Abstract
Multiple sclerosis (MS) may impact quality of life, careers and family plans of the affected individuals. The current treatments with disease modifying therapies aim to prevent people with MS (pwMS) from disability accumulation and progression. Different countries have different reimbursement policies resulting in inequalities in patient care among geographical regions. Access to anti-CD20 therapies for relapsing MS is restricted in Hungary because therapy of individual cases only is reimbursed. In the light of the latest research and national guidelines, 17 Hungarian MS experts agreed on 8 recommendations regarding relapsing pwMS using the Delphi round method. Strong agreement (> 80%) was achieved in all except one recommendation after three rounds, which generated a fourth Delphi round. The experts agreed on treatment initiation, switch, follow-up and discontinuation, as well as on special issues such as pregnancy, lactation, elderly population, and vaccination. Well-defined national consensus protocols may facilitate dialogue between policymakers and healthcare professionals and thus contribute to better patient care in the long run.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-023-02789-0.
Keywords: Consensus, Multiple sclerosis, Recommendation, Treatment
Introduction
MS is a leading cause of disability among young adults. This chronic neurodegenerative and autoimmune disease affects patients in their productive and fertile years. MS may also have an impact on the quality of life, professional career and family planning. Cognitive and physical disability has a significant economic burden and in a worst-case scenario leads to social isolation. The prevalence of MS is rising worldwide. In Hungary, MS prevalence is estimated between 101.4-127.2/100,000 measured by two different methodological approaches [1, 2]. This is similar to the prevalence of the neighboring country Croatia (143.8/100,000) [3]. The worldwide incidence is also increasing, and the highest increase in both incidence and prevalence happened in the elderly populations, especially in women between the age of 50–60 years [4].
The current treatments with disease modifying therapies (DMTs) aim to prevent people with MS (pwMS) from disability accumulation and progression. The use of the current DMTs contributed to decreased prevalence of the late progressive course (secondary progression). The introduction of high-efficacy therapies (HET) early in the disease course may reduce, halt or even reverse disability progression [5, 6]. The difference between reimbursement policies in Denmark and Sweden led to differences in patient outcome measures favoring the start with HET over the escalation model, which starts with platform therapy and switches the treatment to higher efficacy drug when disease activity is shown [7]. Several other recent real-word studies worldwide observed similar advantages of treatment start with HET [8–10]. These data are shaping the treatment strategy of MS, moving from escalation to the early initiation with HET. Such novel strategies are reflected even in national treatment guidelines [11–13].
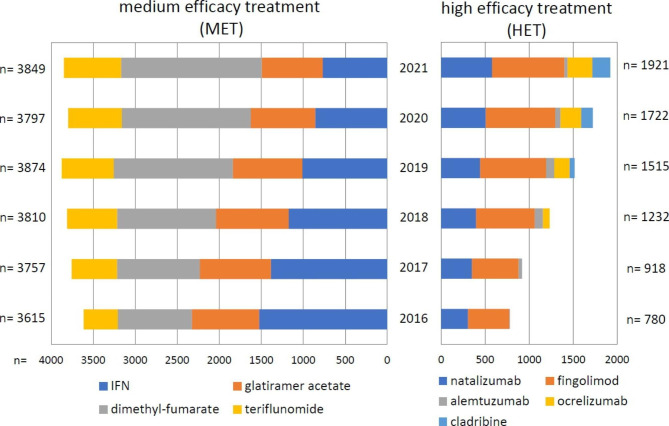
The proportion of pwMS using HET has increased in Hungary as well along with markedly increased annual cost of MS treatments (Fig. 1). Of note, the clinical benefits and reduced disease burden are expected to be seen only in the long run. The measurement of socioeconomic advantages is even more complex.
Fig. 1.
