Abstract
Background
Glucose and non-esterified fatty acids (NEFA) are myocardial fuels whose fasting and post-prandial levels are under different homeostatic regulation. The relationships of fasting and post-load glucose and NEFA with incident heart failure (HF) remain incompletely defined.
Methods
Serum glucose and NEFA were measured during fasting and 2 hours post-oral glucose tolerance test, performed in Cardiovascular Health Study participants not receiving hypoglycemic medication. Participants with prevalent HF or lacking relevant data were excluded. Outcomes were incident HF (primary), and HF with preserved (HFpEF) and reduced (HFrEF) ejection fraction (secondary).
Results
Among 2 238 participants (age 78 ± 4) with a median follow-up of 9.9 years, there were 737 HF events. After adjustment for demographic and lifestyle factors, both fasting (hazard ratio [HR] = 1.11 per SD [95% confidence interval {CI} = 1.01–1.23], p = .040) and post-load (HR = 1.14 per SD [1.05–1.24], p = 0.002) glucose were significantly associated with incident HF. No association was seen for fasting or post-load NEFA. Upon mutual adjustment, only post-load glucose (HR = 1.11 [1.003–1.22], p = .044), but not fasting glucose (HR = 1.06 [0.94–1.20], p = .340), remained associated with HF. Further adjustment for cardiovascular disease and other risk factors in the causal pathway did not affect the association for post-load glucose, but eliminated that for fasting glucose. Associations for fasting and post-load glucose appeared stronger with higher adiposity and were observed specifically for HFrEF but not HFpEF.
Conclusions
Fasting and post-load glucose, but not NEFA, were associated with incident HF. The association was especially robust for post-load glucose, suggesting that pathways involved in post-prandial dysglycemia could offer new targets for HF prevention late in life.
Keywords: Glucose, Heart failure, Non-esterified fatty acids
Heart failure (HF) affects more than 26 million people globally, and this prevalence is climbing (1). HF incidence and prevalence increase with age—up to 80% of those with HF are older adults (2). The disorder’s 2 subtypes, HF with preserved (HFpEF) and reduced ejection fraction (HFrEF), carry a similarly grim prognosis (1).
Aside from age, obesity and glucose dysregulation are major HF risk factors whose prevalence is also increasing worldwide (3,4). Abnormal glucose metabolism is common in older adults, resulting from distinct (patho)physiological changes associated with advancing age (5). Along with diminished physical activity, older adults exhibit increases in fat mass and loss of lean mass. These changes in body composition promote insulin resistance, which, coupled with decreased pancreatic β-cell activity, leads to dysglycemia (6).
Previous work in older adults from the Cardiovascular Health Study (CHS) documented that glucose level 2 hours after an oral glucose tolerance test (OGTT) is more strongly associated with atherosclerotic cardiovascular disease (ASCVD) and mortality than fasting glucose level (7). Several population-based studies of elders have evaluated fasting and post-load glucose in relation to incident HF, with inconsistent findings. Two studies, including an earlier CHS report comprising the mostly White participants enrolled at the study baseline, found that post-load glucose was positively associated with incident HF (8,9). By contrast, two other investigations linked fasting glucose, though not post-load glucose, to an increased risk of HF events (10,11). The foregoing studies were limited by incomplete adjustment for covariates, modest HF numbers, and inability to assess HF subtypes. They also did not undertake a concurrent assessment of fasting and post-load glucose in relation to HF events. Hence, the relative associations of fasting and post-load glucose with incident HF late in life remain unclear.
Like glucose, non-esterified fatty acids (NEFAs) are a principal myocardial fuel source (12). NEFAs are metabolic byproducts of triglyceride hydrolysis whose circulating level reflects the balance between lipolysis and lipogenesis as regulated by insulin, catecholamines, and other hormones (13). In the fasting state, blood NEFA concentration is largely determined by adipose tissue lipolysis, whereas in the post-prandial state, it is primarily governed by the extent of re-esterification by adipocytes of chylomicron-derived NEFA (13). Higher fasting NEFAs have been associated with incident diabetes, as well as HF, in prior work from CHS (14,15). As relates to HF risk, NEFA promotes endothelial dysfunction, and high levels exacerbate myocardial injury under ischemic conditions owing to the greater oxygen requirement of fatty acid as compared with glucose oxidation (16).
We previously showed that circulating NEFA level 2 hours after OGTT, whose decrease is blunted in the setting of obesity or insulin resistance, is preferentially associated with incident diabetes in older adults (17). Although regulation of NEFA differs in the absorptive and post-absorptive state, the relationship of post-load or post-prandial NEFA level with HF has not been examined. We set out to determine the relative contributions of fasting and post-load NEFA, alongside fasting and post-load glucose, to incident HF in community-dwelling adults late in life.
Method
Study Population
Details of the design and procedures for the CHS have been published (18). Briefly, CHS is a prospective investigation of adults ≥ 65 years old recruited from 4 locations in the United States: Washington County, MD; Allegheny County, PA; Sacramento County, CA; and Forsyth County, NC. Medicare eligibility lists in the 4 communities were used to identify potential participants (19). Initial enrollment included 5 201 individuals in 1989–1990, which was followed by the enrollment of 687 mostly African American participants in 1992–1993 (from all locations except Washington County). Eligible participants included those who were able to consent and complete a home interview and 4-hour clinic examination, were planning to reside in the respective county for at least 3 years, and were not wheelchair dependent, receiving cancer treatment, or institutionalized.
Examinations in CHS included a medical history, physical examination, blood collection, and diagnostic testing. The NEFA ancillary study included those with available serum who were eligible for a 2-hour OGTT (ie, not on medical treatment for diabetes mellitus) during the 1996–1997 examination. For the present study, the primary analysis focused on participants free of prevalent HF who had either both fasting and post-load NEFA levels or both fasting and post-load glucose levels.
