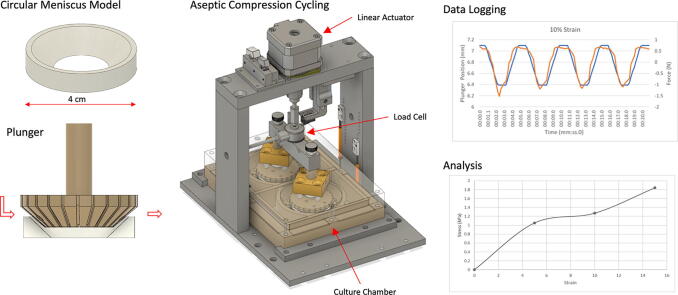
Graphical abstract
Keywords: Meniscus bioreactor, Knee biomechanics, Meniscectomy, Tear, Repair, Tissue engineering
Abstract
Injuries to the meniscus are common and can impair physical activities. Bioprinted meniscal tissue offers an attractive alternative to donor tissue for meniscal repair but achieving the strength of native tissue is a challenge. Here we report the development of a tissue engineering bioreactor designed to apply repetitive force which may lead to an increase in the compressive modulus and durability of bioprinted meniscal tissues. The modular bioreactor system is composed of a sterilizable tissue culture vessel together with a dock that applies and measures mechanical force. The culture vessel allows for simultaneous compression cycling of two anatomically sized menisci. Using a hybrid linear actuator with a stepper motor, the dock can apply up to 300 N of force at speeds up to 20 mm/s, corresponding to the upper limits of anatomical force and motion in the knee. An interchangeable 22 N load cell was mated between the culture vessel and the dock to log changes in force. Both the culture vessel and dock are maintained in a standard cell culture incubator to provide heat and CO2, while the dock is powered and controlled externally using a step motor drive and customized software.
Specifications table
| Hardware name | Meniscus Bioreactor |
|---|---|
| Subject area | Biological sciences (e.g., microbiology and biochemistry) |
| Hardware type | Biological sample handling and preparation |
| Closest commercial analog | CellScale MechanoCulture MCTX |
| Open source license | Creative Commons BY |
| Cost of hardware | $8,000 |
| Source file repository | Available with the article |
Hardware in context
Tissue engineering of replacement tissues and organs for the human body classically considers the interaction of cells, scaffolds, and signals. For engineering of musculoskeletal tissue, an important cellular signal is biomechanical force, which acts to strengthen, enlarge, or organize tissue. For example, bone and muscle maintain mass and grow with mechanical use [1]. Similarly, cartilage is a type of tissue which is strengthened by mechanical stimulation during development, however, mature cartilage has a limited capacity for self-renewal. Fibrous cartilage of the meniscus contains among the fewest cells of all cartilaginous tissue, which necessitates surgical repair or replacement once the meniscus is damaged.
While conventional static-based tissue engineering strategies for the meniscus have not yet produced tissue of physiological strength or structure, approaches which leverage mechanical force have shown that primitive tissue can be ‘matured’ to improve strength and structure prior to transplantation [2], [3]. Promising mechanical approaches include compressive loading, hydrostatic pressure, perfusion, tensile loading, and combinations thereof [4]. Here we developed a computer-controlled mechanical compression bioreactor that applies direct force to bioprinted menisci in culture.
Commercially available bioreactors include the Ebers Medical TC-3F, the FlexCell International FX-5000C, and the CellScale MCTX. These devices are intended to accommodate a range of applications but will not accommodate anatomically sized menisci. Generally, these devices may be capable of approximating the force, displacement, or speed of anatomical meniscus compression, but they are not specifically optimized in combination. Conversely, our device was optimized for long-term culture and compression of menisci, including software routines for measuring the height and strength of individual samples, while providing a streamlined workflow for operation and analysis within a typical cell culture laboratory. Furthermore, the bioreactor is fabricated from durable materials which should allow many years of laboratory use.
Hardware description
-
•
Provides aseptic oscillating compression to soft tissue constructs within CO2 incubators
-
•
Computer controlled strain, force, frequency, and duration
-
•
Live force monitoring and logging
-
•
Post-culture compression analysis
The bioreactor consists of four main components: a cell culture chamber, a dock that applies and measures mechanical force, an external electronics module, and system software. Here, we highlight the main features of each component.
Cell culture chamber
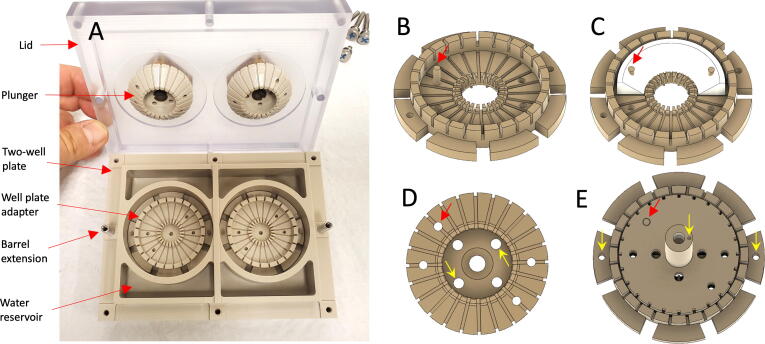
The cell culture chamber is composed of biocompatible and corrosion-resistant materials designed for an aqueous environment. The chamber consists of four sub-components including a two-well cell culture plate which holds media, well plate adapters which position menisci at the center of each well, plungers which apply force to cultured menisci, and a lid which maintains sterility. All culture components were machined out of polyetheretherketone (PEEK) or polyetherimide (PEI) while the lid was machined out of polycarbonate for transparency. Stainless steel hardware secures the lid, while PEEK dowel pins and screws are used for attachment of parts as described below. The entire assembly is autoclavable.
Two-Well cell culture Plate
The two-well cell culture plate was modeled after a conventional multi-well cell culture plate. The plate consists of two 60 × 19 mm wells, sized to accommodate anatomically sized menisci. Water reservoirs adjacent to the culture wells maintain humidity. Each well contains two dowel pin holes for indexing and attachment of adapters. Threaded holes around the perimeter of the plate allow thumbscrew attachment of the lid. Barrel extensions are used for precise alignment of the lid, Fig. 1A.
Fig. 1.
Cell Culture Chamber. (A) Cell culture chamber components include a two well culture plate, two well plate adapters, plungers and a lid; (B) Well plate adapter, arrow indicates optional meniscus dowel pin anchor; (C) Possible sample anchoring; (D) Bottom of Plunger, outer red arrow indicates clearance hole for dowel pin anchor, inner yellow arrows indicate air bleed holes; (E) Assembly shows orientation of well-plate dowel pins and piston dowel pin necessary to index the plunger to the meniscal dowl pin (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Well Plate Lid
Similar to the well plate, the lid was modeled after a traditional multi-well cell culture plate. The recessed lid wraps around the perimeter of the plate to block entry of contaminants while maintaining a 0.5 mm air gap for gas exchange, Fig. 1A. Clearance holes around the perimeter of the lid allow thumbscrew fixation to the well-plate. The lid also has clearance holes for the plungers’ pistons and threaded holes to mount retention caps.
Well Plate Adapter
To provide flexibility, adapters are used to position menisci within the culture plate. The height of the adapter brings the bottom of the menisci up to 16 mm from the top of the well, increasing oxygenation of cultured tissue. Twenty-four continuous channels measuring 1 mm2 along the floor and walls of the adapter allow liquid flow beneath and around cultured menisci. Four removable dowel pins allow medial or lateral menisci to be anchored at pseudo-dorsal and anterior horns, Fig. 1B & 1C. An outer 43 mm diameter wall allows expansion of anatomically sized menisci, while an inner 19.3 mm diameter wall restricts movement or contraction.
