Background:
This study aims to evaluate the efficacy of systemic chemotherapy combined with radiofrequency ablation in the treatment of inoperable colorectal cancer with liver metastasis.
Materials and methods:
The authors conducted a retrospective cohort analysis on 30 patients diagnosed as colorectal cancer with liver metastasis who underwent systemic chemotherapy combined with radiofrequency ablation of the liver lesions from January 2017 to August 2020 at our institution. Responses was evaluated by International Working Group on Image-guided Tumor Ablation criteria, along with progression-free survival.
Results:
The response rate after 4 cycles and 8 cycles of chemotherapy were 73.3% and 85.2%, respectively. All patients achieved responses after of radiofrequency therapy, with the rates of complete response and partial response were 63.3% and 36.7%. The median progression-free survival was 16.7 months. After radiotherapy ablation, all patients had mild to moderate hepatic pain, 10% of patients had fever and increased liver enzymes occurred in 90% of patients.
Conclusions:
Systemic chemotherapy combined with radiofrequency ablation was safe and effective in colorectal cancer with liver metastasis and warrants further large-scale studies.
Keywords: chemotherapy, colorectal cancer, liver metastases, RFA
Introduction
Highlights
Combining systemic chemotherapy with radiofrequency ablation in stage IV colorectal cancer has been shown to have promising recently. However, the results across studies are not consistent.
Our study demonstrated a good response of this method, with the rates of complete and partial response being 63.3% and 36.7%, respectively.
The treatment method was safe, with the majority of patients having mild side effects.
Liver is the most popular metastatic location of colorectal cancer (CRC) because most of venous blood from the digestive tract comes to the liver through portal vein system. At the time of diagnosis, there is ~20–30% of patients who had liver metastasis, and more than 50% of patients will develop liver metastasis during the course of the disease1. CRC with liver metastasis was classified into resectable, potentially resectable and unresectable. Radical surgery results in superior prognosis and offers high quality of life in resectable cases. However, only about 10–20% of patients with CRC liver metastasis are operable2. Systemic chemotherapy is the standard treatment for inoperable patients with liver-metastasis colorectal cancer. Regimens using Oxaliplatin or Irinotecan combined with 5-fluorouracil and Leucovorin improved the response rate from 20 to 50%. Targeted drugs such as Bevacizumab or Cetuximab might increase the response rate up to 70%3.
Radiofrequency ablation (RFA) was introduced in the early 1990s. This technique is a less invasive and safer compared with surgery, especially in hard-to-reach locations. Therefore, RFA is increasingly used in the treatment of colorectal cancer with liver metastasis. Combining systemic chemotherapy with RFA is a multimodality treatment, which has been shown in several studies to be safe, improve responses and prolong survival in comparison with chemotherapy alone4. However, the results across studies are not consistent. Therefore, we conducted this study to evaluate the response rates, progression-free survival and associated factors in CRC patients with liver metastasis treated with liver RFA and chemotherapy.
Materials and methods
Patients
This retrospective cohort study included 30 colorectal cancer patients with liver metastasis treated with chemotherapy plus RFA from January 2017 to August 2020.
We included patients who had colorectal cancer confirmed by histopathology, were diagnosed with de novo liver metastasis and underwent surgery to remove the primary tumour but had no indications for liver tumour resection, or recurrence with liver metastases. These patients had evidence of liver metastases on liver biopsy or imaging methods: computer tomography (CT), magnetic resonance, PET/CT and had no extrahepatic metastases.
Patients in the study are over 18 years old, with Eastern cooperative oncology group (ECOG) score 0–1. The function of liver, kidney and haematological system were adequate for treatment. After chemotherapy, the patients achieved a partial responded or stable disease will get RFA for remaining liver lesions. Patients with progression after first-line chemotherapy and those with liver lesions not amenable for RFA were excluded.
Chemotherapy and radiofrequency ablation
Patients treated with chemotherapy for at least 4 cycles of XELOX regimen (no prior chemotherapy) or XELIRI (previously treated with adjuvant Oxaliplatin–base regimen) with or without Bevacizumab. Evaluation of disease response was based on RECIST 1.1 criteria. Patients who had residual liver lesions after chemotherapy received RFA. The indication and timing of RFA (after 4 or 8 cycles) were determined by multidisciplinary tumour board discussions.
XELOX regimen: Oxaliplatin: 130 mg/m2, IV infusion over 2 h, on day 1, Capecitabine: 1000 mg/m2, twice a day, drink after eating within 30 min, from day 1 to day 14, cycle 21 days.
