Abstract
Background
The population attributable fraction (PAF) of dementia from hearing loss (HL) in the United States is ~2% when incorporating self-reported HL measures. However, self-report might underestimate clinically significant audiometric HL among older adults. Here, we quantified PAFs of dementia from audiometric HL overall and by age, sex, and race/ethnicity groups among a nationally representative sample of community-dwelling older adults in the United States.
Methods
We used cross-sectional data from Round 11 (2021) of the National Health and Aging Trends Study, a prospective cohort study representing the U.S. Medicare population aged 65+ years (N = 2 470). We estimated model-adjusted PAFs of prevalent dementia by audiometric HL (pure-tone averages: normal hearing, <26 dB HL; mild HL, 26–40 dB HL; moderate or greater HL, ≥41 dB HL).
Results
Among eligible participants (34.8% aged ≥80 years; 55.3% female; 82.4% non-Hispanic White), 37.5% had mild, and 28.8% had moderate or greater HL. Dementia prevalence overall was 10.6%, with the PAF predominately driven by moderate or greater HL (PAF = 16.9%; 95% confidence interval [CI]: 4.1–28.7%). The PAF from any degree of HL was larger but with a wider CI (PAF = 18.7%, 95% CI: −5.3% to 40.1%). There was evidence associations differed by sex but not age or race/ethnicity; moderate or greater HL exhibited stronger associations among males (PAF = 40.5%; 95% CI: 19.5% to 57.2%) than females (PAF = 3.2%; 95% CI: −12.7% to 17.9%).
Conclusions
In a nationally representative sample of community-dwelling older adults in the United States, 17% of dementia cases were attributable to moderate or greater audiometric HL, an estimate that is eightfold higher relative to studies relying on self-reported hearing measures only.
Keywords: Cognitive impairment, Modifiable risk factors, Neurology, Public health
Dementia affects millions of Americans and is a leading cause of mortality (1) that is also associated with substantial morbidity and health care–related costs (2,3). With no curative treatments yet available (4,5), clinical and public health efforts are often focused on risk factor reduction for primary dementia prevention (6,7). Over the past decade, strong observational evidence suggests that hearing loss, which is prevalent in two-thirds of adults aged 65 years or older (8), is associated with greater risk of cognitive decline and dementia (6,9). Elucidating the potential impact of hearing loss prevention and treatment on dementia risk in the United States has therefore become a national priority (6,10).
In 2 seminal studies, the population attributable fraction (PAF) of dementia from midlife hearing loss has been estimated at ~8% globally (6) and ~2% in the United States (11). One key challenge faced by the latter analysis, however, is the use of self-reported measures for estimating the prevalence of hearing loss in the population. Although more feasible for data collection in large epidemiologic studies, defining hearing loss via self-report might directly impact the estimation and interpretation of population attributable fraction (PAFs) because self-report can underestimate clinically significant hearing loss among older adults (12). Objectively measured hearing (ie, pure-tone audiometry), however, is considered the reference-standard for defining clinically-relevant peripheral hearing acuity. Yet whether the PAF of dementia from hearing loss in the United States varies when considering solely audiometric hearing loss among a nationally representative sample remains uncertain.
In this study, we quantified the proportion of dementia cases attributable to audiometric hearing loss in late-life among a nationally representative sample of older adults in the United States. We hypothesized that the PAF of dementia from audiometric hearing loss is higher than previous estimates that relied on self-reported hearing. Because the prevalence of hearing loss increases substantially with age and is shown to be higher among men (8,13), we also hypothesized that the PAFs are highest among individuals with hearing loss in the older old age group (≥80 years) and among men compared with those in early late life and females, respectively. And among those with hearing loss, we further examined associations between hearing aid use and dementia, hypothesizing nonhearing aid use would be associated with an excess proportion of dementia cases.
Method
Study Design and Sample
The National Health and Aging Trends Study (NHATS) is an ongoing, prospective cohort study designed to be representative of the U.S. Medicare population aged 65+ years (14). A detailed description of the NHATS cohort and sampling process was published previously (15). Briefly, data are collected annually from sample participant interviews, proxy interviews, or both. The baseline visit occurred in Round 1 (2011), with replenishment of the cohort occurring in Round 5 (2015). Participants that were not community-dwelling at their baseline interview (ie, residing in a residential care community or in a nursing home) were not eligible for participant interviews during subsequent rounds of data collection; in this case, only proxy interviews were collected. Beginning at Round 11 (2021), the interview—conducted in-person at the participant’s home, over telephone, or both—was divided into 2 parts. Part 1 consisted of questionnaires ascertaining history of health conditions and Part 2 contained the cognitive test battery and pure-tone audiometry testing.
In this study, we included all older adults that: (a) were community dwelling at Round 11, (b) completed interview Parts 1 and 2, and (c) had complete cognitive outcome data (n = 2 937). We excluded participants with missing or incomplete hearing (eligible for hearing activity but not attempted, n = 116; hearing activity attempted but missing, n = 306) and covariate data (race/ethnicity, n = 37; education, n = 35; hypertension, n = 1; heart disease, n = 6; lung disease, n = 2; cancer, n = 1). The final analytic sample consisted of n = 2 470 older adults.
