Summary
Background
Endocardial catheter ablation (CA) has limited long-term benefit for persistent and longstanding persistent atrial fibrillation (PersAF/LSPAF). We hypothesized hybrid epicardial-endocardial ablation (HA) would have superior effectiveness compared to CA, including repeat (rCA), in PersAF/LSPAF.
Methods
CEASE-AF (NCT02695277) is a prospective, multi-center, randomized controlled trial. Nine hospitals in Poland, Czech Republic, Germany, United Kingdom, and the Netherlands enrolled eligible participants with symptomatic, drug refractory PersAF and left atrial diameter (LAD) > 4.0 cm or LSPAF. Randomization was 2:1 to HA or CA by an independent statistician and stratified by site. Treatment assignments were masked to the core rhythm monitoring laboratory. For HA, pulmonary veins (PV) and left posterior atrial wall were isolated with thoracoscopic epicardial ablation including left atrial appendage exclusion. Endocardial touch-up ablation was performed 91–180 days post-index procedure. For CA, endocardial PV isolation and optional substrate ablation were performed. rCA was permitted between days 91–180. Primary effectiveness was freedom from AF/atrial flutter/atrial tachycardia >30-s through 12-months absent class I/III anti-arrhythmic drugs except those not exceeding previously failed doses. It was assessed in the modified intention-to-treat (mITT) population who had the index procedure and follow-up data. Major complications were assessed in the ITT population who had the index procedure. Thirty-six month follow-up continues.
Findings
Enrollment began November 20, 2015 and ended May 22, 2020. In 154 ITT patients (102 HA; 52 CA), 75% were male, mean age was 60.7 ± 7.9 years, mean LAD was 4.7 ± 0.4 cm, and 81% had PersAF. Primary effectiveness was 71.6% (68/95) in HA versus 39.2% (20/51) in CA (absolute benefit increase: 32.4% [95% CI 14.3%–48.0%], p < 0.001). Major complications through 30-days after index procedures plus 30-days after second stage/rCA were similar (HA: 7.8% [8/102] versus CA: 5.8% [3/52], p = 0.75).
Interpretation
HA had superior effectiveness compared to CA/rCA in PersAF/LSPAF without significant procedural risk increase.
Funding
AtriCure, Inc.
Keywords: Atrial fibrillation, Persistent atrial fibrillation, Longstanding persistent atrial fibrillation, Hybrid ablation, Epicardial ablation, Endocardial ablation, Catheter ablation, Left atrial appendage, Randomized controlled trial
Research in context.
Evidence before this study
To identify outcomes of ablation for persistent and longstanding persistent atrial fibrillation, PubMed was searched using the terms (“persistent atrial fibrillation” OR “longstanding persistent atrial fibrillation”) AND ablation between January 1, 2015 and November 30, 2022, and results were limited to Clinical Trials and Randomized Clinical Trials. Retrospective studies, non-relevant studies, studies with fewer than 50 patients, and studies that included patients with prior left atrial ablation were excluded. Of 126 hits and two additional hits found by manual searching, 37 total studies (32 RCT and 5 single-arm trials) were comprehensively reviewed. The vast majority of trials utilized radiofrequency and/or cryoballoon catheters for endocardial ablation, with only one trial each evaluating epicardial ablation and single-stage hybrid ablation. Twelve randomized studies compared pulmonary vein isolation (PVI) only to PVI with additional endocardial substrate ablation in non-paroxysmal AF. They reported a wide range of rhythm outcomes after PVI, ranging from 39% to 51% freedom from all atrial arrhythmias off anti-arrhythmic drugs with 12–18 months of follow-up. Further, more than half of the studies revealed no significant benefit of additional endocardial ablation outside of the PVs, which underscores the need for different ablation strategies for non-paroxysmal AF to improve rhythm outcomes.
Added value of this study
CEASE-AF is the largest randomized controlled trial to compare a hybrid ablation treatment approach consisting of combined thoracoscopic epicardial ablation, left atrial appendage exclusion and endocardial catheter ablation to a treatment approach consisting exclusively of endocardial catheter ablation, including clinical indicated repeat catheter ablation within 6 months of the index procedure. The addition of incremental epicardial lesions and left atrial appendage exclusion to endocardial ablation resulted in significantly improved rhythm outcomes in the hybrid arm, without a significant increase in major complications. Furthermore, only 4% of patients who received hybrid ablation required another ablation during the first year of follow-up, which is strikingly lower than repeat ablation rates following endocardial catheter ablation both in the trial’s endocardial catheter arm and published literature.
Implications of all the available evidence
Published literature and consensus guidelines recognize the variability and difficulty to achieve favorable rhythm outcomes after endocardial ablation for non-paroxysmal AF. The CEASE-AF results are important to inform clinical practice wherein epicardial-endocardial hybrid ablation as a treatment approach increases restoration of sinus rhythm through a collaborative heart team approach.
Introduction
Atrial fibrillation (AF) affects 33 million people worldwide and significantly elevates the risks of stroke and heart failure, leading to increased mortality.1, 2, 3 While anti-arrhythmic drug (AAD) therapy remains the first line treatment for AF, it may have significant side effects and poor effectiveness in many patients. Endocardial catheter ablation (CA) has evolved as a treatment option as supported by guidelines.4,5 However, due to advanced electrical and structural remodeling of the left atrium, endocardial CA effectiveness for persistent AF (PersAF) and longstanding PersAF (LSPAF) have been limited.6, 7, 8, 9, 10 The need for repeat CA (rCA) has been documented in a these patients but has failed to demonstrate substantially improved outcomes.11, 12, 13, 14
The rationale for the design of the Combined Endoscopic Epicardial and Percutaneous Endocardial Ablation versus Repeated Catheter Ablation in Patients with PErsistent and Longstanding Persistent Atrial Fibrillation (CEASE-AF) trial was to evaluate the risks and benefits of targeting the left atrial (LA) epicardial substrate in addition to standard endocardial CA to achieve epicardial-endocardial pulmonary vein and LA posterior wall isolation and LA appendage (LAA) exclusion for the treatment of advanced AF. We hypothesized that a two-stage Hybrid Ablation (HA) approach combining endocardial ablation with minimally invasive epicardial ablation including LAA exclusion would have greater clinical effectiveness than single or repeat endocardial CA for PersAF and LSPAF. By reducing lesion gaps, addressing transmurality, and providing a comprehensive lesion set for the treatment of advanced AF, we posed that HA would overcome the limitation of endocardial CA/rCA alone for advanced AF without significantly increasing the procedural complication rate.
