Introduction:
Atopic dermatitis remains a widespread problem affecting various populations globally. While numerous treatment options have been employed, pimecrolimus remains a potent and viable option. Recently, there has been increasing interest in comparing the safety and efficacy of pimecrolimus with its vehicle.
Methods:
The authors conducted a comprehensive search of several databases, including PubMed, COCHRANE, MEDLINE, and Cochrane Central, from inception to May 2022, using a wide search strategy with Boolean operators. The authors also employed backward snowballing to identify any studies missed in the initial search. The authors included randomized controlled trials in our meta-analysis and extracted data from the identified studies. The authors used Review Manager (RevMan) Version 5.4 to analyze the data, selecting a random-effects model due to observed differences in study populations and settings. The authors considered a P-value of 0.05 or lower to be statistically significant.
Results:
The authors initially identified 211 studies, of which 13 randomized controlled trials involving 4180 participants were selected for analysis. Our pooled analysis revealed that pimecrolimus 1% was more effective at reducing the severity of atopic dermatitis than its vehicles. However, no significant difference was observed in adverse effects between pimecrolimus and vehicle, except for pyrexia, nasopharyngitis, and headache, which were increased with pimecrolimus.
Conclusion:
Our meta-analysis showed that pimecrolimus 1% is more effective than vehicle, although the safety profile remains inconclusive. Pimecrolimus reduced the Investigator’s Global Assessment score, Eczema Area and Severity Index score, and severity of pruritus when compared to its vehicle, indicating a higher efficacy profile. This is one of the first meta-analyses to assess the efficacy and safety profile of pimecrolimus 1% against a vehicle and may assist physicians in making informed decisions.
Keywords: atopic dermatitis, elidel, eczema and dermatology, pimecrolimus
Introduction
Highlights
While a multitude of treatment options has been employed to date, pimecrolimus remains a feasible and potent option.
The pooled analysis revealed that Pimecrolimus 1% was more effective at lowering the severity of atopic dermatitis when compared to vehicle.
The differences in all of the three variables were statistically significant and indicated that pimecrolimus was more successful at mitigating the severity of atopic dermatitis compared to vehicle.
Atopic dermatitis (AD), also known as atopic eczema, is a chronic, relapsing, and inflammatory skin disease that affects both adults and children. The term eczema is closely associated with the disease’s clinical manifestation, which involves crusting and serous oozing, as well as the formation of blisters along with erythema and scaling1. Globally, ~2.4% of the population suffersfrom AD. The burden of the disease varies significantly among different countries, with reported prevalence rates of 4.9% in the adult population of the US, 2.1% in Japan, and up to 20% in Sweden2. In developed countries, the incidence of AD is on the rise, affecting ~1–3% of adults and 15–20% of children3.
Topical agents are the cornerstone of AD treatment, often used in combination with systemic treatment or phototherapy for more severe cases. Topical calcineurin inhibitors, which belong to the second class of anti-inflammatory drugs after topical corticosteroids, are naturally produced by streptomyces bacteria and play a pivotal role in inhibiting calcineurin-dependent T-cell activation, resulting in the suppression of AD-associated inflammatory mediators and proinflammatory cytokines4. In two short-term (3–12 weeks) and long-term (up to 12 months) trials, topical tacrolimus ointment (strengths of 0.03 and 0.1%), and pimecrolimus cream (1%) were shown to be superior to vehicle5,6.
Pimecrolimus is recommended for use in children below 2 years of age and in the adult population. However, more recently, countries like Canada have also authorized its use in infants greater than 3 months of age7. The current literature has various randomized controlled trials (RCTs) to evaluate the efficacy and safety of pimecrolimus compared to its vehicle, but these results have not yet been systematically reviewed and pooled. Consequently, this systematic review and meta-analysis is noteworthy since it pools the findings from several RCTs. Given that there is no meta-analysis focusing solely on independent safety and efficacy parameters of pimecrolimus, this meta-analysis provides researchers with essential insight for the clinical use of pimecrolimus. As a result, the objective of this systematic review and meta-analysis was to investigate the efficacy and safety of pimecrolimus.
Methodology
Data Sources and Strategy
This study has been reported in line with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines criteria8, Supplemental Digital Content 1, http://links.lww.com/MS9/A140. The PRISMA flow chart is included in Figure S1, Supplemental Digital Content 3, http://links.lww.com/MS9/A142. The current study is also in compliance with the AMSTAR 2 guidelines, Supplemental Digital Content 2, http://links.lww.com/MS9/A141, and the quality of the current systematic review is observed to be low in accordance with the guideline9.
