Abstract
Our objective was to explore the hypothesis that the risk of leptomeningeal dissemination (LMD) in patients who underwent stereotactic radiosurgery (SRS) for brain metastases is influenced by the site of the primary cancer, the addition of whole brain radiation therapy (WBRT), surgical resection, and control over their systemic disease. We conducted a retrospective cohort analysis of 805 patients who were treated with SRS for brain metastases between 1999 and 2012 at the Wake Forest Baptist Medical Center, and excluded all patients with evidence of LMD before SRS. The primary outcome was LMD. Forty-nine of 795 patients developed LMD with a cumulative incidence of 6.2 % (95 % Confidence Interval (CI), 4.7–8.0). Median time from SRS to LMD was 7.4 months (Interquartile Range (IQR), 3.3–15.4). A colorectal primary site (Hazard Ratio (HR), 4.5; 95 % CI 2.5–8.0; p < 0.0001), distant brain failure (HR, 2.0; 95 % CI 1.2–3.2; p = 0.007), breast primary site (HR, 1.6; 95 % CI 1.0–2.7; p = 0.05), the number of intracranial metastases at time of initial SRS (HR, 1.1; 95 % CI 1.0–1.2; p = 0.02), and age (by 5-year interval) (HR, 0.9; 95 % CI 0.8, 0.9; p = 0.0006) were independent factors associated with LMD. There was no evidence that surgical resection before SRS altered the risk of LMD (HR, 1.1; 95 % CI 0.6–2.0, p = 0.78). In patients who underwent SRS for brain metastases, a colorectal or breast primary site, distant brain failure, younger age, and an increased number of intracranial metastases were independently associated with LMD. Given its relative rarity as an outcome, multi-institutional prospective studies will likely be necessary to validate and quantify these relationships.
Keywords: Leptomeningeal disease, Leptomeningeal metastases, Carcinomatous meningitis, Neoplastic meningitis, Gamma knife
Introduction
The survival of patients with brain metastases has improved over the past decade due to improved systemic therapies [1], improved brain-directed therapies [2], and improved imaging which has led to earlier detection [3]. In fact, outcomes have changed so significantly for patients with brain metastases that novel prognostic models are being developed to account for modern treatment paradigms [4]. Treatment algorithms have recently taken into account the preservation of quality of life in anticipation of improving survival [5]. In spite of the improvements made with brain metastases, leptomeningeal dissemination (LMD) remains nearly universally and rapidly fatal. This condition, also known as leptomeningeal carcinomatosis, leptomeningeal disease, neoplastic meningitis, and carcinomatous meningitis, is characterized by metastatic involvement of the spinal fluid and leptomeninges, and is a known mode of treatment failure after a patient develops brain metastasis. The clinical presentation of LMD classically involves symptoms and signs attributable to multiple levels of the neuaxis (cerebral, posterior fossa, or spine) with 34 % experiencing symptoms at only one level, 39 % experiencing symptoms related to two levels, and 25 % experiencing symptoms at all three levels [6]. Although the most common findings are headache, confusion, nausea/vomiting, diplopia, cerebellar dysfunction, back pain, and leg weakness [6], the most dreaded consequence of LMD is a swift progression into neurologic decline and death.
While several series have characterized patients with brain metastases who later develop LMD [7], few risk factors for development of LMD have been identified. Metastases from breast cancer and melanomas have previously been implicated as higher risk primary sites which can lead to LMD [7–9], while other reports have implicated lung and gastrointestinal cancers as high risk primary sites [8]. With respect to treatment modalities, there has been some argument that surgical resection (specifically piecemeal resection in the posterior fossa) confers a higher risk of LMD when compared to stereotactic radiosurgery (SRS) [8, 10]. With regard to potential modalities that could protect against development of LMD, whole brain radiation therapy (WBRT) has been successful in controlling distant failure [11], but there is still no consensus as to whether it is protective against LMD [7–10, 12].
Several treatment options exist for patients with LMD including intrathecal chemotherapy [13], systemic chemotherapy [14], WBRT (with possible total spinal irradiation) [15], and novel combinations of targeted therapies [16], but nevertheless all of these interventions have proven to be temporizing measures and patients tend to ultimately succumb to their disease [17–19]. Our goal was to identify any risk factors for the development of LMD, with particular attention paid to treatment modalities such as surgery and WBRT, which have been associated with LMD in the past. As LMD affects quality of life and clinical outcomes of patients with intracranial metastases, the identification of risk factors for early failure and protective factors are of great clinical significance, and would have the potential to help dictate management. We have assessed the incidence of, and risk factors for LMD in a large, retrospective, single institution series of patients who have received radiosurgery for brain metastases.