Informed consent was obtained from all participants (or from proxies, where applicable) at each Round, and approved by the local institutional review board (Johns Hopkins University).
Dementia Ascertainment
To classify dementia status, NHATS developed a standardized algorithm that incorporates neurocognitive test scores (assessing 3 cognitive domains: orientation, memory, and executive functioning), self- or proxy-reported physician diagnoses, and informant questionnaires (16). In line with National Institute on Aging-Alzheimer’s Association guidelines, probable dementia is defined by at least 2 cognitive domain scores ≤1.5 standard deviations [SD] below the overall sample mean, or a physician diagnosis of dementia or meeting AD8 Dementia Screening Interview criteria (17). Possible dementia is defined by one cognitive domain score ≤1.5 SD below mean. For the primary analysis, we combined possible and probable dementia to maximize sensitivity (ie, the “broad” definition for dementia; the accuracy of which has been investigated in prior work (16)) for identifying dementia.
Audiometric Hearing Loss
Introduced at NHATS Round 11, air-conduction pure-tone audiometry was conducted in-person during Part 2 using a portable audiometer (SHOEBOX Ltd., Ottawa, Canada) (18). The pure-tone average was calculated as the average of 4 speech frequencies (0.5, 1.0, 2.0, and 4.0 kHz) in the better-hearing ear (BPTA).
For the primary analysis, we used the BPTA to categorize hearing loss in accordance with American Speech-Language-Hearing Association criteria (19): Normal hearing, <26 decibels hearing loss (dB HL); mild hearing loss, 26–40 dB HL; moderate or greater hearing loss, ≥41 dB HL. We explored additional parameterizations, including (a) hearing loss as a binary variable (normal hearing vs. any hearing loss), and (b) revised World Health Organization (WHO) recommendations for defining hearing loss (normal hearing, <20 dB HL; mild hearing loss, 20 through <35 dB HL; moderate or greater hearing loss, ≥35 dB HL) (20).
Covariates
We included demographic and clinical/health behavior variables identified a priori as possible confounders. Demographic variables from the baseline visits included age group (65–69, 70–74, 75–79, 80–84, 85–89, 90+), sex (male, female), self-reported race/ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic other, and Hispanic), and educational attainment (less than high school, high school or equivalent, and greater than high school). Clinical and health behavior variables included baseline (Round 1 or 5) smoking status (ever smoker, never smoker), as well as a summary variable for the total number of prevalent chronic health conditions (self-reported diagnoses by a physician) of diabetes, hypertension, heart attack, heart disease, lung disease, any cancer, and stroke at Round 11 (indicating 0, 1, 2, 3, or 4+ total chronic diseases).
Statistical Analysis
To account for the complex sampling design of NHATS, we applied survey weights to all analyses in accordance with NHATS statistical guidelines (21). We describe demographic and clinical/health behavior characteristics by hearing category in the analytic sample. For all analyses, we used 2-sided tests with an alpha of 0.05 to define statistical significance.
For the primary analysis, we first estimated multivariable-adjusted prevalence ratios (PR) and 95% confidence intervals (CI) of dementia by hearing category using Poisson regression models with robust variance (22). We adjusted the primary inference model for all demographic and clinical covariates.
Next, we leveraged the model-adjusted PRs to estimate the PAF of dementia from audiometric hearing loss among older adults in the U.S. population (23).
where pdi is the prevalence of ith hearing loss category conditioning on dementia. Using individual-level NHATS data enabled us to adapt the Miettinen formula (24), which is appropriate for estimating a PAF when using an adjusted morbidity ratio (25–27). Positing a causal relationship between hearing loss and dementia, no unmeasured confounding or bias, and no expansion of the population at risk for dementia from hearing loss reduction, the PAF provides the absolute upper bounds of the proportion of dementia cases that could potentially be prevented if hearing loss were eliminated in the population (25,26). One strength of NHATS lies in the ability to confidently estimate the prevalence of audiometric hearing loss at the population level; without which, quantifying the PAF would be unreliable.
We obtained nonparametric 95% CIs for the PAF estimates using 10 000 bootstrapped samples. A priori, we also examined whether these associations varied by age group (aged <80 years, 80+ years), sex (male, female), and self-identified race (non-Hispanic White, non-Hispanic Black) by stratifying the models and PAF estimates. In exploratory analyses, we examined: (a) how the magnitude of associations varied when applying the 2021 WHO recommendations to hearing loss categorization, and (b) associations between hearing aid use and prevalent dementia restricted to those with moderate or greater hearing loss.
We performed all analyses in R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria) (28). We used the tableone package to generate descriptive statistics and the survey package for applying survey weights to the data.
NHATS data through Round 11 are publicly available (https://nhats.org/researcher/data-access).