Plungers
Conical plungers were designed to contact and provide 10% strain (compression) across the top surface of cultured menisci. A different plunger design would be required for uniform compression at other strain levels. Like the well plate adapter, there are 24 × 1 mm2 continuous channels in the plunger to allow liquid flow above the meniscus during compression. The oversized 42 mm diameter plunger ensures contact at the outer edge of the 40 mm diameter meniscus despite expansion or slight misalignment. The plunger has clearance holes for dowel pins, as well as ports for media flow and air escape, Fig. 1D. Using a PEEK screw, the plungers are attached to pistons which extend through linear motion bearings mounted in the lid of the culture chamber. The pistons are indexed to the plungers and the tie-bar of the dock using small dowel pins, Fig. 1E.
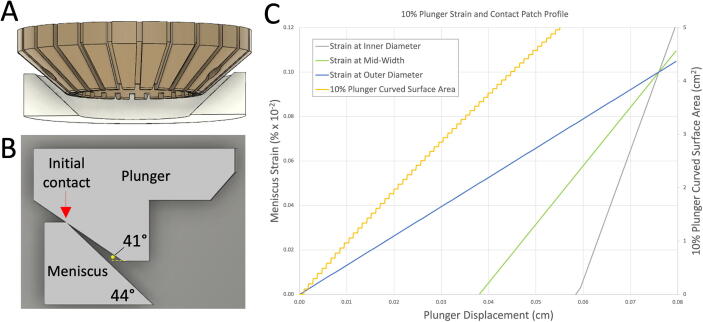
Because the adult meniscus is conical, the plungers were shaped as inverted conical frustums, Fig. 2, with a 41° angle between the top and bottom, Fig. 2B. The chosen geometry allows the plunger to contact the outer diameter of the meniscus first and gradually increase contact towards the inner diameter as the plungers are depressed. Using the chosen geometry, we achieve 10% homogenous strain across the angular height of a 0.76 cm tall meniscus after 0.076 cm of compression, Fig. 2D. The inner and flattest portion of the meniscus is not compressed by design to allow clearance for plunger movement.
Fig. 2.
10% Plunger Geometry. (A&B) Plunger and meniscus profile and contact geometry; (C) Strain profile near the inner, middle and outside diameter of the meniscus shows how the 10 % plunger achieves homogenous strain across the sample surface at 10 %, and non-homogenous strain above and below the 10 % target.
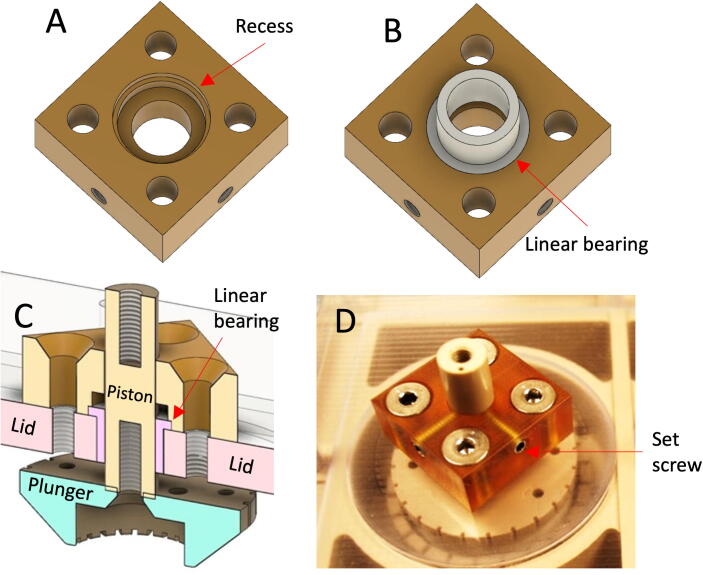
Piston Retention Caps
Two retention caps affixed to the top of the lid are used to immobilize food-grade linear motion bearings mounted in the lid, Fig. 3. The retention caps also house nylon-tipped set screws which temporarily lock the pistons in place during setup or media changes, preventing them from inadvertently contacting menisci or falling out.
Fig. 3.
Piston Retention Cap Assembly. (A) Bottom view of retention cap, (B) Low friction linear bearing fitment, (C) Cross-section of retention cap, bearing and piston assembled in the lid, (D) Set screws for temporary locking of piston.
Motorized Dock
A motorized dock was developed to apply and measure mechanical force transmitted to the culture chamber. Use of the dock requires only a simple thumbscrew connection to the culture chamber. Multiple culture chambers may be sequentially connected and disconnected using a single set of electronics. The dock is intended to be installed on a shelf within a standard CO2 incubator with the external electronics located outside of the incubator. Except for the linear actuator, the dock is composed of corrosion resistant materials, including a stainless sealed load cell, stainless screws, and an aluminum frame which do not require sterilization. Below we detail customization of the sub-components of the dock.
Frame
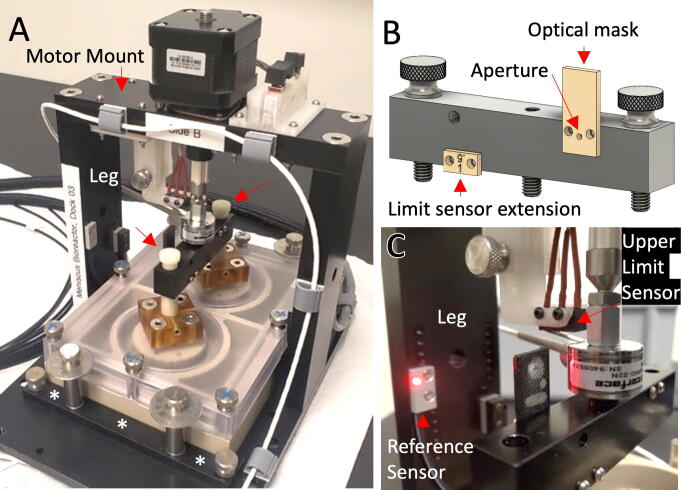
The frame of the dock was machined from 6061 aluminum and anodized black. The frame consists of a base plate, two legs and a motor mount. The base plate has threaded holes for attachment of front and rear alignment bumpers that position and secure the culture chamber beneath the motor mount, Fig. 4A. Using one set of holes, the culture chamber can be centered under the actuator for simultaneous compression of two wells together with a ‘piston tie-bar’ as shown in Fig. 4A & 4B. Using a second set of holes, a single well of the culture chamber can be centered beneath the actuator for independent manipulation and measurement. The culture chamber is easily inserted, affixed, and removed from the baseplate using thumbscrews to secure the bumpers and plunger–pistons.
Fig. 4.
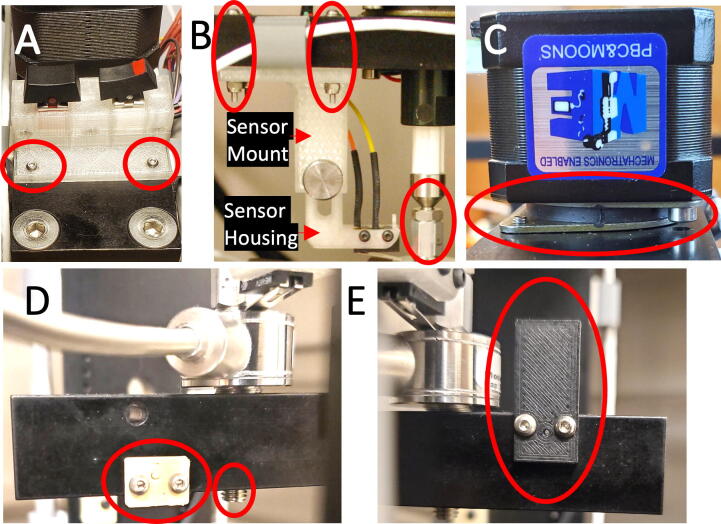
Motorized Dock. (A) The culture chamber is attached to the dock in three-dimensions using removable bumpers and thumbscrews (asterisks show front bumper), while plunger–pistons are attached to a piston tie-bar with thumbscrews (red arrows); (B) A piston tie-bar distributes force to one or two wells and triggers optical reference and lower limit sensors; (C) An upper limit sensor contacts the back of the load cell and is triggered by mechanical movement. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Linear actuator and load cell
A hybrid linear actuator (LC174S-E06008-25-S-150, Applied Motion Products) with a NEMA 17 stepper motor was mounted in the dock with a rubber damper (0008, Applied Motion Products). The actuator has a linear travel of 4 µm per step, a stroke of 25.4 mm, and is capable of 300 N force and 20 mm/s velocity.
