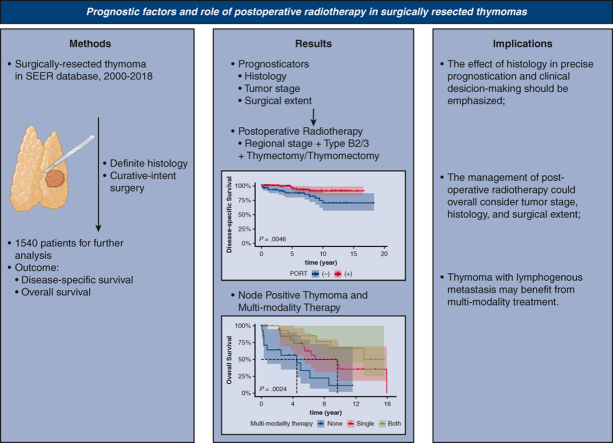
Abstract
Objective
To investigate the prognostic factors in and role of postoperative radiotherapy (PORT) for surgically resected thymomas.
Methods
A total of 1540 patients with pathologically confirmed thymomas undergoing resection between 2000 and 2018 were identified retrospectively from the SEER (Surveillance, Epidemiology, and End Results) database. Tumors were restaged as local (limited to thymus), regional (invasion to mediastinal fat and other neighboring structures), or distant stage. Disease-specific survival (DSS) and overall survival (OS) were estimated by the Kaplan–Meier method and the log-rank test. Adjusted hazard ratios (HRs) with 95% CIs were calculated by Cox proportional hazards modeling.
Results
Tumor stage and histology were independent predictors of both DSS (regional: HR, 3.711; 95% CI, 2.006-6.864; distant: HR, 7.920; 95% CI, 4.061-15.446; type B2/B3: HR, 1.435; 95% CI, 1.008-2.044) and OS (regional: HR, 1.461; 95% CI, 1.139-1.875; distant: HR, 2.551; 95% CI, 1.855-3.509; type B2/B3: HR, 1.409; 95% CI, 1.153-1.723). For patients with regional stage and type B2/B3 thymomas, PORT was associated with better DSS after thymectomy/thymomectomy (HR, 0.268; 95% CI, 0.099-0.727), but the association was not significant after extended thymectomy (HR, 1.514; 95% CI, 0.516-4.44). Among patients with lymph node metastases, those who received PORT (HR, 0.372; 95% CI, 0.146-0.949), chemotherapy (HR, 0.843; 95% CI, 0.303-2.346), or both (HR, 0.296, 95% CI, 0.071-1.236) had a better OS.
Conclusions
The extent of invasion and tumor histology were independent predictors of worse survival following surgical resection of thymoma. Patients with regional invasion and type B2/B3 thymoma who undergo thymectomy/thymomectomy may benefit from PORT, while patients with nodal metastases may benefit from multimodal therapy, including PORT and chemotherapy.
Key Words: thymoma, postoperative radiotherapy, prognosis, histology, SEER
Graphical abstract
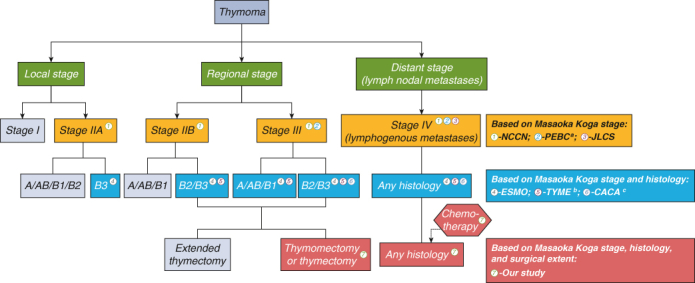
Comparison of clinical guidelines and our study results.
Central Message.
Postoperative radiotherapy for patients with regional invasion and type B2/B3 thymoma who undergo thymectomy/thymomectomy could significantly improve disease-specific survival.
Perspective.
Postoperative radiotherapy (PORT) should be considered in tumors of advanced stage using National Comprehensive Cancer Network guidelines or of aggressive histology according to European Society for Medical Oncology recommendations. The extent of resection also should be considered, as PORT is associated with better survival in thymoma with regional invasion and type B2/B3. Additionally, multimodal therapy should be provided to patients with thymoma with lymphogenous metastases.
Thymic epithelial tumors (TETs) are a series of rare malignancies located in the anterior mediastinum,1 with an incidence of roughly 1.5 to 3.9 cases per 1 million individuals based on different regional reports.2, 3, 4 TETs are composed mainly of thymomas (>80% of cases), thymic carcinomas, and neuroendocrine carcinomas.
Thymomas are considered slow-growing TETs that spread by local extension.5 Nevertheless, recurrence still occurs after complete resection, of which the majority is intrathoracic relapse.6 Local irradiation has been considered as postoperative therapy for improving locoregional tumor control; however, the prognostic role of postoperative radiotherapy (PORT) in thymoma patients has been controversial owing to a paucity of prospective studies, especially for thymomas with regional invasion. In the setting of an R0 resection, European Society for Medical Oncology (ESMO) clinical practice guidelines recommend providing PORT for Masaoka–Koga stage III thymomas and considering PORT for Masaoka–Koga stage II thymomas with aggressive histology (type B2/B3),7 because of numerous studies identifying type B2/B3 thymoma as a significant prognostic factor for recurrence.8,9 However, according to National Comprehensive Cancer Network (NCCN) clinical practice guidelines,10 PORT can be considered for patients with Masaoka–Koga stage II-IV thymoma undergoing R0 resection regardless of histology. Several other studies have also provided varying recommendations for the administration of PORT in clinical practice.11, 12, 13, 14, 15
Thymomas are notably less likely than thymic carcinomas to spread by lymphogenous metastasis.16 Because of this rarity, the prognostic efficacy of treatment other than surgery, such as PORT or systematic therapy, remains vague for patients with regional lymph nodal metastases in the clinical setting.
Our study aimed to investigate the independent risk factors for mortality in surgically resected thymomas and the prognostic impact of PORT in surgically resected thymomas with either regional invasion or lymph node metastases.
Methods
Data Extraction
Data were extracted from the Surveillance, Epidemiology, and End Results (SEER) 18-Registry of the National Cancer Institute, an open-access US nationwide cancer database. The corresponding clinicopathologic and survival data were extracted using SEER∗Stat version 8.3.7 (National Institutes of Health). Based on the value of the primary site variable (thymus = 379), we collected patients diagnosed with thymoma between 2000 and 2018. The Institutional Review Board of Shanghai Pulmonary Hospital has determined that studies using deidentified data, such as SEER, do not require review (June 23, 2022).
Study Population
The study's eligibility criteria were as follows: (1) patients with definite histologic subtypes, as defined by the corresponding International Classification of Diseases codes with the malignant behavior code (/3) (ie, 8581, 8582, 8583, 8584, and 8585 represented type A, type AB, type B1, type B2, and type B3 thymomas, respectively), and (2) patients receiving cancer-directed surgery. A total of 1737 eligible patients were identified.
The exclusion criteria were (1) patients receiving preoperative radiotherapy; (2) patients receiving non–curative-intent surgery, such as debulking surgery, local tumor destruction, and local tumor excision, and patients with unknown information about the surgical extent; and (3) patients whose records contained any conflicting data points. After exclusions, a total of 1540 patients were available for further analysis.
Variable Transformation
Because the patients’ pathologic staging was unknown, we referred to the staging system from the SEER database (see https://seer.cancer.gov/tools/ssm/). We grouped patients into local (limited to the thymus), regional (invasion to the mediastinal fat and other neighboring structures), or distant stage, as was done in previous studies.17,18 The relationship between the staging system and the Masaoka–Koga/eighth edition of the TNM staging system is explained in Table E1. In addition, patients with lymph node metastases and the specific number of lymph nodes containing metastases were identified by the variables that documented whether the regional lymph nodes were involved and the exact number of regional lymph nodes found to contain metastases.
Based on the records in the database, the extent of surgical resection was classified as thymomectomy, thymectomy, or extended thymectomy. Thymomectomy is defined as resection of the tumor; thymectomy, as resection of tumor and the thymus gland; extended thymectomy, as resection of tumor, thymus, and all pericardial fat between the phrenic nerves and other structures if there is evidence of invasion.
Statistical Analysis
Continuous variables were analyzed using the independent-samples Student t test or one-way ANOVA. Categorical variables were compared by the Pearson chi-square test or Fisher exact test (when n < 40). Disease-specific survival (DSS) and overall survival (OS) were the primary and secondary outcomes of interest, respectively. DSS was defined as the time between the diagnosis of cancer and death from thymoma (censored observations: unrelated deaths and unknown causes of death). OS was defined as the time between the diagnosis of cancer and death by any cause. A log-rank test was used to compare survival differences by clinicopathologic characteristics. Cox proportional hazards modeling was used to calculate adjusted hazard ratios (HRs) with 95% CIs, controlling for age, sex, race, surgical extent, malignant history, and other treatments. The Benjamini–Hochberg method was used to control the false discovery rate for multiple comparisons.