Results
Study Sample Characteristics
Among eligible older adults, 34.8% (95% CI: 32.7, 36.9%) were aged ≥80 years, 55.3% (95% CI: 52.8, 57.8%) were female, and 82.4% (95% CI: 79.5, 85.3%) were non-Hispanic White (Table 1). The prevalence of audiometric hearing loss was 66% (mild hearing loss, 37.5% [95% CI: 35.5, 39.5%]; moderate or greater hearing loss, 28.8% [95% CI: 26.7, 30.9%]); prevalence was higher among older age groups (eg, proportion aged 80–84: moderate or greater hearing loss, 24.4% [95% CI: 21.0, 27.7%]; normal hearing, 14.4% [95% CI: 11.9, 16.9%]). Overall, the prevalence of dementia was 10.6% (95% CI: 9.2, 12.0%).
Table 1.
Demographic and Clinical Characteristics of Study Sample, the National Health and Aging Trends Study (2021)
| Overall | Normal Hearing | Mild Hearing Loss | Moderate or Greater Hearing Loss | |
|---|---|---|---|---|
| % (95% CI) | NA | 33.7 (30.7, 36.6) | 37.5 (35.5, 39.5) | 28.8 (26.7, 30.9) |
| Age group, y, % (95% CI) | ||||
| 70–74 | 30.7 (28.2, 33.2) | 43.9 (38.9, 48.9) | 28.7 (24.9, 32.5) | 17.8 (13.5, 22.1) |
| 75–79 | 34.5 (31.7, 37.3) | 38.3 (33.4, 43.2) | 36.3 (32.3, 40.2) | 27.7 (23.0, 32.5) |
| 80–84 | 19.9 (18.2, 21.6) | 14.4 (11.9, 16.9) | 21.3 (18.7, 24.0) | 24.4 (21.0, 27.7) |
| 85–89 | 10.4 (9.3, 11.5) | 2.6 (1.7, 3.6) | 11.1 (9.2, 13.1) | 18.4 (15.6, 21.2) |
| 90+ | 4.6 (4.0, 5.2) | 0.7 (0.3, 1.2) | 2.5 (1.7, 3.4) | 11.7 (9.9, 13.5) |
| Female, % (95% CI) | 55.3 (52.8, 57.8) | 63 (57.4, 68.6) | 54.7 (50.6, 58.9) | 47.0 (42.8, 51.3) |
| Race/ethnicity, % (95% CI) | ||||
| Non-Hispanic White | 82.4 (79.5, 85.3) | 79.3 (74.5, 84.1) | 83.5 (80.2, 86.8) | 84.5 (80.9, 88.1) |
| Non-Hispanic Black | 7.6 (6.4, 8.8) | 9.7 (7.4, 12.1) | 7.9 (6.4, 9.4) | 4.8 (3.8, 5.9) |
| Non-Hispanic other | 3.4 (2.1, 4.7) | 4.1 (1.5, 6.6) | 3.3 (1.5, 5.1) | 2.9 (1.3, 4.5) |
| Hispanic | 6.5 (4.5, 8.6) | 6.9 (3.8, 10.0) | 5.3 (3.1, 7.5) | 7.8 (4.6, 11.0) |
| Education, % (95% CI) | ||||
| <HS | 12.1 (10.4, 13.8) | 7.6 (5.3, 9.9) | 11.7 (9.6, 13.9) | 17.9 (14.2, 21.5) |
| HS or equivalent | 24.4 (22.1, 26.7) | 19.4 (15.7, 23.1) | 25.4 (22.2, 28.6) | 28.8 (25.0, 32.6) |
| >HS | 63.5 (60.6, 66.5) | 73.0 (68.9, 77.3) | 62.8 (58.8, 66.9) | 53.3 (49.3, 57.3) |
| Ever smoker, % (95% CI) | 50.0 (47.6, 52.3) | 43.8 (40.4, 47.2) | 52.6 (48.4, 56.8) | 53.8 (49.3, 58.2) |
| Number of chronic diseases, % (95% CI) | ||||
| 0 | 16.7 (14.8, 18.5) | 18.5 (14.2, 22.8) | 16.4 (13.5, 19.3) | 14.9 (12.1, 17.7) |
| 1 | 35.1 (32.5, 37.7) | 40.1 (36.1, 44.1) | 33.1 (29.2, 37.0) | 31.9 (27.7, 36.2) |
| 2 | 28.8 (26.5, 31.0) | 26.4 (22.4, 30.4) | 28.7 (25.7, 31.7) | 31.5 (27.5, 35.6) |
| 3 | 14.6 (12.8, 16.4) | 12.0 (9.2, 14.9) | 15.8 (12.9, 18.7) | 15.9 (13.1, 18.7) |
| 4+ | 4.9 (4.0, 5.8) | 3.0 (1.4, 4.6) | 5.9 (4.4, 7.4) | 5.8 (4.3, 7.2) |
Notes: Survey-weighted demographic and clinical characteristics of NHATS participants. CI = confidence intervals; HS = high school.
