Abstract
Background
Recent studies have shown that aspirin consumption may reduce the risk of hepatocellular carcinoma (HCC), but their correlation is still not fully understood. This meta-analysis aimed to investigate the correlation between aspirin consumption and HCC.
Methods
A systematic literature search was conducted on PubMed, Scopus, Cochrane Library, EMBASE, and Web of Science databases. The search period was from the establishment of the database to July 1, 2022 with no language restrictions.
Results
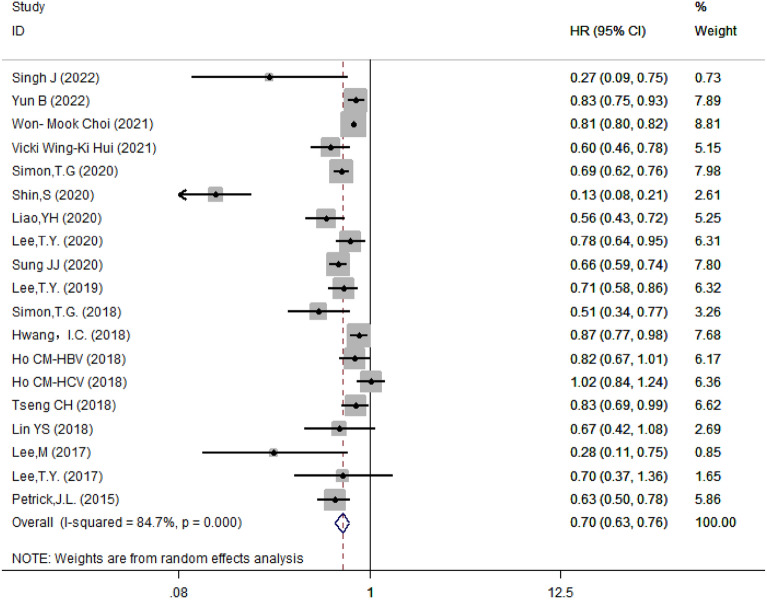
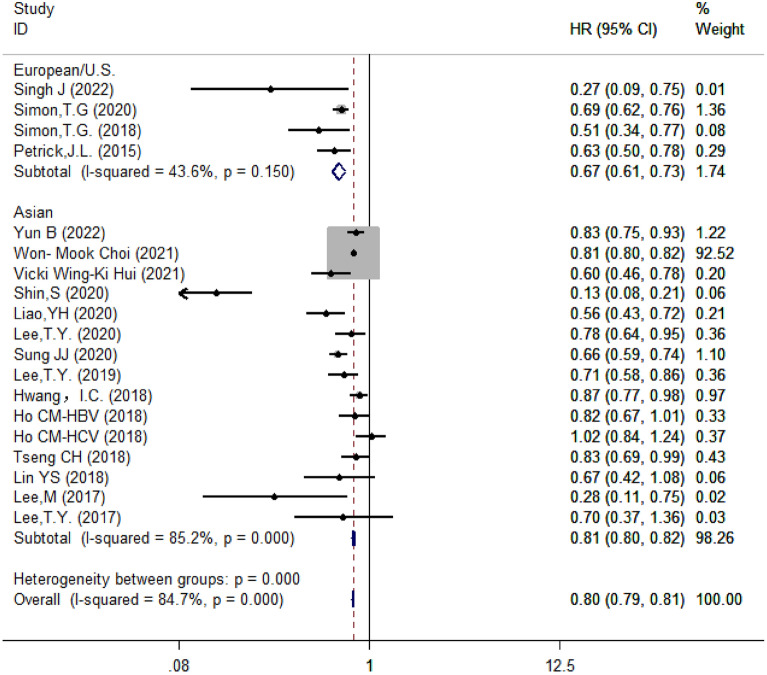
A total of 19 studies including three prospective studies and 16 retrospective ones with 2,217,712 patients were included. Compared with those who did not take aspirin, those who took aspirin had a 30% lower risk of HCC (hazard ratio [HR] = 0.70, 95% confidence interval [CI] 0.63–0.76, I2 = 84.7%, P < 0.001). Subgroup analysis showed that aspirin significantly reduced the risk of HCC by 19% in Asia (HR = 0.81, 95% CI 0.80–0.82, I2 = 85.2%, P < 0.001) and by 33% (HR = 0.67, 95% CI 0.61–0.73, I2 = 43.6%, P = 0.150) in Europe and the U.S with no significant difference. Moreover, in patients with HBV or HCV infection, aspirin reduced 19% and 24% of the risk of HCC, respectively. However, aspirin administration might increase risks of gastrointestinal bleeding in patients with chronic liver disease (HR = 1.14, 95% CI 0.99–1.31, I2 = 0.0%, P = 0.712). Sensitivity analysis showed no significant difference of results after excluding individual studies, suggesting that the results were robust.
Conclusion
Aspirin may reduce the risk of HCC in both healthy population and patients with chronic liver disease. However, attention should be paid to adverse events such as gastrointestinal bleeding in patients with chronic liver disease.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-023-01204-5.
Keywords: Aspirin, Hepatocellular carcinoma, Meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy that accounts for 75–85% of liver cancers. Patients with HCC have poor prognoses and the onset of HCC is insidious. Risk factors of HCC include chronic liver inflammation and liver fibrosis or cirrhosis. In recent years, the global incidence of HCC has increased and it is estimated that more than 1 million patients will die from HCC in 2030.
Most HCC patients don’t have the opportunity for surgery because of advanced stages. Only a small number of patients that are diagnosed at an early stage can receive radical resection [2]. Other therapies such as transcatheter arterial chemoembolization, targeted therapy, or immunotherapy also had limited efficacy [3]. Therefore, there is an urgent need for effective therapies to control the progression of HCC.
Aspirin is a classical non-steroidal anti-inflammatory drug (NSAIDs) that exerts antiplatelet effects by acetylating platelet cyclooxygenase (COX), thereby reducing the risk of myocardial infarction and stroke [4]. Therefore, it is widely used in the prevention and treatment of cardiovascular diseases. In the last few decades, much evidence has shown the potential of aspirin in the prevention and treatment of cancer [5–7]. A randomized trial including 25,570 participants showed that aspirin reduced the risk of death from gastrointestinal cancers, including esophageal, colorectal, and pancreatic cancers, as well as from non-gastrointestinal solid cancers, such as lung, prostate, bladder, and kidney cancers, especially colorectal cancers [8]. Studies have demonstrated that aspirin can reduce the risk of HCC, whereas non-aspirin NSAID does not have this effect [9, 10]. Moreover, compared with irregular aspirin administration, the risk-reducing effect of aspirin is dose- and duration dependent [6]. In in vitro and in vivo experiments, the combination of aspirin and other drugs exhibited a promising efficacy to suppress HCC progression [11–14].