NEFA and Glucose Measurement
For the OGTT, participants had fasting serum collected after an 8-hour overnight fast. They then consumed 75 g of dextrose, and post-load serum was collected 2 hours later. Serum was shipped to the University of Vermont (Burlington, VT) central laboratory for storage at −80°C. Glucose and NEFA measurements were conducted at the central laboratory. Glucose measurements were performed shortly after collection with the Kodak Ektachem 700 analyzer (Eastman Kodak Corp., Rochester, NY). NEFA measurement was performed on never-thawed samples in 2019–2020 with the Wako enzymatic method (Wako Life Sciences, Mountain View, CA) (20). Previous work has shown the stability of serum lipids when stored at −80°C for up to 10 years. The intra-assay coefficient of variation was 5%.
Glycemia Categories
Impaired fasting glucose was defined as fasting glucose of 100–125 mg/dL, and impaired glucose tolerance as post-load glucose of 140–199 mg/dL. Diabetes was variously defined as fasting glucose ≥126 mg/dL, post-load glucose ≥200 mg/dL, or either of these 2 criteria, or as fasting glucose ≥126 mg/dL and/or use of hypoglycemic medication.
Heart Failure Ascertainment
Participant contacts occurred twice a year either by in-person or telephone visits throughout follow-up (21). The primary endpoint was incident HF; the secondary endpoints were HF HFrEF and HFpEF. All potential cases of HF were adjudicated by an events committee (21). HF assessment was determined by the treating physician’s diagnosis, confirmatory signs and symptoms, a current prescription of a diuretic and vasodilator or digitalis, and diagnostic imaging (chest radiography or cardiac imaging) when available (22). HF subtype determination was based on documented EF. HF events were classified as HFpEF when EF ≥50%, as HFrEF when EF <50%, or of unknown subtype. Adjudication of HF extended through June 2015.
Covariates
Covariates were determined at the 1996–1997 examination, or for lipid fractions and height, measured and carried forward from the 1992–1993 examination. Age, race, sex, smoking, and alcohol consumption were assessed by self-report. Height, weight, waist circumference, and blood pressure were measured using established procedures. Physical activity was determined by self-report of type and intensity of activities. Use of pharmacotherapies was documented annually. Serum albumin, C-reactive protein (CRP), and lipid profiles were quantitated at the central laboratory. The estimated glomerular filtration rate (eGFR) was calculated using cystatin C. As was the case for prevalent HF, prevalent coronary heart disease (CHD) and stroke were ascertained through a combination of CHS questionnaires, medical-record review, and physician confirmation upon enrollment, and adjudication of interim events through the baseline exam for the current analysis (21,23). Incident CHD was adjudicated by the events committee during follow-up (21). Prevalent atrial fibrillation (AF) was identified by yearly electrocardiograms, International Classification of Diseases, ninth revision (ICD-9), diagnostic codes, and Center for Medicare and Medicaid Services claims data, as previously described (24). The proportion of missing data for all individual covariates was <5%.
Statistical Analysis
Descriptive summaries of the included sample are provided with the mean ± standard deviation (SD) for continuous variables and counts (%) for categorical variables. The included sample was compared with the remainder of the CHS cohort at the year 1996–1997 using a t-test for continuous variables and a chi-squared test for categorical variables. For all analyses of fasting and post-load glucose and NEFA with incident HF, extreme outliers (≥99th percentile of the distribution of the exposure measures) were removed (n = 23 for glucose measures; n = 21 for NEFA measures).
The cross-sectional associations of fasting and post-load glucose and fasting and post-load NEFAs with continuous covariates were assessed with Pearson correlation coefficients. Linear regression was used to determine the average differences in glucose or NEFA across different levels of categorical covariates.
Cox proportional hazards regression was applied to evaluate the associations of glucose and NEFA measures with incident HF and its subtypes with adjustment for potential confounders. A cause-specific approach was used for modeling HF subtypes, censoring the participant at the time of a diagnosis of HF of the alternative or unknown subtype. Sequential models added covariates selected on the basis of known associations or underlying mechanisms. Model 1 was unadjusted. Model 2 adjusted for age, sex, race, and enrollment site. Model 3 (main model) additionally adjusted for body mass index (BMI), physical activity, smoking, alcohol use, estrogen replacement therapy, serum albumin, and eGFR. Model 4 adjusted for additional covariates that are potentially on the causal pathway, including systolic blood pressure, anti-hypertensive medication use, low-density lipoprotein, high-density lipoprotein (HDL), triglycerides, lipid-lowering medication, prevalent CHD, prevalent stroke, prevalent claudication, and prevalent AF. Restricted cubic splines were examined for departures from linearity. Since relationships were approximately linear, we report associations per SD increment in glucose and NEFA levels. In additional analyses, we assessed whether fasting and post-load glucose measures were independent of one another by including them simultaneously in the models. In exploratory analyses, we examined whether further adjustment for waist circumference, CRP, or interval development of CHD influenced the associations under study. Additional secondary analyses assessed for effect modification of glucose and NEFA measures by each other, and also by sex and BMI, by including appropriate cross-product terms in the main model. We performed a sensitivity analysis to examine associations at a more proximate follow-up of 5 years. In further exploratory analyses, we repeated the assessment of the relationships for glucose measures with HF using fasting and post-load glycemia categories. Last, we conducted a parallel analysis in the larger cohort who attended the 1996–1997 examination and had fasting glucose levels available. We explored how glycemia categories defined only by such levels, with and without hypoglycemic therapy, related to incident HF and its subtypes beyond our primary study sample.
All analyses were conducted with R (R Development Core Team; http://www.r-project.org). A 2-sided p < .05 was considered statistically significant.
