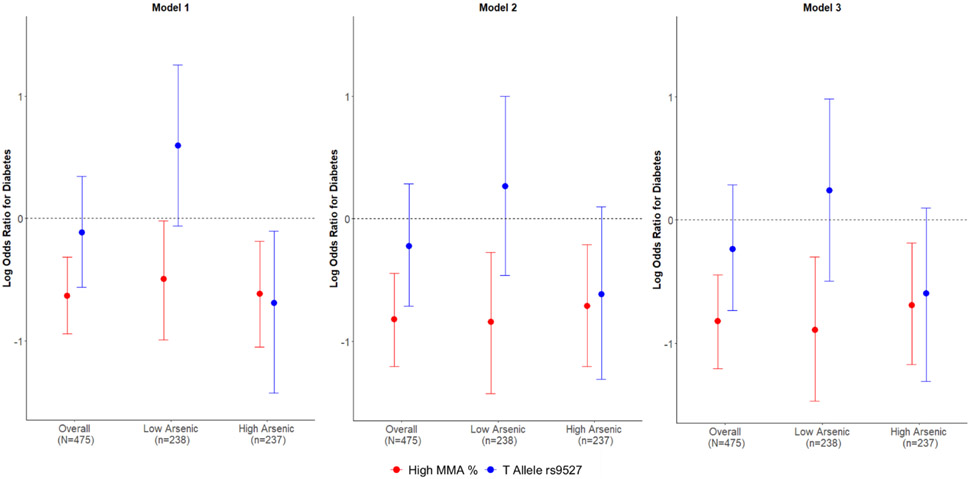
To use the allelic variation in the rs9527 SNP as an instrumental variable for MMA%, we assessed the direct association between binary genotype and MMA% using a linear regression model stratified by total arsenic concentration dichotomized at its median. At higher levels of arsenic exposure, the T allele for rs9527 is associated with a 4% increase in MMA% (2%, 6%), and this estimate is conserved across all levels of confounding control (p<0.001) (Table 3; Fig. 3). Using multivariable logistic regression, log transformed MMA% is associated with 50% reduced odds for type 2 diabetes (0.31, 0.83) and this estimate is conserved for both high and low total arsenic exposures with additional confounding control. Lastly, utilizing rs9527 genotype as an instrumental variable revealed consistent associations with MMA% exposure where the T allele was found to be associated with a 45% reduction in the odds of type 2 diabetes (0.27, 1.10) among those with high total arsenic exposure (p=0.09).
Table 3.
Mendelian Randomization results for prevalent diabetes per increase in urinary proportion of MMA and the T allele of rs9527 genotype (N=475).
| Model | Exposure | Outcome | Overall (N=475) | Low Total Arsenic (n=238) | High Total Arsenic (n=237) | P value Interaction | |||
|---|---|---|---|---|---|---|---|---|---|
| Beta/OR (95% CI) | P value | Beta/OR (95% CI) | P value | Beta/OR (95% CI) | P value | ||||
| Model 1 | rs9527 | MMA % | 0.02 (0.008, 0.04) | 0.003 | 0.01 (−0.009, 0.03) | 0.30 | 0.04 (0.02, 0.06) | <0.001 | 0.08 |
| MMA% | Diabetes | 0.53 (0.39, 0.73) | <0.001 | 0.61 (0.37, 0.98) | 0.04 | 0.54 (0.35, 0.83) | 0.005 | 0.71 | |
| rs9527 | Diabetes | 0.89 (0.57, 1.41) | 0.63 | 1.81 (0.94, 3.50) | 0.08 | 0.50 (0.24, 0.90) | 0.02 | 0.004 | |
| Model 2 | rs9527 | MMA % | 0.03 (0.01, 0.04) | 0.001 | 0.01 (−0.007, 0.03) | 0.20 | 0.04 (0.02, 0.06) | <0.001 | 0.02 |
| MMA% | Diabetes | 0.44 (0.30, 0.64) | <0.001 | 0.43 (0.24, 0.76) | 0.003 | 0.49 (0.30, 0.81) | 0.005 | 0.70 | |
| rs9527 | Diabetes | 0.80 (0.49, 1.33) | 0.40 | 1.30 (0.63, 2.71) | 0.50 | 0.54 (0.27, 1.10) | 0.09 | 0.09 | |
| Model 3 | rs9527 | MMA % | 0.03 (0.01, 0.04) | 0.001 | 0.01 (−0.007, 0.03) | 0.20 | 0.04 (0.02, 0.06) | <0.001 | 0.11 |
| MMA% | Diabetes | 0.44 (0.30, 0.64) | <0.001 | 0.41 (0.23, 0.74) | 0.003 | 0.50 (0.31, 0.83) | 0.007 | 0.63 | |
| rs9527 | Diabetes | 0.79 (0.48, 1.33) | 0.38 | 1.27 (0.61, 2.67) | 0.52 | 0.55 (0.27, 1.10) | 0.09 | 0.13 | |
MMA: Monomethylated arsenic; OR: Odds ratio
Models stratified by creatinine standardized total urinary arsenic.
