Abstract
Monoclonal immunoglobulin deposition disease (MIDD), often associated with plasma cell dyscrasias, predominantly affects the kidneys. In this disease, hematologic response to treatment can be reliably assessed by International Myeloma Working Group (IMWG) consensus criteria, while uniform criteria for assessing renal response are lacking. We report a retrospective analysis of renal outcomes among 34 patients with MIDD. With most patients treated with bortezomib and autologous stem cell transplantation, 26 of 28 (94%) achieved very good partial hematologic response or better. We demonstrate that both IMWG (based on estimated glomerular filtration rate, eGFR) and amyloid (based on proteinuria) criteria are needed to capture renal response: among 28 evaluable patients, 6 (21%) had isolated proteinuria, while 13 (46%) had isolated decreased eGFR. Using both criteria, which were concordant in patients with both decreased eGFR and proteinuria, 22 of 28 patients (79%) achieved a renal response, including 2 of 7 discontinuing dialysis. All 6 patients (100%) with isolated proteinuria and 7 of 13 (54%) with isolated decreased eGFR achieved renal response, suggesting that isolated proteinuria is an early manifestation of MIDD associated with reversible renal damage. Baseline eGFR predicted renal response (p=0.02 by quartile) and survival (p=0.02), while hematologic response (CR vs. non-CR) did not, probably because of high hematologic response rate. With a median follow up of 110 months, the median overall survival was 136 months (95% CI: 79–NR) and median renal survival had not been reached. Prospective studies using uniform renal response criteria are needed to optimize the management of MIDD.
Keywords: monoclonal immunoglobulin deposition disease, multiple myeloma, monoclonal gammopathy of renal significance, IMWG renal response criteria, amyloid criteria, amyloid renal response criteria
Introduction
Randall-type monoclonal immunoglobulin deposition disease (MIDD) is one of the most common entities among monoclonal gammopathy of renal significance (MGRS), a recently named group of renal diseases that have in common the presence of an underlying nephrotoxic monoclonal immunoglobulin protein.1–4 While the precise pathologic mechanisms that lead to the various distinct phenotypes involved in MGRS are diverse and generally poorly understood, characteristics used in the current classification system include the distinct pathologic appearances on light microscopy, electron microscopy, and immunofluorescence, as well as the characteristic deposition sites within the kidney. In addition, distinctive clinical features allow for differentiation of the various phenotypes.
MIDD is typically associated with plasma-cell and lymphoid neoplasms. Its characteristic organ dysfunction is caused by the deposition of pathogenic monoclonal light and/or heavy chain immunoglobulin, most commonly in basement membranes of the kidneys, but also other organs such as lung or liver.5–12 The monoclonal gammopathy may consist of light chains, heavy chains, or both, leading to the designations of light chain deposition disease (LCDD), heavy chain deposition disease (HCDD), or light and heavy chain deposition disease (LHCDD), respectively. Monoclonal protein deposits are visualized through light microscopy as non-organized globular structures and identified by immunofluorescence staining as immunoglobulin proteins. The ultrastructure involves electron-dense, nonfibrillar deposits visible in basement membranes by electron microscopy. Most LCDD cases are associated with kappa light-chain isotype,7,12 and there is a known association with the Vk IV variability subgroup of human monoclonal kappa light chains.
Evidence-based guidelines on the management of MIDD (and MGRS in general) are lacking, and prospective trials have not been conducted. However, since this condition is often associated with an underlying plasma cell dyscrasia, patients have typically been treated with regimens used in multiple myeloma. Data from retrospective cohort series of patients with MIDD treated with these anti-myeloma regimens guide the clinical management of MIDD.7–9,11,12 Several groups have reported on the efficacy of bortezomib,9,13–16 autologous stem cell transplantation,9,17–22 and newer drugs such as daratumumab.23 However, while hematologic responses are well characterized based on the extensively vetted and adopted International Myeloma Working Group (IMWG) hematologic response criteria,24,25 uniform criteria for assessing renal response to treatment of the underlying hematologic condition are not well defined in patients with MIDD, and there is no standard of care. As a result, the assessment of renal function outcomes, the hallmark of MIDD, is not uniformly analyzed in published series. Here we report our experience of treatment outcomes, particularly organ outcomes, in a retrospective cohort study of 34 patients with MIDD at two centers.
