Abstract
Background
Close contacts infected with Mycobacterium tuberculosis are at high risk of tuberculosis (TB) disease and a priority for preventive treatment. Three tests measure infection: two interferon-gamma release assays (IGRAs) and the tuberculin skin test (TST). The objective of our study was to assess the association of positive test results in contacts with infectiousness of the presumed TB source case.
Methods
Contacts in a cohort study at 10 United States sites received both IGRAs (QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB (T-SPOT)) and TST. We defined test conversion as negative for all tests at baseline and positive for at least one on retest. Risk ratios (RR) and 95% confidence intervals (CI) assessed association of positive test results with increased infectiousness of the TB case—defined as acid-fast bacilli (AFB) on sputum microscopy or cavities on chest radiographs— and contact demographics.
Results
Adjusted for contacts’ age, nativity, sex, and race, IGRAs (QFT-GIT RR = 6.1, 95% CI 1.7–22.2; T-SPOT RR = 9.4, 95% CI 1.1–79.1), but not TST (RR = 1.7, 95% CI 0.8–3.7), were more likely to convert among contacts exposed to persons with cavitary TB disease.
Conclusions
Because IGRA conversions in contacts are associated with infectiousness of the TB case, their use may improve efficiency of health department contact investigations by focusing efforts on those likely to benefit from preventive treatment in the United States.
Keywords: Tuberculin skin test, QuantiFERON-TB Gold In-tube, T-SPOT.TB, Close contacts
1. Background
Close contacts of persons with infectious tuberculosis (TB) disease are at high risk of infection and progression to TB disease [1], [2], [3]. Approximately one in seven TB cases in the United States is attributed to recent transmission [4]. Identification and treatment of recently infected close contacts reduces risk of progression to TB disease and further transmission and is one of the key strategies for TB elimination in the United States, defined as < 1 new case per million population annually [5].
Diagnosis of Mycobacterium tuberculosis infection is difficult because the current tests are indirect tests that measure the host’s immune response to infection and do not detect M. tuberculosis directly. The tuberculin skin test (TST) is subject to false-positive results among those who received the Bacillus Calmette-Guérin (BCG) TB vaccine, given at birth and often at school entry to > 80% of the world’s children. It can also cross-react with nontuberculous mycobacteria, is affected by subjectivity of the reading, and requires two visits [6], [7], [8]. In persons infected many years previously whose immune reactivity has waned, it can stimulate an immune response that incorrectly suggests recent infection, a phenomenon known as boosting. Interferon-gamma release blood assays (IGRAs) contain M. tuberculosis antigens absent in BCG [9]. Because they are not affected by BCG antigens or most nontuberculous mycobacteria, require only a single visit, and have standardized interpretation of test results, IGRAs might be preferred over TST for contact investigations [10], [11], [12], [13]. Two IGRAs are available in the United States: QuantiFERON-TB Gold In-Tube (QFT-GIT; Qiagen, Germantown, Maryland, U.S.) and T-SPOT.TB (T-SPOT; Oxford Immunotec, Inc., Marlborough, Massachusetts, U.S.).
One way to compare IGRAs and TST in close contacts is to assess associations between infectiousness of the putative source case and test conversion from negative to positive following exposure. Two established indicators of increased infectiousness are presence of acid-fast bacilli (AFB) in sputum smears and lung cavities on chest radiographs [1]. Because TST-associated boosting phenomenon is more common among non-U.S.–born persons, contact demographics are also relevant [14].
To date, no studies in the United States have evaluated all three tests at baseline and retest simultaneously to assess association of recent infection in close contacts with infectiousness of TB cases. We used a subset of a large cohort study to assess the association of positive TSTs and IGRAs in recently infected contacts with infectiousness of the TB cases and contact demographics.
2. Methods
2.1. Study population and testing
The Centers for Disease Control and Prevention (CDC) funded the Tuberculosis Epidemiologic Studies Consortium (TBESC) to conduct epidemiologic investigations into the diagnosis, treatment, and prevention of M. tuberculosis infection [15], [16]. TBESC collaborators included TB programs and academic institutions and 18 affiliated clinics at 10 sites. Contacts were recruited for a cohort study that compared the abilities of TST, QFT-GIT, and T-SPOT to predict progression to TB disease [15]. A detailed description of recruitment has been previously published [16]. Briefly, from July 2012 through May 2017, participating clinics enrolled 22,131 persons of all ages at high risk for M. tuberculosis infection or progression to TB disease, including non-U.S.–born persons, persons with HIV, and close contacts [15]. Close contacts were defined as persons evaluated for an ongoing site contact investigation who spent ≥ 8 h in one week with a person with infectious TB (TB case) during the estimated infectious period as described in CDC contact investigation guidelines [1].
At study enrollment, each participant had blood drawn for both IGRAs, followed by placement of a TST. Blood samples for QFT-GIT and T-SPOT were processed and reported according to manufacturers’ instructions [17], [18]. All demographic characteristics, including sex, were self-reported and collected during participant interview.
Sites obtained local institutional review board (IRB) approval or deferred to the CDC IRB. All participants provided written informed consent, assent, and/or parental/guardian permission.