Per two-fold increase in arsenobetaine- and creatinine-adjusted MMA%.
Model 1: Base model. Model 2: further adjusted for age and gender. Model 3: Further adjusted for smoking and body mass index.
Fig. 3.
Mendelian randomization results for prevalent type 2 diabetes per increase in monomethyl arsenic (MMA %) and minor T allele of rs9527 genotype. Among those with high total arsenic exposure, both having higher MMA % and having the T allele of rs9527 in the gene encoding arsenic methyl transferase (AS3MT) are associated with a lower prevalence of type 2 diabetes across multiple levels of confounding control. Model 1: base model. Model 2: further adjusted for age and gender. Model 3: further adjusted for smoking and body mass index.
Arsenic Metabolism and Insulin Resistance
To discern potential mechanisms for the association between arsenic metabolism and diabetes pathogenesis, we assessed the associations between arsenic metabolite proportions with continuous glycemic traits among diabetes-free participants. Table 4 summarizes the associations between quartile increases in the proportion of each metabolite with HOMA-IR with both a priori adjustment and the leave-one-out approach. Compared to the lowest quartile of MMA%, the highest quartile had a 22% decrease in HOMA-IR with a significant linear trend (p=0.001). Using the leave-one-out approach, increased MMA% was associated with 34% and 42% reductions in HOMA-IR per decrease in DMA% and iAs%, respectively, with significant linear trends (p=0.0002 and p=0.008, respectively). The highest quartile of DMA% was associated with 67% higher HOMA-IR but only when MMA% was also decreased (p=0.001). Similarly, the highest quartile of iAs% corresponded to higher HOMA-IR compared to the lowest quartile (p=0.006; p=0.009).
Table 4.
Percent difference (95% CI) of HOMA-IR in relation to quartile of urinary arsenic species (N = 414).
| Q1 | Q2 | Q3 | Q4 | P for trend |
|
|---|---|---|---|---|---|
| Multivariable adjusted modela | |||||
| MMA % | ref | −7.57 (−20.93, 8.06) | −17.56 (−29.33, −3.82) | −22.26 (−33.53, −9.07) | 0.001 |
| DMA % | ref | 4.40 (−10.62, 21.93) | 8.24 (−7.40, 26.53) | 14.56 (−2.14, 34.10) | 0.082 |
| iAs % | ref | −0.83 (−15.50, 16.38) | −0.07 (−14.25, 16.44) | −1.24 (−15.48, 15.40) | 0.903 |
| Leave one out method | |||||
| MMA % | |||||
| Per decrease in DMA | ref | −14.18 (−27.95, 2.21) | −26.79 (−38.82, −12.39) | −34.24 (−46.10, −19.75) | <0.0001 |
| Per decrease in iAs | ref | −17.87 (−33.35, 1.22) | −34.37 (−49.00, −15.55) | −42.62 (−57.27, −22.94) | 0.0002 |
| DMA % | |||||
| Per decrease in MMA | ref | 16.79 (−5.05, 43.66) | 33.66 (5.49, 69.36) | 67.48 (23.01, 128.02) | 0.0012 |
| Per decrease in iAs | ref | −8.00 (−23.07, 10.02) | −19.56 (−36.17, 1.37) | −29.53 (−47.54, −5.34) | 0.0183 |
| Inorganic | |||||
| Arsenic % | |||||
| Per decrease in MMA | ref | 19.42 (−3.79, 48.23) | 35.93 (5.59, 75.00) | 52.88 (13.05, 106.74) | 0.0055 |
| Per decrease in DMA | ref | 12.23 (−5.95, 33.93) | 21.10 (1.51, 44.48) | 28.90 (6.13, 56.55) | 0.0089 |
Adjusted for total arsenic, age, sex, education, employment, smoking status, pack year smoking history, body mass index, and alcohol consumption
Matsuda Index
There are limitations to the use of HOMA-IR as a measurement of insulin-glucose homeostasis as it is a static measure of insulin resistance. A subgroup of the diabetes-free sample (n=257) completed OGTTs with glucose and insulin measurements at 0, 15, 30, 60, 90, and 120 minutes after receiving a 75-gram oral glucose load. These measurements were used to calculate the Matsuda Index for insulin sensitivity for which higher values represent greater insulin action and enhanced glucose uptake. The Matsuda Index is a dynamic measure of insulin sensitivity and is a robust estimate for insulin-glucose dynamics. Table 5 shows the associations between arsenic species with the Matsuda Index among the 257 participants with OGTTs. Similar to HOMA-IR, MMA% and DMA% were associated with a 57% increase in insulin sensitivity and a 26% decrease in insulin sensitivity, respectively (p<0.0001; p=0.01). Using the leave-one-out approach, MMA% was associated with a significant 134% and 76% increased Matsuda Index corresponding to a decrease in iAs% and DMA%, respectively (p<0.0001). Corresponding to a decreased MMA%, DMA% had a significant negative association with Matsuda Index with the highest quartile showing a 40% lower Matsuda Index compared to the lowest quartile with a significant linear trend (p=0.02). Conversely, relative to a decrease in iAs%, increased DMA% was associated with a 46% increase in Matsuda Index for the highest quartile compared to the lowest quartile (p=0.01). There was not a significant association between iAs% and Matsuda Index.
