Introduction:
The Chikungunya virus (CHIKV), transmitted via mosquitoes, exhibits clinical manifestations ranging from headaches, myalgia and arthralgia to debilitating systemic malfunctions. Endemic to Africa, CHIKV has seen an increase in cases since it was first recorded in 1950. There has recently been an outbreak in numerous African nations. The authors aim to review the history and epidemiology of CHIKV in Africa, current outbreaks, strategies adopted by governments and/or international organisations to mitigate such an outbreak, and future recommendations that can be employed.
Methodology:
Data were collected from medical journals published on Pubmed and Google Scholar, and from the official World Health Organisation, African and United States of America’s Centres for Disease Control and Prevention websites. All articles considering CHIKV in Africa, including epidemiology, aetiology, prevention and management, were sought after.
Results:
Since 2015, the number of Chikungunya cases in Africa has increased, reaching the highest values ever recorded, especially in 2018 and 2019. Even though numerous vaccination and therapeutic intervention trials are still ongoing, no advancement has been made so far, including drug approval. Current management is supportive, with preventative measures, such as insecticides, repellents, mosquito nets and habitat avoidance, paramount to halting disease spread.
Conclusion:
In light of the recent CHIKV outbreak in Africa, local and global attempts are re-emerging to mitigate the eruption of the case of the lack of vaccines and antivirals, controlling the virus may be an arduous feat. Improving risk assessment, laboratory detection and research facilities should be a priority.
Keywords: aetiology, Africa, CHIKF, Chikungunya virus, CHIKV, epidemiology, management, outbreak, prevention
Introduction
Highlights
Endemic to Africa, Chikungunya virus (CHIKV) has seen an increase in cases since it was first recorded in 1950. There has recently been an outbreak in numerous African nations.
The virus reproduces inside the mosquito’s mid-gut, where following dissemination to the Axillary tissues like salivary glands ensue. Compared to other mosquito-borne viruses, CHIKV can infect a new naïve host more quickly.
A massive global effort and funding are needed to avoid future outbreaks. To start, each country, especially those where CHIKV is endemic, must employ an appropriate risk-assessment unit. Said responsibilities include surveillance of areas known to have the vector, detecting vector-attracting habitats and reporting back to the appropriate units.
Chikungunya is a chronic mosquito-borne viral disease caused by the Chikungunya virus (CHIKV), identified as a member of positive-sense ribonucleic acid (+ssRNA) viruses known as Togaviridae. Chikungunya fever was first discovered in Africa among infected individuals over 68 years ago in 1953 1. Several clusters and sporadic cases of CHIKV outbreak, alongside the current re-occurrence of the virus in varying African nations, (Fig. 1) have been reported2–7 and also in other parts of the world. However, according to a study review of CHIKV outbreak between 1999 and 2020, 13 CHIKV outbreaks were reported from 11 African countries2–8 In Asia 53 CHIKV cases recorded in 15 countries8, which is higher than reported cases in Africa and in Oceania over 1700 suspected cases per 10 000 residency were reported by the Federated state of Micronesia9,10, and the study revealed that Americas and Caribbean recorded 25 cases in 16 countries and 5 outbreak of CHIKV were reported in Europe between 1999 and 20208 (Table 1).
Figure 1.
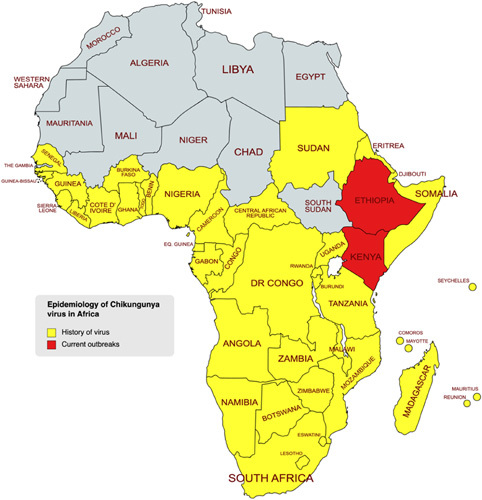
Geographical representation of the spread of the CHIKV virus over time and the current outbreak in domains of Africa. CHIKV, Chikungunya virus.
Table 1.