Study Selection
From the beginning until May 2022, two independent reviewers (S.S. and S.E.A.) conducted an exhaustive electronic search of PubMed, MEDLINE, and Cochrane Central using all available terms for AD and pimecrolimus, as well as MeSH terms and the Boolean operators “AND” and “OR.” The search strategy is included in Supplementary Table S1, Supplemental Digital Content 3, http://links.lww.com/MS9/A142. For our meta-analysis, the following predetermined criteria had to be met: published RCTs; adult patients (>18 years) and pediatric patients including infants and children. Any disagreement over the choice of the studies between the two independent reviewers (S.S. and S.E.A.) was settled by discussion and agreement with a third investigator (S.K.F.). All the relevant articles that were enrolled first on the basis of title and abstract, later full-text read was given to assess for relevance.
Data extraction and quality assessment of studies
Two independent reviewers (S.S. and S.E.A.) cross-checked the studies retrieved by the search method before compiling them in Mendeley Reference Manager (Version 2.77.0) software, where duplicates were checked for and removed. The full texts of the remaining articles were then carefully read to identify the outcomes and calculate their odds ratio (OR) (for most outcomes) or mean difference (MD) [for the Eczema Area and Severity Index (EASI) score]. Furthermore, the references of these full-text publications were manually checked for any relevant research that the automated search may have missed.
The primary focus of this study was to evaluate the safety and efficacy of pimecrolimus in comparison to delivery vehicles in AD. Data pertaining to the outcomes of pimecrolimus versus vehicle were extracted from the selected studies and recorded in Excel spreadsheets. The extracted data included information on the author names; publication date; sample size; Investigator’s Global Assessment (IGA) score, a standardized indicator of AD severity based on clinical examination, which reflects the severity of pruritis and inflammation; EASI score; severity of pruritus; and safety parameters such as pyrexia, nasopharyngitis, headache, respiratory symptoms, gastrointestinal symptoms, and other dermatological manifestations. Additional variables such as control, time duration, age, severity of AD, number of participants, sex, and race were also recorded. The age groups represented in the studies were as follows: four studies included children aged 3–23 months; three studies included children and adolescents aged 2 to 17 years; one study included participants aged 1–17 years; one study included participants aged 3 months to 17 years; one study included participants aged 12 and over; one study included participants aged 2–49 years; and two studies included adults aged 18 years and older.
Quality evaluation of the included studies was conducted using the Cochrane Risk of Bias tool10, which was applied to the studies listed in Supplementary Table S2, Supplemental Digital Content 3, http://links.lww.com/MS9/A142. Two reviewers (S.S. and S.E.A.) performed independent data extraction and quality assessment before including the studies in the review. Continuous data were extracted for the outcome EASI, while dichotomous data were preferred for all other outcomes, including pruritus score, IGA score, and safety outcomes. Baseline participant information was also extracted to reduce the likelihood of selection bias.
Statistical Analysis
The extracted data was assessed using the software Review Manager (RevMan), Version 5.4. (the Cochrane Collaboration). The random-effects model was selected due to the observed differences between the study populations and study settings. A P<0.05 or less was regarded as significant. OR were calculated for the majority of the outcomes, whereas EASI results were presented as a MDs with corresponding 95% CIs.
The heterogeneity of effect sizes was assessed using Higgin’s I 2 statistics, with an I 2 value greater than 50% considered significant11. Additionally, funnel plots (Figure S2–S19, Supplemental Digital Content 3, http://links.lww.com/MS9/A142) were generated and the Begg’s test was performed to check for publication bias. A P<0.05 was considered significant for all analyses mentioned above12.
Results
The study process is encapsulated in the PRISMA flow chart (Figure S1, Supplemental Digital Content 3, http://links.lww.com/MS9/A142). Initially, 211 articles were retrieved from PUBMED, of which 175 were removed after screening for duplicates, titles, abstracts, and full-text. Following the application of inclusion criteria, data from 13 RCTs comparing pimecrolimus 1% with vehicle (n=4180) were collected. While some studies reported both safety and efficacy outcomes, most only reported one or the other. Among the shortlisted studies, 10 reported efficacy outcomes (n=2110), while nine reported safety outcomes (n=2986). Detailed baseline study characteristics, including individual efficacy and safety outcomes reported by the studies, are presented in Table 1 of this manuscript. Patient characteristics of the included studies are presented in Supplementary Table S3, Supplemental Digital Content 3, http://links.lww.com/MS9/A142.
Table 1.