Table 5.
Percent difference (95% CI) of Matsuda index in relation to quartile of urinary arsenic species (N = 257).
| Q1 | Q2 | Q3 | Q4 | P for trend |
|
|---|---|---|---|---|---|
| Traditional multivariable adjusted modela | |||||
| MMA % | ref | 14.94 (−6.13, 40.74) | 37.61 (12.90, 67.72) | 56.66 (28.27, 91.32) | <0.0001 |
| DMA % | ref | −18.95 (−33.41, −1.36) | −15.85 (−31.35, 3.13) | −25.65 (−39.25, −9.00) | 0.010 |
| iAs % | ref | 25.95 (2.72, 54.42) | 7.43 (−11.43, 30.32) | 21.10 (−0.52, 47.41) | 0.144 |
| Leave one out method | |||||
| MMA % | |||||
| Per decrease in DMA | ref | 19.81 (−4.71, 50.63) | 45.41 (14.43, 84.79) | 75.74 (35.41, 128.07) | <0.0001 |
| Per decrease in iAs | ref | 41.77 (7.25, 87.41) | 94.46 (41.00, 168.19) | 134.34 (61.61, 239.81) | <0.0001 |
| DMA % | |||||
| Per decrease in MMA | ref | −30.43 (−46.49, −9.55) | −34.06 (−52.01, −9.40) | −40.21 (−59.65, −11.38) | 0.0175 |
| Per decrease in iAs | ref | 1.84 (−19.17, 28.29) | 24.32 (−6.05, 64.51) | 56.76 (8.26, 126.98) | 0.011 |
| Inorganic | |||||
| Arsenic % | |||||
| Per decrease in MMA | ref | 15.67 (−11.63, 51.39) | −9.31 (−33.33, 23.35) | −21.12 (−46.33, 15.95) | 0.115 |
| Per decrease in DMA | ref | 4.23 (−17.05, 30.98) | −13.73 (−30.76, 7.50) | −15.36 (−33.92, 8.41) | 0.070 |
Adjusted for total arsenic, age, sex, education, employment, smoking status, pack year smoking history, body mass index, and alcohol consumption
HOMA-β and Hemoglobin A1c
Lastly, as HOMA-IR and HOMA-β are computed using the same static fasting glucose and insulin levels, the reduction in HOMA-IR could correspond to a decrease in HOMA-β due to a reduction in insulin secretion. To discern if the associations between arsenic methylation with insulin resistance are due to glucose uptake or due to insulin production, we evaluated the associations of the same arsenic metabolism exposures on HOMA-β and hemoglobin A1c. The results of this analysis are shown in Supplemental Table 1 and 2. A higher proportion of MMA% was associated with a 13% lower HOMA-β for the highest quartile with a significant linear trend both overall using a priori adjustment as well as when corresponding to a reduction in DMA% using the leave-one-out approach (p=0.012; p=0.008, respectively). DMA% and iAs% were associated with a 32% and 22% increase in HOMA-β only when MMA% is decreased (p=0.02; p=0.04). Furthermore, a higher proportion of MMA% was associated with a significant reduction in HbA1c with a significant linear trend corresponding to a decrease in iAs % (p=0.02) and DMA% (p=0.02).