| Region/country | Affected area | Year of outbreak |
|---|---|---|
| Africa | ||
| Cameroon | West (Yaoundé and Dauala) | 2006 |
| Democratic Republic of congo | North-west (Kinshasa) | 1999–2000 2010 2018–2020 |
| Ethiopia | East (Dire Dawa) | 2019 |
| Gabon | North-west (Libreville and Surroundings) | 2006–2007 |
| South-east (Franceville and Surrounding) | 2010 | |
| West – Central (Lambaréné) | 2020–2021 | |
| La Reunion | La Reunion | 2005–2006 |
| Madagascar | East (Toamasina) | 2006 |
| Mayotte | Mayotte | 2005–2006 |
| Republic of Congo | South (Brazzaville) | 2011 |
| West (Diosso) | 2019 | |
| South (Bouenza) | 2019 | |
| West (Kouilou river region) | 2019 | |
| Sudan | East (Kassala) | 2015 |
| East (Kassala), Khartoum, Northern | 2018 | |
| states, and South Darfur | ||
| Senegal | South-east (Kédougou ) South-east (Kédougou ) |
2009 2015 |
| Sierra Leone | South (Bo) | 2012–2013 |
| Kenya | Lamu County | 2004–2005 |
| East County | 2016 | |
| Mombasa County | 2018 and 2022 | |
| America and Caribbean | ||
| Barbados | Southwest (Bridgetown) | 2014 |
| Brazil | North (Amapa) | 2014–2015 |
| North-east (Sergipe) | 2014–2016 | |
| North-east (Feira de Santana and Riachão do Jacuípe, Bahia) | 2015 | |
| South-east (Rio de janeiro) | 2015–2016 | |
| North-east (Ceará) | 2015–2017 | |
| North-east (Ceará) | 2017–2022 | |
| North-east (Piauí) | 2016- 2017 | |
| North-east (Salvador, Bahia) | 2017 | |
| North-east (Alagoas, Amazonas, Bahia, Ceará, Distrito Federal) | 2023 | |
| South region of Brazil | 2023 | |
| Argentina | North (Buenos Aires Province) | 2016, 2023 |
| Bolivia | — | 2015, 2022, 2023 |
| Paraguay | Amambay | 2015–2016, 2018, 2022, 2023 |
| Colombia | North (Corozal and Ovejas, Sucre) | 2014–2015 |
| North (Piedecuesta, Santander) | 2014–2015 | |
| Colombia | 2015, 2023 | |
| Dominica | Dominica | 2013–2014 |
| Dominican Republic | South-east (La Romana) | 2014 |
| Grenada | Grenada | 2014 |
| Haiti | West | 2014 |
| Honduras | Honduras | 2015 |
| Martinique and Guadeloupe | Martinique and Guadeloupe | 2013–2015 |
| Mexico | South (Chiapas) | 2014 |
| South-east (Yucatan) | 2015–2016 | |
| Nicargua | West (Managua) | 2014–2016 |
| Puerto Rico | Puerto Rico | 2014 |
| Saint Martin/Sint Maarten | Saint Martin/Sint Maarten | 2013–2014 |
| Suriname | North (Paramaribo and Commewijne) | 2014–2015 |
| U.S. Virgin Islands | U.S. Virgin Islands | 2014–2015 |
| Venezuela | North (Aragua)- | 2014 2023 |
| Uruguay | North-west (Paysandú) | 2023 |
| Peru | Peru | 2017, 2020 and 2023 |
| Asia | ||
| Bangladesh | Bangladesh (Shibganj, Char Kusahi, Gopalpur) | 2011–2012 |
| East (Dhaka) | 2017 | |
| Bhutan | Southwest (Samtse, Chukha and Thimphu) | 2012 |
| Cambodia | South (Trapeang Roka, Kampong Speu) | 2012 |
| Cambodia (Nationwide) | 2020 | |
| China | South | 2010 |
| Quzhou Zhejiang Province | 2017 | |
| India | East (West Bengal) | 2014–2015 |
| North-east (Assam) | 2015 | |
| West (Pune, Maharashtra) | 2016 | |
| North (Delhi) | 2016 | |
| Central (Madhya Pradesh) | 2016–2018 | |
| India (Nationwide) but Karnataka is most affected cross the country | 2019–2022 | |
| Indonesia | North-west (Sei Suka, North Sumatra) | 2013 |
| North-west (Sumatra) | 2014–2015 | |
| South (Bali) | 2015–2019 | |
| Lao PDR | South (Champassak) | 2012 |
| Malaysia | Malaysia | 2010–2011 |
| Tanjung Sepat | 2017–2019 | |
| Malaysia | 2023 | |
| Nepal | South (Terai) | 2013–2015 |
| Pakistan | Rawalpind | 2016–2017 |
| Philippines | Philippines | 2010–2011 |
| North (San Pablo, Laguna) | 2012 | |
| Central (Cebu) | 2012–2014 | |
| Calabarzon region | 2021 | |
| Metro manila, Central Luzon, Calabarzon and northern Mindanao | 2023 | |
| Thailand | West | 2010–2011 |
| North-east (Bueng Kan) | 2013 | |
| Nationwide | 2021–2023 | |
| Vietnam | Vietnam | 2010–2011 |
| Yemen | West (Al-Hudaydah) | 2010–2012 |
| Europe | ||
| Italy | Central (Lazio) | 2017 |
| France | Provence- Alpes-côte d’Azur | 2010 |
| Montpellier | 2014 | |
| Provence- Alpes-côte d’Azur | 2017 | |
Chikungunya is an acute disease that may cause neurological deformity (i.e. stooped posture) in infected individuals19, as well as various joint issues (i.e. arthralgia lasting more than 2–3 years)20–22, dermatological manifestations, myalgia and fever, all common prodromal symptoms23,24. The transmission of CHIKV to humans is mainly facilitated by environmental factors, the infestation of flavivirus family-infected mosquitoes (i.e. Aedes albopictus and Aedes aegypti), and topographic widespread and sylvatic monkey cyclic transmissions, directly and indirectly, responsible for periodic disease outbreaks and re-emergence in Africa25–28. CHIKV genomic content is approximated to be 11.89 kb with encoded properties of four non-structural proteins (NsP1-4) which enhance viral multiplication and CHIKV pathogenesis29. CHIKV genotypes are frequently classified into three groups: West African, East/Central and South African (ECSA) and Asian.