Methods
Study design
CEASE-AF is a prospective, multicenter, randomized controlled trial (RCT) to compare the outcome of combined epicardial and endocardial ablation versus standard endocardial CA (Clinicaltrials.gov Identifier: NCT02695277). Nine hospitals in Germany, Netherlands, United Kingdom, Czech Republic, and Poland participated. Institutional Review Board or Ethics Committee approval was obtained. A Clinical Events Committee (CEC), composed of independent physicians, reviewed and adjudicated safety events in the trial (Supplementary Appendix). This study was performed in accordance with the Declaration of Helsinki and EN ISO 14155:2011. Data management, monitoring, and statistical analyses were performed by an independent research organization (Cardialysis, Rotterdam, Netherlands).
Participants
Eligible patients were between 18 and 75 years of age; had a history of symptomatic PersAF and a LA diameter (LAD) > 4.0 cm or symptomatic LSPAF; and had failed at least one class I or III AAD. Persistent AF was defined as continuous AF sustained beyond seven days, or lasting greater than 48 h and less than 7 days but necessitating pharmacologic or electrical cardioversion. Longstanding persistent AF was continuous AF of greater than 12 months’ duration. Key exclusion criteria were: previous ablation procedure; paroxysmal AF; LSPAF > 10 years; AF secondary to electrolyte imbalance, thyroid disease, or other reversible cause; need for other cardiac surgery procedures besides AF treatment; or contraindication for CA or epicardial ablation. Complete inclusion and exclusion criteria are in the Supplementary Appendix. Patient sex/gender was self-reported. Written informed consent was obtained from all patients.
Randomisation and masking
Enrolled patients were randomised 2:1 to either staged HA or CA (including repeat CA, collectively referred to hereafter as the CA arm). Randomisation was performed centrally using xClinical’s MARVIN System. Enrolled patients were assigned a sequential identification number at each site. Randomisation sequences were generated by an independent statistician (Cardialysis) using SAS 9.4 and stratified by site. Randomisation assignment was provided to the site through the electronic case report form system.
The treatments were not masked to the participating physicians or patients due to differences in devices and procedures between arms. However, effectiveness endpoints from Holter data were assessed in a blinded fashion by the core lab physician such that they had no knowledge of the patient group.
Procedures
Hybrid ablation arm
Pre-and post-procedure management are described in the Supplementary Appendix. HA was performed in a two-stage epicardial-endocardial procedure. The first stage (index procedure) was comprised of endoscopic epicardial ablation performed by a cardiac surgeon using general anesthesia with a double lumen endotracheal intubation. Port access was gained through bilateral thoracoscopy. The surgical epicardial procedure included a minimum lesion set of pulmonary vein isolation (PVI) and LA posterior wall isolation by means of a “box” lesion (i.e., superior and inferior connecting lines between right and left PVI) using transpolar and bipolar radiofrequency (RF) energy devices (AtriCure Isolator Synergy Clamp [EMR2/EML2] for PVI; Isolator Long Pen TT [MAX5], Coolrail Linear Pen [MCR1], and/or Isolator Linear Pen [MLP1] [AtriCure, Inc, Mason, OH] for linear lesions and mapping/endpoint confirmation). LAA exclusion was performed. Additional epicardial lesions were allowed to be performed based on standard institutional practice.
The second stage of the procedure included endocardial RF CA performed using standard techniques by an electrophysiologist between 91 and 180 days following the epicardial index procedure. PVI and posterior box isolation were verified and completed if necessary. Additional substrate modification could be performed at physician discretion. Follow-up for primary effectiveness began at 180 days post-epicardial index procedure (T0).
Catheter ablation arm
Pre- and post-procedure management are described in the Supplementary Appendix. Endocardial CA was performed using current RF catheter technology including contact force by an electrophysiologist according to institutional standards. PVI was mandatory during the index procedure. Additional ablation strategies were under the physician’s discretion/center’s standard, in accordance with current guidelines. If clinically indicated, a repeat CA was permitted after a 90-day blanking period through 180 days after the endocardial index procedure. Follow-up for primary effectiveness began at 180 days post-endocardial index procedure (T0).
Patients in the CA arm who were a primary effectiveness failure after T0 were permitted to crossover to receive HA per physician recommendation but were deemed an effectiveness failure.
Follow-up visits
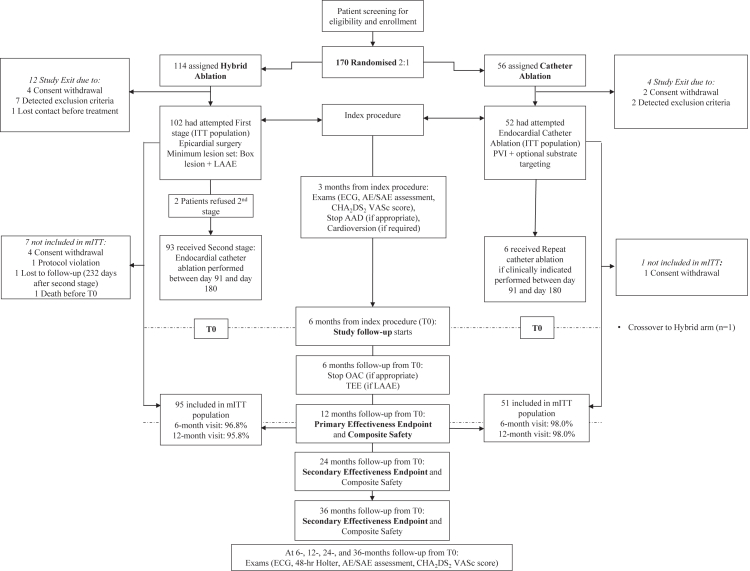
Follow-up visits occurred at 3- and 6-months after the index procedure, then 6- and 12-months after T0 (Fig. 1); T0 was defined as 6 months after the index procedure (first ablation procedure) to allow for staged endocardial ablation in the HA arm or repeat endocardial ablation in the CA arm. Longer-term follow-up visits occurred or will occur at 24 and 36 months from T0. Rhythm status for effectiveness was derived from 48-h Holter monitoring during scheduled visits and symptom-driven monitoring performed during unscheduled hospital visits with a 12-lead ECG and rhythm monitoring of at least 30 s. An independent core laboratory (Banook Group, Nancy, FR) blindly reviewed and interpreted ECG and Holter recordings. A follow-up TEE was performed at the 6-month visit after T0 to confirm LAA exclusion. Other routine diagnostic tests and evaluations were performed according to the assessment schedule (Fig. 1).