However, a randomized controlled trial showed that long-term administration of low-dose aspirin did not reduce the risk of overall cancer, breast cancer, colorectal cancer, or other site-specific cancers [15]. Therefore, it remains controversial whether aspirin can reduce the risk of HCC. This study aimed to investigate the association between aspirin use and HCC through a meta-analysis.
Materials and methods
Literature retrieval strategy
A systematic literature search was conducted on PubMed, Scopus, Cochrane Library, EMBASE and Web of Science databases from the establishment of the database to July 1, 2022, with no language restrictions. A combination of subject terms and free words were used for literature search using Boolean logic operator grouping (Additional file 1).
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) retrospective or prospective cohort studies that explored the relationship between aspirin and the risk of HCC (from 2015 to 2022); (2) reporting the hazard ratio (HR) and 95% confidence interval (CI) of HCC based on different administration of aspirin; (3) the diagnosis of HCC was made based on the diagnostic criteria. Exclusion criteria were as follows: (1) reviews, case reports, meta-analyses, conference abstracts, redundant publications, ecological studies, animal studies, and case–control studies; (2) studies on patients who have been diagnosed with HCC before aspirin use; (3) studies that did not explicitly report any HR or 95% CI.
Evaluation of literature quality
The Newcastle–Ottawa scale (NOS) was used to evaluate literature quality in terms of study population selection, comparability between groups, and outcome measures, with a maximum score of 9. We included high-quality literature with scores greater than or equal to 7 in this study.
Data extraction
Two investigators independently screened the literature and extracted data according to the criteria. In case of disagreements, they discussed or consulted a third party to reach the consensus. During literature screening, the title was first read to exclude irrelevant literature, then the abstract and full text were read to include relevant studies. Corresponding authors were contacted by email to obtain necessary information if needed. The extracted information included (1) first author, year of publication, study region, and study type; (2) study population and whether they had liver disease; (3) the number of participants and cases; (4) reported outcomes, including HR and 95% CI and (5) study-adjusted confounding factors, etc.
Statistical analysis
Stata 14.2 software was used for meta-analysis, and the statistics were presented by HR and 95% CI, with a preference for the corrected values if they were provided in the literature. The Cochran q test (P heterogeneity) and the I2 value were used to evaluate the heterogeneity of the included studies. P ≥ 0.05, 0 ≤ I2 ≤ 50% indicated low heterogeneity, and a fixed effect model was used for analysis; P < 0.05 and I2 > 50% indicated significant heterogeneity, so that a random effect model was used to identify the source of heterogeneity and perform subgroup and sensitivity analyses. Publication bias was evaluated using a funnel plot, Beggs test, and Eggers. P < 0.05 indicated that the difference was statistically significant.
Results
Literature screening process
We retrieved 35, 221, 103, 419, and 19 papers from Pubmed, Scopus, Web of Science, EMBASE, and Cochrane Library, respectively. 240 duplicate papers were excluded. After reading the titles and abstracts and excluding irrelevant literature such as reviews, case reports, meta-analyses, conference abstracts, animal experimental studies, 49 articles were selected for full-text reading. Finally, 18 articles with a total of 2,217,712 participants were enrolled based on the criteria. The literature screening process is shown in Fig. 1.
Fig. 1.
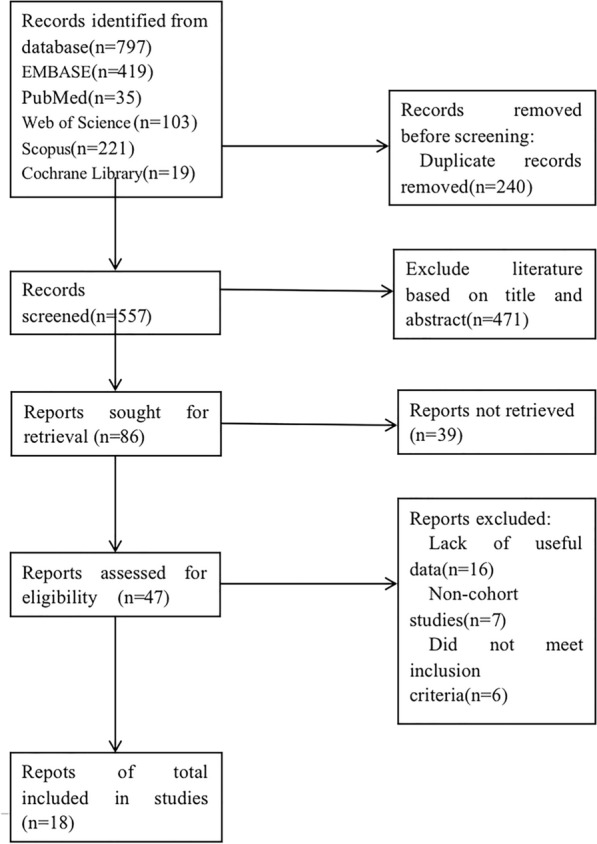
Literature selection process
Basic characteristics and quality evaluation of the included literature
This article included 18 articles containing 19 studies published from 2015 to 2022, in which 13 studies focused on patients with liver disease, 4 studies focused on general population, and the remaining 2 studies focused on specific patients with type 2 diabetes or head and neck squamous cell carcinoma (HNSCC); 3 were prospective studies and 16 were retrospective studies (Table 1). Based on NOS results, all included literature had high quality (Table 2).
Table 1.