Methods
Patients
We performed an institutional review board (IRB)-approved electronic query of pathology records at Memorial Sloan Kettering Cancer Center (MSK) and New York Presbyterian Hospital/Weill Cornell Medical Center (NYP-Cornell) to identify patients with a biopsy-proven diagnosis of MIDD. Patients were eligible for inclusion in this analysis if they received treatment and were subsequently followed at either institution. The demographics of eligible patients, including age, gender and race, were collected from medical records. We also identified hematologic characteristics, including monoclonal immunoglobulin isotype; levels of free light chain (FLC) by FLC assay; levels of serum monoclonal spike by serum protein electrophoresis (SPEP); levels of serum albumin; β2 microglobulin with corresponding staging according to the multiple myeloma International Staging System (ISS); and percentage of bone marrow plasmacytosis as determined by manual cell count of bone marrow aspiration and biopsy before treatment.
Renal characteristics identified included renal function and 24-hour proteinuria. Renal function was assessed by serum creatinine level and estimated glomerular filtration rate (eGFR) calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. Nephrotic-range proteinuria was defined as urine protein ≥3 g/24 h; and hematuria defined as >5 red blood cells/high power field. Treatment characteristics assessed included the induction therapy and combination regimens used, specifically whether bortezomib, lenalidomide, cyclophosphamide and autologous stem cell transplantation (ASCT) were used in first-line therapy.
Renal pathology
Renal biopsies from both institutions were processed according to standard methods and evaluated by light microscopy, immunofluorescence, and electron microscopy at NYPH-Cornell. We extracted renal pathologic data from available pathology reports and a renal pathologist (S.P.S.) reviewed the data for accuracy and confirmation of the diagnosis of MIDD, detailing the following: number of glomeruli; presence or absence of cortex or medulla in the biopsy; presence or absence and percent involvement of global glomerulosclerosis, focal segmental glomerulosclerosis, interstitial fibrosis, and tubular atrophy; grade of arteriosclerosis of renal arteries and arterioles; immunofluorescence staining graded 0 to 3+ for heavy chains (IgG, IgA, IgM) and light chains (kappa and lambda); degree of foot process effacement (percent); confirmation of glomerular and/or tubulointerstitial MIDD; and presence of other non-MIDD renal pathologic findings in the biopsy. Details of the pathologic data are not presented in this paper.
Hematologic and renal response evaluation
We assessed hematologic responses according to the IMWG uniform response criteria.24,25 Renal responses were assessed on the basis of changes in both eGFR and proteinuria because of patients’ diverse disease presentations; although most present with decreased eGFR along with variable degrees of proteinuria, some present with proteinuria and preserved eGFR while others with decreased eGFR without proteinuria. Therefore, the response criteria used were either (i) the IMWG renal response criteria or (ii) amyloid response criteria, respectively, for patients with decreased eGFR <50mL/min/1.73 m2 with or without significant proteinuria (>1 g/24 h), or patients with significant proteinuria (UTP >1g/24 h) with or without preserved eGFR (>50 mL/min/1.73 m2) at presentation. IMWG renal response criteria include: complete response (Renal CR), if eGFR <50 mL/min/1.73 m2 prior to treatment improves to eGFR ≥ 60 mL/min/1.73 m2; partial response (Renal PR), if eGFR <15 mL/min/1.73 m2 prior to treatment improves to 30–59 mL/min/1.73 m2; minor response (Renal MR), if eGFR <15 mL/min/1.73 m2 prior to treatment improves to 15–29 mL/min/1.73 m2 or if eGFR 15–29 mL/min/1.73 m2 prior to treatment improves to 30–59 mL/min/1.73 m2; or renal progression, if there is a > 25% decrease in eGFR or > 50% increase in 24-hour urine protein (to >1 g/24 h).26 Amyloid renal response was defined as either reduced proteinuria by ≥ 50% or a drop in proteinuria to <0.5 g/24 h in those with > 0.5 g/24 h at baseline and stable renal function (i.e., ≤ 25% increase in eGFR).27–29 For patients with eGFR < 50 mL/min/1.73 m2 and proteinuria (>1 g/24 h) as the main presentation of the disease, both sets of criteria defined above were used for response evaluation.