Results
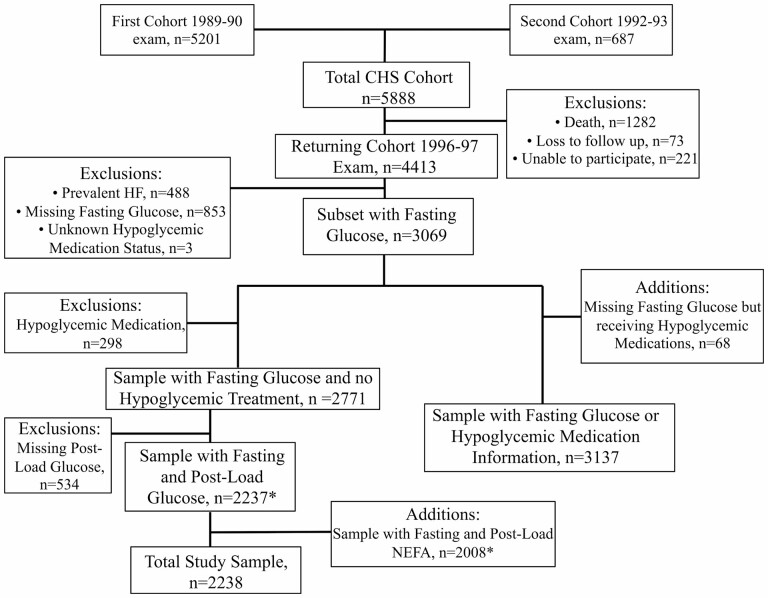
Among the 4 413 participants attending the 1996–1997 exam, 2 238 individuals were free of prevalent HF, had glucose and/or NEFA measurements, and made up the primary study sample (Figure 1). (Of the 488 participants excluded for prevalent HF, cardiac imaging was available for adjudication in 193 cases.) The baseline characteristics of included participants, compared to those not included, are given in Table 1. The study sample was slightly younger with less medical comorbidities, including hypertension, diabetes, prevalent CHD, prevalent stroke, and prevalent AF, than the excluded sample.
Figure 1.
Flow diagram of participants included in the present analysis. * Participants in the top first percentile of glucose or NEFA distributions were excluded from the relevant analyses. For glucose, this corresponded to 23 participants, and for NEFA to 21 participants. NEFA = non-esterified fatty acids.
Table 1.
Baseline Characteristics of Participants Included and Not Included in the Study Cohort
| Characteristics | Included (n = 2 238) | Not Included (n = 2 175) | p Value |
|---|---|---|---|
| Age, years | 77.7 ± 4.4 | 79.5 ± 5.6 | <.001 |
| Male sex, n (%) | 885 (39.5) | 807 (37.1) | .102 |
| Black race, n (%) | 333 (14.9) | 405 (18.6) | .001 |
| High school education or more, n (%) | 1 130 (50.6) | 846 (39.1) | <.001 |
| Body mass index, kg/m2 | 26.7 ± 4.4 | 27.3 ± 5.1 | <.001 |
| Waist circumference, cm | 96.3 ± 12.7 | 98.6 ± 14.2 | <.001 |
| Physical activity score, n (%) | <.001 | ||
| None/little | 479 (21.6) | 721 (41.7) | |
| Moderate | 1 138 (51.4) | 777 (44.9) | |
| Strenuous | 597 (27.0) | 231 (13.4) | |
| Smoking, n (%) | .035 | ||
| Never | 1 069 (48.5) | 875 (46.9) | |
| Former | 957 (43.4) | 795 (42.6) | |
| Current | 180 (8.2) | 196 (10.5) | |
| > 7 alcoholic drinks per week, n (%) | 270 (12.1) | 116 (6.1) | <.001 |
| Estrogen replacement (women), n (%) | 259 (19.2) | 259 (19.2) | <.001 |
| Systolic blood pressure, mmHg | 136.8 ± 20.3 | 137.2 ± 21.3 | .574 |
| Antihypertensive medication, n (%) | 1 138 (50.9) | 1 309 (67.9) | <.001 |
| Diabetes mellitus*, n (%) | 69 (3.1) | 549 (44.2) | <.001 |
| LDL cholesterol, mg/dL | 129.0 ± 32.5 | 126.7 ± 33.7 | .037 |
| HDL cholesterol, mg/dL | 54.3 ± 14.5 | 52.9 ± 14.6 | .001 |
| Triglyceride, mg/dL | 137.6 ± 75.9 | 151.8 ± 96.9 | <.001 |
| Lipid-lowering medication, n (%) | 236 (10.6) | 210 (10.9) | .763 |
| Serum albumin, g/dL | 3.83 ± 0.3 | 3.83 ± 0.3 | .899 |
| Prevalent CHD, n (%) | 418 (18.7) | 747 (34.3) | <.001 |
| Prevalent stroke, n (%) | 102 (4.6) | 256 (11.8) | <.001 |
| Prevalent atrial fibrillation, n (%) | 75 (3.4) | 106 (7.5) | <.001 |
| eGFR, mL/min/1.73 m2 | 72.3 ± 18.3 | 66.8 ± 21.27 | <.001 |
| C-reactive protein, mg/L | 1.2 ± 1.6 | 1.5 ± 1.6 | <.001 |
Notes: CHD = coronary heart disease; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
*Defined as a fasting glucose ≥126 mg/dL, random glucose ≥200 mg/dL, or use of hypoglycemic medication up to and including the 1996–1997 examination.
Both glucose values were available for 2 237 individuals. The mean fasting glucose was 98 ± 15 mg/dL. There were 718 (32.1%) participants with impaired fasting glucose (≥100 mg/dL) and 69 (3.1%) with diabetes (≥126 mg/dL). The mean post-load glucose was 140 ± 52 mg/dL, with 928 (41.5%) participants having impaired glucose tolerance (≥140 mg/dL) and 264 (11.8%) having diabetes (≥200 mg/dL). Both NEFA values were available for 2 008 individuals. The mean fasting NEFA was 0.37 ± 0.16 mEq/L, and the mean post-load NEFA was 0.06 ± 0.05 mEq/L.