A 2-sided P value <.05 was considered to indicate statistical significance. All analyses were performed with SPSS version 26.0 (IBM) and R version 4.1.2 (R Foundation for Statistical Computing).
Results
Baseline Characteristics
Patient demographic and clinicopathologic characteristics are shown in Table 1. Patients were evenly distributed in terms of sex (females, n = 789 [51.2%]; males, n = 751 [48.8%]), with a median age of 60 years (range, 13-89 years). At time of surgical resection, 637, 686, and 217 patients presented with local, regional, and distant stage, respectively. More advanced tumor stage was significantly associated with more aggressive histologic subtype (type B2/B3), more radical surgical extent of surgical resection, and administration of PORT and/or chemotherapy (all P < .001).
Table 1.
Baseline characteristics of all surgically resected thymoma patients (N = 1540)
| Variables | Total (N = 1540) | Local stage (N = 637) | Regional stage (N = 686)∗ | Distant stage (N = 217) | P value |
|---|---|---|---|---|---|
| Age, yr, median (range) | 60 (13-89) | 60 (14-89) | 60 (13-89) | 57 (21-85) | .081† |
| Sex, n (%) | .263 | ||||
| Male | 751 (48.80) | 295 (46.30) | 345 (50.30) | 111 (51.20) | |
| Female | 789 (51.20) | 342 (53.70) | 341 (49.70) | 106 (48.80) | |
| Race, n (%) | .913 | ||||
| Hispanic | 159 (10.30) | 65 (10.20) | 73 (10.60) | 21 (9.70) | |
| Non-Hispanic | 1381 (89.70) | 572 (89.80) | 613 (89.40) | 196 (90.30) | |
| Cancer history, n (%) | .111 | ||||
| No | 1298 (84.30) | 523 (82.10) | 591 (86.30) | 184 (84.80) | |
| Yes | 241 (15.70) | 114 (17.90) | 94 (13.70) | 33 (15.20) | |
| Histologic subtype, n (%) | <.001 | ||||
| Type A | 187 (12.10) | 97 (15.20) | 80 (11.70) | 10 (4.60) | |
| Type AB | 400 (26.00) | 212 (33.30) | 157 (22.90) | 31 (14.30) | |
| Type B1 | 271 (17.60) | 108 (17.00) | 128 (18.70) | 35 (16.10) | |
| Type B2 | 316 (20.50) | 119 (18.70) | 144 (21.00) | 53 (24.40) | |
| Type B3 | 366 (23.80) | 101 (15.90) | 177 (25.80) | 88 (40.60) | |
| Extent of surgery, n (%) | <.001 | ||||
| Extended thymectomy | 342 (22.20) | 41 (6.40) | 192 (28.00) | 109 (50.20) | |
| Thymectomy | 788 (51.20) | 389 (61.10) | 326 (47.50) | 73 (33.60) | |
| Thymomectomy | 410 (26.60) | 207 (32.50) | 168 (24.50) | 35 (16.10) | |
| Postoperative radiotherapy, n (%) | <.001 | ||||
| Yes | 704 (45.70) | 182 (28.60) | 405 (59.00) | 117 (53.90) | |
| No | 836 (54.30) | 455 (71.40) | 281 (41.00) | 100 (46.10) | |
| Chemotherapy, n (%) | <.001 | ||||
| Yes | 283 (18.40) | 37 (5.80) | 142 (20.70) | 104 (47.90) | |
| No | 1257 (81.60) | 600 (94.20) | 544 (79.30) | 113 (52.10) |
Boldface indicates statistical significance.
One patient with missing cancer history data.
One-way analysis of variance.
Prognostic Factors Impacting Survival
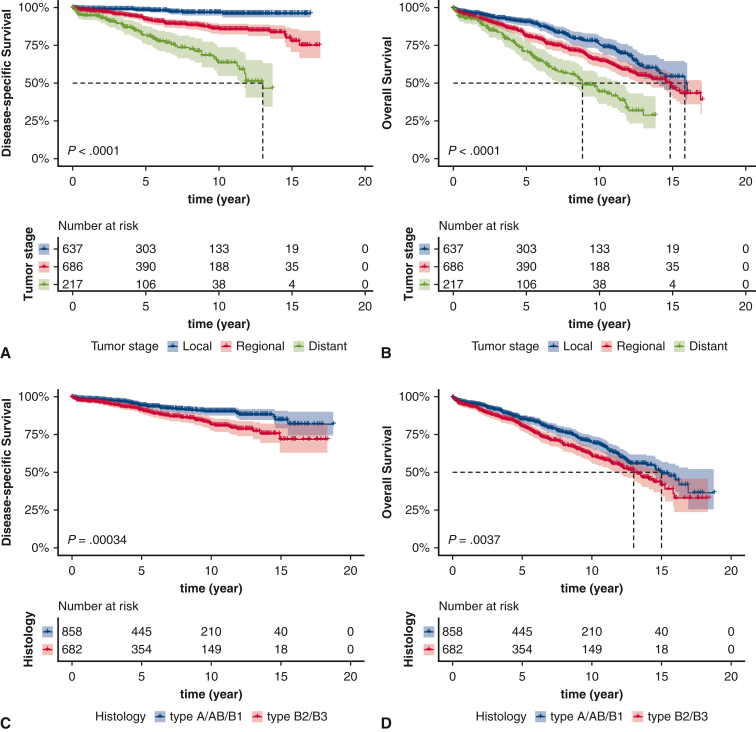
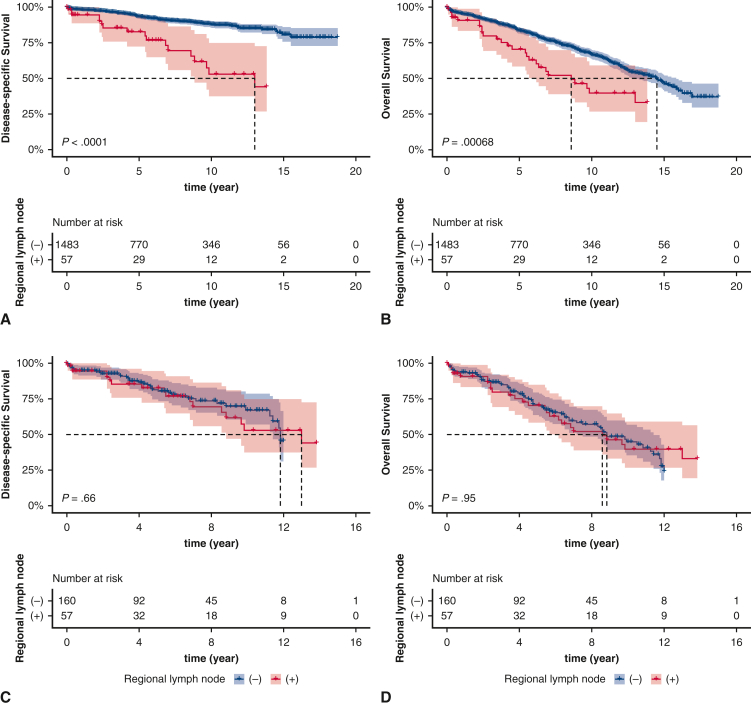
There were significant differences in DSS and OS among different tumor stages and histologic subtypes (tumor stage: DSS, P < .0001; OS, P < .0001; histologic subtype: DSS, P = .00034; OS, P = .0037) (Figure 1, A-D). Patients who presented with lymph node metastases had worse DSS (P < .0001) and OS (P = .00068) among the entire cohort, but this survival difference was nonsignificant in the distant stage cohort (DSS, P = .66; OS, P = .95) (Figure E1, A-D).
Figure 1.
Kaplan–Meier estimates of survival based on tumor stage (A, disease-specific survival [DSS]; B, overall survival [OS]) and histologic subtype of thymoma (C, DSS; D, OS). The shading indicates the range of 95% CI for the corresponding survival curve.
Figure E1.
Kaplan–Meier estimates of survival comparing patients with and without regional lymph nodal metastases in the total cohort (A, disease-specific survival [DSS]; B, overall survival [OS]) and among patients presenting with distant stage (C, DSS; D, OS). The shading indicates the range of 95% CI for the corresponding survival curve.