Population Attributable Fraction of Dementia from Audiometric Hearing Loss
Moderate or greater, but not mild, hearing loss was associated with an increased prevalence of dementia relative to normal hearing (moderate or greater, PR = 1.59 [95% CI: 1.04, 2.45]; mild, PR = 1.13 [95% CI: 0.75, 1.71]; Table 2). The PAF of dementia from moderate or greater hearing loss was 16.9% (95% CI: 4.1, 28.7%); larger than that from mild hearing loss (3.9%; 95% CI: −8.7, 15.8%). Among participants with moderate or greater hearing loss, hearing aid use was associated with lower dementia prevalence (Table 2). In this subpopulation at higher risk for cognitive impairment, the associated proportion of dementia cases attributed to nonhearing aid use was 22.5% (95% CI: 2.7, 41.0%).
Table 2.
Population Attributable Fractions of Dementia Associated With Late-Life Audiometric Hearing Loss Among Older Adults, the National Health and Aging Trends Study (2021)
| Prevalencea, % (95% CI) | PR (95% CI) | PAF, % (95% CI) | |
|---|---|---|---|
| Hearing loss category | |||
| Normal hearing (ref) | 20.5 (14.6, 26.4) | 1 | NA |
| Mild hearing loss | 34.2 (27.7, 40.6) | 1.13 (0.75, 1.71) | 3.89 (−8.65, 15.81) |
| Moderate or greater hearing loss | 45.3 (38.9, 51.7) | 1.59 (1.04, 2.45) | 16.87 (4.06, 28.73) |
| Any hearing loss | 79.5 (73.5, 85.4) | 1.31 (0.88, 1.93) | 18.66 (−5.30, 40.13) |
| Hearing aid use category | |||
| Hearing aid use (ref) | 31.8 (23.5, 40.1) | 1 | NA |
| No hearing aid use | 68.2 (59.9, 76.5) | 1.49 (1.00, 2.23) | 22.51 (2.71, 40.97) |
Notes: PAFs associated with audiometric hearing loss and, separately, no hearing aid use, among older adult participants in the National Health and Aging Trends. Associations of hearing aid use with dementia are assessed among those with moderate or greater hearing loss. CI = confidence interval; PAF = population attributable fraction; PR = prevalence ratio.
aConditional prevalence of hearing loss category among those with dementia.