Baseline study characteristics of included studies
| References | Intervention and control | Setting | Participants | Definition of AD | Outcomes of efficacy | Outcomes of safety | Severity of AD | Age of participants |
|---|---|---|---|---|---|---|---|---|
| Lawrence F Eichenfield 13 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 6 weeks DB, Randomized | 589 | Hanifin and Rajka14 | IGA; EASI | — | Mild to very severe | 3 months–17 years |
| Alexander Kapp15 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 12 months DB, Randomized | 251 Infants | .Seymour et al. 16 | Incidence of flares; TCs requirement; IGA; EASI; Pruritus score; Patient’s global assessment of disease control. |
Nasopharyngitis, Otitis Media, URTI, Cough, Bronchitis, Rhinitis, Diarrhea, Gastroenteritis, Impetigo, Bacterial Infection, Herpes Simplex, Pyrexia. | Mild to very severe | 3–23 months |
| Roland Kaufmann17 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | Four weeks DB, followed by 12 weeks OL | 196 Infants | .Seymour et al. 16 | Onset of effectiveness; Incidence of flares at EOS; EASI; IGA; Caregiver’s assessment of pruritus severity and sleep loss. |
Nasopharyngitis, Cough, Bronchitis, Rhinitis, Diarrhea, Gastroenteritis, Pyrexia. | Mild to very severe | 3–23 months |
| Richard G B Langley13 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 6 wk DB followed by 20 weeks OL | 403 Children and adolescents. | .Williams et al. 18 | IGA; EASI and pruritus assessment. | Nasopharyngitis, Otitis Media, URTI, Bacterial Infection, Influenza. | Mild to very severe | 2–17 years |
| D F MURRELL19 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 6 weeks DB followed by 6 weeks OL | 200 | Hanifin and Rajka14 | IGA; EASI; Pruritus score; Dermatitis score. | Nasopharyngitis, URTI, Herpes simplex, influenza, Headache. | Mild to moderate | 12 years or over |
| D Y M Leung20 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 6 weeks DB, randomized. |
73 | Hanifin and Rajka14 | IGA; EASI; Pruritus score; Patients AD assessment; TLS | — | Mild to severe | 2–49 years |
| Kristine Breuer21 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 4 weeks DB, Randomized followed by 12 weeks OL. | 195 Infants | .Seymour et al. 16 | IGA, EASI, SCORAD | — | Mild to very severe | 3–23 months |
| Lawrence F Eichenfield5 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 6 weeks DB, randomized |
403 Children and adolescents | .Williams et al.18 | IGA; EASI; Pruritus score; Patient’s global assessment of disease control. | Nasopharyngitis, URTI, Application of site burning, Headache | Mild to moderate | 1–17 years |
| Vincent C. HO22 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 6 weeks DB, randomized followed by 20 weeks OL | 186 Infants | .Williams et al.18 | IGA; EASI; Pruritus Score | Nasopharyngitis, URTI, Bronchitis, Rhinitis, Diarrhea, Gastroenteritis, Bacterial Infection, Pyrexia & Influenza. | Mild to moderate | 3–23 months |
| T. LUGER23 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 3 weeks DB, randomized. | 260 Adults | Hanifen and Rajka14 | Adapted EASI; Pruritus score; Patient’s self-assessment of disease control. |
— | Moderate to severe | >18 years |
| B Sigurgeirsson24 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 26 weeks, DB, Randomized | 521 Children | Williams et al.18. |
Number of TCS free days; number of flares. | Nasopharyngitis, Otitis Media, URTI, Cough, Bronchitis, Rhinitis, Gastroenteritis, Impetigo, Pyrexia, Influenza, Application of site burning, Headache. | Mild to moderate | 2–17 years |
| Ulrich Wahn25 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 12 month DB | 711 Children and adolescents. | .Williams et al. 18 | Incidence of flares at 6 and 12 months; TCs requirement; Time to first flare; IGA; EASI. | Impetigo, Bacterial Infection and Herpes Simplex. | Moderate to very severe | 2–17 years |
| Micheal Meurer26 | Pimecrolimus 1% b.i.d and Vehicle b.i.d | 24 weeks DB, randomized | 192 Adults | Rajka and Langland27 | Incidence of flares; TCs requirement; IGA; EASI; Pruritus score; Patient’s global assessment of disease control. |
Application of Site Burning | Moderate to severe | >18 years |
AD, Atopic Dermatitis; B.i.d, Twice daily; DB, Double Bind; EASI, Eczema Area and Severity Index; IGA, Investigators Global Assessment; OL, Open Label; URTI, Upper respiratory tract infection.
Efficacy of Pimecrolimus 1% versus Vehicle
IGA Score with Pimecrolimus versus Vehicle
In our pooled analysis, eight studies5,13,15,19–22,28 comprising 2298 participants reported IGA scores at 6 weeks, which were statistically significant. Pimecrolimus 1% was significantly more effective than the vehicle [OR=2.89 (2.18, 3.82), P<0.00001, I 2=41%; Fig. 1] in reducing the IGA scores to clear or almost clear (IGA 0 or 1). Two studies5,22 comprising 589 participants reported IGA at 3 weeks, which was also statistically significant. Pimecrolimus 1% was significantly more effective than the vehicle [OR=4.18 (2.51, 6.95), P<0.00001, I 2=0%; Fig. 1] in reducing the IGA scores to clear or almost clear (IGA 0 or 1).
Figure 1.