Statistical analysis
Categorical patient characteristics were summarized by frequency (percentage) and continuous characteristics were summarized by median and interquartile range (IQR). Time to renal response (TRR, defined as time between start of therapy to time of best response) and renal survival time (RST, defined as time between start of therapy to hemodialysis) were evaluated by the Kaplan-Meier method. Associations between patient characteristics (including baseline characteristics, treatment characteristics, and hematologic responses) and time to event outcome were assessed by log-rank. The effects of patient and disease characteristics on TRR and RST were estimated by univariate Cox proportional hazard model with p<0.05 being considered significant. All statistical analyses were performed using R.
Results
Patient characteristics
Between January 1999 and January 2016, we identified 54 patients with a diagnosis of MIDD at MSK and NYPH-Cornell, 34 of whom received treatment and met criteria for inclusion in this series. Among these 34 patients, 25 (74%) met criteria for symptomatic multiple myeloma requiring therapy, 3 (9%) met criteria for smoldering multiple myeloma (SMM), and 4 (12%) for monoclonal gammopathy of undetermined significance (MGUS), using IMWG criteria excluding reduced GFR <40 mL/min or serum creatinine >2 mg/dL as diagnostic criterion.30 One (3%) patient had chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and 1 (3%) had Waldenstrom macroglobulinemia in addition to MGUS. One (3%) patient had evidence of extrarenal involvement (cardiac and liver) in addition to renal damage, although investigations to uncover extrarenal involvement were not performed systematically in most patients.
Baseline characteristics of the 34 patients at diagnosis are shown in Table 1. Patients had a median age of 49.5 years (IQR, 44–59), 20 (59%) were male, and 23 (68%) had hypertension. Baseline hematologic characteristics included: kappa light chain isotype in 31 (91%) patients; median involved free light chain level 129.3 mg/dL (IQR, 31.9–291); median serum free-light-chain ratio (involved/uninvolved) 69.3 (IQR, 14.2–206); M-spike detectable by serum protein electrophoresis (median 0; IQR, 0–0.30) in 11 (32%) patients; median bone marrow plasmacytosis 20% (IQR, 12.8–30); evidence of myeloma-related lytic bone disease on imaging in 9 (26%) patients; median serum albumin 3.95 g/dL (IQR, 3.5–4.2); median β2 microglobulin 5.6 mg/L (IQR, 4.5–8.7); and median lactate dehydrogenase 196 U/L (IQR, 168–216). Baseline renal characteristics included: median eGFR by CKD-EPI 23.2 (IQR, 10.9–42.1); median proteinuria 2700 mg/24 h (IQR, 525.2–5840); nephrotic-range proteinuria (≥3 g/24 h) present in 15 (44%) and hematuria in 5 (15%); and dialysis required at presentation prior to initiation of therapy in 7 (21%) patients.
Table 1.
Baseline Characteristics (N=34)
| Age (median, IQR) | 49.5 (44–59) |
|
| |
| Sex, n (%) | |
| Male | 20 (59) |
| Female | 14 (41) |
|
| |
| Race, n (%) | |
| White | 28 (82) |
| African American | 6 (18) |
|
| |
| Hypertension, n (%) | 23 (68) |
|
| |
| Hematologic characteristics | |
|
| |
| Involved serum free light chain isotype, n (%) | |
| Kappa | 31 (91) |
| Lambda | 2 (6) |
| Kappa & Lambda | 1 (3) |
|
| |
| MIDD subtype, n (%) | |
| HCDD | 2 (6) |
| LCDD | 31 (91) |
| LHCDD | 1 (3) |
|
| |
| Involved FLC level, mg/dL (median, IQR) | 129.3 (31.9–291) |
|
| |
| Involved/Uninvolved free light chain ratio (median, IQR) | 69.28 (14.2–206.1) |
|
| |
