Abstract
Objective:
To test vitamin D3 and omega-3 fatty acids (omega-3s) for late-life depression prevention under the National Academy of Medicine framework for indicated (targeting subthreshold depression) and selective (targeting presence of high-risk factors) prevention.
Methods:
The VITamin D and OmegA-3 TriaL (VITAL) is a 2 × 2 factorial trial of vitamin D3 (2,000 IU/d) and/or omega-3s (1 g/d) for cardiovascular and cancer prevention (enrollment: November 2011–March 2014; end date: December 31, 2017). In this targeted prevention study, we included 720 VITAL clinical sub-cohort participants who completed neurobehavioral assessments at baseline and 2 years (91.9% retention). High-risk factors were subthreshold or clinical anxiety, impaired activities of daily living, physical/functional limitation, medical comorbidity, cognitive impairment, caregiving burden, problem drinking, and low psychosocial support. Coprimary outcomes were incident major depressive disorder (MDD), adjudicated using DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition), and change in mood (Patient Health Questionnaire-9 [PHQ-9]). We used exact tests to determine treatment effects on MDD incidence and repeated-measures models to determine treatment effects on PHQ-9.
Results:
A total of 11.1% had subthreshold depression, 60.8% had ≥ 1 high-risk factor, MDD incidence was 4.7% (5.1% among completers), and mean PHQ-9 score change was 0.02 points. Among those with subthreshold depression, the MDD risk ratio (95% confidence interval) was 0.36 (0.06 to 1.28) for vitamin D3 and 0.85 (0.25 to 2.92) for omega-3s, compared to placebo; results were also null among those with ≥ 1 high-risk factor (vitamin D3 vs placebo: 0.63 [0.25 to 1.53]; omega-3s vs placebo: 1.08 [0.46 to 2.71]). There were no significant differences in PHQ-9 score change comparing either supplement with placebo.
Conclusions:
Neither vitamin D3 nor omega-3s showed benefits for indicated and selective prevention of late-life depression; statistical power was limited.
Trial Registration:
ClinicalTrials.gov identifier: NCT01696435 J Clin Psychiatry 2023;84(4):22m14629
Late-life depression (LLD) prevention can be efficiently accomplished by employing the National Academy of Medicine (NAM) framework for prevention of mental disorders; ie, targeting those with subthreshold depression (indicated prevention) or high-risk factors (selective prevention).1 Compared to traditional prevention frameworks (ie, primary, secondary, and tertiary prevention), the NAM framework has high clinical utility in LLD prevention: it defines at-risk populations using evidence-based knowledge of contextual factors and involves targeted interventions among those at highest risk. Presence of subthreshold depression and presence of selective high-risk factors are responsible for 25% and 50%, respectively, of all incident major depressive disorder (MDD) cases occurring during late life.2–4
Older adults with subthreshold depressive symptoms and LLD high-risk factors (eg, medical comorbidity, caregiving strain, low social support) may also have elevated inflammation levels or poor vascular and metabolic health.5,6 Several lines of evidence suggest that vitamin D3 and omega-3 fatty acids (omega-3s) promote mood health by decreasing inflammation and improving metabolic indicators, and also via neuroprotective benefits7–10; if applied among targeted groups who constitute a relatively large proportion of cases, these supplements might offer substantial benefits for LLD prevention. Prior randomized controlled trials (RCTs) of indicated and selective LLD prevention have largely applied psychological interventions; MDD relative risk reductions of up to ~50%–60% were observed.11–14 Vitamin D3 and omega-3 supplements may offer additional advantages for targeted LLD prevention, as they are safe, inexpensive, easily accessible, and highly acceptable to most people due to their simplicity.15
Over the last two decades, only 4 RCTs16–19 examined the effects of vitamin D3 supplementation of ≥ 12 months’ duration for indicated and selective prevention of depression in mid- and/or late-life adults; 3 trials showed no benefit of vitamin D3 for MDD risk or mood among those with subthreshold depression and/or ≥ 1 high-risk factor.16–18 Regarding omega-3s, only 4 RCTs17,20–22 examined effects of supplementation of ≥ 12 months’ duration for indicated and selective prevention of depression in mid- and/or late-life adults; no benefits on MDD were found. Most prior RCTs of nutrient interventions used a single risk factor for addressing selective prevention of MDD (eg, physical/functional impairment or medical comorbidity). Yet, the combination of several selective risk factors appears responsible for the majority of LLD cases.2,3,23 While substantial benefits might be achieved using nutrient supplements for targeted LLD prevention, data from RCTs using such approaches are limited.
VITAL-DEP (VITamin D and OmegA-3 TriaL-Depression Endpoint Prevention; ClinicalTrials.gov identifier: NCT01696435), an ancillary study to VITAL, addressed these knowledge gaps by testing vitamin D3 or omega-3s supplementation for indicated and selective prevention of LLD in a deeply phenotyped sub-cohort of 720 participants who completed repeat in-clinic assessments. In this targeted RCT, we hypothesized that daily supplementation with vitamin D3 or omega-3s, compared to placebos, would show benefits for indicated and selective prevention of LLD over a 2-year follow-up.
METHODS
Trial Design
VITAL randomized 25,871 participants (men aged ≥ 50 and women aged ≥ 55 years) to receive vitamin D3 (2,000 IU/d), omega-3s (1 g/d including 465 mg eicosapentaenoic acid [EPA] and 375 mg docosahexaenoic acid [DHA]) and/or matching placebos in a 2 × 2 factorial design for prevention of cardiovascular disease and cancer (enrollment period: November 2011–March 2014; end of the intervention: December 31, 2017); the protocol was published elsewhere.24 VITAL used a pragmatic, hybrid design that included a nationwide cohort of 25,871 participants and a sub-cohort of 1,054 participants who lived near an affiliated National Institutes of Health (NIH)–sponsored Harvard Catalyst–Clinical Translational Science Center (CTSC) in Boston, Massachusetts, and presented at the CTSC Center for Clinical Investigation for detailed, in-person assessments at baseline and 2 years. All VITAL-CTSC participants were invited to take part in the 45-minute VITAL-DEP neuropsychiatric assessment.25 Participants were eligible for this study if they did not have (1) any of these psychiatric disorders, as determined by the Mini-International Neuropsychiatric Interview (MINI) for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)26: current depression (MDD or major depressive episode), alcohol or substance abuse/dependence in the past 12 months, primary psychotic disorders (eg, schizophrenia) or psychotic mood disorders, bipolar disorder, obsessive-compulsive disorder, or posttraumatic stress disorder; (2) unstable psychiatric symptoms during evaluation (eg, suicidality, psychosis); or (3) dementia-level cognitive impairment determined by norm-based cut-points.27,28 All participants provided written informed consent, and study approvals were obtained from the Institutional Review Board of Mass General Brigham.
