Abstract
Background
A claims-based frailty index (CFI) allows measurement of frailty on a population scale. Our objective was to examine the association of changes in CFI over 12 months with mortality and Medicare costs.
Methods
We used a 5% sample of fee-for-service Medicare beneficiaries. We estimated CFI (range: 0–1: nonfrail (<0.25), mildly frail (0.25–0.34), moderately-to-severely frail (≥0.35) on January 1, 2015 and January 1, 2016. Beneficiaries were categorized as having a large decrease (−<0.045), small decrease (−≤0.045–0.015), stable (±0.015), small increase (>0.015–0.045), or large increase (>0.045). We used Cox proportional hazards model to estimate hazard ratio (HR) for mortality adjusting for age, sex, and 2015 CFI value and compared total Medicare costs from January 1, 2016 to December 31, 2016.
Results
The study population included 995 664 beneficiaries (mean age 77 years, 56.8% female). In nonfrail (n = 906 046), HR (95% confidence interval [CI]) ranged from 0.71 (0.67–0.75) for a large decrease to 2.75 (2.68–2.33) for a large increase. In moderate-to-severely frail beneficiaries (n = 16 527), the corresponding HR (95% CI) ranged from 0.63 (0.57–0.70) to 1.21 (1.06–1.38). The mean total Medicare cost per member per year (standard deviation) was from $12 149 ($83 508) in nonfrail beneficiaries to $61 155 ($345 904) in moderate-to-severely frail beneficiaries.
Conclusions
One-year changes in CFI are associated with elevated mortality risk and health care costs across all levels of frailty.
Keywords: Frailty, Healthcare costs, Medicare claims
With aging, health problems accumulate and precipitate frailty, a vulnerable state driving elevated risks of adverse health outcomes and health care utilization and costs (1,2). Measuring frailty may help clinicians and health systems deliver age-friendly, high-quality geriatric care to older adults (3,4). However, integration of a frailty assessment in the United States health systems has been slow due to time and resource constraints. Use of a claims-based frailty index (CFI) offers an effective and inexpensive method of systematically capturing frailty, by serving as a proxy for underlying deficit accumulation or phenotypic frailty (5,6). The reliance of a CFI on administrative claims data continuously generated from routine clinical encounters may enable longitudinal population health monitoring and inform target populations for resource allocation.
While frailty is a clinically dynamic state (7,8), research on longitudinal changes in frailty and health outcomes has shown mixed results (9). A frailty index derived from electronic health records data was ineffective in capturing an improvement in frailty over time, but worsening frailty was associated with increased mortality (10). In contrast, a frailty index from survey data showed improvement as well as worsening in frailty, yet improvement was not always associated with better health outcomes (11). To date, little is known about whether a CFI is sensitive to changes and whether changes are associated with future health outcomes or health care cost. Establishing this relationship is a prerequisite step to justify use of CFI for population health monitoring.
In this study, we sought to examine annual changes in CFI across different levels of frailty (nonfrailty, mild frailty, and moderate-to-severe frailty) and whether observed changes in CFI were predictive of future mortality and health care costs among Medicare fee-for-service beneficiaries.
Method
Study Data and Sample
This retrospective study used Medicare claims data from a 5% random sample of fee-for-service beneficiaries between January 1, 2014, and December 31, 2016. We used the following files: Medicare summary beneficiary file for enrollment, death, demographic information, chronic conditions, and cost variables. The CFI was calculated from inpatient, outpatient, carrier, skilled nursing facility (SNF), hospice, home health, and durable medical equipment claims data. Changes in CFI were measured between January 1, 2015, and January 1, 2016, and CFI calculation require 1 year of claims data. Therefore, we required that beneficiaries be 67 years or older on January 1, 2016, and continuously enrolled in Medicare Part A and B for the prior 2 years. Beneficiaries were excluded if they died before January 1, 2016, were enrolled in managed care, or had any hospice claims between January 1, 2014, and January 1, 2016. The Hebrew SeniorLife Institutional Review Board approved this study.