A 22 N miniature load cell (WMC-22, Interface Force) was attached to the end of the actuator with an M4 coupler to measure force. This load cell measures tensile and compressive force, which can be used to determine the height and Young’s modulus of cultured menisci. The live hub of the load cell was positioned down and attached to the piston tie-bar which connects with the culture chambers’ plunger pistons, Fig. 4C.
Reference and limit sensors
Two pairs of through-beam optical sensors (E32-T15ZR, Omron), each consisting of a transmitter and receiver, were used to monitor the position of the piston tie-bar. Both pairs of sensors were mounted on the legs of the dock, whereby movement of the tie-bar between the sensors interrupts or allows passage of light.
A reference position was established whereby the plungers are in the culture media, but not in contact with the meniscus. This location is triggered when a hole in the piston tie-bar allows light passage between the transmitter and receiver. A plastic mask with a 1.0 mm aperture was mounted on the tie-bar to refine this position, Fig. 4B & 4C.
A lower limit position was established to prevent crashing of the plungers at the bottom of the culture chamber. The lower limit is triggered when the piston tie-bar interrupts passage of light beneath the bar. A plastic 1.5 mm extension mounted on the tie-bar was used to refine the trigger point, while plastic apertures with 2.0 mm holes were mounted over the transmitter and receiver pair to limit light scattering.
An upper limit position was established to prevent crashing of the plungers with the lid. The location is triggered when the top of the load cell contacts a micro switch (463092370402, Wurth Elektronik) mounted above, Fig. 4C. A plastic mount secures the switch to the dock. For insertion or removal of culture plates, the switch can be temporarily raised to provide additional clearance.
User-Operated Switches:
The actuator can be fully controlled in software, but two mechanical switches mounted on the dock provide a manual control option. A rocker switch (ET01J6AQE2, C&K) was used as a hardware safety to enable or disable the actuator. A momentary rocker switch (400AWMSP4R1BLKM6QE, E-Switch) was used to allow jogging of the actuator up or down. A plastic mount was designed to mount both switches on top of the dock, Fig. 4A.
Dock external electronics
To increase the longevity of electronics and reduce the size of the dock, electronics were mounted in an external enclosure (DRH-N-1, RLH Industries) above the incubator. Connections between the dock and the enclosure were made using 6-foot-long multi-strand wire, quick connectors, and fiber optic cable. A laptop was positioned adjacent the incubator for operation and data acquisition. Below we detail customization of the external electronics.
Step motor drive and power Supply:
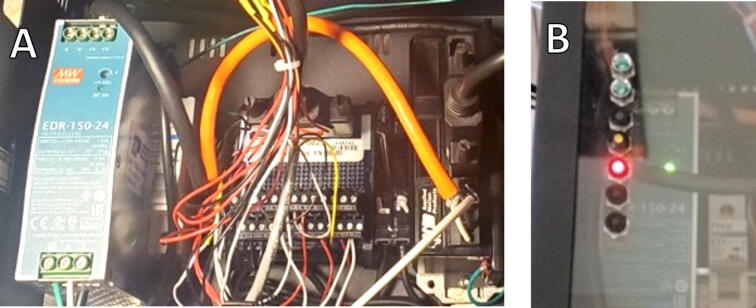
A step motor drive (ST5-Q-NN, Applied Motion Products) and 24-volt power supply (PS150D24, Applied Motion Products) were mounted to the DIN rail of the external enclosure together with a 2-amp circuit breaker (RLH-MCB-2, RLH Industries), Fig. 5A. The step motor drive was connected to the laptop using a USB-to-serial port converter.
All reference and limit sensors as well as rocker switches were connected to inputs on the step motor drive via a breakout box. Outputs from the sensors and drive were also connected to LED status lights mounted on the front of the enclosure for monitoring, Fig. 5B.
Fig. 5.
Dock External Electronics. (A) Left to right: Power supply, circuit breaker, step motor drive breakout box, optical sensor amplifiers, step motor drive; (B) LED status lights.
Optical sensor Amplifiers:
Two optical sensor amplifiers (E3X-HD11 2 M, Omron) corresponding to the reference and lower limit sensor were mounted to the DIN rail of the external enclosure. The reference amplifier was set to ‘L’ mode which outputs 24-volts with continuity of light at the reference position. The lower limit amplifier was set to ‘D’ mode which outputs voltage when light is interrupted by the tie-bar. The threshold settings were set to 400 and 950 for the reference and lower limit, respectively.
Load Cell PC Interface Module:
A single channel USB PC interface module (INF-USB3-C10-2, Interface Force), capable of logging at up to 5,000 Hz, was hardwired and scaled to the load cell by the manufacturer. The module was affixed to the outside of the incubator using adhesive cable routing clamps and plugged into the laptop.
Laptop Computer:
To operate two docks simultaneously over long duration, we used a Lenovo ThinkPad P73 Mobile workstation. The laptop was configured with an Intel Core i7-9750H 2.60 GHz CPU, 16 GB Ram and Windows 10 Pro. We positioned the laptop on a small stand adjacent the incubator and connected two step motor drives and two load cells into 4 USB ports.
Software
Virtual Instrument (VI) files (LabVIEW 2014, National Instruments) were created to operate the dock and perform data acquisition. Dedicated files were created to calibrate and configure hardware, as well as compress and analyze menisci. Each file was exported as an executable program (application) with a graphical user interface (GUI) and on-screen instructions. A synopsis of the programs is provided below, while comprehensive information is available within the Supplementary Operating Software Documentation.
Height Calibration:
A utility was developed to calibrate the position of the plunger and reference sensor. This program moves the actuator down until the plunger contacts an object of known height, which is input by the user. The distance between the plunger and the bottom of the well at the reference sensor position is written to a file on the laptop, while the position of the actuator is assigned to volatile memory in the step motor drive. This program is run primarily at the time of equipment setup.
Hardware Configuration:
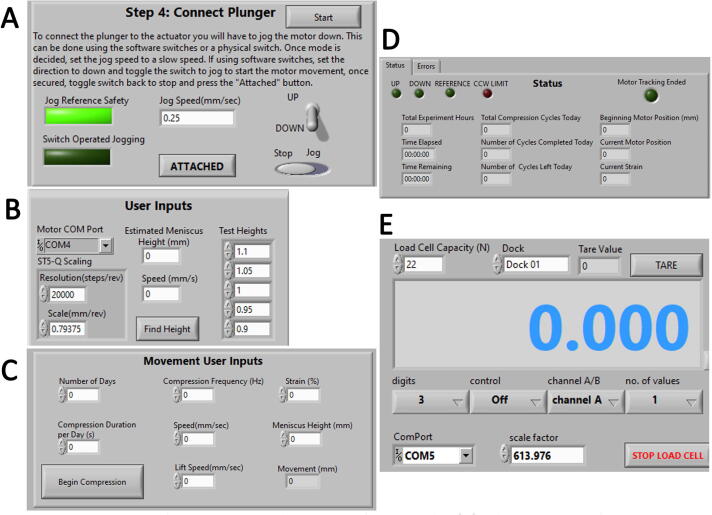
A ‘go-to’ configuration program was developed for routine experiment setup. The critical function of this program is user control of the actuator, whereby the plunger may be arbitrarily moved up or down, Fig. 6A, or commanded to a numerical height. This allows for insertion and removal of the culture chamber, attachment of the pistons to the tie-bar, and movement of the plungers to the tops of menisci prior to experimentation. This program also allows configuration of load cell and motor parameters, such as scaling, calibration, taring, and acceleration rate.
Fig. 6.