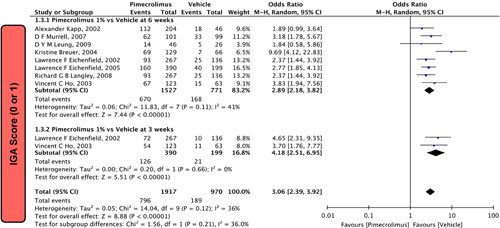
Forest plot of IGA score (0 or 1) of pimecrolimus versus vehicle.
Severity of Pruritus
In our meta-analysis, two studies5,22 involving 589 participants reported the severity of pruritus at 6 weeks, which was statistically significant. Pimecrolimus 1% was significantly more effective than vehicle [OR = 3.49 (1.73,7.04), P=0.0005, I 2=69%; Fig. 2]. Similarly, three studies5,22,23 comprising 677 participants reported the severity of pruritus at 3 weeks, which was also statistically significant. Pimecrolimus 1% was significantly more effective than vehicle [OR=4.27 (2.58,7.05), P<0.00001, I 2=43%; Fig. 2].
Figure 2.
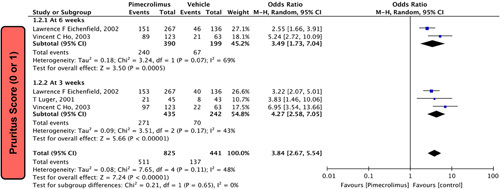
Forest plots evaluating pruritus score (0 or 1) of pimecrolimus versus vehicle.
EASI Score
In our pooled analysis, four studies17,20,21,28 comprising of 784 participants reported EASI that was statistically significant. Pimecrolimus 1% effectively decreased the EASI score compared to the vehicle [MD=−11.31 (−13.38, −9.24); P<0.00001, I 2=5%; Fig. 3].
Figure 3.
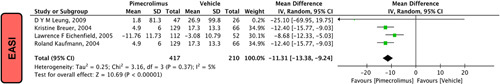
Forest plots evaluating EASI of pimecrolimus versus vehicle.
Safety of Pimecrolimus 1% Versus Vehicle
The use of pimecrolimus resulted in a statistically significant increase in three safety outcomes, namely headache, nasopharyngitis, and pyrexia, compared to vehicle use. However, other adverse effects were not statistically significant in this analysis. Therefore, the safety profile of pimecrolimus has not been fully assessed.
Pyrexia
Our pooled analysis included four studies15,17,22,24 with a total of 1138 participants reporting pyrexia. The results showed that pimecrolimus 1% significantly increased the risk of pyrexia compared to the use of a vehicle [OR = 1.65 (1.03, 2.63), P=0.04, I 2=31%; Fig. 4].
Figure 4.
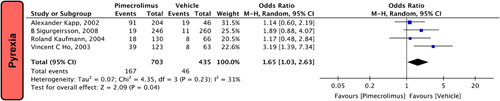
Forest plots evaluating the safety outcomes of pyrexia of pimecrolimus versus vehicle.
Nasopharyngitis
In our pooled analysis, seven studies5,13,15,17,19,22,24 comprising 2145 participants reported nasopharyngitis. Our pooled analysis demonstrates that pimecrolimus 1% significantly increases the risk of nasopharyngitis compared to vehicle [OR = 1.61 (1.24,2.10), P=0.0004, I 2=0%; Fig. 5].
Figure 5.
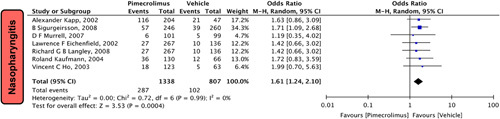
Forest plots evaluating the safety outcomes of nasopharyngitis of pimecrolimus versus vehicle.
Headache
In our pooled analysis, three studies5,19,24 comprising of 1109 participants reported headache. Our pooled analysis demonstrates that pimecrolimus 1% significantly increases the risk of beadache compared to vehicle [OR = 1.70 (1.00,2.87), P=0.05, I 2=0%; Fig. 6].
Figure 6.
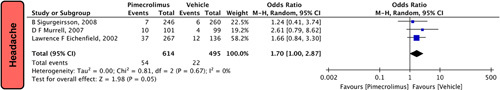
Forest plots evaluating the safety outcomes of headache of pimecrolimus versus vehicle.
Respiratory Symptoms
Pimecrolimus 1% given to patients with AD did not increase the risk of respiratory symptoms such as upper respiratory tract infection [OR=1.19 (0.87, 1.63), P=0.26, I 2=0%] six studies 5,13,15,19,22,24, cough [OR=1.22 (0.73, 2.02), P=0.45, I 2=0%] three studies15,17,24, influenza [OR=1.58 (0.55, 4.50), P=0.39, I 2=46%] four studies13,19,22,24, rhinitis [OR=0.77 (0.39, 1.53) four studies15,17,22,24, P=0.46, I 2=56%], and bronchitis [OR=0.85 (0.52, 1.38), P=0.51, I 2=0%], four studies15,17,22,24 when compared to vehicle (Fig. 7).