After adjusting for patient demographics and clinicopathologic features with the multivariable analyses, tumor stage and histologic subtype remained significant predictors of DSS (regional vs local stage: adjusted HR, 3.711; 95% CI, 2.006-6.864; distant vs local stage: adjusted HR, 7.920; 95% CI, 4.061-15.446; type B2/B3 vs type A/AB/B1: adjusted HR, 1.435; 95% CI, 1.008-2.044) and OS (regional vs local stage: adjusted HR, 1.461; 95% CI, 1.139-1.875; distant vs local stage: adjusted HR, 2.551; 95% CI, 1.855-3.509; type B2/B3 vs type A/AB/B1: adjusted HR, 1.409; 95% CI, 1.153-1.723) (Table E2).
Prognostic Impact of PORT in Patients Presenting with Local and Regional Stages
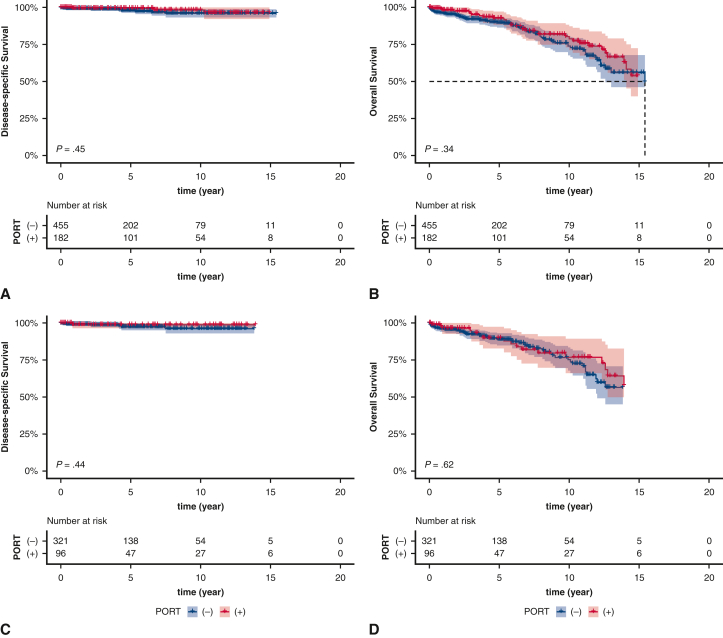
Among patients who presented at a local stage, there were no significant differences in DSS and OS based on receipt of PORT (DSS, P = .45; OS, P = .34) (Figure E2, A and B). The survival differences remained insignificant after stratification based on histologic subtype (Figure E2, C-F).
Figure E2.
Kaplan–Meier estimates of survival based on receipt of PORT among local stage patients (A, disease-specific survival [DSS]; B, overall survival [OS]) and subgroup analysis based on histologic subtypes (C and D, type A/AB/B1 DSS and OS; E and F, type B2/B3, DSS and OS). The shading indicates the range of 95% CI for the corresponding survival curve. PORT, Postoperative radiotherapy.
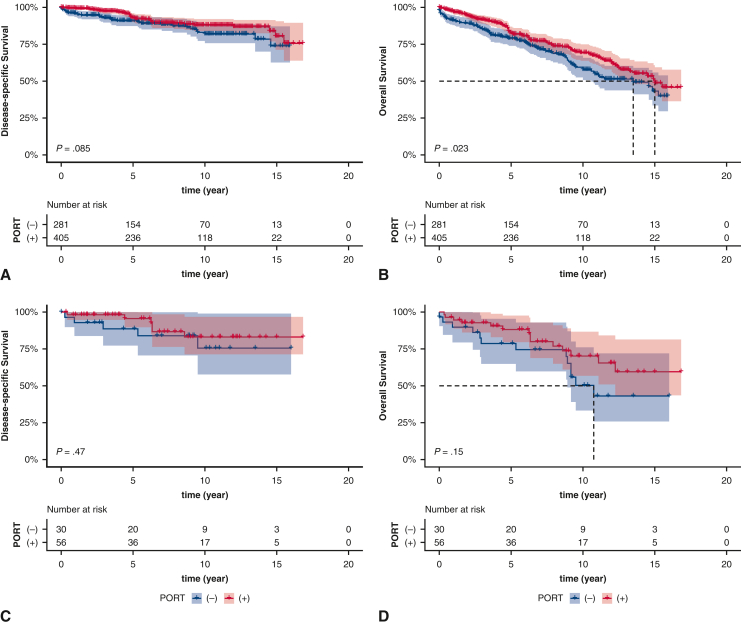
Among patients who presented at a regional stage, there was a significant difference in OS based on receipt of PORT (P = .023), but not in DSS (P = .085) (Figure 2, A and B). However, PORT was not an independent prognostic factor for DSS and OS after adjusting for covariates (DSS: adjusted HR, 0.691; 95% CI, 0.43-1.108; OS: adjusted HR, 0.78; 95% CI, 0.591-1.03) (Table E3).
Figure 2.
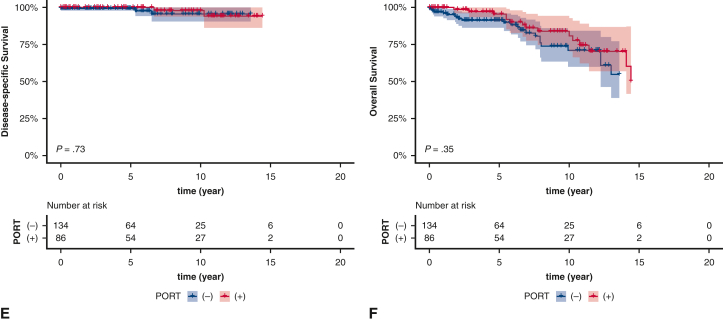
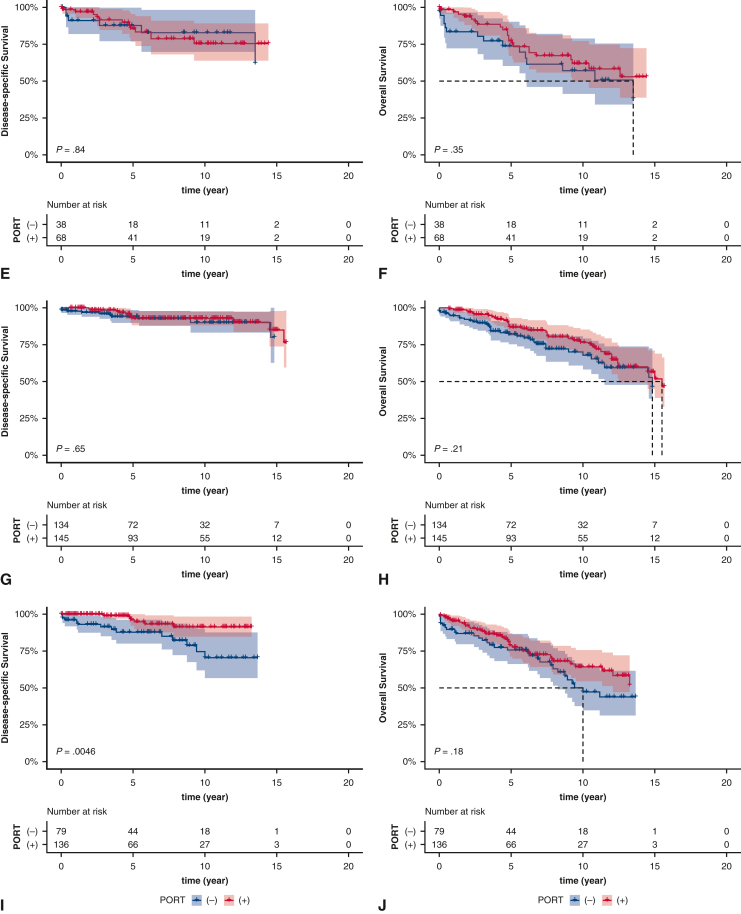
Kaplan–Meier estimates of survival based on receipt of postoperative radiotherapy (PORT) in patients with regional stage (A and B), patients with regional stage and type A/AB/B1 after extended thymectomy (C and D), patients with regional stage and type B2/B3 after extended thymectomy (E and F), patients with regional stage and type A/AB/B1 after thymectomy/thymomectomy (G and H), and patients with regional stage and type B2/B3 after thymectomy/thymomectomy (I and J). The shading indicates the range of 95% CI for the corresponding survival curve.
Because surgical extent has been associated with prognosis,19,20 the prognostic impact of PORT was further explored among the different types of resections. In patients who underwent an extended thymectomy, PORT was not associated with better survival regardless of histologic subtype (Figure 2, C-F). In patients who underwent thymectomy or thymomectomy, PORT did not significantly improve survival for patients with type A/AB/B1 thymoma (Figure 2, G and H); however, in patients with type B2/B3 thymoma, PORT significantly improved DSS (P = .0046) (Figure 2, I), but not OS (P = .18) (Figure 2, J). After adjusting for covariates, PORT was an independent prognostic factor of DSS (adjusted HR, 0.268; 95% CI, 0.099-0.727; adjusted P = .015, Benjamini–Hochberg method) (Table E4).