Operating Software Graphical User Interface Panels. (A) A hardware configuration panel provides instructions for actuator control; (B) Height measurement program panel allows probing of multiple test heights; (C) Strain-based compression program allows for long-duration compression cycling; (D) Compression cycling status display; (E) Load cell reading and tare panel.
Height Measuring:
A height measuring program was created for ‘in-situ’ height determination of the meniscus in the culture chamber well. This program requires a user-estimated height as input, Fig. 6B, which can be probed ± 10 % while logging position and force.
Strain-Based Compression:
A strain-based cyclical compression program was developed to compress samples while logging force. User-adjustable settings include experiment duration (days), compression duration per day (s), strain (ε), speed (mm / s), and frequency (Hz) of compression, Fig. 6C. The user must enter the height of the meniscus or the average height of two menisci. During operation, this program displays live data such as force, plunger position, elapsed and remaining time, Fig. 6D. The live display shows recent force and displacement, which is also logged to file. For convenience, this program includes a media change function that allows the user to interrupt the experiment to remove and replace the culture chamber.
Stress-Hybrid Based Compression:
While most bioreactors offer strain-based compression, we designed a hybrid program that monitors changes in stress and adjusts applied strain. This program is based on the strain-based compression program, however, also requires the user to enter a target force (N). After each day of strain-based compression, the software compares the logged average peak force to the target force, and increments the strain setting for the following day. This update process repeats once per day to maintain a set stress.
Compression Tester:
A compression testing program was created to plot force and position. User input includes displacement, preload, speed, hold time, cycles, and sampling rate. The user can convert the output to stress and strain to determine material properties and Young’s modulus (Ε).
Design files
Design files summary
| Design file name | File type | Open source license | Location of the file |
|---|---|---|---|
| Well Plate | CAD | Creative Commons BA | Available with the article |
| Well Plate Adapter | CAD | Creative Commons BA | Available with the article |
| Well Plate Lid | CAD | Creative Commons BA | Available with the article |
| Piston Retention Cap | CAD | Creative Commons BA | Available with the article |
| Plunger | CAD | Creative Commons BA | Available with the article |
| Piston | CAD | Creative Commons BA | Available with the article |
| Circular Meniscus | STL | Creative Commons BA | Available with the article |
| Dock Base | CAD | Creative Commons BA | Available with the article |
| Alignment Bumper | CAD | Creative Commons BA | Available with the article |
| Dock Leg | CAD | Creative Commons BA | Available with the article |
| Motor Mount | CAD | Creative Commons BA | Available with the article |
| Piston Tie-Bar | CAD | Creative Commons BA | Available with the article |
| Switch Mount | STL | Creative Commons BA | Available with the article |
| Switch Mount Cover | STL | Creative Commons BA | Available with the article |
| Upper Limit Sensor Mount | STL | Creative Commons BA | Available with the article |
| Upper Limit Sensor Housing | 3mf | Creative Commons BA | Available with the article |
| Reference Sensor Aperture | STL | Creative Commons BA | Available with the article |
| Lower Limit Sensor Extension | STL | Creative Commons BA | Available with the article |
| Lower Limit Sensor Mask | STL | Creative Commons BA | Available with the article |
The Well Plate is a two-well cell culture plate that holds meniscal samples, media, and water.
The Well Plate Adapter holds menisci in the center of the Well Plate wells.
The Well Plate Lid keeps the Well Plate sterile and aligns the Pistons and Plunger with the Well Plate Adapter.
The Piston Retention Cap mounts to the Well Plate, holds the linear bearing in place, and secures the Plungers and Pistons.
Plungers contact and transmit force to the top of cultured menisci.
The Piston connects the Plunger to the Piston Tie-Bar.
The Circular Meniscus is a model used to configure and test the bioreactor.
The Dock Base serves as a mount for the Dock Legs and Alignment Bumpers which locate the Well Plate and the actuator.
Alignment Bumpers secure the Well Plate to the Dock Base in XYZ-dimensions.
Dock Legs serve to support the Motor Mount above the Well Plate and provide a mounting surface for the reference and lower limit optical sensors.
The Motor Mount serves as a mount for the actuator, Upper Limit Sensor Mount, and the Switch Mount.
The Piston Tie-Bar distributes force from the actuator between two Pistons and serves to trigger the reference and lower limit optical sensors.
A Switch Mount secures two rocker switches that enable, disable, and jog the actuator.
A Switch Mount Cover conceals wiring and covers the rocker switches.
The Upper Limit Sensor Mount serves to mount a micro switch between the motor mount and load cell.
The Upper Limit Sensor Housing secures a micro switch and slides within its Sensor Mount.
The Reference Sensor Aperture is an optical mask with a 1.0 mm hole used to trigger (reveal) the reference position.
The Lower Limit Sensor Extension is an optical mask mounted to the bottom of the Piston Tie-Bar to trigger (occlude) the lower limit sensor.
A pair of Lower Limit Sensor Masks are mounted over the transmitter and receiver to limit stray light.
Bill of materials.
Bill of materials summary
|
Custom Designed Materials | ||||||
|---|---|---|---|---|---|---|
| Designator | Component | Number | Cost per unit -currency | Total cost -currency | Source of materials | Material type |
| Well Plate | Culture Plate | 1 | $1,000 USD | $1,000 USD | https://www.protolabs.com | PEEK* |
| Well Plate Adapter | Culture Plate | 2 | $400 USD | $800 USD | PEEK* | |
| Well Plate Lid | Culture Plate | 1 | $300 USD | $300 USD | Polycarbonate# | |
| Piston Retention Cap | Culture Plate | 2 | $130 USD | $260 USD | PEI# | |
| Plunger | Culture Plate | 2 | $225 USD | $450 USD | PEEK* | |
| Piston | Culture Plate | 2 | $135 USD | $270 USD | PEEK* | |
| Circular Meniscus | Meniscus | 1 | $10 USD | $10 USD | TPU | |
| Dock Base | Dock | 1 | $400 USD | $400 USD | Aluminum^ | |
| Alignment Bumper | Dock | 2 | $100 USD | $200 USD | Aluminum^ | |
| Dock Leg | Dock | 2 | $150 USD | $300 USD | Aluminum^ | |
| Motor Mount | Dock | 1 | $175 USD | $175 USD | Aluminum^ | |
| Piston Tie-Bar | Dock | 1 | $310 USD | $310 USD | Aluminum^ | |
| Switch Mount | Dock | 1 | $10 USD | $10 USD | PLA | |
| Switch Mount Cover | Dock | 1 | $10 USD | $10 USD | PLA | |
| Upper Limit Sensor Mount | Dock | 1 | $10 USD | $10 USD | PLA | |
| Upper Limit Sensor Housing | Dock | 1 | $10 USD | $10 USD | PLA | |
| Reference Sensor Aperture | Dock | 1 | $10 USD | $10 USD | PLA | |
| Lower Limit Sensor Extension | Dock | 1 | $10 USD | $10 USD | PLA | |
| Lower Limit Sensor Mask | Dock | 2 | $10 USD | $20 USD | PLA | |
Assembly hardware and commercially available materials are provided in Supplemental Bill of Materials.
*PEEK is an engineering-grade thermoplastic chosen for biocompatibility and sterilizability, which is applicable to cGMP manufacturing. For non-clinical applications, less expensive materials or manufacturing methods may be substituted to reduce cost, such as the use of PEI or 3D printing. In prototyping, all components of the Culture Plate were FDM printed in PLA, which were not liquid tight and had poor fitment. While it may be possible to use midrange materials, combined with post-processing, careful consideration must be given to the biocompatibility and sterilizability of 3D printed Culture Plate parts.
#If visualization is not needed, these parts may be made from aluminum or equivalent materials to save cost.
^These components do not need to be biocompatible or sterilized, and thus may be produced from alternate materials. The two most critical components of the Dock are the Motor Mount and Piston Tie-Bar, which should be made from aluminum or equivalently rigid materials to ensure accurate force and load cell readings. The weight of the Piston Tie-Bar should also be kept to a minimum, as heavier materials will influence load cell readings.