2.2. Enrollment of close contacts
As part of routine TB control activities, TB programs attempt to identify and test all close contacts of persons with infectious TB. Ideally, contacts who are negative when first tested are routinely retested 8–10 weeks after last known exposure to the TB case to allow sufficient time for an immune response [1]. Our study protocol did not require an 8–10-week interval for retest after last exposure to the TB case; each health department retested contacts according to its own circumstances, including the responsiveness and availability of the contacts and staffing capacity. Per study protocol, if the health department retested a participant, all three tests were administered, except that an initial positive TST was repeated at the health department’s discretion. Otherwise, each health department followed its own procedures for contact investigations, including determination of whom to test and retest, and which result(s) to consider when deciding whether to retest. The study would not have both baseline and retest results for participants if the contacts (a) were identified and recruited by study staff only at retest; (b) had one or more positive tests at baseline and did not get retested; (c) were lost to follow-up and could not be retested; or (d) were initially tested ≥ 8 weeks after their last exposure to the TB case, so would not need more than one test.
2.3. Definition of a recently infected close contact
We defined a recently infected close contact as a participant negative by TST and both IGRAs on the first (baseline) test and positive on at least one test at retest (conversion); all three retest results had to be positive or negative (valid result). Variables extracted from the medical record included exposure time of ≥ 8 h per week and identification of the TB case, but not place, circumstances, or estimated dates of exposure.
We used the U.S. Food and Drug Administration (FDA)-approved cutoff of ≥ 8 spots for positive T-SPOT result. For QFT-GIT, a result was positive if TB antigen minus nil was ≥ 0.35 IU/mL and ≥ 25% of the nil value. TSTs were read 48–72 h (±4 h) after placement and interpreted as positive if induration was ≥ 5 mm [19]. T-SPOT borderline results, 5–7 spots, were considered invalid and excluded from analysis.
2.4. Identification of TB cases
For each enrolled contact, local health department staff provided the putative source TB patient’s state case number; this was linked to CDC’s National Tuberculosis Surveillance System (NTSS) to obtain information on sputum smear results and cavitary disease.
2.5. Statistical analysis
Quantitative values for TST induration, interferon gamma levels for QFT-GIT, and spot count for T-SPOT were reported with median and interquartile range (IQR) values. We developed models that used either one contact or TB case characteristic at a time (initial models) and multiple characteristics (final models) to assess test conversion outcomes for each of the three TB tests. The initial models had included one TB case characteristic (sputum smear or cavitary disease), or contact characteristic (age, nativity, sex, or race/ethnicity) by test type (TST, QFT-GIT, or T-SPOT). The 3 final models included the same TB case (cavitary disease) and contact variables (age, nativity, sex, and race/ethnicity) and only differed by the test type (TST, QFT-GIT, or T-SPOT). For all models, we used a generalized linear mixed effects model with a Poisson distribution and a log link [20] to account for correlations among (i) multiple contacts exposed to a single TB case, and (ii) participants at the same site. For each of the models, we estimated risk ratios (RR) and 95% confidence intervals (CI) to identify factors associated with test conversion from negative to positive for each of the 3 tests. Because smear status and cavitary disease are indicators of increased infectiousness of TB case and smear status showed no significant association with test conversions in initial models, we used cavitary disease in the final models. Some age categories had no conversions, so we combined age groups 0–5 and 6–14 years. Similarly, for race/ethnicity, we combined Alaska Native/American Indian, Native Hawaiian/Pacific Islander, and ‘Other’ (multiracial) categories. All analyses used SAS (version 9.4, Cary, North Carolina, U.S.).
3. Results
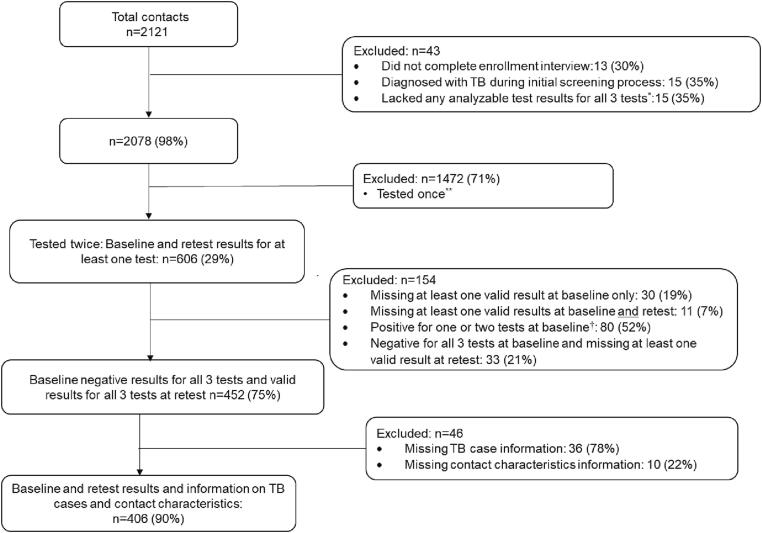
Of 2,121 close contacts enrolled at 16 clinics, 43 (2%) were excluded from further analysis [Fig. 1]. Of the 2,078 remaining, 1,472 (71%) had results from only one set of tests; 606 (29%) had valid baseline and retest results for at least one test type; and 452 of the 606 (75%) (1) were negative for all three tests at baseline and (2) had valid results for all three tests at baseline and retest. Demographic characteristics of participants tested once or twice (baseline and retest) were generally similar [Supplementary material 1].