Thymomas with Lymph Node Metastases
Lymph nodal metastasis was associated with type B3 thymoma, administration of chemotherapy, and a more radical extent of surgical resection (P < .001 for all) (Table E5). Additionally, more radical extent of resection was associated with better lymph node harvest (median of lymph node harvest, extended thymectomy vs thymectomy vs thymomectomy: 0 [interquartile range (IQR), 0-3] vs 0 (IQR, 0-2) versus 0 (IQR, 0-1); P < .001, Kruskal–Wallis test). Fifty-seven patients with nodal metastases were identified, among which most were type B3 thymoma (n = 27; 47.4%) and one lymph node contained metastases (n = 26; 45.6%).
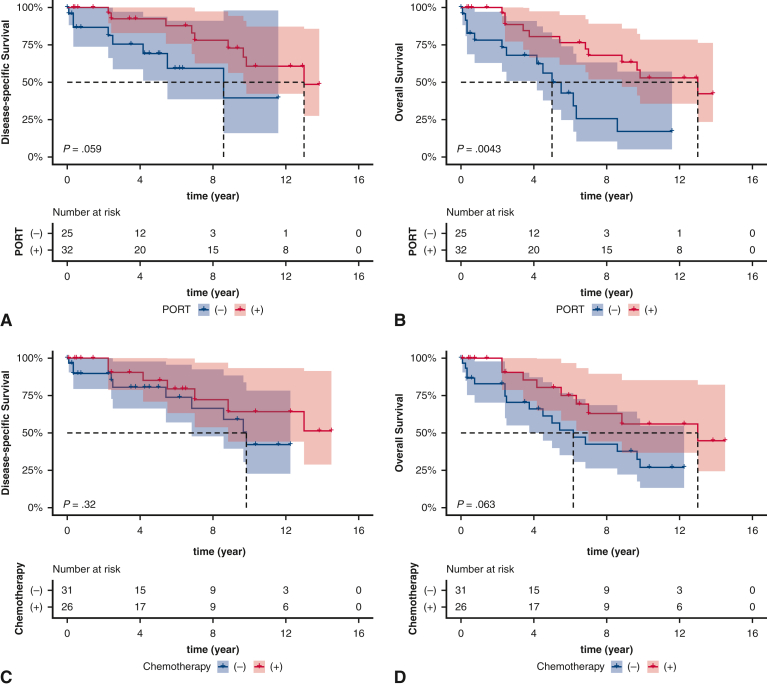
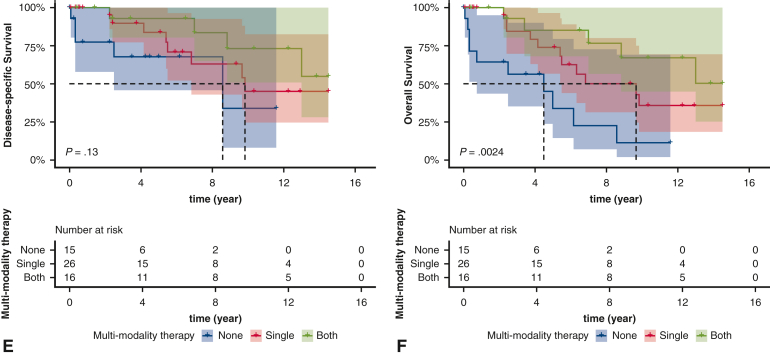
In patients with lymph nodal metastases, PORT or chemotherapy was associated with improved DSS, albeit nonsignificantly so (Figure 3, A-C). Additionally, PORT and chemotherapy were associated with improved OS, although the association with chemotherapy was nonsignificant (Figure 3, B and D). Patients who received a combination of chemotherapy and PORT had improved DSS (P = .13) and OS (P = .0024) compared with those who did not receive either (Figure 3, E and F). The unadjusted and adjusted HR of each of the foregoing comparisons are provided in Table E6.
Figure 3.
Kaplan–Meier estimates of survival based on receipt of postoperative radiotherapy (PORT) (A, disease-specific survival [DSS]; B, overall survival [OS]), chemotherapy (C, DSS; D, OS), and multimodal therapy (E, DSS; F, OS) in patients with regional lymph nodal metastases. The shading indicates the range of 95% CI for the corresponding survival curve.
Discussion
In our analysis, tumor stage and histologic subtype were identified as significant risk factors for DSS and OS after surgical resection of thymoma, and PORT was associated with better DSS in patients with regional stage and type B2/B3 thymomas who underwent either thymectomy or thymomectomy. Multimodal treatment, including PORT and chemotherapy, improved the survival of patients with surgically resected thymoma with lymph node metastases.
Tumor Histology
Compared with type A/AB/B1 thymomas, type B2/B3 thymomas have predominantly atypical epithelial cells21 associated with more aggressive behavior and worse recurrence-free survival.8,22, 23, 24 A meta-analysis reviewing OS among the 5 histologic subtypes based on 2192 patients found that type B2/B3 thymomas were associated with worse OS compared with type A/AB/B1 thymomas.25 In fact, patients with type B2/B3 thymomas usually presented at a more advanced stage and had a higher rate of incomplete resection in the clinical setting.24,26 Owing to the differences in prognosis, it was recognized that thymoma may be distinguished into different subgroups: indolent histologic subtypes (type A/AB/B1) versus aggressive histologic subtypes (type B2/B3).8,22,27 Our data support these findings and indicate that different histologic subtypes potentially could aid clinicians in stratifying thymoma patients, tailoring therapeutic modalities, and surveillance. Up to now, tumor stage and histology have been adopted by several clinical guidelines, such as those of ESMO, China Anti-Cancer Association, and ThYmic MalignanciEs (founded in Italy), as considerations for PORT administration. For more precise prognostic stratification and personalized clinical decision making, histology may be incorporated into new staging system, similar to the American Joint Committee on Cancer’s staging of esophageal carcinoma.28
Extent of Resection
Independent of histologic subtype, we found that patients who underwent an extended thymectomy had worse survival compared to those who underwent thymomectomy or thymectomy (Table E2). We believe that one reason for this is because patients undergoing extended thymectomy were generally found to have more structural invasion before or during the operation. Thymomectomy is considered controversial as a curative approach for thymoma and likely is suitable only for Masaoka–Koga stage I patients without myasthenia gravis.5,7,29 It has been associated with a higher risk of local recurrence.19,20 In the setting of regional stage disease, the extent of resection should not be limited to the thymus according to NCCN, ESMO, and other national clinical guidelines,7,10,30 mainly because regional stage disease was characterized by extrathymic invasion. Consequently, the extent of resection remains uncertain in thymomectomy and thymectomy, and this uncertainty may be associated with a nonnegligible risk of local-regional recurrence.19,20,31 However, a large proportion of regional stage patients underwent thymectomy or thymomectomy in our analysis (n = 494; 72%). The reasons for this may be multifactorial, including surgeon preference and discordance between pathologic and radiologic stage. As reported by Moon and colleagues,32 the concordance rate was only moderate (kappa coefficient = 0.621), and thus the surgical decisions could be influenced by an inaccurate radiologic assessment to some extent.
PORT
In local stage thymomas, similar to what has been found in previous studies,12,15,33 our results demonstrate that PORT is not associated with better survival regardless of the histologic subtype. The prognostic impact of PORT in regional stage thymomas remains controversial, however. Previous studies using the SEER database have supported the role of PORT in regional stage thymomas.18,33,34 The difference between our analysis and others is our exclusion of patients who did not receive surgery or who received other non–curative intent surgery, such as debulking surgery, local tumor destruction, and local tumor excision. We found that although PORT was associated with better OS in regional stage thymomas, this association was not significant on multivariable analysis. Interestingly, results from Europe and the United States have reported PORT to be a favorable prognostic factor,35,36 whereas studies from Japan and China have not.13,37 Previous studies that validated the efficacy of PORT either grouped all thymomas together instead of categorizing them by specific stage or pathologic subtype or used Masaoka–Koga stage as the indication for PORT. In patients with regional stage, we assessed the prognostic impact of PORT among those presenting with different histologic subtypes and undergoing various degrees of surgical resection to better identify which populations would benefit from PORT. In patients with regional stage and type B2/B3 thymoma after thymomectomy or thymectomy, PORT was associated with improved DSS. This benefit was not seen in patients presenting with type A/AB/B1 thymomas. There remains a lack of consensus on the prognostic impact of PORT after varying degrees of surgical resection, stratified by histologic subtype. Subsequently, our data suggest that the use of PORT could be a remedial strategy for regional stage thymomas with an aggressive histology (type B2/B3) after thymectomy or thymomectomy.