Discussion
In the present study, arsenic methylation was found to be associated with diabetes prevalence and alterations in measures of insulin resistance among Mexican Americans living in Starr County, Texas. Starr County residents have been noted to have higher rates of T2D compared to the national average for other Hispanic/Latino groups while also living in communities with moderate arsenic contamination of groundwater. We found that a higher proportion of urinary MMA was associated with a reduction in diabetes prevalence. As this is a cross-sectional study and there are concerns about reverse causality and misclassification, these results were supplemented with a Mendelian Randomization approach to minimize bias and confounding. Our results showed that the minor T allele of the rs9527 genotype, which has been shown to independently predict a higher proportion of urinary MMA, is also associated with reduced diabetes prevalence with comparable point estimates among those with elevated exposure. These data suggest that a higher metabolism efficiency based on genotype, driven by a lower proportion of MMA, is associated with an increased prevalence of T2D, and this association has a low risk of residual confounding, reverse causality, or exposure misclassification. Additionally, in order to elucidate mechanistic evidence for the progression to diabetes, we evaluated the associations between arsenic metabolism and measures of glucose-insulin homeostasis among diabetes-free participants. We found that a higher proportion of MMA is associated with lower HOMA-IR and higher Matsuda Index for insulin sensitivity. In the leave-one-out approach, a higher proportion of MMA was associated with lower HOMA-IR and increased Matsuda Index when both DMA% and iAs% are lower. Additionally, a higher proportion of MMA%, per decrease in either DMA% or iAs%, was associated with a lower HbA1c and HOMA-β. These supplementary results show that the lower insulin resistance and β-cell function also correspond with lower average blood glucose levels, leading us to propose that the associations are driven by insulin sensitivity rather than altered insulin production. In combination, these findings support evidence that among individuals exposed to arsenic, a high arsenic metabolism capacity with a lower proportion of MMA may be associated with higher diabetes prevalence driven by alterations in insulin resistance in this minority population. This study is one of few to evaluate the associations between inorganic arsenic methylation, independent of seafood consumption, with diabetes pathogenesis in a high-risk minority population in the United States using novel strategies to minimize confounding and misclassification.
There is significant epidemiological evidence supporting an association between arsenic exposure and increased T2D risk. A systematic review of experimental and epidemiological evidence by Navas-Acien et al. demonstrated associations with arsenic exposure and increased T2D risk in highly exposed regions, but concluded that further evidence was needed in low-to-moderately exposed populations (Navas-Acien et al., 2006). Since then, additional studies have demonstrated associations between low-to-moderate arsenic exposure and T2D within both cross-sectional and prospective cohorts (Grau-Perez et al., 2017, Gribble et al., 2012, Coronado-Gonzalez et al., 2007, Currier et al., 2014, Weiss et al., 2022).
However, the epidemiological evidence for the impact of arsenic metabolism on diabetes status and traits is conflicting. Studies conducted in Bangladesh found a positive association between arsenic metabolism and T2D with a higher proportion of DMA as the primary metabolite associated with disease prevalence (Kuo et al., 2017). Our study found an association between DMA% and increased insulin resistance but only in the setting of reduced MMA%, whereas MMA% was associated with reduced insulin resistance in the setting of either decreased DMA% or decreased iAs%. Additionally, other studies conducted in Bangladesh have found no association between arsenic metabolism and diabetes prevalence (Nizam et al., 2013). Furthermore, a study in the United States from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) cohort used Mendelian randomization to evaluate arsenic metabolism and diabetes-related traits and found no significant associations (Scannell Bryan et al., 2019). On the other hand, studies conducted in Mexico found that individuals with polymorphisms in arsenic metabolism genes and higher levels of urinary DMA had higher rates of diabetes (Drobna et al., 2013). Despite this, another study using exfoliated urothelial cells to measure arsenic exposure found a positive association between trivalent arsenic (iAsIII and MMAIII) with diabetes prevalence and a negative association between DMA and diabetes prevalence (Currier et al., 2014). Multiethnic studies within the United States using both NHANES and the Strong Heart Study have found associations between arsenic methylation and diabetes. Evidence from the Strong Heart Family Study found strong cross-sectional and prospective associations between arsenic methylation with both increased diabetes incidence and HOMA-IR, driven by a lower proportion of MMA (Grau-Perez et al., 2017, Kuo et al., 2015).
In addition to these studies, there have been several studies examining the pathophysiological effects of arsenic and arsenic metabolites with respect to cancer and cardiovascular disease. Many mechanisms of toxicity have been posited to explain arsenic’s impact on cellular proliferation, and these pathways consistently overlap with insulin signaling. In metabolic tissues, insulin triggers a signaling cascade that, among other events, results in the stimulation of PI3-kinase phosphorylation of Akt, activation of mammalian target of rapamycin (mTOR), and glucose transporter 4 (GLUT4) translocation to the cell membrane (Stump et al., 2003). Laboratory-based studies have shown that during oncogenesis arsenic induces PI3-kinase and other proteins in the Akt pathway, including mTOR (Souza et al., 2001, Wang et al., 2013, Altman and Platanias, 2008). Furthermore, another study using 3T3-L1 adipocytes showed that arsenite exhibited insulin-like effects with increased translocation of GLUT4 glucose transporters and increased uptake of extracellular glucose (Bazuine et al., 2003).