Basics features of the included literature
| Author | Year | Region | Type of Research | Number of participants | Number of cases | Study population | HR(95% CI) | Confounding factors for adjustment |
|---|---|---|---|---|---|---|---|---|
| Yun B [16] | 2022 | Korea | RC | 161673 | 7083 | HBV-infected patients | 0.83 (0.75–0.93) | Age, sex, hypertension, diabetes mellitus, dyslipidemia, cirrhosis, antivirals, metformin, statin, smoking, alcohol consumption, and obesity |
| Singh J [17] | 2022 | USA | RC | 521 | 45 | Liver cirrhosis | 0.266 (0.094–0.755) | Age, sex, and CTP in the multivariable model |
| Won- Mook Choi [18] | 2021 | Korea | RC | 32695 | 6539 | HBV-infected patients | 0.81 (0.80–0.82) | Age, gender, socioeconomic status, diabetes mellitus, hypertension, smoking, alcohol consumption, BMI, ALT, and combination medication |
| Vicki Wing-Ki Hu [19] | 2021 | Hong Kong | RC | 35111 | 1557 | HBV-infected patients | 0.60 (0.46–0.78) | Age, gender, cirrhosis, hypertension, renal replacement therapy, creatinine, diabetes mellitus, platelet, albumin, total bilirubin, ALT, HBeAg + |
| Simon,T.G [20] | 2020 | Sweden | PC | 50275 | 1612 | HBV and HCV infected patients | 0.69 (0.62–0.76) | Gender, consecutive years since diagnosis of hepatitis B or C, severity of liver disease, use of antiviral therapy, presence or absence of diabetes, hypertension, obesity or alcohol abuse, misuse, and use of insulin, metformin and statins |
| Shin,S [21] | 2020 | Korea | RC | 949 | 133 | Alcoholic cirrhosis | 0.13 (0.08–0.21) | Age, gender, Child–Pugh score, MELD score, AST, ALT, albumin, total bilirubin, creatinine, INR and platelet count |
| Liao YH [22] | 2020 | Taiwan | RC | 3822 | 278 | HCV- infected patients | 0.56 (0.43–0.72) | Gender, age, hypertension, diabetes, moderate to severe liver disease, myocardial infarction, congestive heart failure, ischemic stroke, antihypertensives, hypoglycemic agents, coumarins and heparins, other antithrombotic drugs and NSAIDs |
| Lee,T.Y [23] | 2020 | Taiwan | RC | 7434 | 436 | HCV- infected patients | 0.78 (0.64–0.95) | Age, gender, cirrhosis, hepatic decompensation, hyperlipidemia, statin use and interferon therapy |
| Sung JJ [24] | 2020 | Hong Kong | RC | 138966 | 751 | General population | 0.66 (0.59–0.74) | Age, gender, other medications (including H2 antagonists, statins, NSAIDs, and anticoagulants), comorbidities (coronary artery disease, stroke) |
| Lee,T.Y [25] | 2019 | Taiwan | RC | 10615 | 697 | HBV-infected patients | 0.71 (0.58–0.86) | Age, gender, cirrhosis, diabetes, hyperlipidemia, hypertension, statin use, metformin use, and nucleic acid analogue use |
| Simon,T.G [6] | 2018 | USA | PC | 133371 | 108 | General population | 0.51 (0.34–0.77) | Gender, age, race/ethnicity, body mass index, alcohol consumption, smoking status, physical activity, diabetes, hypertension, dyslipidemia, regular use of multivitamins, antidiabetic drugs, statins, routine use of non-aspirin NSAIDs |
| Hwang,I.C [26] | 2018 | Korea | RC | 460755 | 2336 | General population | 0.87 (0.77–0.98) | Age, sex, body mass index, smoking, alcohol consumption, physical activity, concomitant medication, blood pressure category, fasting glucose and total cholesterol, socioeconomic status, and CCI |
| Ho CM- HBV [27] | 2018 | Taiwan | RC | 7724 | 552 | HBV-infected patients | 0.82 (0.67–1.01) | Gender, age, low income, cirrhosis, diabetes, hyperlipidemia, malignancies other than hepatocellular carcinoma, COPD, ESRD, transplantation, alcohol consumption, and concomitant use of ACEIs/ARBs, metformin, and statins |
| Ho CM- HCV [27] | 2018 | Taiwan | RC | 7873 | 503 | HCV- infected patients | 0.55 (0.23–1.28) | Gender, age, low income, cirrhosis, diabetes, hyperlipidemia, malignancies other than hepatocellular carcinoma, COPD, ESRD, transplantation, alcohol consumption, and concomitant use of ACEIs/ARBs, metformin, and statins |
| Tseng CH [28] | 2018 | Taiwan | RC | 43800 | 1750 | Patients with type 2 diabetes | 0.83 (0.69–0.99) | Age, sex, occupation and area of residence, major comorbidities (hypertension, dyslipidemia and obesity), diabetes-related complications, use of antidiabetic medications (insulin, sulfonylureas, meglitinides, acarbose, rosiglitazone and pioglitazone), potential risk factors for cancer (chronic obstructive pulmonary disease, tobacco abuse, alcohol-related diagnoses, gallstones, history of Helicobacter pylori infection, EB virus-related diagnoses, hepatitis B virus infection, hepatitis C virus infection, cirrhosis and other chronic non-alcoholic liver disease) and medications commonly used or that may affect cancer risk in patients with diabetes (ACEI/ARB, calcium channel blockers, statins, betablockers and aspirin) |
| Lin YS [29] | 2018 | Taiwan | RC | 18243 | 110 | HNSCC | 0.67 (0.42–1.08) | Age, gender, urbanization, coronary artery disease, hypertension, diabetes, atrial fibrillation, heart failure, hyperlipidemia, chronic kidney disease, and COX2 and statin use |
| Lee, M [30] | 2017 | Korea | RC | 1674 | 63 | HBV-infected patients | 0.28 (0.11–0.75) | Age, sex, diabetes, cirrhosis, Child–Pugh score, MELD score, HBeAg, ALT, albumin, total bilirubin, Scr, PT and platelet count |
| Lee, T.Y [31] | 2017 | Taiwan | RC | 18080 | 41 | NAFLD | 0.70 (0.37–1.36) | Age, gender, ALT elevation, hypertension, hypercholesterolemia, diabetes, gout, statin use, metformin use |
| Petrick, J.L [32] | 2015 | USA | PC | 1084133 | 679 | General population | 0.63 (0.50–0.78) | Gender, age, race, cohort, BMI, smoking status, alcohol consumption |
HCC hepatocellular carcinoma, TACE transcatheter arterial chemoembolization, NSAIDs, nonsteroidal anti-inflammatory drug, COX cyclooxygenase, MI myocardial infarction, NOS Newcastle–Ottawa scale, HNSCC head and neck squamous cell carcinoma, PC prospective cohort, RC retrospective cohort, HR hazard ratio, BMI body mass index, AST aspartate aminotransferase, ALT alanine aminotransferase, INR international normalized ratio, PT prothrombin time, MELD Model for End-Stage Liver Disease, CCI Chronic Coronary Insufficiency, COPD chronic obstructive pulmonary disease, ESRD end-stage renal disease
Table 2.
