This primer covers TAVR, Impella, intra-aortic balloon pumps, and VADs.
Central Message.
These topics constitute cutting-edge technology in cardiac surgery. This is not comprehensive but rather an introduction to familiarize readers with heart failure and endovascular therapies.
In this final section of the primer series, we close by covering a few more advanced topics in cardiac surgery. These topics address the burgeoning endovascular treatment of aortic stenosis, transcatheter aortic valve replacement (TAVR), and mechanical circulatory systems (MCS). Specifically, we will cover Impella devices, intra-aortic balloon pumps (IABPs), and left and right ventricular assist devices (VADs). Description of the device, its indication of use, and complications will be covered in each section.
Transcatheter Aortic Valve Replacement
Background and Indications
Initially pioneered in 1965, TAVR technology has advanced rapidly.1 Recently, TAVR has become the most commonly performed aortic valve replacement procedure, overtaking surgical aortic valve replacements in 2018.2 The Placement of Aortic Transcatheter Valves (PARTNER) 1, 2, and 3 trials established operative success for this procedure in nonsurgical/high-risk,3 intermediate-risk,4 and low-risk5 patient populations, respectively. The surgical risk is determined by the Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM), which is a multivariable risk model. An STS PROM <4% corresponds to low risk, 4% to 8% is intermediate risk, and >8% constitutes a high risk. As such, determining the STS PROM is a crucial part of the patient workup. The decision between TAVR and surgical aortic valve replacement is multifactorial, involving a joint heart team, patient annular/cusp anatomy, vascular access, and patient preference. The general indications and contraindications are provided in Table 1.6
Table 1.
Indications and contraindications for TAVR
| Indications | Contraindications |
|---|---|
| Severe AS with prohibitive surgical risk (STS PROM >8%) and >1-y expected survival | |
| Equivocal for age 65-80 y | Incompatible aortic annulus size (<18 mm or >29 mm) |
| Preferred in age >80 y | Active endocarditis |
| Short distance between annulus and coronary ostium | |
| Age <65 y∗ | |
| Life expectancy <1 y |
AS, Aortic stenosis; STS PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TAVR, transcatheter aortic valve replacement.
Relative contraindication.
Preoperative evaluation is aimed at determining valvular geometry and calcification, as well as peripheral vasculature to guide access-site selection.7 This is accomplished by gated computed tomography angiogram of the aortic annulus through the iliofemoral system. Specialized software provides orthogonal measurements of the aortic valve, termed the double-oblique image orientation. The parameters measured are the annular perimeter and area, sinus–commissure distances, sinus–sinus distances, and coronary ostia height (Figure 1). Of note, routine preoperative cardiac surgery patients (eg, coronary catheterization and transesophageal echo) are still warranted but not specific to TAVR.
Figure 1.
Example of preoperative measurements for TAVR. A, Major and minor aortic valve axis, perimeter, and area. B, Right coronary ostia height. C, Sinus distances. TAVR, Transcatheter aortic valve replacement.
Aortic annular and cusp calcifications are crucial to evaluate, as they are necessary for proper valve deployment. However, calcification, such as in an infra-annular position, may hinder valve deployment and seating. Coronary ostia height is a critical measurement—if they are less than 8 mm from the sinus of Valsalva, some valve sizes may cause occlusion of the ostia. In such cases, preemptive access of the coronary ostia is obtained with a subsequent stent deployment if occlusion occurs. Vascular access can be obtained via various methods, including femoral, axillary, carotid, transapical, transaortic, and transcaval. Access selection is based on patient factors (eg, several vessel calcification or tortuosity), TAVR device selection, and surgeon preference. Most access will be through the common femoral artery.
The TAVR devices can be broadly split into balloon-expandable and self-expandable types. Some current generation devices are the Sapien 3 (balloon-expandable; Edwards Lifesciences) and the Evolut PRO/R (self-expandable; Medtronic). However, as this technology matures and advances, more devices are and will be coming to market, increasing the cardiac surgeons’ armamentarium.8
Procedure
TAVR procedures are typically carried out in hybrid rooms with both interventional cardiology and cardiothoracic surgeons present. The reason for the use of a hybrid room is that fluoroscopy is first used for deployment of the valve. However, if complications arise necessitating an open conversion, the hybrid room is conducive to this change.
Vascular access is typically gained percutaneously via modified Seldinger technique. Access usually includes 2 arterial access points and 1 venous. One of the arterial sites is used for diagnostic purposes and a pigtail catheter to map annular anatomy. Subsequently, an aortogram is obtained to allow for optimal positioning of the C-arm such that the nadir of all 3 cusps is in the same plane. This facilitates the correct positioning and deployment of the transcatheter heart valve. The aortogram also assess for aortic regurgitation and coronary patency, which is important to compare pre- and postvalve deployment. Through the venous access site, a temporary transvenous pacer is deployed. This is used to pace the heart between 140 and 180 beats per minute during valve deployment; typically, lower rates are used for self-expanding valves and higher rates for balloon-expanding ones. This significantly reduces the cardiac output of the heart, which can cause malpositioning of the valve during deployment, potentially leading to a dislodged or embolized valve. The other arterial access site is used for creating an arterial roadmap and for deployment of the delivery system. The deployment system must cross the aortic valve to deploy the new valve; this is accomplished by first using a stiffer wire once access is gained across the aortic valve. Following deployment of the valve, positioning and functioning is assessed via echocardiography and a repeat aortogram.
It is important to note that most access methods result in a retrograde deployment of the valve. The main exception is with transapical, where the apparatus moves from the left ventricle and then across the aortic valve, deploying the valve in an antegrade fashion.