The number of medication prescription of pwMS on HET between 2016 and 2021 -based on the data from the National Health Insurance Fund [18]. The DMTs are shown in the order of their approval by the national authorities. Cladribine has been accepted since January 2020 without restriction. The reimbursement of anti-CD20 therapies are available with restriction, the only exception is ocrelizumab for primary progressive MS with unrestricted availability since January 2022. Data on DMTs n < 10, e.g. rituximab are not shown
Different countries have different reimbursement policies leading to inequalities between patient care in different geographical regions [14]. In the MS Barometer 2020, Hungary ranked in the middle range with 51 out of 100 points report [15] with relatively satisfying access to reimbursed therapies in 2018. According to the country-specific report [16], improved patient care may be achieved with the establishment of a national registry, guidelines on pediatric MS care, better access of patients to rehabilitation programs and symptomatic treatment. The report also suggested involvement of health care providers in the national reimbursement decision policies. In Hungary, treatments for pwMS approved by the European Medicines Agency (EMA) are usually available without reimbursement restrictions only by a delay of 2–4 years. During these years of restricted access, MS neurologists have to apply to the National Health Insurance Fund for reimbursement in each case. This is a time consuming process (mean evaluation time is 60 days) associated with a high administration load and high rejection rate. In Hungary, ocrelizumab is approved only for primary progressive MS, and individual applications are necessary for reimbursement in cases of relapsing MS. Of note, anti-CD20 therapies rank among the most effective therapies, especially if administered timely [17]. Lack of easily accessible anti-CD20 treatments for relapsing MS in Hungary complicates start with HET, escalation or switch strategies, especially in JCV positive patients with highly active or breakthrough disease (Fig. 1).
In 2018, the ECTRIMS guideline [19] was translated into Hungarian. The Hungarian national MS guideline was prepared in 2013, and a revised version is under development. The National Health Insurance Fund in Hungary uses a financial guideline with terms first and second line therapy, although these concepts are out of date in relation to the novel treatment strategies. Beside insufficient human resources and infrastructure [20], MS centers have to deal with restricted access to MS treatments in Hungary, which affects the quality of MS care.
The aim of our study is to highlight the required changes in Hungary and provide consensus recommendations on the treatment of relapsing MS by experts on a national level using the Delphi method.
Methods
From November 2022 to February 2023, monthly online meetings of the Committee of the Hungarian Neuroimmunology Society addressed the preparation of a state-of-art national treatment recommendation using the Delphi method to achieve consensus [21]. Beside the Committee members of the Hungarian Neuroimmunology Society, MS experts from the largest national MS centers have also been involved in the rounds. The 17 consultant neurologists involved were covering the geographical distribution of Hungary and represented the staff of the largest MS centers in Hungary. The discussion about the recommendation was shaped by the 11 Steering Committee members of the Hungarian Neuroimmunology Society actually updating the Hungarian National MS Guidelines on monthly zoom meetings starting in November 2021 until November 2022, and the first Delphi round started in November 2022.
The participants could express their agreement on a 7-point scale (strongly agree, agree, somewhat agree, neither agree or disagree, somewhat disagree, disagree, strongly disagree). Consensus was achieved by agreements of > 80% on online distributed lists of open ended recommendations in 4 rounds.
Results
Recommendation 1
DMT should be offered to all patients with relapsing MS diagnosed based on the 2017 McDonald criteria [22]. Disease activity and prognostic factors should be considered in the choice of the disease modifying drug (Table 1). High-efficacy therapy (HET) should be offered to pwMS with high disease activity and poor prognostic factors (Table 2).
Table 1.
Prognostic factors influencing MS disease outcome
| Poor prognosis | |
|---|---|
| Demographic and environmental factors |
• older age • male sex • non-White population • low vitamin D levels • smoking • comorbid conditions |
| Clinical factors |
• polysymptomatic onset • early cognitive deficits • brainstem, cerebellar or spinal cord onset • primary progressive disease subtype • poor recovery from the 1st relapse • high relapse rate • short interval between the 1st and 2nd relapses • higher EDSS score at diagnosis |
| Radiological factors |
• high T2 lesion number • high T2 lesion volume • presence of Gd-enhancing lesions • presence of infratentorial lesions • presence of spinal cord lesions • whole brain atrophy • grey matter atrophy |
| Biomarkers |
• presence of IgG and IgM oligoclonal bands in the CSF • retinal nerve fiber layer thinning detected with optical coherence tomography |
adapted from Rotstein 2019 [23]
abbreviations: CSF – cerebrospinal fluids, EDSS – expanded disability status scale, Ig – immunoglobulin
Table 2.