Methods
Data acquisition
The Wake Forest University Health Sciences Institutional Review Board approved this study. Between February 17, 1999 and June 7, 2012, a total of 807 cancer patients received Gamma Knife stereotactic radiosurgery for brain metastases at Wake Forest School of Medicine. Electronic medical records were reviewed to determine patient characteristics and outcomes. Patient characteristics are summarized in Table 1. Patient factors such as age, gender, number of treated metastases at first radiosurgery, histology, prior surgery and WBRT, local and distant brain failures were determined from patients’ electronic medical records. Patients with surgical resection were stratified by whether surgery occurred before or after SRS. Prior surgery was defined as any procedure (including biopsies) that may have seeded the tumor into the meninges, but only one patient had a biopsy without resection. All patients who had surgery before SRS had cavity-directed SRS. The vast majority (>90 %) of tumors that were surgically resected were internally debulked; they were resected in a piecemeal fashion. With regards to age and number of metastases, hazards were modeled for every five-year increase in age, and for each additional metastasis.
Table 1.
Demographic and clinical characteristics of cohort
| Clinical feature | LMD (n = 49) | No LMD (n = 746) | p value† |
|---|---|---|---|
| Age in yearsa [Median (IQR)] | 52 (45, 61) | 60 (51, 68) | 0.0002 |
| Sex | 0.08 | ||
| Male (%) | 18 (37) | 374 (50) | |
| Female (%) | 31 (63) | 372 (50) | |
| Primary site of cancer | <0.0001 | ||
| Lung (%) | 17 (35) | 386 (52) | |
| Breast (%) | 15 (31) | 127 (17) | |
| Skin (melanoma) (%) | 8 (16) | 108 (14) | |
| Genitourinary (%) | 0 (0) | 60 (8) | |
| Colon/rectal (%) | 6 (12) | 40 (5) | |
| Esophageal (%) | 0 (0) | 17 (2) | |
| Gynecologic (%) | 2 (4) | 4 (1) | |
| Unknown/other (%) | 1 (2) | 4 (1) | |
| Number of metastases [Median (IQR)] | 2 (1, 4) | 2 (1, 3) | 0.34 |
| Distant failure (%) | 35 (71) | 311 (42) | <0.0001 |
| Surgery (%) | 17 (35) | 229 (31) | 0.63 |
| Before SRS (%) | 17 (35) | 204 (27) | 0.32 |
| After SRS (%) | 0 (0) | 25 (3) | 0.39 |
| Whole brain radiation (%) | 31 (63) | 377 (51) | 0.10 |
| Time to LMD or censoring (months) [median (IQR)] | 7.4 (3.3, 15.4) | 7.5 (3.6, 17.5) | 0.65 |
IQR interquartile range, SRS stereotactic radiosurgery, LMD leptomeningeal dissemination
On date of first stereotactic radiosurgery
Comparisons between the two groups made with Fisher’s exact test for categorical variables and Wilcoxon rank sum test for continuous variables
Patient follow-up, outcome assessment and salvage therapy
Follow-up assessments were typically conducted at 4–6 weeks after radiosurgery, and subsequently every 3 months for the first year. In cases with intracranial progression of disease following SRS treatment, additional SRS treatment was often offered if patients had previously received WBRT or if patients without previous WBRT did not experience early or numerous (>4) distant brain failure. Distant brain failure was defined as any new metastases that developed outside of the previous radiosurgical target volume. Patients who developed further brain metastases after SRS were generally treated with repeat SRS, while WBRT was generally reserved for salvage of four or more total brain metastases over time or in the setting of short-interval distant brain failure. Local failure was defined as either a pathologically-proven recurrence within the SRS treatment field, or a combination of imaging and clinical characteristics of local treatment failure. Imaging characteristics of treatment failure included an increase in area of enhancement by 25 % on axial slice and/or serial increases in size of enhancement with corresponding increased perfusion on perfusion-weighted imaging.
Our primary outcome of interest was development of LMD, which was determined by contrast-enhanced magnetic resonance imaging (MRI), computerized tomography (CT) scan, or cerebral spinal fluid (CSF) sampling. All neuroradiologist reports on MRI and CT imaging, as well as all pathology reports concerning CSF cytology were reviewed for any mention of LMD (A.H.) and any ambivalent results were reviewed with a faculty radiation oncologist (M.C.) whereupon a decision was made to characterize as LMD or not. The leptomeningeal pattern of enhancement was most commonly seen as abnormal signal enhancement around the sulci or spinal intradural extramedullary tumor deposits on a T1-weighted post-contrast MRI or post-contrast CT scan [20]. More than 90 % of our cohort was first diagnosed by MRI, with the remainder being diagnosed by CT or lumbar puncture. A confirmatory lumbar puncture was ordered in ~25 % of cases that had leptomeningeal signs on imaging, and malignant cells were only found in the CSF in approximately ~40 % of these confirmatory tests. In cases that had negative CSF cytology but unequivocal imaging findings, patients were categorized as having LMD. In cases where the neuroradiologist’s reports included a questionable diagnosis of LMD, and CSF results were negative for malignant cells, patients were not considered to have LMD. Patients with nonspecific markers such as elevated protein, elevated WBCs, increased opening pressure, or low glucose levels in the context of ambiguous imaging results were not considered to have LMD. No patients were included based on clinical examination alone. Neurologic death was defined as progressive neurologic dysfunction in the context of stable systemic disease as previously described by Patchell et al. [21].