| Serum M-protein, g/dL (median, IQR) | 0 (0–0.3) |
|
| |
| Bone marrow plasmacytosis, % (median, IQR) | 20 (12.75–30) |
| Patients with bone marrow plasmacytosis ≧ 10%, n (%) | 26 (77) |
|
| |
| Serum albumin, g/dL (median, IQR) | 3.95 (3.5–4.2) |
|
| |
| β2 microglobulin, mg/L (median, IQR) | 5.6 (4.5–8.65) |
|
| |
| ISS stage, n (%) | |
| I | 5 (15) |
| II | 9 (26) |
| II | 15 (44) |
| Not available | 5 (15) |
|
| |
| Lactate dehydrogenase, U/L (median, IQR) | 196 (168–216) |
|
| |
| Associated hematologic diagnosis, n (%) | |
| Multiple myeloma meeting IMWG criteria aside from eGFR | 25 (74) |
| Smoldering myeloma | 3 (9) |
| MGUS | 4 (12) |
| Chronic lymphocytic leukemia | 1 (3) |
| Waldenstrom macroglobulinemia + plasma cell dyscrasia | 1 (3) |
|
| |
| Patients meeting 2014 IMWG Revised Myeloma Defining Criteria excluding renal criterion, n (%) | |
| Hypercalcemia* | 1 (3) |
| Anemia with hemoglobin <10 g/dL | 15 (44) |
| Anemia with hemoglobin 2 g/dL below normal range** | 3 (9) |
| Osteolytic bone lesions on imaging | 11 (32) |
| Involved/Uninvolved serum FLC ratio >100 | 12 (35) |
| Clonal bone marrow plasmacytosis ≥ 60% | 3 (9) |
| Patients with ≥1 IMWG criteria for MM (excluding renal criterion) | 25 (74) |
|
| |
| Renal characteristics | |
|
| |
| Estimated GFR (eGFR) by CKD-EPI prior to treatment (median, IQR) | 23.2 (10.9–42.1) |
|
| |
| Dialysis prior to treatment, n (%) | 7 (21) |
|
| |
| 24-hr urine protein, mg/24 h (median, IQR) | 2700 (525.15–5840) |
|
| |
| Nephrotic-range proteinuria (≥3 g urine protein/24 h), n (%) | 15 (44) |
|
| |
| Hematuria at diagnosis, n (%) | 5 (15) |
|
| |
| Treatment characteristics | |
|
| |
| Bortezomib-based therapy, n (%) | 19 (56) |
|
| |
| Lenalidomide-based therapy, n (%) | 7 (21) |
|
| |
| Cyclophosphamide, n (%) | 8 (26) |
|
| |
| Dexamethasone alone | 5(15) |
|
| |
| Melphalan + ASCT, n (%) | 23 (68) |
CDK-EPI, Chronic Kidney Disease Epidemiology Collaboration; FLC, free light chain; HCDD, heavy chain deposition disease; IQR, interquartile range; LCDD, light chain deposition disease; LHCDD, light and/or heavy chain deposition disease; MIDD; monoclonal immunoglobulin deposition disease; ISS, International Staging System; MGUS, monoclonal gammopathy of undetermined significance; MGRS, monoclonal gammopathy of renal significance.
serum calcium >0.25 mmol/L (>1 mg/dL) higher than the upper limit of normal or >2.75 mmol/L (>11 mg/dL)
Normal range for hemoglobin: Female 11.2–15.5 and males 12.5–16.2
Among the 28 patients whose renal response could be fully assessed before and after treatment, initial renal presentations included: proteinuria >1 g/24 h with preserved eGFR (n=6, 21%), proteinuria and decreased eGFR (<50 mL/min/1.73 m2) (n=9, 32%), and decreased eGFR <50 mL/min/1.73 m2 without significant proteinuria (>1 g/24 h) (n=13, 46%). Some patients were on dialysis at initial presentation or shortly after prior to therapy (n=7, 25%).
Treatment characteristics
The first-line therapies used included bortezomib (n=19; 56%), lenalidomide (n=7; 21%), cyclophosphamide (n=9; 26%), dexamethasone alone (n=5; 15%), and ASCT as part of consolidation therapy (n=23; 68%) (Table 1). Four patients received solid organ transplantation after their diagnosis with MIDD, including 3 receiving renal allografts and 1 receiving a liver allograft. Two patients required subsequent therapy for recurrent MIDD after organ transplantation but maintained allograft function. Seven patients required hemodialysis at initial diagnosis or shortly after diagnosis but before treatment.