Fig. 1.
CONSORT diagram. In the Hybrid Arm, detected exclusion criteria after randomisation included pre- or intraoperative findings revealed low ejection fraction of 23% (n = 1), a phrenic nerve paresis (n = 1), a left atrial diameter > 6 cm (n = 1), a serial rib fraction with adhesions (n = 1), development of a body mass index > 35 (n = 1), and the need or potential need for open cardiac surgery (n = 3). One patient expressed unwillingness/disability to comply with the follow-up protocol. AAD: anti-arrhythmic drug; AE: adverse event; ECG: electrocardiogram; EP: electrophysiology; ITT: intention-to-treat; LAA: left atrial appendage; mITT: modified intention-to-treat; OAC: oral anticoagulation; PV: pulmonary vein; SAE: serious adverse event; TEE: transesophageal echocardiogram; tx: treatment.
Study endpoints
For primary effectiveness, follow-up began at T0. The primary effectiveness endpoint was freedom from documented AF/atrial flutter (AFL)/atrial tachycardia (AT) episodes >30 s through the 12-months follow-up visit in the absence of Class I or III AADs with the exception of AADs at doses not exceeding previously failed doses. A failure of the primary effectiveness endpoint included any documented AF/AFL/AT lasting >30 s; any previously failed class I or III AAD administered at a dose higher than baseline; any newly introduced class I or III AAD usage; a direct current cardioversion for AF/AFL/AT or any ablation intervention occurring through the 12-month follow-up visit, respectively. Mid-and long-term outcomes of the trial continue to be followed.
The safety endpoint of the trial is the composite major complications during the course of the study, which will be compared between the two study arms. As long-term follow-up is ongoing, the following major complications are reported: i) within 30 days of the index procedure (first stage hybrid epicardial ablation or endocardial CA) and within 30 days of the second stage hybrid endocardial CA or endocardial repeat CA) and ii) through 12-months after the index procedure in each arm. Major complications are defined in Supplementary Appendix Table S1.
Statistical analysis
The sample size calculation was based on a two treatment group continuity corrected chi-squared test, a two-sided significance level of 5% and 80% power to detect the difference between a CA group proportion of 50% compared to 25% in the HA group. Based on a 2:1 randomization, a total of 147 subjects (49 in the CA group and 98 in the HA group) were to be treated. Adjusting for loss to follow-up (anticipated 30% loss at 12 months), the required total sample size was 210 subjects (70 in the CA, and 140 in the HA group). Although not pre-specified in the protocol, due to a lower than anticipated dropout rate, study enrollment was ended after 154 participants were treated and 80% power was maintained. Investigators and Sponsor were blinded to effectiveness endpoints when enrollment was stopped and remained blinded until after data-lock. There was no data monitoring committee, however data were monitored by an independent contract research organization (CRO).
Study endpoints are summarized by randomized treatment group. Continuous variables are summarized by presenting the number of subjects, mean, standard deviation, median, minimum, maximum by procedure group (HA or CA). Student’s t-test was used when comparing means between procedure groups. Tabulation of categorical variables by group includes counts and percentages. When comparing the outcome for categorical variables between procedure groups, two-sided Fisher’s exact test was used.
In all analyses described, the null hypothesis are two-sided tests of no treatment group difference. All statistical tests use a two-sided significance level of α = 0.05. The primary effectiveness endpoint analysis tests the null hypothesis of no treatment group difference, using a 5% two-sided significance level and a Fisher’s exact test. The 95% two-sided confidence interval and p-value are reported. SAS 9.3 was used for statistical analysis.
No interim analyses were performed. Analyses were not adjusted for multiplicity.
Role of funding source
The CEASE-AF trial and the article processing charge were funded by AtriCure, Inc. AtriCure, Inc. participated in trial design and coordination, assisted with the literature search, writing and editing of the manuscript. AtriCure, Inc., was blinded to the study results when enrollment was stopped, remained blinded until after data-lock, and did not perform any analyses or adjudication.
Results
From November 20, 2015 to May 22, 2020, 170 patients were enrolled and randomized to HA (n = 114) or CA (n = 56) (Fig. 1). A total of 102 patients received the index procedure in the HA arm and 52 patients received the index procedure in the CA arm (intention to treat [ITT]). There were no significant differences in patient baseline and demographic characteristics between arms with regards to age, gender, AF classification, AF duration, LAD, BMI, or medications (Table 1).
Table 1.
Patient demographics and clinical characteristics.
| Parameter | Hybrid ablation arm (ITT), n = 102 | Catheter ablation arm (ITT), n = 52 | Total ITT population, N = 154 |
|---|---|---|---|