DMTs in the treatment of MS
| DMTs recommended for pwMS with moderate disease activity, 1st choice in escalation | dose and route of administration |
|---|---|
|
interferon-beta-1b (Betaferon) interferon-beta-1a (Avonex) interferon-beta-1a (Rebif) PEG-interferon-beta-1a (Plegridy) |
8 MIU s.c. every other day 30 ug i.m./week 44 ug s.c. 3 times/week 125 ug s.c. / 2 weeks |
|
glatiramer acetate (Copaxone) |
40 mg s.c. 3 times/week |
|
teriflunomide (Aubagio) |
14 mg/day |
|
dimethyl fumarate (Tecfidera) |
1st week 2 × 120 mg from the 2nd week 2 × 240 mg |
| diroximel fumarate (Vumerity) |
1st week 2 × 231 mg from the 2nd week 2 × 462 mg |
| DMTs recommended for pwMS with high disease activity for escalation or as 1st choice* | dose and route of administration |
| fingolimod (Gilenya) | 0.5 mg/day |
| siponimod (Mayzent) |
Depending on the CYP2C9 genotype: Genotypes *1/*1, *1/2 or *2/*2 Days 1–2: 0.25 mg Day 3: 0.5 mg Day 4: 0.75 mg, then 1 mg/day |
| ozanomid (Zeposia) |
Day 1–4: 0.23 mg Day 5–7: 0.46 mg, then 0.92 mg/day |
| ponezimod (Ponvory) |
Days 1–2: 2 mg Days 3–4: 3 mg Days 5–6: 4 mg Day 7: 5 mg Day 8: 6 mg Day 9: 7 mg. Day 10: 8 mg Day 11: 9 mg, Day 12-14: 10mg, then 20mg/day |
| cladribine (Mavenclad) |
3.5 mg/kg divided into 2 yearly courses 1 course = 2 cycles of 4-5days 23–27 days apart |
| natalizumab (Tysabri) |
300 mg i.v./month or also available in 2 × 150 mg s.c./ month |
| alemtuzumab (Lemtrada) |
2 cycles 12 months apart 1st cycle: 12 mg i.v. for 5 days 2nd cycle: 12 mg i.v. for 3 days |
| ocrelizumab (Ocrevus) | Day 1 and 15: 300 mg i.v., than every 6 months 600 mg i.v. |
| ofatumumab (Kesimpta) |
20 mg s.c. Induction: Day 1, 7 and 14: 20 mg s.c., than every 4 weeks 20 mg s.c. |
*Monoclonal antibody therapies (natalizumab, alemtuzumab, ocrelizumab and ofatumumab) are the most efficient DMTs. (based on the SmPCs of subsequent DMTs)
abbreviations: i.m. – intramuscular, i.v. – intravenous, s.c. – subcutaneous
Agreement achieved in Round 4 (88.24% agreed strongly, 5.88% agreed and 5.88% agreed somewhat).
Recommendation 2
Patients on DMTs should be seen at least every 6 months by an MS specialist in an MS center and followed at least 6-monthly by expanded disability status scale (EDSS), and at least yearly by single digit modality test, 9-hole peg test and 25-feet walking test. For specific follow up on treatments, see the summary of product characteristics.
Agreement achieved in Round 2 (100% strongly agreed).
Recommendation 3
Escalation of the therapy should be considered if the patient while on moderate efficacy therapy has a relapse and/or more than 2 new or enlarging T2 and/or Gd enhancing lesions on the follow-up annual MRI. Lateral switch is not recommended while on moderate efficacy therapy, unless the change of therapy is initiated due to side effects or intolerance or pregnancy planning.
After the 4th round 82.35% of the respondents have strongly agreed, and 17.65% agreed.
Recommendation 4
In case of signs of radiological and/or clinical disease activity while on HET, change of therapy to another HET should be considered. For wash out time see Table 3.
Table 3.
Wash out times for different DMTs
| DMT | Wash-out time |
|---|---|
| GA | not necessary |
| IFN-beta | not necessary |
| DMF | not necessary, but normalization of lymphopenia or trend of normalization is recommended |
| teriflunomide | necessary, can be shortened by cholestyramine |
| fingolimod | ≥ 4 weeks (but not exceeding 8 weeks) |
| natalizumab | ≥ 4–8 weeks (but not exceeding 8 weeks) |
| alemtuzumab/cladribine | ≥ 6–12 months |
| ocrelizumab/ofatumumab/rituximab | ≥ 3–6 months |
based on the data of Bigaut et al. 2021 [24]
abbreviations: DMT- disease modifying treatment, DMF – dimethyl fumarate, GA – glatiramer acetate, IFN- interferon,
In Round 2 the respondents answered with the following answers: 82.35% strongly agreed, 5.88 agreed.