Outcome measurements
The median patient follow-up was 110 months. Hematologic responses, evaluable in 28 of 34 patients at completion of first-line therapy, were sCR (n=15, 54%), CR (n=3, 11%), VGPR (n=8, 29%), and PR (n=2, 7%) (Table 2A).
Table 2A.
Best Hematologic Response to Therapy by IMWG Criteria (n=28)
| Response | n (%) |
|---|---|
| VGPR or better | 26 (93) |
| sCR | 15 (54) |
| CR | 3 (11) |
| VGPR | 8 (29) |
| PR | 2 (7) |
CR, complete response; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
Renal responses were evaluable in 28 patients; 22 (79%, including 7 patients on dialysis at diagnosis) had decreased eGFR at baseline and could be assessed by IMWG renal response criteria, while 6 (21%) had an eGFR >50 mL/min/1.73 m2 at baseline and could not be assessed using these criteria (Table 2B and Figure S1). Among the 22 patients assessed by IMWG criteria, 15 (68%) had a response, including 3 (14%) Renal CR, 4 (18%) Renal PR, and 8 (36%) Renal MR, while 7 (32%) had no response. Among the 7 patients on dialysis at baseline, 3 remained on dialysis despite treatment, while 4 stopped dialysis after treatment, 2 as a result of the treatment (considered responders by IMWG) and 2 after receiving a transplanted renal allograft (considered non-responders by IMWG).
Table 2B.
Best Renal Response to Therapy
| IMWG Renal Response Criteria (n=28) | Total | ||||||
|---|---|---|---|---|---|---|---|
| Not Assessable | Renal CR | Renal PR | Renal MR | No Response | |||
| Amyloid Criteria (n=28) | Not Assessable | 0 | 1 | 3* | 3* | 6** | 13 |
| Response | 6 | 2 | 1 | 5 | 0 | 14 | |
| No Response | 0 | 0 | 0 | 0 | 1 | 1 | |
| Total | 6 | 3 | 4 | 8 | 7 | 28 | |
including 1 patient who came off dialysis
including 5 patients who remained on dialysis
CR, complete response; MR, minor response; PR, partial response.
Among the 28 evaluable patients, 15 (54%) could be assessed using the amyloid criteria since they had significant proteinuria (>1 g/24 h) at baseline, while 13 (46%) could not be assessed using these criteria, including 7 patients on dialysis and 6 without significant proteinuria at baseline (<1 g/24 h) (Table 2B and Figure S1). Among these 15 patients assessed by amyloid criteria, 14 (93%) had a response. Among 9 patients who could be assessed by both criteria, all had concordant results using IMWG or amyloid criteria. A composite response assessment based on both IMWG and amyloid criteria showed that 22 of 28 (79%) assessable patients had a renal response.
Dynamics of renal response and survival
With a median follow up of 110 months, the estimated median overall survival was 136 months (95% CI: 79–NR). Eighteen of 28 patients were alive according to available follow-up at the data cutoff date of May 7, 2021. The median time to best renal response from initiation of treatment was 27 months (95% CI:18–105) (Figure 1A and 1C), showing that, for some patients, the renal function continues to improve for several years after the start of therapy. When censoring patients who died without requiring dialysis, the median renal survival has not been reached (95%CI: NR–NR) at the end of the study. Two patients who were not on dialysis at the start of treatment progressed to end-stage renal disease (ESRD) and required dialysis during the follow-up period. These patients developing ESRD meet this outcome rather early, although the number of events is small in this cohort (Figure 1B and 1C).