Fig. 1.
Flow chart showing the final study population for analysis. *Tested once and results for all three tests (Tuberculin skin test, QuantiFERON-TB Gold In-Tube, and T-SPOT.TB) were either missing, indeterminate, or invalid. **Participants who were tested once were contacts who either: (i) had any positive baseline test and were not retested, (ii) were enrolled into the study only during retesting for the contact investigation, (iii) were enrolled for baseline testing but were lost to follow-up before retest, or (iv) were initially tested after 8–10 weeks since last exposure to the TB case †Contacts with all 3 positive results at baseline will not have had a retest, so these numbers represent contacts with one or 2 baseline positive results. Valid result refers to a positive or negative result (i.e., no missing, indeterminate, invalid or borderline results).
Of the 452 with negative baseline and valid retest results for all three tests, 36 (8%) were missing all information about the TB case to which they were exposed, and 10 (2%) were missing information on contact characteristics. Therefore, the final dataset for analysis included data on 406 participants [Fig. 1].
3.1. Demographics of 406 close contacts and their putative source cases
The 406 close contacts included 228 females (56%), 209 (51%) non-U.S.–born participants, and 117 (29%) participants aged < 15 years Table 1a. They were exposed to 179 TB cases; a median of 2 contacts were exposed to each case (Range 1–10, IQR: 1–3).
Table 1a.
Demographics of 406*contacts with negative baseline results for TST, QFT-GIT, and T-SPOT enrolled in a multi-center study in the United States.
| Contact characteristic | n | (%) |
|---|---|---|
| Female | 228 | (56) |
| Non-U.S.–born | 209 | (51) |
| Race/ethnicity | ||
| Asian | 108 | (27) |
| African American/Black | 49 | (12) |
| White | 54 | (13) |
| Hispanic/Latino | 130 | (32) |
| Other** | 65 | (16) |
| Age group | ||
| ≤14 years | 117 | (29) |
| 15–24 years | 64 | (16) |
| 25–44 years | 140 | (34) |
| 45–64 years | 63 | (16) |
| ≥65 years | 22 | (5) |
*Contacts (n = 406) who had 3 baseline negative results and valid results (positive or negative, i.e., no missing, indeterminate, invalid or borderline results) for all 3 tests at retest, and all information for TB cases and contact demographics.
** ‘Other’ race also includes American Indian/Alaska Native and Native Hawaiian/Pacific Islander groups. All listed races are non-Hispanic. TST = Tuberculin skin test; QFT-GIT = QuantiFERON-TB Gold In-Tube; T-SPOT = T-SPOT.TB.
Seventy-one percent of the TB cases had sputum smears positive for AFB (127/179), 56% had lung cavities (100/179), and 48% (86/179) had both [Table 1b]. Fifty-four percent (218/406) of contacts were exposed to TB cases who were both smear positive and had lung cavities.
Table 1b.
Acid-fast bacillus (AFB) sputum smear and chest radiograph results for 179* TB cases whose contacts were enrolled in a multi-center study in the United States.
| TB case characteristics | n | (%) |
|---|---|---|
| AFB sputum smear | ||
| Positive | 127 | (71) |
| Negative | 52 | (29) |
| Chest radiograph | ||
| Cavitary | 100 | (56) |
| Noncavitary | 79 | (44) |
| Sputum smear-positive and cavitary chest radiograph | 86 | (48) |
| Sputum smear-negative and noncavitary chest radiograph | 38 | (21) |
*Contacts (n = 406) exposed to these 179 TB cases had 3 baseline negative results and valid results (positive or negative, i.e., no missing, indeterminate, invalid or borderline results) for all 3 tests at retest, and all information for TB cases and contact demographics.
3.2. Test results
Of the 406 close contacts, 88% (3 5 8) were negative on all three tests at retest, and 3% (12) were positive on all three [Table 2]. Ten percent (41) of TST, 6% (23) of QFT-GIT, and 3% (14) of T-SPOT tests were positive at retest. The retest interval ranged from 4 to 54 weeks after the baseline test (median = 11 weeks). Almost half of participants (45%) had a retest at 4–10 weeks of baseline test and 90% were retested within 19 weeks of the baseline test. Test conversions occurred at a median of 12 weeks after the baseline test for each of the 3 tests with ranges varying from 6 to 54 weeks for TST, 9 to 24 weeks for QFT-GIT, and 8 to 20 weeks for T-SPOT. U.S.-born contacts were more likely than non-U.S.–born contacts to be negative on all three tests (RR = 1.1, 95% CI 1.1–1.2).
Table 2.