Outcomes
The outcomes of TAVR can be grouped into the hemodynamic performance of the valves over time and the mortality in each patient risk stratum. TAVR valves, as they are constructed from tissue, suffer structural valve degeneration, a process similar to their surgical tissue valve counterparts. Contemporary rates of structural valve deterioration are around 1.6% per 100-patient years.9 It is imperative to note that this is at 5-year follow-up, up where typical structural valve degeneration occurs around the first decade after implantation.10 Along similar lines, TAVR showed a marked and consistent decreased aortic transvalvular gradients, with mean gradients around 11 mm Hg.11
Currently, there are limited data on the long-term outcomes of TAVR in each of the 3 risk strata. The 5-year mortality in the PARTNER 1 trial was 71.8%, compared with 93.6% in the standard treatment group.12 For the PARTNER 2 trial, 5-year follow-up of all-cause death was 46% in the TAVR group compared with 42.1% in the surgical valve cohort; this difference was not significant.13 It is important to note that the composite of death or disabling stoke from TAVR surpassed the surgical valve cohort after the 3-year time point. Longer follow-up is eagerly awaited. Currently, only 2-year follow-up up data exist for the PARTNER 3 trial, which shows a lower mortality of 11.5% compared with 17.4% in the surgical valve cohort, with this difference being significant.14 Note that not covered here, but will become increasingly important, are valve-in-valve15 outcomes and surgical explant of TAVR valves.16
Complications
Despite being a minimally invasive surgery, TAVR is not without its complications. TAVR complications can be grouped into conduction issues, paravalvular leak (PVL), and vascular complications. Conduction issues occur due to increased radial force on the aortic annulus compared to sutured valves. Similarly, PVL occurs due to calcium in the leaflets or problems with valve deployment and positioning, which are not excised. Lastly, vascular complications occur due to gaining endovascular access.
The left bundle branch block (LBBB) is primary source of conduction derangements following TAVR. Current estimates place the incidence of LBBB between 10% and 22.9%.17,18 A significant subset of these patients will require a permanent place maker implantation. Development of am LBBB has been implicated in increasing mortality,19 and new pacemaker requirement carries a preestablished mortality risk.20 Some factors predisposing a patient to develop a LBBB and subsequent permanent place maker are a preexisting right bundle branch block and oversizing the TAVR valve.
Paravalvular leak is a harder entity to assess compared with conduction abnormalities. This is due to the stratification of PVL as mild, moderate, or severe. Although a large proportion of patients who undergo TAVR develop mild PVL, approximately 40%, recent data do not suggest that it worsens short-term outcomes.21 This is in comparison with greater degrees of PVL, approximately a 3% incidence, which have been implicated in increased mortality.11,22
Vascular complications are a general term containing entities such as aortic or annulus rupture, need for blood transfusion, or an unplanned surgical intervention.23 In the contemporary generation, increased understating of preoperative planning and patient selection in conjunction with improved TAVR devices have reduced these complications, as a composite end point, to approximately 6%.24 Note that this list is not exhaustive, but for brevity, only the most common complications were included.
MCS Support Overview
MCS devices are engineered devices that aim to augment or entirely replicate cardiovascular functioning. These devices can be used as destination therapy (if not a transplant candidate), bridge to transplant, bridge to decision, or as a temporizing measure in the short term. Short-term MCS devices are used over a short time period (days) to allow for recovery of the myocardium after a myocardial insult or the effects of cardiopulmonary bypass. If no improvement in myocardial functioning occurs and the cardiac output is still insufficient, then more long-term MCS solutions are warranted. It is important to note that extracorporeal membrane oxygenation (covered in Part I of this series) is a hybrid solution that spans both MCS categories.
Short Term
IABPs and Impella (Abiomed), devices are the main short-term MCS devices used, particularly in patients with end-stage heart failure. Despite differing mechanisms of action and effects on cardiovascular physiology, IABPs and Impella devices are both designed with the intention of providing temporary hemodynamic support and maintaining adequate end-organ perfusion in the setting of acute cardiogenic shock. As such, these devices are often used as temporizing measures while treating the underlying disease etiology or as a bridge to more durable destination therapies, such as surgically implanted VADs.
Intra-aortic balloon pump
Device detail
The main components of an IABP include an intravascular catheter with a balloon mounted at the tip; a protective sheath; tubing; and an external control console (Figure 2, A).25 In addition, the device contains radiopaque markings that are used to verify device placement. When deploying the device, the balloon should be positioned in the proximal descending aorta such that the tip lies distal to the left subclavian artery and the proximal portion ends just above the renal artery branches (Figure 2, B).25,26 The external control console regulates the delivery of helium into the balloon. Timing of inflation and deflation varies throughout the cardiac cycle and is most commonly controlled by electrocardiogram synchronization. This mechanism initiates balloon inflation during the middle of the T-wave (early diastole) and deflation at the R-wave (late diastole). Initially after IABP insertion, the balloon is set to inflate with every cardiac cycle (1:1). Then, as the patient’s hemodynamic status improves, the IABP can be weaned by setting the balloon to inflate every other cardiac cycle (1:2) or every third cardiac cycle (1:3). However, less-frequent balloon inflation promotes stasis and thrombus formation, and these settings are only used temporarily minutes to hours before IABP removal. In patients with dysrhythmias not amenable to reliable electrocardiogram synchronization, arterial blood pressure triggering can be used. In this setting, diastolic inflation is initiated at aortic valve closure (dicrotic notch) and deflation during systolic upstroke. Irrespective of triggering modality, IABPs delivers hemodynamic support from the proximal descending aorta by inflating during diastole and deflating during systole. This mechanism is known as diastolic counterpulsation.
Figure 2.
Schematic and position of an IABP. A, Schematic of the key components of an IABP. B, Graphical representation and radiograph of the chest showing proper IABP placement. IABP, Intra-aortic balloon pump.