This article addresses a secondary aim of the VITAL-DEP study protocol.25 VITAL and VITAL-DEP were designed on the basis of an a priori assumption of no interaction between agents, and the prespecified primary analyses examined separately the main effects of each agent. However, the 2 × 2 factorial design of the study allowed for exploratory analyses addressing potential interaction; results for the interaction between treatment agents were reported in subgroup analyses elsewhere, and there was no evidence of interaction between agents.29,30
Procedures
Per the study protocol,25 baseline assessment was used to identify participants eligible for follow-up for MDD at 2 years and to determine at-risk groups for indicated and selective prevention. The MINI was used to achieve valid, time-efficient determinations of eligibility and MDD outcomes.26 Study psychiatrists immediately evaluated participants presenting with unstable psychiatric symptoms (eg, suicidality, manic symptoms, psychosis) to determine their safety and ability to participate; see Supplementary Appendix 1 for details.
Indicated Prevention
We identified participants with subthreshold depression—ie, clinically meaningful depressive symptoms without meeting DSM-IV criteria for MDD or dysthymia on the MINI—by leveraging both detailed neurobehavioral data from baseline CTSC visits and concurrent self-reported depression measures in the VITAL baseline questionnaires. Subthreshold depression was defined by the presence of any of the following31,32: (1) Patient Health Questionnaire-9 (PHQ-9)33 score or PHQ-834 score between 5 and 9 points; (2) core features of depression (anhedonia and/or dysphoria) present at least “more than half the days” for ≥ 2 weeks in the past 2 years; (3) subsyndromal depression including minor depression on the MINI (at least 2 but fewer than 5 depression module symptoms) or the PHQ (at least 2 but fewer than 5 symptoms present at least “more than half the days” for ≥ 2 weeks); or (4) dysthymic symptoms, defined as self-report of depressed mood most of the time for ≥ 2 consecutive years, with symptoms active in the past 1 year.
Selective Prevention
We determined participants with ≥ 1 high-risk factor by leveraging robust literature on LLD risk architecture.2,3,23 We used detailed mood, neuropsychiatric, and well-being measures in the CTSC and self-reported physical and health measures on VITAL baseline questionnaires to classify high-risk participants; see Supplementary Appendix 1 for descriptions of measures. High-risk factors were subthreshold anxiety (Generalized Anxiety Disorder-2 [GAD-2] score ≥ 3 or GAD-7 score ≥ 5)35,36 or clinical anxiety (DSM-IV anxiety disorders); impaired activities of daily living (instrumental activities of daily living [IADL] score ≥ 1)37; problem/hazardous drinking (Alcohol Use Disorders Identification Test-Concise [AUDIT-C] score ≥ 5)38; physical/functional limitation (determined using the 10-item Physical Functioning scale [PF-10] from the Medical Outcomes Short Form-36)39; medical comorbidity (≥ 1 chronic disease); cognitive impairment (below Modified Mini-Mental State Exam [3MS]40 norm-based cutoff scores that factored age, sex, education, race and ethnicity)41: < 92 among non-Hispanic White men, < 95 among non-Hispanic White women, < 89 among Black and Hispanic participants, < 91 among participants with other race or ethnicity); caregiving burden (Zarit brief burden interview score ≥ 10)42; and low psychosocial support (Duke Social Support Index [DSSI]43,44 score ≤ 26).
Coprimary Outcomes
The two coprimary outcomes were (1) risk of incident MDD and (2) 2-year change in mood score. Incident MDD was defined per DSM-IV criteria using the MINI at 2 years. The PHQ-9 was used to ascertain mood score at baseline and 2 years (higher scores indicate worse mood; range, 0–27 points); the minimal clinically important difference (MCID) for change in PHQ-9 score was 0.5 points.
Secondary Outcome
We addressed a composite incident depression outcome that leveraged both in-person CTSC diagnoses and, as previously described,25 depression outcomes ascertained from main VITAL study questionnaires. This outcome, used for a secondary analysis of selective prevention, included DSM-IV MDD, PHQ-9 score ≥ 10, and incident case of depression in the main VITAL study within the 2-year CTSC follow-up period.30
Statistical Analyses
Analyses of coprimary outcomes.
Prior RCTs showed ~50%–60% relative risk (RR) reductions (ie, an RR of 0.4–0.5) using indicated and selective prevention strategies.11–13 Power calculations were based on an expected N of 855 and 2-year absolute risk of MDD of 35% among those with subthreshold depression and 25% among those with ≥ 1 high-risk factor. Based on these estimates, power was ≥ 80% to detect RRs of ≤ 0.60 and ≤ 0.50, respectively, for indicated and selective LLD prevention. Regarding 2-year change in PHQ-9 score, power was 90% and > 99% to detect the MCID in risk groups of indicated and selective prevention, respectively. Analysis of treatment effects among the 720 eligible participants in this study was based on intention-to-treat. Differences between the original statistical analysis plan and current procedures are detailed in Supplementary Appendix 1.
Participants’ characteristics were compared across the 4 treatment groups (vitamin D3, omega-3s, both agents, or both placebos). We used χ2 statistics to determine effects of treatment agents versus placebos on risk of incident MDD among those with versus without subthreshold depression or with versus without high-risk factors for depression; RR and exact 95% confidence intervals (CIs) are reported. The Zelen exact test was used as an interaction test to determine whether treatment effects on incident MDD differed across risk groups of indicated and selective prevention.
In examining 2-year change in mood score, general linear models of response profiles were used to estimate the means and were adjusted for age, sex, and concurrent randomized agent, and time was modeled as an indicator variable; missing outcome data were assumed to be missing at random.45 The mean difference between treatment versus placebo groups in change in PHQ-9 score was estimated using a time × treatment interaction. Models were fitted using maximum likelihood, correlations within participants were modeled using an unstructured covariance pattern, and statistical tests used the Wald test.
Secondary analyses.
Exact χ2 statistics were used to determine effects of treatment agents, compared to placebos, on the composite depression outcome among those with versus without ≥ 1 high-risk factor; the Zelen exact test was used to determine whether treatment effects differed across risk group of selective prevention.
Non-prespecified and post hoc analyses.
First, effects of treatment agents versus placebos on MDD risk and change in PHQ-9 score were estimated in the full sample of 720 participants, rather than separately by indicated and selective prevention risk group. Second, as physical/functional limitation is one of the largest single contributors to LLD risk,2,3,23 we performed additional validation checks for the self-reported PF-10 (available in 94% of sample) by comparing it to the concurrent gold-standard objective physical performance tests (available in a smaller subset of the sample [n ~500]) (see Supplementary Appendix 1).