Figure 7.
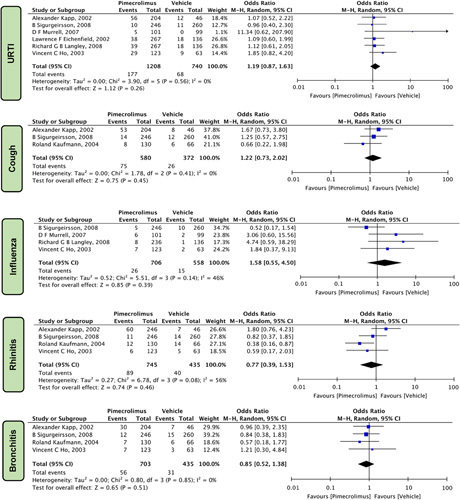
Forest plots evaluating the safety outcomes of pimecrolimus versus vehicle for respiratory symptoms.
Gastrointestinal Symptoms
Upon evaluating the safety profile of pimecrolimus 1% and the vehicle regarding gastrointestinal symptoms, it was revealed that pimecrolimus did not significantly increase the risk of diarrhea [OR=1.27 (0.56, 2.88), P=0.58, I 2=30%] three studies15,17,22 and gastroenteritis [OR=0.95 (0.44, 2.05), P=0.89, I 2=37%] four studies15,17,22,24 when compared to the vehicle (Fig. 8).
Figure 8.
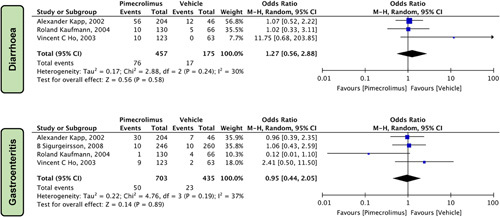
Forest plots evaluating the safety outcomes of pimecrolimus versus vehicle for gastrointestinal symptoms.
Dermatological Manifestations
In our pooled analysis, dermatological manifestation reported by different studies revealed that Pimecrolimus 1% when compared to vehicle (Fig. 9) did not significantly increase the risk of Impetigo [OR=0.77 (0.18, 3.26), P=0.73, I 2= 87%] three studies15,24,25and Application site burning [OR=0.87 (0.44, 1.72), P=0.69, I 2= 33%] three studies5,24,26.
Figure 9.
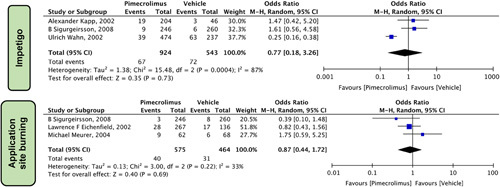
Forest plots evaluating the safety outcomes of pimecrolimus versus vehicle for dermatological manifestations.
In our pooled analysis, the dermatological manifestations reported by different studies revealed that when compared to vehicle (Fig. 9), pimecrolimus 1% did not significantly increase the risk of impetigo [OR = 0.77 (0.18, 3.26), P=0.73, I 2= 87%] three studies15,24,25 and application site burning [OR = 0.87 (0.44, 1.72), P=0.69, I 2= 33%] three studies5,24,26.
Other Symptoms
Five other symptoms were reported, which did not fit into the above categories and revealed a statistically nonsignificant association with the use of pimecrolimus 1% and the vehicle in patients with AD (Fig. 10). These symptoms included bacterial infection [OR=0.76 (0.24, 2.44), P=0.64, I 2= 32%] four studies13,15,22,25, otitis media [OR=1.02 (0.59, 1.75), P=0.95, I 2=0%] three studies13,15,24, and herpes simplex virus (HSV) [OR=0.79 (0.37, 1.71), P=0.55, I 2=0%] three studies15,19,25.
Figure 10.
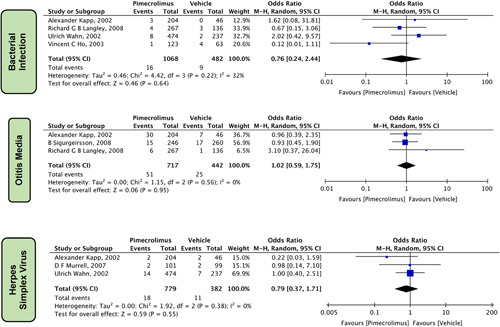
Forest plots evaluating the other safety outcomes of pimecrolimus versus vehicle.