The radiographic image is courtesy of Dr Fahad Dilawez Rathore, Radiopaedia.org, rID: 74994.
Device use
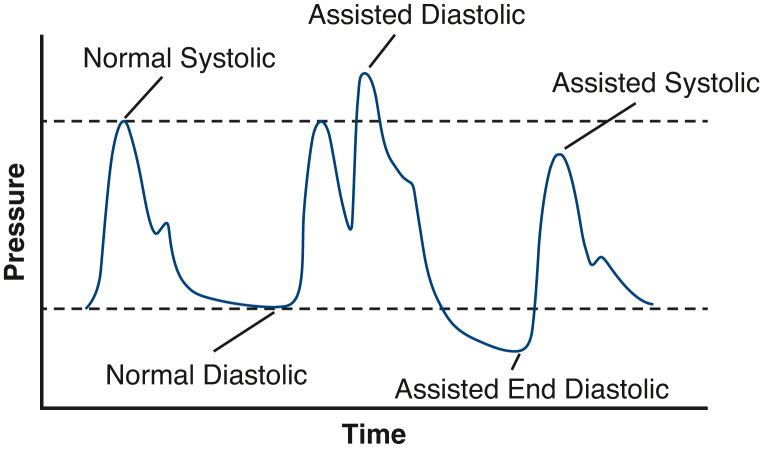
The 2 direct physiologic effects of the IABP are increased coronary perfusion pressure (CPP) and decreased left ventricular afterload. CPP is defined as the pressure gradient between the coronary sinuses in the aortic root and the left ventricle at end diastole and is an important determinant of coronary blood flow.27 During normal diastole, the aortic valve closes, and arterial blood pressure is maintained by aortic elastic recoil. In patients supported with IABPs, diastolic inflation of the device displaces blood toward the aortic root, thereby increasing diastolic blood pressure and CPP. IABP-mediated increases in CPP are thought to drive increased coronary blood flow and myocardial oxygen supply in the failing heart.28,29 During ventricular systole, IABP deflation creates a suction-like effect that reduces left ventricular afterload. This afterload reduction leads to decreased wall stress and lowered myocardial oxygen demand. Taken together, IABP counterpulsation indirectly improves cardiac output by augmenting coronary flow, reducing left ventricular afterload, and lowering myocardial oxygen demand. A schematic representation of unassisted and IABP-assisted aortic pressure changes is shown in Figure 3.
Figure 3.
Representative blood pressure tracing with and without intra-aortic balloon pump support.
Impella (CP/5.5/RV)
Device detail
The components of an Impella include an intravascular pigtail catheter with a microaxial flow pump composed of an inflow area, cannula, outflow area, and motor unit; plastic suture hubs; a protective sheath; tubing; and an external control console (Figure 4, A).30 In addition to radiopaque markers, the Impella also contains pressure transducers to determine appropriate positioning. The Impella device is positioned in the heart such that the pigtail tip and pump inflow area are within the left ventricular cavity (Figure 4, B).30,31 The cannula sits across the aortic valve, and the outflow area and motor unit are within the aortic root lumen. Although most Impella family devices were designed with the intention of supporting left ventricular function, the Impella RP is a more recent device specifically configured to support the failing right ventricle.32,33 For this device, the pump inflow is positioned in the inferior vena cava, the catheter passes into the heart and traverses the tricuspid and pulmonary valves, and the outflow area and motor unit are within the main pulmonary artery lumen. There are multiple versions of the Impella device and each provide a different level of maximal flow support (eg, Impella 2.5 = 2.5 L/min, Impella CP = 4.0 L/min, Impella R = 4.0 L/min, Impella 5.0 = 5.0 L/min).30, 34
Figure 4.
Impella device schematic and echocardiographic placement. A, Representation of the key components of the Impella device. B, Transesophageal echocardiography representative image of proper Impella placement.
Image taken from Abiomed30 Clinical Reference Manual.
Device use
The Impella serves as a pump that supplements antegrade flow across the aortic valve and into the aorta. Impaired left ventricular contractility decreases cardiac output and promotes dilation of the affected chamber. The Impella directly increases net cardiac output and simultaneously reduces the volume load and myocardial oxygen demand of the left ventricle.35 These hemodynamic changes significantly enhance cardiac performance and end-organ perfusion.35 It is important to note that an Impella device can be used as a left ventricle vent for patients on venoarterial extracorporeal membrane oxygenation. These devices use a fluid buffer that is run through the device and into the patient. This buffer serves as a physical barrier between the surface of the device and the blood. This fluid is typically heparin, but institutions are trialing bicarbonate. In either case, the device calculates the volume given to the patient, which is necessary for proper patient management.
Device deployment
Both IABP and Impella devices are traditionally inserted into the common femoral artery either percutaneous or surgical cut down techniques.15 In patients with unsuitable femoral anatomy, axillary or subclavian arteries may serve as alternative access sites. As previously mentioned, the IABP is positioned in the proximal descending aorta. Confirmation of IABP position can be evaluated by chest radiography (balloon tip 2-3 cm below the aortic knob) or fluoroscopy (Figure 2, B).15 For the Impella, the pump inflow area should be positioned ∼3.5 cm below the aortic valve annulus (Figure 4, B).36 The Impella should be placed such that it passes through the center of the aortic valve leaflets and sits in the middle of the left ventricular lumen. Fluoroscopy and transesophageal echocardiography are used to guide proper device placement.
Indications and complications
Given the numerous etiologies of cardiogenic shock and the diverse ways in which temporary mechanical circulatory support devices are used in clinical practice, the indications for IABP and Impella use have grown over time.37, 38, 39 A list of the indications and contraindications for these devices is shown in Table 2.