XELIRI regimen: Irinotecan: 240 mg/m2, IV infusion over 90 min, on day 1, Capecitabine: 1000 mg/m2, twice a day, drink after eating within 30 min, from day 1 to day 14, cycle 21 days.
Bevacizumab: 7.5 mg/kg, IV infusion over 30 min, on day 2, cycle 21 days.
RFA is performed percutaneously under ultrasound guidance or laparoscopically, lesions will be re-evaluated after a month of RFA by contrast-enhanced abdominal CT/MRI. RFA response assessment according to the International Working Group on Image-guided Tumor Ablation5.
Data analysis
Data on clinical characteristics, systemic chemotherapy treatment, method, timing and response of RFA and progression-free survival were recorded through patients’ medical record. Progression-free survival was calculated from the completion of RFA to progression or death from any causes.
Data analysis was done using SPSS software, version 20.0. We used Kaplan–Meier algorithm to assess progression-free survival time, χ2 test, Fisher test to test the association between survival and clinical factors. The clinical factors were chosen based on literature review. P values less than 0.05 were considered to be statistically significant.
This work has compliant with the STROCSS 2021 criteria6.
Results
Characteristics of patients in the study
In this study, there were more male patients, with the male: female ratio of 2.3:1, the mean age was 57.7 years old. All patients had an Eastern cooperative oncology group (ECOG) score of 0 or 1. In 30 patients of the study, there were 18 patients (60%) with synchronous liver metastasis and 12 patients (40%) with metachronous liver metastasis. There were 24 patients (80%) experiencing elevated Carcinoma embryonic antigen (CEA) levels before treatment. Most patients had 1–3 liver tumours. There were 11 patients with tumour size before treatment larger than 3 cm, in which the largest tumour size was 4 cm. (Table 1).
Table 1.
Clinical characteristics of patients.
| No. patients, n (%) | |
|---|---|
| Sex | |
| Male | 21 (70) |
| Female | 9 (30) |
| Mean age ± SD (years) | 57.7 ± 10.0 |
| ECOG | |
| 0 | 25 (83.3) |
| 1 | 5 (16.7) |
| Primary tumour location | |
| Colon | 17 (56.7) |
| Rectal | 13 (43.3) |
| Liver metastasis | |
| Synchronous | 18 (60) |
| Metachronous | 12 (40) |
| Serum concentration of CEA | |
| ≤ 5 ng/ml | 6 (20) |
| > 5 ng/ml | 24 (80) |
| Liver tumour location | |
| Right lobe | 22 (73.3) |
| Left lobe | 3 (10) |
| Both lobes | 5 (16.7) |
| Number of liver tumours before treatment | |
| 1–3 | 28 (93.3) |
| > 3 | 2 (6.7) |
| The largest tumour diameter before treatment | |
| ≤ 3 cm | 19 (63.3) |
| > 3 cm | 11 (36.7) |
carcinoma embryonic antigen; ECOG, eastern cooperative oncology group.
Characteristics of chemotherapy and RFA techniques
The regimen used in the study was XELOX or XELIRI regimens with or without the anti-angiogenic drug (Bevacizumab), for at least 4 cycles. There were 3 patients treated with 4 cycles, while 27 remaining patients were treated with 8 cycles. Patients were evaluated for response after 4 and 8 cycles. Most patients received percutaneous RFA under ultrasound guidance, only one patient received laparoscopy. The patient who underwent laparoscopic RFA had three liver metastases, including a 2 cm tumour in the segment IVb, which was close to the gallbladder. Therefore, this patient had laparoscopic RFA to approach the lesion easier as well as isolate the gallbladder from the burning area. The mean time of RFA for a patient was 16.3 min. (Table 2), (Table 3).
Table 2.
Characteristics of chemotherapy and RFA techniques.
| No. patients, n (%) | |
|---|---|
| Regimen | |
| XELOX ± Bevacizumab | 14 (46.7) |
| XELIRI ± Bevacizumab | 16 (53.3) |
| No. cycles | |
| 4 cyles | 3 (10) |
| 8 cycles | 27 (90) |
| Method of RFA | |
| Percutaneous | 29 (96.7) |
| Laparoscopy | 1 (3.3) |
| Mean time of RFA (min) | 16.3 ± 5.4 |
radiofrequency ablation.
Table 3.