Assessment of Change in Frailty
Frailty was measured using a CFI, which estimates a deficit-accumulation frailty index (range: 0–1; higher scores indicate more severe frailty) based on International Classification of Diseases diagnosis codes, Current Procedural Terminology codes, and Healthcare Common Procedure Coding System codes over 1 year (the programming codes for statistical software are available at https://dataverse.harvard.edu/dataverse/cfi) (6,12). The CFI has been validated against clinical frailty (frailty phenotype and frailty index), physical performance (eg, gait speed and grip strength), and severe disability assessments. We measured CFI at 2 time points: January 1, 2015, and January 1, 2016. Baseline frailty category was defined based on CFI as of January 1, 2015, using standard cut points: nonfrail (<0.25), mildly frail (0.25–0.34), moderately-to-severely frail (≥0.35) (5,12). The 1-year change in CFI (CFI2016 − CFI2015) was categorized as large decrease (−<0.045), small decrease (−≤0.045–0.015), stable (±0.015), small increase (>0.015–0.045), or large increase (>0.045). These cut points were chosen based on previous literature identifying the minimum clinically important difference in a frailty index as 0.03 (11,13,14).
Mortality and Health Care Costs
Beneficiaries were followed until the earliest of death, disenrollment from fee-for-service Medicare, or December 31, 2016. We also examined the mean annualized Medicare total costs and component costs during the 1-year follow-up period.
Baseline Characteristics
We measured age, sex, race, ethnicity, and Chronic Conditions Data Warehouse conditions (15) including: acute myocardial infarction, Alzheimer’s disease and related disorder, anemia, asthma, atrial fibrillation, cataracts, chronic kidney disease, chronic obstructive pulmonary disease, depression, diabetes, glaucoma, heart failure, hip or pelvic fracture, ischemic heart disease, osteoporosis, rheumatoid arthritis or osteoarthritis, stroke or transient ischemic attack, and cancers of breast, endometrial, colorectal, prostate, and lung. Chronic conditions were ascertained by any history of coding, as well as coding within the past calendar year to compare coding of chronic comorbidities from 2015 to 2016.
Statistical Analysis
Baseline demographic characteristics and comorbidities were compared across baseline frailty categories using analysis of variance or Pearson’s chi-square test. We estimated Kaplan–Meier survival curves by baseline frailty category and change in CFI category. To estimate the association of change in CFI on mortality, we fitted Cox proportional hazards regression model to estimate the hazard ratio (HR) and 95% confidence interval (CI) for CFI change categories within each baseline frailty category, adjusting for age, sex, and baseline CFI (continuous variable). We also estimated HR (95% CI) for 0.1-unit change in CFI. We described the mean, standard deviation (SD), median, and interquartile range (IQR) of annualized Medicare total cost by baseline frailty category and change in CFI. Because the CFI accounts for the past year of claims and capture of acute versus chronic conditions may vary and thus change CFI, we explored potential differences in medical condition coding from 2015 to 2016. Analyses were conducted using SAS and Stata, version 16.1 (StataCorp, College Station, TX). A 2-sided p value <.05 was considered statistically significant.
Results
Baseline Characteristics
Our cohort included 995 664 beneficiaries, who were 56.8% female, 87.5% White, and mean 77.0 years of age (SD 7.5; Table 1). Mean CFI (SD) for the overall cohort in 2015 was 0.16 (0.06), with 91.0% nonfrail (n = 906 046), 7.3% mildly frail (n = 73 091), and 1.7% moderate-to-severely frail (n = 16 527). Compared to nonfrail beneficiaries, those who were moderate-to-severely frail tended to be older (mean age 81.9 [8.1] vs 76.6 [7.3] years), and female (68.6% vs 54.9%). They also had higher prevalence of chronic conditions, such as Alzheimer’s disease and related disorders (65.7% vs 6.3%), heart failure (73.1% vs 17.2%), depression (74.4% vs 22.1%), and chronic kidney disease (67.6% vs 18.6%).
Table 1.