Table 2.
Indications and contraindications for IABP and Impella devices
| Considerations | IABP | Impella |
|---|---|---|
| Duration | Days | 4-6 d |
| Indications | ||
| LV failure | LV failure within 2 d of MI or heart surgery | |
| Prophylactic support for high-risk PCI | Acute LV failure from cardiomyopathy | |
| Prophylactic support for severe mitral regurgitation or preoperative low cardiac output state | ||
| Postoperative uncontrolled myocardial ischemia | ||
| Contraindications | ||
| Significant aortic valve regurgitation | Significant aortic valve regurgitation | |
| Aortic aneurysm or dissection | Mechanical aortic valve prosthesis | |
| Significant peripheral vascular disease | Significant peripheral vascular disease | |
| Uncontrolled sepsis | Mural thrombus in left ventricle | |
| Uncontrolled bleeding | Ventricular septal defect | |
| LV myocardial rupture | ||
| Refractory respiratory failure requiring oxygenator | ||
| Cardiac tamponade |
IABP, Intra-aortic balloon pump; LV, left ventricle; MI, myocardial infarction; PCI, percutaneous coronary intervention.
The most common complications for IABPs and Impellas include vascular access-site bleeding, any vascular bleeding, thromboembolic events causing limb ischemia, and device migration.40, 41, 42 Although the risk of bleeding complications is greatest during device insertion and removal, these events can occur at any time during the support period. The risk of bleeding is exacerbated by the fact that patients who receive IABPs are often on therapeutic anticoagulation and may develop device-related thrombocytopenia.43 Similarly, patients supported by Impellas require anticoagulation and commonly experience device-related hemolysis.44 Therefore, it is critical that the access site is continually evaluated for the presence of active bleeding, hematoma formation, pseudoaneurysm development, or ecchymosis in addition to ordering daily complete blood counts. With regard to thromboembolic complications, judicious assessment of distal pulses before and throughout the duration of device use is essential. To ensure appropriate device position, daily monitoring of IABP position using chest radiography should be performed. The positioning of Impella devices can be determined by evaluating signal waveforms on the controller unit. Suspected migration of the Impella should be evaluated by fluoroscopy or echocardiography. Finally, a rare but severe complication of IABP devices is balloon rupture (<2% of patients). This manifests as blood within the balloon catheter and should prompt a helium leak alarm from the controller console. When this occurs, the IABP should be immediately stopped and promptly removed to avoid device thrombosis.
Long Term
Ventricular assist devices
VADs supplement the cardiac output of either the left or right ventricle. A left ventricular assist device (LVAD) flows blood from the left ventricle and flows it directly into the ascending aorta. A right ventricular assist device (RVAD) flows blood from the right atrium, or rarely the right ventricle, into the pulmonary arteries. RVADs are most commonly used in conjunction with an LVAD, commonly termed a biventricular assist device. As these are the most durable MCS devices, they can be used in bridge to transplant, destination therapy, bridge to decision, and bridge to recovery situations. LVADs represent the majority of VADs used.
Device detail
The current generation of LVADs use a centrifugal pump design, as it has shown superior outcomes compared to an axil-flow device, with a reduction in pump thrombosis being a major improvement.45 An important consideration of centrifugal pumps is that they are preload and afterload sensitive since they do not directly move a volume of blood but establish a pressure gradient. The LVAD has a low profile with a metal cannula used for LV insertion. The outflow of the LVAD is connected to Dacron tubing, which is anastomosed with the ascending aorta. Connected to the LVAD is a powerline that is tunneled through the skin to outside of the body. The most used model currently is the HeartMate 3 (Abbott).
RVADs have a different design owning to their differences in deployment. Because they are not directly fixed to the ventricle, the motor is typically outside of the patient, with tunneled or intravascular lines connecting to the right atrium and the pulmonary artery; these motors are still centrifugal. Some examples of an RVAD are ProtekDuo (TandemLife/LivaNova), TandemHeart (Cardiac Assist Inc), and CentriMag (Abbott).
Device use
The decision to use VAD devices is often a challenging one, necessitating a multidisciplinary team to evaluate each patient; indications and contraindications are presented in Table 3. This is particularly important as recent United Network for Organ Sharing allocation policy has decreased priority for patients with a VAD.46 In general, LVADs are used in the failing left ventricle when maximum medical therapy is unable to abate symptoms. Typically, the Interagency Registry for Mechanically Assisted Circulatory Support profile is used to help stratify patients and direct device allocation.47 Key considerations involve the current RV function (when considering a LVAD), presence of valvular disease, and psychosocial evaluation, as these devices require routine and vital maintenance by the patient. These considerations are also valid for those being evaluated for a RVAD. The most common reason for RVAD use is when right ventricular dysfunction occurs following LVAD implantation. Other indications are with acute right ventricular dysfunction refractory to medical management.48
Table 3.
Indications and contraindications for ventricular assist devices
| Indications | Contraindications |
|---|---|
| NYHA IV | Right ventricular dysfunction∗ |
| LVEF ≤25% | Inadequate social support system |
| Systolic BP <80 mm Hg (cardiac index <2.0) | Anatomical incompatibility |
| Pulmonary capillary wedge pressure ≥20 mm Hg | Terminal comorbidity |
| Aforementioned symptoms despite maximum medical therapy | Neurologic compromise |
| Small body surface area (<1.2) | |
| Bleeding/thrombocytopenia |
NYHA, New York Heart Association, LVEF, left ventricular ejection fraction; BP, blood pressure.
Applicable for left ventricular assist device only.