Interventions after LMD were defined as procedures that were specific to LMD, such as intrathecal chemotherapy administration via lumbar puncture or an Ommaya Reservoir, cranial-spinal radiation, or systemic chemotherapy thought to benefit selected patients with LMD such as capecitabine [14].
Statistical analysis
Data was managed and analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC). Cumulative incidence was assessed using its % CIF macro using death as a competing risk. For demographic and clinical variables, normality was assessed using Shapiro-Wilkes test. We tested assumptions with an observed score process (“assess” step) for proportional hazards, and employed cumulative martingale residual plots (assess model step) for modeling of continuous variables. Comparisons between the leptomeningeal failure group were done using the Wilcoxon Rank-Sum test or two-sided T test for continuous variables, or Chi squared or Fischer Exact tests for categorical variables.
To identify predictors of LMD, Cox proportional hazards models were developed using PROC PHREG. The proportional hazards and modeling assumptions were evaluated using the “assess” function of PROC PHREG. WBRT and distant brain failure were time-varying covariates and thus were modeled using the Andersen-Gill approach. All variables were assessed with univariate analysis, then stepwise selection was used to build the multivariable model. Any variables with p values <0.3 were added to the model, and these were kept if their p-values remained below 0.15. In addition, we employed a cause-specific competing risk analysis as there was a high occurrence of death in this cohort. Robust covariance matrixes were used to construct 95 % confidence intervals.
To assess the association between LMD and surgery to the posterior fossa, we identified all patients in our cohort who underwent surgical resection of one or more brain metastases. We excluded patients who developed LMD before surgical resection as well as patients who underwent surgery to both an infratentorial and supratentorial lesion. Infratentorial structures included the cerebellum, medulla, and pons. Fischer’s exact test was used as our statistical test.
Results
Incidence and characteristics of patients with leptomeningeal failure
