“We cannot solve our problems with the same thinking we used when we created them.”
–Albert Einstein
“For things to reveal themselves to us, we need to be ready to abandon our views about them.”
–Thich Nhat Hahn
Proposal for standard-risk neonates with HLHS. Hybrid approach for high-risk neonates as per individual institution's approach.
Central Message.
Rapid bPAB within 48 hours of birth will mitigate the reduction of systemic oxygen delivery due to circulatory maldistribution that occurs immediately during the transitional circulation in HLHS.
Since the description of hypoplastic left heart syndrome (HLHS) by Noonan and Nadas in 1958, and the seminal publication of surgical intervention by Norwood, Lang, and Hansen in 1982,1 outcomes have improved remarkably from a universally fatal lesion to one where there are now survivors in their fourth decade of life.2 Key elements in the maturation of surgical therapies and medical management have included modifications of the original Norwood operation, including techniques for arch reconstruction and options to provide pulmonary blood flow3; improved anesthesia and cardiopulmonary bypass (CPB)4; improved understanding of the multidistribution circulation5; hybrid palliation for high-risk patients6, 7, 8, 9, 10, 11, 12; a “deferred Norwood” strategy13 (bilateral pulmonary artery banding [PAB] and prostaglandin); improvements in interstage monitoring14,15; an interim superior cavopulmonary connection (SCPC)16; modifications of the Fontan operation17, 18, 19; and a better understanding of the longer-term consequences of the Fontan circulation/total cavopulmonary connection.20, 21, 22 Finally, although neonatal heart transplantation remains a viable surgical option, limited donor availability renders this strategy as a primary mode of surgical therapy impractical for the majority of patients.
In the context of these remarkable improvements in the past 40+ years, several management controversies currently exist and are subject to considerable debate and disagreement among practitioners caring for these neonates—both between and within cardiac programs. Most importantly, these include type of initial procedure (hybrid vs Norwood); optimal timing of the Norwood operation; practices to maximize systemic oxygen delivery, both before and after surgery; and the routine use of delayed sternal closure, among others. Many of these controversies have evolved due to the unstable physiology present, particularly during the 2 most vulnerable periods for these babies—immediately after birth and immediately after surgery.
Current Results of Intervention for HLHS and Variants
It must be emphasized that any comparison of surgical mortality rates is confounded by the risk profile of the patients who undergo the various surgical options. With this important caveat in mind, the cumulative mortality rate for the Fontan pathway—the classic Norwood operation during the neonatal period, combining early (∼10%),23 interstage (∼10%),2 SCPC/bidirectional Glenn, and Fontan (1%-6% and 1%-3%) have resulted in longer-term survival rates that have plateaued around 60%.3,24,25,E1 The hybrid operation, used at some centers as a primary procedure for all patients, as well as at many centers for patients at increased risk, has been reported at approximately 78% survival at 10 years (see Table 1).E13, E14, E15 The deferred Norwood strategy, most commonly utilized in Japan, has been reported to have a 6-year transplant-free survival rate of 84.5%.E16
Table 1.
Key outcomes of Norwood and hybrid stage I
| Norwood procedure | Hybrid stage I | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | STSE2 7/1/16-6/30/20 |
PC4∗,E3 1/18-12/21 |
NPCQIC†,E4 | SVR trialE5, E6, E7 | Other literatureE8, E9, E10, E11 | STS | PC4E3 1/18-12/21 |
NPCQIC∗,‡,E4 | Other literature12,E8,E9,E12 |
| Hospital mortality (%) | 12 (0-100) | 10.5 | 4.7 | 16 | 26 | 15 | 1.8-13.2 | ||
| Unplanned reintervention (%) | Surgical 16% Catheterization 5.4 |
Surgical 15% Catheterization 16 |
Surgical Range 3-40 Catheterization Range 6-20 |
Surgical 22 Catheterization 6.5 |
Surgical 15% Catheterization 16 |
||||
| Cardiac arrest (%) | 14 | 15 | 18 | 23 | 15 | ||||
| ECMO (%) | 17 | 10 | 10 | 19 | 10 | ||||
| Renal replacement therapy (%) | 3.0 | 3.6 | 5.6 | 3.6 | |||||
| Neurological injury (%) | 7.9 | 6.9 | 7.90 | ||||||
| Seizure (%) | 11 | 15 | |||||||
| Stroke (%) | 6.6 | 9.0 | |||||||
| Vocal cord injury (%) | 21 | 9-34 | 14 | 20 | |||||
| Nasogastric or gastrostomy tube at discharge (%) | 61 | 68 | 28 | 61 | |||||
| Tracheostomy (%) | 2.2 | 3.4 | |||||||
| Postoperative hospital length of stay (d) | Mean 59 | Median 47 | Note: 9.4% in hospital until SCPC | Median 60 | |||||
STS, Society of Thoracic Surgeons; PC4, Pediatric Cardiac Critical Care Consortium; NPCQIC, National Pediatric Cardiology Quality Improvement Collaborative; SVR, single ventricle reconstruction; ECMO, extracorporeal membrane oxygenation; SCPC, superior cavopulmonary connection.
PC4 specific definitions: newer PC4 sites contributed data for less than the specified date range; Norwood procedure and hybrid stage I included if index operation; cardiac arrest, ECMO, renal replacement therapy, neurologic injury, vocal cord injury all postoperative; renal replacement therapy only for acute kidney injury not prophylaxis; vocal cord injury before February 1, 2019, only diagnosed while still in cardiac intensive care unit, diagnosis is endoscopic; hypoxic ischemic encephalopathy included with stroke.
NPCQIC data combines Norwood and hybrid data, and excludes anyone who transitioned to biventricular physiology, or who was deemed not a candidate for Comprehensive Stage II palliation. Mortality is Norwood-specific.
Mortality is hybrid-specific.
In addition to among the highest surgical mortality rates in congenital cardiac surgery, most of these patients typically have long hospitalizations, with the median length of hospitalization between >7-8 weeks,E17, E18 including some with longer lengths of stay that include the SCPC on the same admission. Complications are common, including consequences of invasive catheters, mechanical ventilation, injury to the recurrent laryngeal and phrenic nerves, infection, necrotizing enterocolitis, unplanned procedures, and many more (see Table 1). In addition to these short-term consequences, these complications can also contribute to long-term challenges such as ventricular dysfunction,E19, E20, E21, E22 atrioventricular valve regurgitation,E23, E24, E25 vascular access limitations, neurodevelopmental challenges,E26, E27, E28 chronic kidney disease,E29, E30, E31, E32, E33 hypertension,E34 proteinuria,E34 gastrointestinal complications,E8, E35, E36, E37 and abnormal lung function.E20, E38, E39 Considering the high mortality and morbidity subsequent to surgical intervention for HLHS, the need for a fundamental change in the management of these patients should be considered, utilizing the components of all 3 currently used approaches, with a specific emphasis on minimizing morbidity as well as improving short-term survival.