Point estimates of the PR and PAF were similar, albeit attenuated, when hearing loss was categorized using updated WHO recommendations (Supplementary Table 1).
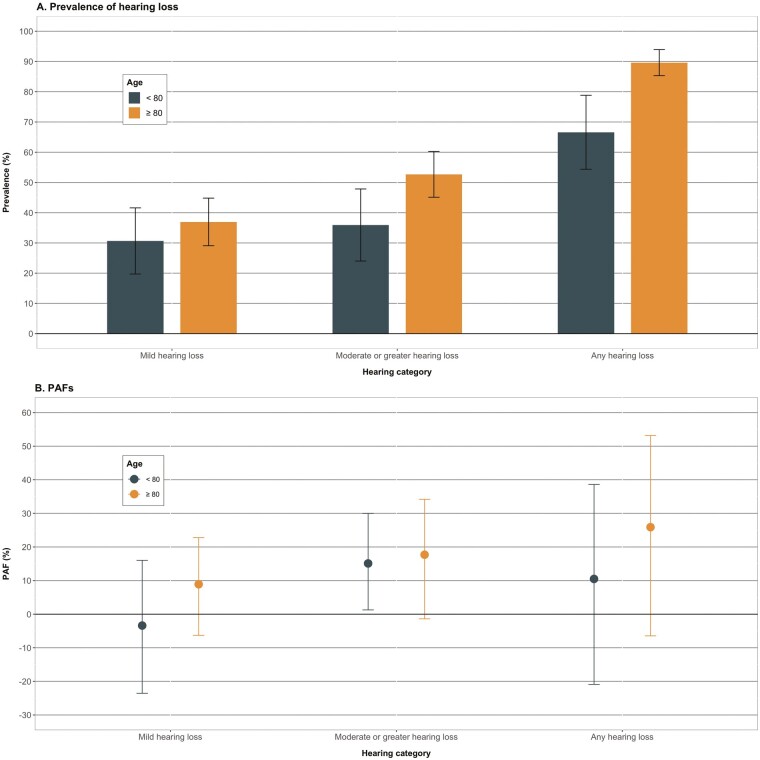
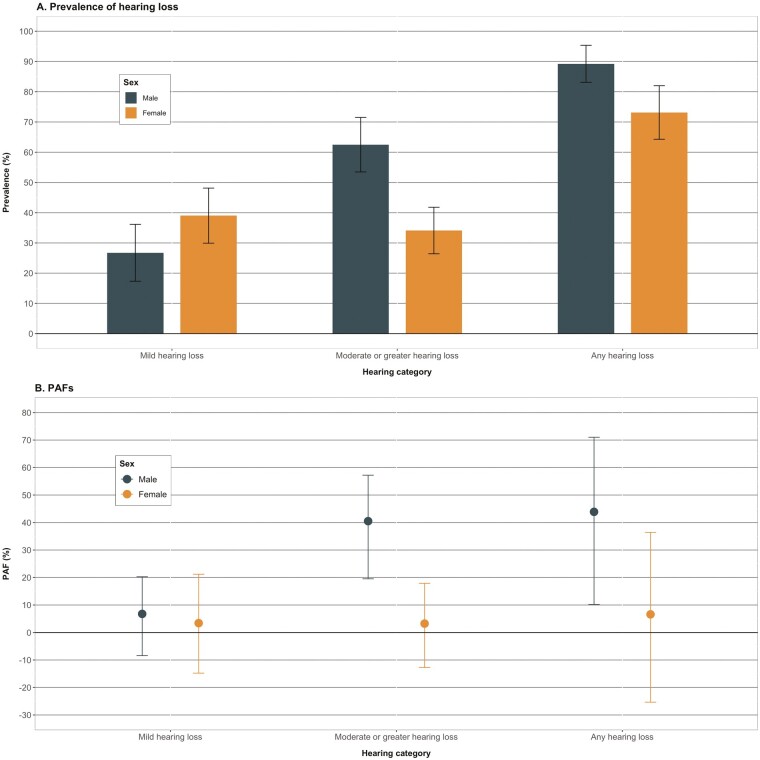
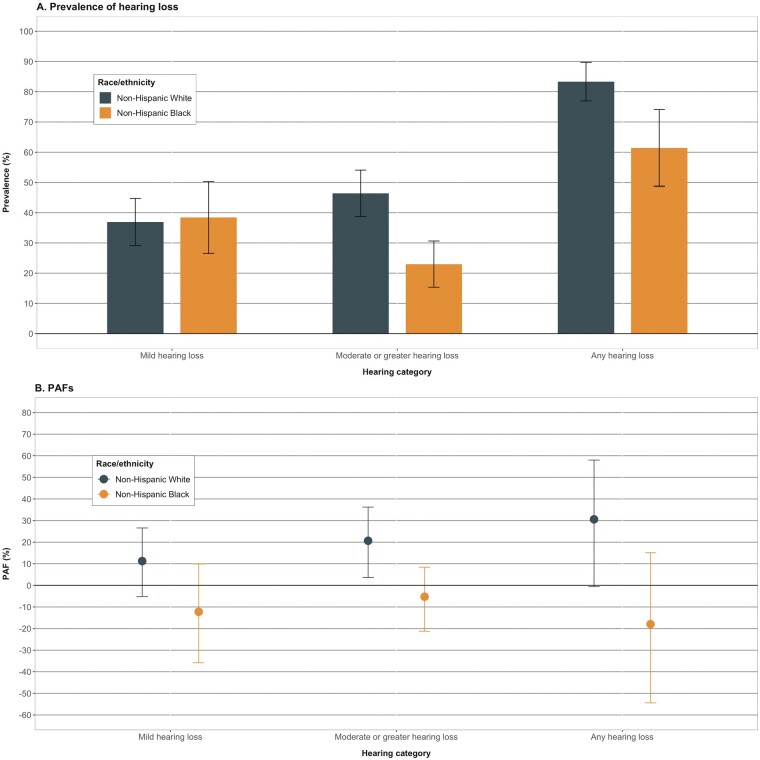
Stratified by age, associations between hearing loss and dementia were similar among those aged ≥80 years relative to those aged <80 (Figure 1, Supplementary Table 2; moderate or greater hearing loss: <80 years, PAF = 15.1% [95% CI: 1.3, 30.0%]; ≥80 years, PAF = 17.7% [95% CI: −1.4, 34.2%]). There were, however, stronger associations among males than females for moderate or greater hearing loss (males, PAF = 40.5% [95% CI: 19.5, 57.2%]; females, PAF = 3.2% [95% CI: −12.7, 17.9%]; Figure 2, Supplementary Table 3). By race/ethnicity, PAFs from moderate or greater hearing loss were higher among non-Hispanic White participants compared with non-Hispanic Black participants, however, the PAFs for Black participants were null with wide CIs (Figure 3, Supplementary Table 4; non-Hispanic White, PAF = 20.6% [95% CI: 3.6, 36.2%]; non-Hispanic Black, PAF < 0).
Figure 1.
Prevalence of hearing loss and population attributable fractions of dementia associated with late-life hearing loss, stratified by age, The National Health and Aging Trends Study, 2021. (A) Displays the weighted prevalence and 95% CIs of hearing loss categories among those with dementia by age groups. (B) Displays the adjusted population attributable fractions and nonparametric 95% CIs of dementia associated with hearing loss. The prevalence of audiometric hearing loss was higher among those in the older age group (≥80 years) than the younger age group (<80 years). PAFs were tended to be larger among those aged ≥80 years relative to <80 years; however, the magnitude of the difference was not strong. For both age groups, PAFs were strongest for moderate or greater hearing loss. CI = confidence interval; PAF = population attributable fraction.