While these studies suggest that arsenic may exert insulin-like effects or promote signaling through intermediates of the insulin signaling pathway, there is also strong evidence to support the opposite as well as additional adverse mechanisms by which arsenic can modulate insulin-glucose homeostasis. Studies have shown that inorganic arsenic inhibits glucose-induced insulin secretion and impairs β-cell functioning in both in vivo and in vitro models (Dover et al., 2018, Liu et al., 2014, Wei et al., 2020). Furthermore, other experimental studies have found that arsenic induces glucose intolerance and contributes to insulin resistance (Jia et al., 2020, Kirkley et al., 2018, Sargis, 2014). There are few experimental studies that have looked at the impact of arsenic metabolites on diabetes pathophysiology. Of the few studies that have been done, trivalent forms of arsenic were found to impair insulin-stimulated glucose uptake in 3T3-L1 adipocytes and glucose-induced insulin secretion in mice islets (Walton et al., 2004, Douillet et al., 2013). Given the complexities of these findings, this current work contributes to the growing understanding of how arsenic and its metabolites modulate glucose homeostasis across tissues and over the diverse spectrum of human exposures.
The impact of arsenic methylation is further complicated when considering all potential mediators and confounders. There is evidence that arsenic metabolism varies by both sex, age, diet, smoking status, genetic polymorphisms, and the gut microbiome. Women, never smokers, and those with higher B vitamin intake methylate arsenic more efficiently, resulting in higher levels of DMA% (Tseng, 2009). There is also data that polymorphisms in arsenic metabolizing genes, AS3MT, and genes associated with one-carbon metabolism are associated with increased methylation efficiency and modify the relationship between arsenic metabolism and disease (Pierce et al., 2012, Spratlen et al., 2018, Pierce et al., 2013). Lastly, there is evidence to suggest that arsenic alters the microbiome, and that specific microbiota reciprocally alter arsenic metabolism (Yang et al., 2021). Collectively, these data suggest that clarifying arsenic’s impact on glucose homeostasis will require careful consideration of these and likely other additional factors.
This study has many strengths. Primarily, this study evaluated the association between arsenic metabolism and diabetes prevalence using a robust Mendelian randomization approach to minimize confounding and misclassification bias. Furthermore, to increase the robustness and elucidate potential mechanisms of arsenic’s metabolic toxicity, we evaluated the association between arsenic methylation with both dynamic and steady-state measures of insulin resistance among individuals without diabetes. This study was performed in a known high-risk, commonly underserved, minority population with known elevated groundwater arsenic levels. Additionally, we conducted robust confounding control for fish consumption and other sources of organic arsenic exposure, and we utilized well-established, advanced statistical modeling methods to assess the associations of arsenic metabolism and disease. Lastly, our results are consistent with previous studies, supporting evidence that more efficient arsenic methylation is associated with increased T2D risk. Despite these strengths, this study has several limitations. First, the use of Mendelian Randomization relies upon several assumptions: (1) the genetic variant, rs9527, is associated with the exposure, specifically arsenic methylation; (2) the relationship of rs9527 with diabetes is not confounded; and (3) there is exclusive mediation between rs9527 with diabetes through arsenic methylation. We tested the first assumption by showing that the rs9527 genotype is associated with a higher proportion of MMA. This is also biologically plausible since rs9527 has been identified as an intronic variant in the gene encoding the enzyme arsenic methyltransferase, which methylates arsenic. The second assumption is more difficult to test since unmeasured confounding remains a risk; however, we took steps to assess the robustness of our associations using a stepwise adjustment approach for several known predictors of T2D. Furthermore, it is possible that people do not choose their partners at random but rather based on particular characteristics that are reflective of underlying genotype. This non-random mating would violate the independence assumption of MR. Lastly, the final MR assumption is not fully testable, but we note the robust use of this variant across the literature for the effects of arsenic metabolism that generate similar findings (DiGiovanni et al., 2020, Scannell Bryan et al., 2019). In addition to the assumptions of MR, this is a cross-sectional cohort study with limited evidence of temporality, which therefore limits inference of causality for the assessment of the individual arsenic metabolites. Another limitation is the use of single spot urinary arsenic measurements as they only represent short-term exposure. Finally, as a consequence of the observational nature of this study and despite thorough control of measured confounders, there is potential risk for residual confounding due to unmeasured dietary differences and potential misclassification of measured confounders due to participant self-reporting.