Distribution of retest results by nativity among 406 close contacts enrolled in a multi-center study in the United States.
| Test combination | Retest results n = 406 |
Non-U.S.–born n = 209 |
U.S.-born n = 197 |
|||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Triple positive (+++) | 12 | (3) | 11 | (5) | 1 | (1) |
| Triple negative ( − − −) | 358 | (88) | 173 | (83) | 185 | (94) |
| Isolated TST+ (+−−) | 24 | (6) | 18 | (9) | 6 | (3) |
| Isolated QFT-GIT+ (−+−) | 5 | (1) | 3 | (1) | 2 | (1) |
| Isolated T-SPOT+ (−−+) | 1 | (0.2) | 1 | (0.5) | 0 | (0) |
| Isolated TST− (−++) | 1 | (0.2) | 1 | (0.5) | 0 | (0) |
| Isolated QFT-GIT− (+−+) | 0 | (0) | 0 | (0) | 0 | (0) |
| Isolated T-SPOT− (++−) | 5 | (1) | 2 | (1) | 3 | (2) |
The 406 contacts had negative results for all three tests at baseline, valid results (positive or negative, i.e., no missing, indeterminate, invalid or borderline results) for all three tests at retest, and all information on TB cases and contact characteristics. TST = Tuberculin skin test; QFT-GIT = QuantiFERON-TB Gold In-Tube; T-SPOT = T-SPOT.TB. The order of test results (TST, QFT-GIT, T-SPOT); + = positive, − = negative.
3.3. Association of a positive test with TB case and contact characteristics
In the models that accounted for correlations among multiple contacts exposed to a single TB case and participants at the same site, only QFT-GIT conversions were associated with contacts’ exposure to TB cases with cavitary disease compared to those without cavities (RR = 4.2, 95% CI 1.1–16.8; RR for TST = 1.8, 95% CI 0.8–3.8; RR for TSPOT = 7.2, 95% CI 0.2–212.9). Test conversions were not associated with exposure to smear-positive cases: QFT-GIT RR = 3.8, 95% CI 0.7–20); T-SPOT RR = 4.0, 95% CI 0.1–212.1); TST RR = 1.8, 95% CI 0.8–4.6. Compared to U.S. birth, birth outside the U.S. was associated with TST conversions (RR = 2.6, 95% CI 1.2–5.5) but not with QFT-GIT (RR = 2.2, 95% CI 0.8–6.2) or T-SPOT (RR = 24.1, 95% CI 0.6–896.6) conversions. Age, sex, and race/ethnicity were not associated with test conversions [Supplementary material 2].
After adjustment for contacts’ age, nativity, sex, and race/ethnicity, exposure to TB cases with cavitary disease was associated with conversion by QFT-GIT (RR = 6.1, 95% CI 1.7–22.2) and T-SPOT (RR = 9.4, 95% CI 1.1–79.1), but not by TST (RR = 1.7, 95% CI 0.8–3.7) [Table 3]. Birth outside the U.S. was associated with test conversion by TST (RR = 2.6, 95% CI 1.1–6.0) and T-SPOT (RR = 13.5, 95 %CI 1.4–133.0), but not by QFT-GIT (RR = 2.1, 95% CI 0.7–6.1). Contacts’ age, sex and race/ethnicity were not associated with test conversion [Table 3].
Table 3.
Final models Adjusted risk ratios showing association of test conversions* among 406 contacts with triple negative baseline results enrolled in a multi-center study in the United States.
| TST | QFT-GIT | T-SPOT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive n (%) |
RR** | [95% CI] | Positive n (%) |
RR** | [95% CI] | Positive n (%) |
RR** | [95% CI] | |
| TB case characteristics | |||||||||
| Chest radiograph (n) | |||||||||
| Cavitary (2 4 3) | 30 (12) | 1.7 | (0.8–3.7) | 20 (8) | 6.1 | (1.7–22.2) | 13 (5) | 9.4 | (1.1–79.1) |
| Noncavitary (1 6 3) | 11 (7) | Ref | 3 (2) | Ref | 1 (0.6) | Ref | |||
| Contact characteristic | |||||||||
| Nativity (n) | |||||||||
| Non-U.S.–born (2 0 9) | 31 (15) | 2.6 | (1.1–6.0) | 17 (8) | 2.1 | (0.7–6.1) | 13 (6) | 13.5 | (1.4–133.0) |
| U.S.-born (1 9 7) | 10 (5) | Ref | 6 (3) | Ref | 1 (0.5) | Ref | |||
| Sex (n) | |||||||||
| Female (2 2 8) | 20 (9) | 0.7 | (0.4–1.4) | 14 (6) | 1.2 | (0.5–3.1) | 8 (4) | 0.9 | (0.3–3.1) |
| Male (1 7 8) | 21 (12) | Ref | 9 (5) | Ref | 6 (3) | Ref | |||
| Age (years) (n) | |||||||||
| ≤14 (1 1 7) | 6 (5) | Ref | 3 (3) | Ref | 1 (1) | Ref | |||
| 15–24 (64) | 9 (14) | 2.1 | (0.7–6.6) | 5 (8) | 2.7 | (0.6–12.5) | 1 (2) | 1.3 | (0.1–24.0) |
| 25–44 (1 4 0) | 18 (13) | 1.3 | (0.4–4.1) | 10 (7) | 1.2 | (0.3–4.8) | 9 (6) | 2.6 | (0.3–26.6) |
| 45–64 (63) | 6 (10) | 1.4 | (0.4–5.3) | 4 (6) | 1.9 | (0.4–9.8) | 2 (3) | 2.3 | (0.1–34.2) |
| ≥65 (22) | 2 (9) | 1.2 | (0.2–7.1) | 1 (5) | 0.9 | (0.1–10.3) | 1 (5) | 2.5 | (0.1–57.0) |
| Race/ethnicity (n) | |||||||||
| Asian (1 0 8) | 15 (14) | Ref | 10 (9) | Ref | 6 (6) | Ref | |||