Figure 2.
Prevalence of hearing loss and population attributable fractions of dementia associated with late-life hearing loss, stratified by sex, The National Health and Aging Trends Study, 2021. (A) Displays the weighted prevalence and 95% CIs of hearing loss categories among those with dementia stratified by sex. (B) Displays the adjusted population attributable fractions and nonparametric 95% CIs of dementia associated with hearing loss. Given a high prevalence, the PAF associated with moderate or greater hearing loss was 10-fold greater among males relative to females. CI = confidence interval; PAF = population attributable fraction.
Figure 3.
Prevalence of hearing loss and population attributable fractions of dementia associated with late-life hearing loss, stratified by race/ethnicity, The National Health and Aging Trends Study, 2021. (A) Displays the weighted prevalence and 95% CIs of hearing loss categories among those with dementia stratified by race/ethnicity. (B) Displays the adjusted population attributable fractions and nonparametric 95% CIs of dementia associated with hearing loss. The PAF associated with moderate or greater hearing loss tended to be more strongly supportive among non-Hispanic White participants relative to non-Hispanic Black participants. CI = confidence interval; PAF = population attributable fraction.
Discussion
In this study, we estimated the associated proportion of dementia cases attributable to audiometric hearing loss using a sample representative of the community-dwelling older adult Medicare beneficiary population aged 65 years and older in the United States. Our analysis indicated up to 17% of dementia cases in the United States were attributable to moderate or greater audiometric hearing loss in late-life, and there was evidence for a greater proportion attributable among males relative to females. These results underscore 2 key points. First, the fraction of all dementia cases that can be attributed to clinically significant hearing loss—a treatable risk factor—is sizeable and is comparable or greater to that of modifiable vascular risk factors after adjustment for individual-level demographic and clinical characteristics. Second, studies estimating the PAF of dementia that leverage self-reported hearing loss measures likely underestimate the total contribution of hearing loss to dementia at the population level.
Overall, the adjusted PAFs we observed are nearly eightfold higher than current estimates and highlights the importance of choosing the appropriate hearing measure for the scientific question of interest. Previous studies estimated hearing loss contributes to ~8% of dementia cases globally (6), and 2%–9% in the United States (11,29). Because the strength of the PRs for hearing loss is largely consistent with pooled risk ratios leveraged for the previously-cited studies (6,30), the divergence in PAF estimates is likely attributed in large part to differences in the estimates of the prevalence of hearing loss used (prevalence estimates of hearing loss ranged from 7% to 11% among adults aged 45+ years in the United States for 2 studies (11,29), and 32% among adults in the United Kingdom aged 55+ years (31) for another study (6)). Two factors likely impacted these differences in prevalence. First is the age at which hearing loss was ascertained, which will necessarily affect prevalence given how sharply peripheral hearing loss increases with age. Second, the use of self-report hearing loss measures, which are not sensitive to clinically significant hearing loss among older adults (12). However, these purportedly benign differences directly impact on clinical and public health recommendations on prioritization of specific modifiable risk factors for dementia prevention. As an illustration, the adjusted PAF from audiometric hearing loss of 17% in this nationally representative sample, is comparable in strength to the largest associated PAF of 18% from midlife obesity in recent work (11). The urgent question of which modifiable risk factors have the highest associated PAFs should therefore continue to be thoughtfully examined.
Although these data support hearing loss as an important contributor to brain health, there are several caveats in the interpretation. We used audiometric hearing loss ascertained among a group of older individuals. These data might be better interpreted as the proportion of dementia cases attributable to hearing loss, as, without accounting for the competing risk of death, a PAF from hearing loss of 17% among a group of adults aged 70+ years is perhaps not indicative of the total preventative potential of hearing loss reduction on dementia. The impact of hearing loss on dementia, conceptualized by increasing cognitive load and social isolation as well as alteration of brain structure and function that contribute downstream to poor cognitive health (32), is likely a gradual process mirroring the insidious onset of both chronic conditions. Intervening on hearing loss in late-life, therefore, might not confer as significant of a benefit to brain health as that of primary prevention earlier in the life course. However, because milder hearing loss tends to display weaker associations with dementia relative to moderate or greater hearing loss (borne out in a recent analysis within NHATS (33), and consistent with prior longitudinal studies in the United States (34,35)), it might be possible that preventing the transition from mild-to-moderate hearing loss, even in late life, could still yield cognitive benefits before the full brunt from more severe hearing loss is borne out in the brain. It is also noteworthy that in this nationally representative sample we found evidence for an excess proportion of dementia associated with nonhearing aid use among those with moderate or greater hearing loss, which reinforces the need for randomized studies to investigate the impact of hearing loss treatment on dementia risk.
