The mitochondria, apart from balancing optimal cellular homeostasis and energetics to sustain life, also mediate pathophysiological mechanisms leading to injury and death. In this regard, mitochondrial transplantation is emerging as a novel therapeutic strategy to circumvent deleterious effects of mitochondrial dysfunction in various pathological disorders. The transplantation of mitochondria involves the process of isolation, selection of optimal mitochondria, transfer, and internalization of exogenous intact mitochondria into the target cells or tissue. In the myocardium, metabolic and ionic changes secondary to the lack of oxygen are major contributors to mitochondrial damage. Mitochondrial transplantation has been demonstrated to protect against cardiac ischemia-reperfusion injury1,2,3,4 as well as to rescue the diabetic heart,5 with improved cardiac function and a reduction in infarct size.
In their study published in this issue of Molecular Therapy,6 Zhang et al. demonstrated the benefits of mitochondrial transplantation in two different models of heart failure, namely an anthracycline-induced form and a genetically driven form of dilated cardiomyopathy. The authors started by testing different mitochondrial isolation methods in four different cell types, namely the human colon carcinoma cell line (R-mito), mouse fibroblast (F-mito), mouse skeletal muscle (M-mito), and mouse neonatal myocytes (H-mito). Mitochondrial isolation efficiency in terms of mitochondrial number as well as mitochondrial activity such as membrane potential and superoxide production were assessed. At 24 hours post-injection of human cell line-derived mitochondria into murine hearts, both human and mouse mtDNA were detected in the mouse hearts, confirming uptake of mitochondria, albeit cross-species, as well as upregulation of host mtDNA replication as early as 2 hours post-transplantation. Intramyocardial mitochondrial transplantation into murine hearts prevented doxorubicin-induced cardiotoxicity via prevention of apoptosis and restoration of mitochondrial respiration. Although the protection against death was associated with an increased mitochondrial amount, restoration of contractile function in the doxorubicin-challenged neonatal mouse ventricular myocytes relies on the presence of metabolically matched transplanted mitochondria. In addition, only the transplantation of H-mito induced maximal respiration alongside upregulated mitochondrial fusion protein Opa1.
In the genetically driven model of dilated cardiomyopathy (DCM), the authors chose to utilize human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) from Duchenne muscular dystrophy patients. Transplanted isogenic mitochondria at the onset of reperfusion into ischemic hiPSC-CMs restored mitochondrial membrane potential. Isogenic mitochondrial transplantation into DCM hiPSC-CMs improved contractile velocity, albeit only limited to ventricular hiPSC-CMs, despite equal mitochondrial uptake rates. Similar to the doxorubicin-challenged neonatal myocytes, H-mito transplantation into the DCM hiPSC-CMs also increased maximal mitochondrial respiration. Single-cell RNA-seq by the authors revealed an induction of gene networks associated with cell cycle status (mitosis, cell cycle, and cell division) and structural reinforcement (cytoskeleton, actin binding, and muscle protein) in these H-mito-transplanted ventricular DCM hiPSC-CMs. In the atrial DCM hiPSC-CMs, however, cytoskeletal remodeling and mitochondrial respiration pathways remain unchanged, thereby signifying that the restoration of mitochondrial respiration by mitochondrial transplantation may be the major factor underlying the benefits observed in the ventricular hiPSC-CMs.