Statistical analyses were performed using SAS 9.4 (SAS Institute; Cary, NC). Tests were 2-sided; for an α level of .05 with two coprimary outcomes, P < .025 was used for statistical significance for each outcome after Bonferroni correction. Results regarding secondary, non-prespecified, and post hoc analyses should be interpreted with caution.
RESULTS
Figure 1 summarizes the recruitment and disposition of participants. Among eligible 720 participants, 58 (8.1%) were lost to-follow-up, refused follow-up MINI assessment, or withdrew from the study; 662 (91.9%) completed MINI assessments at 2 years.
Figure 1. Randomization and Follow-Up of Participants.
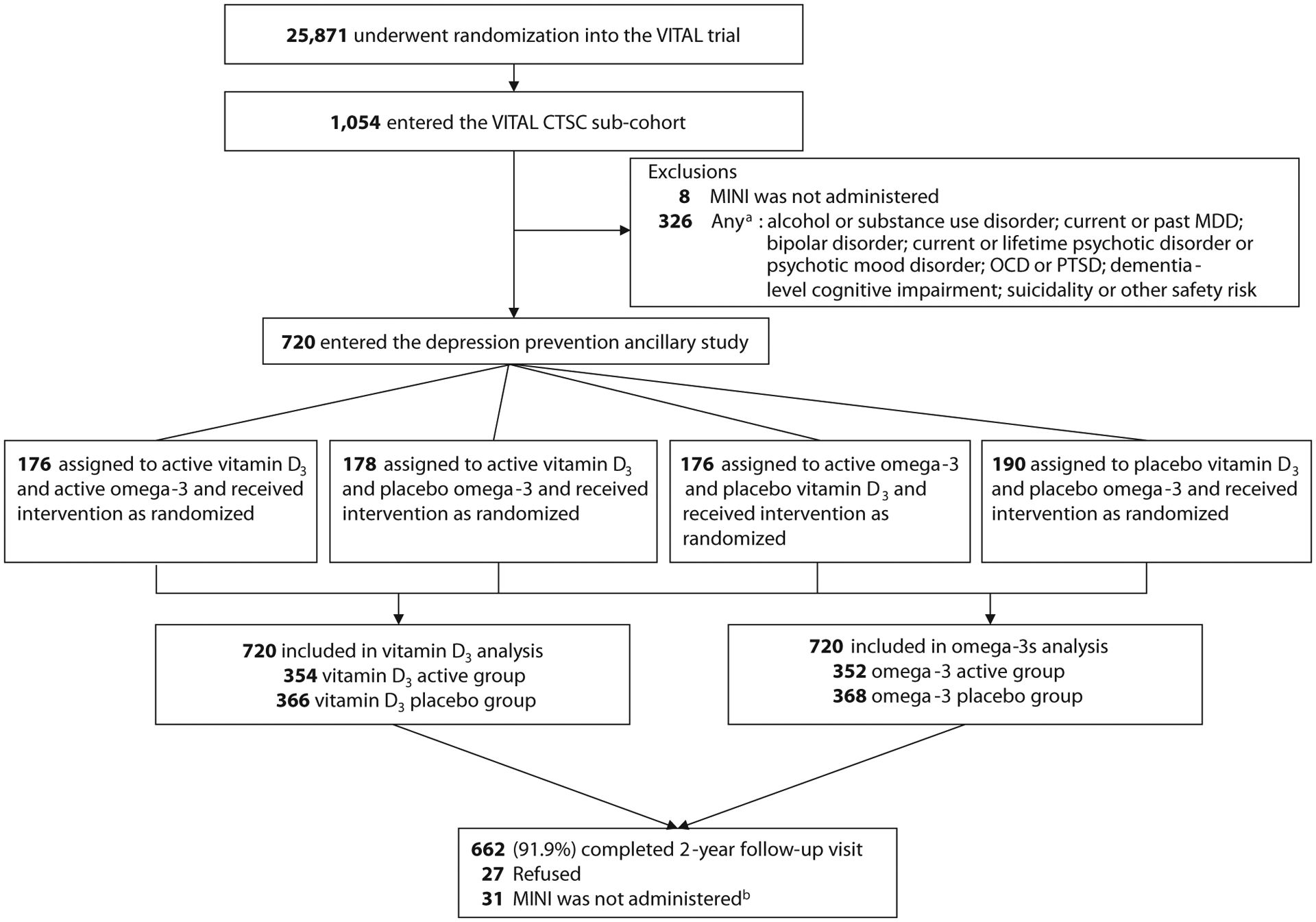
aParticipants could have had more than one exclusion condition.
bFollow-up MINI assessments were not administered due to lost to follow-up, refusal of the MINI assessment at the follow-up CTSC visit, or study withdrawal.
Abbreviations: CTSC = Clinical and Translational Science Center, MDD = major depressive disorder, MINI = Mini-International Neuropsychiatric Interview,26 OCD = obsessive-compulsive disorder, PTSD = posttraumatic stress disorder, VITAL = VITamin D and OmegA-3 TriaL.24
In VITAL, participants answered study pill adherence questions at 6 months and 1 year post-randomization and annually thereafter. At 2-year follow-up, the percentages of participants who reported taking at least two-thirds of study capsules were 95.4% and 94.4%, respectively, in vitamin D3 and placebo groups and 95.0% and 95.3%, respectively, in omega-3s and placebo groups (Supplementary Table 1). The mean (SD) age of participants was 65.4 (6.5) years; 44.4% were female, and 15% were racial and/or ethnic minorities. Characteristics were balanced across randomization groups (Table 1). Of the 720 participants, there were 80 (11.1%) with subthreshold depression and 438 (60.8%) with ≥ 1 high-risk factor. Among those with subthreshold depression, presence of high-risk factors for LLD was common (Supplementary Figure 1). High-risk factors were balanced across randomization groups, except for slightly higher prevalence of cognitive impairment and physical/functional limitation among those randomized to active omega-3s. Additional characteristics are provided in Supplementary Table 2.
Table 1.