The response after chemotherapy.
| After 4 cycles | After 8 cycles | |||||
|---|---|---|---|---|---|---|
| Partial response, N (%) | Stable disease, N (%) | P | Response disease, N (%) | Stable disease, N (%) | P | |
| XELOX | 11 (78.6) | 3 (21.4) | 0.689 | 11 (91.7) | 1 (8.3) | 0.605 |
| XELIRI | 11 (68.8) | 5 (31.2) | 12 (80) | 3 (20) | ||
| Total | 22 (73.3) | 8 (26.7) | 23 (85.2) | 4 (14.8) | ||
Treatment results
The response after treatment
After 4 or 8 cycles of chemotherapy, all patients achieved partial response or stable disease. Patients treated with XELOX regimen (Bevacizumab can be added) had a response rate after 4 or 8 cycles of 78.6% and 91.7%, respectively, while these proportions in the XELIRI group were 68.8% and 80%. There was no significantly statistic difference in response between the two regimens. (Table 4).
Table 4.
The response after radiofrequency ablation.
| Factors | Complete respone, n (%) | Partial response, n (%) | P | |
|---|---|---|---|---|
| No. liver tumours | 1 | 13 (72.2) | 5 (27.8) | 0.181 |
| 2 | 6 (60) | 4 (40) | ||
| 3 | 0 | 2 (100) | ||
| Total | 19 (63.3) | 11 (36.7) | ||
All patients achieved a response after RFA, of which 19 patients (63.3%) achieved a complete response, 11 patients achieved a partial response and there was no association between the response after RFA and the number of tumours (P=0.181). (Table 5).
Table 5.
The progressive and recurrent locations after complete response.
| No. patients | Rate (%) | |
|---|---|---|
| Recurrence of primary liver lesions | 5 | 29.4 |
| New lesions in liver | 9 | 52.9 |
| New extrahepatic lesions | 3 | 17.7 |
After chemotherapy and radiofrequency ablation
Among the 19 complete responders, there was 13 patients progressed or relapsed, in which there were two patients having both recurrence at the site of RFA and new lesions in the liver. One patient suffered a relapse at the location of RFA and new pulmonary metastases. Another patient developed both new liver lesions and lung metastases. The progression in the liver with new lesions is the most common, accounting for 52.9%. (Fig. 1), (Table 6).
Figure 1.
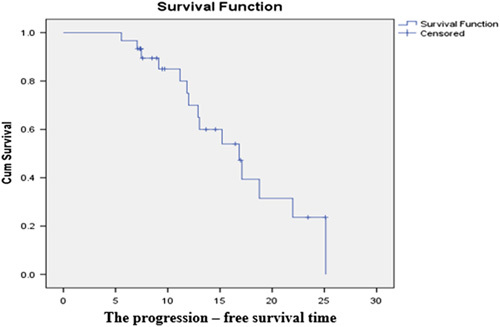
The progression-free survival time.
Table 6.
Factors associated with progression-free survival.
| Subgroups | Median progressive-free survival (months) | P |
|---|---|---|
| Age | ||
| ≤ 70 (n=26) | 17.9 ± 1.4 | 0.0002 |
| > 70 (n=4) | 7.6 ± 0.9 | |
| Sex | ||
| Male (n=21) | 14.0 ± 1.3 | 0.109 |
| Female (n=9) | 20.5 ± 2.0 | |
| ECOG | ||
| 0 (n=25) | 18.1 ± 1.5 | 0.002 |
| 1 (n=5) | 10.4 ± 1.3 | |
| Initial number of liver tumours | ||
| 1–2 (n=23) | 17.0 ± 1.9 | 0.56 |
| ≥ 3 (n=7) | 16.2 ± 1.9 | |
| Initial largest liver tumour diameter | ||
| ≤ 3 cm (n=19) | 17.2 ± 2.2 | 0.872 |
| > 3 cm (n=11) | 16.6 ± 1.6 | |
| Liver tumour diameter before RFA | ||
| ≤ 2 cm (n=22) | 17.2 ± 1.8 | 0.572 |
| 2-3 cm (n=8) | 15.0 ± 1.6 | |
| Time of liver metastasis | ||
| Synchronous metastasis (n=18) | 17.6 ± 1.6 | 0.301 |
| Metachorous metastasis (n=12) | 14.7 ± 2.0 | |
| Regimen chemotherapy | ||
| XELOX ± Bevacizumab (n=14) | 22.0 ± 2.2 | 0.591 |
| XELIRI ± Bevacizumab (n=16) | 16.5 ± 2.3 | |
| Initial concentration of CEA | ||
| ≤ 30 ng/ml | 18.4 ± 1.6 | 0.023 |
| > 30 ng/ml | 12.1 ± 1.8 | |
carcinoma embryonic antigen; ECOG, eastern cooperative oncology group; RFA, radiofrequency ablation.