Characteristics of Medicare Fee-For-Service Beneficiaries by Baseline Frailty Status on January 1, 2015
| Characteristics | Total n (%) |
Nonfrail n (%) |
Mildly Frail n (%) |
Moderate-to-Severely Frail n (%) |
p Value |
|---|---|---|---|---|---|
| Sample size | 995 664 | 906 046 | 73 091 | 16 527 | |
| Age, mean (SD) | 77.0 (7.5) | 76.6 (7.3) | 81.2 (8.0) | 81.9 (8.1) | <.001 |
| Female | 565 549 (56.8) | 433 028 (54.9) | 105 430 (63.1) | 27 091 (68.6) | <.001 |
| Race | <.001 | ||||
| White | 871 082 (87.5) | 793 674 (87.6%) | 63 410 (86.8%) | 13 998 (84.7%) | |
| Black | 66 995 (6.7) | 59 391 (6.6%) | 5 912 (8.1%) | 1 652 (10.0%) | |
| Other | 57 627 (5.8) | 52 981 (5.8%) | 3 769 (5.2%) | 877 (5.3%) | |
| Hispanic | 12 089 (1.2) | 10 284 (1.1%) | 1 400 (1.9%) | 405 (2.5%) | <.001 |
| CFI on January 1, 2015 (SD) | 0.16 (0.06) | 0.15 (0.04) | 0.29 (0.03) | 0.40 (0.05) | <.001 |
| CFI on January 1, 2016 (SD) | 0.17 (0.07) | 0.16 (0.06) | 0.27 (0.07) | 0.34 (0.09) | <.001 |
| Acute myocardial infarction | 40 711 (4.1%) | 30 524 (3.4%) | 7 918 (10.8%) | 2 269 (13.7%) | <.001 |
| Alzheimer’s disease and related disorders | 99 212 (10.0%) | 57 449 (6.3%) | 30 912 (42.3%) | 10 851 (65.7%) | <.001 |
| Anemia | 476 998 (47.9%) | 400 549 (44.2%) | 61 246 (83.8%) | 15 203 (92.0%) | <.001 |
| Asthma | 117 293 (11.8%) | 92 870 (10.3%) | 19 096 (26.1%) | 5 327 (32.2%) | <.001 |
| Atrial fibrillation | 134 638 (13.5%) | 105 237 (11.6%) | 23 028 (31.5%) | 6 373 (38.6%) | <.001 |
| Cancer* | 151 857 (15.3%) | 134 022 (14.8%) | 14 465 (19.8%) | 3 370 (20.4%) | <.001 |
| Cataract | 656 309 (65.9%) | 583 186 (64.4%) | 59 435 (81.3%) | 13 688 (82.8%) | <.001 |
| Chronic kidney disease | 218 435 (21.9%) | 168 589 (18.6%) | 38 678 (52.9%) | 11 168 (67.6%) | <.001 |
| Chronic obstructive pulmonary disease | 215 572 (21.7%) | 168 383 (18.6%) | 36 909 (50.5%) | 10 280 (62.2%) | <.001 |
| Depression | 255 784 (25.7%) | 199 794 (22.1%) | 43 697 (59.8%) | 12 293 (74.4%) | <.001 |
| Diabetes | 335 390 (33.7%) | 279 102 (30.8%) | 44 776 (61.3%) | 11 512 (69.7%) | <.001 |
| Glaucoma | 229 409 (23.0%) | 202 078 (22.3%) | 22 120 (30.3%) | 5 211 (31.5%) | <.001 |
| Heart failure | 210 450 (21.1%) | 155 975 (17.2%) | 42 392 (58.0%) | 12 083 (73.1%) | <.001 |
| Hip/pelvic fracture | 28 850 (2.9%) | 18 641 (2.1%) | 7 421 (10.2%) | 2 788 (16.9%) | <.001 |
| Hypertension | 769 990 (77.3%) | 682 364 (75.3%) | 71 292 (97.5%) | 16 334 (98.8%) | <.001 |
| Ischemic heart disease | 431 844 (43.4%) | 359 733 (39.7%) | 57 810 (79.1%) | 14 301 (86.5%) | <.001 |
| Osteoporosis | 206 301 (20.7%) | 172 169 (19.0%) | 26 943 (36.9%) | 7 189 (43.5%) | <.001 |
| Rheumatoid arthritis or osteoarthritis | 529 308 (53.2%) | 455 379 (50.3%) | 59 599 (81.5%) | 14 330 (86.7%) | <.001 |
| Stroke or transient ischemic attack | 124 209 (12.5%) | 91 255 (10.1%) | 25 213 (34.5%) | 7 741 (46.8%) | <.001 |
Notes: CFI = claims-based frailty index; SD = standard deviation.
*Cancer includes breast, endometrial, colorectal, prostate, and lung cancer.
