Abstract
Background:
Many adverse pregnancy outcomes (APOs) are associated with elevated cardiovascular disease (CVD) risk. However, APO data in the context of pre-existing CVD risk factors, and from diverse populations, are limited. We assessed the occurrence of APOs among individuals with and without prepregnancy CVD risk factors, overall and by race/ethnicity.
Methods:
We conducted a retrospective study using electronic medical record data from a large urban safety-net hospital. Individuals with prenatal care and delivery between 2016 and 2018 at the hospital were included, and data from prenatal intake through the delivery hospitalization were captured. The exposure, prepregnancy CVD risk factors (hypertension, diabetes, tobacco use, and obesity), and the outcome, APOs (hypertensive disorders of pregnancy, gestational diabetes, preterm delivery, low birth weight, and stillbirth), were identified from electronic medical records.
Results:
We identified 3760 unique delivering individuals, of whom 55.1% self-identified as Black non-Hispanic and 17% as Hispanic. Prepregnancy CVD risk factor prevalence was 45.6%, most commonly obesity (26.6%). APO prevalence was 35.6%, most commonly a hypertensive disorder of pregnancy (20.1%). Overall, 45.7% of APOs occurred in the absence of recognized prepregnancy CVD risk factors, representing 16.3% of the total sample. Among individuals without prepregnancy CVD risk factors, APO prevalence was 30.0% and did not vary by race/ethnicity.
Conclusions:
In this racially and ethnically diverse hospital-based sample, APOs were present in one in three parous individuals without prepregnancy CVD risk factors—a group with potentially elevated CVD risk who might otherwise be missed by traditional CVD risk factor screening.
Keywords: preconception, hypertension, diabetes, cardiovascular disease risk, adverse pregnancy outcomes
Introduction
Many adverse pregnancy outcomes (APOs) are indicators of latent or subclinical cardiovascular disease (CVD) risk.1 For instance, hypertensive disorders of pregnancy, preterm delivery, and gestational diabetes each affect up to 10% of pregnancies, and are individually associated with a doubling or more of 10-year CVD risk.1,2
Because traditional CVD risk factors such as chronic hypertension are less prevalent than APOs during the reproductive years, APOs may often be the first sign that a person is at increased risk for CVD. However, the number of individuals who may be identified as having elevated CVD risk on the basis of APOs alone is not well described.
Furthermore, persons of color are disproportionately affected by both APOs and CVD but underrepresented in CVD prevention research. The American Heart Association has called for more research to understand and address racial and ethnic disparities in APOs and CVD risk.2
Given limited published information on the co-occurrence of CVD risk factors and APOs and the recognized lack of data for persons of color,2 we assessed prepregnancy CVD risk factors and APOs in an urban safety-net hospital serving a racially diverse patient population. Our primary aim was to assess the occurrence of APOs among individuals with and without prepregnancy CVD risk factors, to estimate the prevalence of APOs being potentially the first indication of elevated CVD risk. Our secondary aim was to assess the co-occurrence of prepregnancy CVD risk factors and APOs by race/ethnicity.
Methods
We conducted a retrospective electronic medical record-based study of individuals delivering at a large urban safety-net hospital, 2016–2018. The study was approved by the Institutional Review Board.
The dataset included demographic information, reproductive history, prepregnancy conditions, pregnancy diagnoses, delivery information, and routinely collected clinical data such as blood pressure and weight. Self-reported race, ethnicity, language, and birthplace were assessed from specific fields in the medical record, which limited racial identity to one group per individual. Race and ethnicity were combined into five categories: Black, Non-Hispanic; White, Non-Hispanic; Hispanic; Asian/Asian Indian; and Other/Unknown. The “Other” race category included American Indian/Native American, Middle Eastern, Native Hawaiian/Pacific Islander, and Other. Standard prenatal and delivery care at the hospital includes comprehensive medical history review.
We defined our sample as individuals who delivered at the hospital at ≥20 weeks of gestation and therefore at risk of developing the APOs of interest. We further restricted our analysis to individuals who were ≥18 years old at the time of delivery and who received prenatal care at the hospital, since they were more likely to have complete medical history data compared with those receiving all of their prenatal care at another site. We excluded subsequent deliveries to individuals with more than one delivery during the study period, thereby only including one pregnancy per individual. Eligible individuals were identified, and data were extracted from the medical record, by the hospital's Clinical Data Warehouse.