Eight hundred seven (807) cancer patients who received stereotactic radiosurgery were identified between February 17, 1999 and June 7, 2012, and their characteristics are detailed in Table 1. In this cohort, 61 patients were diagnosed with LMD but 12 had evidence of LMD prior to receiving stereotactic radiosurgery and were excluded from the analysis (Fig. 1). Forty-nine (49) of the 795 remaining patients had evidence of LMD. The cumulative incidence of LMD, adjusted for a competing risk of death, was 3.9 % (95 % CI 2.7–5.4) at 1-year, 5.5 % (95 % CI 4.1–7.3) at 2-years, and 5.9 % (95 % CI 4.4–7.7 %) at 3-years. Total adjusted cumulative incidence was 6.2 % (95 % CI 4.7–8.0).
Fig. 1.
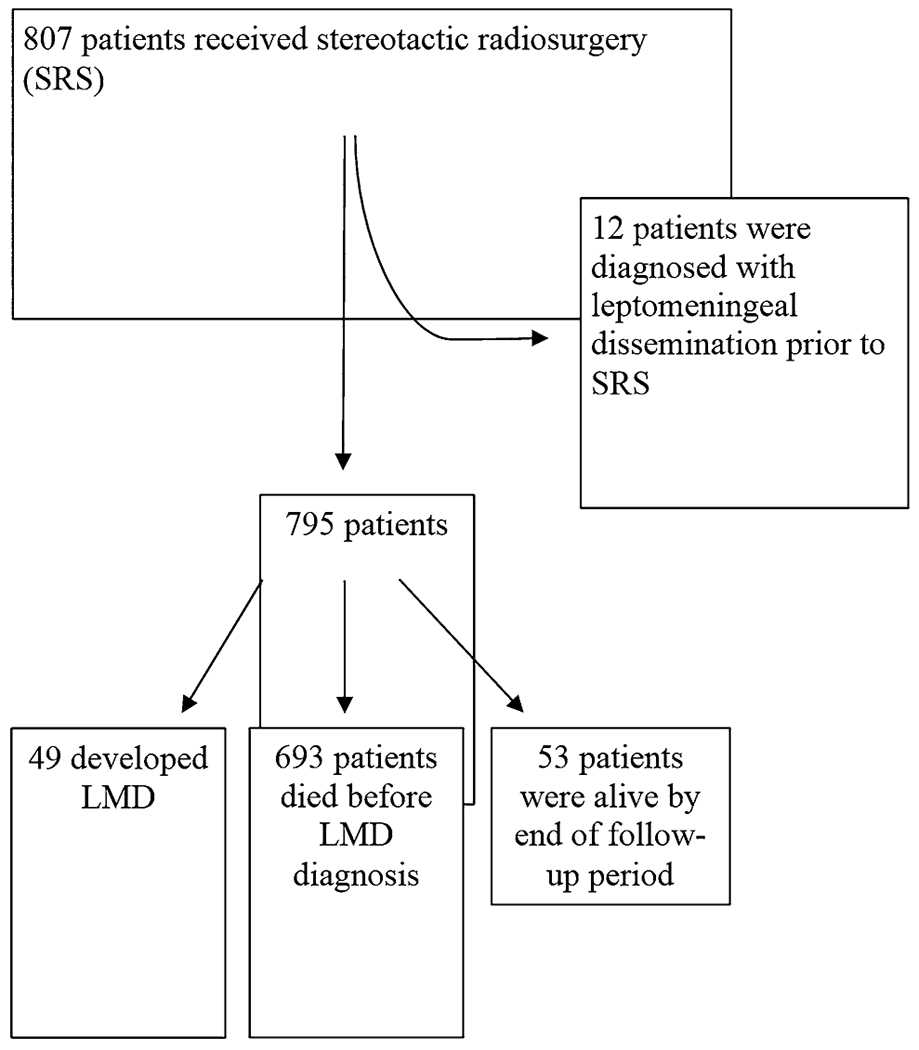
Flow diagram of cohort
Clinical presentation of leptomeningeal failure
Presenting symptoms were available in 60 of the 61 total patients who developed LMD. In order of the most to least common presenting symptom, our cohort experienced headaches (n = 16, 26.7 %), lower extremity weakness (n = 14, 23.3 %), decreased cognition (n = 10, 16.7 %), changes in vision (n = 9, 15 %), changes in gait (n = 9, 15 %), decreased sensation/paresthesias in the lower extremity (n = 7, 11.7 %), nausea (n = 7, 11.7 %), fatigue (n = 7, 11.7 %), changes in bowel/bladder function (n = 7, 11.7), and decreased sensation/paresthesias in the upper extremity (n = 6, 10 %). Eight patients (13.3 %) were neurologically asymptomatic when LMD was diagnosed. These eight were diagnosed with LMD by routine follow-up MRI or imaging ordered as part of routine imaging after known brain metastases. Less common (n < 6) presenting symptoms were upper extremity weakness, changes in coordination, pain in the torso, changes in speech, changes in hearing, dizziness, back pain, loss of consciousness, facial droop, and facial numbness.
Risk factors for development of leptomeningeal failure
Factors that may play a role in the development of LMD are outlined in Table 2. In univariate analysis, age (HR, 0.9; 95 % CI 0.8–1.0; p = 0.006), a colorectal primary site (HR, 2.9; 95 % CI 1.3–6.7 p = 0.01), number of metastases (HR, 1.1; 95 % CI 1.0–1.3; p = 0.005), distant brain failure (HR, 4.1; 95% CI 2.1–7.9; p < 0.0001), and WBRT (HR, 2.4; 95 % CI 1.4–4.4; p = 0.003) were associated with the development of LMD. It should be noted that of the 60 patients with a genitourinary primary cancer, none developed LMD and the p values were incalculable, making it ineligible for inclusion in both the univariate and multivariable models. Gender, other primary histologies excluding colorectal or genitourinary tumors, and prior surgical resection were not significant predictors of LMD.
Table 2.