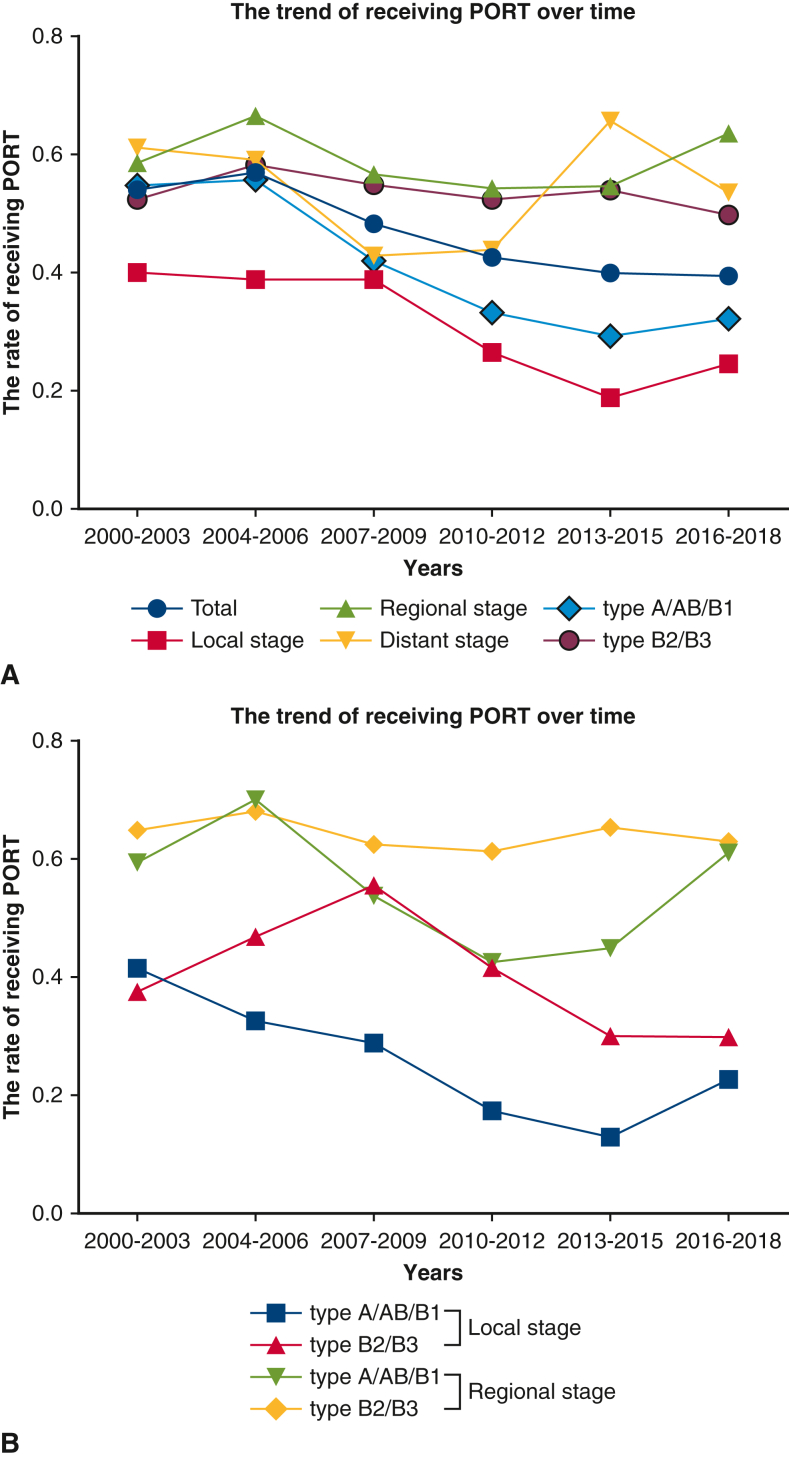
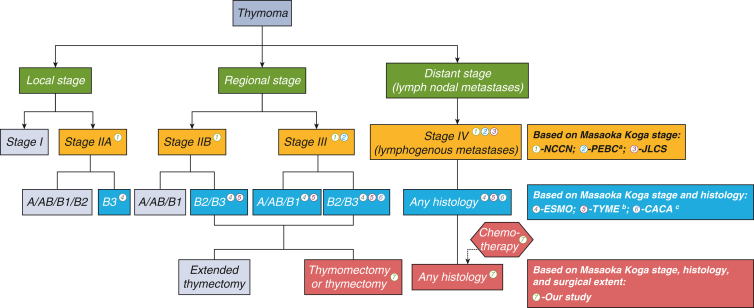
We also summarized the trend of receiving PORT over time. Based on the reduction in the use of PORT in local stage or type A/AB/B1 thymoma, we found that over time, specialists simultaneously considered tumor histology and stage for using PORT in clinical scenarios (Figure E3). PORT management is compared among the different guidelines in Figure 4. Compared with other clinical guidelines, our study may provide guidance for the administration of PORT with overall consideration of stage, tumor histology, and surgical extent (Figure 4).
Figure E3.
The trend of receiving PORT over time. A, Rate of PORT in the entire cohort, by stage or by histology. B, Rate of PORT grouped by stage and histology. PORT, Postoperative radiotherapy.
Figure 4.
Comparison of postoperative radiotherapy (PORT) management under R0 resection among different guidelines. The colored bubbles represent the patients in whom PORT may be recommended by guidelines. NCCN, National Comprehensive Cancer Network; JLCS, Japan Lung Cancer Society; ESMO, European Society for Medical Oncology. a, A program in evidence-based care (PEBC) was developed by Ontario Health (Cancer Care Ontario, Canada) suggesting that PORT is recommended for TNM stage III-IV thymomas. b, ThYmic MalignanciEs (TYME) was founded by the Italian collaborative group. c, The China Anti-Cancer Association (CACA) suggested that PORT be recommended for TNM stage II-IIIa thymomas with type B2/B3 and all TNM stage IV thymomas.
Thymoma with Lymphogenous Metastasis
The presence of lymph node metastases has been associated with a poor prognosis, and numerous studies have focused on the necessity of lymph node dissection or sampling during thymoma surgery.38, 39, 40 In our analysis, patients with lymph node metastases had the worst prognosis among the entire cohort and similar survival as patients with other metastases. Lymph node metastases were frequently observed in our patients with type B3 thymomas, as was also demonstrated in other studies.41, 42, 43 Only a few studies have assessed outcomes among patients with regional node metastases after thymoma resection. Weksler and colleagues38 reported that PORT improved survival in patients with regional node–positive disease (145 months vs 62 months), but the difference was not significant. In our study, PORT was an independent prognosticator of OS among patients with thymoma with lymph node metastases, and patients who received chemotherapy had better OS than those who did not, albeit not significantly so. Active multimodal treatment, including PORT and chemotherapy, may have a favorable prognostic role in treating surgically resected thymoma with lymph node metastases. It should be noted, however, that the sequence of surgical resection followed by chemotherapy was documented in only 17 patients and unknown in the remaining 9, which means that not every patient with lymph node metastases might have received postoperative chemotherapy in our analysis, and further study is needed.
Limitations
Our study has several limitations. First, the study is limited by the inherent shortcomings of any retrospective database research, including potential selection bias and limited data availability. Second, data on resection margins, chemotherapy agents, comorbidities, patient performance status, PORT techniques and target regions, sites of recurrence, and subsequent treatments were not available in the database, limiting our ability to adjust for these confounders. Third, the patients in our analysis were divided into only 3 groups based on stage from the SEER database, and the tumor stage reflects only the natural course of solid tumor invasion. Fourth, the database lacks details specifying which anatomic structures are invaded in patients presenting at a regional stage, and this heterogeneity limited our ability to explore the impact of PORT in detail. In the analysis for thymoma with lymphogenous metastasis, a major limitation is that the limited sample size constrained the statistical power to some extent. Finally, given our use of one database, it remains to be seen whether our conclusions can be applied to patients from other regions.
Conclusions
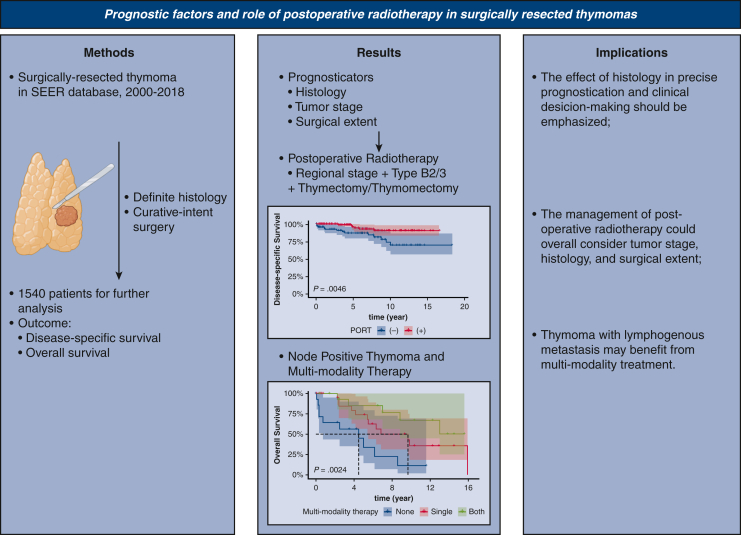
In conclusion (Figure 5), greater extent of invasion and histologic B2/B3 subtype portend worse DSS and OS in surgically resected thymomas, and the use of PORT was associated with better DSS in patients with type B2/B3 thymoma with regional invasion who received thymectomy or thymomectomy. Furthermore, the effect of histology on prognosis and personalized clinical decision making might indicate that histology could be integrated as a predictor into new staging systems. Active multimodal treatment including PORT and chemotherapy may improve survival in surgically resected thymoma patients with lymph node metastases.