Literature quality evaluation
| Inclusion in the literature | Crowd selection | Comparability between group | Measurement results | NOS score |
|---|---|---|---|---|
| Yun B [16] | 4 | 2 | 3 | 9 |
| Singh J [17] | 4 | 1 | 3 | 8 |
| Won- Mook Choi [18] | 4 | 2 | 2 | 8 |
| Vicki Wing-Ki Hui [19] | 4 | 2 | 3 | 9 |
| Simon, T.G. [20] | 4 | 2 | 3 | 9 |
| Shin, S [21] | 4 | 1 | 3 | 8 |
| Liao YH [22] | 4 | 2 | 3 | 9 |
| Lee, T.Y. [23] | 4 | 2 | 3 | 9 |
| Sung JJ [24] | 4 | 2 | 3 | 9 |
| Lee, T.Y. [25] | 4 | 2 | 3 | 9 |
| Simon, T.G. [6] | 4 | 2 | 3 | 9 |
| Hwang, I.C. [26] | 4 | 2 | 3 | 9 |
| Ho CM-HBV [27] | 4 | 2 | 3 | 9 |
| Ho CM-HCV [27] | 4 | 2 | 3 | 9 |
| Tseng CH [28] | 4 | 2 | 3 | 9 |
| Lin YS [29] | 3 | 2 | 2 | 7 |
| Lee, M [30] | 4 | 1 | 3 | 8 |
| Lee, T.Y. [31] | 4 | 2 | 3 | 9 |
| Petrick, J.L. [32] | 4 | 2 | 3 | 9 |
Aspirin was associated with reduced risk of HCC
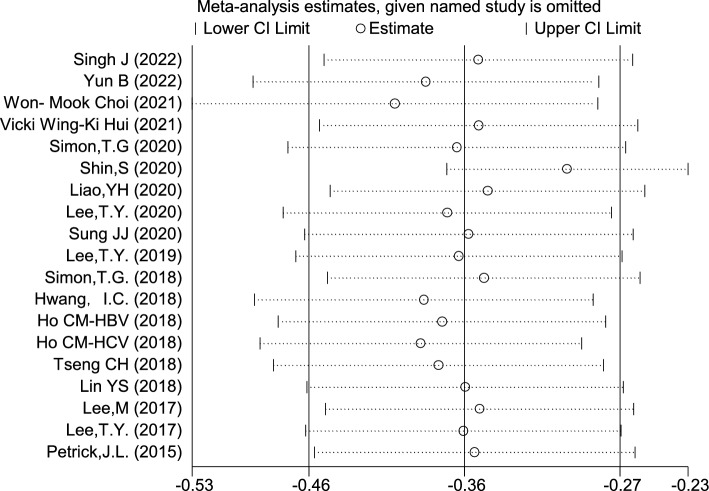
The 19 included studies showed a high heterogeneity, therefore, random effect model was adopted and showed that the risk of HCC was 30% lower in those taking aspirin compared with those not taking aspirin (HR = 0.70, 95% CI 0.63–0.76, I2 = 84.7%, P < 0.001) (Fig. 2). The results showed that there was no significant difference of HR after excluding individual studies (Fig. 3), suggesting that the results were robust.
Fig. 2.
Forest plot of correlation between aspirin and the risk of hepatocellular carcinoma
Fig. 3.
Sensitivity analysis results
Subgroup analysis of the association between aspirin and the risk of HCC
Subgroup analysis based on regions
Since aspirin and the risk of HCC. The studies were divided into two subgroups based on regions: the Asia group contained 15 studies whereas the Europe and the United States group had 4 studies. The results showed that aspirin reduced the risk of HCC by 19% in Asia (HR = 0.81, 95% CI 0.80–0.82, I2 = 85.2%, P < 0.001) and by 33% in Europe and the U.S. (HR = 0.67, 95% CI 0.61–0.73, I2 = 43.6%, P = 0.150) (Fig. 4).
Fig. 4.
Subgroup analysis of forest plots based on regions
Subgroup analysis based on study design
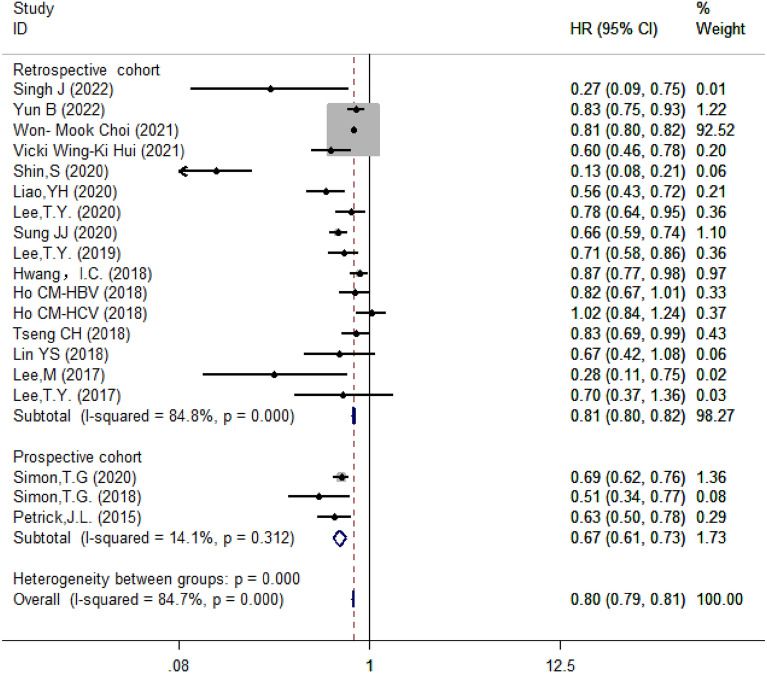
Moreover, based on study design, these studies were classified into prospective (n = 3) and retrospective (n = 16) studies. The results showed that aspirin reduced the risk of HCC in the retrospective studies (HR = 0.81, 95% CI 0.80–0.82, I2 = 84.8%, P < 0.001) (Fig. 5).
Fig. 5.