Despite continued efforts to create suitable and efficient Chikungunya fever vaccinations30,31, there is no current recommended curative or therapy for Chikungunya. This review, thereby, discussed the Chikungunya outbreak in Africa by reviewing the epidemiology and associated issues in combating Chikungunya. We also highlight Chikungunya in Africa and ongoing efforts alongside novel and strategic recommendations being made for the future.
Epidemiology and outbreak of CHIKV in Africa
In some nations, including Africa, Indonesia, the Southern United States, India and the West Indies, weakening polyarthralgia and fever became more common in the eighteenth and nineteenth centuries. Although, CHIKV could not be accurately associated with said outbreaks before the virus advent in the mid-1950s32,33. Previous incidents before the virus’s discovery may have been caused by the virus itself or another virus that was already found in the afflicted areas, such as the o’nyong’nyong virus in Africa which exhibits similar clinical manifestations34.
Since the advent of CHIKV, multiple minor incidences of the virus have occurred in Africa34. Recent cases, which started as far back as 2004, have affected many areas in Africa alongside other continents. Between 1952 and 1953, Chikungunya was isolated initially following the original epidemic in Tanzania. There was a very high transmission of the virus in many African countries35 (Table 2). Following the outbreak in said countries, the spread of Chikungunya ceased between 1999 and 2000, when ~50 000 individuals in Kinshasa, Democratic Republic of Congo (DRC), were affected38. CHIKV isolates originated from the ECSA gene, which is more common in Central Africans than in East-South Africans. The isolate of the virus was obtained more than 20 years earlier, which gave room for the hypothesis of a dogged and unknown viral spread38, later proven true via CHIKV virological diagnosis among unconfirmed yellow fever patients in the DRC between 2003 and 201239.
Table 2.
| Region/country | Area | Year of outbreak | Viral lineage |
|---|---|---|---|
| Cameroon | West (Yaounde) | 2006 | ECSA |
| Democratic Republic of Congo | North-west (Kinshasha) | 1999–2000 | ECSA |
| Gabon | North-west (Libreville and surroundings) | 2006–2007 | ECSA |
| La Reunion | La Reunion | 2005–06 | ECSA |
| Madagascar | East (Toamasina) | 2006 | NR |
| Mayotte | Mayotte | 2005–2006 | NR |
| Senegal | South-east (Kedougou) | 2009 | West African |
| Sudan | East (Kassala) | 2005 | NR |
| Kenya | — | 2004 | — |
East/Central and South African; NR, not reported.
The years 2002 and 2006 demonstrated a few reports of CHIKV infection being documented in Equatorial Guinea40 in addition to the period of increased yellow fever incidence in Sudan in 200541. The massive epidemic that struck Kenya’s coastal area in 2004 sickened over 13 500 people. This had a major impact on public health post-transmission to countries like Seychelles, La Réunion, Comoros and Mauritius Island of the Indian Ocean, including the City of Mombasa in 2005–200642.
After 2005, Chikungunya (or dengue) virus outbreaks occurred in several African nations. These outbreaks were primarily caused by Aedes albopictus (Asian tiger mosquito or Forest Mosquito), which by 2006 and 2007 had taken over as the primary vector for the dissemination of Chikungunya in Gabon and Cameroon43.
At least 20 000 individuals were affected in Libreville during said serious incident in the Gabonese Republic in 200743. The spread continued till 2010 when it reached the southern deep forest region, affecting villages throughout. In the Republic of Congo (RC), Brazzaville, there was a great outbreak that affected over 8000 people19. CHIKV was reported to cause fever in 8.3% of victims during an eighteen-month prospective study conducted in Kenya on pyrexial children8,44. There have been serious cases of CHIKV (with an ~80% attack rate in Mandera) in Kenya, including the Somalia border, where the first reported case indicated a growth in the capacity of their laboratory. In the years 2017–2018, there was another incidence in Kenya, with Mombasa County having 453 individuals affected8,22, said cases were confirmed in the laboratory22. In August 2018, over 13 000 doubtable cases were found in Sudan in a great epidemic that occurred in August 20188,22.
A very great epidemic occurred that caused over 10,500 doubtable cases in RC and an exceeding 1000 cases in the DRC in November 2018. This was due to said experiences in Kinshasa and Brazzaville, the capitals of the DRC and RC, and a newly recorded epidemic that caused over 40 500 doubtable victims and 300 confirmed cases in Ethiopia in 2019 and 2022, respectively. Meanwhile, Kenya recorded 83 cases in 20228,22,45 (Table 1), and following the outbreak of CHIKV infection globally, Europe, Asia and Australia and the Pacific reported no cases of CHIKV in 2022 apart from Paraguay with cases recorded cases of CHIKV and dengue as of 1 December 202237.
However, as of 9 March 2023 more than 110 000 cases of CHIKV and 43 deaths were recorded and the majority of cases and deaths were from Paraguay with 82 240 cases and 43 deaths and 30 386 cases were recorded in Brazil, 655 cases in Argentina, Thailand had 259 cases and Bolivia documented 300 cases while Africa have not reported cases of CHIKV as of 9 March 202313 (Table 1).