Characteristics of Participants at Baseline, According to Randomized Assignment to Treatment Agent and/or Placeboa
| Characteristic | Active Vitamin D3 and Active Omega-3s (n = 176) | Active Vitamin D3 and Placebo Omega-3s (n = 178) | Placebo Vitamin D3 and Active Omega-3s (n = 176) | Placebo Vitamin D3 and Placebo Omega-3s (n = 190) |
|---|---|---|---|---|
| Age at CTSC visit, mean (SD) | 65.3 (6.3) | 65.2 (6.2) | 65.5 (7.0) | 65.4 (6.7) |
| Sex | ||||
| Male | 96 (54.5) | 94 (52.8) | 98 (55.7) | 112 (58.9) |
| Female | 80 (45.5) | 84 (47.2) | 78 (44.3) | 78 (41.1) |
| Self-reported race/ethnicity | ||||
| Non-Hispanic White | 144 (81.8) | 154 (86.5) | 146 (83.0) | 168 (88.4) |
| Black | 12 (6.8) | 9 (5.1) | 16 (9.1) | 13 (6.8) |
| Hispanic | 6 (3.4) | 1 (0.6) | 3 (1.7) | 5 (2.6) |
| Othersb | 14 (8.0) | 14 (7.9) | 11 (6.3) | 4 (2.1) |
| Subthreshold depression | ||||
| Yesc | 20 (11.4) | 22 (12.4) | 22 (12.5) | 16 (8.4) |
| No | 156 (88.6) | 156 (87.6) | 154 (87.5) | 174 (91.6) |
| 1+ High-risk factors for late-life depression | ||||
| Yesd | 106 (60.2) | 111 (62.4) | 114 (64.8) | 107 (56.3) |
| No | 70 (39.8) | 67 (37.6) | 62 (35.2) | 83 (43.7) |
| Total no. of high-risk factors for late-life depression | ||||
| 0 | 70 (39.8) | 67 (37.6) | 62 (35.2) | 83 (43.7) |
| 1 | 62 (35.2) | 71 (39.9) | 66 (37.5) | 71 (37.4) |
| 2 | 35 (19.9) | 26 (14.6) | 34 (19.3) | 25 (13.2) |
| 3+ | 9 (5.1) | 14 (7.9) | 14 (8.0) | 11 (5.8) |
| Individual High-Risk Group | ||||
| Subthreshold or clinical anxietye | ||||
| Yes | 13 (7.4) | 15 (8.4) | 11 (6.3) | 13 (6.9) |
| No | 163 (92.6) | 163 (91.6) | 164 (93.7) | 176 (93.1) |
| Impaired activities of daily livingf | ||||
| Yes | 7 (4.0) | 3 (1.7) | 7 (4.0) | 4 (2.1) |
| No | 169 (96.0) | 175 (98.3) | 169 (96.0) | 185 (97.9) |
| Problem drinkingg | ||||
| Yes | 26 (14.8) | 28 (15.7) | 32 (18.2) | 30 (15.8) |
| No | 150 (85.2) | 150 (84.3) | 144 (81.8) | 160 (84.2) |
| Physical/functional limitationh | ||||
| Yes | 36 (21.8) | 29 (17.2) | 41 (25.3) | 30 (16.5) |
| No | 129 (78.2) | 140 (82.8) | 121 (74.7) | 152 (83.5) |
| Medical comorbidityi | ||||
| Yes | 23 (13.1) | 25 (14.0) | 22 (12.5) | 23 (12.1) |
| No | 153 (86.9) | 153 (86.0) | 154 (87.5) | 167 (87.9) |
| Cognitive impairmentj | ||||
| Yes | 48 (27.4) | 37 (20.9) | 51 (29.0) | 38 (20.1) |
| No | 127 (72.6) | 140 (79.1) | 125 (71.0) | 151 (79.9) |
| Caregiving burdenk | ||||
| Yes | 3 (1.7) | 13 (7.3) | 12 (6.8) | 7 (3.7) |
| No | 173 (98.3) | 164 (92.7) | 164 (93.2) | 181 (96.3) |
| Low psychosocial supportl | ||||
| Yes | 5 (2.9) | 15 (8.5) | 10 (5.9) | 9 (4.8) |
| No | 170 (97.1) | 162 (91.5) | 160 (94.1) | 180 (95.2) |
Values are shown as n (%) unless otherwise noted. Values for percentages may not add to 100.0 due to rounding.
Others race/ethnicity group included Asians and Pacific Indians, American Indians, Native Hawaiian, other unknown, and missing reported race/ethnicity participants.
Indicated prevention targeted participants with subthreshold depression, but who did not meet DSM-IV criteria for current major depressive disorders or dysthymia, at baseline.
Selective prevention targeted participants with ≥ 1 high-risk factor for late-life depression at baseline; see details in Supplementary Appendix 1 (under E. Approach for selective prevention).
Subthreshold or clinical anxiety was assessed based on core features of anxiety (GAD-2 score ≥ 3 or GAD-7 score ≥ 5) and/or adjudicated anxiety disorder diagnoses in administered diagnostic interviews.36
Impaired activities in daily living was determined based on IADL score (1+ points) reported on OARS multidimensional functional assessment questionnaire.37
Problem drinking was defined based on VITAL-DEP CTSC scoresheet alcohol gating questions and/or AUDIT-C score cutoff ≥ 5.38
Physical/functional function limitation was assessed using the PF-10 of the SF-36.39
Medical comorbidity was derived from the count of major chronic diseases.
Cognitive impairment was determined using published 3MS (range, 0–100) norm-based cut-points, accounting for age, sex, race/ethnicity, and education, for identifying presence of cognitive impairment; for non-Hispanic White men, < 92; for non-Hispanic White women, < 95; for Black and Hispanic participants, < 89; and for Other race/ethnicity participants, < 91.41
Caregiving burden was assessed using Zarit Burden Interview scale42; a cutoff of ≥ 10 and/or self-reported rating of moderately or higher caregiving burden were used to define presence of caregiving burden. The denominator for this group included participants who either did not report caregiver burden or were not providing any information on ill-caregiving.
Psychosocial support was measured using 10-item DSSI43; a cutoff of ≤ 26 for DSSI score was used to define low psychosocial support.44
Abbreviations: 3MS = Modified Mini-Mental State Exam, AUDIT-C = Alcohol Use Disorders Identification Test-Concise, CTSC = Clinical Translational Science Center, DSSI = Duke Social Support Index, GAD = generalized anxiety disorder, IADL = instrumental activities of daily living, OARS = Older Americans Resources and Services, PF-10 = 10-item physical functioning scale, SF-36 = Short Form-36, VITAL-DEP = VITamin D and OmegA-3 TriaL Depression Endpoint Prevention.
Results for Primary Analyses
The DSM-IV MDD incidence rate was 4.7% (34/720) at 2-year follow-up; the rate was 5.1% (34/662) among study completers. The absolute risk of incident MDD was 3-fold higher in participants with versus without subthreshold depression (12.5% vs 3.8%; Fisher exact P value = .002); no such difference in absolute risk was observed among those with versus without ≥ 1 high-risk factor. The mean 2-year change in PHQ-9 score was 0.02 points.