Some patient-related risk factors for mortality and short- and long-term morbidity are nonmodifiable, such as low weight, prematurity, additional congenital anomalies, and genetic syndromes. However, there are 2 particularly high-risk periods for a neonate with HLHS where a change in strategy is modifiable and may improve outcome: during the transitional circulation and during the early postoperative period. The currently utilized care strategies during these time frames vary considerably across care teams, and lend themselves to both standardization and patient-specific application.
Thus, given the suboptimal short- and long-term results, we are proposing a fundamental change in strategy to minimize morbidity and mortality during these two time frames, utilizing currently available techniques: rapid bilateral PAB (bPAB) during the first 24 to 48 hours of life with continued use of Prostaglandin, and abandoning the Norwood procedure in the neonatal period whenever possible. This approach is proposed, in part, due to a better understanding of the vulnerability during the transitional circulation, coupled with an in-depth understanding of the developmental biology of the heart as well as all other organ systems in the neonate.
Developmental Physiology Following Birth in Neonates With Structurally Normal Hearts
Heart
After birth, following the fall in pulmonary vascular resistance (PVR) and the closure of the ductus arteriosus, the afterload of the right ventricle (RV) decreases significantly, the RV dominance regresses rapidly in the first 48 hours of life, and RV mass is expected to stabilize around age 3 to 4 months.E40, E41 During the first 2 weeks after birth, as pressure and volume workload change, the systemic left ventricle accommodates pressure changes via cellular hyperplasia and angiogenesis (cardiomyocyte proliferation) rather than hypertrophy. Animal studies also suggest a maximal enhancement in contractility and relaxation within the first 8 postnatal days.E42 In summary, the ability to respond to an afterload stress with physiologic hyperplasia and angiogenesis (rather than pathologic hypertrophy) is limited to the very early neonatal period, during the transitional circulation.
Brain
The production and migration of neurons are largely prenatal events, reaching a peak at 28 weeks’ gestation.E43 However, proliferation and migration of glial progenitors and differentiation of astrocytes and oligodendrocytes continues for an extended period after birth.E44 Apoptosis begins in the second trimester, but continues throughout the first year of life, as does synaptogenesis (ie, connectivity), oligodendrogenesis, axon growth, and myelination.E45 These metabolically active areas of brain growth are particularly susceptible to hypoxic-ischemic injury.
Kidneys
At birth, there are rapid changes in plasma flow to the kidney, filtration at the glomerular level (ie, glomerular filtration rate [GFR], drug metabolism, and toxin elimination). Renal function is low at birth, (GFR ∼20-39 mL/min/1.73 m2) with a continued rise during the first 4 weeks.E46, E47 Factors contributing to the maturational increase in GFR include increase in filtration surface area and glomerular permeability as well as an increase in arterial pressure and renal blood flow. The literature shows high variability in the estimates of both effective renal plasma flow as well as GFR.E48, E49, E50 In general, GFR doubles during the first 5 days after birth, from 19.6 to 40.6 mL/min per 1.73 m2, and then more gradually increases to 59.4 mL/min per 1.73 m2 by age 4 weeks. Adult levels of GFR may not be reached until 1 year of age.E51, E52, E53
It is beyond the scope of this article to discuss all of the other important functions of neonatal kidneys, such as maximum osmolality concentration, salt and fluid homeostasis, and response to injury; suffice it to say that the neonatal kidneys are considerably less developed and resilient compared with the renal function seen in infants.
Gut
The first 1 to 2 weeks after birth are crucial in the development of the intestine, gut microbiota, immune tolerance, and fluid and nutritional transport. The gut undergoes profound changes shortly after birth, particularly with the change from maternally derived nutrition to the introduction of colostrum and breast milk. In piglets, the intestinal DNA concentration increases between 84% and 154% in 24 hours.E54 By day three, mucosal mass increases by 115%, intestinal length by 24%, and intestinal diameter by 15%.E55
In a human fetus, the intestine is filled with sterile amniotic fluid and the initiation of microbial colonization begins with oral intake of milk on postnatal day one. This first exposure to microorganisms and environmental endotoxins is followed by a crucial sequence of active events necessary for immune tolerance and homeostasis. The initial gut colonization influences the future gut microbiota, and the gut microbiome demonstrates accelerated maturation during the first year.E56, E57 During the first 2 weeks, the intestinal epithelium develops to tolerate colonizing bacteria, such as secretion of neonate-specific antimicrobial peptides and constitutive active downregulation of the innate immune TLR4 pathway.E58 Goblet cells, which serve an innate barrier function, do not reach the tips of the villi for at least 1 week after birth. There is a close interplay between the major cell types lining the intestine, nutrients, and the microbiota, which are all constituents of the intestinal ecosystem.E59 Thus, the risk of intestinal injury and permeability are at their highest shortly after birth, and are particularly susceptible to abnormalities in blood flow, oxygen delivery, and lack of enteral nutrition.
Lung
The lung parenchyma and vasculature are both immature at birth. Although the number of airway generations is nearly complete at birth, the morphology of the pulmonary parenchyma subsequently transforms greatly in later infancy and childhood. Only approximately 85% of the alveoli are formed after birth,E60 and the lung parenchyma contains several generations of transitory ducts that end in saccules, which will later develop into alveoli. Parallel to the augmented alveoli formation, alveolar surface area increases significantly within the first year of life and the increased number of alveoli per unit area is also confined to early infancy.E61 In addition to the gas-exchange function of the lungs, the extremely rapid changes in pulmonary vascular resistance have been well described, and will not be repeated here. Although not measured in human newborn infants, the fall in PVR is measured in seconds—not hours or days—in newborn lambs.26
The Fetal and Early Transitional Circulation in Neonates With HLHS
During fetal life, the combined cardiac output (CCO) from the single RV is only mildly decreased compared with the CCO in a fetus with a structurally normal heart.E62, E63 In contrast to a neonate with a structurally normal heart where the RV and left ventricle outputs are equal after ductal closure, the RV must support the multidistribution cirulation: systemic blood flow, plus the pulmonary blood flow, plus any tricuspid regurgitant volume (if present).5 Thus, in HLHS, CCO increases rapidly during the first few days of life, by a combined increase in stroke volume and heart rate.E64 In contrast to a normal heart, the relative contribution and changes from hyperplasia to hypertrophy early after birth in HLHS is unknown. However, this acute change leads to a volume-mass mismatch, and we speculate that this may be a factor contributing to an increase in tricuspid regurgitation in some neonates. Due to the concomitant changes in heart rate and stroke volume, the volume workload of the RV increases immediately, whereas the acquisition of ventricular mass is delayed. Although not studied in neonates with HLHS, rapid increases in RV mass due to increased afterload have been demonstrated within 4 days in lambs,E65 and a similar finding of rapid mass acquisition of the left ventricle has been observed in neonates undergoing the rapid 2-stage arterial switch operation for transposition of the great arteries.E66
Pulmonary blood flow continues to increase as PVR falls, whereas the opposite occurs with systemic blood flow. Recent work by Eckersley and colleaguesE64 demonstrated that systemic blood flow is abnormally low within 6 hours of postnatal life, and continuously decreases to about half of the normal values during the first 4 days of postnatal life. During this period, significantly higher middle cerebral artery and celiac artery pulsatile indexes are also observed, consistent with elevated cerebral and intestinal vascular resistance and reduced cerebral and intestinal blood flow, confirmed by decreased flow in the superior vena cava.E64
Indeed, cerebral blood flow and oxygen delivery falls, and cerebral oxygen extraction rises daily during the transition while awaiting surgery,E67, E68 and most likely contributes to the long-term neurodevelopmental disabilities so common in this population.E69, E70
In neonates with HLHS, although systemic blood flow is low almost immediately after birth, the clinical manifestations of low systemic blood flow—including tachycardia, tachypnea, lower blood pressure, rising lactic acid values, falling near-infrared spectroscopy values, increased risk of necrotizing enterocolitis, and decreasing urine output—only become apparent over the subsequent 2 to 7 days or so.E64, E71, E72, E73, E74 Concurrently, the increased pulmonary blood flow can result in tachypnea, carbon dioxide retention, respiratory failure, and pulmonary edema. A variety of interventions can be performed to redistribute the combined cardiac output preferentially to the systemic circulation, including endotracheal intubation and mechanical ventilation with sedation and neuromuscular blockade to decrease oxygen consumption, increasing positive end-expiratory pressure, and other techniques to increase PVR such as subatmospheric oxygen and hypercarbic gas mixtures, blood transfusions to increase oxygen delivery, and vasoactive agents such as milrinone for reducing systemic vascular resistance. All of these interventions carry potential morbidity, and very few of these have been studied under randomized conditions during the preoperative period. Nonetheless, a number of centers routinely use these strategies during the preoperative period.