Subgroup analysis of forest plots based on study design
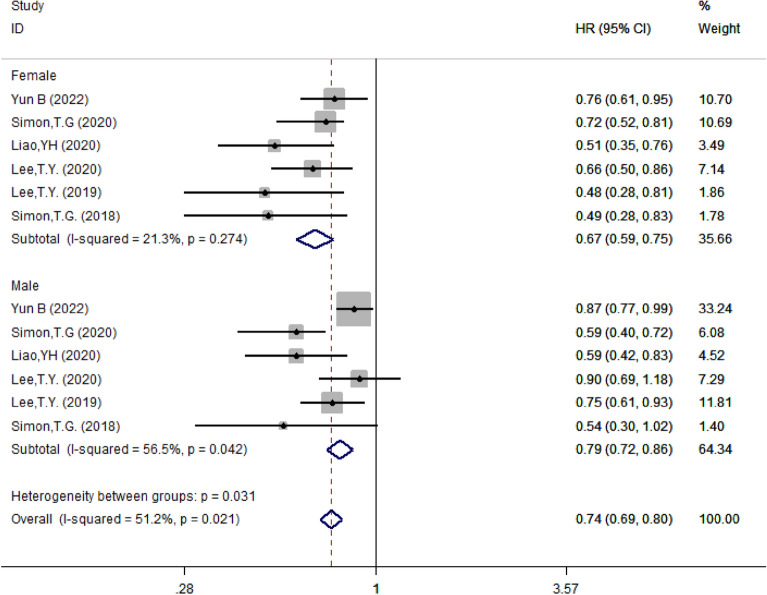
Subgroup analysis based on gender
According to genders, studies were divided into female and male groups, with a total of five papers reporting the HR for both female and male. The results showed that aspirin reduced the risk of HCC by 33% in female (HR = 0.67, 95% CI 0.59–0.75, I2 = 21.3%, P = 0.274) and by 21% in male (HR = 0.79, 95% CI 0.72–0.86, I2 = 56.5%, P = 0.042) (Fig. 6).
Fig. 6.
Subgroup analysis of forest plots based on gender
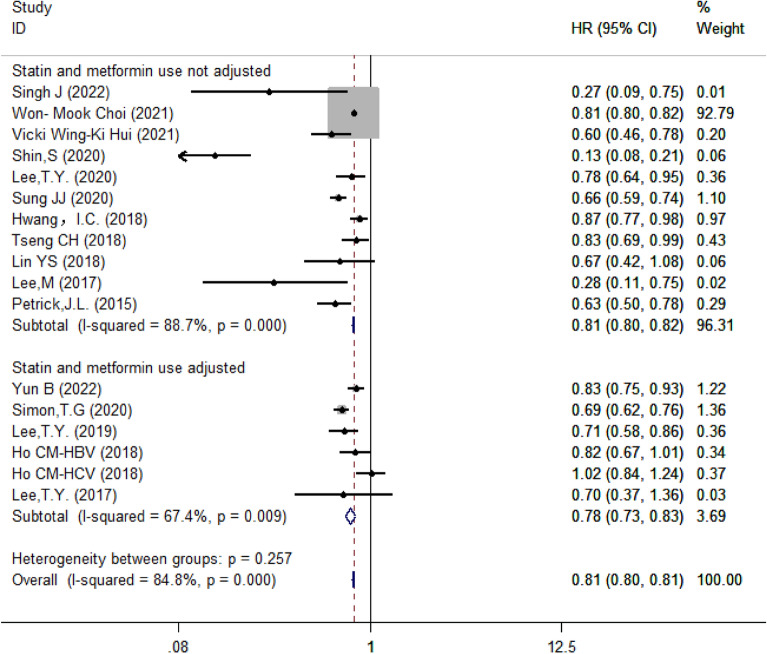
Subgroup analysis based on whether results were adjusted by statins and metformin
Then we categorized studies according to whether the results were adjusted by statins and metformin. There were 5 papers with 6 studies adjusted results for both statins and metformin and 11 papers without adjustment. The remaining two papers only adjusted results for glucose-lowering drugs, so that they were excluded. The results showed that aspirin was associated with reduced risks of HCC when the results were adjusted for statins and metformin (HR = 0.78, 95% CI 0.73–0.83, I2 = 67.4%, P = 0.009) or not (HR = 0.81, 95% CI 0.80–0.82, I2 = 88.7%, P < 0.001); however, there was no no significant difference between the two groups (P = 0.257) (Fig. 7).
Fig. 7.
Subgroup analysis of forest plots depending on whether statins and metformin were adjusted
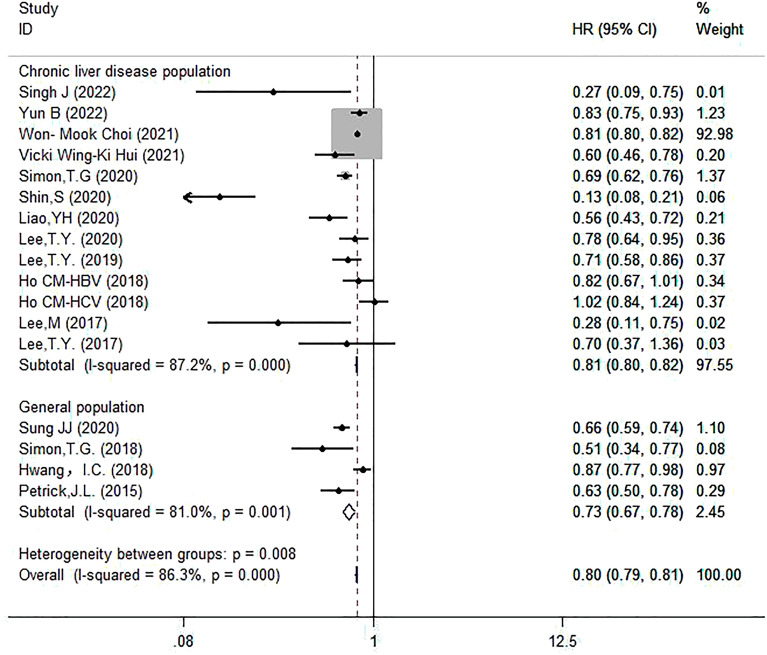
Subgroup analysis based on study population
Based on different populations, divided into two subgroups: patients with chronic liver disease (n = 13) and general population (n = 4). The remaining two studies were excluded. The results showed that aspirin reduced the risk of HCC in patients with chronic liver disease (HR = 0.81, 95% CI 0.80–0.82, I2 = 87.2%, P < 0.001) (Fig. 8).
Fig. 8.