Recommendation 5
Regardless of age, de-escalation/discontinuation of treatment needs a cautious approach. In patients with progressive disease and EDSS ≥ 7, de-escalation/discontinuation can be considered. In non-progressive disease without clinical and MRI activity for years, de-escalation/discontinuation always needs individual assessment and is not routinely recommended. This should be based on the age, adverse events, potential risks of AEs, comorbidities and their treatment, radiological and clinical activity in the preceding 5 years, the ongoing DMT (caution particularly with natalizumab and fingolimod), and the effects/AEs of previous DMTs.
Agreement achieved in Round 2 (88.24% agreed strongly and 11.76% agreed).
Recommendation 6
MS does not affect fertility; it has no adverse effect on pregnancy or neonatal outcomes. Women with family plans are advised to postpone the pregnancy until their disease is stable. Hormonal contraception is not contraindicated in MS. In case of IVF, GnRH agonists should be avoided. Both obstetric and neurological follow-up is recommended before, during and after the pregnancy. The risk of MS relapse decreases during pregnancy but increases in the first few months after birth. In case of relapses during pregnancy, indication of the corticosteroid treatment (500-1000 mg methylprednisolone (or equivalent) daily for 3–5 days) should be decided on a case-by-case basis. MRI without gadolinium contrast agent is permitted if necessary. MS professionals should consider the safety of a treatment during pregnancy when prescribing DMT to women of childbearing age. Administration of interferon-beta, glatiramer acetate, and natalizumab can be considered during pregnancy. Dimethyl fumarate and anti-CD20 therapies can be continued until pregnancy. For more details regarding DMT use in pregnancy and breastfeeding, see Table 4. During breastfeeding, short corticosteroid pulse therapy is permitted if necessary and should be decided individually.
Table 4.
DMT use and family planning
| DMT | Discontinuation before pregnancy | Use in pregnancy | Breastfeeding |
|---|---|---|---|
| glatiramer acetate | not necessary | compatible | compatible |
| INF-β | not necessary | compatible | compatible |
| dimethyl-fumarate / diroximel-fumarate | not necessary | contraindicated | contraindicated |
| teriflunomide | accelerated elimination | contraindicated | contraindicated |
| fingolimod | at least 2 months | contraindicated | contraindicated |
| siponimod | at least 10 days | contraindicated | contraindicated |
| ozanimod | at least 3 months | contraindicated | contraindicated |
| ponesimod | at least 1 week | contraindicated | contraindicated |
| cladribine | at least 6 months | contraindicated | 7 days after the drug intake breastfeeding is compatible |
| natalizumab | not necessary | compatible in very active disease; stop at 30–34 GW; extended interval dosing (6 weeks) | compatible, no interval between infusion and next breastfeeding |
| ocrelizumab | until pregnancy | contraindicated | probably compatible, wait at least 4 h after infusion |
| ofatumumab | until pregnancy | contraindicated | compatible (except the first days after birth) |
| alemtuzumab | last dose 4 months before conception | contraindicated |
probably compatible EMA: 4 months after the last infusion |
based on SMPCs and Krysko et al. 2023 [25] abbreviations: EMA – European Medicine Agency, GW – gestational week
The responses in Round 2 were strongly agree: 82.24%, agree: 5.88% and somewhat disagree 5.88%.
Recommendation 7
PwMS should be informed about optimal individualized immunization at diagnosis but latest before starting DMTs. Vaccines not containing live pathogens can be administered 3–5 days after short course of high dose corticosteroid treatment if the patient is in remission; live attenuated vaccines can be given 3 months after the steroid treatment. Ideally, vaccines not containing live pathogens should be administered at least 2–4 weeks before the start of the DMT (depending on the DMT), and the administration of the live attenuated vaccines (varicella, mumps, measles, rubella) should be completed 4–6 weeks before the start of the therapy. In case the initiation of DMT is urgent, one dosage from the varicella vaccine consisting of two dosages may be administered before the start of the therapy.
Agreement achieved in Round 2 (94.12% strongly agreed and 5.88% agreed).