The goal of implantation of an LVAD is proper positioning of the inflow canula at the apex of the left ventricle.49 Improper positioning can decrease the preload to the device and cause suck-down events, where the cannula makes contact with the left ventricular cavity or interventricular septum.50 An anchoring ring is first sutured onto the left ventricular apex. This serves as a fixation point of the inflow canula, allowing for better positioning and fixation of the entire device. Once the motor and inflow tract are secured to the anchoring ring, the outflow graft is sutured to the aorta. The typical placement is 2 cm from the sinotubular junction with the graft beveled between 45° and 60°. This facilitates a good lay of the graft, reducing redundancy and angulation in the graft. For brevity, and the nuances with each RVAD, the implantation techniques have been omitted and readers are directed to the following articles.51,52
Outcomes
The outcomes of patients with LVADs cannot be summarized by a single metric, as their indication for LVAD implant significantly changes their trajectory (eg, destination therapy vs bridge to recovery). Further complicating matters, institutional preference can differ on the categorization of patients and their indication for LVAD implantation. Unsurprisingly, the best results are found in patients who are bridge to recovery, showing LV function comparable with healthy control patients.53 The percentage of patients who reach this milestone ranges from 5% to 24%, likely owning to differences in institutional protocols. For patients bridging to transplant, 1-year survival has been reported at 84%.54 In destination therapy patients, the 2-year survival has been steadily improving and is now 60%.55 Biventricular assist device placement (LVAD plus RVAD) has shown worse outcomes compared with LVAD alone, with a short-term mortality as high as 45%.56, 57, 58 For isolated RVAD implantation, 30-day mortality has been reported at 50%.59 However, it must be noted that this represents a small proportion of RVAD use. With technological improvements, better patient selection, and improvements of concomitant medical therapy, VAD outcomes have seen substantial improvement and likely will continue to do so.
Complications
The most catastrophic complication of LVAD use is the development of right ventricular failure following implantation. Recent estimates place the incidence of this complication at 13%, with patients requiring inotropic support or RVAD implantation.60 Given the severity of this complication, efforts have focused on preoperative stratification of patients right ventricular functioning and likelihood of developing right ventricular failure. In general, right ventricular metrics indicating good functioning (eg, cavity size, interventricular septum movement, and tricuspid annular plane systolic excursion) have been shown to be protective.61,62
Following right ventricular dysfunction, thromboembolic events are another potentially devastating complication, particularly if they result in a stroke. Due to the blood contacting surface of VADs, clotting and embolization can occur despite anticoagulation. Recent data place the incidence of stroke as low as 12% and as high as approximately 30%.63,64 Pump thrombosis can serve as a nidus for strokes and has a reported incidence between 8.1% and 11%.65,66 Management includes increased anticoagulation, facilitating fibrinolysis, up to a complete pump exchange. Overall, these complications have not seen much progress despite improvements in VAD technology and patient selection, illustrating the fundamental issue with blood contacting surfaces.
On the other end of the spectrum, bleeding is also a complication of VAD use. Although anticoagulation predisposes patients to bleeding, the cause of bleeding in VAD patients is more complex. Bleeding typically occurs in the gastrointestinal system and affects up to 30% of patients.67 In addition to the anticoagulation, hemostasis is aggravated by a Heyde-like syndrome, due to the continuous flow of current generation VADs, and impaired platelet aggregation.68,69
All current-generation VADs are powered with an external energy source. As such, a permanent break in the skin occurs from the tunneled drive lines; this results in a continual infection risk. The estimated prevalence of driveline infections is 20% and is responsible for 8.1% of deaths at 3 months.70,71 Preventative measure are key are reducing infection, including correct bandage changes and patient education. Unfortunately, transplantation is the only effective solution, with other methods prolonging survival without cessation of the infection.
Lastly, the development of aortic insufficiency (AI) can be challenging in this patient population. When AI develops after LVAD implantation, the actual systemic cardiac output of the LVAD can be compromised, increasing mortality—this is due to a circular flow pattern retrograde into the lower pressured LV.72 LVADs can both cause de novo AI or worsen preexisting AI. When stratified based on preoperative AI severity, mild AI had the highest development of significant AI (36.4%), with de novo severe AI occurring in 10% of patients.73 The treatment of AI, both pre- and post-LVAD is inconsistent across institutions. Preexisting AI can be treated by suturing the valve closed or replacing it at time of LVAD implant.74 New AI can similarly be treated surgically or with endovascular valve replacement or with closure devices.75
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Davies H. Catheter-mounted valve for temporary relief of aortic insufficiency. Lancet. 1965;285:250. [Google Scholar]