Changes in Frailty
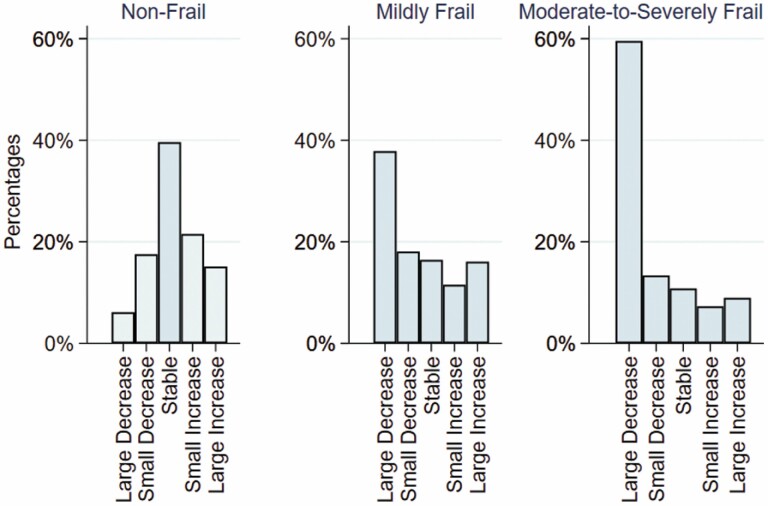
Over 1 year, large increases and decreases in CFI were observed among all baseline frailty categories (Figure 1). For the nonfrail group, beneficiaries most commonly had stable CFI (359 006 [39.6%]), with a small proportion having a large increase in frailty (137 223 [15.1%]). For the mildly frail group, 1-year CFI change across categories was more evenly distributed, although a large decrease was most common (27 664 [37.8%]). Lastly, in the moderate-to-severely frail group a large decrease in CFI was most common (9 843 [59.6%]), followed by a small decrease (2 211 [13.4%]). Comparing coding of medical conditions in 2015 versus 2016 the majority increased in prevalence (Supplementary Table 1). However, acute conditions that decreased in prevalence in the moderate-to-severely frail group including acute myocardial infarction (3.9% in 2015 to 2.0% in 2016) and stroke or transient ischemic attack (21.7% in 2015 to 11.2% in 2016).
Figure 1.
Distribution of 1-year CFI change by baseline frailty category. Baseline frailty category was defined as nonfrail (CFI<0.25), mildly frail (CFI 0.25–0.34), and moderately-to-severely frail (CFI≥0.35). The 1-year change in CFI (CFI2016 − CFI2015) was categorized as large decrease (−<0.045), small decrease (−≤0.045–0.015), stable (±0.015), small increase (>0.015–0.045), or large increase (>0.045). CFI = claims-based frailty index.
Mortality by Baseline Frailty Category and Change in Frailty
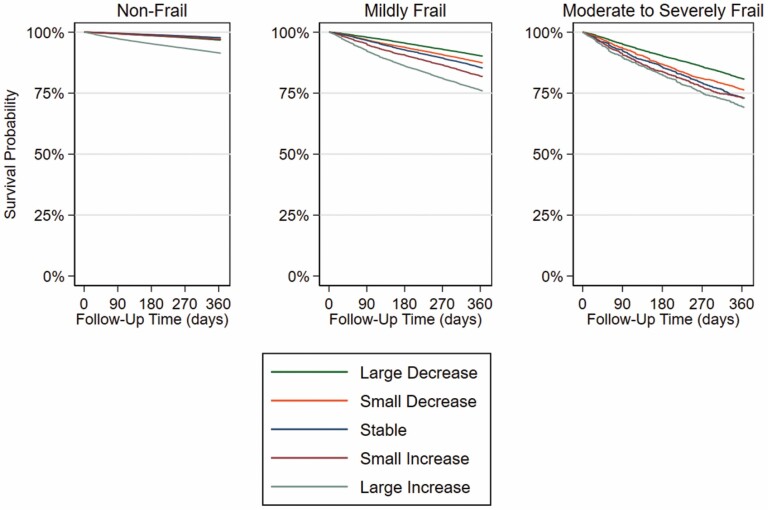
Over the 1-year follow-up 44 716 (4.5%) died and 27 900 (2.8%) were disenrolled from Medicare Fee-For-Service Part A and/or B. The mortality rate (per 100 person-years) ranged from 3.5 in the nonfrail group, 15.5 in the mildly frail group, and 25.3 in the moderate-to-severely frail group (Table 2). Kaplan–Meier curves were the most distinct across change in frailty categories among those with moderate-to-severe frailty at baseline (Figure 2). For any level of baseline frailty, the highest mortality rates were observed among those who had a large increase in their CFI (nonfrail: 9.1 per 100 person-years; mildly frail: 27.9 per 100 person-years; moderate-to-severely frail: 37.4 per 100 person-years). Within each baseline frailty group after adjusting for age, sex, and baseline CFI, HRs (95% CI) for large decrease to large increase categories versus stable CFI were 0.71 (0.67, 0.75) to 2.75 (2.26, 2.33) for the nonfrail group, 0.62 (0.59, 0.66) to 1.71 (1.61, 1.82) for the mildly frail group, and 0.63 (0.57, 0. 70) to 1.21 (1.06, 1.38) for the moderate-to-severely frail group. The HR (95% CI) per 0.1-unit increase in CFI was 2.30 (2.26, 2.33), 1.68 (1.64, 1.72), and 1.39 (1.34, 1.45) for the nonfrail, mildly frail, and moderate-to-severely frail groups, respectively.