| Age (years), Mean ± SD | 60.8 ± 8.1 | 60.6 ± 7.4 | 60.7 ± 7.9 |
| Male, n (%) | 77 (75.5) | 38 (73.1) | 115 (74.7) |
| BMI (kg/m2), Mean ± SD | 29.7 ± 3.5 | 29.8 ± 3.1 | 29.7 ± 3.4 |
| AF Classification, n (%) | |||
| Persistent | 81 (79.4) | 43 (82.7) | 124 (80.5) |
| LSPAF | 21 (20.6) | 9 (17.3) | 30 (19.5) |
| mEHRA Class, n (%) | |||
| 1 | 1 (1.0) | 3 (5.8) | 4 (2.6) |
| 2a | 17 (16.7) | 8 (15.4) | 25 (16.2) |
| 2b | 40 (39.2) | 22 (42.3) | 62 (40.3) |
| 3 | 42 (41.2) | 18 (34.6) | 60 (39.0) |
| 4 | 2 (2.0%) | 1 (1.9) | 3 (1.9) |
| NYHA Class, n (%) | |||
| I | 16 (15.7) | 6 (11.5) | 22 (14.3) |
| II | 45 (44.1) | 29 (55.8) | 74 (48.1) |
| III | 16 (15.7) | 6 (11.5) | 22 (14.3) |
| IV | 1 (1.0) | 1 (1.9) | 2 (1.3) |
| No heart failure | 24 (23.5) | 10 (19.2) | 34 (22.1) |
| CHA2DS2-VASc score, n (%) | |||
| 0 | 16 (15.7) | 10 (19.2%) | 26 (16.9) |
| 1 | 28 (27.5) | 13 (25.0) | 41 (26.6) |
| 2 | 24 (23.5) | 17 (32.7) | 41 (26.6) |
| 3 | 22 (21.6) | 7 (13.5) | 29 (18.8) |
| 4 | 5 (4.9) | 4 (7.7) | 9 (5.8) |
| 5 | 5 (4.9) | 1 (1.9) | 6 (3.9) |
| 6 | 2 (2.0) | 0 (0.0) | 22 (1.3) |
| LA size (cm) | |||
| Mean ± SD | 4.7 ± 0.5 | 4.7 ± 0.4 | 4.7 ± 0.4 |
| Median (Q1–Q3) | 4.70 (4.3, 4.9) | 4.65 (4.3, 5.0) | 4.70 (4.3, 5.0) |
| Min–Max | 4.0–6.0 | 4.0–5.6 | 4.0–6.0 |
| LVEF (%) | |||
| Mean ± SD | 58.3 ± 9.0 | 57.8 ± 8.5 | 58.1 ± 8.8 |
| Median (Q1–Q3) | 60.00 (55.0, 65.0) | 60.00 (53.5, 64.0) | 60.00 (54.0, 65.0) |
| Min–Max | 31.0–77.0 | 30.0–75.0 | 30.0–77.0 |
| History of, n (%) | |||
| Cardioversion | 95 (93.1) | 50 (96.2) | 145 (94.2) |
| Arterial hypertension | 54 (52.9) | 28 (53.8) | 82 (53.2) |
| Diabetes mellitus | 14 (13.7) | 4 (7.7) | 18 (11.7) |
| Sleep apnea | 2 (2.0) | 0 (0.0) | 2 (1.3) |
| Thromboembolism | 1 (1.0) | 0 (0.0) | 1 (0.6) |
| TIA/Stroke | 7 (6.9) | 3 (5.8) | 10 (6.5) |
| Number of years in AF (Mean ± SD) | 2.94 ± 3.29 | 3.34 ± 3.52 | 3.08 ± 3.36 |
| Medication use at baseline, n (%) | |||
| AADs (Class I/III) | 55 (53.9) | 30 (57.7) | 85 (55.2) |
| Oral anticoagulation | 95 (93.1) | 45 (86.5) | 140 (90.9) |
AF, atrial fibrillation; BMI, body mass index; LA, left atrial; LSPAF, longstanding persistent AF; LVEF, left ventricular ejection fraction; ITT, intention-to-treat; NYHA, New York Heart Association; mEHRA, modified European Heart Rhythm; Max, maximum; Min, minimum; Q, quartile; SD, standard deviation; TIA, transient ischemic attack; AADs: Anti-arrhythmic drugs.
In the CA arm, all 52 patients received PV ablation. Where entrance and exit block were assessed, endpoint achievement for PVI was confirmed in 89.4% of patients. Additional ablation beyond PVI by means of targeting the LA posterior wall (roof line, posterior wall box, complex fractionated atrial electrograms, and/or voltage-guided ablation) was performed in 40.4% (21/52). Out of those who received a complete endocardial posterior wall box lesion, the endpoint bidirectional block was achieved in 57%. Cavotriscuspid isthmus (CTI) ablation was performed in 13.5% (7/52). Details of additional endocardial ablation during the CA index procedures and repeat procedures are summarized in Supplementary Appendix Tables S2 and S3, respectively.
In the HA arm, LAA exclusion was performed by epicardial clip (AtriClip PRO1/PRO2, AtriCure, Inc.) in 92.2% (94/102) of patients. Of those patients, 87 had available post-operative TEE, which showed an effective exclusion rate of 100%, with no residual stump >10 mm and no residual flow between the left atrium and LAA. Eight patients (7.8%) had stapler excision. Bidirectional block after epicardial ablation was assessed using pacing and sensing. Depending on whether exit block was assessable, bidirectional or entrance block could be confirmed in 98% for PVI and 94% for posterior wall box (Supplementary Appendix Table S4). Details of additional epicardial ablation during the HA index procedures are summarized in Supplementary Appendix Table S5. The details of the second stage endocardial lesions of the HA arm are shown in Supplementary Appendix Table S6.
Total procedure duration was significantly longer with HA (336.4 ± 97 min) compared to CA (251.9 ± 114 min, p < 0.001) (Table 2). Fluoroscopy time with HA was significantly lower than with CA (16.0 ± 13 versus 24.3 ± 19, p = 0.001). In the CA arm, the index electrophysiology procedural duration was 74 min longer than the endocardial portion of the HA arm (p < 0.001).
Table 2.
Procedural durations.
| Hybrid arm ITT | Catheter arm ITT | Two-sided p-value | |
|---|---|---|---|
| Total procedure duration (min), mean ± SD (n) | 336.4 ± 97 (102) | 251.9 ± 114 (52) | <0.001 |
| Index procedure (min), mean ± SD (n) | 192.4 ± 51 (102) | 232.2 ± 98 (52) | |
| 2nd stage endocardial procedure (min), mean ± SD (n) | 158.0 ± 80 (93) | N/A | |
| Repeat catheter ablation pre-T0 (min), mean ± SD (n) | N/A | 170.5 ± 75 (6) | |
| Total fluoroscopy duration (min), mean ± SD (n) | 16.0 ± 13 (93) | 24.3 ± 19 (52) | 0.001 |
| Index procedure (min), mean ± SD (n) | N/A | 21.8 ± 15 (52) | |
| 2nd stage endocardial procedure (min), mean ± SD (n) | 16.0 ± 13 (93) | N/A | |
| Repeat catheter ablationpre T0 (min) mean ± SD (n) | N/A | 22.2 ± 16 (6) |
ITT: intention to treat; N/A: not applicable; SD: standard deviation.