Randomization to vitamin D3, compared to placebo, did not affect risk of incident MDD among those with versus without subthreshold depression or those with versus without ≥ 1 high-risk factor; Zelen tests showed no differences in treatment effects across risk groups (Figure 2). Regarding indicated prevention, although the estimate was in the direction of more than a 60% reduction in MDD risk among participants with subthreshold depression randomized to vitamin D3 versus placebo, results were not statistically significant. Regarding selective prevention, no significant effects of vitamin D3, compared to placebo, on risk of incident MDD were observed. The mean difference in 2-year change in PHQ-9 score comparing vitamin D3 and placebo was not statistically significant among those with versus without subthreshold depression or those with versus without ≥ 1 high-risk factor; estimates were lower than the prespecified MCID of 0.5 points (Table 2).
Figure 2. Effect of Vitamin D3 or Omega-3s, Compared to Their Matching Placebos, on Risk of DSM-IV Incident MDD at 2 Years by Risk Group of Indicated and Selective Preventiona.
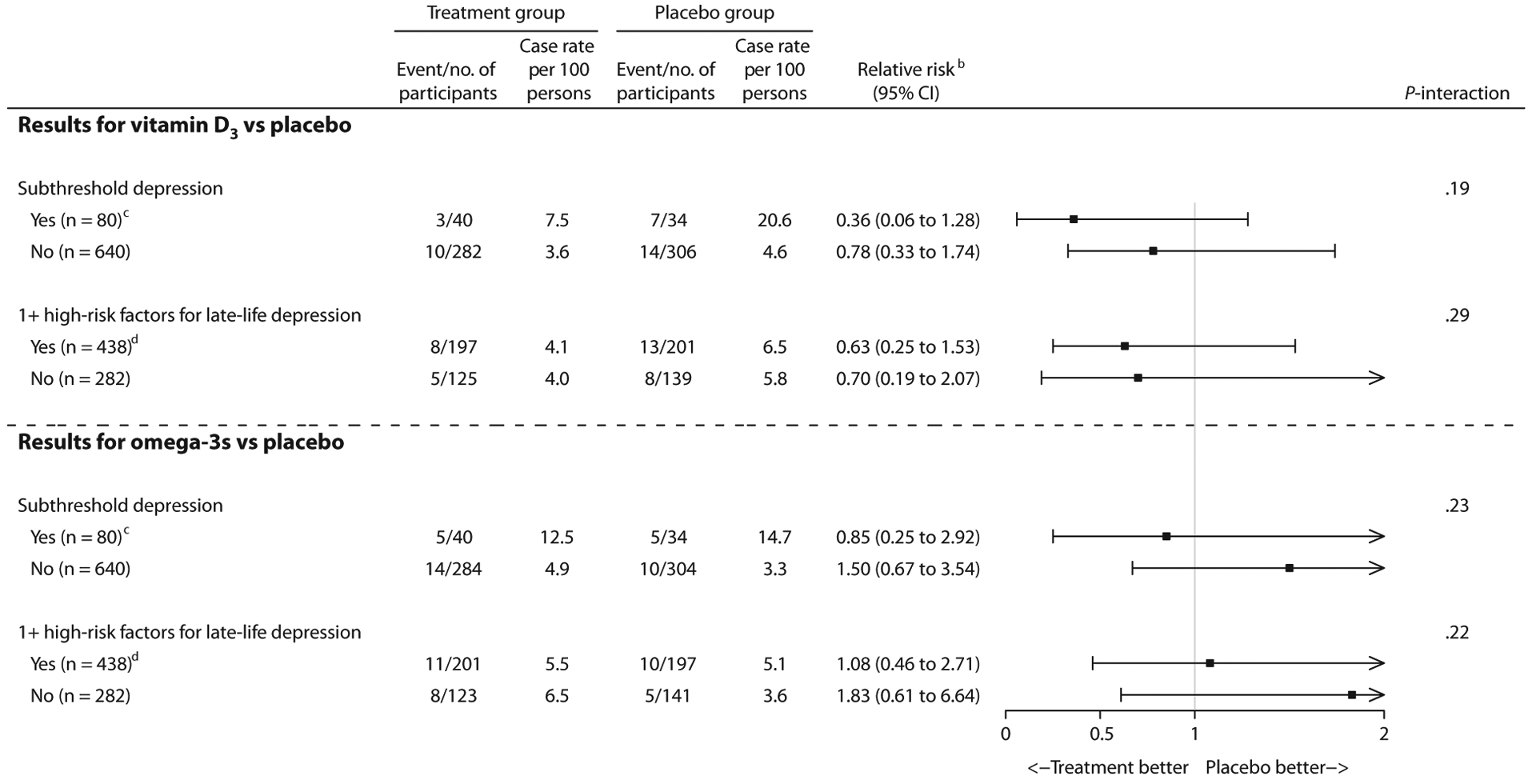
aFrom a total 720 eligible participants, 662 completed follow-up at year 2. For the coprimary outcome of incident MDD, P < .025 was considered as threshold for overall statistical significance.
bRelative risk and its CI were based on exact tests using the exact χ2 score statistic.
cIndicated prevention targeted participants with subthreshold depression, but who did not meet DSM-IV criteria for current major depressive disorders or dysthymia, at baseline.
dSelective prevention targeted participants with ≥ 1 high-risk factor for late-life depression at baseline; see details in Supplementary Appendix 1 (under E. Approach for selective prevention).
eP-interaction was calculated using the Zelen exact test for equal odds ratios. The Zelen exact test was performed to determine whether effects of vitamin D3 or omega-3s, compared to placebo, differ across risk groups of indicated and selective prevention.
Table 2.
Adjusted Mean Difference in Change in PHQ-9 Score at 2-Year Follow-Up Comparing Vitamin D3 and Placebo Groups by Risk Group of Indicated and Selective Preventiona
| Vitamin D3 vs Placebo | |||
|---|---|---|---|
| Risk Group | Eligible Participants, n | Mean Difference (95% CI)b | P-Interactionc |
| Subthreshold depression | .86 | ||
| Yesd | 80 | −0.02 (−1.26 to 1.21) | |
| No | 640 | −0.11 (−0.29 to 0.08) | |
| 1+ High-risk factors for LLD | .53 | ||
| Yese | 438 | −0.14 (−0.45 to 0.17) | |
| No | 282 | −0.01 (−0.28 to 0.25) | |
For this coprimary outcome, P < .025 was considered as threshold for statistical significance. The prespecified MCID(minimally clinically important difference) in PHQ-9 score was 0.5 points.
Results are from repeated measures model; all participants contributed to the repeated measure analysis at one and/or both time points. Models were controlled for age, sex, and marine omega-3 fatty acids randomization group.