Table 2.
Association of Frailty Category and 1-Year CFI Change With 1-Year Mortality
| Frailty Category* | Sample Size n (%) |
Deaths n (%) |
Mortality Rate (per 100 person-years) [95% CI] | Adjusted Hazard Ratio HR [95% CI]* |
|---|---|---|---|---|
| Nonfrail | 906 046 | 30 951 (3.4%) | 3.5 [3.5–3.6] | |
| Large decrease | 55 612 (6.1%) | 1 773 (3.2%) | 3.3 [3.1–3.4] | 0.71 [0.67–0.75] |
| Small decrease | 159 034 (17.6%) | 3 686 (2.3%) | 2.4 [2.3–2.4] | 0.78 [0.75–0.81] |
| Stable (reference) | 359 006 (39.6%) | 8 148 (2.3%) | 2.3 [2.3–2.4] | Reference |
| Small increase | 195 171 (21.5%) | 5 694 (2.9%) | 3.0 [2.9–3.1] | 1.23 [1.19–1.27] |
| Large increase | 137 223 (15.1%) | 11 650 (8.5%) | 9.1 [8.9–9.2] | 2.75 [2.68–2.84] |
| Per 0.1-point increase | n/a | 2.30 [2.26–2.33] | ||
| Mildly frail | 73 091 | 10 259 (14.0%) | 15.5 [15.2–15.8] | |
| Large decrease | 27 664 (37.8%) | 2 664 (9.6%) | 10.3 [9.9–10.7] | 0.62 [0.59–0.66] |
| Small decrease | 13 222 (18.1%) | 1 637 (12.4%) | 13.5 [12.9–14.2] | 0.86 [0.80–0.92] |
| Stable (reference) | 12 015 (16.4%) | 1 715 (14.3%) | 15.8 [15.1–16.6] | Reference |
| Small increase | 8 429 (11.5%) | 1 491 (17.7%) | 20.1 [19.1–21.2] | 1.25 [1.17–1.34] |
| Large increase | 11 761 (16.1%) | 2 752 (23.4%) | 27.9 [26.9–29.0] | 1.71 [1.61–1.82] |
| Per 0.1-point increase | n/a | 1.68 [1.64–1.72] | ||
| Moderate-to-severely frail | 16 527 | 3 569 (21.6%) | 25.3 [24.5–26.1] | |
| Large decrease | 9 843 (59.6%) | 1 843 (18.7%) | 21.4 [20.4–22.3] | 0.63 [0.57–0.70] |
| Small decrease | 2 211 (13.4%) | 510 (23.1%) | 27.2 [24.9–29.6] | 0.85 [0.75–0.97] |
| Stable (reference) | 1 786 (10.8%) | 466 (26.1%) | 31.8 [29.0–34.8] | Reference |
| Small increase | 1 205 (7.3%) | 312 (25.9%) | 32.1 [28.8–35.9] | 1.04 [0.90–1.20] |
| Large increase | 1 482 (9.0%) | 438 (29.6%) | 37.4 [34.1–41.1] | 1.21 [1.06–1.38] |
| Per 0.1-point increase | n/a | 1.39 [1.34–1.45] |
Notes: Baseline frailty category was defined as nonfrail (CFI<0.25), mildly frail (CFI 0.25–0.34), and moderately-to-severely frail (CFI≥0.35). The 1-year change in CFI (CFI2016 − CFI2015) was categorized as large decrease (−<0.045), small decrease (−≤0.045–0.015), stable (±0.015), small increase (>0.015–0.045), or large increase (>0.045). CFI = claims-based frailty index; CI = confidence interval.