After identifying our sample, we defined each individual's exposure status as having no versus any recognized prepregnancy CVD risk factors. We assessed the following four prepregnancy CVD risk factors: chronic hypertension, pregestational diabetes, tobacco use, and obesity. Chronic hypertension and pregestational diabetes were identified based on International Classification of Disease, 10th Revision (ICD-10) codes. Tobacco use (current, former, never) was determined from self-reported smoking status in the medical record. Obesity was defined by body mass index (BMI) ≥30 kg/m2, calculated from prepregnancy or early pregnancy (<20 weeks of gestation) height and weight measurements. To address the large proportion of individuals without recorded prepregnancy height and weight data, we included early pregnancy BMI measurements, since gestational weight gain trajectories do not begin to increase uniformly until the late second trimester.3 Other traditional CVD risk factors, such as cholesterol and family history, were not included as these data were not routinely collected.
We then assessed for the development of the outcome, any APO associated with CVD risk. APOs of interest included hypertensive disorders of pregnancy, gestational diabetes, preterm delivery, low birth weight (restricted to term deliveries), and stillbirth. For hypertensive disorders of pregnancy, we included diagnoses indicating hypertension of new onset or with significant clinical worsening during pregnancy (i.e., gestational hypertension, preeclampsia, eclampsia, and superimposed preeclampsia on chronic hypertension) using ICD-10 diagnosis codes4,5 documented in the medical record (Supplementary Table S1 for codes and more detailed methods). ICD-10 codes were also used to identify gestational diabetes.5,6 Preterm delivery was defined as delivery before 37 weeks of gestation, and low birth weight at term was defined as the delivery of an infant <2500 g among infants born after 37 weeks of gestation.
For the data analysis, we first examined the demographic and reproductive characteristics (i.e., parity, single or multiple gestation, and mode of delivery) of the sample. We described the overall sample and then stratified by those with and without any prepregnancy CVD risk factor. We calculated the prevalence of APOs in the sample overall, then by prepregnancy CVD risk factor status (none/any). We calculated the relative risk of an APO for those with versus without a prepregnancy CVD risk factor using log binomial models, adjusting for maternal age as a potentially important confounder. We did not adjust for other potentially important confounders due to the descriptive nature of our analysis. However, we did stratify analyses to examine CVD risk factor and APO prevalence between groups defined by race and ethnicity.
Finally, we conducted a sensitivity analysis, in which we calculated CVD risk factor and APO prevalence in the subsample of nulliparous (i.e., first-time delivering) individuals only. The purpose of the sensitivity analysis was to assess the robustness of our findings in a subsample without a history of APOs in prior pregnancies.
Data were analyzed in March 2021-April 2022 using RStudio, Version 1.2.5042.
Results
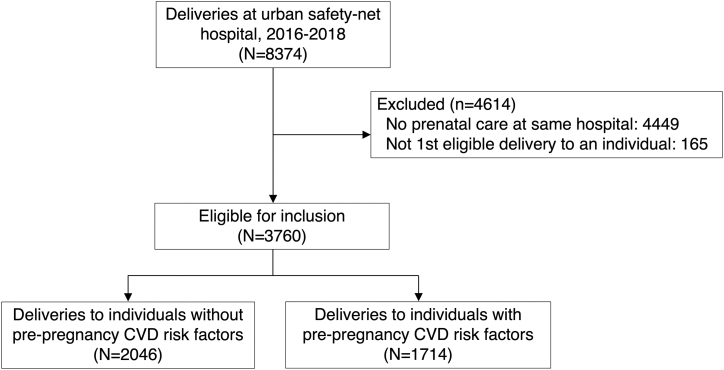
Of a total of 8374 deliveries to 8037 individuals at the hospital between 2016 and 2018, we identified 3760 eligible delivering individuals (Fig. 1). Their average age was 30.5 years (standard deviation 5.7 years), and one-quarter were ≥35 years old at the time of delivery (Table 1). A majority identified as Black non-Hispanic (55.1%), and ∼17% identified as Hispanic and 17% identified White non-Hispanic. More than half were born outside of the US (56.1%), and at least 23% reported a preferred language other than English, most commonly Haitian Creole (10.0%) or Spanish (9.0%). Over 70% were publicly insured.
FIG. 1.
Study inclusion flowsheet. CVD, cardiovascular disease.
Table 1.