P-interaction is from the test of the prevention category × treatment × time interaction term in the model.
Indicated prevention targeted participants with subthreshold depression, but who did not meet DSM-IV criteria for current major depressive disorder or dysthymia, at baseline.
Selective prevention targeted participants with ≥ 1 high-risk factor for LLD at baseline; see details in Supplementary Appendix 1 (under E. Approach for selective prevention).
Abbreviations: LLD = late-life depression, PHQ-9 = Patient Health Questionnaire-9.
Randomization to omega-3s, compared to placebo, did not affect risk of incident MDD among those with versus without subthreshold depression or those with versus without ≥ 1 high-risk factor; Zelen tests showed no differences in treatment effects across risk groups (Figure 2). Regarding indicated and selective prevention, no significant effects of omega-3s, compared to placebo, on risk of incident MDD were observed. The mean difference in 2-year change in PHQ-9 comparing omega-3s and placebo was not statistically significant among those with versus without subthreshold depression (Table 3). Regarding selective prevention, there appeared to be worse mean change over 2 years in PHQ-9 score, comparing omega-3s with placebo (0.35 points; 95% CI, 0.04 to 0.66); however, the test for multiplicative interaction was nonsignificant after Bonferroni correction, indicating no differential effect of omega-3s on change in PHQ-9 score among those with versus without ≥ 1 high-risk factor; estimates were lower than the prespecified MCID of 0.5 points.
Table 3.
Adjusted Mean Difference in Change in PHQ-9 Score at 2-Year Follow-Up Comparing Omega-3s and Placebo Groups by Risk Group of Indicated and Selective Preventiona
| Omega-3s vs Placebo | |||
|---|---|---|---|
| Risk Group | Eligible Participants, n | Mean Difference (95% CI)b | P-Interactionc |
| Subthreshold depression | .23 | ||
| Yesd | 80 | 0.84 (−0.36 to 2.03) | |
| No | 640 | 0.11 (−0.08 to 0.30) | |
| 1+ High-risk factors for LLD | .05 | ||
| Yese | 438 | 0.35 (0.04 to 0.66) | |
| No | 282 | −0.05 (−0.31 to 0.21) | |
For this coprimary outcome, P < .025 was considered as threshold for statistical significance. The prespecified MCID (minimally clinically important difference) in PHQ-9 score was 0.5 points.
Results are from repeated measures model; all participants contributed to the repeated measure analysis at one and/or both time points. Models were controlled for age, sex, and vitamin D3 randomization group.
P-interaction is from the test of the prevention category × treatment × time interaction term in the model.
Indicated prevention targeted participants with subthreshold depression, but who did not meet DSM-IV criteria for current major depressive disorder or dysthymia, at baseline.
Selective prevention targeted participants with ≥ 1 high-risk factor for LLD at baseline; see details in Supplementary Appendix 1 (under E. Approach for selective prevention).
Abbreviations: LLD = late-life depression, PHQ = Patient Health Questionnaire-9.
Results for Secondary Analyses
Of the 720 participants, 48 (6.7%) developed the composite depression outcome (7.3% [48/662] among study completers). Effects of vitamin D3 or omega-3s, versus placebos, on MDD incidence did not differ across risk groups for selective prevention (Supplementary Tables 3 and 4); neither supplement showed significant effects among those with ≥ 1 high-risk factor.
Results for Non-Prespecified and Post hoc Analyses
These results showed the following: (1) Neither supplement showed significant effects on risk of DSM-IV incident MDD in the full sample (Supplementary Table 5); the overall adjusted mean difference in change in PHQ-9 score at 2 years was not significant, comparing vitamin D3 or omega-3s versus placebos (Supplementary Table 6). (2) Validation of the PF-10 in our sample showed significant correlations with objective physical performance tests and significant discrimination of Short Physical Performance Battery scores among those with versus without impairment based on the PF-10 (see Supplementary Tables 7 and 8 and Supplementary Appendix 1).
DISCUSSION
In this RCT including 720 older adults at elevated risk for depression due to presence of subthreshold depression or high-risk factors for late-life depression, neither vitamin D3 nor omega-3s, compared with placebos, significantly affected risk of incident MDD or change in mood scores at 2 years; however, statistical power was limited by low MDD case rates (~5%).
Older persons at elevated risk for depression may have higher inflammation levels and poor vascular and metabolic health.5,6 Vitamin D3 and omega-3s may lower LLD risk by improving inflammatory profiles and enhancing vascular and metabolic health, as well as by improving neurotrophic and neuroprotective factors.7–10 Our null findings regarding vitamin D3 supplementation are consistent with results from shorter-term and/or smaller-sample RCTs of vitamin D3 ≥ 800 IU/d that showed no benefit for indicated and selective prevention.16–18,46 For example, a prior RCT (n = 155; MDD incidence = 3.4%)16 found no effect of 12-month supplementation of vitamin D3 (1,200 IU/d) on MDD risk or mood scores among older adults with subthreshold depressive symptoms, physical/functional limitation, and low vitamin D3 levels. An RCT by Bot and colleagues (n = 1,025; MDD incidence = 10%)17 found no beneficial effects of 12-month supplementation with a multinutrient agent containing 800 IU/d of vitamin D3 and 1.4 g/d of omega-3s (EPA:DHA = 3:1) for MDD incidence or change in mood score over 1 year among midlife adults with overweight/obesity and subthreshold depression. Our study is consistent with these findings and has a 2-year treatment duration and higher dose of vitamin D3 (2,000 IU/d), along with high follow-up (92%) and compliance rates (95%).
Similarly, our null findings regarding omega-3s supplementation for indicated and selective prevention of LLD are consistent with most published RCTs.17,20–22 However, Sinn and colleagues (n = 50; mean age = 73 years)47 found that EPA and DHA supplements, compared to placebos, have been associated with lower mood scores at 6-month follow-up in older adults with mild cognitive impairment. Overall, future trials might add clarity by integrating biomarkers and genetics when classifying at-risk populations; this approach may shed light on mechanism as well as persons most likely to benefit from treatment.
Study strengths include a well-characterized sample, factorial trial design, testing two modalities of prevention using the NAM framework, rigorously adjudicated endpoints, use of well-validated measures for characterizing risk groups, and high follow-up and adherence rates.