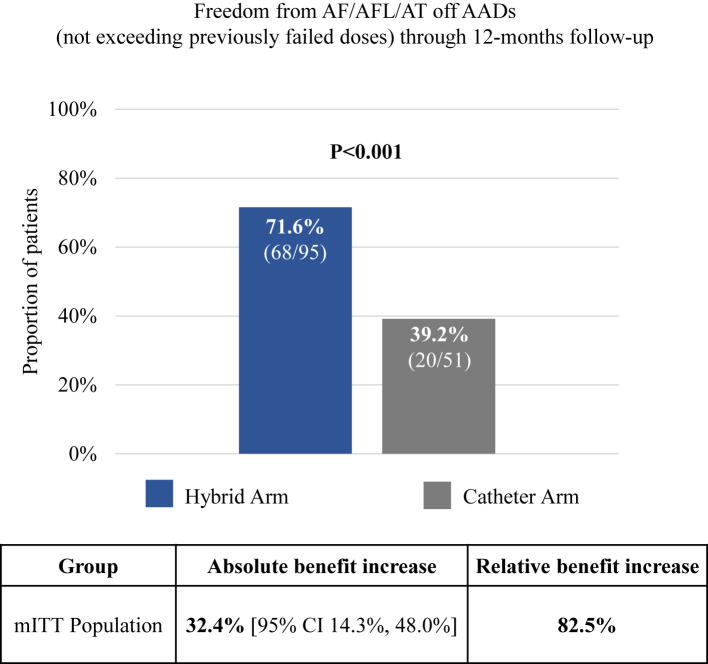
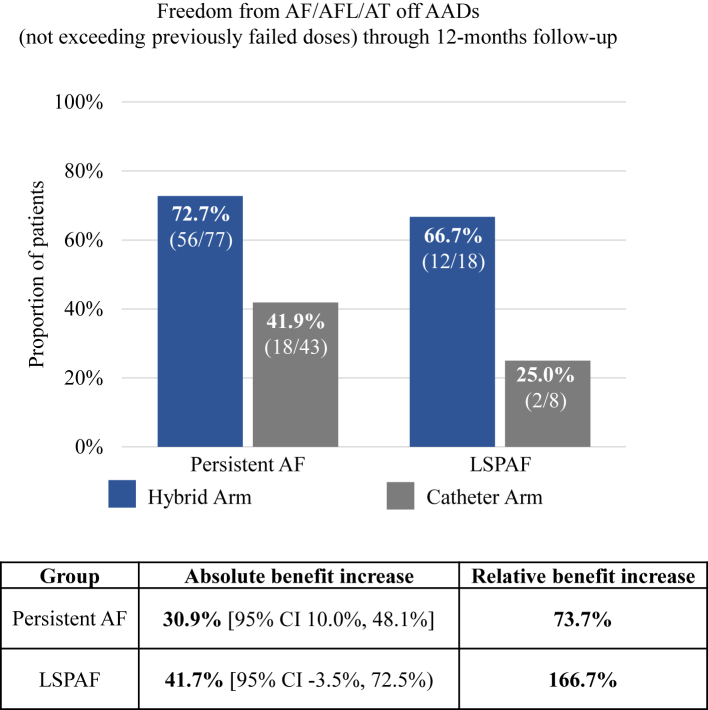
There were 146 patients (95 HA and 51 CA) in the modified ITT (mITT) population, defined as patients who had an attempted epicardial or endocardial index procedure and had available effectiveness endpoint data after the T0 visit. Through 12-months follow-up from T0, 71.6% (68/95) of HA patients were free from AF/AFL/AT in the absence of new AADs or increased doses of previously failed AADs versus 39.2% (20/51) treated with CA alone (absolute benefit increase of 32.4% [95% CI 14.3%–48.0%], p < 0.001; Fig. 2). The distribution of arrhythmia recurrences is shown in Supplementary Appendix Table S7. Of the 95 HA mITT patients, 77 had PersAF and 18 had LSPAF. Similarly, of 51 mITT patients, 43 had PersAF and 8 had LSPAF. In PersAF patients, primary effectiveness was 72.7% (56/77) with HA versus 41.9% (18/43) with CA (absolute benefit increase 30.9% [95% CI 10.0%–48.1%]; p = 0.002) (Fig. 3). A similar trend was observed in the LSPAF subgroup, with primary effectiveness of 66.7% (12/18) with HA compared to 25.0% (2/8) with CA (absolute benefit increase 41.7% [95% CI −3.5% to 72.5%]) (Fig. 3). However, the study was not powered for these subgroup analyses.
Fig. 2.
Effectiveness outcomes of Hybrid Ablation (HA, blue) and Catheter Ablation (CA, gray) off new/increase dose previously failed Class I or III anti-arrhythmic drugs (AADs) in the overall modified intention-to-treat population. Relative benefit increase is the ratio of the absolute benefit increase (the difference in success percentage between the HA and CA groups) and the CA group success percentage. P-values reflect Fisher’s exact test. CI: confidence interval.
Fig. 3.
Effectiveness outcomes of Hybrid Ablation (HA, blue) and Catheter Ablation (CA, gray) off new/increase dose previously failed Class I or III anti-arrhythmic drugs (AADs) in the persistent and longstanding persistent atrial fibrillation (LSPAF) subgroups. Relative benefit increase is the ratio of the absolute benefit increase (the difference in success percentage between the HA and CA groups) and the CA group success percentage. P-values reflect Fisher’s exact test. CI: confidence interval.
Freedom from AF/AFL/AT regardless of AADs through 12 months was 74.7% (71/95) in the HA arm and 41.2% (21/51) in the CA arm (absolute benefit increase of 33.6% [95% CI 15.9%–49.1%]; p < 0.001) (Supplementary Appendix Fig. S1). Freedom from AF/AFL/AT off any Class I/III AAD through 12 months was 63.2% (60/95) in the HA arm and 35.3% (18/51) in the CA arm (absolute benefit increase of 27.9% [95% CI 7.0%–43.8%]; p = 0.002).
Primary effectiveness was also significantly greater in the HA arm compared to the CA arm when the ITT population was evaluated (66.7% [68/102] versus 40.4% [21/52]), with missing follow-up data imputed as failure in the HA arm and success in the CA arm. The absolute benefit increase was 26.3% [95% CI 6.9%–42.0%], p = 0.003).
In the CA arm, additional ablation of the LA posterior wall did not improve primary effectiveness compared to PVI only (40.0% (8/20) versus 38.7% (12/31)). Further, the absolute benefit increase of HA over this CA subgroup was 31.6% (95% CI 0.9%–53.4%). The study was not randomised or powered for this analysis.
Composite major complication rates within 30-days after the index procedure and 30-days after the second stage HA or rCA were 7.8% (8/102) and 5.8% (3/52) in the HA and CA arms (p = 0.751) (Table 3). Thirty-day composite major complication rates post-index procedure were 4.9% (5/102) in HA versus 5.8% (3/52) in CA (p = 1.000). Through 12-months post-index procedure, composite major complications occurred in 8.8% (9/102) of patients treated with HA versus 5.8% (3/52) of patients treated with CA (p = 0.752) (Table 4). The number of major complications per 100 patient-years was 10.23 versus 9.77 in HA versus CA, respectively (Supplementary Appendix Table S8). One non-disabling, ischemic stroke without hemorrhagic transformation that resolved without sequalae occurred in the HA arm on day 25 post-index procedure in a patient who had a baseline CHA2DS2-VASc score of 3. There was one death in the HA arm at 93 days post-index procedure, prior to endocardial ablation, due to acute myocardial infarction. The CEC adjudicated this event as related to underlying ischemic heart disease and severe coronary artery atheroma, unrelated to the procedure or devices. In the CA arm, a mapping catheter caused severe damage to the mitral valve requiring surgical intervention by means of operative mitral valve repair in one patient.