Aetiology of Chikungunya
Chikungunya is a word originating from the Tanzanian Makonde language meaning to “bend up”. This describes the bending posture of the patient that is affected by the severity of the disease1,8,22,46. Since the virus outbreak, CHIKV has been identified in ~40 countries, including Africa, Asia, Europe and most recently, America33. The virus is a member of the Togoviridae family and the genus Alphavirus. There are 30 species of the arthropod-borne alphavirus, otherwise known as arbovirus, in the genus, which descend from arthropod-borne viruses that share seven distinct antigenic complexes47.
The first outbreak of CHIKV was isolated, and the epidemic was reported in Tangankiya province, now Tanzania, from 1952 to 1953. The patient had incapacitating arthralgia, high fever and rash1,46,48. Infected Aedes mosquito bites, particularly those of Aedes aegypti and Aedes albopictus, are the primary vectors for the transmission of this disease of high mortality to humans49. The virus is transmitted via an infected mosquito as it feeds on a viraemic human who has the virus circulating in its blood, which starts the cycle. The virus may then spread to a new, unaware victim when the mosquito feeds, a brief period of internal virus replication ensuing. When a mosquito bites an individual who possesses an active virus in their blood, the infection begins to spread. Infection of the mosquito that then consumes a blood meal from the infected host can start a new cycle of transmission46.
The virus reproduces inside the mosquito’s mid-gut, where following dissemination to the Auxillary tissues like salivary glands ensue. Compared to other mosquito-borne viruses, CHIKV can infect a new naive host more quickly. Laboratory tests demonstrate that CHIKV may be observed in saliva as soon as 2–3 days after subsequent blood meal50. This illustrates that it can take less than a week for the full transmission cycle to occur from a human to a mosquito and back again. Once infected, it is believed that the mosquito can continue to spread viruses for the remainder of its life47. The virus then replicates in the skin and fibroblasts where dissemination through the blood to the lymphoid tissue, brain, liver and joints occurs23,24,48. Although CHIKV infection is linked to a low mortality rate, the primary syndrome of acute CHIKV infection is a high fever (39–40 °C) which may persist for seven days in two phases51. Post-pyrexia, sequelae such as “Post Chikungunya Chronic Polyarthralgia (pCHIKV-CPA)” may occur. This poses a profound negative impact on the quality of life of infected individuals, causing crippling arthralgia lasting several months to years52. Other signs and symptoms include asthenia, arthritis, conjunctivitis, myalgia, gestational distress and pruritic or maculopapular rash (Table 3).
Table 3.
Current CHIKV outbreak in Africa.
| Countries | Date | Total cases | Confirmed cases | Note |
|---|---|---|---|---|
| Democratic Republic of Congo | 2019 2011, 2020 |
6149 | — — |
The outbreak is still ongoing and has spread to eight of the twelve health departments of the country. |
| Sudan | 2015 2018 |
13 978 | — — |
Seven states (Kassala, Red Sea, Al Gadaref, River Nile, Northern State, South Darfur and Khartoum) have been affected by the outbreak. |
| Gabon | 2010 | — | — | The outbreak was declared in South-east (Franceville and surroundings) |
| Senegal | 2010, 2015 | 10 | — | The outbreak was declared in the region of kedougou. |
| Republic of Congo | 2011 2019–2020 |
— — |
— — |
South (Brazzaville) West (Diosso) |
| Sierra Leone | 2012–2013 | — | — | The outbreak was declared in the region of South (Bo) |
| Kenya | 2016 2018 2022 |
1792 453 83 |
— — 5 |
The outbreak was confined to the Mandera East sub-county The outbreak has spread to three sub-counties (Changamwe, Jomvu, Kissauni, Likoni, Mvita and Nyali) of Mombasa and one of Kilifi: with the majority of suspected cases reported from Mvita and Likoni in Mombasa. |
| Ethiopia | 2019 2022 |
— 311 |
— 3 |
The outbreak was declared in East (Dire Dawa) |
CHIKV, Chikungunya virus.
Recent medical advances in Chikungunya diagnosis
However, because Chikungunya’s clinical symptoms are arthralgia, myalgia, headache, vomiting, backache and diffuse maculopapular rashes53, like that of Dengue and Zika viruses, it is challenging to diagnose Chikungunya only from its clinical manifestations. For that reason, laboratory tests are needed for diagnosis. These include serology testing, CHIKV isolation and applying reverse transcription–polymerase chain reaction (RT-PCR) for detecting viral RNA54. Diagnosis mainly concerns the time of specimen collection, as CHIKV replicates rapidly, reaching high RNA titres. Viruses are usually detected by real-time RT-PCR during the first seven days of infection, post-clinical symptom presentation54. However, although rapid diagnostic kits are available, their sensitivity rarely correlates with that of RT-PCR. That is because such kits detect host-derived anti-CHIKV immunoglobulin M antibodies. In addition, the detection of immunoglobulin M antibodies is usually less sensitive than antigen detection55.