Maternal Sociodemographic and Clinical Characteristics by Presence/Absence of Prepregnancy Cardiovascular Disease Risk Factors at an Urban Safety-Net Hospital 2016–2018
| Maternal characteristics | Total (n = 3760) | Prepregnancy CVD risk factora |
|
|---|---|---|---|
| None (n = 2046) | Any (n = 1714) | ||
| Age in years, mean ± SD | 30.5 (5.71) | 30.1 (5.60) | 31.0 (5.80) |
| Age, years, n (%) | |||
| 18–24 | 595 (15.8) | 352 (17.2) | 243 (14.2) |
| 25–29 | 1023 (27.2) | 557 (27.2) | 466 (27.2) |
| 30–34 | 1189 (31.6) | 688 (33.6) | 501 (29.2) |
| ≥35 | 953 (25.3) | 449 (21.9) | 504 (29.4) |
| Race/Ethnicity, n (%) | |||
| Black, Non-Hispanic | 2071 (55.1) | 1130 (55.2) | 941 (54.9) |
| White, Non-Hispanic | 641 (17.0) | 273 (13.3) | 368 (21.5) |
| Hispanic | 650 (17.3) | 352 (17.2) | 298 (17.4) |
| Asian/Asian Indian | 147 (3.9) | 125 (6.1) | 22 (1.3) |
| None of the above/unknown | 251 (6.7) | 166 (8.1) | 85 (5.0) |
| Language, n (%) | |||
| English | 2670 (71.0) | 1298 (63.4) | 1372 (80.0) |
| Spanish | 337 (9.0) | 210 (10.3) | 127 (7.4) |
| Haitian Creole | 375 (10.0) | 246 (12.0) | 129 (7.5) |
| Cape Verdean/Portuguese Creole | 111 (3.0) | 83 (4.1) | 28 (1.6) |
| Other/unknown | 267 (7.1) | 209 (10.2) | 58 (3.4) |
| Birthplace, n (%) | |||
| US | 1635 (43.5) | 648 (31.7) | 987 (57.6) |
| Outside of US | 2110 (56.1) | 1389 (67.9) | 721 (42.1) |
| Unknown//missing | 15 (0.4) | 9 (0.4) | 6 (0.4) |
| Insurance, n (%) | |||
| Public | 2684 (71.4) | 1380 (67.4) | 1304 (76.1) |
| Private | 911 (24.2) | 566 (27.7) | 345 (20.1) |
| Other/none/unknown | 165 (4.4) | 100 (4.9) | 65 (3.8) |
| Parity including current, n (%) | |||
| 0 (Nulliparous) | 1546 (41.1) | 981 (47.9) | 565 (33.0) |
| 1 | 1154 (30.7) | 624 (30.5) | 530 (30.9) |
| 2 | 634 (16.9) | 288 (14.1) | 346 (20.2) |
| ≥3 | 426 (11.3) | 153 (7.5) | 273 (15.9) |
| Multiple gestation | 108 (2.9) | 57 (2.8) | 51 (3.0) |
| Caesarean delivery | 1457 (38.8) | 691 (33.8) | 766 (44.7) |
Defined by having ≥1 of the following: chronic hypertension, pregestational diabetes, pre-/early pregnancy obesity (BMI ≥30 kg/m2 at <20 weeks of gestation), current or prior tobacco use.
BMI, body mass index; CVD, cardiovascular disease; SD, standard deviation.
The overall prevalence of prepregnancy CVD risk factors in our sample was 45.6% (Table 2). The most common CVD risk factors were obesity (26.6%) and tobacco use (ever, 20.9%), followed by hypertension (8.4%) and diabetes (3.2%). Those with versus without prepregnancy CVD risk factors had a higher proportion with maternal age ≥35 years (29.4% vs. 21.9%), White non-Hispanic race/ethnicity (21.5% vs. 13.3%), English as their primary language (80.0% vs. 63.4%), United States as their birth country (57.6% vs. 31.7%), and multiparity (67.0% vs. 52.1%; Table 1).
Table 2.