*HRs were estimated from Cox proportional hazards models for each baseline frailty category and adjusted for age, sex, and baseline CFI (continuous variable).
Figure 2.
Kaplan–Meier curves of 1-year mortality according to baseline frailty category and 1-year CFI change. Baseline frailty category was defined as nonfrail (CFI<0.25), mildly frail (CFI 0.25–0.34), and moderately-to-severely frail (CFI≥0.35). The 1-year change in CFI (CFI2016 − CFI2015) was categorized as large decrease (−<0.045), small decrease (−≤0.045–0.015), stable (±0.015), small increase (>0.015–0.045), or large increase (>0.045). CFI = claims-based frailty index.
Annualized Medicare Costs by Baseline Frailty Category and Change in Frailty
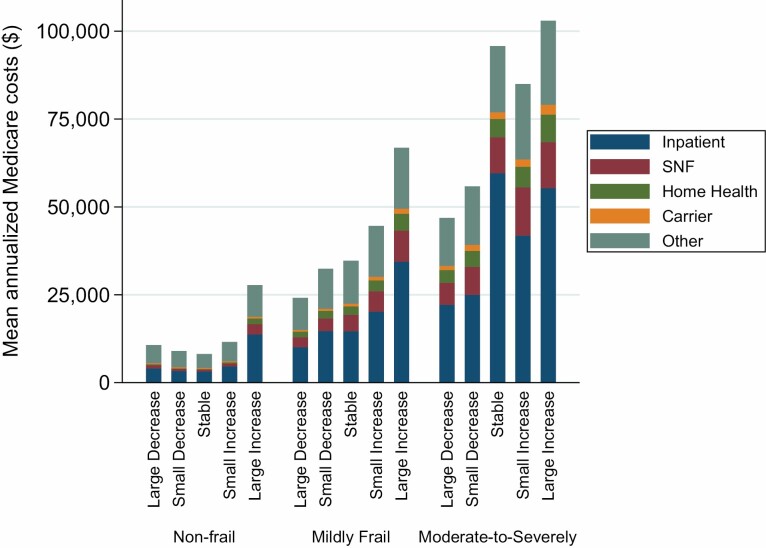
The mean annualized total Medicare cost (SD) increased with the severity of frailty at baseline, from $12 149 ($83 508) in nonfrail group to $61 155 ($345 904) in moderate-to-severely frail group (Figure 3 and Supplementary Table 2). Median and IQRs are presented in Supplementary Table 3, as these data were heavily right-skewed. The mean annualized cost also varied by the CFI change category, with a large decrease generally corresponding to lower costs and a large increase corresponding to higher costs within each frailty category (large decrease vs large increase in CFI: $10 695 [$41 373] vs $27 737 [$180 302] in the nonfrail group; $24 138 [$60 552] vs $66 812 [$199 069] in the mildly frail group; $46 864 [$163 207] vs $102 964 [$350 734] in the moderate-to-severely frail group). Across all groups, inpatient costs accounted for the largest proportion of total cost, followed by SNF, then home health. However, SNF costs tended to become more prominent with observed increases in baseline frailty and change in CFI.
Figure 3.
Mean annualized Medicare costs according to baseline frailty category and 1-year CFI change. Baseline frailty category was defined as nonfrail (CFI<0.25), mildly frail (CFI 0.25–0.34), and moderately-to-severely frail (CFI≥0.35). The 1-year change in CFI (CFI2016 − CFI2015) was categorized as large decrease (−<0.045), small decrease (−≤0.045–0.015), stable (±0.015), small increase (>0.015–0.045), or large increase (>0.045). SNF = skilled nursing facility.
Discussion
In this study of Medicare fee-for-service beneficiaries, we found that change in CFI over 1 year was common, with both increases and decreases in frailty observed in all baseline frailty groups. Both baseline frailty and a 1-year change in frailty were associated with future mortality risk and total Medicare costs. At each level of baseline frailty, increasing frailty was associated with increased mortality risk and higher costs. Decreasing CFI was associated with decreased mortality risk and lower costs. The highest mortality and total costs were observed among those with moderate-to-severe frailty who had a large increase in CFI. Our results suggest that CFI is sensitive to change over time with change in health status and may be useful in monitoring overall population health and identifying high-cost beneficiaries.