Analysis of cerebrospinal fluid composition is also used to aid the detection of CHIKV presence in the central nervous system. This cerebrospinal fluid composition analysis examines how CHIKV alters specific central nervous system components. Examples of these factors are marginally elevated protein, mildly lowered glucose, pleocytosis and mildly elevated lactate levels. These changes help aid the diagnosis of CHIKV. The outcomes of this method, though, might be the same for many virus types. Hence, the clinical findings and immunological analyses should be combined with this information to make a clear diagnosis56.
The most recent diagnostic technique is the application of rapid diagnostic immuno-chromatography (IC) testing kits. These IC testing kits use anti-CHIKV monoclonal antibodies derived from mice53. The rate of disease detection via IC testing kits is 6 days post-fever onset since the testing kits detect viral envelop protein, which usually reduces after 4–5 days post-infection. Furthermore, the IC testing kit detection rates are the best method of diagnosis when compared to other diagnostic techniques53 since the majority of patients typically contact a doctor to examine the condition causing their symptoms early.
Current efforts to mitigate Chikungunya in Africa
Chikungunya is often self-limiting, with a resolution of symptoms spontaneous and full recovery observed. However, currently, no targeted antiviral medication or treatment to mitigate symptomatic Chikungunya is in circulation12. Conservative management of affected individuals comprises immunological boosting through vitamin administration, hydration and rest12. The second main goal is to treat arthralgia by lowering the temperature with non-steroidal anti-inflammatory medicines like Ibuprofen. Additionally, any infected individual works as an incubating agent as illness begins, and patient isolation while avoiding additional mosquito bites help to interrupt the cycle of CHIKV transmission57. Taking further precautions against contracting the virus includes purifying water to kill larvae and decreasing the mosquito’s natural and artificial habitat by spraying pesticides on surfaces and containers46. It is also essential to protect against the Aedes aegypti mosquito bite by applying repellents and using insecticide-treated mosquito nets46. There has not been a CHIKV vaccine created as of yet. However, several vaccines have advanced to late phases of clinical testing and, in terms of both safety and immunogenicity, are suitable to prevent the emergence of the disease58.
The challenges and obstacles in fighting Chikungunya in Africa
Due to several outbreaks, interest in CHIKV has surged recently. In 2019, an outbreak was declared in Congo where 6149 cases had been reported and eight of the country’s twelve health departments had been affected by this outbreak. Also in 2018, 13 978 cases have been documented in Sudan, in which seven states have been affected, and Kenya has reported around 453 cases12. Due to climate change and greater travel, many diseases have unexpectedly spread, especially throughout tropical and subtropical regions but even to temperate zones59.
A possible method of disease prevention is the creation of vaccinations or specialised antiviral medication therapy regimens to fight against and eradicate the CHIKV12. It is crucial for the creation of efficient vaccinations since, in most cases, the virus was brought back by travellers from impacted areas59.
Many challenges and obstacles were faced to eradicate CHIKV. Unfortunately, vaccines and antivirals were not available until now. Symptom-relieving medications are the mainstay of treatment, such as analgesics and antipyretics12. The inability to quickly deploy clinical trials during outbreaks, unpredictable disease epidemiology and regulatory processes are impeding the development of desperately needed vaccinations59.
The effective use of resources is aided by surveillance, but if misused, it could compromise an individual’s privacy. Quarantine effectively slows the spread of disease despite sacrificing human rights and liberty. Restrictions on travel are burdensome. Despite screening initiatives, they will not have much of an impact on CHIKV’s spread; infected people will still spread the virus even if they appear healthy during the initial 4–10 days of the viral incubation period57.
Along with unprecedented population growth in developing countries, drug-resistant infections, unregulated urbanisation in tropical regions where vector-borne diseases are most common, and insecticide-resistant vectors are all factors contributing to the globalisation of diseases caused by arboviruses.
Ecosystems are altered by human activities in the environment, such as population pressure and agriculture. Additionally, in underdeveloped nations, anarchic urbanisation is frequently accompanied by social behaviours that favour the growth of vectors. For example, abandoned auto tires in residential areas that gather rainwater are perfect mosquito breeding grounds that help spread the virus58.
In addition to affecting mobility and overall health, and after generating explosive epidemics on a global level after decades of regionally confined outbreaks, tremendous suffering and significant economic costs, as well as financial strain in impacted regions, were established59.
Future recommendations
Because Chikungunya is a vector-borne virus, a variety of vaccines (i.e. virus-like particles59,60, chimeric, sub-unit60 and DNA vaccines61,62) are currently being tested. No vaccine has been approved22,61,63. Some antivirals and other compounds were studied as well, with no progress58. Given the potential for chronic symptoms, ongoing research is necessary46. Current management includes supportive treatment such as anti-inflammatories, antipyretics and analgesics19,64. Basic prevention consists of avoiding water reservoirs and containers (which are known to attract the mosquito vector), insect repellent, mosquito nets and conservative clothing47. However, the virus is still spreading despite these precautions. A massive global effort and funding are needed to avoid future outbreaks (Fig. 2). To start, each country, especially those where CHIKV is endemic, must employ an appropriate risk-assessment unit. Said responsibilities include (1) surveillance of areas known to have the vector, (2) detecting vector-attracting habitats and (3) reporting back to the appropriate units66. Furthermore, more clinical research is needed to better understand the virus’s transmission, pathogenicity and strain diversity to impede epidemics65. Additional investments involve affordable, easily available diagnostic tests (i.e. serology, virology) that help increase the level of reporting65. Since some African countries suffer economically, not all cases may be discovered. Not to mention that other vector-borne diseases (i.e., Dengue virus) are present similarly22. Extra care is necessitated to increase lab capacity and capability, in addition to any required technical support. This might be accomplished by boosting financing under the control of international organisations that are actively researching strategies to halt the spread of CHIKV46. Lastly, and of especially equal importance, is public awareness concerning the vector, the virus, the mechanism of transmission, symptoms, habitat and preventative measures.