Hazard ratios for hazard of developing leptomeningeal dissemination
| Univariate analysis | ||
|---|---|---|
|
| ||
| Clinical feature | Univariate hazard ratio (95 % CI) | p value |
| Age (5-year interval) | 0.9 (0.8, 1.0) | 0.006 |
| Female Sex – (male, referent) | 1.4 (0.8, 2.5) | 0.24 |
| Primary site of cancera | ||
| Breast | 1.5 (0.8, 2.8) | 0.16 |
| Lung | 0.6 (0.3, 1.0) | 0.07 |
| Melanoma | 1.3 (0.6, 2.9) | 0.47 |
| Colon/rectal | 2.9 (1.2, 6.7) | 0.01 |
| Genitourinary | ‡ | |
| Otherb | 1.4 (0.4, 4.9) | 0.57 |
| Number of metastases | 1.1 (1.0, 1.3) | 0.005 |
| Distant Failure | 4.1 (2.1, 7.9) | <0.0001 |
| Surgical resection before SRS | 1.1 (0.6, 2.0) | 0.78 |
| Surgical resection after SRS | ‡ | |
| Whole brain radiation therapy | 2.4 (1.4, 4.4) | 0.003 |
| Stepwise multivariable model | ||
|
| ||
| Clinical feature | Adjusted hazard ratio(95 % CI) | p value |
|
| ||
| Colorectal primary sitea | 4.5 (2.5, 8.0) | <0.0001 |
| Number of metastases | 1.1 (1.0, 1.2) | 0.0091 |
| Distant failure | 2.0 (1.2,3.2) | 0.0065 |
| Age (5-year interval) | 0.9 (0.8, 0.9) | 0.0006 |
| Breast cancer primary sitea | 1.6 (1.0, 2.7) | 0.05 |
CI confidence interval, SRS stereotactic radiosurgery
Each primary site was compared against all other sites combined
Includes “other and unknown”, gynecologic, and esophagus
No leptomeningeal dissemination observed
In our multivariable model (Table 2), a colorectal primary site (Hazard Ratio (HR), 4.5; 95 % Confidence Interval (CI), 2.5–8.0; p < 0.0001), distant brain failure (HR, 2.0; 95 % CI 1.2–3.2; p = 0.007), breast primary site (HR, 1.6; 95 % CI 1.0–2.7; p = 0.05), the number of intracranial metastases at time of initial SRS (HR, 1.1; 95 % CI 1.0–1.2; p = 0.02), and age (by 5-year interval) (HR, 0.9; 95 % CI 0.8, 0.9; p = 0.0006) were independent factors associated with LMD. The occurrence of LMD with relation to time is shown in the Kaplan—Meier plot in Fig. 2a, with all 49 occurrences of LMD occurring within 38 months of the patient’s first GK. The occurrence of LMD related to a breast cancer primary site is separated from the cohort in Fig. 2b, illustrating its differing plateau time.
Fig. 2.
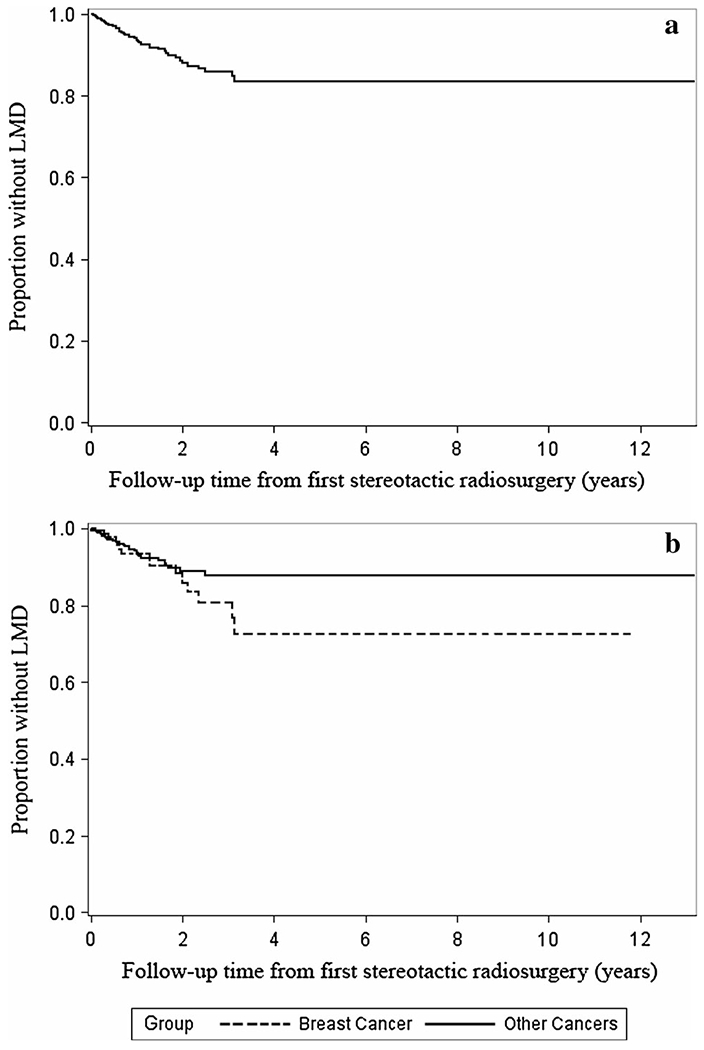
a Kaplan–Meier estimates for the development of LMD, all. b Kaplan–Meier estimates for the development of LMD, div
Outcomes of patients who developed leptomeningeal disease
In Table 3, we briefly outline the time course of patients with LMD. Those who underwent cranial spinal radiation, intrathecal chemotherapy, or capecitabine after LMD lived longer (Median, 3.4 months; IQR, 1.4–5.8) compared to those who did not undergo intervention (Median, 1.6 months; IQR, 0.9–3.5; p = 0.01). One patient underwent a ventriculoperitoneal shunt for his hydrocephalus; no evidence of peritoneal seeding was encountered. Those who had developed LMD from a breast primary site also had longer overall survival from time of LMD (Median, 4.0 months; IQR, 1.6–7.2) than all other primary sites (Median, 1.8 months; IQR, 1.0–3.8; p = 0.03). Death from a neurologic cause was frequently observed in the patient population that experienced LMD. Of those who developed LMD and had a documented cause of death, 32/46 (69.6 %) succumbed to neurologic causes; of those who did not have LMD and had a documented cause of death, 243/612 (39.7 %) succumbed to neurologic causes (p < 0.0001). In Kaplan—Meier analysis, we did not observe a significant difference in the time from SRS to neurologic death between those who eventually developed LMD (Median 11.3 months; IQR 4.5–21.1) and those who did not (Median 7.3 months; IQR 3.9–15.4; p = 0.12).