Conclusion
In a study of a richly phenotyped Mexican American cohort, enhanced capacity to fully metabolize arsenic via its methylation, as reflected by lower proportions of MMA or higher proportions of DMA, was associated with increased prevalence of T2D and with increased steady-state and dynamic measures of insulin resistance among individuals without diabetes. These findings support existing epidemiological studies of arsenic metabolism that have concluded that a higher methylation capacity for arsenic may be diabetogenic. Further work is now required to evaluate potential confounding, mediation, and interdependence by diet, supplements, and microbiome in order to fully understand how this complex and ubiquitous chemical influences diabetes risk.
Supplementary Material
Funding
This work was supported by the National Institutes of Health (R01 ES028879 and R21 ES030884 supporting RMS; P30 ES027792 supporting MA and RMS; UL1 TR002003 supporting MA and RMS via the UIC Center for Clinical and Translational Science; F30 ES033510 and the University of Illinois at Chicago’s Medical-Scientist Training Program (T32 GM079086) supporting MS; 5P30 CA023108-41 supporting BPJ via the Dartmouth Cancer Center NCI Cancer Center Support Grant, and ROls HL102830, DK116378, and DK118631 supporting GJ, DA, ELB and CLH.
Footnotes
CRediT authorship contribution statement
Margaret C. Weiss: Methodology, Software, Formal analysis, Writing – original draft, Visualization. Yu-Hsuan Shih: Methodology, Software, Writing – review & editing. Molly Scannell Bryan: Software, Supervision, Writing – review & editing. Brian P. Jackson: Data curation, Investigation, Writing – review & editing. David Aguilar: Data curation, Writing – review & editing. Eric L. Brown: Data curation, Writing – review & editing. Goo Jun: Data curation, Writing – review & editing. Craig L. Hanis: Supervision, Data curation, Writing – review &editing. Maria Argos: Supervision, Methodology, Conceptualization, Writing – review & editing. Robert M. Sargis: Supervision, Methodology, Conceptualization, Project administration, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. RMS declares he has received honoraria from CVS/Health and the American Medical Forum, neither of which relate to the present study. All other authors declare they have no competing interests.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.envadv.2023.100361.
Data availability
Data will be made available on request.
References
- Kuo CC, Moon KA, Wang SL, Silbergeld E, Navas-Acien A, 2017. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ. Health Perspect 125 (8), 087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung TC, Huang JW, Guo HR., 2015. Association between arsenic exposure and diabetes: a meta-analysis. Biomed. Res. Int 2015, 368087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Razo LM, Garcia-Vargas GG, Vargas H, Albores A, Gonsebatt ME, Montero R, et al. , 1997. Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch. Toxicol 71 (4), 211–217. [DOI] [PubMed] [Google Scholar]
- Schulz MC, Sargis RM., 2021. Inappropriately sweet: Environmental endocrine-disrupting chemicals and the diabetes pandemic. Adv. Pharmacol 92, 419–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanis CL, Ferrell RE, Barton SA, Aguilar L, Garza-Ibarra A, Tulloch BR, et al. , 1983. Diabetes among Mexican Americans in Starr County, Texas. Am. J. Epidemiol 118 (5), 659–672. [DOI] [PubMed] [Google Scholar]
- Johnson TD, Belitz K., 2017. Domestic well locations and populations served in the contiguous U.S.: 1990. Sci. Total Environ 607 (608), 658–668. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E., 2008. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 300 (7), 814–822. [DOI] [PubMed] [Google Scholar]
- Grau-Perez M, Kuo CC, Cribble MO, Balakrishnan P, Jones Spratlen M, Vaidya D, et al. , 2017. Association of low-moderate arsenic exposure and arsenic metabolism with incident diabetes and insulin resistance in the strong heart family study. Environ. Health Perspect 125 (12), 127004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Razo LM, Garcia-Vargas GG, Valenzuela OL, Castellanos EH, Sanchez-Pena LC, Currier JM, et al. , 2011. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environ. Health 10, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribble MO, Crainiceanu CM, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. , 2013. Body composition and arsenic metabolism: a cross-sectional analysis in the strong heart study. Environ. Health 12, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno K, Gamboa-Loira B, Lopez-Carrillo L, Cebrian ME., 2022. Prevalence of type 2 diabetes mellitus in relation to arsenic exposure and metabolism in Mexican women. Environ. Res 210, 112948. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Howard BV, Umans JG, Cribble MO, Best LG, Francesconi KA, et al. , 2015. Arsenic exposure, arsenic metabolism, and incident diabetes in the strong heart study. Diabetes Care. 38 (4), 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Chen Y, Navas-Acien A, Garabedian ML, Coates J, Newman JD., 2021. Arsenic exposure, arsenic metabolism, and glycemia: results from a clinical population in New York City. Int. J. Environ. Res. Public Health 18 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BL, Kibriya MG, Tong L, Jasmine F, Argos M, Roy S, et al. , 2012. Genome-wide association study identifies chromosome 10q24.32 variants associated with arsenic metabolism and toxicity phenotypes in Bangladesh. PLos Genet. 8 (2), e1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiovanni A, Demanelis K, Tong L, Argos M, Shinkle J, Jasmine F, et al. , 2020. Assessing the impact of arsenic metabolism efficiency on DNA methylation using Mendelian randomization. Environ. Epidemiol 4 (2), e083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Butterworth A, Thompson SC., 2013. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol 37 (7), 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N, Giri A, Chakraborty S, Bhattacharjee P., 2016. Association of single nucleotide polymorphism with arsenic-induced skin lesions and genetic damage in exposed population of West Bengal, India. Mutat. Res. Genet. Toxicol. Environ. Mutagen 809, 50–56. [DOI] [PubMed] [Google Scholar]
- Gao S, Mostofa MG, Quamruzzaman Q, Rahman M, Rahman M, Su L, et al. , 2019. Gene-environment interaction and maternal arsenic methylation efficiency during pregnancy. Environ. Int 125, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanis CL, Redline S, Cade BE, Bell GI, Cox NJ, Below JE, et al. , 2016. Beyond type 2 diabetes, obesity and hypertension: an axis including sleep apnea, left ventricular hypertrophy, endothelial dysfunction, and aortic stiffness among Mexican Americans in Starr County, Texas. Cardiovasc. Diabetol 15, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun G, Aguilar D, Evans C, Burant CF, Hanis CL., 2020. Metabolomic profiles associated with subtypes of prediabetes among Mexican Americans in Starr County, Texas, USA. Diabetologia 63 (2), 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC., 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28 (7), 412–419. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA., 1999. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 22 (9), 1462–1470. [DOI] [PubMed] [Google Scholar]
- Scheer J, Findenig S, Goessler W, Francesconi KA, Howard B, Umans JG, et al. , 2012. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal. Methods 4 (2), 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B., 2015. Fast ion chromatography-ICP-QQQ for arsenic speciation. J. Anal. At. Spectrom 30 (6), 1405–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MR, Tellez-Plaza M, Vaidya D, Grau M, Francesconi KA, Goessler W, et al. , 2016. Estimation of Inorganic Arsenic Exposure in Populations With Frequent Seafood Intake: Evidence From MESA and NHANES. Am. J. Epidemiol 184 (8), 590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michigan Imputation Server [Available from: https://imputationserver.sph.umich.edu.
- Rahman M, Vahter M, Sohel N, Yunus M, Wahed MA, Streatfield PK, et al. , 2006. Arsenic exposure and age and sex-specific risk for skin lesions: a population-based case-referent study in Bangladesh. Environ. Health Perspect 114 (12), 1847–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AL, Sohel N, Rahman M, Persson LA, Vahter M, 2010. Impact of smoking and chewing tobacco on arsenic-induced skin lesions. Environ. Health Perspect 118 (4), 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB., 2015. Association of arsenic exposure with smoking, alcohol, and caffeine consumption: data from NHANES 2005-2010. Environ. Toxicol. Pharmacol 39 (2), 651–658. [DOI] [PubMed] [Google Scholar]
- Willett WC, Howe GR, Kushi LH., 1997. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr 65 (4), 9S–31S. Suppl1220S-8Sdiscussion. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E., 2006. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ. Health Perspect 114 (5), 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble MO, Howard BV, Umans JG, Shara NM, Francesconi KA, Goessler W, et al. , 2012. Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am. J. Epidemiol 176 (10), 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado-Gonzalez JA, Del Razo LM, Garcia-Vargas G, Sanmiguel-Salazar F, Escobedo-de la Pena J, 2007. Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ. Res 104 (3), 383–389. [DOI] [PubMed] [Google Scholar]