Build instructions
Assembly instructions for each of the three main hardware components are provided below. Consult the Supplemental Bill of Materials for information about the assembly hardware for each section.
Cell culture chamber
Assembly of the culture chamber is an intuitive process, whereby the major components only fit together one way. However, due to the precise fit between parts and manufacturing tolerance, fabricated parts will require sanding. Here we provide details to guide assembly.
Two-Well cell culture Plate:
-
1.
Locate the custom manufactured Culture Plate and two Well Plate Adapters. From the Supplemental Bill of Materials, locate two Barrel Extensions and two PEEK dowel pins.
-
2.
Gently install the Barrel Extensions into the Culture Plate in the middle holes at the ends of the plate as shown in Fig. 1A. Do not use tools to tighten the extensions as they will break if overtightened.
-
3.
Test fit each of the dowel pins into the clearance holes in each well of the Culture Plate. If the dowel pins do not easily fit, gently sand until they fit by hand. Repeat for both ends of the dowel pins so they fit in either orientation.
-
4.
Test fit each of the dowel pins into the two clearance holes of the Well Plate Adapters, Fig. 1B. If the pins do not fit easily, take note the orientation of the pins and gently sand until they fit. Maintaining the orientation of the pin, install two dowel pins into each well of the Culture Plate.
Well Plate Adapter:
-
1.
Locate the two Well Plate Adapters and four PEEK dowel pins.
-
2.
Test fit each of the dowel pins into the four clearance holes in each Well Plate Adapter. If the dowel pins do not easily fit, gently sand until they fit by hand.
-
3.
Test fit each Well Plate Adapter into the wells of the Culture Plate. If they do not fit easily all the way to the bottom, gently sand around the outer perimeter.
Plungers:
-
1.
Locate two Plungers, two Pistons, two PEEK screws, and two PEEK dowel pins.
-
2.
Insert a dowel pin into one end of each piston, then gently push the end of the pistons into the plungers. Gently secure the pistons to the plungers using the PEEK screw.
Well Plate Lid:
-
1.
Locate the Lid, two Retention Caps, two Linear Bearings, four set screws, and eight flat head screws.
-
2.
Begin by inserting the linear bearings into the top of the lid, Fig. 3C, without the lid cap. If the bearings do not fit, the holes in the lid need to be sanded until they fit easily by hand. Wrap a strip of 200-grit sandpaper around a pen or pencil of similar diameter to the bearing to sand the holes in the lid evenly. The bearings need to fit very easily, but without free play.
-
3.
After installing the bearings into the Lid, install the Retention Caps onto the lid to capture the bearing. If the Retention Caps fit tightly over the bearing flange, sand the recess inside the Retention Cap gently using the pencil trick until they fit easily, Fig. 3A. Gently secure the Retention Caps to the Lid with flat head screws, but do not tighten them yet, Fig. 3D.
-
4.
Insert the piston–plunger assembly through the bottom of the lid and then gently tighten the flat head screws on the Retention Caps. Check the movement of the piston through the lid and gently sand the piston at the top or bottom if it becomes tight at either end. Use a drill to turn the piston to ensure even sanding. If the piston is tight everywhere, sand the inside of the bearing using the pencil trick instead of sanding the pistons. Be certain the pistons move completely free.
-
5.
Insert two set screws into each Retention Cap to gently secure the pistons in place, Fig. 3D.
-
6.
Gently place the Lid onto the Culture Plate and avoid excess force on the barrel extensions. Secure with the 7/8″ long thumbscrews around the perimeter, and two ¼” long thumbscrews at the barrel extensions.
Motorized Dock
While assembly of the Dock is intuitive, there are configuration options for mounting the actuator as well as adjusting the heights of sensors. Here we provide details to guide assembly using a single actuator with sensor heights appropriate for 7.6 mm tall meniscal samples and 0.76 mm actuator displacement.
Frame:
-
1.
Locate: two Legs, two Lower Limit Sensor Masks, four optical sensors, four M2 button screws and four M2x4 flat head screws (included with the sensors).
-
2.
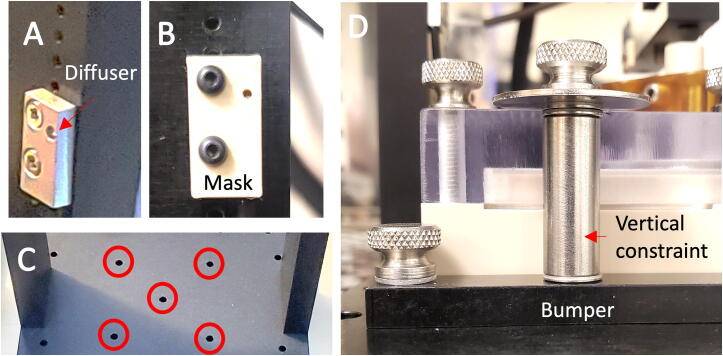
Reference sensor assembly: Observe the inner and outer columns of seven threaded holes on each Leg. Orient the Leg with the outer column of holes above the inner column. Using flat head screws, attach a sensor in the rightmost outer column of holes in one of the Legs, leaving four open holes exposed above the top of the sensor, Fig. 7A. Position the sensor with the optical diffuser in the rightmost position with the wire running down. This is now the right Leg. Assemble a left Leg with the sensor in the leftmost column with the diffuser in the leftmost position so that the Legs become mirror images.
-
3.
Lower limit sensor assembly: Continuing with the left Leg, attach a sensor to the lower column of holes on the right side of the Leg, leaving two open holes exposed above the top of the sensor, Fig. 7B. Position a sensor mask in front of the sensor and attach with 12 mm long button head screws. Repeat the process for the right Leg.
-
4.
Locate: Base Plate and four 1/4-20″ flat-head screws.
-
5.
Mount the Legs: Find the five holes in the shape of an ‘X’ nearest the shorter end of the Base Plate, Fig. 7C. Attach the two legs to the plate on either side of the ‘X’, with the sensors facing each other and the sensor wires running down towards the plate. The legs should be oriented such that the lower limit sensors with optical masks are closest the shorter end of the Base Plate.
-
6.
Locate two Alignment Bumpers, four 1″ standoffs, four 1″ washers, twelve 1 mm shims, four #10–32 flat-head screws and four #10–32 × 3/8″ long thumbscrews.
-
7.
Assemble two Alignment Bumpers: Using the inner pair of holes on each bumper, assemble the vertical constraint according to Fig. 7D. The assembly sequence from bottom-up is: flat-head screw > bumper > shim > standoff > shim > shim > 1″ washer > thumbscrew.
-
8.
Attach the two bumpers to the Base Plate using four thumbscrews (#10–32, 7/16″ long) according to Figs. 7D and Fig. 8A.
Fig. 7.
Dock Assembly. (A) Reference sensor mounting position on the right leg; (B) Lower limit sensor with mask mounted on the left leg; (C) ‘X’ pattern holes centered beneath the actuator; (D) The alignment bumpers lock the culture chamber to the dock.
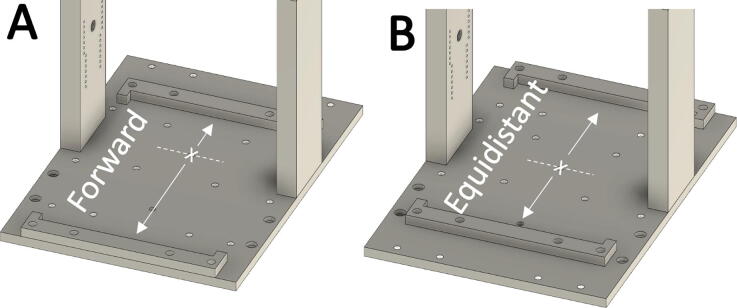
Fig. 8.
Positioning of Alignment Bumpers. (A) Position of the alignment bumpers for compression in a single culture well directly beneath the actuator (indicated by’X’), or (B) Position of the alignment bumpers for compression of two culture wells equidistant the center of the actuator.
Motor Mount Assembly:
-
1.