Figure 2.

Poster highlighting CHIKV outbreak in Africa46,63–65. CHIKV, Chikungunya virus.
Conclusion
Chikungunya is caused by CHIKV, a mosquito-borne virus known to cause fever, myalgia, arthralgia and headaches. Rarely, manifestations include chronic neurological or cardiovascular sequelae. The vector, mosquitoes belonging to Aedes spp. (Aedes aegypti and Aedes albopictus), is endemic on the African continent. The first instance was identified in Tanzania in 1952, and since then, it has spread to several African nations as well as the Indian subcontinent and South Asia. The most recent outbreak was in Kenya and Ethiopia and is currently ongoing. Despite the current high number of instances, likely, there are still more cases than are being reported due to the poor economic situation in many African nations, the absence of competent, accessible and affordable laboratory tests, as well as the lack of technical equipment. The need for additional funding and investment is made clear by this, both for better detection and to fund additional research on transmission, strains and vaccines. Other than conservative measuring, there is yet no vaccine or treatment that is effective.
Ethical approval
Ethics approval was not required for this review.
Consent
Informed consent was not required for this review.
Source of funding
NA.
Author contribution
Conceptualization of ideas: all authors. Critical reviews with comments: all authors. Final Draft: all authors approved the final manuscript.
Conflicts of interest disclosure
NA.
Research registration unique identifying number (UIN)
NA.
Guarantor
Abubakar Nazir.
Data availability statement
NA.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 19 June 2023
Contributor Information
Stanley Chinedu Eneh, Email: Stanleyeneh234@gmail.com.
Olivier Uwishema, Email: uwolivier1@ktu.edu.tr.
Abubakar Nazir, Email: ABU07909@GMAIL.COM.
Elissa El Jurdi, Email: Elissa_Jurdi@hotmail.com.
Omotayo Faith Olanrewaju, Email: omotayofaith0@gmail.com.
Zahraa Abbass, Email: zahraa.abbass123456@gmail.com.
Mubarak Mustapha Jolayemi, Email: mustaphamubarakjolayemi@gmail.com.
Nour Mina, Email: nouramina2000@gmail.com.
lea kseiry, Email: leakseiry27@gmail.com.
Helen Onyeaka, Email: h.onyeaka@bham.ac.uk.
References
- 1. Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond 1956;54:177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pialoux G, Gauzere B-A, Jaureguiberry S, et al. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 2007;7:319–327. [DOI] [PubMed] [Google Scholar]
- 3. Zuckerman AJ, Banatvala JE, Pattison JR, et al. Principle and practice of Clinical Virology, 5th ed.. J Wiley & Sons, Ltd; 2005. [Google Scholar]
- 4. Aubry M, Teissier A, Roche C, et al. Chikungunya outbreak, French Polynesia, 2014. Emerg Infect Dis 2015;21:724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nhan TX, Musso D. The burden of Chikungunya in the Pacific. Clin Microbiol Infect 2015;21:e47–e48. [DOI] [PubMed] [Google Scholar]
- 6. Leparc-Goffart I, Nougairede A, Cassadou S, et al. Chikungunya in the Americas. Lancet 2014;383:514. [DOI] [PubMed] [Google Scholar]
- 7. Venturi G, De Luca M, Fortuna C, et al. Detection of a Chikungunya outbreak in Central Italy, August to September 2017. Eur Surveill 2017;22:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bettis AA, L’Azou Jackson M, Yoon I-K, et al. The global epidemiology of Chikungunya from 1999 to 2020: a systematic literature review to inform the development and introduction of vaccines. PLoS Negl Trop Dis 2022;16:e0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pastula DM, Hancock WT, Bel M, et al. Chikungunya virus disease outbreak in Yap State, Federated States of Micronesia. PLoS Negl Trop Dis 2017;11:e0005410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aubry M, Teissier A, Huart M, et al. Seroprevalence of dengue and Chikungunya virus antibodies, French Polynesia, 2014–2015. Emerg Infect Dis 2018;24:558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vairo F, Haider N, Kock R, et al. Chikungunya: epidemiology, pathogenesis, clinical features, management, and prevention. Infect Dis Clin North Am 2019;33:1003–1025. [DOI] [PubMed] [Google Scholar]
- 12. Vairo F, Aimè Coussoud-Mavoungou MP, Ntoumi F, et al. On Behalf Of The Pandora-Id-Net Consortium Chikungunya Outbreak Group Taskforce. Chikungunya Outbreak in the Republic of the Congo, 2019-Epidemiological, Virological and Entomological Findings of a South-North Multidisciplinary Taskforce Investigation. Viruses 2020;12:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. European Centre for Disease Prevention and Control. Chikungunya worldwide overview and situation report 2023. https://www.ecdc.europa.eu/en/chikungunya-monthly
- 14. Selhorst P, Makiala-Mandanda S, De Smet B, et al. Molecular characterization of Chikungunya virus during the 2019 outbreak in the Democratic Republic of the Congo. Emerg Microbes Infect 2020;9:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bower H, el Karsany M, Adam AAAH, et al. “Kankasha” in Kassala: A prospective observational cohort study of the clinical characteristics, epidemiology, genetic origin, and chronic impact of the 2018 epidemic of Chikungunya virus infection in Kassala, Sudan. PLoS Negl Trop Dis 2021;15:e0009387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. PAHO/WHO. Epidemiological Alert Increase in cases and deaths from chikungunya in the Region of the Americas (2023) https://www3.paho.org/data/index.php/en/mnu-topics/chikv-en/550-chikv-weekly-en.html [Google Scholar]