Although improvements in frailty are possible clinically and have been observed in prospective cohort studies where frailty was assessed in-person, the sensitivity to change of frailty index derived from health care databases (electronic health records and claims data) has not been well demonstrated. Due to the nature of electronic health records and claims data, diagnoses tend to only accumulate, which makes it difficult to capture improvements. For example, Stow et al. found only increases in their frailty index based on cumulative information in the electronic health records (16). In comparison, our CFI, which uses claims data only in the previous 1 year, could capture conditions that were active enough to receive medical treatments and health care services, thereby reflecting the current or more recent health status. In our supplemental analyses, some comorbidities, in fact, decreased in coding from 2015 to 2016 (Supplementary Table 1); acute myocardial infarction among moderate-to-severely frail (3.9%–2.0%). It is important to note that the CFI is ultimately an attempt to measure the underlying construct of frailty itself, and that some of the observed changes may be related to measurement error due to undercoding rather than resolution of a condition or regression to the mean after a year of excessive health care utilization. This is particularly relevant on the extremes of the distributions (ie, very low CFI or very high CFI), where floor or ceiling effects can happen. We observed small numbers of nonfrail patients who had a large decrease in CFI and moderate-to-severely frail patients with a large increase in CFI.
Our findings confirm previous work that demonstrated an increased risk of mortality with worsening frailty over 1 year (11). Importantly, we demonstrate that an improvement in CFI was associated with decreased mortality risk compared to stable CFI, and that worsening CFI was associated with increased mortality risk. This dose–response relationship supports the clinical utility of measuring CFI and its change for prognostication. Health care systems can apply our CFI algorithm to the ubiquitous administrative claims data as a means to target high-risk beneficiaries to allocate resources for population management efforts, care management, and delivery of specific care pathways (eg, geriatric comanagement services). Moreover, researchers may consider using CFI as an outcome in a comparative study of interventions to reverse or prevent frailty in aging populations.
In addition to mortality, we found that CFI change was associated with health care costs. Previous studies have only demonstrated the association of frailty measured at a single time point with increase in health care costs (17,18). However, because frailty is a dynamic health state, it is important to also show that increase in CFI was associated with higher health care costs, whereas a decrease in frailty was associated with lower health care costs. Moreover, we observed a substantially larger variation in the mean total Medicare cost across CFI change categories in the moderate-to-severely frail group (large decrease to large increase, $46 864–$102 964) than the mildly frail group ($24 138–$66 812) and nonfrail group ($8 134–$27 737). The increase in costs were largely driven by increases in inpatient and SNF costs. Given these results, interventions to reduce hospitalizations and SNF stays among moderate-to-severely frail individuals who have a large increase in CFI from the prior year may yield considerable reductions in Medicare total costs.
Our study has important limitations. First, our cohort included Medicare fee-for-service beneficiaries enrolled in Part A and B during a 2-year period. Therefore, our population is relatively healthier, as all participants had to survive for 1 year. Also, how well CFI change predicts mortality and health care costs in beneficiaries enrolled in Medicare Advantage Plan or private insurance plans remains uncertain. Second, our population was predominantly of non-Hispanic ethnicity and White race and underestimates the distribution of racial and ethnic minorities on Medicare. Third, frailty measured from claims data is only a proxy for underlying frailty, and this specific CFI is based on deficit accumulation. Thus, it is not as accurate as a clinician’s bedside assessment of frailty, which often involves assessment of physical performance (eg, walking speed). However, our CFI has been widely validated and shown to outperform other claims-based frailty algorithms (5,19). The major advantage of CFI over clinical assessments is that it allows a population-scale measurement, which is not feasible for clinical assessments. Whether CFI changes represent true changes in frailty should be explored in future work comparing CFI and in-person frailty assessment. Finally, estimating annualized Medicare costs to account for a different follow-up duration assumes that the mean costs per a unit time (eg, monthly cost) remain constant over 12 months. This approach may overestimate total cost for those with episodic high spending or are near the end of life.
In conclusion, our study demonstrates that the utility of tracking CFI over time at the population level to identify older adults at risk for death and high total Medicare spending in the following year. These findings support the ability of health systems to effectively target high-risk and high-cost individuals for high-quality geriatric care interventions and care management. Researchers may also consider using CFI change as an outcome measure of overall health status from administrative claims data in which other patient-reported outcome measures are not available.
Supplementary Material
Acknowledgments
An earlier version of this work was presented at the American Geriatrics Society Scientific Meeting in Orlando, FL, in May 2022.