The median progression-free survival time of the study group was 16.7 months, in which the differences of age, Eastern cooperative oncology group (ECOG) score and Carcinoma embryonic antigen (CEA) concentration subgroups were factors affecting progression-free survival. (Fig. 2).
Figure 2.
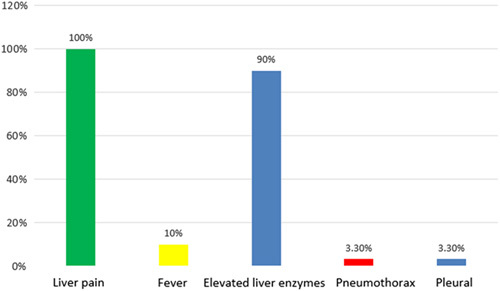
Complications and side effects of radiofrequency ablation.
Complications and side effects of radiofrequency ablation
After RFA, all patients complained of mild or moderate liver pain (according to WHO score). The elevated liver enzymes were seen in 90% patient only in mild level, 10% patients experienced fever and a patient had effusion and pneumothorax. All patients were stable in the afterward treatment process without intervention with procedures and there was no patient died from complications of RFA.
Discussion
Liver tumours in the right lobe (73.3%) were more common than in the left lobe (10%), while lesions of both lobes were witnessed in 16.7%. RFA procedure for right-sided tumours was more convenient than left-sided ones, since they could be approached more easily. In addition, the large amount of healthy parenchymal tissue in the right lobe makes the needle puncture process to be more convenient than that of the left liver tumour. Prior chemotherapy will reduce the size and number of liver tumours, creating favourable conditions for RFA afterwards. In our study, the proportion of patients with more than 3 liver tumours at baseline was 6.7% and after chemotherapy, all patients had 3 or less liver tumours. The efficacy of RFA is superior in cases with 3 or less liver metastases, none of which was larger than 3 cm and the patients in our study were suitable for this procedure7,8
We assessed tumour response after RFA by the International Working Group on Image-guided Tumor Ablation criteria5. Accordingly, the response after treatment is divided into complete response and partial response based on signs of angiogenesis on contrast-enhanced CT scan. Our study has the complete response rate after RFA of 63.3% and a partial response rate of 36.7%. Analysis of the association between response rate and the number of liver metastases did not show any statistically significant differences. Some studies also analyzed factors affecting response rate after RFA, although their patients had larger number and size of hepatic tumours. For instance, the study of Ruers and colleagues conducted RFA for patients with 1–9 liver tumours, while Choi and colleagues selected patients with tumour size of 1.7–13 cm. These studies showed that the rate of complete response in patients with tumour size of 3 cm or less is higher than in patients with tumour size over 3 cm and there was no difference in the complete response rate between groups with different number of tumours. Complete response rate after RFA with liver metastases in some study ranged from 52 to 98%9-11. The complete response rates varied between studies not only because of the differences in the size of the liver lesions of the patients in the studies, but also related to the technical equipment of the centre and the experience of the doctor conducting the RFA.
The average follow-up time in our study was 13 ± 5.7 months, with 13 patients progressing or relapsing out of a total of 19 patients with complete response, accounting for 68.4%. In which, local progression or recurrence accounted for 29.4%, new liver lesions accounted for 52.9% and extrahepatic metastatic lesions were 17.7%. A notable problem of RFA liver tumours is the high rate of recurrence at the RFA location and the appearance of a new mass in the liver. Local recurrence often occurs at the margins of RFA-affected lesions, which makes it difficult to detect recurrence on ultrasound, even on CT scans. The size of the liver tumour is related to the risk of recurrence, because the large size of the liver tumour makes it difficult for the tumour to be completely necrosis after RFA. Generally, liver tumours of 3 cm or less are considered the most suitable for RFA because of their high rate of complete necrosis and lower recurrence rate12,13,
Progression-free survival reflects the long-term effectiveness of treatment. Ruers et al.7 showed that progression-free survival in the RFA with chemotherapy group was 16.8 months, while that in the chemotherapy group was only 9.9 months (P= 0.025) . In our study, the median progression-free survival was 16.7 months. A study of Nguyen Viet Long conducted on 61 patients, each patient had from 1 to 5 liver metastatic tumours. The largest tumour diameter was 5 cm, with a progression-free survival of 14.21 ± 1.34 months14. Solbiati et al.10 conducted a study on 117 patients with the number of liver tumours from 1 to 4, in which the size of liver tumours from 0.9 to 9.6 cm, had a median progression-free survival of 12 months. When analyzing factors associated with progression-free survival, our study showed that there was a statistically significant difference in progression-free survival between patients under 70 years versus over 70 years of age, Eastern cooperative oncology group (ECOG) scores 0 versus 1, Carcinoma embryonic antigen (CEA) at baseline less than or equal to 30 ng/ml versus greater than or equal to 30 ng/ml.