Locate: Switch Mount and Cover, rocker switch, momentary rocker switch, three M2 × 12 mm screws
-
2.
Insert the rocker switch and the momentary rocker switch into the Switch Mount shown in Fig. 9A, then fasten the cover to the switch using M2 screws.
-
3.
Locate: Motor Mount, Upper Limit Sensor Mount and Housing, Switch Mount, eight M2 screws with washers and locknuts, and one #8–32 × 7/8″ long thumbscrew.
-
4.
Using the M2 hardware, attach the Switch Mount to the top of the Motor Mount (the side with countersunk holes), Fig. 9A.
-
5.
Continuing with M2 hardware, attach the Upper Limit Sensor Mount to the underside of the Motor Mount (farthest and diagonal the Switch Mount). Install the Upper Limit Sensor Housing into the Mount and secure with the thumbscrew, Fig. 9B.
-
6.
Locate: Motor Mount, linear actuator w/vibration mount, two thread locking M3x6 and M3x16 screws.
-
7.
Attach the vibration damper mount to the linear actuator using the two M3x6 screws, then attach the vibration mount to the Motor Mount using two M3x16 screws, Fig. 9C.
-
8.
Locate: load cell, M4x3 standoff, M4x10 coupler.
-
9.
Screw the M4x3 nut all the way onto the actuator threads, then screw the M4x10 coupler all the way onto the dead end of the load cell (the side opposite the live hub). Next, screw the bottom of the load cell onto the actuator as far as possible while keeping the couplers in place, as seen in Fig. 9B. Leave the assembly loose for now.
-
10.
Locate: Motor Mount, Piston Tie-Bar, one #10–32 set screw, four ¼-20″ flat head screws, and M4x0.1 shims.
-
11.
Take note the orientation of the Piston Tie-Bar according to Fig. 9D and screw the #10–32 set screw all the way into the bottom. Then, gently thread the top of the Tie-Bar into the load cell (the live hub side) but do not tighten.
-
12.
Loosely attach the Motor Mount to the Legs of the Dock using the flat head screws and position the load cell so the wire is pointing directly towards one of the Legs without making contact. Fig. 4C shows proper orientation of the load cell wire towards the left Leg. After orientation, use two open-ended wrenches to jam the M4x3 nut against the M4x10 standoff to temporarily immobilize the load cell position.
-
13.
Piston Tie-Bar shimming: Note the position of the Tie-Bar in relation to the Legs and Culture Chamber in Fig. 4A. If the Tie-Bar is not close to parallel with the Legs and Culture Chamber, take note of the position and then remove / reinstall the Tie-Bar with a 0.100 mm shim between the load cell and Tie-Bar. Repeat this process and continue adding or removing shims until the Tie-Bar is as close to parallel as possible with the Legs. After shimming, loosen the M4 jam nut above the load cell and reposition the load cell to get the Tie-Bar as parallel as possible, then re-tighten the M4 jam nut assembly using the two wrenches.
Fig. 9.
Dock Assembly Con’t. (A) Momentary move and enable rocker switch assembly; (B) Upper limit sensor mounting and actuator jam-nut assembly; (C) Linear actuator mounted with vibration damper; (D) Lower limit sensor extension and single piston threading; (E) Reference sensor mask.
Reference and Limit Sensors:
-
1.
Locate: Reference Sensor Aperture, Lower Limit Sensor Extension, micro switch, four M2x4 screws, two M2x12 screws.
-
2.
Using two M2x4 screws, install the Reference Sensor Aperture onto the Piston Tie-Bar such that the Aperture is in the light path between the reference sensors, Fig. 9E. The Piston Tie-Bar is symmetrical about the central access, thus only one out of two possible mounting locations will be used.
-
3.
Using additional M2x4 screws, install the Lower Limit Sensor Extension onto the Piston Tie-Bar such that it extends 1.5 mm down from the bottom of the Tie-Bar between the lower limit sensors. In the correct position, the number ‘2′ will be upside down, Fig. 9D. If it is desired to raise the lower limit by 0.5 mm (to decrease displacement 0.5 mm), flip the Extension so that the number ‘2′ is right-side up. Alternatively, the sensors can be physically lowered or raised in larger 3.75 mm increments using the different mounting holes.
-
4.
Using the longer M2x12 screws, install the micro switch onto the Upper Limit Sensor Housing as shown at the bottom of Fig. 9B. The lever of the micro switch should extend downwards towards the back of the load cell.
Dock external electronics
The electronics module is made from commercially available parts and requires electrical skills to ensure wiring safety. We direct the reader to review the documentation included with the components prior to assembly. In the event of superseded products, numerous hardware substitutions are possible using different step motor drives, actuators, sensors, and switches so long the basic operation of the actuator and sensors are preserved. Here we provide an overview of the wiring used to operate the dock, which may be used as a reference.
Step Motor Drive Connections:
-
1.Make the following connections between the linear actuator and the step motor drive:
-
a.Motor blue wire to STC B-
-
b.Motor red wire to STC B+
-
c.Motor green wire to STC A-
-
d.Motor black wire to STC A+
-
a.
-
2.Make the following connections between the power supply and the step motor drive:
-
a.24 V + to DB-25 Pin 8 [XCOM] input common for reference sensor
-
b.24 V + to DB-25 Pin 22 [X7] for upper limit sensor
-
c.24 V + to DB-25 Pin 24 [X8] for lower limit sensor
-
d.24 V- to DB-25 Pin 17 [YCOM] output common
-
e.24 V + to STC V + main power
-
f.24 V- to STC V- main power
-
a.
-
3.Make the following connections between the rocker switches and the step motor drive:
-
a.Momentary rocker end 1 (either end) to DB-25 Pin 4 [X6]
-
b.Momentary rocker end 2 (opposite end) to DB-25 Pin 5 [X5]
-
c.Enable rocker end (either end) to DB-25 Pin 7 [X3]
-
a.
-
4.Make the following connections between the LED Status Lights and the step motor drive:
-
a.Blue motion LED- to DB-25 Pin 15 [Y2] hardware movement
-
b.Green command up LED- to DB-25 Pin 14 [Y1]
-
c.Yellow command down LED- to DB-25 Pin 16 [Y3]
-
a.
-
5.
Connect the middle lug of the upper limit micro switch to DB-25 Pin 23 [X7]
-
6.
Connect the RS-232 serial cable from the drive to a USB-to-serial port converter and plug the USB cable into the laptop computer.
Optical Sensor Amplifiers:
-
1.Make the following connections between the output of the power supply and the inputs on both optical sensor amplifiers:
-
a.24 V + to brown sensor wire
-
b.24 V- to blue sensor wire
-
a.
-
2.
Make the following connections between the output of the optical sensor amplifiers to both the step motor drive and the corresponding LED status lights:
-
a.
Lower limit optical sensor amplifier black wire to DB-25 Pin 25 [X8] and the red lower limit LED-
-
b.
Reference optical sensor amplifier black wire to DB-25 Pin 6 [X4] and the red reference LED-
Load Cell PC Interface Module:
-
1.
Connect the USB plug directly to the Laptop Computer
LED Status Lights:
-
1.
Make the following connections between the output of the power supply and the LED status lights:
-
a.
24 V- to Red upper limit sensor LED-
-
b.
24 V- to Blue enable LED-
-
c.
24 V + to Red lower limit LED+
-
d.
24 V + to Red reference LED+
-
e.
24 V + to Blue motion LED+
-
f.
24 V + to Green command up LED+
-
g.
24 V + to Yellow command down LED+
-
2.
Connect the Red upper limit LED + to the inner lug of the upper limit micro switch
-
3.
Connect the Blue enable LED + to the middle lug of the enable rocker switch
Operation instructions
Below we provide instructions for routine operation of the bioreactor. Comprehensive software instructions are provided in the Supplementary Software Documentation.
First-Time Software Setup
Following assembly of the hardware, the following operations must be completed in the software prior to operation:
-
1.
Establish software communication between the step motor drive and the laptop computer according to the instructions included with the drive and as detailed in section 1.1 of the Supplementary Software Documentation.