The cross-sectional associations between glucose and NEFA measures with baseline characteristics are presented in Table 2. Both fasting and post-load glucose were related to measures of adiposity and hypertension, triglycerides, serum albumin, and CRP (all positively), as well as strenuous physical activity, HDL cholesterol, and eGFR (all negatively). The two glucose measures showed directionally different associations with male sex, and current versus former smoking. Only post-load glucose was related to age and prevalent stroke (both positively), while only fasting glucose was associated with estrogen replacement therapy (negatively). As for NEFA measures, both were associated with adiposity, hypertension, triglycerides, serum albumin (all positively), and current smoking (negatively). The two NEFA measures showed opposite associations with alcohol consumption and HDL cholesterol (positive for fasting and negative for post-load NEFA). Only fasting NEFA showed associations with age (positive), and male sex, moderate/strenuous physical activity, former smoking and prevalent CHD (all negative). By contrast, only post-load NEFA was related to eGFR (negatively).
Table 2.
Cross-sectional Associations of Glucose and NEFA Measures With Baseline Characteristics
| Covariates | Fasting Glucose (β or r) | Post-load Glucose (β or r) | Fasting NEFA (β or r) | Post-load NEFA (β or r) |
|---|---|---|---|---|
| Age | −0.01 | 0.06* | 0.08* | −0.04 |
| Male sex | 3.28* | −6.72* | −0.10* | 0.003 |
| Black race | 1.02 | 1.02 | −0.003 | 0.003 |
| BMI | 0.27* | 0.16* | 0.09* | 0.15* |
| Waist circumference | 0.27* | 0.15* | 0.05* | 0.17* |
| Physical activity | ||||
| Moderate | −0.33 | −3.08 | −0.03* | −0.003 |
| Strenuous | −1.56* | −8.74* | −0.06* | −0.004 |
| Smoking | ||||
| Former | 1.82* | −3.00 | −0.04* | −0.001 |
| Current | 0.80 | −11.53* | −0.04* | −0.007* |
| Alcohol consumption | 1.26 | −6.72 | 0.03* | −0.01* |
| Estrogen replacement therapy | −4.46* | 3.78 | 0.04* | 0.01* |
| Systolic blood pressure | 0.01 | 0.07* | 0.14* | 0.05* |
| Antihypertensive medication | 2.73* | 9.15* | 0.01 | 0.003* |
| Diabetes | 38.26* | 98.75* | 0.03 | 0.04* |
| LDL cholesterol | 0.03 | 0.02 | 0.01 | −0.01 |
| HDL cholesterol | −0.22* | −0.09* | 0.23* | −0.18* |
| Triglycerides | 0.13* | 0.17* | 0.08* | 0.33* |
| Lipid lowering medication | 0.004 | 4.62 | −0.01 | 0.004 |
| Serum albumin | 4.89* | 0.07* | 0.08* | 0.09* |
| Prevalent coronary heart disease | 0.70 | 1.30 | −0.03* | −0.001 |
| Prevalent stroke | 1.39 | 10.99* | 0.02 | −0.004 |
| eGFR | −0.05* | −0.07* | −0.01 | −0.09* |
| C-reactive protein | 0.10* | 0.17* | 0.09* | 0.17* |
Notes: β = average increase in the outcome associated with the participant characteristic or per unit of the characteristic; r = correlation coefficient provided for those characteristics which are continuous; BMI = body mass index; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; LDL = low-density lipoprotein; NEFA = non-esterified fatty acids.
*Indicates cross-sectional associations with a p value <.05.
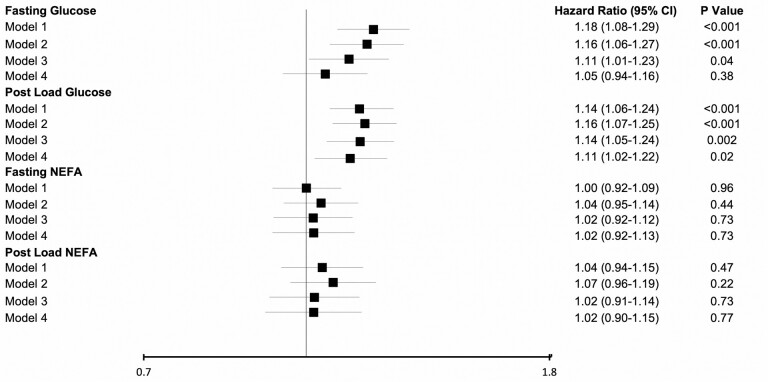
During the median (interquartile range) follow-up of 9.9 (5.3–15.1) years, 737 cases of incident HF occurred. Lost to follow up was 1.6%. CHD preceded or coincided with HF in 410 of incident cases. HF was classified as HFpEF in 257 cases, and as HFrEF in 196 cases, the rest were undefined. Both fasting and post-load glucose showed a positive association with incident HF in the minimally adjusted and main models (Models 2 and 3), but no association was present for either fasting or post-load NEFAs at either level of adjustment (Figure 2). Upon further adjustment for factors at least partly in the causal pathway, the relationship for fasting glucose was substantially attenuated and became nonsignificant, while that for post-load glucose persisted with only slight attenuation.
Figure 2.