Recommendation 8
The clinical phenotype of late-onset MS may be different from early-onset MS, and is characterized by higher percentage of progressive course, pyramidal and cerebellar symptoms, while sensory symptoms are less prevalent. However, patients with late-onset MS reach disability later and prognostic factors may be similar. Therefore, the type of disease course may be more important than the age of onset. In aging MS patients the clinical and radiological activity declines, while comorbidities become more common. Although treatment choice is not different in active late-onset MS or aging MS, comorbidities may be important (e.g. risk of malignancy, lymphopenia, hypogammaglobulinemia, hypertension, infection and PML).
Agreement achieved in Round 2 (94.12% strongly agreed and 5,88% agreed).
Conclusion
As our knowledge on MS pathomechanism is broadening, principles of treatment strategies change. Accordingly, treatment recommendations should be regularly updated. Several national guidelines are available with partial overlap. Definition of the disease activity and escalation strategy is somewhat different in the distinct guidelines (Table 5), probably reflecting adjustment to the different healthcare systems and additional factors at national level. Well-defined national consensus protocols may facilitate dialogue between policymakers and healthcare professionals and thus contribute to better patient care in the long run.
Table 5.
Different approaches to therapy escalation
| relapse | MRI | progression (EDSS) | remarks | |
|---|---|---|---|---|
| Rio score (21) | ≥ 1 | ≥ 3 new T2 lesions | ≥ 1 point | 〈risk if the Rio score ≥ 2 points |
| MAGNIMS score (22) | ≥ 1 | ≥ 3 new T2 lesions | - | 〈risk if the MAGNIMS score ≥ 2 points |
| NEDA 3 | ≥ 1 | ≥ 1 new T2 lesions | ≥ 1 point | better for positive predictive value |
| EAN/ECTRIMS g. 4 | ≥ 1 | 1 or 2 new T2 lesions |
if ↑ (not defined) |
combination of clinical and MRI parameters |
| AAN guideline 5 | ≥ 1 | ≥ 2 new T2 lesions |
if ↑ (not defined) |
tolerability and adherence |
| Canadian recommendation 6 |
≥ 1 major ≥ 2 minor |
≥ 3 new T2 lesions | > 1 point | close monitoring if there is any sign of disease activity |
| German recommendation 7 | ≥ 1 | ≥ 2–3 new T2 lesions | 0.5-1 point | in line with NEDA concept |
| Croatian recommendation 8 | ≥ 1 | ≥ 3 new T2 lesions | - | combination of clinical and MRI parameters |
It is important to note that the consensus recommendations are not intended to replace critical thinking or individualization of the choice of DMTs and patient care.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- CSF
cerebrospinal fluids
- DMT
disease modifying therapy
- ECTRIMS
European Committee for Research and Treatment in Multiple Sclerosis
- EDSS
expanded disability status scale
- EMA
European Medicines Agency
- GW
gestational week
- Ig
immunoglobulin
- i.m.
intramuscular
- i.v.
intravenous
- MS
multiple sclerosis
- pwMS
people with MS
- s.c.
subcutaneous
Author contributions
Study concept and design: ZI, CsR, GL, TC, ZM, AM and CR. CR,CsR, AM, GL, ZM, GJ, PÁ, GR, MS, ZJ, ZB, AT, IP, KM, ID, ZI and TC took part in the Delphi survey. Analysis and interpretation of data: CR, ZI, CsR, AM, GL, TC, GJ. CR drafted the manuscript, which was revised by TC and ZI. CR, CsR, AM, GL, ZM, GJ, PÁ, GR, MS, ZJ, ZB, AT, IP, KM, ID, ZI and TC commented on and approved the final form. All authors read and approved the final manuscript.
Funding
University of Szeged Open Access Fund Grant number 6071.
Open access funding provided by University of Szeged.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
CR participated as clinical investigator and/or received consultation and/or speaker fees and/or conference travel grants from: Biogen, Merck, Novartis, Sanofi Genzyme, Roche, Teva. IZ has served on scientific advisory boards, received support for congress participation, speaker honoraria, or for being member of clinical endpoint committees in clinical trials, and received research support for his laboratory from Biogen, Merck, Roche, Sanofi Genzyme, Novartis, Alexion, and Bristol Myers Squibb. CsR received speaker fees, conference honoraria from Biogen, Novartis, Merck, Roche, Sanofi.