Relationship of Developmental Immaturity, Preoperative Management of the Transitional Circulation, and CPB on Postoperative Outcomes
In neonates and infants, a low cardiac output state has been demonstrated for at least 24 to 36 hours after surgery.E75, E76 In addition to the low GFR due to preexisting immaturity of the kidneys discussed above, GFR has been shown to be even lower (<30 mL/min/m2) after CPB in patients with biventricular circulations. Although GFR has not been measured following stage 1 Norwood surgery, the incidence of acute kidney injury is high. Central nervous system abnormalities are common, such as a 10% to 20% risk of seizures,E77 increased white matter injury,E78 stroke,E79, E80 and hemorrhageE80as described in Table 1.
From a developmental perspective, it is not surprising that—in addition to among the highest surgical mortality rates in the entire spectrum of congenital heart disease—there is a significant amount of cumulative morbidity following either the hybrid or Norwood procedures in the neonate. In particular, the effects of the Norwood operation with CPB, and either regional cerebral perfusion or hypothermic circulatory arrest, include a considerable risk to the myocardium, brain, kidneys, and gut. The Norwood procedure during the early neonatal period superimposes a period of myocardial ischemia as well as the systemic effects of CPB on immature organs with little to no improvement in the underlying physiology—a circulation with complete mixing, hypoxemia, and maldistribution of flow.5 It is therefore not surprising that there is a high frequency of low cardiac output syndrome, arrhythmias, cardiac arrest, extracorporeal membrane oxygenation, anasarca, acute kidney injury, seizures, and necrotizing enterocolitis. Enteral/oral feeding is often delayed, and tube feedings are necessary after discharge for at least half of the hospital survivors. The median length of hospital stay is about 2 months, but may be much longer, considering the superior cavopulmonary connection takes place during the same hospitalization in ∼10% of patients (see Table 1). Familial well-being and in particular maternal mental health may also be seriously compromised, with lifelong effects.
Proposal
We propose early bPAB within 24 to 48 hours of life and deferral of the Norwood procedure beyond the neonatal period. We speculate that near-immediate or rapid bPAB will significantly improve the circulatory maldistribution, which should then allow for improved organ maturation, including appropriate ventricular maturation and myocardial hyperplasia/hypertrophy,E66, E81 less tricuspid regurgitation,E82 and less congestive heart failure during the early neonatal period.E83 Additional benefits will include the early institution of neurodevelopmental care and maternal bonding,E84 a lower risk of seizures after CPB,E85 improved family well-being, an increase in renal blood flowE83 and GFR, oral feeding, improved nutritional status, maturation of the immune system, and more.
Compared with bPAB in a classic hybrid approach, with an anticipated period of 4 to 6 months before a comprehensive stage 2, the bPAB for a deferred Norwood should be considerably more restrictive if a period of only 4 to 6 weeks of banding is anticipated. This tight bPAB approach is likely to improve circulatory maldistribution more rapidly, but result in earlier progression of hypoxemia as the baby grows.
Given the emerging data on the rapidity of onset of end-organ ischemia during the early transitional circulation,E64 we believe that rapid restriction of the pulmonary flow is warranted. This strategy should minimize the documented fall in global systemic blood flow, and improve oxygen delivery to the coronary arteries, myocardium, brain, kidney, and gut.
Additionally, rapid bPAB will provide near-normal systemic oxygen delivery during the time that is necessary to evaluate the anatomy and myocardial function, the brain, all other organs, perform a genetic consultation if indicated, further counsel parents and obtain informed consent, while decision making is undertaken for the next steps. We speculate that this approach will decrease the likelihood of intensive care unit interventions frequently employed before surgery; such as intubation, mechanical ventilation, change in inspired gases, inodilators, lack of enteral feeding, central venous access, and parenteral nutrition. There is evidence that the performance of bPAB (age 5-7 days), even in a heterogeneous population of neonates with HLHS, can be accomplished with early survival rates above 95% and extremely low morbidity.12 There is every reason to believe that even earlier banding will have similar success.
Compared with the recovery from a Norwood procedure in the first week of life, postponing the Norwood procedure with CPB and myocardial ischemia beyond the neonatal period allows the recovery to take place in a baby with more physiologic stability, having received healthy newborn infant care, and developing organ maturity. In a small series, with a deferred Norwood following bPAB at a mean of age 7 days, Ota and colleaguesE86 reported lower peak lactate values and higher urine output after the deferred Norwood compared with those who had a primary Norwood during the neonatal period.