Associations of fasting and post-load glucose and non-esterified fatty acids (NEFA) with incident heart failure. Standard deviations for exposure measures are as follows. Fasting glucose, SD = 15 mg/dL; post-load glucose, SD = 52 mg/dL; fasting NEFA, SD = 0.16 mmol/L; post-load NEFA, SD = 0.04 mmol/L. Model 1. Unadjusted model. Model 2. Adjusted for age, sex, race, and enrollment site. Model 3. Adjusted for Model 1 covariates, as well as body mass index, physical activity, smoking, alcohol use, estrogen replacement therapy, serum albumin, and estimated glomerular filtration rate. Model 4. Adjusted for Model 2 covariates, as well as systolic blood pressure, anti-hypertensive medication use, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, lipid-lowering medication, prevalent coronary heart disease, prevalent stroke, prevalent claudication, and prevalent atrial fibrillation.
When fasting and post-load glucose were adjusted for each other in the main model, only post-load glucose (hazard ratio [HR] per SD = 1.11 [95% confidence interval {CI} = 1.00–1.22], p = .044) remained associated with incident HF, while fasting glucose (HR per SD = 1.06 [95% CI = 0.94–1.20], p = .340) was no longer significantly so.
In exploratory analyses, additional adjustment for waist circumference had no meaningful impact on the risk estimates (Supplementary Table 1). Further adjustment for CRP slightly attenuated the associations for both glucose measures, but that for post-load glucose remained significant (HR per SD = 1.09 [95% CI = 1.00–1.20], p = .049). Additional adjustment for intercurrent CHD led to minimal further attenuation of the association, which became nonsignificant (HR per SD = 1.08 [95% CI = 0.97–1.19], p = .174). The sensitivity analysis truncating follow-up at 5 years revealed a strengthening of risk estimates for all glucose and NEFA measures (Supplementary Table 1), but the findings were broadly similar to those with full follow-up.
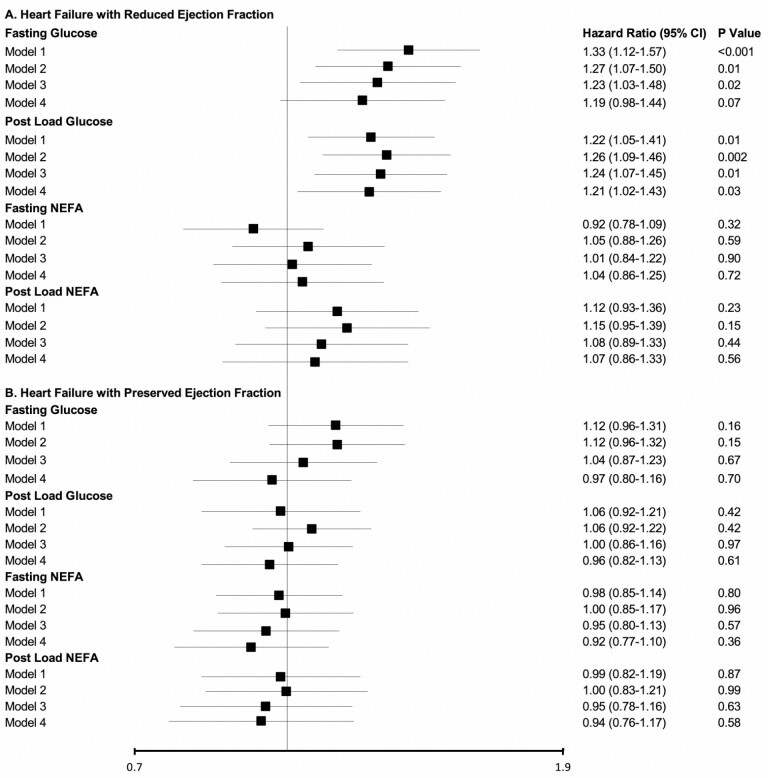
Assessment of HF subtypes showed that both fasting and post-load glucose had associations with incident HFrEF, though these were not noted for fasting or post-load NEFA (Figure 3A). Similar relationships were seen in the minimally adjusted and main models. These associations were not meaningfully altered after adjustment for putative causal intermediates in Model 4. There were no associations for either glucose or NEFA measure with HFpEF (Figure 3B).
Figure 3.
Associations of fasting and post-load glucose and non-esterified fatty acids with incident heart failure subtypes. (A) Heart failure with reduced ejection fraction. (B) Heart failure with preserved ejection fraction. Standard deviations for exposure measures, models, and abbreviations are as given in Figure 2.
Exploratory analysis of fasting and post-load glycemia categories revealed significant associations with incident HF for impaired fasting glucose and diabetes based on post-load glucose (with or without fasting glucose) in early models, which persisted only for diabetes by post-load glucose across levels of adjustment (Supplementary Table 2). There were small numbers in certain categories and broad and largely overlapping 95% CIs, which limited assessment. Analysis of fasting glycemia categories in both the study sample and the larger cohort showed significant graded associations with HF for impaired fasting glucose and diabetes, both with and without the inclusion of hypoglycemic therapy, through the main model (Supplementary Table 3). These associations were attenuated across successive models, remaining significant only for diabetes upon adjustment for potential intermediate factors. Risk estimates were numerically higher for untreated diabetes in the study sample than the larger cohort, or treated versus untreated diabetes, there was a large overlap of 95% CIs. Exploration of HF subtypes in the larger cohort showed significant associations for diabetes, both untreated and treated, with HFrEF at higher levels of adjustment, but these were not seen for HFpEF (Supplementary Table 4).