DISCUSSION
In this meta-analysis of over 4000 patients, we compared the safety and efficacy of pimecrolimus versus a vehicle for treating AD in adult and pediatric populations. To assess pimecrolimus’ efficacy, we compared IGA scores, EASI scores, and the severity of pruritus among the 13 studies included in the meta-analysis. The differences in all three variables were statistically significant and indicated that pimecrolimus was more successful than the vehicle in mitigating the severity of AD. We found a significant difference in the incidence of three outcomes; headache, nasopharyngitis, and pyrexia, which were more likely to occur with pimecrolimus use than with vehicle use, among the various safety outcomes evaluated. However, the additional adverse effects evaluated were not statistically significant in our study, making it impractical to assess pimecrolimus’s safety profile. The severity of AD cases ranged from mild to very severe in this pooled analysis, which included both adults and children, implying that the findings held true for all age groups and severity levels. However, there is limited research analyzing the long-term effects of AD medical therapy25. Hence, more research is necessary to evaluate pimecrolimus’ safety profile.
The mechanism of action of pimecrolimus 1% is to inhibit T lymphocyte activation and the synthesis of proinflammatory cytokines such as interleukin-2 and interferon-gamma. This helps to reduce inflammation and pruritus in the affected skin, thereby reducing the severity of AD symptoms29. Decreased levels of IGA reflect a reduction in skin itch and inflammation. Pimecrolimus 1% has been shown in clinical studies to significantly reduce the IGA score in individuals with mild to severe AD, compared to placebo medication5. Another meta-analysis of RCTs comparing pimecrolimus 1% cream to a control group or alternative therapies for AD patients was conducted by Ashcroft et al. 30 in 2007, which revealed that pimecrolimus was more effective in reducing IGA scores than vehicles.
The IGA and the EASI scores, in particular, have suggested that pimecrolimus improves clinical outcomes and reduces pruritus. This meta-analysis consistently shows a superior effectiveness profile of pimecrolimus when compared to a vehicle (also known as a nonactive control). Clinically, pimecrolimus is recommended for use in mild to moderate AD4. Another systematic review concluded that pimecrolimus is more effective than the vehicle in terms of efficacy31. In short-term (3–12 weeks) and long-term (up to 12 months) studies on adults and children with active disease, topical calcineurin inhibitors, such as pimecrolimus, have been shown to be more effective than the vehicle4.
Other reviews and meta-analyses have confirmed our findings regarding the efficacy of pimecrolimus over vehicle30–32. This was evident even at a pimecrolimus concentration of 0.2%, as demonstrated by a dose-finding study conducted by Luger et al.23. Interestingly, a 26-week trial by Langley et al. 13 suggested that pimecrolimus is more effective in treating AD in the face and neck regions than in other parts of the body. However, this study has limited generalizability since it was only conducted on children aged 12–17. In an infant trial, the onset of action of pimecrolimus was reported to be as early as day 4 of use17. Individually, none of the 13 studies included in this meta-analysis reported significant differences in the incidence of adverse events between pimecrolimus and vehicle groups. Still, collectively, our results indicate otherwise. Furthermore, although our meta-analysis found no significant differences in the frequency of HSV infections between pimecrolimus and vehicle groups, a 2009 review found that pimecrolimus use was linked to a slightly higher incidence of HSV infections32. The present meta-analysis sought to provide an overview of the safety and efficacy of pimecrolimus versus vehicle in AD. While our goal was indeed to garner an in-depth understanding of these, we did not seek to evaluate the safety and efficacy across the various severity grades. Therefore, we have not included a sub-sectional analysis of the treatments across varying grades of severity.
This meta-analysis has certain shortcomings. Firstly, our results are limited by patient variability, including variations in age, sex, and ethnicity. Due to heterogeneity, the random-effects model was used for statistical analysis. Secondly, the effect of pimecrolimus could not be assessed separately for the pediatric and adult population due to a lack of data comparing pimecrolimus and vehicle in the respective age groups. Therefore, trends regarding the age of patients with AD could not be analyzed. Further prospective studies are required to study the efficacy of pimecrolimus in the pediatric and adult population separately. Thirdly, the safety profile of pimecrolimus could not be completely assessed, as our analysis found most adverse effects to be not statistically significant. Further data from studies are required to evaluate the safety profile of the drug. Fourthly, our meta-analysis excluded studies conducted in any language other than English. Furthermore, this meta-analysis only considered short-term trials. The effectiveness or safety of therapies for chronic illnesses like AD may not be fully reflected by short-term trials, rendering it another limitation of this study. Therefore, long-term study data are required to evaluate the effect of pimecrolimus in all ages. Lastly, the studies included in this meta-analysis were not classified according to the severity of AD. Since the treatment of AD varies according to the severity of AD, the results of this meta-analysis regarding the efficacy of pimecrolimus were only generalized and not specific to different grades of severity of AD. Moreover, data from multicenter studies are required that stratify AD according to severity grades and analyze the efficacy of pimecrolimus and vehicle. However, this meta-analysis is novel since it was the first to assess the safety profile of pimecrolimus in comparison to a vehicle.