Prepregnancy Cardiovascular Disease Risk Factors and Adverse Pregnancy Outcomes at an Urban Safety-Net Hospital 2016–2018
| Diagnoses, n (%) | Total (n = 3760) | Prepregnancy CVD risk factor |
||
|---|---|---|---|---|
| None (n = 2046) | Any (n = 1714) | Relative riska (95% CI) | ||
| ≥1 Prepregnancy CVD risk factor | 1714 (45.6) | N/A | 1714 (100) | N/A |
| Hypertension | 316 (8.4) | N/A | 316 (18.4) | N/A |
| Diabetes (type 1 or 2) | 121 (3.2) | N/A | 121 (7.1) | N/A |
| Obesity, pre-/early pregnancyb | 999 (26.6) | N/A | 999 (58.3) | N/A |
| Tobacco use, ever | 785 (20.9) | N/A | 785 (45.8) | N/A |
| ≥1 APO | 1340 (35.6) | 613 (30.0) | 727 (42.4) | 1.70 (1.48–1.95) |
| Hypertensive disorder of pregnancyc | 754 (20.1) | 346 (16.9) | 408 (23.8) | 1.53 (1.30–1.80) |
| Gestational hypertension | 461 (12.3) | 222 (10.9) | 239 (13.9) | 1.35 (1.11–1.64) |
| Preeclampsia | 293 (7.8) | 124 (6.1) | 169 (9.9) | 1.65 (1.29–2.10) |
| Superimposed preeclampsia | 61 (1.6) | 0 (0) | 61 (3.6) | N/A |
| Preterm delivery | 455 (12.1) | 185 (9.0) | 270 (15.8) | 1.85 (1.52–2.26) |
| Early (<34 weeks of gestation) | 157 (4.2) | 59 (2.9) | 98 (5.7) | 1.99 (1.43–2.77) |
| Late (34–<37 weeks of gestation) | 298 (7.9) | 126 (6.2) | 172 (10.0) | 1.69 (1.33–2.15) |
| Low birth weight | 139 (3.7) | 54 (2.6) | 85 (5.0) | 1.96 (1.38–2.78) |
| Gestational diabetes | 276 (7.3) | 136 (6.6) | 140 (8.2) | 1.15 (0.90–1.47) |
| Stillbirth | 29 (0.8) | 13 (0.6) | 16 (0.9) | 1.42 (0.68–2.97) |
| Combination of prepregnancy CVD risk factors and APOs | ||||
| No CVD risk factor and no APO | 1433 (38.1) | 1433 (70.0) | N/A | N/A |
| No CVD risk factor and ≥1 APO | 613 (16.3) | 613 (30.0) | N/A | N/A |
| ≥1 CVD risk factor and no APO | 987 (26.3) | N/A | 987 (57.6) | N/A |
| ≥1 CVD risk factor and ≥1 APO | 727 (19.3) | N/A | 727 (42.4) | N/A |
Relative risk of the corresponding diagnosis for those with any versus no prepregnancy CVD risk factors, adjusting for maternal age.
Defined by BMI ≥30 kg/m2 at <20 weeks of gestation, with 19.3% missing (n = 756).
Not including chronic hypertension without superimposed preeclampsia.
APO, adverse pregnancy outcome; CI, confidence interval; N/A, not applicable.
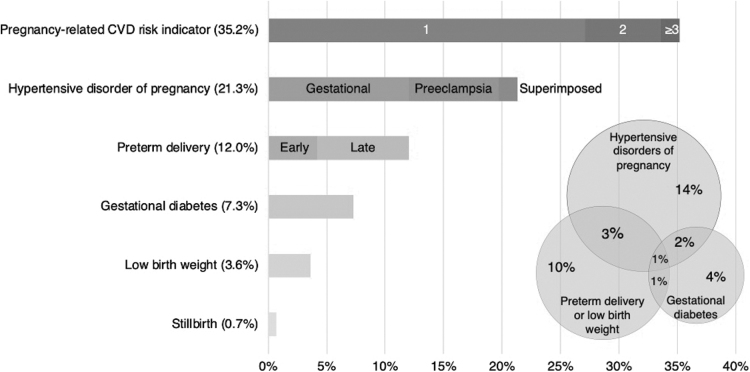
Over one-third (35.6%) of our sample developed an APO (Table 2). Hypertensive disorders of pregnancy were the most common APO (20.1%; Table 2, Fig. 2). Nine percent of delivering individuals had ≥2 APOs, most often a hypertensive disorder of pregnancy and preterm delivery (n = 121, 3.2% of total sample). The co-occurrence of other APOs is illustrated in Figure 2 inset, with circles drawn to approximate scale and percentages based on the total sample.
FIG. 2.
Prevalence of APOs at an urban safety-net hospital 2016–2018 (n = 3760). Inset 1: distribution of number of APOs. Inset 2: co-occurrence of top 4 adverse pregnancy outcomes. Definitions: early preterm, delivery at <34 weeks' gestation; late preterm, delivery at 34–<37 weeks' gestation; low birth weight, <2500 g term delivery. Notes on Inset 2: circles drawn to approximate scale, and percentages based on the total sample; preterm delivery and low birth weight combined for ease of visual display and interpretation. APO, adverse pregnancy outcome; Gestational, gestational hypertension; Superimposed, superimposed preeclampsia on chronic hypertension.