Assessment for interaction showed significant effect modification by BMI of the relationships of both glucose measures with incident HF. The interaction with continuous BMI was such that the associations were stronger with higher adiposity for both fasting glucose (pinteraction = .025) and post-load glucose (pinteraction = .002). Supplementary Table 5 shows the associations of fasting and post-load glucose with incident HF stratified by standard BMI categories, illustrating the gradient of increasing risk at higher adiposity. Such risk was especially pronounced in the obese category, particularly for post-load glucose. There was no corresponding interaction with BMI for either NEFA measure (pinteraction ≥ .553). No evidence of effect modification by sex was detected for fasting or post-load glucose or NEFA (pinteraction ≥ .409), nor of glucose and NEFA measures with each other (pinteraction ≥ .126)
Discussion
Main Results
In this community-dwelling cohort of older adults, we found that fasting and post-load glucose, but not fasting or post-load NEFA, were associated with an increased risk of HF events. Although both glucose measures were related to HF incidence after adjustment for potential confounders, only post-load glucose retained this association after adjustment for putative causal intermediates or upon concurrent adjustment with fasting glucose. In secondary analyses, both fasting and post-load glucose were associated with HFrEF, but not HFpEF, although associations became nonsignificant on mutual adjustment. Additional analyses documented consistent associations for impaired fasting glucose and diabetes with HF and HFrEF in the larger sample that did not undergo OGTT, supporting the validity of the fasting glucose measure for the broader cohort. Last, exploratory assessments showed evidence of effect modification by adiposity, such that the relationships for fasting and post-load glucose each, but not for either NEFA measure, were stronger with increasing BMI.
Prior Studies
Diabetes has long been associated with the development of HF (25). This relationship has also been documented for dysglycemia in the prediabetic range, with impaired fasting glucose and impaired glucose tolerance, each showing a significant association with HF incidence in a recent meta-analysis (26). The design and sample types of included studies varied, however, and many relied on HF outcomes from administrative data (26). Among older adults, several population-based studies have previously examined the relationships of fasting and post-load glucose with incident HF. In the Kuopio and Health ABC studies, fasting glucose, but not post-load glucose, was associated with incident HF, whereas in an earlier analysis of CHS and the Uppsala Longitudinal Study of Adult Men, only post-load glucose showed a significant association with this outcome (8–11). These prior studies, however, often included a modest number of incident HF events. They did not fully account for important potential confounders such as BMI, lifestyle factors, or eGFR, nor did they assess the impact of thorough adjustment for putative causal intermediates. Notably, these previous studies did not evaluate fasting and post-load glucose concurrently or HF subtypes.
Fewer longitudinal studies have evaluated circulating NEFA and HF. Previous data from CHS showed fasting NEFA to be associated with an increased risk of HF events (15). No association between fasting NEFA and incident HF was observed in middle-aged to older adults enrolled in the Multi-Ethnic Study of Atherosclerosis (27). Like the earlier CHS investigation, there was no opportunity to analyze post-load NEFA, as OGTT was not performed.
To our knowledge, this is the largest investigation to evaluate both fasting and post-load glucose in relation to HF in a prospective cohort, and the first to do so concurrently with fasting and post-load NEFA. The current study newly shows that fasting and post-load glucose are positively associated with incident HF in older adults after accounting for potential confounders, while failing to detect corresponding associations for either fasting or post-load NEFA. Moreover, the present analyses are the first to highlight that upon concurrent adjustment, or after accounting additionally for putative causal intermediates, post-load, but not fasting, glucose retained its association with incident HF, attesting to a more robust association for this measure. Secondary analyses provide novel details to these associations, showing that the relationship with HF for fasting and post-load glucose were each more pronounced at higher BMI, and occurred with HFrEF, but not HFpEF.
Potential Explanations and Mechanisms
Among older adults, 2-hour glucose level post-OGTT uncovers a larger proportion of dysglycemia—both pre-diabetes and diabetes—than fasting glucose or glycated hemoglobin (28). While fasting glucose predominantly reflects hepatic insulin resistance, post-load glucose is determined primarily by skeletal muscle insulin resistance and impaired pancreatic β-cell secretory reserve (7). The latter are cardinal features of dysglycemia in older adults, explaining their greater contribution to the dysglycemic burden in this age group (6).
The observation that 2-hour glucose bore a relationship with incident HF independent of fasting glucose or a range of putative intermediates aligns with the more robust associations identified previously in relation to ASCVD and all-cause mortality in this cohort (7). This finding suggests that derangements in post-prandial glucose regulation or the consequences of such glycemic excursions may be of particular relevance to HF pathogenesis, as they are for ASCVD and death.
That post-load dysglycemia is a particularly strong marker of HF risk in older adults may owe to its relationship with skeletal muscle (29). In the presence of sarcopenia, the reduced glucose disposal into the organ would be manifest through elevated post-load rather than fasting glucose (28). This would have especially adverse consequences for HF susceptibility because exercise intolerance in HF is dependent as much on impaired cardiac filling as it is on impaired skeletal muscle function (30). In fact, impaired skeletal muscle quality has been previously associated with incident HF in older adults (31).
Additionally, there are data to suggest that acute glucose swings, such as occur post-prandially, result in more oxidative stress than more stably elevated glucose levels, as seen in the post-absorptive state (32). The consequences of amplification of oxidative stress for the heart and vasculature could account for the more robust association observed for post-load glucose and incident HF herein (33).
Analyses of interaction showed that post-load and fasting glucose bore stronger associations with HF at higher BMI, particularly among participants with obesity. These findings, though secondary, are consistent with the role of adiposity in the development of insulin resistance in older adults, as well as its proinflammatory influences and contribution to volume expansion (34,35). Such actions would serve to only compound the relations of glycemic measures, both fasting and post-load, with HF risk.
Interestingly, both fasting and post-load glucose were related to incident HFrEF, but not HFpEF. The relationship between post-load glucose and HF persisted after adjustment for intercurrent CHD, suggesting that this was not a function of ischemic myocardial injury. Yet, the hyperinsulinemia that would be a preceding or ongoing feature of the observed dysglycemic abnormalities can heighten oxidative and endoplasmic reticulum stress, and lead to the cardiomyocyte injury and cardiac systolic impairment that would account for the association observed (36). Dysglycemia and related features have also, however, been implicated in the pathophysiology of HFpEF (37). It is unclear why similar associations with HFpEF were not detected, but these secondary findings will require additional investigation.