Subgroup analysis of forest plots based on the study population
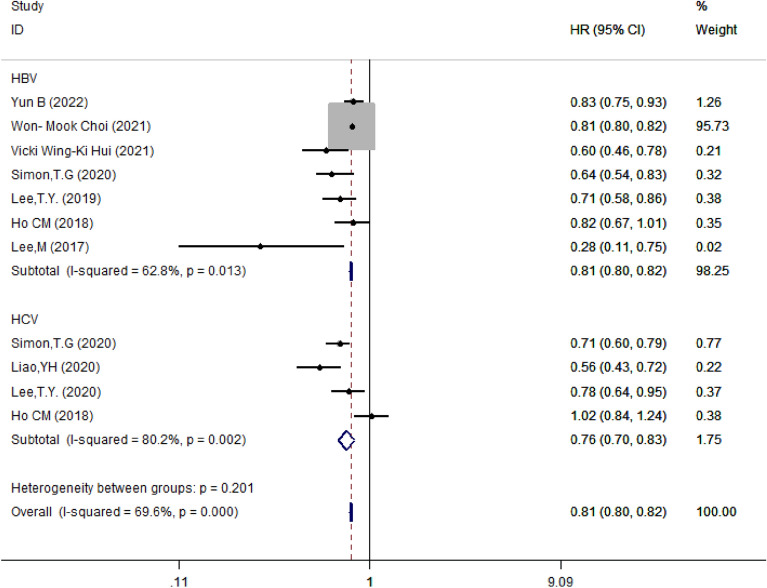
Subgroup analysis based on hepatitis subtypes
Further, in patients with liver disease, we divided studies into HBV (n = 7) and HCV (n = 4) groups based on different hepatitis viruses. The results showed that the risk of HCC was reduced by 19% in HBV-infected patients (HR = 0.81, 95% CI 0.80–0.82, I2 = 62.8%, P = 0.013) and by 24% in HCV-infected patients (HR = 0.76, 95% CI 0.70–0.83, I2 = 80.2%, P = 0.002). There was no significant difference between the two groups (P = 0.201) (Fig. 9).
Fig. 9.
Subgroup analysis of forest plots depending on the type of infected virus
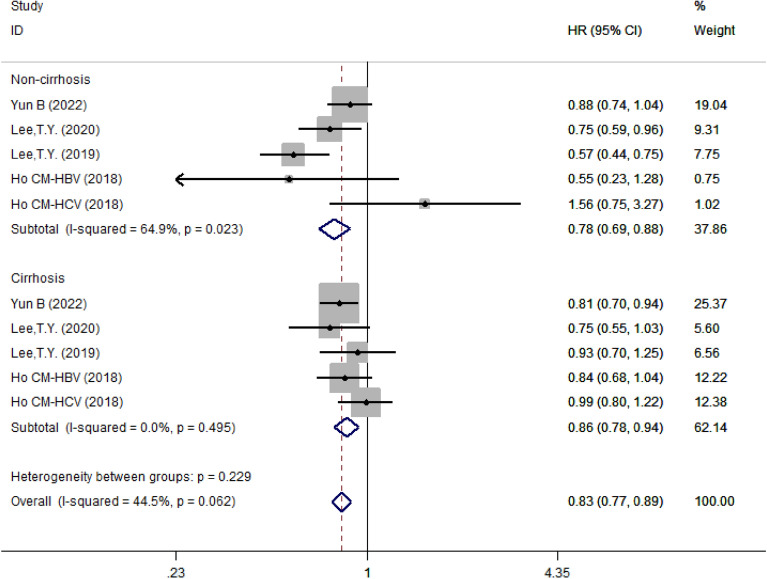
Subgroup analysis based on liver cirrhosis status
Also, we divided patients with liver disease into liver cirrhosis and non-cirrhosis groups. The results showed that aspirin was a protective factor in either cirrhosis (HR = 0.78, 95% CI 0.69–0.88, I2 = 64.9%, P = 0.023) and non-cirrhosis (HR = 0.86, 95% CI 0.78–0.94, I2 = 0.0%, P = 0.495) groups (Fig. 10).
Fig. 10.
Subgroup analysis of forest plots depending on presence or absence of cirrhosis
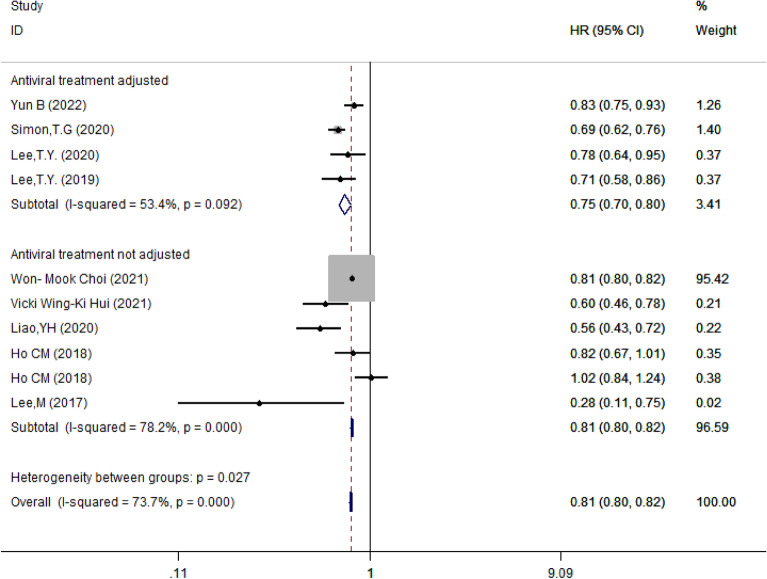
Subgroup analysis based on whether results were adjusted by antiviral drugs
When studies were classified based on whether the results were adjusted by antiviral drugs, we found that aspirin remained to be a protective factor in either adjusted (HR = 0.75, 95% CI 0.70–0.80, I2 = 53.4%, P = 0.092) and 0.81 (95% CI 0.80–0.82, I2 = 78.2%, P < 0.001) respectively. There was a significant difference between groups (P = 0.027) (Figs. 11).
Fig. 11.
Subgroup analysis of forest plots depending on whether results were adjusted based on antiviral drugs
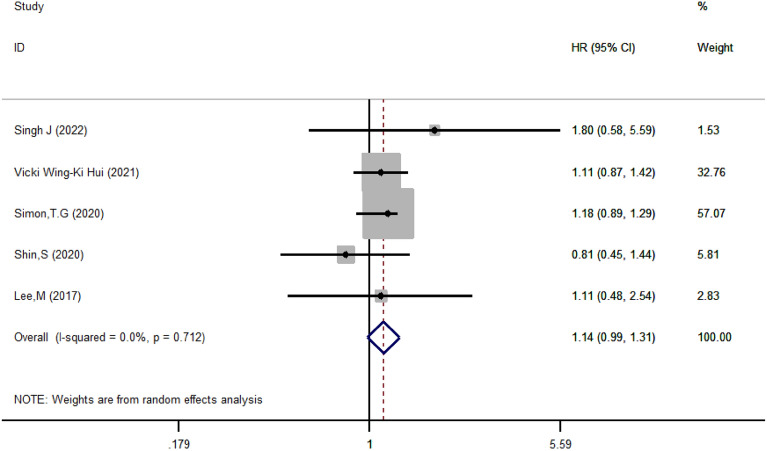
Analysis of bleeding risk associated with aspirin
To determine whether aspirin increased the risk of bleeding, we analyzed 5 papers and found that aspirin was associated with an increased risk of gastrointestinal bleeding in patients with chronic liver disease (HR = 1.14, 95% CI 0.99–1.31, I2 = 0.0%, P = 0.712) (Fig. 12).