| African American/ | |||||||||
| Black (49) | 7 (14) | 0.9 | (0.3–3.0) | 4 (8) | 1.0 | (0.3–3.6) | 3 (6) | 2.0 | (0.4–10.3) |
| White (54) | 4 (7) | 0.8 | (0.3–3.0) | 1 (2) | 0.2 | (0.0–2.1) | 1 (2) | 1.3 | (0.1–14.8) |
| Hispanic (1 3 0) | 13 (10) | 0.8 | (0.3–2.3) | 5 (4) | 0.4 | (0.1–1.3) | 3 (2) | 0.5 | (0.1–2.3) |
| Other† (65) | 2 (3) | 0.2 | (0.1–1.1) | 3 (5) | 0.6 | (0.1–2.3) | 1 (2) | 0.4 | (0.0–4.3) |
*Contacts with negative results at baseline who converted to positive results at retest and having all information for TB cases and contact demographics are included here. **Adjusted for correlations among multiple contacts exposed to a TB case and clinic and contacts’ age, nativity, sex and race/ethnicity, three models were run separately (TST, QFT-GIT and T-SPOT). † ‘Other’ race also includes American Indian/Alaska Native and Native Hawaiian/Pacific Islander groups. All listed races are non-Hispanic. AFB = acid-fast bacillus; TST = Tuberculin skin test; QFT-GIT = QuantiFERON-TB Gold In-Tube; T-SPOT = T-SPOT.TB; RR = Risk ratio; CI = Confidence interval; Ref = Reference group.
3.4. Quantitative test values for contacts whose tests converted to positive
The median quantitative retest values for TST conversions were 1 to 3 times higher than the cutoff value (≥5 mm) for a positive test. For QFT-GIT, the median retest values for converters were 3 to 14 times higher than the cutoff value (≥0.35 IU/ml), and for T-SPOT, they were 2 to 6 times higher than the cutoff value (≥8 spots) [Table 4].
Table 4.
Median quantitative test values of converters* among 406 contacts with triple negative baseline results enrolled in a multi-center study in the United States.
| TST | QFT-GIT | T-SPOT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (Median (IQR), mm) | (Median (IQR), IU/ml) | (Median (IQR), spots) | |||||||
| n | Baseline | Retest | n | Baseline | Retest | n | Baseline | Retest | |
| TB case characteristics | |||||||||
| Chest radiograph | |||||||||
| Cavitary | 30 | 0 (0–0) | 12 (9–15) | 20 | 0 (0–0.1) | 2.3 (0.7–3.4) | 13 | 0 (0–2) | 32 (19–50) |
| Noncavitary | 11 | 0 (0–0) | 10 (6–15) | 3 | 0.1 (0–0.1) | 1.1 (0.4–1.2) | 1 | 1 (1–1) | 27 (27–27) |
| Contact characteristics | |||||||||
| Nativity | |||||||||
| Non-U.S.–born | 31 | 0 (0–0) | 12 (8–15) | 17 | 0 (0.01–0.1) | 2.4 (0.8–3.7) | 13 | 1 (0–2) | 32 (20–50) |
| U.S.-born | 10 | 0 (0–0) | 10 (7–12) | 6 | 0 (-0.01–0.1) | 1.2 (0.4–2.3) | 1 | 0 (0–0) | 17 (17–17) |
| Sex | |||||||||
| Female | 20 | 0 (0–0) | 14 (8–17) | 14 | 0 (0.01–0.1) | 1.8 (0.8–2.8) | 8 | 1 (0–2) | 26 (20–35) |
| Male | 21 | 0 (0–0) | 11 (10–13) | 9 | 0.1 (0–0.1) | 2.3 (0.6–3.7) | 6 | 0 (0–2) | 45 (17–50) |
| Age (years) | |||||||||
| ≤14 | 6 | 0 (0–0) | 7 (6–11) | 3 | 0 (0.0–0.1) | 2.8 (0.4–10) | 1 | 0 (0–0) | 50 (50–50) |
| 15–24 | 9 | 0 (0–0) | 11 (9–14) | 5 | 0 (0.0–0.1) | 1.1 (0.4–1.2) | 1 | 0 (0–0) | 17 (17–17) |
| 25–44 | 18 | 0 (0–0) | 13 (10–19) | 10 | 0.1 (0–0.3) | 2.5 (0.8–3.7) | 9 | 1 (0–3) | 32 (20–50) |
| 45–64 | 6 | 0 (0–0) | 15 (11–17) | 4 | 0 (0–0.1) | 1.2 (0.6–5.7) | 2 | 0 (0–0) | 28 (19–37) |
| ≥65 | 2 | 0 (0–0) | 11 (10–12) | 1 | 0 (0–0) | 3.1 (3.1–3.1) | 1 | 1 (1–1) | 24 (24–24) |
| Race/ethnicity | |||||||||
| Asian | 15 | 0 (0–0) | 11 (8–19) | 10 | 0.1 (0–0.1) | 1.2 (0.78–2.5) | 6 | 0 (0–2) | 38 (19–50) |
| African American/Black | 7 | 0 (0–0) | 13 (6–15) | 4 | 0.1 (0–0.2) | 5.0 (0.4–9.8) | 3 | 3 (0–4) | 32 (9–50) |
| White | 4 | 0 (0–0) | 11 (10–15) | 1 | 0 (0–0) | 2.3 (2.3–2.3) | 1 | 0 (0–0) | 17 (17–17) |
| Hispanic | 13 | 0 (0–0) | 12 (1–14) | 5 | 0 (0–0) | 2.8 (1.7–2.8) | 3 | 1 (0–1) | 27 (20–50) |
| Other** | 2 | 0 (0–0) | 11 (10–12) | 3 | 0.1 (0–0.4) | 1.9 (0.4–3.1) | 1 | 1 (1–1) | 24 (24–24) |
*Contacts with negative results at baseline who converted to positive results at retest and having all information for TB cases and contact demographics are included here. ** ‘Other’ race also includes Alaska Native/American Indian, and Native Hawaiian/Pacific Islander groups. All listed races are non-Hispanic. AFB = acid-fast bacillus; TST = tuberculin skin test; QFT-GIT = QuantiFERON-TB Gold In-Tube; T-SPOT = T-SPOT.TB; IQR = Interquartile range, mm = millimeter, IU/mL = international units per milliliter.