Turning to the absence of association documented for NEFA, the finding for fasting NEFA is at odds with the association with HF documented at an earlier CHS exam (15). The previous analysis was larger, at 4 248 participants with 1 286 HF events. But, the upper bound of our 95% CI (1.12) for fasting NEFA excludes the HR = 1.15 observed therein, such that the difference is unlikely to be primarily due to power. A more probable explanation may involve the different populations included. Unlike the prior study, the present analysis excluded participants with medically treated diabetes, since such participants were ineligible for OGTT. Accordingly, the proportion with prevalent diabetes in the previous analysis was 14.3% (15), as compared with 3.1% herein. While the previous association was not altered after adjustment for diabetes, it is likely that fasting NEFA level therein was reflective of more pronounced metabolic perturbations that may not have been fully accounted for in the previous analyses. This comparison also applies to the previous null findings for fasting NEFA and HF in the Multi-Ethnic Study of Atherosclerosis, a younger population with a diabetes prevalence of 6.5%, although the number of incident HF events (323) therein was more modest (27).
Another consideration for our null findings for fasting and post-load NEFA is that these measures encompass total circulating NEFAs, not all of which necessarily bear adverse associations with HF. Indeed, in a prior analysis of fasting individual NEFAs, several emerged as positively associated, and others as inversely associated with the outcome, such that investigation of individual NEFA associations postprandially could be more revealing (38).
Clinical Implications
Because it is more cumbersome to perform than fasting glucose or glycated hemoglobin, OGTT is not routinely recommended for the diagnosis of dysglycemia in older adults. But our findings in adults largely 75 and older suggest that investigation into the basis for the especially robust the risk of HF associated with post-load over fasting glucose could potentially reveal novel mechanisms amenable to intervention. This is of particular importance as this is the age group at greatest risk for both dysglycemia and HF (2,5). There is some suggestive evidence that antihyperglycemic strategies targeting post-prandial glucose may be especially effective at cardiovascular risk reduction in older adults, although this requires further study (39). Notably, the therapeutic class lately documented to be most effective for HF prevention in diabetes, the sodium-glucose co-transporter-2 inhibitors, have not been shown to achieve their benefits specifically through actions on post-prandial glucose (40). But it is possible that these agents’ ability to tamp down post-prandial glucose excursions could be an element of their therapeutic success. The present findings suggest that strategies centered on regulation of post-prandial glucose excursions deserve additional study.
Limitations
Several limitations must be acknowledged. The observational design does not permit determination of causality, nor can residual confounding be excluded. Adjudication and subphenotyping of HF cases lacked cardiac imaging in a substantial proportion of cases, and detailed information on etiology was not collected. There was also no systematic use of information on natriuretic peptides, routine clinical adoption of which did not occur until the latter part of the follow-up period. As such, HF adjudications may have been subject to misclassification. The findings apply to participants who attended the study visit and are not necessarily generalizable to all older adults, or to different age or race/ethnic groups. Because participants receiving hypoglycemic therapy were excluded, this may have enriched individuals with post-prandial hyperglycemia since diagnosis of diabetes at the time depended preferentially on fasting glucose. Last, 2-hour NEFA concentration reflected an oral glucose load; whether NEFA level post-prandially has a different association with HF will require separate study.
Conclusion
In this cohort of participants surviving to advanced old age, post-load glucose was more robustly associated with incident HF than fasting glucose, but neither fasting nor post-load NEFA were related to this outcome. Additional research into pathways regulating post-prandial glucose and therapeutic strategies targeting dysglycemia in the absorptive state could be of value for improved HF prevention late in life.
Supplementary Material
Contributor Information
Adam Oesterle, Department of Medicine, Division of Cardiology San Francisco VA & University of California San Francisco, San Francisco, California, USA.
Petra Buzkova, Department of Biostatistics, University of Washington, Seattle, Washington, USA.
Cara N Pellegrini, Department of Medicine, Division of Cardiology San Francisco VA & University of California San Francisco, San Francisco, California, USA.
Calvin Hirsch, Department of Medicine, University of California Davis, Davis, California, USA.
Russell P Tracy, Department of Pathology and Laboratory Medicine, University of Vermont, Burlington, Vermont, USA.
David S Siscovick, New York Academy of Medicine, New York, New York, USA.
Luc Djousse, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Ken J Mukamal, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Jorge R Kizer, Department of Medicine, Division of Cardiology San Francisco VA & University of California San Francisco, San Francisco, California, USA.
Funding
This work was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 and R01AG053325 from the National Institute on Aging, and K24 HL135413 from NHBLI. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Conflict of Interest
J.R.K. reports stock ownership in Abbott, Bristol-Myers Squibb, Johnson & Johnson, Medtronic, Merck, and Pfizer. The other authors declare no conflict of interest.
Author Contributions
A.O., L.D., K.J.M., and J.R.K. designed the research project. A.O. and J.R.K. drafted the manuscript. P.B. performed the statistical analyses. C.N.P., along with L.D., K. J. M., and the other coauthors, critically reviewed and edited the manuscript. P.B. takes responsibility for the contents of the article.