Fig. 12.
Forest plot of aspirin use and bleeding risk
Publication bias analysis
Funnel plots were drawn and exhibited asymmetric, indicating the existence of publication bias. The Beggs and Eggers tests showed consistent results (Beggs: P = 0.093; Eggers: P = 0.011) (Fig. 13).
Fig. 13.
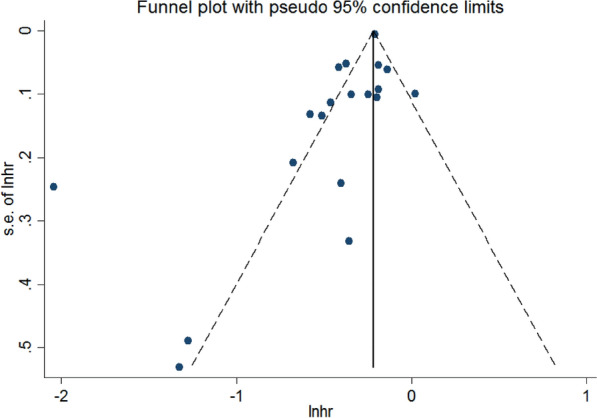
Funnel plot of the association between aspirin and the risk of hepatocellular carcinoma
Discussion
In recent years, many studies have demonstrated the anti-HCC effect of aspirin, which is believed to reduce the incidence of HCC [2]. However, the mechanism through which aspirin inhibits the development of HCC remains unclear. Aspirin may exert anti-HCC activity by several mechanisms [33]. For example, aspirin is able to inhibit pro-inflammatory molecule COX-2, whose inhibitor is shown to reduced liver fibrosis, portal hypertension, and hepatoma cell proliferation [37]. Also, aspirin can inhibit the activation of NF-κB pathway [34–36]. Moreover, as aspirin may induce autophagy-related cell death and interference with glucose uptake by downregulating the expression of glucose transporter protein 1 and activating Bcl-2/Bax signaling pathway [38]. In addition, aspirin can reverse sorafenib resistance in HCC and has a synergistic antitumor effect in combination with sorafenib [14].
This meta-analysis including 2,217,714 participants provides a systematic evaluation of the relationship between aspirin and the risk of HCC. Our results indicated that aspirin reduced the risk of HCC by 30% compared with that in patients who did not use aspirin. Since considerable heterogeneity was detected in the included study, we performed subgroup analyses according to study region, study type, sex, adjusted medication, and study population. Results indicated that use was associated with a reduced risk of HCC independent of these factors. As for patients with liver disease, we concluded consistent results regardless of the presence or absence of cirrhosis and adjustment for administration of antiviral drugs. In addition, the adjusted results showed a diminished effect of aspirin in reducing the risk of HCC compared with that in aspirin users who were not adjusted for both statins and metformin, suggesting that the combination of aspirin and these drugs may have a synergistic anti-HCC effect. Our results also suggest that aspirin is associated with an increased risk of gastrointestinal bleeding in patients with chronic liver disease.
Compared with previous studies, our study has a large sample size with a long follow-up period. However, there are several limitations. First, the heterogeneity of included studies was obvious, which may be due to different study designs, different and highly variable sample sizes across studies, different inclusion and exclusion criteria, the presence of more confounding factors, inconsistent control of confounding factors across studies, and varied lengths of follow-up. In addition, the presence of confounding factors in this study should be considered. For example, aspirin is often concomitantly administered with statins, lipid-lowering agents, and clopidogrel. Studies suggested that statin and clopidogrel may reduce the risk of HCC [39, 40]. Therefore, additional studies are needed to exclude the influence of confounding factors when exploring the benefit of aspirin in HCC. In addition, although aspirin has some potential benefits for patients with liver disease, its consistent use may cause some adverse events, such as gastrointestinal and intracranial hemorrhage. Therefore, it remains controversial whether aspirin should be advocated in high-risk patients such as those with peptic ulcers and esophagogastric fundic varices. Simon et al. showed that the administration of low-dose aspirin did not increase the risk of gastrointestinal bleeding [6]. Therefore, additional studies are required to determine the optimal dose and duration of aspirin to exert clinical benefit for HCC patients without increasing risks of adverse events.
Conclusion
Aspirin may reduce the risk of HCC in both healthy population and patients with chronic liver disease. However, attention should be paid to adverse events such as gastrointestinal bleeding in patients with chronic liver disease.
Supplementary Information
Additional file 1. : A combination of subject terms and free words were used for literature search using Boolean logic operator grouping.
Acknowledgements
Not applicable.