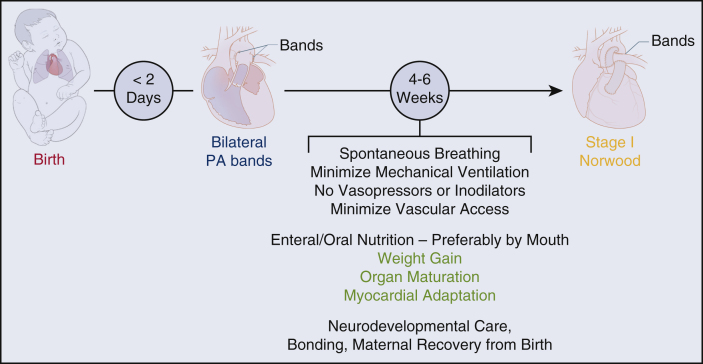
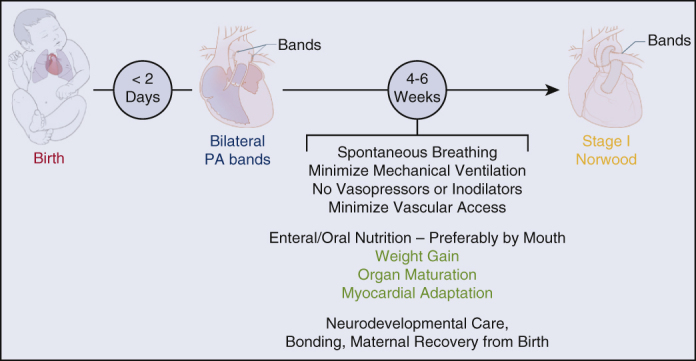
Finally, it is important to emphasize that, following rapid bPAB, either the hybrid approach or deferred Norwood approach may still be taken, no “bridges are burned”. The next-step decision can be based on patient factors, cardiologist or surgeon preference, and/or institutional experience (see Figure 1).
Figure 1.
Proposed management strategy for neonates with hypoplastic left heart syndrome and standard risk profile. For high-risk patients, a hybrid approach is recommended (stent as neonate, comprehensive stage 2 at age 4-6 months). PA, Pulmonary artery.
Potential Consequences and Unanswered Questions
Our proposed approach is, admittedly, theoretical at this point, and there are a number of unknowns to be considered. By surgically placing PABs in all patients, the Norwood by definition becomes a re-do operation. Will this result in a more technically challenging procedure? Will surgical adhesions result in more bleeding, or more frequent injury to the recurrent laryngeal and/or phrenic nerves? Will CPB times be increased? Will the cerebral and coronary circulations be adequately maintained on prostaglandin, particularly with aortic atresia? Although there are the many proposed benefits from early PAB and delayed Norwood operation described above, what will be the unintended consequences of 4 to 6 weeks in hospital? All of these are testable hypotheses.
It should be mentioned that an additional possibility to achieve the same physiologic result as we propose would be the use of catheter-based PA flow restrictors. This approach would allow the Norwood to be performed as a first operation/sternotomy; however, the effects of the flow restrictors on physiology and PA growth would need to be similarly assessed. We need consistent and standardized assessments of cardiac, cerebral, renal, and intestinal function before and after the Norwood procedure as these new approaches on a heterogeneous patient population are implemented.
Conclusions and Next Steps
The current short- and long-term results of surgical intervention for HLHS, although gratifying in the historical sense, remain unsatisfying. We can and must do better with respect to short- and long-term survival rates, minimizing cumulative hospital morbidity, and improving quality of life for patients and their families over the long-term. As we learn more about the fetal circulation in HLHS, the immediate consequences of the transitional circulation, and revisit the well-described developmental immaturity present at birth, we believe that our proposal of rapid bPAB within 48 hours and a delayed Norwood procedure are the next incremental steps in the management of HLHS.
As we continue to search for changes in medical and surgical strategies to improve the outcome for babies born with HLHS, we must emphasize that correct, best-practice, patient-specific approaches will only be determined through short- and long-term multicenter collaboration. No single center will have sufficient patient volume to definitively answer these questions. One size will not fit all.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank medical illustrator Sofia S. Hanabergh, MSc, for the Figure 1.
Footnotes
Drs Wernovsky and Ozturk contributed equally to the article.
Drs Wernovsky and Ozturk share the co-first authorship.
References
- 1.Norwood W.I., Lang P., Hansen D.D. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983;308:23–26. doi: 10.1056/NEJM198301063080106. [DOI] [PubMed] [Google Scholar]
- 2.Ghanayem N.S., Allen K.R., Tabbutt S., Atz A.M., Clabby M.L., Cooper D.S. Interstage mortality after the Norwood procedure: results of the multicenter single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newburger J.W., Sleeper L.A., Gaynor J.W., Hollenbeck-Pringle D., Frommelt P.C., Li J.S., et al. Transplant-free survival and interventions at 6 years in the SVR trial. Circulation. 2018;137:2246–2253. doi: 10.1161/CIRCULATIONAHA.117.029375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg C.S. Deep hypothermic circulatory arrest and regional cerebral perfusion in pediatric cardiac surgery. Prog Pediatr Cardiol. 2010;29:67–71. [Google Scholar]
- 5.Wernovsky G., Tweddell J.S. In: Anderson’s Pediatric Cardiology. Fourth Edition. Wernovsky G., Anderson R.H., Kumar K., Mussatto K.A., Redington A.N., Tweddell J.S., editors. Elsevier Publishing; Philadelphia: 2020. Physiologic Principles to Maximize Outcome in Patients With a Functionally Univentricular Heart. Chapter 70, pp 1261-1272. [Google Scholar]
- 6.Ozturk M., d’Udekem Y., Yerebakan C. Hypoplastic left heart syndrome-pushing the limits. J Thorac Cardivoasc Surg Tech. 2022;13:165–166. doi: 10.1016/j.xjtc.2022.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yerebakan C., Tongut A., Ozturk M., Ceneri N.M., d'Udekem Y. The technique of bilateral pulmonary artery banding in high-risk patients with hypoplastic left heart syndrome. Operat Tech Thorac Cardiovasc Surg. June 12, 2022 doi: 10.1053/j.optechstcvs.2022.06.004. [Epub ahead of print]. [DOI] [Google Scholar]
- 8.Ceneri N.M., Desai M.H., Tongut A., Ozturk M., Ramakrishnan K., Staffa S.J., et al. Hybrid strategy in neonates with ductal-dependent systemic circulation and multiple risk factors. J Thorac Cardiovasc Surg. 2022;164:1291–1303.e6. doi: 10.1016/j.jtcvs.2021.11.103. [DOI] [PubMed] [Google Scholar]
- 9.Akintuerk H., Michel-Behnke I., Valeske K., Mueller M., Thul J., Bauer J., et al. Stenting of the arterial duct and banding of the pulmonary arteries: basis for combined Norwood stage I and II repair in hypoplastic left heart. Circulation. 2002;105:1099–1103. doi: 10.1161/hc0902.104709. [DOI] [PubMed] [Google Scholar]
- 10.Galantowicz M., Cheatham J.P., Phillips A., Cua C.L., Hoffman T.M., Hill S.L., et al. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg. 2008;85:2063–2071. doi: 10.1016/j.athoracsur.2008.02.009. discussion 2070-1. [DOI] [PubMed] [Google Scholar]
- 11.Michel-Behnke I., Akintürk H., Schranz D. Fate of the stented arterial duct. Circulation. 2000;102:E178. doi: 10.1161/01.cir.102.22.e178. [DOI] [PubMed] [Google Scholar]