Figure 5.
Graphical abstract showing the methods, results, and implications of our study. SEER, Surveillance, Epidemiology, and End Results; PORT, postoperative radiotherapy.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Drs Yang and Dai contributed equally to this work as co-first authors.
This research was supported by the National Natural Science Foundation of China (Grant 82172848), Shanghai Rising-Star Program (Grant 19QA1407400), Shanghai Municipal Education Commission “Chen Guang” project (19CG19), Shanghai “Rising Stars of Medical Talent” Youth Development Program Youth Medical Talents-Specialist Program, Shanghai Pulmonary Hospital Fund for Excellent Young Scholars (fkyq1908), and the Shanghai Pulmonary Hospital Fund (fkzr2105 and fkyq1908).
Appendix 1
Table E1.
Tumor staging comparing Masaoka–Koga/TNM stages with the stage groupings assigned using tumor information from SEER data
| Tumor stage | Masaoka–Koga stage | TNM stage 8 of TETs | Staging system from SEER database |
|---|---|---|---|
| Local stage | I-IIA | T1aN0M0-confined to thymus | Localized only (localized, NOS): confined to thymus, NOS; no mediastinal or pleura involvement or unknown if involved |
| Regional stage | IIB-III (with resectable structure invasion) | T1aN0M0-mediastinum fat-T3N0M0 | Regional by direct extension only: confined to thymus with mediastinal or pleural involvement; direct invasion of pericardium; brachiocephalic vein; chest wall; extrapericardial pulmonary artery or vein; lung; phrenic nerve; superior vena cava |
| Distant stage | III (with unresectable structure invasion)-IV | T4N0M0; TxN1-2M0 | Regional lymph node(s) involved only: ascending aorta/para-aortic; cervical (low anterior); hilar; internal mammary; lower jugular; mediastinal (lower, middle, NOS); paratracheal (lower, upper, NOS); perithymic/perithyroid/pericardial; phrenic (inferior, superior); precricoid/delphian; pretracheal/prevascular; subaortic/aortopulmonary window; subcarinal; supraclavicular/venous angle: confluence of internal jugular and subclavian vein; regional lymph node(s), NOS |
| TxNxM1a-b | Distant site(s)/lymph node(s) involved: distant site(s) (including further contiguous extension), including extrathoracic sites or separate pleural or pericardial nodule(s); distant lymph node(s), NOS; distant metastasis, NOS, including carcinomatosis, distant metastasis with or without distant lymph node(s), or with pleural or pericardial nodule(s) metastasis |
NOS, Not otherwise specified; SEER, Surveillance, Epidemiology, and End Results.
Table E2.
Univariable and multivariable analyses of DSS and OS in all patients with surgically resected thymoma
| Variables | DSS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||
| HR (95% CI) | P value | Adjusted HR (95% CI) | P value | HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Age | 1.009 (0.997-1.021) | .138 | 1.017 (1.004-1.03) | .011 | 1.043 (1.035-1.051) | <.001 | 1.047 (1.038-1.055) | <.001 |
| Sex (female, reference) | 1.039 (0.742-1.454) | .824 | 0.929 (0.661-1.306) | .671 | 1.1 (0.905-1.336) | .339 | 1.088 (0.893-1.326) | .401 |
| Race (Hispanic, reference) | 0.605 (0.363-1.007) | .053 | 0.548 (0.326-0.919) | .023 | 0.775 (0.556-1.079) | .131 | 0.642 (0.46-0.896) | .009 |
| Histologic subtype | ||||||||
| Type A/AB/B1 | 1 | 1 | 1 | 1 | ||||
| Type B2/B3 | 1.849 (1.314-2.603) | <.001 | 1.435 (1.008-2.044) | .045 | 1.331 (1.096-1.617) | .004 | 1.409 (1.153-1.723) | .001 |
| Tumor stage | ||||||||
| Local stage | 1 | 1 | 1 | 1 | ||||
| Regional stage | 4.106 (2.271-7.424) | <.001 | 3.711 (2.006-6.864) | <.001 | 1.444 (1.143-1.825) | .002 | 1.461 (1.139-1.875) | .003 |
| Distant stage | 12.044 (6.565-22.095) | <.001 | 7.92 (4.061-15.446) | <.001 | 2.667 (2.021-3.519) | <.001 | 2.551 (1.855-3.509) | <.001 |
| Extent of surgery | ||||||||
| Extended thymectomy | 1 | 1 | 1 | 1 | ||||
| Thymectomy | 0.293 (0.199-0.432) | <.001 | 0.544 (0.362-0.82) | .004 | 0.603 (0.48-0.758) | <.001 | 0.794 (0.62-1.017) | .067 |
| Thymomectomy | 0.437 (0.282-0.678) | <.001 | 0.759 (0.479-1.2) | .238 | 0.816 (0.63-1.057) | .124 | 0.995 (0.754-1.313) | .974 |
| PORT (no, reference) | 0.805 (0.574-1.129) | .209 | 0.573 (0.406-0.809) | .002 | 0.797 (0.655-0.969) | .023 | 0.709 (0.58-0.868) | .001 |
| Chemotherapy (none, reference) | 3.021 (2.147-4.25) | <.001 | 1.951 (1.333-2.854) | .001 | 1.297 (1.03-1.634) | .027 | 1.313 (1.019-1.691) | .035 |
| Cancer history (none, reference) | 1.604 (1.064-2.419) | .024 | 1.557 (1.004-2.414) | .048 | 1.673 (1.321-2.120) | <.001 | 1.16 (0.904-1.487) | .243 |
DSS, Disease-specific survival; OS, overall survival; HR, hazard ratio; PORT, postoperative radiotherapy.
Table E3.
Univariable and multivariable analyses of DSS and OS among regional stage thymoma patients
| Variable | DSS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||
| HR (95% CI) | P value | Adjusted HR (95% CI) | P value | HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Age | 1 (0.984-1.016) | .987 | 1.007 (0.99-1.024) | .404 | 1.034 (1.023-1.045) | <.001 | 1.04 (1.028-1.051) | <.001 |
| Female sex (reference) | 0.955 (0.597-1.528) | .847 | 0.911 (0.565-1.471) | .704 | 0.93 (0.707-1.223) | .602 | 0.974 (0.737-1.287) | .853 |
| Hispanic race (reference) | 0.495 (0.259-0.945) | .033 | 0.54 (0.275-1.058) | .073 | 0.745 (0.478-1.161) | .194 | 0.644 (0.408-1.017) | .059 |
| Histologic subtype | ||||||||
| A/AB/B1 | 1 | 1 | 1 | 1 | ||||
| B2/B3 | 1.596 (0.995-2.562) | .053 | 1.519 (0.942-2.449) | .087 | 1.415 (1.075-1.861) | .013 | 1.597 (1.208-2.113) | .001 |
| Extent of surgery | ||||||||
| Extended thymectomy | 1 | 1 | 1 | 1 | ||||
| Thymectomy | 0.424 (0.244-0.735) | .002 | 0.507 (0.289-0.891) | .018 | 0.794 (0.578-1.091) | .155 | 0.857 (0.618-1.189) | .356 |
| Thymomectomy | 0.844 (0.469-1.517) | .57 | 0.897 (0.492-1.636) | .723 | 1.075 (0.744-1.555) | .7 | 1.091 (0.748-1.591) | .651 |
| PORT (negative, reference) | 0.665 (0.416-1.062) | .088 | 0.691 (0.43-1.108) | .125 | 0.729 (0.554-0.959) | .024 | 0.78 (0.591-1.03) | .08 |
| Chemotherapy (none, reference) | 3.052 (1.9-4.903) | <.001 | 2.723 (1.668-4.445) | <.001 | 1.498 (1.1-2.041) | .01 | 1.639 (1.192-2.254) | .002 |
| Cancer history (none, reference) | 1.312 (0.688-2.501) | .41 | 1.347 (0.688-2.636) | .384 | 1.393 (0.959-2.024) | .082 | 1.01 (0.686-1.488) | .959 |
DSS, Disease-specific survival; OS, overall survival; HR, hazard ratio; PORT, postoperative radiotherapy.