Author contributions
SW and LZ participated in the study design and coordination and the manuscript preparation. ZL, ZY, and RC were involved in the study design and conducted the search. YX critically reviewed the manuscript and provided valuable discussions and criticisms. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Jilin Province Finance Department Project, No.2021SCZ32.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuai Wang and Lijuan Zuo are contributed equally to this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 3.Galle PR, Tovoli F, Foerster F, et al. The treatment of intermediate stage tumours beyond TACE: from surgery to systemic therapy. J Hepatol. 2017;67(1):173–183. doi: 10.1016/j.jhep.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Xia H, Hui KM. Emergence of aspirin as a promising chemopreventive and chemotherapeutic agent for liver cancer. Cell Death Dis. 2017;8(10):e3112. doi: 10.1038/cddis.2017.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808–14. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon TG, Ma Y, Ludvigsson JF, et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 2018;4(12):1683–1690. doi: 10.1001/jamaoncol.2018.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li JH, Wang Y, Xie XY, et al. Aspirin in combination with TACE in treatment of unresectable HCC: a matched-pairs analysis. Am J Cancer Res. 2016;6(9):2109–2116. [PMC free article] [PubMed] [Google Scholar]
- 8.Rothwell PM, Fowkes FGR, Belch JFF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 9.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain MA, Kim DH, Jang JY, et al. Aspirin induces apoptosis in vitro and inhibits tumor growth of human hepatocellular carcinoma cells in a nude mouse xenograft model. Int J Oncol. 2012;40(4):1298–1304. doi: 10.3892/ijo.2011.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitia G, Aiolfi R, Di Lucia P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci USA. 2012;109(32):E2165–E2172. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao M, Kong Q, Hua H, et al. AMPK-mediated up-regulation of mTORC2 and MCL-1 compromises the anti-cancer effects of aspirin. Oncotarget. 2016;7(13):16349–16361. doi: 10.18632/oncotarget.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YX, Feng JY, Sun MM, et al. Aspirin inhibits the proliferation of hepatoma cells through controlling GLUT1-mediated glucose metabolism. Acta Pharmacol Sin. 2019;40(1):122–132. doi: 10.1038/s41401-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Dai W, Mo W, et al. By inhibiting PFKFB3, aspirin overcomes sorafenib resistance in hepatocellular carcinoma. Int J Cancer. 2017;141(12):2571–2584. doi: 10.1002/ijc.31022. [DOI] [PubMed] [Google Scholar]
- 15.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the women’s health study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 16.Yun B, Ahn SH, Yoon JH, et al. Clinical indication of aspirin associated with reduced risk of liver cancer in chronic hepatitis B: a nationwide cohort study. Am J Gastroenterol. 2022;117(5):758–768. doi: 10.14309/ajg.0000000000001725. [DOI] [PubMed] [Google Scholar]
- 17.Singh J, Wozniak A, Cotler SJ, et al. Combined use of aspirin and statin is associated with a decreased incidence of hepatocellular carcinoma. J Clin Gastroenterol. 2022;56(4):369–373. doi: 10.1097/MCG.0000000000001546. [DOI] [PubMed] [Google Scholar]
- 18.Choi Won-Mook, Kim Hyo Jeong, Jo Ae Jeong, et al. Association of aspirin and statin use with the risk of liver cancer in chronic hepatitis B: a nationwide population-based study. Liver Int. 2021;41(11):2777–2785. doi: 10.1111/liv.15011. [DOI] [PubMed] [Google Scholar]
- 19.Hui Vicki Wing-Ki, Yip Terry Cheuk-Fung, Wong Vincent Wai-Sun, et al. Aspirin reduces the incidence of hepatocellular carcinoma in patients with chronic hepatitis B receiving oral nucleos(t)ide analog. Clin Transl Gastroenterol. 2021;12(3):e00324. doi: 10.14309/ctg.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon TG, Duberg AS, Aleman S, et al. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med. 2020;382(11):1018–1028. doi: 10.1056/NEJMoa1912035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin S, Lee SH, Lee M, et al. Aspirin and the risk of hepatocellular carcinoma development in patients with alcoholic cirrhosis. Medicine. 2020;99(9):e19008. doi: 10.1097/MD.0000000000019008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao YH, Hsu RJ, Wang TH, et al. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: a nationwide cohort study [J] BMC Gastroenterol. 2020;20(1):6. doi: 10.1186/s12876-020-1158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee TY, Hsu YC, Tseng HC, et al. Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2020;18(12):2784–92. doi: 10.1016/j.cgh.2020.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Sung JJ, Ho JM, Lam AS, et al. Use of metformin and aspirin is associated with delayed cancer incidence. Cancer Epidemiol. 2020;69:101808. doi: 10.1016/j.canep.2020.101808. [DOI] [PubMed] [Google Scholar]
- 25.Lee TY, Hsu YC, Tseng HC, et al. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern Med. 2019;179(5):633–640. doi: 10.1001/jamainternmed.2018.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang IC, Chang J, Kim K, et al. Aspirin use and risk of hepatocellular carcinoma in a national cohort study of Korean adults. Sci Rep. 2018;8(1):4968. doi: 10.1038/s41598-018-23343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho CM, Lee CH, Lee MC, et al. Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in chemoprevention of hepatocellular carcinoma: a nationwide high-risk cohort study. BMC Cancer. 2018;18(1):401. doi: 10.1186/s12885-018-4292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018;38(11):2018–2027. doi: 10.1111/liv.13872. [DOI] [PubMed] [Google Scholar]
- 29.Lin YS, Yeh CC, Huang SF, et al. Aspirin associated with risk reduction of secondary primary cancer for patients with head and neck cancer: a population-based analysis. PLoS ONE. 2018;13(8):e0199014. doi: 10.1371/journal.pone.0199014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee M, Chung GE, Lee JH, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66(5):1556–1569. doi: 10.1002/hep.29318. [DOI] [PubMed] [Google Scholar]
- 31.Lee TY, Wu JC, Yu SH, et al. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer. 2017;141(7):1307–1314. doi: 10.1002/ijc.30784. [DOI] [PubMed] [Google Scholar]
- 32.Petrick JL, Sahasrabuddhe VV, Chan AT, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the liver cancer pooling project. Cancer Prev Res. 2015;8(12):1156–1162. doi: 10.1158/1940-6207.CAPR-15-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alfonso L, Ai G, Spitale RC, et al. Molecular targets of aspirin and cancer prevention. Br J Cancer. 2014;111(1):61–7. doi: 10.1038/bjc.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Cai W, Chu ESH, et al. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene. 2017;36(31):4415–4426. doi: 10.1038/onc.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paik YH, Kim JK, Lee JI, et al. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut. 2009;58(11):1517–1527. doi: 10.1136/gut.2008.157420. [DOI] [PubMed] [Google Scholar]
- 36.Kern MA, Schubert D, Sahi D, et al. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36(4 Pt 1):885–894. doi: 10.1053/jhep.2002.36125. [DOI] [PubMed] [Google Scholar]
- 37.Gao JH, Wen SL, Yang WJ, et al. Celecoxib ameliorates portal hypertension of the cirrhotic rats through the dual inhibitory effects on the intrahepatic fibrosis and angiogenesis. PLoS ONE. 2013;8(7):e69309. doi: 10.1371/journal.pone.0069309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Yu Y, Liu L. Influence of aspirin use on clinical outcomes of patients with hepatocellular carcinoma: a meta-analysis. Clin Res Hepatol Gastroenterol. 2021;45(6):101545. doi: 10.1016/j.clinre.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Kim G, Jang SY, Nam CM, et al. Statin use and the risk of hepatocellular carcinoma in patients at high risk: a nationwide nested case-control study [J] J Hepatol. 2018;68(3):476–484. doi: 10.1016/j.jhep.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Lee M, Chung GE, Lee JH, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment [J] Hepatology. 2017;66(5):1556–1569. doi: 10.1002/hep.29318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. : A combination of subject terms and free words were used for literature search using Boolean logic operator grouping.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.