- 12.Schranz D., Bauer A., Reich B., Steinbrenner B., Recla S., Schmidt D., et al. Fifteen-year single center experience with the “Giessen Hybrid” approach for hypoplastic left heart and variants: current strategies and outcomes. Pediatr Cardiol. 2015;36:365–373. doi: 10.1007/s00246-014-1015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoashi T., Imai K., Okuda N., Komori M., Kurosaki K., Ichikawa H. Intermediate-term outcomes of deferred Norwood strategy. Eur J Cardio Thorac Surg. 2022;62 doi: 10.1093/ejcts/ezac099. [DOI] [PubMed] [Google Scholar]
- 14.Ghanayem N., Hoffman G., Mussatto K., Cava J., Frommelt P., Rudd N., et al. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg. 2003;126:1367–1375. doi: 10.1016/s0022-5223(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 15.Oster M.E., Kelleman M., McCracken C., Ohye R.G., Mahle W.T. Association of digoxin with interstage mortality: results from the pediatric heart network single ventricle reconstruction trial public use dataset. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norwood W.I., Jacobs M.L. Fontan's procedure in two stages. Am J Surg. 1993;166:548–551. doi: 10.1016/s0002-9610(05)81151-1. [DOI] [PubMed] [Google Scholar]
- 17.Bridges N.D., Mayer J.E., Lock J.E., Jonas R.A., Hanley F.L., Keane J.F., et al. Effect of baffle fenestration on outcome of the modified Fontan operation. Circulation. 1992;86:1762–1769. doi: 10.1161/01.cir.86.6.1762. [DOI] [PubMed] [Google Scholar]
- 18.de Leval M.R., Kilner P., Gewillig M., Bull C., McGoon D.C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. J Thorac Cardiovasc Surg. 1988;96:682–695. [PubMed] [Google Scholar]
- 19.Laks H., Ardehali A., Grant P.W., Permut L., Aharon A., Kuhn M., et al. Modification of the Fontan procedure. Superior vena cava to left pulmonary artery connection and inferior vena cava to right pulmonary artery connection with adjustable atrial septal defect. Circulation. 1995;91:2943–2947. doi: 10.1161/01.cir.91.12.2943. [DOI] [PubMed] [Google Scholar]
- 20.d'Udekem Y., Iyengar A.J., Galati J.C., Forsdick V., Weintraub R.G., Wheaton G.R., et al. Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation. 2014;130:S32–S38. doi: 10.1161/CIRCULATIONAHA.113.007764. [DOI] [PubMed] [Google Scholar]
- 21.Dennis M., Zannino D., du Plessis K., Bullock A., Disney P.J., Radford D.J., et al. Clinical outcomes in adolescents and adults after the Fontan procedure. J Am Coll Cardiol. 2018;71:1009–1017. doi: 10.1016/j.jacc.2017.12.054. [DOI] [PubMed] [Google Scholar]
- 22.Iyengar A.J., Shann F., Cochrane A.D., Brizard C.P., d’Udekem Y. The Fontan procedure in Australia: a population-based study. J Thorac Cardiovasc Surg. 2007;134:1353–1354. doi: 10.1016/j.jtcvs.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Mascio C.E., Irons M.L., Ittenbach R.F., Gaynor J.W., Fuller S.M., Kaplinski M., et al. Thirty years and 1663 consecutive Norwood procedures: has survival plateaued? J Thorac Cardiovasc Surg. 2019;158:220–229. doi: 10.1016/j.jtcvs.2018.12.117. [DOI] [PubMed] [Google Scholar]
- 24.Mayer J.E., Hill K., Jacobs J.P., Overman D.M., Kumar S.R. The society of Thoracic Surgeons Congenital Heart Surgery Database: 2020 update on outcomes and research. Ann Thorac Surg. 2020;110:1809–1818. doi: 10.1016/j.athoracsur.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Iyengar A.J., Winlaw D.S., Galati J.C., Wheaton G.R., Gentles T.L., Grigg L.E., et al. The extracardiac conduit Fontan procedure in Australia and New Zealand: hypoplastic left heart syndrome predicts worse early and late outcomes. Eur J Cardio Thorac Surg. 2014;46:465–473. doi: 10.1093/ejcts/ezu015. discussion 473. [DOI] [PubMed] [Google Scholar]
- 26.Dawes G.S., Mott J.S., Widdicombe J.G., Wyatt D.G. Changes in the lungs of the newborn lamb. J Physiol. 1953;121:141–162. doi: 10.1113/jphysiol.1953.sp004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
E-References
- Ohye R.G., Sleeper L.A., Mahony L., Newburger J.W., Pearson G.D., Lu M., et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.R., Mayer J.E., Overman D.M., Shashidharan S., Wellnitz C., Jacobs J.P. The society of Thoracic Surgeons Congenital Heart Surgery Database: 2021 Update on outcomes and Research. Ann Thorac Surg. 2021;112:1753–1762. doi: 10.1016/j.athoracsur.2021.10.002. [DOI] [PubMed] [Google Scholar]
- Pediatric Cardiac Critical Care Consortium. Improving outcomes and quality through collaboration. https://pc4quality.org/
- Luna A.O., Kuhnell P., Wooton S., Handler S.S., Wright G., Hammel J., et al. Factors associated with inability to discharge after stage 1 palliation for single ventricle heart disease: an analysis of the national Pediatric Cardiology Quality Improvement Collaborative Database. Pediatr Cardiol. 2022;43:1298–1310. doi: 10.1007/s00246-022-02852-w. [DOI] [PubMed] [Google Scholar]
- Tabbutt S., Ghanayem N., Ravishankar C., Sleeper L.A., Cooper D.S., Frank D.U., et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: a report from the pediatric heart network single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:882–895. doi: 10.1016/j.jtcvs.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohye R.G., Schonbeck J.V., Eghtesady P., Laussen P.C., Pizarro C., Shrader P., et al. Cause, timing, and location of death in the single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:907–914. doi: 10.1016/j.jtcvs.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali S.K., Ohye R.G., Lu M., Kaltman J., Caldarone C.A., Pizarro C., et al. Variation in perioperative care across centers for infants undergoing the Norwood procedure. J Thorac Cardiovasc Surg. 2012;144:915–921. doi: 10.1016/j.jtcvs.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R.R., Carver S.W., Schmidt R., Keskeny H., Hoch J., Pizarro C. Gastrointestinal complications after stage I Norwood versus hybrid procedures. Ann Thorac Surg. 2013;95:189–196. doi: 10.1016/j.athoracsur.2012.05.130. discussion 195-6. [DOI] [PubMed] [Google Scholar]
- Prodhan P., Agarwal A., El Hassan N.O., Bolin E.H., Beam B., Garcia X., et al. Tracheostomy among infants with hypoplastic left heart syndrome undergoing cardiac operations: a multicenter analysis. Ann Thorac Surg. 2017;103:1308–1314. doi: 10.1016/j.athoracsur.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Prodhan P., Tang X., Gossett J., Beam B., Simsic J., Ghanayem N., et al. Gastrostomy tube placement among infants with hypoplastic left heart syndrome undergoing stage 1 palliation. Congenit Heart Dis. 2018;13:519–527. doi: 10.1111/chd.12610. [DOI] [PubMed] [Google Scholar]