Table E4.
Univariable and multivariable analyses of DSS among regional stage type B2/B3 thymoma patients who receive thymectomy or thymomectomy
| Variable | DSS |
|||
|---|---|---|---|---|
| Univariable |
Multivariable |
|||
| HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Age | 1.027 (0.994-1.061) | .109 | 1.036 (1-1.074) | .05 |
| Sex (female, reference) | 0.529 (0.201-1.393) | .197 | 0.48 (0.171-1.341) | .161 |
| Race (Hispanic, reference) | 0.562 (0.163-1.934) | .361 | 1.114 (0.265-4.681) | .883 |
| Histologic subtype | ||||
| Type B2 | 1 | 1 | ||
| Type B3 | 0.874 (0.355-2.155) | .771 | 0.798 (0.308-2.069) | .643 |
| Extent of surgery | ||||
| Thymectomy | 1 | 1 | ||
| Thymomectomy | 1.576 (0.634-3.921) | .328 | 1.365 (0.506-3.679) | .539 |
| PORT (no, reference) | 0.271 (0.103-0.714) | .008 | 0.268 (0.099-0.727) | .01 |
| Chemotherapy (none, reference) | 2.93 (1.177-7.293) | .021 | 3.759 (1.428-9.896) | .007 |
| Cancer history (none, reference) | 1.3 (0.375-4.51) | .679 | 1.032 (0.265-4.028) | .964 |
DSS, Disease-specific survival; HR, hazard ratio; PORT, postoperative radiotherapy.
Table E5.
Baseline characteristics of patients with and without lymph node metastases
| Variable | Regional lymph node negative/unknown (N = 1483)∗ | Regional lymph node positive (N = 57) | P value |
|---|---|---|---|
| Age, y, median (range) | 60 (13-89) | 59 (24-84) | .695 |
| Sex, n (%) | .094 | ||
| Male | 717 (48.3) | 34 (59.6) | |
| Female | 766 (51.7) | 23 (40.4) | |
| Race, n (%) | .695 | ||
| Hispanic | 154 (10.4) | 5 (8.8) | |
| Non-Hispanic | 1329 (89.6) | 52 (91.2) | |
| Cancer history, n (%) | .731 | ||
| No | 1249 (84.3) | 49 (86.0) | |
| Yes | 233 (15.7) | 8 (14.0) | |
| Histologic subtype, n (%) | <.001 | ||
| Type A | 183 (12.3) | 4 (7.0) | |
| Type AB | 393 (26.5) | 7 (12.3) | |
| Type B1 | 261 (17.6) | 10 (17.5) | |
| Type B2 | 307 (20.7) | 9 (15.8) | |
| Type B3 | 339 (22.9) | 27 (47.4) | |
| Extent of surgery, n (%) | <.001 | ||
| Extended thymectomy | 315 (21.2) | 27 (47.4) | |
| Thymectomy | 770 (51.9) | 18 (31.6) | |
| Thymomectomy | 398 (26.8) | 12 (21.1) | |
| PORT, n (%) | .107 | ||
| Yes | 811 (54.7) | 32 (56.1) | |
| No | 672 (45.3) | 25 (43.9) | |
| Chemotherapy, n (%) | <.001 | ||
| Yes | 257 (17.3) | 26 (45.6) | |
| No | 1226 (82.7) | 31 (54.4) | |
| Positive lymph nodes, n (%) | - | ||
| 1 | - | 26 (45.6) | |
| 2 | - | 6 (10.5) | |
| 4 | - | 1 (1.8) | |
| 5 | - | 2 (3.5) | |
| Unknown | - | 22 (38.6) |
Boldface indicates statistical significance.
PORT, Postoperative radiotherapy.
Including 280 patients with missing data on regional lymph nodal metastases status and 1 patient with missing cancer history data.
Table E6.
Unadjusted and adjusted HR of PORT, chemotherapy, and multimodal therapy in thymoma with lymph node metastases
| Comparison | DSS |
OS |
||
|---|---|---|---|---|
| HR (95% CI) | Adjusted HR (95% CI)∗ | HR (95% CI) | Adjusted HR (95% CI)∗ | |
| PORT(+) vs PORT(−) | 0.395 (0.146-1.071) | 0.290 (0.08-1.053) | 0.328 (0.147-0.730) | 0.372 (0.146-0.949) |
| Chemotherapy (+) vs chemotherapy (−) | 0.611 (0.229-1.634) | 0.705 (0.213-2.335) | 0.469 (0.207-1.062) | 0.843 (0.303-2.346) |
| Chemotherapy and PORT vs neither of them | 0.263 (0.066-1.040) | 0.202 (0.032-1.277) | 0.178 (0.058-0.544) | 0.296 (0.071-1.236) |
| Either chemotherapy or PORT vs neither of them | 0.485 (0.154-1.525) | 0.345 (0.082-1.445) | 0.352 (0.147-0.845) | 0.451 (0.151-1.343) |
DSS, Disease-specific survival; HR, hazard ratio; PORT, postoperative radiotherapy.
Controlling for age, sex, race, surgical extent, cancer history, and histologic subtype in a Cox proportional hazards model.
References
- 1.Carter B.W., Marom E.M., Detterbeck F.C. Approaching the patient with an anterior mediastinal mass: a guide for clinicians. J Thorac Oncol. 2014;9:S102–S109. doi: 10.1097/JTO.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad U. The eighth edition TNM stage classification for thymic tumors: what do I need to know? J Thorac Cardiovasc Surg. 2021;161:1524–1529. doi: 10.1016/j.jtcvs.2020.10.131. [DOI] [PubMed] [Google Scholar]
- 3.Siesling S., van der Zwan J.M., Izarzugaza I., Jaal J., Treasure T., Foschi R., et al. Rare thoracic cancers, including peritoneum mesothelioma. Eur J Cancer. 2012;48:949–960. doi: 10.1016/j.ejca.2012.02.047. [DOI] [PubMed] [Google Scholar]
- 4.Fang W., Fu J., Shen Y., Wei Y., Tan L., Zhang P., et al. Management of thymic tumors - consensus based on the Chinese alliance for research in thymomas multi-institutional retrospective studies. J Thorac Dis. 2016;8:641–645. doi: 10.21037/jtd.2016.03.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falkson C.B., Bezjak A., Darling G., Gregg R., Malthaner R., Maziak D.E., et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol. 2009;4:911–919. doi: 10.1097/jto.0b013e3181a4b8e0. [DOI] [PubMed] [Google Scholar]
- 6.Hamaji M., Ali S.O., Burt B.M. A meta-analysis of surgical versus nonsurgical management of recurrent thymoma. Ann Thorac Surg. 2014;98:748–755. doi: 10.1016/j.athoracsur.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Girard N., Ruffini E., Marx A., Faivre-Finn C., Peters S., Committee E.G. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v40–v55. doi: 10.1093/annonc/mdv277. [DOI] [PubMed] [Google Scholar]
- 8.Liu H., Gu Z., Qiu B., Detterbeck F.C., Roden A.C., Ruffini E., et al. A recurrence predictive model for thymic tumors and its implication for postoperative management: a Chinese alliance for research in thymomas database study. J Thorac Oncol. 2020;15:448–456. doi: 10.1016/j.jtho.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Gao L., Wang C., Fang W., Zhang J., Lv C., Fu S. Outcome of multimodality treatment for 188 cases of type B3 thymoma. J Thorac Oncol. 2013;8:1329–1334. doi: 10.1097/JTO.0b013e31829ceb50. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology. Thymomas and Thymic Carcinoma Version 1.2022. 2022. https://www.nccn.org/
- 11.Rimner A., Yao X., Huang J., Antonicelli A., Ahmad U., Korst R.J., et al. Postoperative radiation therapy is associated with longer overall survival in completely resected stage II and III thymoma-an analysis of the international thymic malignancies interest group retrospective database. J Thorac Oncol. 2016;11:1785–1792. doi: 10.1016/j.jtho.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes A.T., Shinohara E.T., Guo M., Mitra N., Wilson L.D., Rengan R., et al. The role of radiation therapy in malignant thymoma: a Surveillance, Epidemiology, and End Results database analysis. J Thorac Oncol. 2010;5:1454–1460. doi: 10.1097/JTO.0b013e3181e8f345. [DOI] [PubMed] [Google Scholar]
- 13.Omasa M., Date H., Sozu T., Sato T., Nagai K., Yokoi K., et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer. 2015;121:1008–1016. doi: 10.1002/cncr.29166. [DOI] [PubMed] [Google Scholar]
- 14.Zhou D., Deng X.F., Liu Q.X., Zheng H., Min J.X., Dai J.G. The effectiveness of postoperative radiotherapy in patients with completely resected thymoma: a meta-analysis. Ann Thorac Surg. 2016;101:305–310. doi: 10.1016/j.athoracsur.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 15.Singhal S., Shrager J.B., Rosenthal D.I., LiVolsi V.A., Kaiser L.R. Comparison of stages I–II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg. 2003;76:1635–1642. doi: 10.1016/s0003-4975(03)00819-1. [DOI] [PubMed] [Google Scholar]
- 16.Kondo K., Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg. 2003;76:1859–1864. doi: 10.1016/s0003-4975(03)01017-8. [DOI] [PubMed] [Google Scholar]