Our study has notable strengths. In contrast to most prior work (6,11) NHATS collected objectively measured hearing acuity, was designed to be representative of the community-dwelling older adult population in the United States and oversampled vulnerable older adults that might otherwise be left out of population-based samples. In a similar vein, relative to many large population-based studies with available auditory data, NHATS visits are conducted in the participant’s home, allowing greater inclusion of older adults who may be excluded from clinic-based or population-based studies where travel to a clinic visit is required. This provides less of a reason to believe this is a select sample of only healthy older adults able to attend clinic visits. Leveraging individual-level data also bolstered our analysis in 2 important ways. First, this enabled us to use an adjusted PAF formula over the unadjusted Levin formula (36), the latter of which is frequently applied (6,11,29). Broadly speaking unadjusted formulas yield a downwardly biased PAF in the presence of positive confounding of exposure-outcome associations (23,27). And second, we could stratify our models to obtain age-, sex-, and race/ethnicity- (non-Hispanic white and black) specific PRs and PAFs.
We acknowledge several limitations to this analysis. In this cross-sectional analysis, we could not account for the competing risk of death, which would enhance the interpretation of PAFs as the preventative potential from hearing loss reduction. Randomized trials of hearing loss interventions among older adults will provide good evidence for a causal interpretation (37), however, it should be emphasized that future carefully designed longitudinal observational studies will still be necessary to complement these data to quantify PAFs over a longer duration. Second, given insufficient numbers we could not obtain stratified estimates for Hispanic and non-Hispanic Asian or American Indian/Alaskan Native race/ethnicity subgroups. Future work should prioritize work in these populations. And lastly, because the cognitive test battery and pure-tone audiometry were only administered to community-dwelling residents, we could not examine associations among older adults residing in nursing homes or residential care facilities.
Conclusion
Nearly 1 in 5 dementia cases among community-dwelling older adults in the United States are attributable to late-life hearing loss, and the proportion varied by sex. When measured objectively, the PAFs from audiometric hearing loss are of a similar or greater magnitude to those associated with modifiable vascular risk factors such as midlife obesity, indicating that stakeholders should still prioritize auditory health as a potential preventative strategy for dementia risk reduction.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the National Health and Aging Trends Study for their important contributions.
Contributor Information
Jason R Smith, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Cochlear Center for Hearing and Public Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Alison R Huang, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Cochlear Center for Hearing and Public Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Frank R Lin, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Cochlear Center for Hearing and Public Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Nicholas S Reed, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Cochlear Center for Hearing and Public Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Jennifer A Deal, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Cochlear Center for Hearing and Public Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Funding
This study was supported by the NIH (grant numbers K23AG065443 [N.S.R.] and K01AG054693 [J.A.D]). J.R.S. was supported by the Epidemiology and Biostatistics of Aging Pre-Doctoral Training Grant (NIA 5T32AG000247-27) and the Cochlear Center Epidemiology Scholarship for Sensory Loss in Aging.
Conflict of Interest
F.R.L. is a consultant to Frequency Therapeutics and Apple Inc. and is the director of a research center funded in part by a philanthropic gift from Cochlear Ltd to the Johns Hopkins Bloomberg School of Public Health. N.S.R. reports serving on the scientific advisory boards for Neosensory. The other authors declare no conflict.
Author Contributions
J.R.S. was responsible for conceptualization, formal analysis, investigation, writing of the original manuscript draft, and editing and review. A.R.H. was responsible for investigation and manuscript editing and review. F.R.L. was responsible for investigation and manuscript editing and review. N.S.R. was responsible for investigation and manuscript editing and review. J.A.D. was responsible for conceptualization, funding acquisition, investigation, supervision, and manuscript editing and review.
References
- 1. Stokes AC, Weiss J, Lundberg DJ, et al. Estimates of the association of dementia with US mortality levels using linked survey and mortality records. JAMA Neurol 2020;77(12):1543–1550. doi: 10.1001/jamaneurol.2020.2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700–789. doi: 10.1002/alz.12638 [DOI] [PubMed] [Google Scholar]
- 3. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM.. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. doi: 10.1056/nejmsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panza F, Lozupone M, Logroscino G, Imbimbo BP.. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15(2):73–88. doi: 10.1038/s41582-018-0116-6 [DOI] [PubMed] [Google Scholar]
- 5. Lancet T. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022;400(10367):1899. doi: 10.1016/s0140-6736(22)02480-1 [DOI] [PubMed] [Google Scholar]
- 6. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 Report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Isaacson RS, Ganzer CA, Hristov H, et al. The clinical practice of risk reduction for Alzheimer’s disease: a precision medicine approach. Alzheimers Dement 2018;14(12):1663–1673. doi: 10.1016/j.jalz.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin FR, Niparko JK, Ferrucci L.. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171(20):1851–1852. doi: 10.1001/archinternmed.2011.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA.. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2018;144(2):115–126. doi: 10.1001/jamaoto.2017.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Department of Health and Human Services. National Plan to Address Alzheimer’s Disease: 2021 Update. https://aspe.hhs.gov/sites/default/files/documents/66904c18bb1f0843c3c113d7099e98c1/napa-national-plan-2021-update.pdf.2021 (2022, November 18, date accessed).