Table 3.
Time course for patients who developed LMD
| Interval (months) | Median | 1st Quartile | 3rd Quartile |
|---|---|---|---|
| Time from 1st Brain Met to LMD | 13.7 | 7.9 | 25.0 |
| Time from SRS to LMD | 7.4 | 3.3 | 15.4 |
| Time from SRS to Death | 11.5 | 7.3 | 23.0 |
| Time from LMD to Death | 2.5 | 1.2 | 4.9 |
| Time from LMD to Death (Intervention, n = 27) | 3.4 | 1.4 | 5.8 |
| Time from LMD to Death (No Intervention, n = 22) | 1.6 | 0.9 | 3.5 |
Leptomeningeal disease after craniotomy
Of our original 807 patient cohort, 252 patients underwent surgery for an intracranial metastasis. Fifty-five patients underwent one or more surgeries for infratentorial metastases, while 197 underwent one or more surgeries for supratentorial metastases. Patients who underwent surgery to an infratentorial metastasis had a higher rate of developing LMD (n = 9, 16.4 %) compared to patients who underwent surgery to a supratentorial metastasis (n = 12, 6.1%; Relative Risk, 2.7; 95 % CI 1.2–6.0; p = 0.024).
Discussion
In our series of patients who received SRS for their brain metastases from solid tumors, we found there were several independent factors that correlated with an increased risk of LMD: primary tumor type (colorectal or breast cancer), distant brain failure, younger age, and an increased number of intracranial metastases at first SRS. These findings largely agree with the current body of published literature as previous reports have described a higher risk of LMD in patients who had brain metastases from breast primary sites [7–9], and also in patients who had higher number of brain metastases [8, 9]. In addition, our secondary analysis showing an increased risk of LMD after surgical intervention of infratentorial metastases supports prior literature on the subject [8, 22–26]. Furthermore, our study found no LMD events in those with genitourinary cancers, which is congruent with previous series on patients with genitourinary cancers that also demonstrate a low association with the development of LMD [27, 28]. However, in contrast to several studies that had no LMD events in those with colorectal primaries [8, 9, 29], our study found an increased incidence of LMD in those with colorectal primaries.
The rate of LMD with respect to time is illustrated in Fig. 2a. This suggests that the risk of LMD remains near constant with time after the first SRS, then plateaus after ~37 months, possibly due to survivorship bias. These findings complement the patterns of incidence seen in the similarly large (n = 827) cohorts from Suki et al. in 2009, and Jo et al. in 2012. However, the observed plateau time in Suki et al. 2009 was ~15 months, which may reflect a difference in the makeup of the studied cohorts; only 10 % of patients were breast cancer patients in Suki et al., whereas in both Jo et al. and the current study, 16.6 and 17 % respectively were breast cancer patients [8, 9]. Breast cancer patients appear to live longer, and thus are driving the plateau of survivorship bias longer in the current series when compared to the 2009 Suki publication. In Fig. 2a, we separate breast cancer patients to illustrate how both breast and non-breast sites appear to yield the same yearly rate of LMD, but those with breast cancer primaries sustain this rate longer, similarly to the way Atalar et al’s cohort behaved in their study [7]. Though it would seem breast cancer’s longer natural history may be responsible for its higher rate of LMD, a longer burden of disease does not necessarily lead to LMD. For example, patients with our lowest risk (genitourinary) primary site had a longer time to LMD/death/censoring (Median, 8.3 months, IQR 4.6–21.8) than patients with our highest risk (colorectal) primary site (Median, 7.2 months; IQR 2.2–14.1; p = 0.03).