- Currier JM, Ishida MC, Gonzalez-Horta C, Sanchez-Ramirez B, Ballinas-Casarrubias L, Gutierrez-Torres DS, et al. , 2014. Associations between arsenic species in exfoliated urothelial cells and prevalence of diabetes among residents of Chihuahua, Mexico. Environ. Health Perspect 122 (10), 1088–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MC, Shih YH, Bryan MS, Jackson BP, Aguilar D, Hanis CL, et al. , 2022. Relationships between urinary metals and diabetes traits among Mexican Americans in Starr County, Texas, USA. Biol. Trace Elem. Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizam S, Kato M, Yatsuya H, Khalequzzaman M, Ohnuma S, Naito H, et al. , 2013. Differences in urinary arsenic metabolites between diabetic and non-diabetic subjects in Bangladesh. Int. J. Environ. Res. Public Health 10 (3), 1006–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell Bryan M, Sofer T, Mossavar-Rahmani Y, Thyagarajan B, Zeng D, Daviglus ML, et al. , 2019. Mendelian randomization of inorganic arsenic metabolism as a risk factor for hypertension- and diabetes-related traits among adults in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) cohort. Int. J. Epidemiol 48 (3), 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, Del Razo LM, Garcia-Vargas GG, Sanchez-Pena LC, Barrera-Hernandez A, Styblo M, et al. , 2013. Environmental exposure to arsenic, AS3MT polymorphism and prevalence of diabetes in Mexico. J. Expo. Sci. Environ. Epidemiol 23 (2), 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS., 2003. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc. Natl. Acad. Sci. U S A 100 (13), 7996–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza K, Maddock DA, Zhang Q, Chen J, Chiu C, Mehta S, et al. , 2001. Arsenite activation of P13K/AKT cell survival pathway is mediated by p38 in cultured human keratinocytes. Mol. Med 7 (11), 767–772. [PMC free article] [PubMed] [Google Scholar]
- Wang F, Liu S, Xi S, Yan L, Wang H, Song Y, et al. , 2013. Arsenic induces the expressions of angiogenesis-related factors through PI3K and MAPK pathways in SV-HUC-1 human uroepithelial cells. Toxicol. Lett 222 (3), 303–311. [DOI] [PubMed] [Google Scholar]
- Altman JK, Platanias LC., 2008. Exploiting the mammalian target of rapamycin pathway in hematologic malignancies. Curr. Opin. Hematol 15 (2), 88–94. [DOI] [PubMed] [Google Scholar]
- Bazuine M, Ouwens DM, Gomes de Mesquita DS, Maassen JA., 2003. Arsenite stimulated glucose transport in 3T3-L1 adipocytes involves both Glut4 translocation and p38 MAPK activity. Eur. J. Biochem 270 (19), 3891–3903. [DOI] [PubMed] [Google Scholar]
- Dover EN, Patel NY, Styblo M, 2018. Impact of in vitro heavy metal exposure on pancreatic beta-cell function. Toxicol. Lett 299, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Guo X, Wu B, Yu H, Zhang X, Li M., 2014. Arsenic induces diabetic effects through beta-cell dysfunction and increased gluconeogenesis in mice. Sci. Rep 4, 6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Qiu T, Yao X, Wang N, Jiang L, Jia X, et al. , 2020. Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. J. Hazard. Mater 384, 121390. [DOI] [PubMed] [Google Scholar]
- Jia X, Qiu T, Yao X, Jiang L, Wang N, Wei S, et al. , 2020. Arsenic induces hepatic insulin resistance via mtROS-NLRP3 inflammasome pathway. J. Hazard. Mater 399, 123034. [DOI] [PubMed] [Google Scholar]
- Kirkley AG, Carmean CM, Ruiz D, Ye H, Regnier SM, Poudel A, et al. , 2018. Arsenic exposure induces glucose intolerance and alters global energy metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol 314 (2), R294–R303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargis RM., 2014. The hijacking of cellular signaling and the diabetes epidemic: mechanisms of environmental disruption of insulin action and glucose homeostasis. Diabetes Metab. J 38 (1), 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau, 2020. Hispanic or Latino Origin. American Community Survey [Internet]. [Google Scholar]
- Walton FS, Harmon AW, Paul DS, Drobna Z, Patel YM, Styblo M, 2004. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol. Appl. Pharmacol 198 (3), 424–433. [DOI] [PubMed] [Google Scholar]
- Douillet C, Currier J, Saunders J, Bodnar WM, Matousek T, Styblo M., 2013. Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol. Appl. Pharmacol 267 (1), 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CH., 2009. A review on environmental factors regulating arsenic methylation in humans. Toxicol. Appl. Pharmacol 235 (3), 338–350. [DOI] [PubMed] [Google Scholar]
- Spratlen MJ, Grau-Perez M, Umans JG, Yracheta J, Best LG, Francesconi K, et al. , 2018. Arsenic, one carbon metabolism and diabetes-related outcomes in the Strong Heart Family Study. Environ. Int 121 (Pt 1), 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BL, Tong L, Argos M, Gao J, Farzana J, Roy S, et al. , 2013. Arsenic metabolism efficiency has a causal role in arsenic toxicity: Mendelian randomization and gene-environment interaction. Int. J. Epidemiol 42 (6), 1862–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Chi L, Lai Y, Hsiao YC, Ru H, Lu K, 2021. The gut microbiome and arsenic-induced disease-iAs metabolism in mice. Curr. Environ. Health Rep 8 (2), 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.