- 2.Carroll J.D., Mack M.J., Vemulapalli S., Herrmann H.C., Gleason T.G., Hanzel G., et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 3.Leon M.B., Smith C.R., Mack M., Miller D.C., Moses J.W., Svensson L.G., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 4.Leon M.B., Smith C.R., Mack M.J., Makkar R.R., Svensson L.G., Kodali S.K., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 5.Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 6.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., Gentile F., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 7.Francone M., Budde R.P.J., Bremerich J., Dacher J.N., Loewe C., Wolf F., et al. CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR) Eur Radiol. 2020;30:2627–2650. doi: 10.1007/s00330-019-06357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eikelboom R., Moran R.M., Yan W., Yamashita M., Patel A., Reardon M., et al. Current and future transcatheter aortic valve replacement valves. Curr Opin Cardiol. 2022;37:173–179. doi: 10.1097/HCO.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 9.Pibarot P., Ternacle J., Jaber W.A., Salaun E., Dahou A., Asch F.M., et al. Structural deterioration of transcatheter versus surgical aortic valve bioprostheses in the PARTNER-2 trial. J Am Coll Cardiol. 2020;76:1830–1843. doi: 10.1016/j.jacc.2020.08.049. [DOI] [PubMed] [Google Scholar]
- 10.Sellers S.L., Blanke P., Leipsic J.A. Bioprosthetic heart valve degeneration and dysfunction: focus on mechanisms and multidisciplinary imaging considerations. Radiol Cardiothorac Imaging. 2019;1 doi: 10.1148/ryct.2019190004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodali S., Thourani V.H., White J., Malaisrie S.C., Lim S., Greason K.L., et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J. 2016;37:2252–2262. doi: 10.1093/eurheartj/ehw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapadia S.R., Leon M.B., Makkar R.R., Tuzcu E.M., Svensson L.G., Kodali S., et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–2491. doi: 10.1016/S0140-6736(15)60290-2. [DOI] [PubMed] [Google Scholar]
- 13.Makkar R.R., Thourani V.H., Mack M.J., Kodali S.K., Kapadia S., Webb J.G., et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. 2020;382:799–809. doi: 10.1056/NEJMoa1910555. [DOI] [PubMed] [Google Scholar]
- 14.Leon M.B., Mack M.J., Hahn R.T., Thourani V.H., Makkar R., Kodali S.K., et al. Outcomes 2 Years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77:1149–1161. doi: 10.1016/j.jacc.2020.12.052. [DOI] [PubMed] [Google Scholar]
- 15.Giordana F., Bruno F., Conrotto F., Saglietto A., D'Ascenzo F., Grosso Marra W., et al. Incidence, predictors and outcomes of valve-in-valve TAVI: a systematic review and meta-analysis. Int J Cardiol. 2020;316:64–69. doi: 10.1016/j.ijcard.2020.05.058. [DOI] [PubMed] [Google Scholar]
- 16.Fukuhara S., Brescia A.A., Shiomi G.M., Rosati C.M., Yang B., Kim K.M., et al. Surgical explantation of transcatheter aortic bioprostheses. Circulation. 2020;142:2285–2287. doi: 10.1161/CIRCULATIONAHA.120.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nazif T.M., Dizon J.M., Hahn R.T., Xu K., Babaliaros V., Douglas P.S., et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv. 2014;8:60–69. doi: 10.1016/j.jcin.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Arri S.S., Myat A., Malik I., Curzen N., Baumbach A., Gunning M., et al. New onset left bundle branch block after transcatheter aortic valve implantation and the effect on long-term survival—a UK wide experience. Eur Heart J. 2020;41 [Google Scholar]
- 19.Regueiro A., Abdul-Jawad Altisent O., Del Trigo M., Campelo-Parada F., Puri R., Urena M., et al. Impact of new-onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;9 doi: 10.1161/CIRCINTERVENTIONS.115.003635. [DOI] [PubMed] [Google Scholar]
- 20.Glaser N., Persson M., Dalén M., Sartipy U. Long-term outcomes associated with permanent pacemaker implantation after surgical aortic valve replacement. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.16564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thourani V.H., Kodali S., Makkar R.R., Herrmann H.C., Williams M., Babaliaros V., et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. doi: 10.1016/S0140-6736(16)30073-3. [DOI] [PubMed] [Google Scholar]
- 22.Kodali S.K., Williams M.R., Smith C.R., Svensson L.G., Webb J.G., Makkar R.R., et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 23.Kappetein A.P., Head S.J., Généreux P., Piazza N., van Mieghem N.M., Blackstone E.H., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document. J Thorac Cardiovasc Surg. 2012;145:6–23. doi: 10.1016/j.jtcvs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Reardon M.J., Van Mieghem N.M., Popma J.J., Kleiman N.S., Søndergaard L., Mumtaz M., et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 25.González L.S., Chaney M.A. Intraaortic balloon pump counterpulsation, part I: history, technical aspects, physiologic effects, contraindications, medical applications/outcomes. Anesth Analg. 2020;131:776–791. doi: 10.1213/ANE.0000000000004954. [DOI] [PubMed] [Google Scholar]
- 26.Gajanan G., Brilakis E.S., Siller-Matula J.M., Zolty R.L., Velagapudi P. The intra-aortic balloon pump. J Vis Exp. 2021 doi: 10.3791/62132. [DOI] [PubMed] [Google Scholar]
- 27.van de Hoef T.P., Siebes M., Spaan J.A., Piek J.J. Fundamentals in clinical coronary physiology: why coronary flow is more important than coronary pressure. Eur Heart J. 2015;36:3312–3319a. doi: 10.1093/eurheartj/ehv235. [DOI] [PubMed] [Google Scholar]
- 28.Williams D.O., Korr K.S., Gewirtz H., Most A.S. The effect of intraaortic balloon counterpulsation on regional myocardial blood flow and oxygen consumption in the presence of coronary artery stenosis in patients with unstable angina. Circulation. 1982;66:593–597. doi: 10.1161/01.cir.66.3.593. [DOI] [PubMed] [Google Scholar]
- 29.Kern M.J., Aguirre F.V., Tatineni S., Penick D., Serota H., Donohue T., et al. Enhanced coronary blood flow velocity during intraaortic balloon counterpulsation in critically ill patients. J Am Coll Cardiol. 1993;21:359–368. doi: 10.1016/0735-1097(93)90676-r. [DOI] [PubMed] [Google Scholar]