- Skinner M.L., Halstead L.A., Rubinstein C.S., Atz A.M., Andrews D., Bradley S.M. Laryngopharyngeal dysfunction after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;130:1293–1301. doi: 10.1016/j.jtcvs.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Miyata H., Hirahara N., Murakami A., Kado H., Sakamoto K., et al. Long-term results of bilateral pulmonary artery banding versus primary Norwood procedure. Pediatr Cardiol. 2018;39:111–119. doi: 10.1007/s00246-017-1735-1. [DOI] [PubMed] [Google Scholar]
- Galantowicz M., Yates A.R. Improved outcomes with the comprehensive stage 2 procedure after an initial hybrid stage 1. J Thorac Cardiovasc Surg. 2016;151:424–429. doi: 10.1016/j.jtcvs.2015.10.023. [DOI] [PubMed] [Google Scholar]
- Yerebakan C., Valeske K., Elmontaser H., Yörüker U., Mueller M., Thul J., et al. Hybrid therapy for hypoplastic left heart syndrome: myth, alternative, or standard? J Thorac Cardiovasc Surg. 2016;151:1112–1121. doi: 10.1016/j.jtcvs.2015.10.066. 1123.e1-5. [DOI] [PubMed] [Google Scholar]
- Dave H., Rosser B., Knirsch W., Hubler M., Pretre R., Kretschmar O. Hybrid approach for hypoplastic left heart syndrome and its variants: the fate of the pulmonary arteries. Eur J Cardio Thorac Surg. 2014;46:14–19. doi: 10.1093/ejcts/ezt604. [DOI] [PubMed] [Google Scholar]
- Hoashi T., Imai K., Okuda N., Komori M., Kurosaki K., Ichikawa H. Intermediate-term outcomes of deferred Norwood strategy. Eur J Cardio Thorac Surg. 2022;62 doi: 10.1093/ejcts/ezac099. [DOI] [PubMed] [Google Scholar]
- Michielon G., DiSalvo G., Fraisse A., Carvalho J.S., Krupickova S., Slavik Z., et al. In-hospital interstage improves interstage survival after the Norwood stage 1 operation. Eur J Cardio Thorac Surg. 2020;57:1113–1121. doi: 10.1093/ejcts/ezaa074. [DOI] [PubMed] [Google Scholar]
- Spigel Z.A., Kalustian A., Ghanayem N., Imamura M., Adachi I., McKenzie E.D., et al. Predictors of transplant-free survival after the Norwood procedure. Ann Thorac Surg. 2021;112:638–644. doi: 10.1016/j.athoracsur.2020.06.024. [DOI] [PubMed] [Google Scholar]
- Moon J., Shen L., Likosky D.S., Sood V., Hobbs R.D., Sassalos P., et al. Relationship of ventricular morphology and atrioventricular valve function to long-term outcomes following Fontan procedures. J Am Coll Cardiol. 2020;76:419–431. doi: 10.1016/j.jacc.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychik J., Atz A.M., Celermajer D.S., Deal B.J., Gatzoulis M.A., Gewillig M.H., et al. American Heart Association Council on Cardiovascular Disease in the Young and Council on Cardiovascular and Stroke Nursing Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation. 2019;140 doi: 10.1161/CIR.0000000000000696. [DOI] [PubMed] [Google Scholar]
- Chowdhury S.M., Graham E.M., Taylor C.L., Savage A., McHugh K.E., Gaydos S., et al. Diastolic dysfunction with preserved ejection fraction after the Fontan procedure. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.024095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui W., Abd El Rahman M.Y., Schuck R., Rentzsch A., Yigitbasi M., Ovroutski S., et al. Diastolic asynchrony and myocardial dysfunction in patients with univentricular heart after Fontan operation. J Echocardiogr. 2013;11:130–137. doi: 10.1007/s12574-013-0191-z. [DOI] [PubMed] [Google Scholar]
- Tseng S.Y., Siddiqui S., Di Maria M.V., Hill G.D., Lubert A.M., Kutty S., et al. Atrioventricular valve regurgitation in single ventricle heart disease: a common problem associated with progressive deterioration and mortality. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G., Ayer J., Celermajer D., Zentner D., Justo R., Disney P., et al. Atrioventricular valve failure in Fontan palliation. J Am Coll Cardiol. 2019;73:810–822. doi: 10.1016/j.jacc.2018.12.025. [DOI] [PubMed] [Google Scholar]
- King G., Buratto E., Celermajer D.S., Grigg L., Alphonso N., Robertson T., et al. Natural and modified history of atrioventricular valve regurgitation in patients with Fontan circulation. J Am Coll Cardiol. 2022;79:1832–1845. doi: 10.1016/j.jacc.2022.02.022. [DOI] [PubMed] [Google Scholar]
- Goldberg C.S., Mussatto K., Licht D., Wernovsky G. Neurodevelopment and quality of life for children with hypoplastic left heart syndrome: current knowns and unknowns. Cardiol Young. 2011;21:88–92. doi: 10.1017/S104795111100165X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahle W.T., Wernovsky G. Neurodevelopmental outcomes in hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:39–47. doi: 10.1053/j.pcsu.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Tabbutt S., Nord A.S., Jarvik G.P., Bernbaum J., Wernovsky G., Gerdes M., et al. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics. 2008;121:476–483. doi: 10.1542/peds.2007-1282. [DOI] [PubMed] [Google Scholar]
- Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T.W., Tan Y., Krawczeski C.D., Spencer J.D., Bai S., Phelps C., et al. Incidence and impact of acute kidney injury in patients with hypoplastic left heart syndrome following the hybrid stage 1 palliation. Cardiol Young. 2021;31:414–420. doi: 10.1017/S1047951120004199. [DOI] [PubMed] [Google Scholar]
- Chawla L.S., Kimmel P.L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- Garcia R.U., Natarajan G., Walters H.L., Delius R.E., Aggarwal S. Acute kidney injury following first-stage palliation in hypoplastic left heart syndrome: hybrid versus Norwood palliation. Cardiol Young. 2018;28:261–268. doi: 10.1017/S1047951117001809. [DOI] [PubMed] [Google Scholar]
- Madsen N.L., Goldstein S.L., Frøslev T., Christiansen C.F., Olsen M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. 2017;92:751–756. doi: 10.1016/j.kint.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Hessey E., Melhem N., Alobaidi R., Ulrich E., Morgan C., Bagshaw S.M., et al. Acute kidney injury in critically ill children is not all acute: lessons over the last 5 years. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.648587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldt R.H., Driscoll D.J., Offord K.P., Cha R.H., Perrault J., Schaff H.V., et al. Protein-losing enteropathy after the Fontan operation. J Thorac Cardiovasc Surg. 1996;112:672–680. doi: 10.1016/S0022-5223(96)70051-X. [DOI] [PubMed] [Google Scholar]