It should be noted that in our cohort there was no evidence that the addition of surgical resection before SRS (HR, 1.1; p = 0.78) significantly altered the risk of LMD even though the vast majority of tumors were removed in the same piecemeal fashion that previous studies implicated as a risk factor for LMD [8, 10, 30]. In contrast, it appeared that LMD was more likely to be a result of reseeding of the central nervous system from progressive systemic disease, given the correlation to distant brain failure [4, 31].
Our data on the effect of WBRT on developing LMD was inconclusive due to its confounding relationship with both an increased number of intracranial metastases and distant brain failure. These confounding factors led to WBRT being dropped from the multivariable model. The previous literature concerning the effect of WBRT on LMD is also inconclusive with some studies finding WBRT to be protective against LMD [9] and others finding no effect [8, 12]. The relevance of this finding is that WBRT in patients who undergo surgery for brain metastases may not prevent LMD. Instead, alternative adjuvant therapy such as cavity-directed radiosurgery, which will avoid the toxicities of WBRT [5], may be reasonable. NCCTGN107C [32] is currently evaluating whether WBRT or SRS after resection of 1-3 brain metastases provides significant differences in outcome.
The strength of our study lies in our relatively large sample size as well as the long follow-up time of our patients; 742 of 795 patients were followed until death. This allowed us to capture the majority of potential LMD events, the cause of death, and also minimized censoring in our study. Like all previous single-center studies, the limitations of our analysis are primarily centered around the lack of statistical power afforded by the low incidence of LMD as well as the high competing risk of death. While our center had the greatest raw number of events of published series, once stratification into multiple categories occurred, statistical power diminished considerably.
Another limitation of our study was that we did not record data on the specific intervention to each supratentorial or infratentorial metastasis in patients with multiple metastases, thus it was ambiguous whether SRS was performed on an infra- or supratentorial lesion in patients with both. Because of this, we chose not to separate patients with infratentorial metastases in our main analysis. Lastly, our data on outcomes after a diagnosis of LMD is provided only for completeness sake because the selection bias that occurred when considering candidates for post-LMD intervention likely accounts for the difference in survival time.
Conclusion
In patients who underwent SRS for brain metastases, a colorectal or breast primary site, occurrence of distant brain failure, younger age, and an increased number of intracranial metastases were independently associated with LMD. Given its relative rarity as an outcome, multi-institutional prospective studies will likely be necessary to validate and quantify these relationships.
Footnotes
Disclosure All authors have nothing to disclose.
Contributor Information
Andrew J. Huang, Department of Radiation Oncology, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA
Karen E. Huang, Department of Radiation Oncology, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA
Brandi R. Page, Department of Radiation Oncology, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA
Diandra N. Ayala-Peacock, Department of Radiation Oncology, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA
John T. Lucas, Jr., Department of Radiation Oncology, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA
Glenn J. Lesser, Department of Medicine (Hematology & Oncology), Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA
Adrian W. Laxton, Department of Neurosurgery, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA
Stephen B. Tatter, Department of Neurosurgery, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA
Michael D. Chan, Department of Radiation Oncology, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA
References
- 1.Cochran DC, Chan MD, Aklilu M et al. (2012) The effect of targeted agents on outcomes in patients with brain metastases from renal cell carcinoma treated with Gamma Knife surgery. J Neurosurg 116:978–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen CA, Chan MD, McCoy TP et al. (2011) Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg 114:1585–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loganathan AG, Chan MD, Alphonse N et al. (2012) Clinical outcomes of brain metastases treated with Gamma Knife radiosurgery with 3.0 T versus 1.5 T MRI-based treatment planning: have we finally optimised detection of occult brain metastases? J Med Imaging Radiat Oncol 56:554–560 [DOI] [PubMed] [Google Scholar]
- 4.Ayala-Peacock DN, Peiffer AM, Lucas JT, et al. (2014) A nomogram for predicting distant brain failure in patients treated with gamma knife stereotactic radiosurgery without whole brain radiotherapy. Neuro Oncol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD (2012) Radiation-induced brain injury: a review. Front Oncol 2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM (2010) Leptomeningeal metastases in the MRI era. Neurology 74:1449–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atalar B, Modlin LA, Choi CY et al. (2013) Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys 87:713–718 [DOI] [PubMed] [Google Scholar]