- 11. Nianogo RA, Rosenwohl-Mack A, Yaffe K, Carrasco A, Hoffmann CM, Barnes DE.. Risk factors associated with Alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol 2022;79(6):584–591. doi: 10.1001/jamaneurol.2022.0976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamil RJ, Genther DJ, Lin FR.. Factors associated with the accuracy of subjective assessments of hearing impairment. Ear Hear. 2015;36(1):164–167. doi: 10.1097/AUD.0000000000000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharma RK, Lalwani AK, Golub JS.. Prevalence and severity of hearing loss in the older old population. JAMA Otolaryngol Head Neck Surg 2020;146(8):762–763. doi: 10.1001/jamaoto.2020.0900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The National Health & Aging Trends Study: At-a-Glance. https://www.nhats.org/researcher/about.
- 15. Freedman VA, Kasper JD.. Cohort profile: The National Health and Aging Trends Study (NHATS). Int J Epidemiol. 2019;48(4):1044–1045. doi: 10.1093/ije/dyz109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasper JD, Freedman VA, Spillman B.. Classification of Persons by Dementia Status in the National Health and Aging Trends Study. Technical Paper #5. Johns Hopkins University School of Public Health, 2013. www.NHATS.org (2022, November 18, date accessed). [Google Scholar]
- 17. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The National Health and Aging Trends Study (NHATS) Vision and Hearing Activities User Guide. (2022). https://www.nhats.org/sites/default/files/2022-07/NHATS%20Vision%20and%20Hearing%20Activities%20User%20Guide.pdf (2022, November 18, date accessed).
- 19. Clark JG. Uses and abuses of hearing loss classification. ASHA. 1981;23(7):493–500. [PubMed] [Google Scholar]
- 20. World Report on Hearing (2021). License: CC BY-NC-SA 3.0 IGO. World Health Organization. [Google Scholar]
- 21. Accounting for Sample Design in NHATS and NSOC Analyses: Frequently Asked Questions. (2022). https://www.nhats.org/sites/default/files/2022-06/Accounting%20for%20the%20NHATS%20NSOC%20Design%20in%20Analyses%20FAQ_v2_0.pdf (2022, November 18, date accessed).
- 22. Barros AJD, Hirakata VN.. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3(1):21. doi: 10.1186/1471-2288-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10(3):195–216. doi: 10.1177/096228020101000303 [DOI] [PubMed] [Google Scholar]
- 24. Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974;99(5):325–332. doi: 10.1093/oxfordjournals.aje.a121617 [DOI] [PubMed] [Google Scholar]
- 25. Rockhill B, Newman B, Weinberg C.. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–19. doi: 10.2105/ajph.88.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rothman KJ, Greenland S, Lash TL. (2015). Modern Epidemiology. http://public.eblib.com/choice/publicfullrecord.aspx?p=2032120 (2022, November 18, date accessed).
- 27. Darrow LA, Steenland NK.. Confounding and bias in the attributable fraction. Epidemiology. 2011;22(1):53–58. doi: 10.1097/EDE.0b013e3181fce49b [DOI] [PubMed] [Google Scholar]
- 28. R Core Team (2021). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- 29. Lee M, Whitsel E, Avery C, et al. Variation in population attributable fraction of dementia associated with potentially modifiable risk factors by race and ethnicity in the US. JAMA Netw Open 2022;5(7):e2219672. doi: 10.1001/jamanetworkopen.2022.19672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ford AH, Hankey GJ, Yeap BB, Golledge J, Flicker L, Almeida OP.. Hearing loss and the risk of dementia in later life. Maturitas. 2018;112:1–11. doi: 10.1016/j.maturitas.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 31. Scholes S, Mindell J. (2014). Health Survey for England 2014: health, social care and lifestyles. In: Craig R, Fuller E, Mindell J, eds. Chapter 4: Hearing. Health and Social Care Information Centre. https://files.digital.nhs.uk/publicationimport/pub19xxx/pub19295/hse2014-ch4-hear.pdf (2022, November 18, date accessed). [Google Scholar]
- 32. Powell DS, Oh ES, Lin FR, Deal JA.. Hearing impairment and cognition in an aging world. J Assoc Res Otolaryngol. 2021;22(4):387–403. doi: 10.1007/s10162-021-00799-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang AR, Jiang K, Lin FR, Deal JA, Reed NS.. Hearing loss and dementia prevalence in older adults in the US. JAMA. 2023;329(2):171–173. doi: 10.1001/jama.2022.20954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L.. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–220. doi: 10.1001/archneurol.2010.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deal JA, Betz J, Yaffe K, et al. Hearing impairment and incident dementia and cognitive decline in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2017;72(5):703–709. doi: 10.1093/gerona/glw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum 1953;9(3):531–541. [PubMed] [Google Scholar]
- 37. Deal JA, Goman AM, Albert MS, et al. Hearing treatment for reducing cognitive decline: design and methods of the aging and cognitive health evaluation in elders randomized controlled trial. Alzheimers Dement (N Y) 2018;4:499–507. doi: 10.1016/j.trci.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.