Table 3.
Composite major complication rates within 30 days of Hybrid index and second stage procedures and Catheter index and repeat ablations.
| Characteristic | Hybrid arm ITT | Catheter arm ITT | Two-sided p-value |
|---|---|---|---|
| Major complications (composite endpoint, % pts. with complications)b | 7.8% (8/102) | 5.8% (3/52) | 0.751a |
| Total events reported, n/N | 9/102 | 5/52 | |
| Death (all-cause) | 0/102 | 0/52 | |
| Stroke (non-disabling) | 1/102 | 0/52 | |
| Transient ischemic attack | 0/102 | 1/52 | |
| Myocardial infarction | 1/102 | 0/52 | |
| Pericarditis | 1/102 | 1/52 | |
| Bleeding (at vascular access site) | 1/102 | 0/52 | |
| Wound infection at surgical site or puncture sites requiring surgical intervention | 0/102 | 0/52 | |
| Major vascular access complications | 1/102 | 1/52 | |
| Atrio-esophageal fistula | 0/102 | 0/52 | |
| Esophageal injury | 0/102 | 0/52 | |
| Permanent phrenic nerve paralysis | 0/102 | 0/52 | |
| Permanent pacemaker implantation (SSS) | 1/102 | 0/52 | |
| Pulmonary vein stenosis | 0/102 | 0/52 | |
| Cardiac tamponade/cardiac perforation | 0/102 | 1/52 | |
| Empyema | 0/102 | 0/52 | |
| Pneumothorax requiring intervention | 1/102 | 0/52 | |
| Pneumonia | 1/102 | 0/52 | |
| Aspiration after nose bleeding caused by insertion of a nasopharyngeal airway | 1/102 | 0/52 | |
| Mitral valve leaflet injury requiring surgical intervention | 0/102 | 1/52 |
Fisher’s exact test.
Hybrid arm: one patient had two major complications: myocardial infarction and stroke. Catheter arm: one patient had three major complications: TIA, pericarditis and mitral valve leaflet injury requiring surgical intervention. The mitral valve leaflet injury was due to dislocation of a mapping catheter.
Table 4.
Major complications through 365 days post-index procedure.
| Characteristic | Hybrid arm ITT | Catheter arm ITT | Two-sided p-value |
|---|---|---|---|
| Major complications (composite endpoint, % pts. with complications)b | 8.8% (9/102) | 5.8% (3/52) | 0.752a |
| Total events reported, n/N | 10/102 | 5/52 | |
| Deathc (all-cause) | 1/102 | 0/52 | |
| Stroke (non-disabling) | 1/102 | 0/52 | |
| Transient ischemic attack | 0/102 | 1/52 | |
| Myocardial infarction | 1/102 | 0/52 | |
| Pericarditis | 1/102 | 1/52 | |
| Bleeding (at vascular access site) | 1/102 | 0/52 | |
| Wound infection at surgical site or puncture sites requiring surgical intervention | 0/102 | 0/52 | |
| Major vascular access complications | 1/102 | 1/52 | |
| Atrio-esophageal fistula | 0/102 | 0/52 | |
| Esophageal injury | 0/102 | 0/52 | |
| Permanent phrenic nerve paralysis | 0/102 | 0/52 | |
| Permanent pacemaker implantation (SSS) | 1/102 | 0/52 | |
| Pulmonary vein stenosis | 0/102 | 0/52 | |
| Cardiac tamponade/cardiac perforation | 0/102 | 1/52 | |
| Empyema | 0/102 | 0/52 | |
| Pneumothorax requiring intervention | 1/102 | 0/52 | |
| Pneumonia | 1/102 | 0/52 | |
| Aspiration after nose bleeding caused by insertion of a nasopharyngeal airway | 1/102 | 0/52 | |
| Mitral valve leaflet injury requiring surgical intervention | 0/102 | 1/52 |
ITT: intention to treat; NA: not applicable; SSS: sick sinus syndrome.
Fisher’s exact test.
Hybrid arm: One patient had two major complications: myocardial infarction and stroke. Catheter arm: one patient had three major complications: TIA, pericarditis and mitral valve leaflet injury requiring surgical intervention. The mitral valve leaflet injury was due to dislocation of a mapping catheter.
Death occurred 93 days post-index procedure prior to endocardial ablation; was unrelated to procedure per Clinical Events Committee adjudication.
Between T0 and the 12-month follow-up visit, 4.2% (4/95) of patients in the HA arm and 35.3% (18/51) patients in the CA arm had additional ablation (p < 0.001). Cardioversions (pharmaceutical and electrical) were performed in 11.6% (11/95) of HA patients and 25.5% (13/51) of CA patients during this time frame (p = 0.037).
Discussion
The primary effectiveness results of CEASE-AF showed that HA resulted in a 32.4% absolute and 82.5% relative benefit increase compared to CA/rCA through 12-months follow-up in a patient population with advanced AF. To our knowledge, this is the largest multi-center RCT comparing effectiveness and safety of staged HA including LAA exclusion to endocardial CA including repeat ablation, in patients with PersAF and enlarged LA or LSPAF. This population is currently deemed to have a high failure rate of interventional treatment or are not even considered for rhythm-control strategies.