- 30.Abiomed . Abiomed; 2022. Impella Ventricular Support Systems for Use During Cardiogenic Shock and High-Risk PCI Instructions for Use and Clinical Reference Manual. [Google Scholar]
- 31.Anderson B.B., Collard C.D. Images in anesthesiology: proper positioning of an Impella 2.5 and CP heart pump. Anesthesiology. 2017;127:1014. doi: 10.1097/ALN.0000000000001791. [DOI] [PubMed] [Google Scholar]
- 32.Akhmerov A., Ramzy D. Mechanical circulatory support in right ventricular failure. Interv Cardiol Clin. 2021;10:185–194. doi: 10.1016/j.iccl.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Welker C., Huang J., Nunez-Gil I.J., Villavicencio M.A., Ramakrishna H. Percutaneous right ventricular mechanical circulatory support: analysis of recent data. J Cardiothorac Vasc Anesth. 2022;36:2783–2788. doi: 10.1053/j.jvca.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 34.Burzotta F., Trani C., Doshi S.N., Townend J., van Geuns R.J., Hunziker P., et al. Impella ventricular support in clinical practice: collaborative viewpoint from a European expert user group. Int J Cardiol. 2015;201:684–691. doi: 10.1016/j.ijcard.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 35.Gottula A.L., Shaw C.R., Milligan J., Chuko J., Lauria M., Swiencki A., et al. Impella in transport: physiology, mechanics, complications, and transport considerations. Air Med J. 2022;41:114–127. doi: 10.1016/j.amj.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Tran T., Mudigonda P., Mahr C., Kirkpatrick J. Echocardiographic imaging of temporary percutaneous mechanical circulatory support devices. J Echocardiogr. 2022;20:77–86. doi: 10.1007/s12574-022-00563-y. [DOI] [PubMed] [Google Scholar]
- 37.González L.S., Chaney M.A. Balloon pump counterpulsation part II: perioperative hemodynamic support and new directions. Anesth Analg. 2020;131:792–807. doi: 10.1213/ANE.0000000000004999. [DOI] [PubMed] [Google Scholar]
- 38.Balthazar T., Vandenbriele C., Verbrugge F.H., Den Uil C., Engström A., Janssens S., et al. Managing patients with short-term mechanical circulatory support: JACC review topic of the week. J Am Coll Cardiol. 2021;77:1243–1256. doi: 10.1016/j.jacc.2020.12.054. [DOI] [PubMed] [Google Scholar]
- 39.Zhou A.L., Etchill E.W., Giuliano K.A., Shou B.L., Sharma K., Choi C.W., et al. Bridge to transplantation from mechanical circulatory support: a narrative review. J Thorac Dis. 2021;13:6911–6923. doi: 10.21037/jtd-21-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Jong M.M., Lorusso R., Al Awami F., Matteuci F., Parise O., Lozekoot P., et al. Vascular complications following intra-aortic balloon pump implantation: an updated review. Perfusion. 2018;33:96–104. doi: 10.1177/0267659117727825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ancona M.B., Montorfano M., Masiero G., Burzotta F., Briguori C., Pagnesi M., et al. Device-related complications after Impella mechanical circulatory support implantation: an IMP-IT observational multicentre registry substudy. Eur Heart J Acute Cardiovasc Care. 2021;10:999–1006. doi: 10.1093/ehjacc/zuab051. [DOI] [PubMed] [Google Scholar]
- 42.Papolos A.I., Barnett C.F., Tuli A., Vavilin I., Kenigsberg B.B. Impella management for the cardiac intensivist. ASAIO J. 2022;68:753–758. doi: 10.1097/MAT.0000000000001680. [DOI] [PubMed] [Google Scholar]
- 43.Vonderheide R.H., Thadhani R., Kuter D.J. Association of thrombocytopenia with the use of intra-aortic balloon pumps. Am J Med. 1998;105:27–32. doi: 10.1016/s0002-9343(98)00128-4. [DOI] [PubMed] [Google Scholar]
- 44.Badiye A.P., Hernandez G.A., Novoa I., Chaparro S.V. Incidence of hemolysis in patients with cardiogenic shock treated with Impella percutaneous left ventricular assist device. ASAIO J. 2016;62:11–14. doi: 10.1097/MAT.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 45.Mehra M.R., Goldstein D.J., Uriel N., Cleveland J.C., Yuzefpolskaya M., Salerno C., et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378:1386–1395. doi: 10.1056/NEJMoa1800866. [DOI] [PubMed] [Google Scholar]
- 46.Liu J., Yang B.Q., Itoh A., Masood M.F., Hartupee J.C., Schilling J.D. Impact of new UNOS allocation criteria on heart transplant practices and outcomes. Transplant Direct. 2021;7:e642. doi: 10.1097/TXD.0000000000001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevenson L.W., Pagani F.D., Young J.B., Jessup M., Miller L., Kormos R.L., et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2008;28:535–541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Kapur N.K., Esposito M.L., Bader Y., Morine K.J., Kiernan M.S., Pham D.T., et al. Mechanical circulatory support devices for acute right ventricular failure. Circulation. 2017;136:314–326. doi: 10.1161/CIRCULATIONAHA.116.025290. [DOI] [PubMed] [Google Scholar]
- 49.Adamson R.M., Mangi A.A., Kormos R.L., Farrar D.J., Dembitsky W.P. Principles of HeartMate II implantation to avoid pump malposition and migration. J Card Surg. 2014;30:296–299. doi: 10.1111/jocs.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han J.J., Acker M.A., Atluri P. Left ventricular assist devices. Circulation. 2018;138:2841–2851. doi: 10.1161/CIRCULATIONAHA.118.035566. [DOI] [PubMed] [Google Scholar]