- Rychik J., Dodds K.M., Goldberg D., Glatz A.C., Fogel M., Rossano J., et al. Protein losing enteropathy after Fontan operation: glimpses of clarity through the lifting fog. World J Pediatr Congenit Heart Surg. 2020;11:92–96. doi: 10.1177/2150135119890555. [DOI] [PubMed] [Google Scholar]
- ElHassan N.O., Tang X., Gossett J., Zakaria D., Ross A., Kona S.K., et al. Necrotizing enterocolitis in infants with hypoplastic left heart syndrome following stage 1 palliation or heart transplant. Pediatr Cardiol. 2018;39:774–785. doi: 10.1007/s00246-018-1820-0. [DOI] [PubMed] [Google Scholar]
- Turquetto A.L.R., Canêo L.F., Agostinho D.R., Oliveira P.A., Lopes M.I.C.S., Trevizan P.F., et al. Impaired pulmonary function is an additional potential mechanism for the reduction of functional capacity in clinically stable Fontan patients. Pediatr Cardiol. 2017;38:981–990. doi: 10.1007/s00246-017-1606-9. [DOI] [PubMed] [Google Scholar]
- Hawkins S.M., Taylor A.L., Sillau S.H., Mitchell M.B., Rausch C.M. Restrictive lung function in pediatric patients with structural congenital heart disease. J Thorac Cardiovasc Surg. 2014;148:207–211. doi: 10.1016/j.jtcvs.2013.07.080. [DOI] [PubMed] [Google Scholar]
- Joyce J.J., Dickson P.I., Qi N., Noble J.E., Raj J., Baylen B.G. Normal right and left ventricular mass development during early infancy. Am J Cardiol. 2004;93:797–801. doi: 10.1016/j.amjcard.2003.11.063. [DOI] [PubMed] [Google Scholar]
- Anversa P., Olivetti G., Loud A.V. Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. I. Hypertrophy, hyperplasia, and binucleation of myocytes. Circ Res. 1980;46:495–502. doi: 10.1161/01.res.46.4.495. [DOI] [PubMed] [Google Scholar]
- Schiffmann H., Flesch M., Häuseler C., Pfahlberg A., Böhm M., Hellige G. Effects of different inotropic interventions on myocardial function in the developing rabbit heart. Basic Res Cardiol. 2002;97:76–87. doi: 10.1007/s395-002-8390-1. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T., de Courten-Myers G.M., Petetot J.M.C., Xi G., de los Reyes E. Human cortex development. J Neuropathol Exp Neurol. 1996;55:320–328. [PubMed] [Google Scholar]
- Stiles J., Jernigan T.L. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M., Dubois J., Yu Q., Mukherjee P., Huang H. Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage. 2019;185:836–850. doi: 10.1016/j.neuroimage.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basalely A., Liu D., Kaskel F.J. Big equation for small kidneys: a newly proposed model to estimate neonatal GFR. Pediatr Nephrol. 2020;35:543–546. doi: 10.1007/s00467-019-04465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubhaju L., Sutherland M.R., Horne R.S.C., Medhurst A., Kent A.L., Ramsden A., et al. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Renal Physiol. 2014;307:F149–F158. doi: 10.1152/ajprenal.00439.2013. [DOI] [PubMed] [Google Scholar]
- Schwartz G.J., Brion L.P., Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- Madise-Wobo A.D., Disu E.A., Gbelee O.H., Solarin A., Animasahun B.A. Estimated glomerular filtration rate in apparently healthy term neonates in Nigeria. African J Nephrol. 2018;21:34–38. [Google Scholar]
- Monem A., Hamid M., Abdel-Aal H., Soliman S. Glomerular filtration rate changes in the first week of healthy full-term newborns. Alex J Pediatr. 2021;34:125. [Google Scholar]
- Smeets N.J., IntHout J., van der Burgh M.J., Schwartz G.J., Schreuder M.F., de Wildt S.N. Maturation of GFR in term-born neonates: an individual participant data meta-analysis. J Am Soc Nephrol. 2022;33:1277–1292. doi: 10.1681/ASN.2021101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobelli S., Guignard J.P. Maturation of glomerular filtration rate in neonates and infants: an overview. Pediatr Nephrol. 2021;36:1439–1446. doi: 10.1007/s00467-020-04632-1. [DOI] [PubMed] [Google Scholar]
- Salem F., Johnson T.N., Hodgkinson A.B.J., Ogungbenro K., Rostami-Hodjegan A. Does “birth” as an event impact maturation trajectory of renal clearance via glomerular filtration? Reexamining data in preterm and full-term neonates by avoiding the creatinine bias. J Clin Pharmacol. 2021;61:159–171. doi: 10.1002/jcph.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.J., Mellor D.J., Tungthanathanich P., Birtles M.J., Reynolds G.W., Simpson H.V. Growth and morphological changes in the small and the large intestine in piglets during the first three days after birth. J Dev Physiol. 1992;18:161–172. [PubMed] [Google Scholar]
- Widdowson E.M. Cellular growth and function. Proc Nutr Soc. 1976;35:357–362. doi: 10.1079/pns19760056. [DOI] [PubMed] [Google Scholar]
- Torow N., Marsland B., Hornef M., Gollwitzer E. Neonatal mucosal immunology. Mucosal Immunol. 2017;10:5–17. doi: 10.1038/mi.2016.81. [DOI] [PubMed] [Google Scholar]
- Penders J., Thijs C., Vink C., Stelma F.F., Snijders B., Kummeling I., et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- Tourneur E., Chassin C. Neonatal immune adaptation of the gut and its role during infections. Clin Dev Immunol. 2013;2013:270301–270307. doi: 10.1155/2013/270301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J. Gastrointestinal maturation and implications for infant feeding. Early Hum Dev. 2007;83:767–775. doi: 10.1016/j.earlhumdev.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Burri P.H. Fetal and postnatal development of the lung. Annu Rev Physiol. 1984;46:617–628. doi: 10.1146/annurev.ph.46.030184.003153. [DOI] [PubMed] [Google Scholar]
- Thurlbeck W.M. Postnatal human lung growth. Thorax. 1982;37:564–571. doi: 10.1136/thx.37.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N., Gai J., Bost J., Donofrio M.T. Alterations in cardiac output in fetuses with congenital heart disease. Prenat Diagn. 2022;42:1133–1141. doi: 10.1002/pd.6193. [DOI] [PubMed] [Google Scholar]