An optimal endocardial lesion set or ablation strategy for advanced AF has yet to be defined. Catheter ablation and repeat CA for PersAF and LSPAF may still prove ineffective in the long-term. In 5-year follow-up of the Hamburg study, single procedure success of sequential endocardial CA for LSPAF was 20% and 45% after median 2 (range 1–5) procedures regardless of AADs.10 In the CEASE-AF CA arm, 12% (6/51) of patients received rCA within 180 days after their index CA, in line with what could be expected in clinical practice. Clinical indications for repeat catheter ablations were generally applied in line with current recommendations including patient being symptomatic, having failed anti-arrhythmic medication and/or cardioversion and patient giving consent for a second intervention. It is notable that also in the HA arm there were patients who refused endocardial touch up ablation. In PRECEPT, which included PersAF patients (<12 months of continuous AF and mean LA size of 4.2 cm) 5.7% and 7.8% of patients received repeat ablations during and after the 180-day blanking period, respectively, through 15- months follow-up after CA.15 In STOP-Persistent AF (<6 months of continuous AF and normal LA size), 12% of patients received repeat ablation through 12-months.16 In STAR AF-II, which included patients with PersAF and LSPAF with a mean LA diameter of 4.4 cm, repeat ablation was performed in 21–33% of patients through 18 months, depending on index endocardial ablation strategy.14
CEASE-AF validates observational studies and meta-analysis on improved outcomes after combined epicardial and endocardial HA for non-paroxysmal AF.17 Besides observational studies, the CONVERGE RCT also demonstrated improved effectiveness in PersAF/LSPAF using a combined epicardial- and endocardial ablation approach but different ablation strategies.18 Therefore, HA extends the portfolio for advanced AF treatment through a collaborative heart team approach among electrophysiologists and cardiac surgeons that is supported by guidelines.4
The serious adverse event rates were numerically higher in HA but not statistically different compared to CA. In both study arms, the serious adverse event rates were similar to those in published literature.14,18, 19, 20 Known complications of AF ablation such as atrioesophageal fistulae or phrenic nerve injury did not occur in either arm. One non-disabling stroke (1%) was reported in HA in a patient with elevated risk of stroke and one cardiac tamponade (1.9%) was reported in CA. The 30-day safety rate for both HA stages are within the expected range for repeated CA and balanced by a significant rhythm benefit, which sustained through the duration of the follow-up.12,21
Although the total HA procedural time was longer, this did not impact procedural safety. Further, endocardial touch-up ablation after epicardial ablation was less time-consuming than the endocardial index procedure in CA. This resulted in significantly decreased electrophysiology procedure time, decreased fluoroscopy time, and lower radiation exposure in HA.
The improved effectiveness of HA over CA may result from several factors, including LAA management. While the specific driving force is not yet clear, the collective components of HA achieve greater success than endocardial ablation alone. HA may provide more durable, transmural lesions than epicardial-only or endocardial-only ablation and addresses epicardial-endocardial dissociation.22,23 Further, the HA treatment approach allows for easy access and targeting of structures that are known to potentially play a role in the pathological mechanisms of AF as well as the risk of thromboembolic events such as the LAA. All patients in the HA arm had LAA management but the incremental effectiveness benefit of LAA exclusion in the HA arm was not evaluated. The HA arm required a box lesion, which is the commonly applied lesion set in epicardial ablation and associated with significantly improved rhythm outcomes compared to PVI and roof line.24 While a posterior wall box can be created with CA as well, it is limited by high reconnection rates, potential esophageal thermal injury risk, and lack of supportive RCT data.6,8 A subgroup analysis of our results revealed no benefit of posterior wall ablation in addition to PVI for endocardial CA. This is in line with findings of the randomized CAPLA trial that showed endocardial posterior wall isolation did not improve atrial arrhythmia-free survival compared to PVI alone in patients with persistent AF and longstanding persistent AF less than 3 years of duration, which confirms the limitation of efficaciously targeting the posterior wall endocardially.25
Since there is no consensus nor conclusive evidence on the benefit of ablation strategies beyond PVI with CA,24 PVI remains the cornerstone of endocardial ablation.5 The applied lesion sets in CEASE-AF were not consistent in the two arms. However, the goal of this trial was to evaluate the incremental benefit of the epicardial lesions in addition to endocardial lesions by comparing two different ablation strategies for advanced AF as they are currently applied. Therefore, a minimal lesion set was defined for each arm that accounted for guideline recommendations as well as for the variability in CA techniques according to institutional clinical practices. The systematic literature search alongside this study revealed that endocardial ablations beyond PVI were performed in approximately 40% if various endocardial ablation strategies were allowed.15,26,27 This rate is in line with what had been applied in the CA arm of CEASE-AF.
Another limitation in the context of HA and CA is comparison of different overall numbers of procedures. However, by definition, HA should be considered as one treatment approach consisting of an epicardial and endocardial part. This can be performed in two stages, as was done in CEASE-AF, or in a single stage/procedure.28 The investigators chose a staged HA approach for several reasons. Staging allows for shorter anesthesia runs, acute edema resolution, and epicardial lesion maturity before the endocardial procedure. Since two physician specialties are involved in HA, staging helps with better alignment, scheduling logistics, and catheter lab/operating room productivity. Similarly, CA can be performed as a single procedure, or may be repeated subsequently. In this trial, rCA in the CA arm prior to T0 was only applied when clinically indicated.
Symptom-driven ECG monitoring was performed at unscheduled visits, which could have underestimated actual failure rates in both arms. However, scheduled 48-h Holter monitoring at 6- and 12-month follow-up was more intensive than 24-h Holter monitoring that is currently recommended by the 2017 HRS consensus statement.
Taken together, the two treatment approaches, minimal lesion sets, and allowance of repeated CA before T0 complied with standard clinical practices at nine investigational sites in five countries and as such should accurately reflect treatment paradigms for non-paroxysmal AF.
CEASE-AF is the largest prospective, multi-center RCT that demonstrated superior freedom from atrial arrhythmias for staged HA compared to endocardial CA including repeat ablation in patients with advanced AF. This is further evidence that supports combined epicardial-endocardial ablation should be more effective than endocardial only ablation and emphasizes the role of a collaborative heart team approach in the treatment of non-paroxysmal AF.
Contributors
ND, DK, BvP, AB, AM, GM, JS, SH, MW, BvP, NR, PN, TO, and PS contributed to data collection. All authors contributed to data interpretation. TW reviewed the literature search. ND, TW, and PS wrote the manuscript. TW, DK, BvP, AB, GM, SH, MW, and PS edited the manuscript. All authors reviewed the manuscript and approved the final version. ND and TW accessed and verified the data.
Data sharing statement
The primary results of CEASE-AF will be made available on clinicaltrials.gov (NCT02695277) as required by FDAAA 801. Beginning 5 years after completion of the trial (last patient’s 12-month follow-up visit), partial data may be provided to qualified researchers through a direct request, including proposed research question(s), to MedAffairs@atricure.com, as determined by representatives of the Sponsor’s Scientific, Clinical, and Medical Affairs departments. Patient level data will not be made available.