Contributor Information
Sandra Miao Shi, Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Boston, Massachusetts, USA; Division of Gerontology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Nessa Steinberg, Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Boston, Massachusetts, USA.
Gahee Oh, Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Boston, Massachusetts, USA.
Brianne Olivieri-Mui, Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Boston, Massachusetts, USA.
Stephanie Sison, Division of Gerontology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Ellen P McCarthy, Division of General Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; Bouvé College of Health Sciences, The Roux Institute, Northeastern University, Boston, Massachusetts, USA.
Dae Hyun Kim, Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Boston, Massachusetts, USA; Division of Gerontology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Funding
This study was funded by grant R01AG071809 from National Institute on Aging - National Institutes of Health (NIH) to D.H.K.
Conflict of Interest
D.H.K. is a consultant to Alosa Health and VillageMD. The other authors declare no disclosures.
Author Contributions
Study design: all authors. Analysis: G.O., N.S., S.S., D.H.K. Data interpretation: all authors. Manuscript drafting: N.S., S.S. Manuscript editing: all authors.
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/s0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pajewski NM, Lenoir K, Wells BJ, Williamson JD, Callahan KE. Frailty screening using the electronic health record within a Medicare accountable care organization. J Gerontol A Biol Sci Med Sci. 2019;74(11):1771–1777. doi: 10.1093/gerona/glz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. doi: 10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring frailty in administrative claims data: comparative performance of four claims-based frailty measures in the United States Medicare data. J Gerontol A Biol Sci Med Sci. 2020;75(6):1120–1125. doi: 10.1093/gerona/glz224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166(4):418–423. doi: 10.1001/archinte.166.4.418 [DOI] [PubMed] [Google Scholar]
- 8. Mendonça N, Kingston A, Yadegarfar M, et al. Transitions between frailty states in the very old: the influence of socioeconomic status and multi-morbidity in the Newcastle 85+ cohort study. Age Ageing. 2020;49(6):974–981. doi: 10.1093/ageing/afaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orkaby AR, Nussbaum L, Ho Y-L, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002–2012. J Gerontol A Biol Sci Med Sci. 2018;74(8):1257–1264. doi: 10.1093/gerona/gly232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stow D, Matthews FE, Hanratty B. Frailty trajectories to identify end of life: a longitudinal population-based study. 2018;16(1):171. doi: 10.1186/s12916-018-1148-x [DOI] [PMC free article] [PubMed]
- 11. Shi SM, Olivieri-Mui B, McCarthy EP, Kim DH. Changes in a frailty index and association with mortality. J Am Geriatr Soc. 2021;69(4):1057–1062. doi: 10.1111/jgs.17002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the health and retirement study. J Gerontol A Biol Sci Med Sci. 2019;74(8):1271–1276. doi: 10.1093/gerona/gly197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jang IY, Jung HW, Lee HY, Park H, Lee E, Kim DH. Evaluation of clinically meaningful changes in measures of frailty. J Gerontol A Biol Sci Med Sci. 2020;75(6):1143–1147. doi: 10.1093/gerona/glaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Theou O, van der Valk AM, Godin J, et al. Exploring clinically meaningful changes for the frailty index in a longitudinal cohort of hospitalized older patients. J Gerontol A Biol Sci Med Sci. 2020;75(10):1928–1934. doi: 10.1093/gerona/glaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Condition Categories—Chronic Conditions Data Warehouse. Accessed February 24, 2022. https://www2.ccwdata.org/web/guest/condition-categories.
- 16. Stow D, Matthews FE, Barclay S, et al. Evaluating frailty scores to predict mortality in older adults using data from population based electronic health records: case control study. Age Ageing. 2018;47(4):564–569. doi: 10.1093/ageing/afy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Figueroa JF, Maddox KEJ, Beaulieu N, Wild RC, Jha AK. Concentration of potentially preventable spending among high-cost Medicare subpopulations. Ann Intern Med. 2017;167(10):706–713. doi: 10.7326/M17-0767 [DOI] [PubMed] [Google Scholar]
- 18. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi: 10.1016/s0140-6736(19)31785-4 [DOI] [PubMed] [Google Scholar]
- 19. Festa N, Shi SM, Kim DH. Accuracy of diagnosis and health service codes in identifying frailty in Medicare data. BMC Geriatr. 2020;20(1):329. doi: 10.1186/s12877-020-01739-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.