- Sun L., Macgowan C.K., Portnoy S., Sled J.G., Yoo S.J., Grosse-Wortmann L., et al. New advances in fetal cardiovascular magnetic resonance imaging for quantifying the distribution of blood flow and oxygen transport: potential applications in fetal cardiovascular disease diagnosis and therapy. Echocardiography. 2017;34:1799–1803. doi: 10.1111/echo.13760. [DOI] [PubMed] [Google Scholar]
- Eckersley L.G., Mills L., Hirose A., Khoo N.S., Wernovsky G., Hornberger L.K. The pperinatal transition and early neonatal period in hypoplastic left heart syndrome is associated with reduced systemic and cerebral perfusion. Can J Cardiol. 2021;37:1923–1933. doi: 10.1016/j.cjca.2021.07.002. [DOI] [PubMed] [Google Scholar]
- Katayama H., Krzeski R., Frantz E.G., Ferreiro J.I., Lucas C.L., Ha B., et al. Induction of right ventricular hypertrophy with obstructing balloon catheter. Nonsurgical ventricular preparation for the arterial switch operation in simple transposition. Circulation. 1993;88:1765–1769. doi: 10.1161/01.cir.88.4.1765. [DOI] [PubMed] [Google Scholar]
- Jonas R.A., Giglia T.M., Sanders S.P., Wernovsky G., Nadal-Ginard B., Mayer J.E., et al. Rapid, two-stage arterial switch for transposition of the great arteries and intact ventricular septum beyond the neonatal period. Circulation. 1989;80:I203–I208. [PubMed] [Google Scholar]
- Lynch J.M., Gaynor J.W., Licht D.J. Brain injury during transition in the newborn with congenital heart disease: hazards of the preoperative period. Semin Pediatr Neurol. 2018;28:60–65. doi: 10.1016/j.spen.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Lynch J.M., Ko T., Busch D.R., Newland J.J., Winters M.E., Mensah-Brown K., et al. Preoperative cerebral hemodynamics from birth to surgery in neonates with critical congenital heart disease. J Thorac Cardiovasc Surg. 2018;156:1657–1664. doi: 10.1016/j.jtcvs.2018.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sananes R., Goldberg C.S., Newburger J.W., Hu C., Trachtenberg F., Gaynor J.W., et al. PHN Investigators Six-year neurodevelopmental outcomes for children with single-ventricle physiology. Pediatrics. 2021;147 doi: 10.1542/peds.2020-014589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J., Ridout D., Johnson S., Hoskote A., Sheehan K., Wellman P., et al. Postoperative morbidities with infant cardiac surgery and toddlers' neurodevelopment. Arch Dis Child. 2022;107:922–928. doi: 10.1136/archdischild-2021-322756. [DOI] [PubMed] [Google Scholar]
- McElhinney D.B., Hedrick H.L., Bush D.M., Pereira G.R., Stafford P.W., Gaynor J.W., et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics. 2000;106:1080–1087. doi: 10.1542/peds.106.5.1080. [DOI] [PubMed] [Google Scholar]
- Theilen U., Shekerdemian L. The intensive care of infants with hypoplastic left heart syndrome. Arch Dis Child Fetal Neonatal Ed. 2005;90:F97–F102. doi: 10.1136/adc.2004.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. Physiology, diagnosis and clinical profile of the hypoplastic left heart syndrome. Prog Pediatr Cardiol. 1996;5:19–22. [Google Scholar]
- Hoshino K., Ogawa K., Hishitani T., Kitazawa R., Uehara R. Hypoplastic left heart syndrome: duration of survival without surgical intervention. Am Heart J. 1999;137:535–542. doi: 10.1016/s0002-8703(99)70503-x. [DOI] [PubMed] [Google Scholar]
- Wernovsky G., Wypij D., Jonas R.A., Mayer J.E., Hanley F.L., Hickey P.R., et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- Hoffman T.M., Wernovsky G., Atz A.M., Bailey J.M., Akbary A., Kocsis J.F., et al. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. Am Heart J. 2002;143:15–21. doi: 10.1067/mhj.2002.120305. [DOI] [PubMed] [Google Scholar]
- Naim M.Y., Gaynor J.W., Chen J., Nicolson S.C., Fuller S., Spray T.L., et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150:169–180. doi: 10.1016/j.jtcvs.2015.03.045. discussion 178-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beca J., Gunn J.K., Coleman L., Hope A., Reed P.W., Hunt R.W., et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–979. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- Chen J., Zimmerman R.A., Jarvik G.P., Nord A.S., Clancy R.R., Wernovsky G., et al. Perioperative stroke in infants undergoing open heart operations for congenital heart disease. Ann Thorac Surg. 2009;88:823–829. doi: 10.1016/j.athoracsur.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A.J., Fox C.K., Ichord R.N., Almond C.S., Bernard T.J., Beslow L.A., et al. Stroke in children with cardiac disease: report from the international pediatric stroke study group symposium. Pediatr Neurol. 2015;52:5–15. doi: 10.1016/j.pediatrneurol.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin C., Jonas R.A., Sanders S.P., Wernovsky G., Mone S.M., Colan S.D. Rapid two-stage arterial switch operation. Acquisition of left ventricular mass after pulmonary artery banding in infants with transposition of the great arteries. Circulation. 1994;90:1304–1309. doi: 10.1161/01.cir.90.3.1304. [DOI] [PubMed] [Google Scholar]
- Carrillo S.A., Texter K.M., Phelps C., Tan Y., McConnell P.I., Galantowicz M. Tricuspid valve and right ventricular function throughout the hybrid palliation strategy for hypoplastic left heart syndrome and variants. World J Pediatr Congenit Heart Surg. 2021;12:9–16. doi: 10.1177/2150135120947692. [DOI] [PubMed] [Google Scholar]
- Hsu C.H., Kurtz T.W., Waldinger T.P. Cardiac output and renal blood flow in glycerol-induced acute renal failure in the rat. Circ Res. 1977;40:178–182. doi: 10.1161/01.res.40.2.178. [DOI] [PubMed] [Google Scholar]
- Wray J., Ridout D., Jones A., Davis P., Wellman P., Rodrigues W., et al. Morbidities after cardiac surgery: impact on children's quality of life and parents' mental health. Ann Thorac Surg. 2021;112:2055–2062. doi: 10.1016/j.athoracsur.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R.J., Mayne E.W., Sandoval Karamian A.G., Iqbal M., Purington N., Ryan K.R., et al. Evaluation of seizure risk in infants after cardiopulmonary bypass in the absence of deep hypothermic cardiac arrest. Neurocrit Care. 2022;36:30–38. doi: 10.1007/s12028-021-01313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota N., Murata M., Tosaka Y., Ide Y., Tachi M., Ito H., et al. Is routine rapid-staged bilateral pulmonary artery banding before stage 1 Norwood a viable strategy? J Thorac Cardiovasc Surg. 2014;148:1519–1525. doi: 10.1016/j.jtcvs.2013.11.053. [DOI] [PubMed] [Google Scholar]