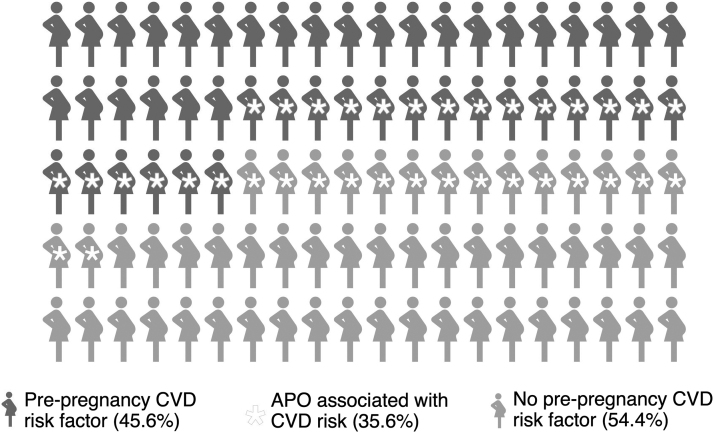
The risk of developing an APO was higher among those with versus without recognized prepregnancy CVD risk factors (42.4% vs. 30.0%; relative risk 1.70, 95% confidence interval: 1.48–1.95). Nevertheless, a large proportion of those without prepregnancy CVD risk factors still developed an APO. Nearly half (45.7%) of all APOs occurred in the absence of recognized prepregnancy CVD risk factors, representing 16.3% of the total sample (Table 2, Fig. 3).
FIG. 3.
Overlap of prepregnancy CVD risk factors and APOs at an urban safety-net hospital 2016–2018 (n = 3760). Prepregnancy CVD risk factors: chronic hypertension, pregestational diabetes, pre-/early pregnancy obesity (BMI ≥30 kg/m2 at <20 weeks' gestation), and current or prior tobacco use. APOs associated with CVD risk: hypertensive disorders of pregnancy (gestational hypertension, preeclampsia/eclampsia, and superimposed preeclampsia on chronic hypertension), preterm delivery, low birth weight, gestational diabetes, and stillbirth. BMI, body mass index.
The prevalence of APOs among those without prepregnancy CVD risk factors was similar across racial/ethnic groups: non-Hispanic Black, 28.2%; non-Hispanic White, 27.5%; Hispanic, 33.0%; Asian, 30.4%; and Other/Unknown, 39.2%. Meanwhile, the prevalence of APOs among those with prepregnancy CVD risk factors was higher in groups of individuals who identified as non-Hispanic Black, non-Hispanic White, or Hispanic (20.1%–20.6%), and lower among those who identified as Asian (7.5%) (Table 3, Supplementary Table S2).
Table 3.
Prepregnancy Cardiovascular Disease Risk Factors and Adverse Pregnancy Outcomes, by Race/Ethnicity, at an Urban Safety-Net Hospital 2016–2018
| Diagnoses, n (%) | Race/Ethnicity |
||||
|---|---|---|---|---|---|
| Black, non-Hispanic (n = 2071) | White, non-Hispanic (n = 641) | Hispanic (n = 650) | Asian/Asian Indian (n = 147) | Other/unknown (n = 251) | |
| ≥1 Prepregnancy CVD risk factor | 941 (45.4) | 368 (57.4) | 298 (45.8) | 22 (15.0) | 85 (33.9) |
| Hypertension | 198 (9.6) | 47 (7.3) | 46 (7.1) | 4 (2.7) | 21 (8.4) |
| Diabetes (type 1 or 2) | 67 (3.2) | 8 (1.2) | 34 (5.2) | 3 (2.0) | 9 (3.6) |
| Obesity, pre-/early pregnancya | 638 (30.8) | 104 (16.2) | 192 (29.5) | 13 (8.8) | 52 (20.7) |
| Tobacco use, ever | 331 (16.0) | 300 (46.8) | 130 (20.0) | 6 (4.1) | 18 (7.2) |
| ≥1 APO | 735 (35.5) | 207 (32.3) | 247 (38.0) | 49 (33.3) | 102 (40.6) |
| Hypertensive disorder of pregnancyb | 440 (21.2) | 126 (19.7) | 124 (19.1) | 20 (13.6) | 44 (17.5) |
| Gestational hypertension | 264 (12.7) | 88 (13.7) | 66 (10.2) | 16 (10.9) | 27 (10.8) |
| Preeclampsia | 176 (8.5) | 38 (5.9) | 58 (8.9) | 4 (2.7) | 17 (6.8) |
| Preeclampsia superimposed on chronic hypertension | 42 (2.0) | 7 (1.1) | 7 (1.1) | 0 (0) | 5 (2.0) |
| Preterm delivery | 232 (11.2) | 69 (10.8) | 101 (15.5) | 18 (12.2) | 35 (13.9) |
| Early (<34 weeks of gestation) | 83 (4.0) | 22 (3.4) | 34 (5.2) | 3 (2.0) | 15 (6.0) |
| Late (34–<37 weeks of gestation) | 149 (7.2) | 47 (7.3) | 67 (10.3) | 15 (10.2) | 20 (8.0) |
| Low birth weight | 93 (4.5) | 24 (3.7) | 13 (2.0) | 4 (2.7) | 5 (2.0) |
| Gestational diabetes | 120 (5.8) | 25 (3.9) | 75 (11.5) | 22 (15.0) | 34 (13.5) |
| Stillbirth | 16 (0.8) | 4 (0.6) | 5 (0.8) | 0 (0) | 4 (1.6) |
| Combination of prepregnancy CVD risk factors and APOs | |||||
| No CVD risk factor and no APO | 811 (39.2) | 198 (30.9) | 236 (36.3) | 87 (59.2) | 101 (40.2) |
| No CVD risk factor and ≥1 APO | 319 (15.4) | 75 (11.7) | 116 (17.8) | 38 (25.9) | 65 (25.9) |