This study also has limitations. First, the assessment interval was 2 years; as more than 50% of MDD cases may spontaneously remit by 12 months,48 some cases that occurred and fully remitted between assessments may not have been ascertained. Second, among those generally healthy participants, a lower-than-expected eligible sample (720 vs 855) and low case rates limited power to detect significant effects; however, observed MDD incidence was comparable to that in recent RCTs of similar community-based participants.16,17 To mitigate risk of type II error, we secondarily addressed selective prevention using a broader composite depression outcome; however, results were unchanged. Third, evidence suggests that doses of ≥ 1.5 g/d omega-3s may be necessary for LLD prevention49; however, we did not test effects of high-dose omega-3s or of different balances of EPA versus DHA. Fourth, generalizability is a potential issue; the sample included generally healthy adults and only 15% racial/ethnic minority participation.
CONCLUSIONS
Among 720 older men and women at elevated risk for depression due to presence of subthreshold depression or established high-risk factors, neither vitamin D3 nor omega-3s, compared with placebos, significantly affected risk of incident major depressive disorder or change in mood scores over a treatment duration of 2 years; however, statistical power was limited by low MDD incidence rates. Findings do not support use of supplemental vitamin D3 or omega-3s for indicated and selective prevention of late-life depression.
Supplementary Material
Clinical Points.
Limited trial evidence exists on whether supplementations of vitamin D3 (2,000 IU/d) and/or marine omega-3 fatty acids (1 g/d) are beneficial for prevention of late-life depression among those at higher risk (ie, those with subthreshold depression or with ≥ 1 high-risk factor for depression).
Daily supplementation of vitamin D3 and marine omega-3 fatty acids did not show an advantage over placebo among at-risk participants for late-life depression.
Acknowledgments:
We acknowledge the invaluable contributions and dedication of the 25,871 participants in VITAL and the entire staff of the VITAL study. VITAL-DEP has been approved by the Institutional Review Board of Partners Healthcare/Brigham and Women’s Hospital, and the VITAL study agents have received Investigational New Drug Approval from the US Food and Drug Administration. Voting members of the Data and Safety Monitoring Board for VITAL and ancillary studies, including VITAL-DEP, included Lawrence S. Cohen, MD; Theodore Colton, ScD; Mark A. Espeland, PhD; Craig Henderson, MD; Alice H. Lichtenstein, ScD; Rebecca A. Silliman, MD, PhD; and Nanette Wenger, MD (chair). Ex-officio members include Josephine Boyington, PhD, MPH; Rebecca Costello, PhD; Cindy Davis, PhD; Peter Greenwald, MD; and Wendy Weber, PhD. VITAL and VITAL-DEP are registered at clinicaltrials.gov (VITAL: NCT01169259; VITAL-DEP: NCT01696435). The VITAL website is www.vitalstudy.org.
Funding/Support:
VITAL-DEP is supported by R01 MH091448 and R56 MH091448 from NIMH. VITAL is supported by grants R01 AT011729, U01 CA138962, and R01 CA138962, which includes support from the National Cancer Institute; National Heart, Lung and Blood Institute (NHLBI); Office of Dietary Supplements; National Institute of Neurological Disorders and Stroke; and the National Center for Complementary and Integrative Health of the National Institutes of Health (NIH). The VITAL ancillary studies and Clinical and Translational Science Center (CTSC) component are supported by grants DK088078 and R01 DK088762 from the National Institute of Diabetes and Digestive and Kidney Diseases; R01 HL101932 and R01 HL102122 from NHLBI; R01 AG036755 from the National Institute on Aging (NIA); R01 AR059086 and R01 AR060574 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; and R01 MH091448 from NIMH). This work was conducted with support from the Harvard Catalyst CTSC (UL1TR001102 from the National Center for Advancing Translational Sciences). Dr Reynolds’ participation also received support from P30 MH090333 from NIMH and the University of Pittsburgh Medical Center Endowment in Geriatric Psychiatry. Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma/BASF of Norway (Omacor fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs.
Role of the Funders/Sponsors:
The NIH, Harvard Catalyst, US FDA, Pharmavite LLC, and Pronova BioPharma/BASF had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Relevant Financial Relationships:
Dr Vyas has received research support from Nestlé-Purina Petcare Company. Dr Mischoulon has received research support from Nordic Naturals and Heckel Medizintechnik GmbH. He has received honoraria for speaking from the Massachusetts General Hospital (MGH) Psychiatry Academy; Peerpoint Medical Education Institute, LLC; and Harvard blog. He also works with the MGH Clinical Trials Network and Institute (CTNI), which has received research funding from multiple pharmaceutical companies and the National Institute of Mental Health (NIMH). Dr Chang receives royalties from Up-to-Date. Dr Buring’s spouse was on the Scientific Advisory Board of Pharmavite LLC during the trial. Dr Manson has received research support from Mars Edge. Dr Reynolds receives payment from the American Association of Geriatric Psychiatry as Editor-in-Chief of The American Journal of Geriatric Psychiatry and royalty income for intellectual property as co-inventor of the Pittsburgh Sleep Quality Index and, in the past, received a one-time honorarium from Merck for consultation on care pathways for insomnia. Dr Reynolds also received royalty income from Oxford University Press and from Up-to-Date. Dr Okereke receives royalties from Springer Publishing for a book on late-life depression prevention. No other authors have disclosures to report.
Footnotes
Supplementary Material: Available at Psychiatrist.com.