CONCLUSION
This meta-analysis highlights the efficacy and safety of pimecrolimus 1% in treating patients with AD (eczema), enabling clinicians to anticipate the course of the condition. The meta-analysis demonstrates a strong correlation between IGA, pruritus score, and EASI, indicating the potency of pimecrolimus in reducing AD compared to the vehicle. However, several notable associations were discovered in the safety profile. Pimecrolimus induced pyrexia, nasopharyngitis, and headaches (in the short-term, given the short follow-up time of individual studies), whereas other side effects were inconclusive in the analysis; therefore, the safety of pimecrolimus remains inconclusive. Our comprehensive analysis found limited data to answer clinically essential questions about how pimecrolimus and the vehicle compare in terms of effectiveness (particularly as a choice for a long-term treatment strategy), adverse events, tolerability, and financial cost to existing clinically recommended therapies. Future meta-analyses and randomized controlled studies, conducted on larger sample sizes and of longer duration, must bridge the information gap by focusing on additional pimecrolimus safety and long-term effectiveness outcomes across age groups, as well as assessing treatment according to the severity of AD.
Ethical approval
NA.
Consent
NA.
Sources of funding
NA.
Author contribution
S.E.A., S.S., S.K.F., and R.J.: conceived the idea, designed the study, and drafted the manuscript; A.K.S., M.A.N., and A.A.: conducted comprehensive literature search, screened the studies for relevant content, and created the literature review table; H.I., K.A.K., and A.A.H.: revised the manuscript critically and refined the literature review table; H.K.A., T.F., A.T., A.T., and S.S.: drafted the discussion part of the manuscript, revised the final version of the manuscript critically based on the reviewer and editorial comments; A.A.P., T.A., A.A., and A.A.: conceived the initial study idea, diagnosed the case, and gave the final approval for publication.
Conflicts of interest disclosure
NA.
Research registration unique identifying number (UIN)
NA.
Guarantor
Saad Shakil. Ziauddin University. E-mail: saadshakil07@gmail.com
Provenance and peer review
Not commissioned, externally peer-reviewed.
Data availability statement
NA.
Disclosures
NA.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.annalsjournal.com.
Published online 22 May 2023
Contributor Information
Sareema E. Akhtar, Email: sareemaeman@gmail.com.
Saad Shakil, Email: saadshakil07@gmail.com.
Sabeeh K. Farooqui, Email: Sabeeh24farooqui@gmail.com.
Tarek Khedro, Email: tarek.khedro@gmail.com.
Ahmad L. Alzufairi, Email: ahmadalzufairi@gmail.com.
Muhammad A. Niaz, Email: muhamedaliniaz@gmail.com.
Saifullah Syed, Email: saifullahsyed@rcsi.com.
Riaz Jiffry, Email: riazjiffry19@gmail.com.
Abdulla K. Alsubai, Email: alsubaiabdulla@gmail.com.
Abdullah Almesri, Email: AbdullahAlmesri@rcsi.ie.
Hebatalla Ismail, Email: hebaismail@rcsi.com.
Kashif A. Khan, Email: k.khan2@nuigalway.ie.
Ahmed Al-Hindawi, Email: 18236928@rcsi-mub.com.
Hussein K. Ali, Email: Hussein_k_ali@hotmail.com.
Timothy Falodun, Email: timifalodun@gmail.com.
Aysa Tabassi, Email: Aysa.mtabassi.22@gmail.com.
Aylin Tabassi, Email: a.tabassi14@gmail.com.
Talal Almas, Email: talalalmas.almas@gmail.com.