| ≥1 CVD risk factor and no APO | 525 (25.4) | 236 (36.8) | 167 (25.7) | 11 (7.5) | 48 (19.1) |
| ≥1 CVD risk factor and ≥1 APO | 416 (20.1) | 132 (20.6) | 131 (20.2) | 11 (7.5) | 37 (14.7) |
Defined by BMI ≥30 kg/m2 at <20 weeks of gestation, with 19.3% missing (n = 756).
Not including chronic hypertension without superimposed preeclampsia.
In sensitivity analyses restricting to nulliparous individuals (n = 1546), CVD risk factor and APO prevalence estimates were similar to those of the total sample (Supplementary Table S3, Supplementary Fig. S1 for detailed results).
Discussion
In this racially diverse patient sample at an urban safety-net hospital, we demonstrated a high prevalence of APOs, commonly occurring in delivering persons both with and without prepregnancy CVD risk factors (42% and 30%, respectively).
A central finding from our study is that APOs are early markers of CVD risk—often occurring before the development of traditional CVD risk factors—which may allow for more timely recognition of elevated CVD risk. The relationship between APOs and future CVD is well established; the American College of Cardiology and American Heart Association have recognized preeclampsia and preterm delivery as CVD risk-enhancing factors.7 However, most studies on APOs and CVD risk do not examine APOs in the context of pre-existing CVD risk factors. Here, we examine the occurrence of APOs in persons both with and without identified prepregnancy CVD risk. We show that 46% of APOs occur in the absence of recognized (i.e., clinically diagnosed) prepregnancy CVD risk factors, thus signaling potentially elevated CVD risk in one in three individuals who might have otherwise been missed because they lacked traditional CVD risk factors.
Another contribution to the literature is our examination of prepregnancy CVD risk factors and APOs by race/ethnicity. In our sample, prepregnancy CVD risk factor prevalence, as well as APO prevalence among those with ≥1 prepregnancy CVD risk factor, varied by race/ethnicity. Meanwhile, the proportion of individuals without prepregnancy CVD risk factors who subsequently developed an APO was similar across all racial and ethnic groups. This finding underscores the potential value of APOs for early CVD risk detection in diverse populations, regardless of race/ethnicity. It also suggests that prepregnancy conditions may be an important driver of observed racial/ethnic disparities in APOs and CVD risk. Our findings should be investigated in larger datasets with detailed CVD risk factor data from diverse patient populations.
CVD prevention guidelines recommend that all parous individuals be screened for a history of APOs, and that CVD risk screening and counseling ideally begin within the first year after an APO.8–10 Indeed, timely intervention after APOs can lead to sustained improvements in CVD risk.11,12 Unfortunately, many individuals with APOs do not receive adequate information or follow-up to address their CVD risk.13,14 In a large nationwide survey of postpartum individuals, including 20% with APOs, most did not report receiving basic CVD prevention counseling on diet, exercise, and smoking at their postpartum visit.15 In an anonymous survey of 118 internists, only 5% reported routinely screening for a history of preeclampsia, and only 9% counseled patients with a known history of preeclampsia on their individual CVD risks.16 Interventions to support the integration of APO history into CVD prevention efforts are urgently needed,2,17 across a range of clinical specialties, including obstetrics, primary care, and cardiology.