- 8.Suki D, Abouassi H, Patel AJ, Sawaya R, Weinberg JS, Groves MD (2008) Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg 108:248–257 [DOI] [PubMed] [Google Scholar]
- 9.Jo KI, Lim DH, Kim ST et al. (2012) Leptomeningeal seeding in patients with brain metastases treated by gamma knife radiosurgery. J Neurooncol 109:293–299 [DOI] [PubMed] [Google Scholar]
- 10.Siomin VE, Vogelbaum MA, Kanner AA, Lee SY, Suh JH, Barnett GH (2004) Posterior fossa metastases: risk of leptomeningeal disease when treated with stereotactic radiosurgery compared to surgery. J Neurooncol 67:115–121 [DOI] [PubMed] [Google Scholar]
- 11.Aoyama H, Shirato H, Tago M et al. (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491 [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto K, Narita Y, Miyakita Y et al. (2011) Comparison of clinical outcomes of surgery followed by local brain radiotherapy and surgery followed by whole brain radiotherapy in patients with single brain metastasis: single-center retrospective analysis. Int J Radiat Oncol Biol Phys 81:e475–e480 [DOI] [PubMed] [Google Scholar]
- 13.Clatot F, Philippin-Lauridant G, Ouvrier MJ et al. (2009) Clinical improvement and survival in breast cancer leptomeningeal metastasis correlate with the cytologic response to intrathecal chemotherapy. J Neurooncol 95:421–426 [DOI] [PubMed] [Google Scholar]
- 14.Rogers LR, Remer SE, Tejwani S (2004) Durable response of breast cancer leptomeningeal metastasis to capecitabine monotherapy. Neuro Oncol 6:63–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasserstrom WR, Glass JP, Posner JB (1982) Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 49:759–772 [DOI] [PubMed] [Google Scholar]
- 16.Vincent A, Lesser G, Brown D et al. (2013) Prolonged regression of metastatic leptomeningeal breast cancer that has failed conventional therapy: a case report and review of the literature. J Breast Cancer 16:122–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riess JW, Nagpal S, Iv M et al. (2014) Prolonged survival of patients with non-small-cell lung cancer with leptomeningeal carcinomatosis in the modern treatment era. Clin Lung Cancer 15(3):202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwak HS, Joo J, Kim S et al. (2013) Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J Thorac Oncol 8:599–605 [DOI] [PubMed] [Google Scholar]
- 19.Le Rhun E, Taillibert S, Zairi F et al. (2013) Prolonged survival of patients with breast cancer-related leptomeningeal metastases. Anticancer Res 33:2057–2063 [PubMed] [Google Scholar]
- 20.Freilich RJ, Krol G, DeAngelis LM (1995) Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol 38:51–57 [DOI] [PubMed] [Google Scholar]
- 21.Patchell RA, Tibbs PA, Regine WF et al. (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280:1485–1489 [DOI] [PubMed] [Google Scholar]
- 22.DeAngelis LM, Mandell LR, Thaler HT et al. (1989) The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery 24:798–805 [DOI] [PubMed] [Google Scholar]
- 23.Dosoretz DE, Blitzer PH, Russell AH, Wang CC (1980) Management of solitary metastasis to the brain: the role of elective brain irradiation following complete surgical resection. Int J Radiat Oncol Biol Phys 6:1727–1730 [DOI] [PubMed] [Google Scholar]
- 24.Kitaoka K, Abe H, Aida T, Satoh M, Itoh T, Nakagawa Y (1990) Follow-up study on metastatic cerebellar tumor surgery—characteristic problems of surgical treatment. Neurol Med Chir (Tokyo) 30:591–598 [DOI] [PubMed] [Google Scholar]
- 25.Norris LK, Grossman SA, Olivi A (1997) Neoplastic meningitis following surgical resection of isolated cerebellar metastasis: a potentially preventable complication. J Neurooncol 32:215–223 [DOI] [PubMed] [Google Scholar]
- 26.van der Ree TC, Dippel DW, Avezaat CJ, Sillevis Smitt PA, Vecht CJ, van den Bent MJ (1999) Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry 66:225–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yust-Katz S, Mathis S, Groves MD (2013) Leptomeningeal metastases from genitourinary cancer: the University of Texas MD Anderson Cancer Center experience. Med Oncol 30:429. [DOI] [PubMed] [Google Scholar]
- 28.Orphanos G, Ardavanis A (2010) Leptomeningeal metastases from prostate cancer: an emerging clinical conundrum. Clin Exp Metastasis 27:19–23 [DOI] [PubMed] [Google Scholar]
- 29.Suki D, Hatiboglu MA, Patel AJ et al. (2009) Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 64:664–674 discussion 674–666 [DOI] [PubMed] [Google Scholar]
- 30.Ahn JH, Lee SH, Kim S et al. (2012) Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg 116:984–993 [DOI] [PubMed] [Google Scholar]
- 31.Kress MA, Oermann E, Ewend MG, Hoffman RB, Chaudhry H, Collins B (2013) Stereotactic radiosurgery for single brain metastases from non-small cell lung cancer: progression of extracranial disease correlates with distant intracranial failure. Radiat Oncol 8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown PD. Stereotactic radiosurgery or whole-brain radiation therapy in treating patients wiht brain metastases that have been removed by surgery. In: (US) NLoM, editor. Bethesda (MD) [Google Scholar]