- 17.Forquer J.A., Rong N., Fakiris A.J., Loehrer P.J., Sr., Johnstone P.A. Postoperative radiotherapy after surgical resection of thymoma: differing roles in localized and regional disease. Int J Radiat Oncol Biol Phys. 2010;76:440–445. doi: 10.1016/j.ijrobp.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Lim Y.J., Kim H.J., Wu H.G. Role of postoperative radiotherapy in nonlocalized thymoma: propensity-matched analysis of Surveillance, Epidemiology, and End results database. J Thorac Oncol. 2015;10:1357–1363. doi: 10.1097/JTO.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 19.Guerrera F., Falcoz P.E., Moser B., van Raemdonck D., Bille' A., Toker A., et al. Thymomectomy plus total thymectomy versus simple thymomectomy for early-stage thymoma without myasthenia gravis: a European Society of Thoracic Surgeons Thymic Working Group Study. Eur J Cardio Thorac Surg. 2021;60:881–887. doi: 10.1093/ejcts/ezab224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa K., Yokoi K., Nakajima J., Tanaka F., Maniwa Y., Suzuki M., et al. Is thymomectomy alone appropriate for stage I (T1N0M0) thymoma? Results of a propensity-score analysis. Ann Thorac Surg. 2016;101:520–526. doi: 10.1016/j.athoracsur.2015.07.084. [DOI] [PubMed] [Google Scholar]
- 21.Marx A., Chan J.K., Coindre J.M., Detterbeck F., Girard N., Harris N.L., et al. The 2015 World Health Organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol. 2015;10:1383–1395. doi: 10.1097/JTO.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harnath T., Marx A., Strobel P., Bolke E., Willers R., Gripp S. Thymoma-a clinico-pathological long-term study with emphasis on histology and adjuvant radiotherapy dose. J Thorac Oncol. 2012;7:1867–1871. doi: 10.1097/JTO.0b013e3182745f73. [DOI] [PubMed] [Google Scholar]
- 23.Guerrera F., Rendina E.A., Venuta F., Margaritora S., Ciccone A.M., Novellis P., et al. Does the World Health Organization histological classification predict outcomes after thymomectomy? Results of a multicentre study on 750 patients. Eur J Cardio Thorac Surg. 2015;48:48–54. doi: 10.1093/ejcts/ezu368. [DOI] [PubMed] [Google Scholar]
- 24.Meurgey A., Girard N., Merveilleux du Vignaux C., Maury J.M., Tronc F., Thivolet-Bejui F., et al. Assessment of the ITMIG statement on the WHO histological classification and of the eighth TNM staging of thymic epithelial tumors of a series of 188 thymic epithelial tumors. J Thorac Oncol. 2017;12:1571–1581. doi: 10.1016/j.jtho.2017.06.072. [DOI] [PubMed] [Google Scholar]
- 25.Marchevsky A.M., Gupta R., McKenna R.J., Wick M., Moran C., Zakowski M.F., et al. Evidence-based pathology and the pathologic evaluation of thymomas: the World Health Organization classification can be simplified into only 3 categories other than thymic carcinoma. Cancer. 2008;112:2780–2788. doi: 10.1002/cncr.23492. [DOI] [PubMed] [Google Scholar]
- 26.Rena O., Papalia E., Maggi G., Oliaro A., Ruffini E., Filosso P., et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer. 2005;50:59–66. doi: 10.1016/j.lungcan.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Rea F., Marulli G., Girardi R., Bortolotti L., Favaretto A., Galligioni A., et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardio Thorac Surg. 2004;26:412–418. doi: 10.1016/j.ejcts.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 28.Rice T.W., Ishwaran H., Ferguson M.K., Blackstone E.H., Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12:36–42. doi: 10.1016/j.jtho.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa K., Asamura H., Sakurai H., Watanabe S.I., Tsuta K. Does the mode of surgical resection affect the prognosis/recurrence in patients with thymoma? J Surg Oncol. 2014;109:179–183. doi: 10.1002/jso.23499. [DOI] [PubMed] [Google Scholar]
- 30.Imbimbo M., Ottaviano M., Vitali M., Fabbri A., Leuzzi G., Fiore M., et al. Best practices for the management of thymic epithelial tumors: a position paper by the Italian collaborative group for ThYmic MalignanciEs (TYME) Cancer Treat Rev. 2018;71:76–87. doi: 10.1016/j.ctrv.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Ruffini E., Filosso P.L., Guerrera F., Lausi P., Lyberis P., Oliaro A. Optimal surgical approach to thymic malignancies: new trends challenging old dogmas. Lung Cancer. 2018;118:161–170. doi: 10.1016/j.lungcan.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Moon J.W., Lee K.S., Shin M.H., Kim S., Woo S.Y., Lee G., et al. Thymic epithelial tumors: prognostic determinants among clinical, histopathologic, and computed tomography findings. Ann Thorac Surg. 2015;99:462–470. doi: 10.1016/j.athoracsur.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 33.Patel S., Macdonald O.K., Nagda S., Bittner N., Suntharalingam M. Evaluation of the role of radiation therapy in the management of malignant thymoma. Int J Radiat Oncol Biol Phys. 2012;82:1797–1801. doi: 10.1016/j.ijrobp.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Weksler B., Shende M., Nason K.S., Gallagher A., Ferson P.F., Pennathur A. The role of adjuvant radiation therapy for resected stage III thymoma: a population-based study. Ann Thorac Surg. 2012;93:1822–1829. doi: 10.1016/j.athoracsur.2012.03.004. discussion 1828-9. [DOI] [PubMed] [Google Scholar]
- 35.Ruffini E., Detterbeck F., Van Raemdonck D., Rocco G., Thomas P., Weder W., et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardio Thorac Surg. 2014;46:361–368. doi: 10.1093/ejcts/ezt649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson M.W., Palma D.A., Camidge D.R., Jones B.L., Robin T.P., Sher D.J., et al. The impact of postoperative radiotherapy for thymoma and thymic carcinoma. J Thorac Oncol. 2017;12:734–744. doi: 10.1016/j.jtho.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q., Gu Z., Yang F., Fu J., Shen Y., Wei Y., et al. The role of postoperative radiotherapy for stage I/II/III thymic tumor-results of the ChART retrospective database. J Thorac Dis. 2016;8:687–695. doi: 10.21037/jtd.2016.03.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weksler B., Pennathur A., Sullivan J.L., Nason K.S. Resection of thymoma should include nodal sampling. J Thorac Cardiovasc Surg. 2015;149:737–742. doi: 10.1016/j.jtcvs.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 39.Clermidy H., Maury J.M., Collaud S., Drevet G., Ginoux M., Chalabreysse L., et al. Lymph node dissection in thymoma: is it worth it? Lung Cancer. 2021;157:156–162. doi: 10.1016/j.lungcan.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Hwang Y., Kang C.H., Park S., Lee H.J., Park I.K., Kim Y.T., et al. Impact of lymph node dissection on thymic malignancies: multi-institutional propensity score matched analysis. J Thorac Oncol. 2018;13:1949–1957. doi: 10.1016/j.jtho.2018.08.2026. [DOI] [PubMed] [Google Scholar]
- 41.Fang W., Wang Y., Pang L., Gu Z., Wei Y., Liu Y., et al. Lymph node metastasis in thymic malignancies: a Chinese multicenter prospective observational study. J Thorac Cardiovasc Surg. 2018;156:824–833.e1. doi: 10.1016/j.jtcvs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 42.Hwang Y., Park I.K., Park S., Kim E.R., Kang C.H., Kim Y.T. Lymph node dissection in thymic malignancies: implication of the ITMIG lymph node map, TNM stage classification, and recommendations. J Thorac Oncol. 2016;11:108–114. doi: 10.1016/j.jtho.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Brascia D., De Palma A., Schiavone M., De Iaco G., Signore F., Panza T., et al. Lymph nodes involvement and lymphadenectomy in thymic tumors: tentative answers for unsolved questions. Cancers. 2021;13:5085. doi: 10.3390/cancers13205085. [DOI] [PMC free article] [PubMed] [Google Scholar]