It is notable that our sample has a 36% prevalence of APOs, which is higher than published estimates, both prepregnancy (20%–30%18) and lifetime (10%–15%,2 33%1). In particular, the observed 20% prevalence of hypertensive disorders of pregnancy in our sample exceeds previously reported prevalence estimates of 5%–10%.2,18 Such differences may be explained, in part, by our patient population (56% born overseas and 70% publicly insured), who may have had limited access to health care before pregnancy; in this context, some APOs diagnosed during pregnancy may actually represent undiagnosed prepregnancy CVD risk factors. In addition, there are prevailing racial inequities in APOs (e.g., preeclampsia is 60% more common in populations who identify as Black vs. White19), and 55% of our sample self-identifies as Black. Other findings from our study, for example, the 26% prevalence of prepregnancy obesity, are consistent with published national data.20
Our study has limitations. First, we have incomplete data on some CVD risk factors (e.g., family history, prepregnancy BMI), and for others (i.e., hypertension and diabetes) we rely on ICD-10 codes. Thus, underdiagnosis or misclassification of prepregnancy CVD risk factors is possible. In addition, the blood pressure threshold for hypertension diagnosis was lowered in 2017,21 making hypertension diagnoses potentially more common in the latter part of our study period. Our internal data suggest that prepregnancy and pregnancy-related hypertension diagnoses rose similarly after 2017, therefore we expect the effect of the guideline change on our results to be minimal. Second, again due to our reliance on ICD-10 codes rather than direct assessment from physical or laboratory values, there is the potential for misclassification or underdiagnosis of certain APOs, specifically hypertensive disorders of pregnancy and gestational diabetes. We are also unable to distinguish between spontaneous and medically indicated preterm deliveries, which is relevant because medically indicated preterm deliveries appear to be more strongly associated with incident CVD.22 Nevertheless, both spontaneous and medically indicated preterm deliveries are positively associated with future CVD23 and therefore of interest in our study. Third, more generally, we relied on medical record data input by clinicians and other clinical staff members, which is subject to data entry error. The medical record also limits individuals to a single race, preventing us from analyzing outcomes for individuals who may in reality identify with multiple races. Finally, our findings are based on a hospital-based sample from a single urban institution, notable for a high degree of racial/ethnic diversity and many overseas-born patients, and therefore may not be generalizable to other populations or settings.
Our study also has many strengths. Our racially diverse sample provides much-needed data on minoritized populations, who face increased risks of both APOs and CVD,2,19,24 but are underrepresented in CVD risk cohort studies.2 A second strength is our use of medical record data, which—despite limitations—provide information on variables such as tobacco use, height, weight, language, and birthplace that are not commonly available from administrative data. Finally, our inclusive sampling strategy (i.e., including multiparous individuals, multiple gestations, and stillbirths) enables our study to capture pregnancies affected by APOs better than other studies with more exclusions.
APOs may be able to inform earlier CVD risk counseling and prevention in as many as one in three parous individuals. Given the pervasive disconnect between obstetric care and long-term CVD risk screening and counseling, important directions for future work include clinician training to better incorporate obstetric history into routine CVD risk assessment17 and interventions to improve individualized CVD risk counseling for patients with APOs.
Supplementary Material
Authors' Contributions
M.E.M.H.: conceptualization, methodology, formal analysis, writing—original draft, writing—review and editing, visualization, and project administration; C.A.P.: writing—review and editing; T.A.B.: methodology, supervision, and resources; A.T.A.: data curation, and formal analysis; C.V.E.: writing—review and editing; E.J.B.: writing—review and editing and supervision; C.D.Y.: conceptualization, writing—review and editing; S.E.P.: conceptualization, methodology, formal analysis, writing—original draft, resources, project administration, and funding acquisition.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
M.E.M.H. was supported by the Boston University School of Medicine, Boston University School of Medicine Department of Medicine Career Investment Award, and the American Heart Association Career Development Award 937987. E.J.B. was supported by grants from the NIH 2R01 HL092577; 1R01 HL141434; 2U54HL120163; 1R01 AG066010; 1R01 AG066914; and the American Heart Association, 18SFRN34110082. C.D.Y. and S.E.P. were jointly supported by a grant from the NIH 1R01HL158864. S.E.P. was additionally supported by the Boston University School of Public Health Early Career Catalyst Award and the NIH K01HL133600.