- 51.Abdelshafy M., Caliskan K., Guven G., Elkoumy A., Elsherbini H., Elzomor H., et al. Temporary right-ventricular assist devices: a systematic review. J Clin Med. 2022;11:613. doi: 10.3390/jcm11030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Potapov E., Starck C., Falk V., Eulert-Grehn J.J. Mechanical circulatory support: technical tips for the implantation of a right ventricular assist device. JTCVS Open. 2021;8:37–40. doi: 10.1016/j.xjon.2021.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jakovljevic D.G., Yacoub M.H., Schueler S., MacGowan G.A., Velicki L., Seferovic P.M., et al. Left ventricular assist device as a bridge to recovery for patients with advanced heart failure. J Am Coll Cardiol. 2017;69:1924–1933. doi: 10.1016/j.jacc.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slaughter M.S., Pagani F.D., McGee E.C., Birks E.J., Cotts W.G., Gregoric I., et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2012;32:675–683. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Rogers J.G., Butler J., Lansman S.L., Gass A., Portner P.M., Pasque M.K., et al. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J Am Coll Cardiol. 2007;50:741–747. doi: 10.1016/j.jacc.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 56.Reid G., Mork C., Gahl B., Appenzeller-Herzog C., von Segesser L.K., Eckstein F., et al. Outcome of right ventricular assist device implantation following left ventricular assist device implantation: systematic review and meta-analysis. Perfusion. 2021;37:773–784. doi: 10.1177/02676591211024817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda K., Naka Y., Yang J.A., Uriel N., Colombo P.C., Jorde U.P., et al. Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J Heart Lung Transplant. 2014;33:141–148. doi: 10.1016/j.healun.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 58.Yoshioka D., Takayama H., Garan R.A., Topkara V.K., Han J., Kurlansky P., et al. Contemporary outcome of unplanned right ventricular assist device for severe right heart failure after continuous-flow left ventricular assist device insertion. Interact Cardiovasc Thorac Surg. 2017;24:828–834. doi: 10.1093/icvts/ivw409. [DOI] [PubMed] [Google Scholar]
- 59.Bernhardt A.M., De By T.M., Reichenspurner H., Deuse T. Isolated permanent right ventricular assist device implantation with the HeartWare continuous-flow ventricular assist device: first results from the European Registry for Patients with Mechanical Circulatory Support. Eur J Cardiothorac Surg. 2015;48:158–162. doi: 10.1093/ejcts/ezu406. [DOI] [PubMed] [Google Scholar]
- 60.Kormos R.L., Teuteberg J.J., Pagani F.D., Russell S.D., John R., Miller L.W., et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–1324. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 61.Argiriou M., Kolokotron S.-M., Sakellaridis T., Argiriou O., Charitos C., Zarogoulidis P., et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis. 2014;6 Suppl 1(Suppl 1):S52–S59. doi: 10.3978/j.issn.2072-1439.2013.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frankfurter C., Molinero M., Vishram-Nielsen J.K.K., Foroutan F., Mak S., Rao V., et al. Predicting the risk of right ventricular failure in patients undergoing left ventricular assist device implantation. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.006994. [DOI] [PubMed] [Google Scholar]
- 63.Rogers J.G., Pagani F.D., Tatooles A.J., Bhat G., Slaughter M.S., Birks E.J., et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376:451–460. doi: 10.1056/NEJMoa1602954. [DOI] [PubMed] [Google Scholar]
- 64.Kirklin J.K., Naftel D.C., Pagani F.D., Kormos R.L., Stevenson L.W., Blume E.D., et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Najjar S.S., Slaughter M.S., Pagani F.D., Starling R.C., McGee E.C., Eckman P., et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;33:23–34. doi: 10.1016/j.healun.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Uriel N., Han J., Morrison K.A., Nahumi N., Yuzefpolskaya M., Garan A.R., et al. Device thrombosis in HeartMate II continuous-flow left ventricular assist devices: a multifactorial phenomenon. J Heart Lung Transplant. 2013;33:51–59. doi: 10.1016/j.healun.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Cushing K., Kushnir V. Gastrointestinal bleeding following LVAD placement from top to bottom. Dig Dis Sci. 2016;61:1440–1447. doi: 10.1007/s10620-016-4123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heyde E. Gastrointestinal bleeding in aortic stenosis. N Engl J Med. 1958;259:196. [Google Scholar]
- 69.Suarez J., Patel C.B., Felker G.M., Becker R., Hernandez A.F., Rogers J.G. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Heart Fail. 2011;4:779–784. doi: 10.1161/CIRCHEARTFAILURE.111.962613. [DOI] [PubMed] [Google Scholar]
- 70.Kirklin J.K., Naftel D.C., Kormos R.L., Pagani F.D., Myers S.L., Stevenson L.W., et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014;33:12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 71.Trachtenberg B.H., Cordero-Reyes A., Elias B., Loebe M. A review of infections in patients with left ventricular assist devices: prevention, diagnosis and management. Methodist Debakey Cardiovasc J. 2015;11:28–32. doi: 10.14797/mdcj-11-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Truby L.K., Garan A.R., Givens R.C., Wayda B., Takeda K., Yuzefpolskaya M., et al. Aortic insufficiency during contemporary left ventricular assist device support: analysis of the INTERMACS registry. JACC Heart Fail. 2018;6:951–960. doi: 10.1016/j.jchf.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kagawa H., Aranda-Michel E., Kormos R.L., Keebler M., Hickey G., Wang Y., et al. Aortic insufficiency after left ventricular assist device implantation: predictors and outcomes. Ann Thorac Surg. 2020;110:836–843. doi: 10.1016/j.athoracsur.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 74.Atkins B.Z., Hashmi Z.A., Ganapathi A.M., Harrison J.K., Hughes G.C., Rogers J.G., et al. Surgical correction of aortic valve insufficiency after left ventricular assist device implantation. J Thorac Cardiovasc Surg. 2013;146:1247–1252. doi: 10.1016/j.jtcvs.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 75.Kar B., Prathipati P., Jumean M., Nathan S.S., Gregoric I.D. Management of aortic insufficiency using transcatheter aortic valve replacement in patients with left ventricular assist device support. ASAIO J. 2020;66:e82–e86. doi: 10.1097/MAT.0000000000001053. [DOI] [PubMed] [Google Scholar]