- 17. National Center for Vector Borne Diseases Control (NCVBDC). Chikungunya situation in India 2017-2022. https://ncvbdc.mohfw.gov.in/index4.php?lang=1&level=0&linkid=486&lid=3765
- 18. Adam A, Jassoy C. Epidemiology and laboratory diagnostics of dengue, yellow fever, zika, and Chikungunya virus infections in Africa. Pathogens 2021;10:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moyen N, Thiberville S-D, Pastorino B, et al. First reported Chikungunya fever outbreak in the Republic of Congo, 2011. PLoS ONE 2014. ;. 9:e115938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schilte C, Staikowsky F, Couderc T, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis 2013;7:e2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goupil BA, Mores CN. A review of Chikungunya virus-induced arthralgia: clinical manifestations, therapeutics, and pathogenesis. Open Rheumatol J 2016;10:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo G, Subissi L, Rezza G. Chikungunya fever in Africa: a systematic review. Pathog Glob Health 2020;114:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tritsch SR, Encinales L, Pacheco N, et al. Chronic joint pain 3 years after Chikungunya virus infection largely characterized by relapsing-remitting symptoms. J Rheumatol 2020;47:1267–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nyamwaya DK, Otiende M, Omuoyo DO, et al. Endemic Chikungunya fever in Kenyan children: a prospective cohort study. BMC Infect Dis 2021;21:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Althouse BM, Guerbois M, Cummings DAT, et al. Role of monkeys in the sylvatic cycle of Chikungunya virus in Senegal. Nat Commun 2018;9:1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eastwood G, Sang RC, Guerbois M, et al. Enzootic circulation of Chikungunya virus in East Africa: serological evidence in non-human Kenyan primates. Am J Trop Med Hyg 2017;97:1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 2015;372:1231–1239. [DOI] [PubMed] [Google Scholar]
- 28. Wahid B, Ali A, Rafique S, et al. Global expansion of Chikungunya virus: mapping the 64-year history. Int J Infect Dis 2017;58:69–76. [DOI] [PubMed] [Google Scholar]
- 29. Voss JE, Vaney MC, Duquerroy S, et al. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 2010;468:709–712. [DOI] [PubMed] [Google Scholar]
- 30. Rezza G, Weaver SC. Chikungunya as a paradigm for emerging viral diseases: evaluating disease impact and hurdles to vaccine development. PLoS Negl Trop Dis 2019;13:e0006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gouglas D, Thanh Le T, Henderson K, et al. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob Health 2018;6:e1386–e1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Halstead SB. Reappearance of Chikungunya, formerly called dengue, in the Americas. Emerg Infect Dis 2015;21:557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan American Health Organization [PAHO/WHO]. Chikungunya [Internet]. 2022 Jul6. https://www.paho.org/en/topics/chikungunya</underline> . [Google Scholar]
- 34. Petersen LR, Powers AM. Chikungunya: epidemiology. F1000Res 2016;5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Powers AM, Logue CH. Changing patterns of Chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 2007;88(Pt 9:2363–2377. [DOI] [PubMed] [Google Scholar]
- 36. Waggoner J, Brichard J, Mutuku F, et al. Malaria and Chikungunya detected using molecular diagnostics among Febrile Kenyan Children. Open Forum Infect Dis 2017;4:ofx110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sadiq Safi ur Rehman, Eneh Stanley C, Nazir Abubakar, et al. Tackling Chikungunya and dengue crisis in Paraguay amidst COVID-19: an epidemiological alert—a correspondence. Int J Surg 2023;109:624–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pastorino B, Muyembe-Tamfum JJ, Bessaud M, et al. Epidemic resurgence of Chikungunya virus in democratic Republic of the Congo: identification of a new central African strain. J Med Virol 2004;74:277–282. [DOI] [PubMed] [Google Scholar]
- 39. Makiala-Mandanda S, Ahuka-Mundeke S, Abbate JL, et al. Identification of dengue and Chikungunya cases among suspected cases of yellow fever in the Democratic Republic of the Congo. Vector Borne Zoonotic Dis (Larchmont, NY) 2018;18:364–370. [DOI] [PubMed] [Google Scholar]
- 40. Collao X, Negredo AI, Cano J, et al. Different lineages of Chikungunya virus in Equatorial Guinea in 2002 and 2006. Am J Trop Med Hyg 2010;82:505–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gould LH, Osman MS, Farnon EC, et al. An outbreak of yellow fever with concurrent Chikungunya virus transmission in South Kordofan, Sudan, 2005. Trans R Soc Trop Med Hyg 2008;102:1247–1254. [DOI] [PubMed] [Google Scholar]
- 42. World Health Organization [WHO]. Chikungunya in La Réunion (France), Mayotte, Maurice, Seychelles and India. 2006 https://www.who.int/emergencies/disease-outbreak-news/item/2006_03_17-en
- 43. Leroy EM, Nkoghe D, Ollomo B, et al. Concurrent Chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis 2009;15:591–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zavala-Colon M A, Gonzalez-Sanchez J. Engohang-Ndong J. History and geographic distribution of Chikungunya virus. Chikungunya Virus - A Growing Global Public Health Threat. IntechOpen; 2022. [Google Scholar]
- 45. European Centre for disease prevention and control, June 2022.