Supplementary Material
References
- 1. Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease in later life: Umbrella review. BMJ 2020;371:m3502; doi: 10.1136/bmj.m3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: Unique opportunities for cardiovascular disease prevention in women: A scientific statement from the American Heart Association. Circulation 2021;143(18):e902–e916; doi: 10.1161/CIR.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 3. Santos S, Eekhout I, Voerman E, et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med 2018;16(1):201; doi: 10.1186/s12916-018-1189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Labgold K, Stanhope KK, Joseph NT, et al. Validation of hypertensive disorders during pregnancy: ICD-10 codes in a high-burden Southeastern United States Hospital. Epidemiology 2021;32(4):591–597; doi: 10.1097/EDE.0000000000001343 [DOI] [PubMed] [Google Scholar]
- 5. Ko JY, DeSisto CL, Simeone RM, et al. Adverse pregnancy outcomes, maternal complications, and severe illness among US delivery hospitalizations with and without a coronavirus disease 2019 (COVID-19) diagnosis. Clin Infect Dis 2021;73(Supplement_1):S24–S31; doi: 10.1093/cid/ciab344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stanhope KK, Joseph NT, Platner M, et al. Validation of ICD-10 codes for gestational and pregestational diabetes during pregnancy in a large, public hospital. Epidemiology 2021;32(2):277–281; doi: 10.1097/EDE.0000000000001311 [DOI] [PubMed] [Google Scholar]
- 7. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11); doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(5):1545–1588; doi: 10.1161/01.str.0000442009.06663.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 212: Pregnancy and heart disease. Obst Gynecol 2019;133(5):e320; doi: 10.1097/AOG.0000000000003243 [DOI] [PubMed] [Google Scholar]
- 10. Murray Horwitz ME, Fisher MA, Prifti CA, et al. Primary care–based cardiovascular disease risk management after adverse pregnancy outcomes: A narrative review. J Gen Intern Med 2022;1–10; doi: 10.1007/s11606-021-07149-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kitt JA, Fox RL, Cairns AE, et al. Short-term postpartum blood pressure self-management and long-term blood pressure control: A randomized controlled trial. Hypertension 2021;78(0):469–479; doi: 10.1161/HYPERTENSIONAHA.120.17101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aroda VR, Christophi CA, Edelstein SL, et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: The diabetes prevention program outcomes study 10-year follow-up. J Clin Endocrinol Metab 2015;100(4):1646–1653; doi: 10.1210/jc.2014-3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roth H, LeMarquand G, Henry A, et al. Assessing knowledge gaps of women and healthcare providers concerning cardiovascular risk after hypertensive disorders of pregnancy—A scoping review. Front Cardiovasc Med 2019;6; doi: 10.3389/fcvm.2019.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seely EW, Celi AC, Chausmer J, et al. Cardiovascular health after preeclampsia: Patient and provider perspective. J Womens Health 2020;30(3):305–313; doi: 10.1089/jwh.2020.8384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanhope KK, Kramer MR. Variation in the content of postpartum visits by maternal race/ethnicity, preconception, and pregnancy-related cardiovascular disease risk, PRAMS, 2016–2017. Public Health Rep 2021;00333549211005814; doi: 10.1177/00333549211005814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Young B, Hacker MR, Rana S. Physicians' knowledge of future vascular disease in women with preeclampsia. Hypertens Pregnancy 2012;31(1):50–58; doi: 10.3109/10641955.2010.544955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murray Horwitz ME, Molina RL, Battaglia TA. Preventing chronic diseases after complicated pregnancies in the COVID-19 era: A call to action for PCPs. J Gen Intern Med 2021;36(7):2127–2129; doi: 10.1007/s11606-021-06734-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown HL, Smith GN. Pregnancy complications, cardiovascular risk factors, and future heart disease. Obst Gynecol Clin N Am 2020;47(3):487–495; doi: 10.1016/j.ogc.2020.04.009 [DOI] [PubMed] [Google Scholar]
- 19. Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery Hospitalizations Involving Preeclampsia and Eclampsia, 2005–2014: Statistical Brief #222. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Agency for Healthcare Research and Quality (US): Rockville, MD, USA; 2006. [PubMed] [Google Scholar]
- 20. Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016–2019. NCHS Data Brief 2020;392:1–8. [PubMed] [Google Scholar]
- 21. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol 2018;71(19):e127–e248; doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 22. Hastie CE, Smith GCS, Mackay DF, et al. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: Retrospective cohort study of 750 350 singleton pregnancies. Int J Epidemiol 2011;40(4):914–919; doi: 10.1093/ije/dyq270 [DOI] [PubMed] [Google Scholar]
- 23. Minissian MB, Kilpatrick S, Eastwood J-A, et al. Association of spontaneous preterm delivery and future maternal cardiovascular disease. Circulation 2018;137(8):865–871; doi: 10.1161/CIRCULATIONAHA.117.031403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anonymous. Heart disease and stroke statistics-2021 update a report from the American Heart Association. Circulation 2021:E254–E743; doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.