AM participated as clinical investigator and/or received consultation and/or speaker fees and/or conference travel grants from Biogen, Merck, Novartis, Sanofi Genzyme, Roche, Teva. GL received speaker fees and honoraria form Biogen, Novartis, Merck, Roche, Sanofi. ZM received speaker fees and conference/travel grants from: Biogen, Merck, Novartis, Roche and Sanofi Genzyme. ÁP received received investigator and speaker fees from: Sanofi Genzyme, Roche. GJ, GR, AT, KM and ID reported no conflict of interest. MS participated as clinical investigator and received consultation / speaker fees and conference travel grants from: Biogen, Merck, Novartis, Sanofi Genzyme, Roche, Teva. ZJ received consultation fees, speaker fees and conference travel grants from: Biogen, Merck, Novartis, Sanofi Genzyme, Roche. ZB received congress support from Biogen, Merck, Novartis, Sanofi-Genzyme and Roche. IP participated as clinical investigator, and received consultation, and speaker fees, and conference travel grants from: Biogen, Merck, Novartis, Sanofi Genzyme. TC has received personal honoraria for speaking, advisory boards and travel expenses from Biogen, Novartis, Roche, Sanofi-Genzyme, Teva.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iljicsov A, Milanovich D, Ajtay A, Oberfrank F, Bálint M, Dobi B, Bereczki D, Simó M. Incidence and prevalence of multiple sclerosis in Hungary based on record linkage of nationwide multiple healthcare administrative data. PLoS ONE. 2020;15:e0236432. doi: 10.1371/journal.pone.0236432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biernacki T, Sandi D, Fricska-Nagy Z, Kincses ZT, Füvesi J, Laczkó R, Kokas Z, Klivényi P, Vécsei L, Bencsik K. Epidemiology of multiple sclerosis in Central Europe, update from Hungary. Brain Behav. 2020;10:e01598. doi: 10.1002/brb3.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjak T, Štefančić V, Draušnik Ž, Cerovečki I, Roginić D, Habek M, et al. Prevalence of multiple sclerosis in Croatia: data from national and non-governmental organization registries. Croat Med J. 2018;59:65–70. doi: 10.3325/cmj.2018.59.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentzen J, Flachs EM, Stenager E, Brønnum-Hansen H, Koch-Henriksen N. Prevalence of multiple sclerosis in Denmark 1950–2005. Mult Scler. 2010;16:520–5. doi: 10.1177/1352458510364197. [DOI] [PubMed] [Google Scholar]
- 5.Kalincik T, Diouf I, Sharmin S, Malpas C, Spelman T, Horakova D, Havrdova EK, Trojano M, Izquierdo G, Lugaresi A, Prat A, Girard M, Duquette P, Grammond P, Jokubaitis V, van der Walt A, Grand’Maison F, Sola P, Ferraro D, Shaygannejad V, Alroughani R, Hupperts R, Terzi M, Boz C, Lechner-Scott J, Pucci E, Van Pesch V, Granella F, Bergamaschi R, Spitaleri D, Slee M, Vucic S, Ampapa R, McCombe P, Ramo-Tello C, Prevost J, Olascoaga J, Cristiano E, Barnett M, Saladino ML, Sanchez-Menoyo JL, Hodgkinson S, Rozsa C, Hughes S, Moore F, Shaw C, Butler E, Skibina O, Gray O, Kermode A, Csepany T, Singhal B, Shuey N, Piroska I, Taylor B, Simo M, Sirbu CA, Sas A, Butzkueven H. MSBase Study Group. Effect of Disease- modifying therapy on disability in relapsing-remitting multiple sclerosis over 15 years. Neurology. 2021;96:e783–e97. doi: 10.1212/WNL.0000000000011242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JWL, Coles A, Horakova D, Havrdova E, Izquierdo G, Prat A, Girard M, Duquette P, Trojano M, Lugaresi A, Bergamaschi R, Grammond P, Alroughani R, Hupperts R, McCombe P, Van Pesch V, Sola P, Ferraro D, Grand’Maison F, Terzi M, Lechner-Scott J, Flechter S, Slee M, Shaygannejad V, Pucci E, Granella F, Jokubaitis V, Willis M, Rice C, Scolding N, Wilkins A, Pearson OR, Ziemssen T, Hutchinson M, Harding K, Jones J, McGuigan C, Butzkueven H, Kalincik T, Robertson N. MSBase Study Group. Association of initial disease-modifying therapy with later Conversion to secondary progressive multiple sclerosis. JAMA. 2019;321:175–87. doi: 10.1001/jama.2018.