References
- 1. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Díez-Villanueva P, Alfonso F. Heart failure in the elderly. J Geriatr Cardiol. 2016;13:115–117. doi: 10.11909/j.issn.1671-5411.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 4. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - Global burden of disease and forecasted trends. J Epidemiol Glob Health 2020;10:107–111. doi: 10.2991/jegh.k.191028.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029. doi: 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 6. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444–452. doi: 10.2337/dc16-1732 [DOI] [PubMed] [Google Scholar]
- 7. Brutsaert EF, Shitole S, Biggs ML, et al. . Relations of postload and fasting glucose with incident cardiovascular disease and mortality late in life: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2016;71:370–377. doi: 10.1093/gerona/glv106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA 2005;294:334–341. doi: 10.1001/jama.294.3.334 [DOI] [PubMed] [Google Scholar]
- 9. Banerjee D, Biggs ML, Mercer L, et al. . Insulin resistance and risk of incident heart failure: Cardiovascular Health Study. Circ Heart Fail. 2013;6:364–370. doi: 10.1161/CIRCHEARTFAILURE.112.000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalogeropoulos A, Georgiopoulou V, Harris TB, et al. . Glycemic status and incident heart failure in elderly without history of diabetes mellitus: the Health, Aging, and Body Composition Study. J Card Fail. 2009;15:593–599. doi: 10.1016/j.cardfail.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J, Sarnola K, Ruotsalainen S, et al. . The metabolic syndrome predicts incident congestive heart failure: a 20-year follow-up study of elderly Finns. Atherosclerosis 2010;210:237–242. doi: 10.1016/j.atherosclerosis.2009.10.042 [DOI] [PubMed] [Google Scholar]
- 12. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009 [DOI] [PubMed] [Google Scholar]
- 13. Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients 2015;7:9453–9474. doi: 10.3390/nu7115475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Djoussé L, Khawaja O, Bartz TM, et al. . Plasma fatty acid-binding protein 4, nonesterified fatty acids, and incident diabetes in older adults. Diabetes Care. 2012;35:1701–1707. doi: 10.2337/dc11-1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Djoussé L, Benkeser D, Arnold A, et al. . Plasma free fatty acids and risk of heart failure: the Cardiovascular Health Study. Circ Heart Fail. 2013;6:964–969. doi: 10.1161/CIRCHEARTFAILURE.113.000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steinberg HO, Tarshoby M, Monestel R, et al. . Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–1239. doi: 10.1172/JCI119636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boston RC, Moate PJ. NEFA minimal model parameters estimated from the oral glucose tolerance test and the meal tolerance test. Am J Physiol Regul Integr Comp Physiol. 2008;295:R395–R403. doi: 10.1152/ajpregu.90317.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fried LP, Borhani NO, Enright P, et al. . The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w [DOI] [PubMed] [Google Scholar]
- 19. Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9 [DOI] [PubMed] [Google Scholar]
- 20. Jeevanandam M, Hsu YC, Ramias L, Schiller WR. A rapid, automated micromethod for measuring free fatty acids in plasma/serum. Clin Chem. 1989;35:2228–2231. [PubMed] [Google Scholar]
- 21. Ives DG, Fitzpatrick AL, Bild DE, et al. . Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9 [DOI] [PubMed] [Google Scholar]
- 22. Al-Kindi SG, Buzkova P, Shitole SG, et al. . Soluble CD14 and risk of heart failure and its subtypes in older adults. J Card Fail. 2020;26:410–419. doi: 10.1016/j.cardfail.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Psaty BM, Kuller LH, Bild D, et al. . Methods of assessing prevalent cardiovascular disease in the cardiovascular health study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8 [DOI] [PubMed] [Google Scholar]
- 24. Macheret F, Bartz TM, Djousse L, et al. . Higher circulating adiponectin levels are associated with increased risk of atrial fibrillation in older adults. Heart 2015;101:1368–1374. doi: 10.1136/heartjnl-2014-307015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avery CL, Loehr LR, Baggett C, et al. . The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2012;60:1640–1646. doi: 10.1016/j.jacc.2012.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cai X, Liu X, Sun L, et al. . Prediabetes and the risk of heart failure: a meta-analysis. Diabetes Obes Metab. 2021;23:1746–1753. doi: 10.1111/dom.14388 [DOI] [PubMed] [Google Scholar]
- 27. Nomura SO, Karger AB, Weir NL, Lima JAC, Thanassoulis G, Tsai MY. Free fatty acids and heart failure in the Multi-Ethnic Study of Atherosclerosis (MESA). J Clin Lipidol. 2021;15:608–617. doi: 10.1016/j.jacl.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. 2021;17:534–548. doi: 10.1038/s41574-021-00512-2 [DOI] [PubMed] [Google Scholar]
- 29. Faerch K, Borch-Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009;52:1714–1723. doi: 10.1007/s00125-009-1443-3 [DOI] [PubMed] [Google Scholar]
- 30. Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537–546. doi: 10.1161/CIRCHEARTFAILURE.109.903773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L, Bartz TM, Santanasto A, et al. . Body composition and incident heart failure in older adults: results from 2 prospective cohorts. J Am Heart Assoc. 2022;11:e023707. doi: 10.1161/JAHA.121.023707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monnier L, Mas E, Ginet C, et al. . Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681 [DOI] [PubMed] [Google Scholar]
- 33. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Basu R, Breda E, Oberg AL, et al. . Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738 [DOI] [PubMed] [Google Scholar]
- 35. Packer M. Leptin-aldosterone-neprilysin axis: identification of its distinctive role in the pathogenesis of the three phenotypes of heart failure in people with obesity. Circulation 2018;137:1614–1631. doi: 10.1161/CIRCULATIONAHA.117.032474 [DOI] [PubMed] [Google Scholar]
- 36. Packer M. Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart Fail. 2021;9:535–549. doi: 10.1016/j.jchf.2021.05.019 [DOI] [PubMed] [Google Scholar]
- 37. Shah SJ, Borlaug BA, Kitzman DW, et al. . Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute working group summary. Circulation 2020;141:1001–1026. doi: 10.1161/CIRCULATIONAHA.119.041886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Djousse L, Biggs ML, Matthan NR, et al. . Serum individual nonesterified fatty acids and risk of heart failure in older adults. Cardiology 2021;146:351–358. doi: 10.1159/000513917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Avogaro A. Postprandial glucose: marker or risk factor? Diabetes Care. 2011;34:2333–2335. doi: 10.2337/dc11-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hasan FM, Alsahli M, Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res Clin Pract. 2014;104:297–322. doi: 10.1016/j.diabres.2014.02.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.