-
2.
Configure the load cell according to the manufacturer’s instructions as detailed in section 1.2 of the Supplementary Software Documentation.
-
3.
Calibrate the height of the actuator by running the Height Calibration Program and following the on-screen instructions as detailed in section 2.1 of the Supplementary Documentation.
Preparing the Cell Culture Chamber
Prior to running aseptic assays, the Cell Culture Chamber must be cleaned and sterilized as follows:
-
1.
Clean each of the Cell Culture Chamber components with a dilute Alconox or equivalent laboratory detergent and gently brush clean. Thoroughly rinse all components with deionized water.
-
2.
Autoclave the Cell Culture Chamber components as follows:
-
a.
Completely remove the Well Plate Adapters and Plungers w/Pistons from the Well Plate and Well Plate Lid.
-
b.
Attach the Well Plate Lid to the Well Plate and loosely secure with thumbscrews to allow for thermal expansion. Likewise, loosen the eight screws securing the Piston Retention Caps to the Well Plate Lid, but do not remove them.
-
c.
Place all components into an autoclavable tray, cover with aluminum foil, and autoclave using the liquid cycle.
-
d.
After the autoclave cycle, allow the autoclave to cool to 60° C prior to opening the door (1 h is usually sufficient after completion of the cycle). After cooling, place the Cell Culture Chamber components into a biosafety cabinet to dry.
-
3.
Working aseptically in the biosafety cabinet, push the Pistons all the way into the Well Plate Lid to align the Piston Retention Caps and tighten the eight screws securing the Caps. Then tighten the set screws to immobilize the Pistons.
-
4.
Place the Well Plate Adapters back into the Well Plate.
-
5.
Position samples into the Well Plate Adapters, then place the Lid on the Well Plate and secure with thumbscrews.
Cell culture chamber attachment to the Dock
The Culture Chamber must be secured to the Dock in either a single or dual piston configuration. In Single Piston Mode, a single culture well is positioned directly below the actuator for manipulation and measurement using only one plunger. For compression cycling or analysis of a single sample, such as measuring height or mechanical properties, use the Single Piston configuration. In Dual Piston Mode, the two culture wells are positioned equidistant from the actuator and force is distributed through the Piston Tie-Bar. For compression cycling of both wells, use Dual Piston configuration.
-
1.Configure the Alignment Bumpers for Single or Dual Piston operation:
-
a.For Single Piston configuration: position the Alignment Bumpers closest to the front of the dock, according to Fig. 8A, and loosely fasten two thumbscrews on the rear Alignment Bumper.
-
b.For Dual Piston configuration: position the Alignment Bumpers on the dock equidistant the actuator, according to Fig. 8B, and loosely fasten two thumbscrews on the rear Alignment Bumper.
-
a.
-
2.
Seat the Culture Chamber into the Dock pushing against the rear Alignment Bumper, then loosely secure the front Alignment Bumper with two thumbscrews.
***If there is insufficient clearance to insert the Culture Chamber beneath the Piston Tie-Bar, then either lower the Pistons or raise the Piston Tie-Bar. The Pistons may be lowered by loosening the set screws in the Retention Caps. The Tie-Bar may be raised using the Hardware Configuration Program detailed in section 2.2 of the Supplementary Documentation***
-
3.
Run the Hardware Configuration Program and follow the on-screen instructions to connect the Pistons to the Tie-Bar, as detailed in section 2.2 of the Supplementary Documentation. Terminate and close the program only after the Pistons are attached.
-
a.
For Single Piston configuration: loosen the set screws on the appropriate Piston Retention Cap to allow the piston to turn and thread onto the Tie-Bar.
-
b.
For Dual Piston configuration: attach the Pistons to the Piston Tie-Bar using two #10–32 × ¾” thumbscrews before loosening the set screws on the Piston Retention Caps.
-
4.
Run one of the following compression programs. Follow the on-screen instructions and review the relevant section of the Supplementary Software Documentation:
-
a.
Height Measuring Program (Section 2.4 of Supplementary Documentation)
-
b.
Strain-Based Compression Program (Section 2.5 of Supplementary Documentation)
-
c.
Stress-Strain Hybrid Based Compression Program (Section 2.6 of Supplementary Documentation)
-
d.
Compression Tester Program (Section 2.8 of Supplementary Documentation)
Validation and characterization
Compression of a thermoplastic polyurethane (TPU) meniscus
We 3D-printed a TPU meniscus to serve as a model for validation and characterization. To approximate the stiffness of a bioprinted hydrogel meniscus, we printed the TPU model with 12.5 % infill and no perimeters. After printing, we ran our custom Height Measurement, Strain-Based Compression, and Compression Tester programs. Video of real-time piston movement is shown in Supplemental Video 1.
Height measuring Program:
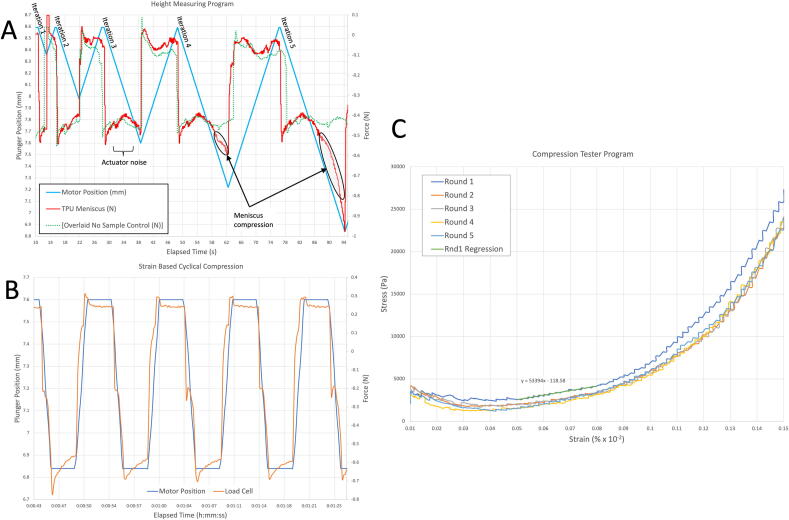
After running the program without any sample and with a single 7.6 mm tall TPU meniscus, data was plotted in Excel for analysis Fig. 10A. The up and downward movement of the actuator appears linear, with the actuator moving the plunger from a starting height of 8.6 mm to progressively lower positions, returning to the starting height after each iteration. There is a level shift in the load cell output when the actuator changes direction. Following each change of direction, oscillations in the load cell are visible due to noise and vibration measuring ≤ 0.1 N in amplitude.
Compression of the TPU meniscus is evident at the end of the fourth iteration when the plunger moves below 7.6 mm, and can be seen more prominently in the fifth iteration where a large consistent decrease in force is evident below 7.6 mm. The small force change expected upon initial contact between the plunger and the meniscus did not generate a data feature sufficient for precise determination of height. However, compression of the meniscus is evident, and therefore a higher load cell data sampling rate or a reduction in background noise would likely enable this determination in future work.
Fig. 10.
Compression of a thermoplastic polyurethane (TPU) meniscus. Plotted data from the (A) Height measurement program, (B) Strain-based compression program, and (C) Compression tester program.
Strain-Based compression program:
Using an estimated sample height of 7.6 mm, we chose to perform a 10% strain, necessitating 0.76 mm of actuator displacement, Fig. 10B. The maximum height of the plunger is set to the height of the sample so that the plunger is in constant contact with the meniscus. This contact reduces vibration noise in the actuator. Level shifts in the force data are present when actuator movement is initiated, but reliable compression data is obtained after settling. The plunger is held at each position based on the chosen compression frequency. At full compression, positive creep due to stress relaxation of the meniscus is visible at the bottom of each cycle.