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spelman T, Magyari M, Piehl F, Svenningsson A, Rasmussen PV, Kant M, Sellebjerg F, Joensen H, Hillert J, Lycke J. Treatment escalation vs Immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol. 2021;78:1197–204. doi: 10.1001/jamaneurol.2021.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buron MD, Chalmer TA, Sellebjerg F, Barzinji I, Christensen JR, Christensen MK, Hansen V, Illes Z, Jensen HB, Kant M, Papp V, Petersen T, Rasmussen PV, Schäfer J, Theódórsdóttir Á, Weglewski A, Sorensen PS, Magyari M. Initial high-efficacy disease-modifying therapy in multiple sclerosis: a nationwide cohortstudy. Neurology. 2020;95:e1041–51. doi: 10.1212/WNL.0000000000010135. [DOI] [PubMed] [Google Scholar]
- 9.Filippi M, Danesi R, Derfuss T, Duddy M, Gallo P, Gold R, Havrdová EK, Kornek B, Saccà F, Tintoré M, Weber J, Trojano M. Early and unrestricted access to high-efficacy disease-modifying therapies: a consensus to optimize benefits for people living with multiple sclerosis. J Neurol. 2022;269:1670–7. doi: 10.1007/s00415-021-10836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montalban X, Matthews PM, Simpson A, Petrie JL, Sammon C, Ramagopalan S, Disanto G, Kuhle J. Real-world evaluation of ocrelizumab in multiple sclerosis: a systematic review. Ann Clin Transl Neurol. 2023;2. 10.1002/acn3.51732. Epub ahead of print. PMID: 36728340. [DOI] [PMC free article] [PubMed]
- 11.Freedman MS, Devonshire V, Duquette P, Giacomini PS, Giuliani F, Levin MC, Montalban X, Morrow SA, Oh J, Rotstein D, Yeh EA, Canadian MS, Working Group Treatment optimization in multiple sclerosis: canadian MS Working Group Recommendations. Can J Neurol Sci. 2020;47:437–55. doi: 10.1017/cjn.2020.66. [DOI] [PubMed] [Google Scholar]
- 12.Wiendl H, Gold R, Berger T, Derfuss T, Linker R, Mäurer M, Aktas O, Baum K, Berghoff M, Bittner S, Chan A, Czaplinski A, Deisenhammer F, Di Pauli F, Du Pasquier R, Enzinger C, Fertl E, Gass A, Gehring K, Gobbi C, Goebels N, Guger M, Haghikia A, Hartung HP, Heidenreich F, Hoffmann O, Kallmann B, Kleinschnitz C, Klotz L, Leussink VI, Leutmezer F, Limmroth V, Lünemann JD, Lutterotti A, Meuth SG, Meyding-Lamadé U, Platten M, Rieckmann P, Schmidt S, Tumani H, Weber F, Weber MS, Zettl UK, Ziemssen T, Zipp F. Multiple sclerosis therapy Consensus Group’ (MSTCG). Multiple sclerosis therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper) Ther Adv Neurol Disord. 2021;14:17562864211039648. doi: 10.1177/17562864211039648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habek M, Adamec I, Barun B, Bašić Kes V, Bogoje Raspopović A, Duka Glavor K, et al. Treatment of relapsing multiple sclerosis - recommendations of the croatian neurological society. Croat Med J. 2022;63:379–88. doi: 10.3325/cmj.2022.63.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coetzee T, Thompson AJ, Atlas of MS. 2020: Informing global policy change. Mult Scler J 2020;26:1807–8. 10.1177/1352458520968811. [DOI] [PubMed]
- 15.https://www.emsp.org/wp-content/uploads/2021/03/MS-Barometer2020-Final-Full-Report-Web.pdf, accessed 3rd November 2022.
- 16.https://www.healthpolicypartnership.com/app/uploads/MS-Barometer-factsheet-Hungary.pdf, accessed 3rd November 2022.