4. Discussion
Our study of recent infection in 406 close contacts is the first in the United States to evaluate the association of test conversion with TB case infectiousness for all three tests. These contacts had negative baseline results for all 3 tests and valid retest results for all 3 tests, so we could identify those with likely recent infection. In the final models, conversion from negative IGRA at baseline to positive IGRA at retest was associated with contacts’ exposure to cavitary disease in the TB case; conversion by TST was not. Birth outside the United States was associated with conversion by TST and T-SPOT, although the confidence intervals were wide for T-SPOT due to few conversion events.
While other studies in similar low TB incidence settings have compared IGRAs with TST in close contacts [11], [21], [22], [23], our study includes baseline and retest results for all three tests and relates the results to the TB case’s infectiousness. Grinsdale et al. [11] assessed the correlation of positive results with intensity, proximity, and duration of TB case exposure in 1,291 non-U.S.–born contacts who received either TST or QFT and concluded that QFT correlated better with measures of exposure. A German study of 812 close contacts that concluded IGRAs were more accurate gave QFT-GIT and T-SPOT only to TST-positive contacts and did not have baseline results for comparison [21]. Gonzślez-Moreno et al. [22] assessed concordance of TST, and QFT-GIT given simultaneously to 413 contacts but did not relate the results to cases’ infectiousness. A Dutch study gave QFT-GIT and T-SPOT to 433 immigrants with positive TST results and concluded that IGRA results are related to TB exposure in the countries of origin [23]. Although smear positivity and cavitary disease are primary indicators of TB patients’ infectiousness, we found an association of IGRA conversions only with cavitary disease. Marks et al. [14] found contacts exposed to highly smear-positive patients were more likely to have positive TSTs. We did not collect information on the degree of smear positivity. Another factor could be the known low sensitivity of sputum smears, which also depends on the quality of specimen collected [24]. Further, a study of household contacts in a high TB incidence setting showed that cough aerosols are a better predictor of TB transmission compared to sputum smear microscopy or culture. Even TB cases with AFB grade ≥ 3 + were aerosol negative and only high aerosol status was associated with TST conversion [25]. In our study, although more contacts were exposed to smear positive cases (n = 295/406, 73%) compared to TB cases with cavitary disease (n = 243/406, 60%) the proportion of conversions for the 3 tests were similar or slightly lower among contacts of smear positive cases [Supplementary material 2]. Together, these could explain the lack of association of smear status with test conversions and highlight cavitary disease as a better indicator of infectiousness. Jones-Lopez et al. [26] attributed discordance in TST and IGRA positivity among contacts to delayed IGRA conversions which could occur as late as 14–22 weeks after exposure [27]. However, the retest interval of 4–54 weeks after the baseline test for contacts in our study reflects public health practice that substantiates the validity of fewer IGRA conversions and is a strength of this study. Follow up of contacts for retest is a time-consuming activity and most health departments are not usually staffed to full capacity. Lack of a viable reminder system for retesting contacts and problems motivating contacts to return for retest could account for the great variation between baseline and retest intervals identified in this study [2].
Although retest interval is based on last known exposure to an infectious TB case (standard practice), we used the date of baseline test to calculate the retest interval. Therefore, a contact initially tested a few weeks after the last known exposure to the TB case, common in contact investigations, would have a shorter retest interval. Because the United States is a low TB incidence country and the risk of unknown exposure to TB is low, we do not attribute the test conversions after the standard 8–10-week interval to re-exposure.
Repeat TSTs among non-U.S.–born persons can stimulate waning immunity (boosting) related either to a previous M. tuberculosis infection or to receipt of BCG vaccine. Marks et al [14] reported that twice as many non-U.S.–born contacts converted by TST compared to U.S.-born contacts and attributed this to prior infection or boosting. We found a similar association with TST conversion at retest in non-U.S.–born compared to U.S.-born persons. We also compared median quantitative values at retest among converters with triple negative baseline results as previous studies suggest interferon gamma levels are associated with increased risk of TB [28]. Previous studies of quantitative IGRA values for contacts with a positive result lacked baseline values [21], [22].