The current study supports previous findings from other groups that transplantation of the mitochondria may exert protective benefits to the recipient myocardium. In this regard, the novelty of the current study lies in (1) the comparison of mitochondrial isolation efficiency and resulting mitochondrial function between different mitochondrial isolation methods applied to different cellular sources, (2) successful transplantation of human cell line-derived mitochondria into the murine heart and neonatal mouse mitochondria into hiPSC-CMs, (3) maintenance of cardiac function in an in vivo murine model of doxorubicin-mediated heart failure post-mitochondrial transplantation, as well as (4) sustaining contraction and respiration in ventricular myocytes in an hiPSC-CM model of mutation-driven DCM. Although the current study puts forth an additional facet of utilizing mitochondrial transplantation to circumvent cardiac failure, there are some observations that may further help put the research findings into perspective. The models used in the study, although converging in cardiac failure, were two different models of in vivo and in vitro heart failure, with different time points of mitochondrial transplantation (pre-doxorubicin challenge in mice versus direct transplantation in DCM hiPSC-CMs). In the mouse model used, M-mito transplantation was carried out a day before the start of doxorubicin administration, and hearts were retrieved after at the sixth week after 4 weeks of doxorubicin administration. Assessing the proportion of the transplanted M-mito in the hearts after removal via fluorescent labeling will allow an evaluation of the sustainability of the transplanted mitochondria over time. This will help in allaying the concern whether the increased mitochondrial abundance via transplantation or other factors such as maintenance of mitochondrial homeostasis and upregulation of certain pro-survival kinases are responsible for the beneficial effects observed. The translational aspect of this study is also complicated in that the doxorubicin model utilizes mice and isolated murine cardiomyocytes, while genetically driven DCM was based on hiPSC-CMs derived from patients. The murine cardiomyocytes received allogenic mitochondria, while the hiPSC-CMs received mitochondria from CRISPR-corrected isogenic cells to assess cardioprotective effects following ischemia-reperfusion and H-mito to examine contractile function. The immunological responses, if any, in the recipient cells was not clearly stated. Other alternatives will be to compare the effects of human (R-mito) mitochondrial transplantation in doxorubicin-challenged mice or standardize by using hiPSC-CMs for both models of heart failure.
In addition, the effects of differing mitochondrial DNA (mtDNA) haplotypes between humans and mice, the resulting manifested phenotypes, if any, remain to be clarified. Fusion of the mitochondria has been previously demonstrated to dilute effects of mtDNA mutations7 as well as to support respiratory capability.8 It is plausible that the observed increase in Opa1 post-H-mitochondrial transplantation in the doxorubicin-challenged cardiomyocytes may support respiratory function in the recipient cells. Examining the morphological phenotype of the host and transplanted mitochondria in both the neonatal myocytes as well as DCM hiPSC-CMs may further support this notion. Another caveat lies in the intramyocardial delivery method of mitochondria. Refining the study by exploring a more clinically relevant delivery method of external mitochondria9,10 at different time points (e.g., during ischemia versus post-reperfusion) may further enhance the clinical value of the findings.
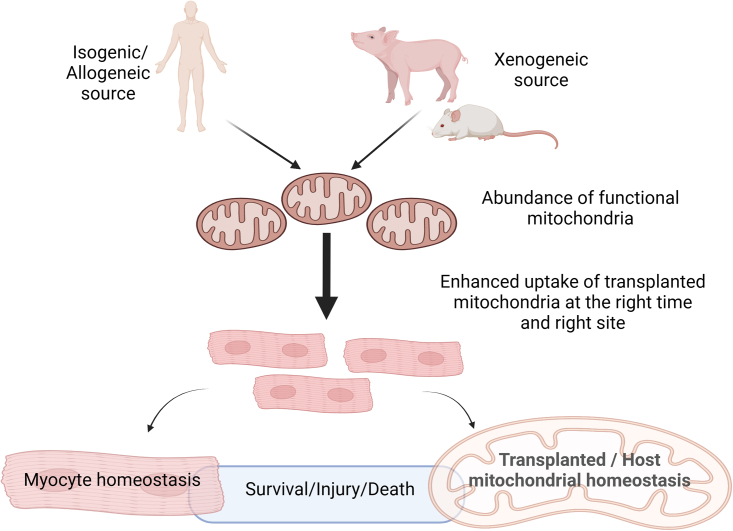
To conclude, the study puts forth an additional notion of utilizing exogenous mitochondria to prevent doxorubicin- and mutation-induced heart failure in the murine heart and hiPSC-CMs respectively. Further investigation in large animal models may be warranted upon refinement of the methodology. Ultimately, the ability to transplant mitochondria with optimal function, in relative abundance, at the right time, into the human heart at the targeted site of injury or even to rejuvenate the myocardium will remain the crux of research efforts in harnessing complementary benefits of external mitochondria in the diseased heart (Figure 1).
Figure 1.