References
- 1. Hadi HA, Tarmizi AI, Khalid KA, et al. The epidemiology and global burden of atopic dermatitis: a narrative review. Life (Basel). 2021;11:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Urban K, Chu S, Giesey RL, et al. The global, regional, and national burden of atopic dermatitis in 195 countries and territories: an ecological study from the global burden of disease study 2017. JAAD Int 2021;2:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. https://www.ajmc.com/view/overview-of-atopic-dermatitis-article Overview of Atopic Dermatitis [Internet]. Accessed 8 September 2022. [Google Scholar]
- 4. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: Part 2: management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014;71:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eichenfield LF, Lucky AW, Boguniewicz M, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol 2002;46:495–504. [DOI] [PubMed] [Google Scholar]
- 6. Kang S, Lucky AW, Pariser D, et al. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. J Am Acad Dermatol 2001;44(1 suppl):S62–S63. [DOI] [PubMed] [Google Scholar]
- 7. Luger T, Paller AS, Irvine AD, et al. Topical therapy of atopic dermatitis with a focus on pimecrolimus. J Europ Aca Dermatol Venereol 2021;35:1505–1518. [DOI] [PubMed] [Google Scholar]
- 8. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. https://pubmed.ncbi.nlm.nih.gov/7786990/ Operating characteristics of a rank correlation test for publication bias - PubMed [Internet]. Accessed 8 September 2022. [Google Scholar]
- 13. Langley RGB, Eichenfield LF, Lucky AW, et al. Sustained efficacy and safety of pimecrolimus cream 1% when used long-term (up to 26 weeks) to treat children with atopic dermatitis. Pediatr Dermatol 2008;25:301–307. [DOI] [PubMed] [Google Scholar]
- 14. Larsen FS, Hanifin JM. Epidemiology of atopic dermatitis. Immunol All Clin 2002;22:1–24. [Google Scholar]
- 15. Kapp A, Papp K, Bingham A, et al. Long-term management of atopic dermatitis in infants with topical pimecrolimus, a nonsteroid anti-inflammatory drug. J Allergy Clin Immunol 2002;110:277–284. [DOI] [PubMed] [Google Scholar]
- 16. Seymour JL, Keswick BH, Hanifin JM, et al. Clinical effects of diaper types on the skin of normal infants and infants with atopic dermatitis. J Am Acad Dermatol 1987;17:988–997. [DOI] [PubMed] [Google Scholar]
- 17. Kaufmann R, Fölster-Holst R, Höger P, et al. Onset of action of pimecrolimus cream 1% in the treatment of atopic eczema in infants. J Allergy Clin Immunol 2004;114:1183–1188. [DOI] [PubMed] [Google Scholar]
- 18. WILLIAMS HC, JBURNEY PG, PEMBROKE AC, et al. The U.K. working party’s diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol 1994;131:406–416. [DOI] [PubMed] [Google Scholar]
- 19. Murrell DF, Calvieri S, Ortonne JP, et al. A randomized controlled trial of pimecrolimus cream 1% in adolescents and adults with head and neck atopic dermatitis and intolerant of, or dependent on, topical corticosteroids. Br J Dermatol 2007;157:954–959. [DOI] [PubMed] [Google Scholar]
- 20. Leung DYM, Hanifin JM, Pariser DM, et al. Effects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle-controlled trial. Br J Dermatol 2009;161:435–443. [DOI] [PubMed] [Google Scholar]
- 21. Breuer K, Braeutigam M, Kapp A, et al. Influence of pimecrolimus cream 1% on different morphological signs of eczema in infants with atopic dermatitis. Dermatology 2004;209:314–320. [DOI] [PubMed] [Google Scholar]
- 22. Ho VC, Gupta A, Kaufmann R, et al. Safety and efficacy of nonsteroid pimecrolimus cream 1% in the treatment of atopic dermatitis in infants. J Pediatr 2003;142:155–162. [DOI] [PubMed] [Google Scholar]
- 23. Luger T, van Leent EJM, Graeber M, et al. SDZ ASM 981: an emerging safe and effective treatment for atopic dermatitis. Br J Dermatol 2001;144:788–794. [DOI] [PubMed] [Google Scholar]
- 24. Sigurgeirsson B, Ho V, Ferrándiz C, et al. Effectiveness and safety of a prevention-of-flare-progression strategy with pimecrolimus cream 1% in the management of paediatric atopic dermatitis. J Eur Acad Dermatol Venereol [Internet] 2008;22:370–371. [DOI] [PubMed] [Google Scholar]
- 25. Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics 2002;110(1 Pt 1):e2. [DOI] [PubMed] [Google Scholar]
- 26. Meurer M, Fartasch M, Albrecht G, et al. Long-term efficacy and safety of pimecrolimus cream 1% in adults with moderate atopic dermatitis. Dermatology 2004;208:365–372. [DOI] [PubMed] [Google Scholar]
- 27. Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:13–14. [DOI] [PubMed] [Google Scholar]
- 28. Eichenfield LF, Lucky AW, Langley RGB, et al. Use of pimecrolimus cream 1% (Elidel) in the treatment of atopic dermatitis in infants and children: the effects of ethnic origin and baseline disease severity on treatment outcome. Int J Dermatol 2005;44:70–75. [DOI] [PubMed] [Google Scholar]
- 29. Grassberger M, Steinhoff M, Schneider D, et al. Pimecrolimus – an anti-inflammatory drug targeting the skin. Exp Dermatol 2004;13:721–730. [DOI] [PubMed] [Google Scholar]
- 30. Ashcroft DM, Dimmock P, Garside R, et al. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials. BMJ 2005;330:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El-Batawy MMY, Bosseila MAW, Mashaly HM, et al. Topical calcineurin inhibitors in atopic dermatitis: a systematic review and meta-analysis. J Dermatol Sci 2009;54:76–87. [DOI] [PubMed] [Google Scholar]
- 32. Werfel T. Topical use of pimecrolimus in atopic dermatitis: update on the safety and efficacy. JDDG 2009;7:739–742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NA.