Our study had limitations. We did not collect information about place of exposure or length or intensity of exposure, all of which affect transmission risk [11], [29]. Consistent with data that show only about 5% of close contacts convert on retest, our study saw few conversions, which resulted in wide IGRA confidence intervals [2], [14]. The study used a subset of a larger cohort study and was not independently powered to achieve the outcome. Study contacts were not a representative sample, because (1) they were recruited only from TBESC-affiliated clinics and (2) they included only persons who consented to be in the study. This may affect generalizability. We developed separate models for each test type and did not consider the correlation among the three test results within the same patient, which may affect the estimate of the standard errors of the parameters. We used QFT-GIT, which has since been replaced by QFT-Plus. However, we previously compared QFT-Plus with QFT-GIT in 500 participants in the larger TBESC study and found 94% concordance [30].
CDC guidelines have recommended either TST or IGRAs for recent contacts [31]. The use of IGRAs has been shown to be cost-effective in certain groups, including contacts [32].
5. Conclusion
Our study adds support for use of IGRAs to identify recently infected close contacts in low TB incidence setting where the risk of unknown exposure to TB is low. Tests for TB infection that more reliably identify recently infected contacts would allow health departments to focus scarce resources on those most likely to benefit from preventive treatment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors thank all study participants, site project coordinators, principal investigators, and co-investigators. The authors also thank Steve Kammerer, Division of Tuberculosis Elimination, CDC, for assistance and advice on statistical methods.
Source of Funding
The study was funded by contracts between the Centers for Disease Control and Prevention and participating sites (ClinicalTrials.gov: NCT01622140).
The findings and conclusions are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. References in this manuscript to any specific commercial products, process, service, manufacturer, or company does not constitute its endorsement or recommendation by the U.S. Government or CDC.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2023.100386.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.CDC. Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association and CDC. MMWR 2005; 54 (No.RR-17):1–47. [PubMed]
- 2.Reichler M.R., Reves R., Bur S., et al. Contact Investigation Study Group. Evaluation of investigations conducted to detect and prevent transmission of tuberculosis. JAMA. 2002;287(8):991–995. doi: 10.1001/jama.287.8.991. [DOI] [PubMed] [Google Scholar]
- 3.Reichler MR, Khan A, Sterling TR, et al., Tuberculosis Epidemiologic Studies Consortium Task Order 2 Team. Risk and Timing of Tuberculosis Among Close Contacts of Persons with Infectious Tuberculosis. J Infect Dis. 2018; 218(6):1000–8. 10.1093/infdis/jiy265. [DOI] [PMC free article] [PubMed]
- 4.Yuen C.M., Kammerer J.S., Marks K., Navin T.R., France A.M., Sreevatsan S. Recent Transmission of Tuberculosis—United States, 2011–2014. PLoS One. 2016;11(4):e0153728. doi: 10.1371/journal.pone.0153728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LoBue P.A., Mermin J.H. Latent tuberculosis infection: the final frontier of tuberculosis elimination in the USA. Lancet Infect Dis. 2017 Oct 1;17(10):e327–e333. doi: 10.1016/S1473-3099(17)30248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farhat M., Greenaway C., Pai M., Menzies D. False-positive tuberculin skin test: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuber Lung Dis. 2006;10:1192–12046. [PubMed] [Google Scholar]
- 7.Wang L., Turner M.O., Elwood R.K., et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;2002(57):804–809. doi: 10.1136/thorax.57.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pouchot J., Grasland A., Collet C., et al. Reliability of tuberculin skin test measurement. Ann Intern Med. 1997;126(3):210–214. doi: 10.7326/0003-4819-126-3-199702010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Mori T., Sakatani M., Yamagishi F., Takashima T., Kawabe Y., Nagao K., et al. Specific detection of tuberculosis infection: An interferon-γ-based assay using new antigens. Am J Respir Crit Care Med. 2004;170(1):59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- 10.Stauffer W.M., Peterson P.K., Baker C.A., Thomas W., Tsukayama D.T. Serial testing of refugees for latent tuberculosis using the QuantiFERON-gold in-tube: effects of an antecedent tuberculin skin test. Am J Trop Med Hyg. 2009;80(4):628–633. [PubMed] [Google Scholar]
- 11.Grinsdale J.A., Ho C.S., Banouvong H., Kawamura L.M. Programmatic impact of using QuantiFERON-TB Gold in routine contact investigation activities. Int J Tuberc Lung Dis. 2011;15(12):1614–1620. doi: 10.5588/ijtld.11.0102. [DOI] [PubMed] [Google Scholar]
- 12.Walters J.K., Sullivan A.D. Impact of routine QuantiFERON testing on latent tuberculosis diagnosis and treatment in refugees in Multnomah County, Oregon, November 2009-October 2012. J Immigr Minor Health. 2016;18(2):292–300. doi: 10.1007/s10903-015-0187-z. [DOI] [PubMed] [Google Scholar]
- 13.Shah M., DiPietro D., Greenbaum A., Ketemepi S., Martins-Evora M., Marsiglia V., et al. Programmatic impact of QuantiFERON-TB Gold In-Tube implementation on latent tuberculosis diagnosis and treatment in a public health clinic. PLoS One. 2012;7(5):e36551. doi: 10.1371/journal.pone.0036551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks SUZANNE M., Taylor ZACHARY, Qualls NOREEN L., Shrestha-kuwahara ROBIN J., Wilce MAUREEN A., Nguyen CRISTY H. Outcomes of contact investigations of infectious tuberculosis patients. Am J Respir Crit Care Med. 2000;162(6):2033–2038. doi: 10.1164/ajrccm.162.6.2004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. Tuberculosis Epidemiologic Studies Consortium: https://www.cdc.gov/tb/topic/research/tbesc/default.htm [Accessed on 3-26-20].