See supplementary material for this article at Psychiatrist.com
REFERENCES
- 1.Committee on Prevention of Mental Disorders. Institute of Medicine. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. Washington, DC: National Academy Press; 1994. [PubMed] [Google Scholar]
- 2.Schoevers RA, Smit F, Deeg DJ, et al. Prevention of late-life depression in primary care: do we know where to begin? Am J Psychiatry. 2006;163(9):1611–1621. [DOI] [PubMed] [Google Scholar]
- 3.Smits F, Smits N, Schoevers R, et al. An epidemiological approach to depression prevention in old age. Am J Geriatr Psychiatry. 2008;16(6):444–453. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz RF, Cuijpers P, Smit F, et al. Prevention of major depression. Annu Rev Clin Psychol. 2010;6(1):181–212. [DOI] [PubMed] [Google Scholar]
- 5.Lee SM, Te S, Breen EC, et al. Circulating versus lipopolysaccharide-induced inflammatory markers as correlates of subthreshold depressive symptoms in older adults. World J Biol Psychiatry. 2020;21(8):634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64A(4):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertone-Johnson ER. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev. 2009;67(8):481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherniack EP, Troen BR, Florez HJ, et al. Some new food for thought: the role of vitamin D in the mental health of older adults. Curr Psychiatry Rep. 2009;11(1):12–19. [DOI] [PubMed] [Google Scholar]
- 9.Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13–25. [DOI] [PubMed] [Google Scholar]
- 10.Su KP, Matsuoka Y, Pae CU. Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci. 2015;13(2):129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van’t Veer-Tazelaar PJ, van Marwijk HW, van Oppen P, et al. Stepped-care prevention of anxiety and depression in late life: a randomized controlled trial. Arch Gen Psychiatry. 2009;66(3):297–304. [DOI] [PubMed] [Google Scholar]
- 12.Sriwattanakomen R, Ford AF, Thomas SB, et al. Preventing depression in later life: translation from concept to experimental design and implementation. Am J Geriatr Psychiatry. 2008;16(6):460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rovner BW, Casten RJ, Hegel MT, et al. Preventing depression in age-related macular degeneration. Arch Gen Psychiatry. 2007;64(8):886–892. [DOI] [PubMed] [Google Scholar]
- 14.Cuijpers P, Pineda BS, Quero S, et al. Psychological interventions to prevent the onset of depressive disorders: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2021;83:101955. [DOI] [PubMed] [Google Scholar]
- 15.Okereke OI, Lyness JM, Lotrich FE, et al. Depression in late-life: a focus on prevention. Focus Am Psychiatr Publ. 2013;11(1):22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Koning EJ, Lips P, Penninx BWJH, et al. Vitamin D supplementation for the prevention of depression and poor physical function in older persons: the D-Vitaal study, a randomized clinical trial. Am J Clin Nutr. 2019;110(5):1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bot M, Brouwer IA, Roca M, et al. ; MooDFOOD Prevention Trial Investigators. Effect of multinutrient supplementation and food-related behavioral activation therapy on prevention of major depressive disorder among overweight or obese adults with subsyndromal depressive symptoms: the MooDFOOD randomized clinical trial. JAMA. 2019;321(9):858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant AM, Avenell A, Campbell MK, et al. ; RECORD Trial Group. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621–1628. [DOI] [PubMed] [Google Scholar]
- 19.Jorde R, Sneve M, Figenschau Y, et al. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599–609. [DOI] [PubMed] [Google Scholar]
- 20.Andreeva VA, Galan P, Torrès M, et al. Supplementation with B vitamins or n-3 fatty acids and depressive symptoms in cardiovascular disease survivors: ancillary findings from the SUpplementation with FOLate, vitamins B-6 and B-12 and/or OMega-3 fatty acids (SU.FOL.OM3) randomized trial. Am J Clin Nutr. 2012;96(1):208–214. [DOI] [PubMed] [Google Scholar]
- 21.Giltay EJ, Geleijnse JM, Kromhout D. Effects of n-3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. Am J Clin Nutr. 2011;94(6):1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmer R, Riemer T, Rauch B, et al. ; OMEGA-Study Group. Effects of 1-year treatment with highly purified omega-3 fatty acids on depression after myocardial infarction: results from the OMEGA trial. J Clin Psychiatry. 2013;74(11):e1037–e1045. [DOI] [PubMed] [Google Scholar]
- 23.Chang SC, Pan A, Kawachi I, et al. Risk factors for late-life depression: a prospective cohort study among older women. Prev Med. 2016;91:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okereke OI, Reynolds CF 3rd, Mischoulon D, et al. The VITamin D and OmegA-3 TriaL-Depression Endpoint Prevention (VITAL-DEP): Rationale and design of a large-scale ancillary study evaluating vitamin D and marine omega-3 fatty acid supplements for prevention of late-life depression. Contemp Clin Trials. 2018;68:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33, quiz 34–57. [PubMed] [Google Scholar]
- 27.Brown LM, Schinka JA, Mortimer JA, et al. 3MS normative data for elderly African Americans. J Clin Exp Neuropsychol. 2003;25(2):234–241. [DOI] [PubMed] [Google Scholar]
- 28.Bassuk SS, Murphy JM. Characteristics of the Modified Mini-Mental State Exam among elderly persons. J Clin Epidemiol. 2003;56(7):622–628. [DOI] [PubMed] [Google Scholar]
- 29.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okereke OI, Reynolds CF 3rd, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324(5):471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Judd LL, Rapaport MH, Paulus MP, et al. Subsyndromal symptomatic depression: a new mood disorder? J Clin Psychiatry. 1994;55(suppl):18–28. [PubMed] [Google Scholar]
- 32.Rapaport MH, Judd LL, Schettler PJ, et al. A descriptive analysis of minor depression. Am J Psychiatry. 2002;159(4):637–643. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–173. [DOI] [PubMed] [Google Scholar]
- 35.Plummer F, Manea L, Trepel D, et al. Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry. 2016;39:24–31. [DOI] [PubMed] [Google Scholar]
- 36.Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 37.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36(4):428–434. [DOI] [PubMed] [Google Scholar]
- 38.Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 39.Haley SM, McHorney CA, Ware JE Jr. Evaluation of the MOS SF-36 physical functioning scale (PF-10), I: unidimensionality and reproducibility of the Rasch item scale. J Clin Epidemiol. 1994;47(6):671–684. [DOI] [PubMed] [Google Scholar]
- 40.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 41.Ryan J, Woods RL, Britt C, et al. ; ASPREE Investigator Group. Normative performance of healthy older individuals on the Modified Mini-Mental State (3MS) examination according to ethno-racial group, gender, age, and education level. Clin Neuropsychol. 2019;33(4):779–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higginson IJ, Gao W, Jackson D, et al. Short-form Zarit Caregiver Burden Interviews were valid in advanced conditions. J Clin Epidemiol. 2010;63(5):535–542. [DOI] [PubMed] [Google Scholar]
- 43.Koenig HG, Westlund RE, George LK, et al. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993;34(1):61–69. [DOI] [PubMed] [Google Scholar]
- 44.Strodl E, Kenardy J. The 5-item Mental Health Index predicts the initial diagnosis of nonfatal stroke in older women. J Womens Health (Larchmt). 2008;17(6):979–986. [DOI] [PubMed] [Google Scholar]
- 45.Fitzmaurice GM, Laird NM, Ware JH. Modelling the mean: analyzing response profiles. In: Fitzmaurice GM, Laird NM, Ware JH, eds. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004:103–139. [Google Scholar]
- 46.Okereke OI, Singh A. The role of vitamin D in the prevention of late-life depression. J Affect Disord. 2016;198:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinn N, Milte CM, Street SJ, et al. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr. 2012;107(11):1682–1693. [DOI] [PubMed] [Google Scholar]
- 48.Whiteford HA, Harris MG, McKeon G, et al. Estimating remission from untreated major depression: a systematic review and meta-analysis. Psychol Med. 2013;43(8):1569–1585. [DOI] [PubMed] [Google Scholar]
- 49.Bai ZG, Bo A, Wu SJ, et al. Omega-3 polyunsaturated fatty acids and reduction of depressive symptoms in older adults: a systematic review and meta-analysis. J Affect Disord. 2018;241:241–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