- 46.World Health Organization [WHO] https://www.who.int/news-room/fact-sheets/detail/chikungunya [Google Scholar]
- 47. Caglioti C, Lalle E, Castilletti C, et al. Chikungunya virus infection: an overview. N Microbiol 2013;36:211–227. [PubMed] [Google Scholar]
- 48. Lumsden WH. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. II. General description and epidemiology. Trans R Soc Trop Med Hyg 1955;49:33–57. [DOI] [PubMed] [Google Scholar]
- 49. Centers for Disease Control and Prevention [CDC]. Transmission [Internet]. 2022. Jul 6. Available from: https://www.cdc.gov/chikungunya/transmission/index.html
- 50. Dubrulle M, Mousson L, Moutailler S, et al. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One 2009;4:e5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ng KW, Chow A, Win MK, et al. Clinical features and epidemiology of Chikungunya infection in Singapore. Singapore Med J 2009;50:785–790. [PubMed] [Google Scholar]
- 52. Consuegra-Rodríguez MP, Hidalgo-Zambrano DM, Vásquez-Serna H, et al. Post-Chikungunya chronic inflammatory rheumatism: follow-up of cases after 1 year of infection in Tolima, Colombia. Travel Med Infect Dis 2018;21:62–68. [DOI] [PubMed] [Google Scholar]
- 53. Jain J, Okabayashi T, Kaur N, et al. Evaluation of an immunochromatography rapid diagnosis kit for detection of chikungunya virus antigen in India, a dengue-endemic country. Virol J 2018;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Johnson BW, Russell BJ, Goodman CH. Laboratory diagnosis of chikungunya virus infections and commercial sources for diagnostic assays. J Infect Dis 2016;214(suppl 5):S471–S474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mello CDS, Cabral-Castro MJ, Faria LCS, et al. Use of cerebrospinal fluid for the diagnosis of neuroinvasive dengue, zika, and Chikungunya: a 19-year systematic review. Rev Soc Bras Med Trop 2021;54:e0891 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rianthavorn P, Wuttirattanakowit N, Prianantathavorn K, et al. Evaluation of a rapid assay for detection of IgM antibodies to Chikungunya. Southeast Asian J Trop Med Public Health 2010;41:92–96. [PubMed] [Google Scholar]
- 57.Chikungunya Virus on the Move Special edition on infectious disease. Harvard University; 2014. https://sitn.hms.harvard.edu/flash/special-edition-on-infectious-disease/2014/chikungunya-virus-on-the-move/ [Google Scholar]
- 58. Devaux CA. Emerging and re-emerging viruses: a global challenge illustrated by Chikungunya virus outbreaks. World J Virol 2012;1:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schrauf Sabrina, Tschismarov Roland, Tauber Erich, et al. Current efforts in the development of vaccines for the prevention of zika and Chikungunya virus infections. Front Immunol 2020;11:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oqbazgi Merhawi. “Treatment and prevention of Chikungunya fever: current status and prospective” in chikungunya virus: a growing global public health threat. Jean Engohang-Ndong. London: IntechOpen,; 2021. [Google Scholar]
- 61. Powers Ann M. Vaccine and Therapeutic options to control Chikungunya virus.. Clin Microbiol Rev 2017;31:e00104–e00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Akahata W, Yang ZY, Andersen H, et al. A virus-like particle vaccine for epidemic chikungunya virus protects nonhuman primates against infection. Nat Med 2010;16:334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Metz SW, Geertsema C, Martina BE, et al. Functional processing and secretion of chikungunya virus E1 and E2 glycoproteins in insect cells. Virol J 2011;8:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ferraro B, Morrow MP, Hutnick NA, et al. Clinical applications of DNA vaccines: current progress. Clin Infect Dis 2011;53:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. World Health Organization [WHO]. Chikungunya – Kenya. 2016 https://www.afro.who.int/news/chikungunya-kenya [Google Scholar]
- 66. Sallberg M, Frelin L, Ahlen G, et al. Electroporation for therapeutic DNA vaccination in patients. Med Microbiol Immunol 2015;204:131–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NA.