- 16.Ho C.S., Feng P.F., Narita M., et al. Comparison of three tests for latent tuberculosis infection in high-risk people in the USA: an observational cohort study. Lancet Infect Dis. 2022;22:85–96. doi: 10.1016/S1473-3099(21)00145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://www.quantiferon.com/us/wp-content/uploads/sites/13/2019/03/L1075116-QuantiFERON-TB-Gold-ELISA-IFU-FDA-rev04.pdf [Accessed on 3-26-20].
- 18.http://www.oxfordimmunotec.com/north-america/wp-content/uploads/sites/2/TB1.pdf [Accessed on 3-26-20].
- 19.CDC. https://www.cdc.gov/tb/publications/factsheets/testing/skintestresults.htm [Accessed on 3-26-20].
- 20.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Diel R., Loddenkemper R., Meywald-Walter K., Gottschalk R., Nienhaus A. Comparative performance of tuberculin skin test, QuantiFERON-TB-Gold In Tube assay, and T-Spot.TB test in contact investigations for tuberculosis. Chest. 2009;135(4):1010–1018. doi: 10.1378/chest.08-2048. [DOI] [PubMed] [Google Scholar]
- 22.Gonzślez-Moreno J., García-Gasalla M., Gállego-Lezaun C., Fernández-Baca V., Mir Viladrich I., Cifuentes-Luna C., et al. Role of QuantiFERON(®)-TB Gold In-Tube in tuberculosis contact investigation: experience in a tuberculosis unit. Infect Dis (Lond) 2015;47(4):244–251. doi: 10.3109/00365548.2014.987813. [DOI] [PubMed] [Google Scholar]
- 23.Kik S.V., Franken W.P., Arend S.M., et al. Interferon-gamma release assays in immigrant contacts and effect of remote exposure to Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009;13(7):820–828. [PubMed] [Google Scholar]
- 24.Mccarter Y.S., Robinson A. Quality Evaluation of Sputum Specimens for Mycobacterial Culture. Am J Clin Pathol. 1996;105(6):769–773. doi: 10.1093/ajcp/105.6.769. [DOI] [PubMed] [Google Scholar]
- 25.Jones-López E.C., Namugga O., Mumbowa F., Ssebidandi M., Mbabazi O., Moine S., et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: a household contact study. Am J Respir Crit Care Med. 2013;187(9):1007–1015. doi: 10.1164/rccm.201208-1422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones-López E.C., White L.F., Kirenga B., et al. Cough Aerosol Cultures of Mycobacterium tuberculosis: Insights on TST / IGRA Discordance and Transmission Dynamics. PLoS One. 2015 Sep 22;10(9):e0138358. doi: 10.1371/journal.pone.0138358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S.W., Oh D.K., Lee S.H., Kang H.Y., Lee C.T., Yim J.J. Time interval to conversion of interferon-gamma release assay after exposure to tuberculosis. Eur Respir J. 2011 Jun;37(6):1447–1452. doi: 10.1183/09031936.00089510. [DOI] [PubMed] [Google Scholar]
- 28.Winje BA, White R, Syre H, et al. Stratification by interferon-γ release assay level predicts risk of incident TB. Thorax. 2018 Apr 5:thoraxjnl-2017-211147. 10.1136/thoraxjnl-2017-211147. [DOI] [PubMed]
- 29.Reichler MR, Khan A, Yuan Y, et al. Tuberculosis Epidemiologic Studies Consortium Task Order 2 Team. Duration of exposure among close contacts of patients with infectious tuberculosis and risk of latent tuberculosis infection. Clin Infect Dis. 2020; Feb 11. pii: ciz1044. 10.1093/cid/ciz1044. [DOI] [PMC free article] [PubMed]
- 30.Venkatappa T.K., Punnoose R., Katz D.J., Higgins M.P., Banaei N., Graviss E.A., et al. Comparing QuantiFERON-TB Gold Plus with other tests to diagnose Mycobacterium tuberculosis infection. J Clin Microbiol. 2019;57(11) doi: 10.1128/JCM.00985-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CDC. Updated Guidelines for Using Interferon Gamma Release Assays to Detect Mycobacterium tuberculosis Infection — United States, 2010]. MMWR 2010;59 (No. RR-5): [1–25]. [PubMed]
- 32.Linas B.P., Wong A.Y., Freedberg K.A., Horsburgh C.R., Jr. Priorities for screening and treatment of latent tuberculosis infection in the United States. Am J Respir Crit Care Med. 2011;184(5):590–601. doi: 10.1164/rccm.201101-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.