Declaration of interests
This study was sponsored by AtriCure, Inc. The following authors report disclosures in addition to the funding on this study from AtriCure, Inc.: Dr. Doll—Medtronic, Inc. (advisory board [consulting, expert testimony]); AtriCure, Inc. (proctoring), AtriCure, Inc. (lecture fees), AtriCure, Inc. (congress travel support); Dr. Weimar—AtriCure, Inc. (contract, stock/stock options); Dr. Kosior—Pfizer (lecture honoraria, travel support); Boehringer Ingelheim (lecture honoraria; travel support); Bayer (lecture honoraria); Dr. van Putte–AtriCure, Inc. (consultant fees and honoraria); Dr. Wijffels–Microport (presentation); AtriCure (consultant fees); Dr. Rüb–Medtronic (advisory board); Bristol-Myers-Squibb, Prospitalia Institute, Abbott, Astra Zeneca (lecture honoraria); Johnson & Johnson, BSI (travel support); European Society of Cardiology (question writing committee); Dr. Mönnig–Abbott, Medtronic (lecture honoraria); Dr. Sahu—Bayer, Pfizer (speaker honoraria); Dr. Hunter–AtriCure, Inc. (consulting fees, payment for educational events); Prof. Suwalski–AtriCure, Inc., Medtronic (consulting fees); Edwards (honoraria). The following authors report no disclosures other than funding on this study from AtriCure, Inc.: Drs. Nemec, Ostrizek, Mokracek, Bulava.
Acknowledgements
The authors thank the CEASE-AF patients and investigators. The authors would like to sincerely thank Erik Fransen, MSc, PhD (AtriCure Europe BV) for his excellent project leadership. Kristen Plasseraud, PhD, and Latoya M. Mitchell, PhD, CMPP (AtriCure, Inc) provided medical writing assistance under the authors’ direction.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102052.
Appendix A. Supplementary data
References
- 1.Benjamin E.J., Wolf P.A., D'Agostino R.B., Silbershatz H., Kannel W.B., Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 2.Blackshear J.L., Odell J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 3.Chugh S.S., Havmoeller R., Narayanan K., et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H., Hindricks G., Cappato R., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiyagarajah A., Kadhim K., Lau D.H., et al. Feasibility, safety, and efficacy of posterior wall isolation during atrial fibrillation ablation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.118.007005. [DOI] [PubMed] [Google Scholar]
- 7.Bai R., Di Biase L., Mohanty P., et al. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm. 2016;13:132–140. doi: 10.1016/j.hrthm.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Markman T.M., Hyman M.C., Kumareswaran R., et al. Durability of posterior wall isolation after catheter ablation among patients with recurrent atrial fibrillation. Heart Rhythm. 2020;17:1740–1744. doi: 10.1016/j.hrthm.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Pothineni N.V.K., Lin A., Frankel D.S., et al. Impact of left atrial posterior wall isolation on arrhythmia outcomes in patients with atrial fibrillation undergoing repeat ablation. Heart Rhythm O2. 2021;2:489–497. doi: 10.1016/j.hroo.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilz R.R., Rillig A., Thum A.M., et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg sequential ablation strategy. J Am Coll Cardiol. 2012;60:1921–1929. doi: 10.1016/j.jacc.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Darby A.E. Recurrent atrial fibrillation after catheter ablation: considerations for repeat ablation and strategies to optimize success. J Atr Fibrillation. 2016;9:1427. doi: 10.4022/jafib.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah S., Barakat A.F., Saliba W.I., et al. Recurrent atrial fibrillation after initial long-term ablation success. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.005785. [DOI] [PubMed] [Google Scholar]
- 13.Al-Hijji M.A., Deshmukh A.J., Yao X., et al. Trends and predictors of repeat catheter ablation for atrial fibrillation. Am Heart J. 2016;171:48–55. doi: 10.1016/j.ahj.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Verma A., Jiang C.Y., Betts T.R., et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 15.Mansour M., Calkins H., Osorio J., et al. Persistent atrial fibrillation ablation with contact force-sensing catheter: the prospective multicenter PRECEPT trial. JACC Clin Electrophysiol. 2020;6:958–969. doi: 10.1016/j.jacep.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Su W.W., Reddy V.Y., Bhasin K., et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: results from the multicenter STOP persistent AF trial. Heart Rhythm. 2020;17:1841–1847. doi: 10.1016/j.hrthm.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Varzaly J.A., Lau D.H., Chapman D., Edwards J., Worthington M., Sanders P. Hybrid ablation for atrial fibrillation: a systematic review and meta-analysis. JTCVS Open. 2021;7:141–154. doi: 10.1016/j.xjon.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLurgio D.B., Crossen K.J., Gill J., et al. Hybrid convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation: results of CONVERGE clinical trial. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.009288. [DOI] [PubMed] [Google Scholar]
- 19.Shrestha S., Plasseraud K.M., Makati K., et al. Hybrid convergent ablation for atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm O2. 2022;3:396–404. doi: 10.1016/j.hroo.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summary of safety and effectiveness data (SSED) - THERMOCOOL SMARTTOUCH SF Bi-directional navigation catheter; THERMOCOOL SMARTTOUCH SF Uni-Directional Navigation catheter. https://www.accessdata.fda.gov/cdrh_docs/pdf3/P030031S100B.pdf2020 Available from:
- 21.Deshmukh A., Patel N.J., Pant S., et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013;128:2104–2112. doi: 10.1161/CIRCULATIONAHA.113.003862. [DOI] [PubMed] [Google Scholar]
- 22.Gharaviri A., Bidar E., Potse M., et al. Epicardial fibrosis explains increased endo-epicardial dissociation and epicardial breakthroughs in human atrial fibrillation. Front Physiol. 2020;11:68. doi: 10.3389/fphys.2020.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim H.S., Hocini M., Dubois R., et al. Complexity and distribution of drivers in relation to duration of persistent atrial fibrillation. J Am Coll Cardiol. 2017;69:1257–1269. doi: 10.1016/j.jacc.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Voeller R.K., Bailey M.S., Zierer A., et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg. 2008;135:870–877. doi: 10.1016/j.jtcvs.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 25.Kistler P.M., Chieng D., Sugumar H., et al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the CAPLA randomized clinical trial. JAMA. 2023;329:127–135. doi: 10.1001/jama.2022.23722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valderrabano M., Peterson L.E., Swarup V., et al. Effect of catheter ablation with vein of marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the VENUS randomized clinical trial. JAMA. 2020;324:1620–1628. doi: 10.1001/jama.2020.16195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo M., Nair D., Mansour M., et al. Contact force catheter ablation for the treatment of persistent atrial fibrillation: results from the PERSIST-END study. J Cardiovasc Electrophysiol. 2022;34(2):279–290. doi: 10.1111/jce.15742. [DOI] [PubMed] [Google Scholar]
- 28.van der Heijden C.A.J., Weberndorfer V., Vroomen M., et al. Hybrid ablation versus repeated catheter ablation in persistent atrial fibrillation: a randomized controlled trial. JACC Clin Electrophysiol. 2023 doi: 10.1016/j.jacep.2022.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.