Compression Tester Program:
This program allows full manual control of the actuator speed and displacement to evaluate mechanical properties of samples (see section 2.8 of the Supplementary Operating Software Documentation). Similar to the output of the Strain-Based Compression Program, the output of this program consists of plunger position, force, and elapsed time. However, by calculating the contact surface area between the plunger and the meniscus, the force can be expressed as stress to generate a stress–strain curve, Fig. 10C. Briefly, the contact surface area is calculated as the Curved Surface Area (CSA) of the frustum of a cone (or a hollow lampshade) as follows:
The stress–strain curve shows that stress decreased from 1 to 3% strain and then increased from 4 to 15%. We believe the initial decrease may be due to limitations in determining the precise height of soft samples and correspondingly inaccurate stress–strain calculations based upon the point of initial plunger contact. In our validation model, the plunger–meniscus contact surface area increased from 0 to 0.68 cm2 with increasing strain. Given that initial contact occurs over an infinitesimally small surface area, an inaccurate force yields an artificially high stress in this model, which is what we observe. Thus, calculation of the modulus is likely to be more accurate at strains of 4% and above, where the contact surface area is higher and the relative error is smaller. Correspondingly, we calculated a Young’s modulus of 53 kPa between 5 and 8% strain, and a modulus of 71 kPa at 10% strain for the 3D printed TPU meniscus consisting of only 12.5% infill without perimeters.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded entirely with federal funds under award HU0001-19-2-0022. The Uniformed Services University of the Health Sciences (USU), 4301 Jones Bridge Rd, Bethesda, MD 20814-4799 is the awarding and administering office of this award. The information or content and conclusions do not necessarily represent the official position or policy of, nor should any official endorsement be inferred on the part of, USU, the Department of Defense, or the U.S. Government.
Biographies

Joseph R. Loverde earned a joint Ph.D. in Biomedical Engineering at New Jersey Institute of Technology and Rutgers Biomedical and Health Sciences at New Jersey Medical School. Working as a Research Scientist in the Department of Chemistry and Life Science at the U.S. Military Academy, Dr. Loverde has published on the use of biomechanics and biomaterials in tissue engineering and has created a number of devices pertaining to neurological and musculoskeletal tissue engineering. 1. Loverde J.R., Tolentino, R.E., Soteropoulos, P., Pfister, B.J. (2020). Biomechanical forces regulate gene transcription during stretch-mediated growth of mammalian neurons. Frontiers in Neuroscience, section Neurogenomics 14: 600136. 2. Mitropoulos A.N., Kiesewetter, K.T., Horne, E., Butler, J., Loverde, J.R., Wickiser, J.K. (2020). Uniform wet-Spinning Mechanically Automated (USMA) fiber device. HardwareX 8:e00124.3. Loverde, J.R., Ozoka, V.C., Aquino, R., Lin, L., Pfister, B.J. (2011). Live imaging of axon stretch growth in embryonic and adult neurons. Journal of neurotrauma 28(11): 2389-2403. 4. Loverde, J.R., Tolentino, R.E., Pfister, B.J. (2011). Axon stretch growth: the mechanotransduction of neuronal growth. Journal of visualized experiments: JoVE(54). http://www.jove.com/video/2753/ axonstretch-growth-the-mechanotransduction-of-neuronal-growth?status=a4759k
Ms. Maria Piroli earned an M.S. in Biomedical Engineering from the University of South Carolina. Maria works as a Laboratory Technician in the Department of Chemistry and Life Science at the U.S. Military Academy. She has extensive experience in cell culture working with mesenchymal stem cells, primary neurons, and in the use of wet-spinning and bioprinting for the development of implantable collagen-based scaffolds.

George J. Klarmann is a Senior Research Scientist at the Uniformed Services University 4D Bio3 Center for Biotechnology and an employee of the Geneva Foundation. George received his B.S. degree in Molecular and Cell Biology from the University of Connecticut, and his Ph.D. in Biochemistry from the University of Utah where he studied the molecular mechanisms of HIV-1 reverse transcription. George has spent the last decade working on solutions to address military orthopedic injuries. His research interests include orthopedic repairs, 3D printing and 3D cell culture models and engineering and purification of proteins.

J. Kenneth Wickiser earned his Bachelor of Science in Engineering Physics from West Point, his Master of Science from the University of Alabama in Mechanical Engineering, and his Doctorate in Philosophy from Yale University in Molecular Biophysics and Biochemistry. He completed his postdoctoral research work at the Rockefeller University before serving as West Point faculty, eventually achieving the rank of Professor of Biochemistry and Associate Dean for Research. In 2021, Dr. Wickiser left federal service to help found the Global Alliance for Preventing Pandemics, a center at Columbia University dedicated to technical workforce development, standardization of best practices, capacity building, and the democratization of science and technology in low- and middle-income countries.

Jason Barnhill is an Associate Professor and the Program Director for the Life Science Program in the Dept. of Chemistry and Life Science at West Point. He began his career as an Infantry officer and transferred to the Microbiology specialty area after his first assignment at West Point. He has completed assignments as a clinical and research microbiologist and is currently focusing his research efforts on 3 dimensional bioprinting, prolonged field care, and regenerative medicine.

Kristin H. Gilchrist is the Associate Director of Research for Uniformed Services University 4D Bio3 Center for Biotechnology and an employee of the Geneva Foundation. She completed her undergraduate education in biomedical engineering at Vanderbilt University and received her masters and doctoral degrees in electrical engineering from Stanford University. At 4D Bio3, she manages projects focused on application of bioprinting and biomanufacturing to enhance the readiness and health of America’s Warfighters. These efforts include development of organ-on-chip models to study military-relevant exposures and tissue engineering solutions for musculoskeletal injuries.

Vincent B. Ho, M.D., M.B.A., is Director of the 4D Bio3 Center for Biotechnology, and Chair and Professor of the Department of Radiology and Radiological Sciences at the Uniformed Services University in Bethesda, Maryland. He is a graduate of the University of Michigan (Ann Arbor, MI; B.S., Biomedical Sciences; and M.D.) and completed his internship and diagnostic radiology residency at Walter Reed Army Medical in Washington, D.C. He has co-authored over 95 peer-reviewed publications, 210 scientific abstracts and 4 books. With 18 U.S. patent assignments, Dr. Ho has a long history of biotechnology innovation. He has held leadership roles for a variety of societies and currently serves on the National Advisory Council for the National Institute for Biomedical Imaging and Bioengineering.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ohx.2023.e00433.
Contributor Information
George J. Klarmann, Email: GKlarmann@genevausa.org.
Vincent B. Ho, Email: vincent.ho@usuhs.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplemental Bill of Materials
Supplementary Operating Software Documentation
References
- 1.Chen M., Guo W., Gao S., Hao C., Shen S., Zhang Z., Wang Z., Li X., Jing X., Zhang X., Yuan Z., Wang M., Zhang Y., Peng J., Wang A., Wang Y., Sui X., Liu S., Guo Q. Biomechanical Stimulus Based Strategies for Meniscus Tissue Engineering and Regeneration. Tissue Eng Part B Rev. 2018;24:392–402. doi: 10.1089/ten.TEB.2017.0508. [DOI] [PubMed] [Google Scholar]
- 2.Martin R.B. The Importance of Mechanical Loading in Bone Biology and Medicine. J Musculoskelet Neuronal Interact. 2007;7(1):48–53. https://www.ncbi.nlm.nih.gov/pubmed/17396005 [PubMed] [Google Scholar]
- 3.Petri M., Ufer K., Toma I., Becher C., Liodakis E., Brand S., Haas P., Liu C., Richter B., Haasper C., von Lewinski G., Jagodzinski M. Effects of Perfusion and Cyclic Compression on in Vitro Tissue Engineered Meniscus Implants. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):223–231. doi: 10.1007/s00167-011-1600-3. [DOI] [PubMed] [Google Scholar]
- 4.Puetzer J.L., Bonassar L.J. Physiologically Distributed Loading Patterns Drive the Formation of Zonally Organized Collagen Structures in Tissue-Engineered Meniscus. Tissue Eng Part A. 2016;22:907–916. doi: 10.1089/ten.TEA.2015.0519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Bill of Materials
Supplementary Operating Software Documentation