- 17.Samjoo IA, Worthington E, Drudge C, Zhao M, Cameron C, Häring DA, Stoneman D, Klotz L, Adlard N. Comparison of ofatumumab and other disease-modifying therapies for relapsing multiple sclerosis: a network meta-analysis. J Comp Eff Res. 2020;9:1255–74. doi: 10.2217/cer-2020-0122. [DOI] [PubMed] [Google Scholar]
- 18.http://www.neak.gov.hu/felso_menu/szakmai_oldalak/publikus_forgalmi_adatok/gyogyszer_forgalmi_adatok accessed 3rd November 2022.
- 19.Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler J. 2018;24:96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 20.Kokas Z, Sandi D, Fricska-Nagy Z, Füvesi J, Biernacki T, Köves Á, Fazekas F, Birkás AJ, Katona G, Kovács K, Milanovich D, Dobos E, Kapás I, Jakab G, Csépány T, Bense E, Mátyás K, Rum G, Szolnoki Z, Deme I, Jobbágy Z, Kriston D, Gerócs Z, Diószeghy P, Bors L, Varga A, Kerényi L, Molnár G, Kristóf P, Nagy Z, Sátori M, Imre P, Péntek S, Klivényi P, Kincses ZT, Vécsei L, Bencsik K. Do hungarian multiple sclerosis care units fulfil international criteria? PLoS ONE. 2022;17:e0264328. doi: 10.1371/journal.pone.0264328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu CC, Sandford B. The Delphi technique: making sense of Consensus. Practical Assess Res Evaluation. 2007;12:1–8. [Google Scholar]
- 22.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoré M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–73. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 23.Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol. 2019;15:287–300. doi: 10.1038/s41582-019-0170-8. [DOI] [PubMed] [Google Scholar]
- 24.Bigaut K, Cohen M, Durand-Dubief F, Maillart E, Planque E, Zephir H, Lebrun-Frenay C, de Seze J. French Group for Recommendations in multiple sclerosis (France4MS) and the Société Française de la Sclérose En plaques (SFSEP). How to switch disease-modifying treatments in multiple sclerosis: guidelines from the french multiple sclerosis society (SFSEP) Mult Scler Relat Disord. 2021;53:103076. doi: 10.1016/j.msard.2021.103076. [DOI] [PubMed] [Google Scholar]
- 25.Krysko KM, Dobson R, Alroughani R, Amato MP, Bove R, Ciplea AI, Fragoso Y, Houtchens M, Jokubaitis VG, Magyari M, Abdelnasser A, Padma V, Thiel S, Tintore M, Vukusic S, Hellwig K. Family planning considerations in people with multiple sclerosis. Lancet Neurol. 2023;22:350–66. doi: 10.1016/S1474-4422(22)00426-4. [DOI] [PubMed] [Google Scholar]
- 26.Río J, Comabella M, Montalban X. Predicting responders to therapies for multiple sclerosis. Nat Rev Neurol., Castilló J, Rovira A, Tintoré M, Sastre-Garriga J, Horga A, Nos C, Comabella M, Aymerich X, Montalbán X. Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler. 2009;15:848 – 53. [DOI] [PubMed]
- 27.Sormani MP, Gasperini C, Romeo M, Rio J, Calabrese M, Cocco E, Enzingher C, Fazekas F, Filippi M, Gallo A, Kappos L, Marrosu MG, Martinelli V, Prosperini L, Rocca MA, Rovira A, Sprenger T, Stromillo ML, Tedeschi G, Tintorè M, Tortorella C, Trojano M, Montalban X, Pozzilli C, Comi G, De Stefano N. MAGNIMS study group. Assessing response to interferon-β in a multicenter dataset of patients with MS. Neurology. 2016;87:134–40. doi: 10.1212/WNL.0000000000002830. [DOI] [PubMed] [Google Scholar]
- 28.Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015;72:152–8. doi: 10.1001/jamaneurol.2014.3537. [DOI] [PubMed] [Google Scholar]
- 29.Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, Haboubi M, Halper J, Hosey JP, Jones DE, Lisak R, Pelletier D, Potrebic S, Sitcov C, Sommers R, Stachowiak J, Getchius TSD, Merillat SA, Pringsheim T. Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:789–800. Erratum in: Neurology. 2019 Oct 22;93(17):769. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.