The post-RFA syndrome is common including fever, liver pain and vomiting. We rate pain according to the WHO pain scale. In our study, all patients had mild and moderate liver pain, 10% of patients had fever and none of them vomited after RFA. These symptoms all resolve on their own or after medical treatment with common antipyretic and analgesic. A patient in the study had effusion, pneumothorax after RFA. This patient presented with liver pain and right chest pain, lasting 8 days, then, the patient underwent abdominal ultrasound and chest X-ray, found a right pleural effusion with a thickness of 24 mm and a small right pneumothorax. The patient cleared effusion, pneumothorax after medical treatment and did not require intervention. As in this patient, this is a possible complication when the liver tumour is located close to the diaphragm. Therefore, when a patient presents with chest pain, shortness of breath after RFA, chest X-ray or CT scan should be considered for early diagnosis and treatment. Results from our study as well as many other studies have shown that percutaneous and laparoscopic RFA for liver tumours is a safe minimally invasive procedure9. Our study has some limitations, including the small sample size, being conducted in a single centre and its retrospective nature. There should be a larger, prospective study to better analyze the effectiveness, safety, as well as determine the prognosis factors of this treatment method.
Conclusions
Systemic chemotherapy in combination with RFA is a safe and effective method in the treatment of inoperable colorectal cancer with liver metastases.
Ethical approval
This study was approved by the ethics committee of the Vietnam National Cancer Hospital.
Consent
This study was retrospective in nature, written consent from the patients was not required.
Sources of funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
T.T.: methodology, supervision. H.T.N.: data analysis, writing—original draft. G.H.P.: data analysis, writing—original draft. C.T.H.: methodology, supervision. Q.D.V.: data analysis, methodology.
Conflicts of interest disclosure
The authors declare no conflict of interest.
Research registration unique identifying number (UIN)
researchregistry8917.
Guarantor
Thang Tran.
Data availability statement
Datasets generated during and/or analyzed during the current study are publicly available, available upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 12 June 2023
Contributor Information
Thang Tran, Email: tranthangncc@gmail.com.
Hoa Thi Nguyen, Email: hoanguyen230@gmail.com.
Giang Hoang Pham, Email: giangpham33.hmu@gmail.com.
Cuc Thi Hoang, Email: hoangcuccuc@gmail.com.
Quan Duc Vu, Email: lhtvuducquan@gmail.com.
Referrence
- 1. Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol 2011;17:4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feliberti EC, Wagman LD. Radiofrequency ablation of liver metastases from colorectal carcinoma. Cancer Control 2006;13:48–51. [DOI] [PubMed] [Google Scholar]
- 3. Eng C, Shalan N. Biological agents versus chemotherapy in the treatment of colorectal cancer. Expert Opin Pharmacother 2006;7:1251–1271. [DOI] [PubMed] [Google Scholar]
- 4. Ruers T, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst 2017;109:djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmed M, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 2014;273:241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mathew G, et al. Strocss 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg Open 2021;37:100430. [DOI] [PubMed] [Google Scholar]
- 7. Ruers T, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012;23:2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shady W, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology 2016;278:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mulier S, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg 2002;89:1206–1222. [DOI] [PubMed] [Google Scholar]
- 10. Solbiati L, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 2001;221:159–166. [DOI] [PubMed] [Google Scholar]
- 11. Van Tilborg A, et al. Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: a potentially curative intervention. Br J Radiol 2011;84:556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ou S, et al. Radiofrequency ablation with systemic chemotherapy in the treatment of colorectal cancer liver metastasis: a 10-year single-center study. Cancer Manag Res 2018;10:5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Govaert KM, et al. Does radiofrequency ablation add to chemotherapy for unresectable liver metastases. Curr Colorectal Cancer Rep 2012;8:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thai Doan K, et al. Prognostic factors of radiofrequency ablation plus systemic chemotherapy for unresectable colorectal cancer with liver metastasis. Int J Hepatol 2020;2020:8836922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated during and/or analyzed during the current study are publicly available, available upon reasonable request.