Hypothetical optimal approach to harnessing mitochondrial transplantation to confer cardioprotection
Isogenic, allogeneic, or even xenogeneic mitochondria can be isolated and transplanted to myocytes or hiPSC-CMs to determine the benefits with regard to cellular death reduction and prolonging survival. The mitochondrial to be transplanted have to be abundant and functional even after transplantation. Transplantation techniques have to be clinically relevant and robust, while taking into account the proper timing of transplantation during the course of disease. Optimal homeostatic mechanisms in both the recipient cells as well as the host/transplanted mitochondria are key to maintaining optimal cellular survival. Created with Biorender.com.
Acknowledgments
Declaration of interests
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Contributor Information
Yaozu Xiang, Email: yaozu.xiang@tongji.edu.cn.
Hao Zhou, Email: zhouhao@plagh.org.
Dachun Xu, Email: xdc77@tongji.edu.cn.
Sang-Ging Ong, Email: sangging@uic.edu.
Sang-Bing Ong, Email: sangbingong@cuhk.edu.hk.
References
- 1.Masuzawa A., Black K.M., Pacak C.A., Ericsson M., Barnett R.J., Drumm C., Seth P., Bloch D.B., Levitsky S., Cowan D.B., McCully J.D. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H966–H982. doi: 10.1152/AJPHEART.00883.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowan D.B., Yao R., Akurathi V., Snay E.R., Thedsanamoorthy J.K., Zurakowski D., Ericsson M., Friehs I., Wu Y., Levitsky S., et al. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLOS One. 2016;11:e0160889. doi: 10.1371/journal.pone.0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaza A.K., Wamala I., Friehs I., Kuebler J.D., Rathod R.H., Berra I., Ericsson M., Yao R., Thedsanamoorthy J.K., Zurakowski D., et al. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J. Thorac. Cardiovasc. Surg. 2017;153:934–943. doi: 10.1016/j.jtcvs.2016.10.077. [DOI] [PubMed] [Google Scholar]
- 4.Blitzer D., Guariento A., Doulamis I.P., Shin B., Moskowitzova K., Barbieri G.R., Orfany A., del Nido P.J., McCully J.D. Delayed transplantation of autologous mitochondria for cardioprotection in a porcine model. Ann. Thorac. Surg. 2020;109:711–719. doi: 10.1016/J.ATHORACSUR.2019.06.075. [DOI] [PubMed] [Google Scholar]
- 5.Doulamis I.P., Guariento A., Duignan T., Orfany A., Kido T., Zurakowski D., Del Nido P.J., McCully J.D. Mitochondrial transplantation for myocardial protection in diabetic hearts. Eur. J. Cardiothorac. Surg. 2020;57:836–845. doi: 10.1093/EJCTS/EZZ326. [DOI] [PubMed] [Google Scholar]
- 6.Zhang A., Liu Y., Pan J., Pontanari F., Chia-Hao Chang A., Wang H., Gao S., Wang C., Chang A.C. Delivery of mitochondria confers cardioprotection through mitochondria replenishment and metabolic compliance. Mol. Ther. 2023 doi: 10.1016/j.ymthe.2023.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Vermulst M., Wang Y.E., Chomyn A., Prolla T.A., McCaffery J.M., Chan D.C. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agier V., Oliviero P., Lainé J., L’Hermitte-Stead C., Girard S., Fillaut S., Jardel C., Bouillaud F., Bulteau A.L., Lombès A. Defective mitochondrial fusion, altered respiratory function, and distorted cristae structure in skin fibroblasts with heterozygous OPA1 mutations. Biochim. Biophys. Acta. 2012;1822:1570–1580. doi: 10.1016/j.bbadis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y., Sun X., Gao R., Zhang L., Chen H., Lv Y., Wei X., Zou Y., Hu K., Sun A., Ge J. Transplantation of mitochondria encapsulated in hydrogel ameliorates myocardial ischemia-reperfusion injury. Chem. Eng. J. 2023;460:141799. doi: 10.1016/J.CEJ.2023.141799. [DOI] [Google Scholar]
- 10.Shin B., Saeed M.Y., Esch J.J., Guariento A., Blitzer D., Moskowitzova K., Ramirez-Barbieri G., Orfany A., Thedsanamoorthy J.K., Cowan D.B., et al. A novel biological strategy for myocardial protection by intracoronary delivery of mitochondria: safety and efficacy. JACC. Basic Transl. Sci. 2019;4:871–888. doi: 10.1016/J.JACBTS.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]