Figure 1:
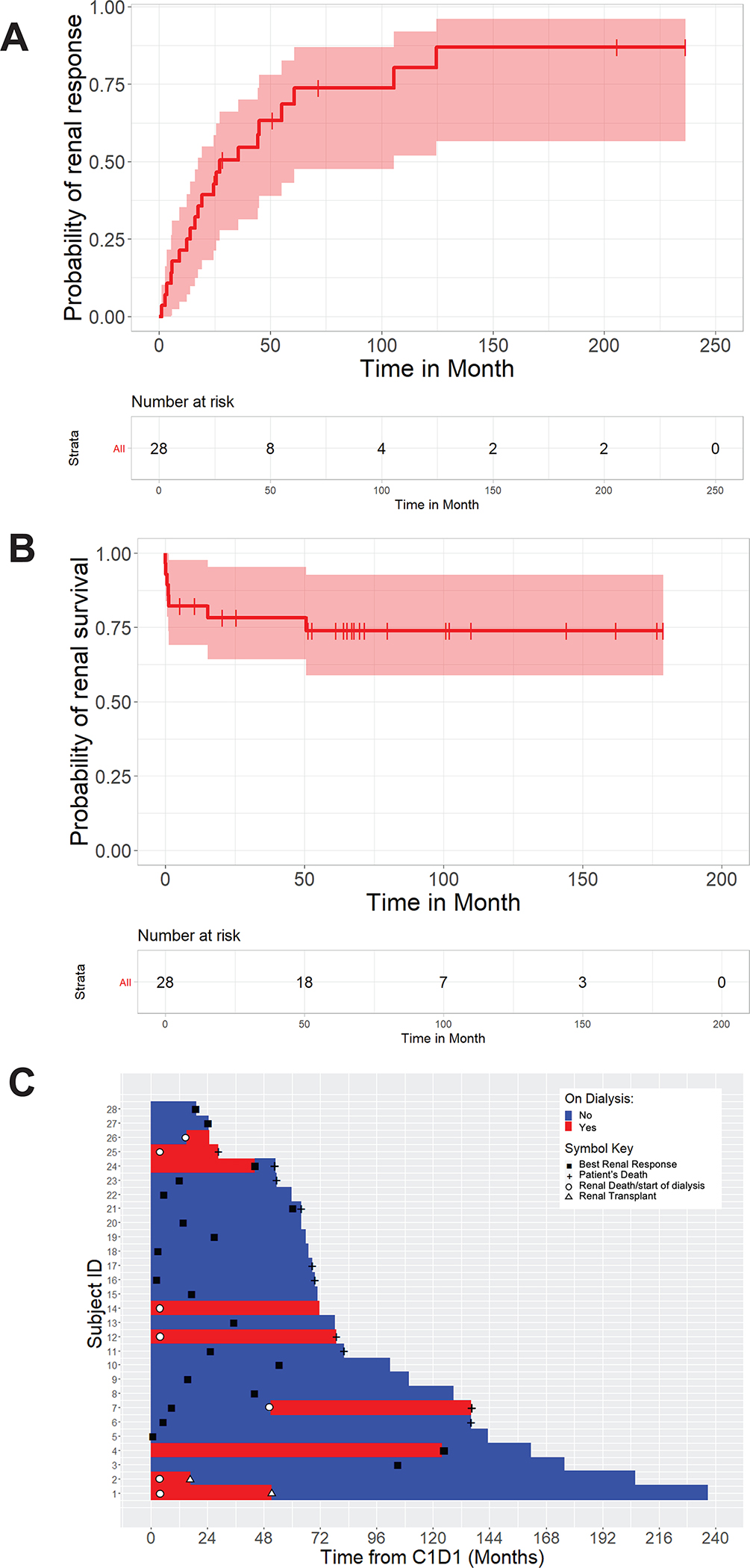
Renal response. (A) Time to best response from start of therapy. (B) Duration of response from start of therapy. (C) Swimmer plot illustrating, for each patient, time to best renal response, time to renal death, and time to last assessment or patient’s death.
By univariate analysis baseline beta-2 microglobulin (β2M) at diagnosis was a factor associated with renal response (p=0.04). eGFR at diagnosis was a factor with suggestive evidence (p<0.07) with all three quartiles having similar positive effect on renal response compared to the first quartile (eGFR level below 10.92). When combining quartiles two, three, and four into a single category, eGFR was associated with renal response (p=0.02). Likewise, beta-2 microglobulin and eGFR at diagnosis were factors strongly associated with renal survival (p=0.01 and p=0.02, respectively). Hematologic response (CR vs. non-CR) was not associated with renal response or survival in this cohort (Table 3).
Table 3.
Factors Associated with Renal Response
| Variable | HR | P-value |
|---|---|---|
|
| ||
| Age (Quartiles) | 0.86 | |
| 45.5–50 | 0.67 (0.21,2.15) | |
| 50–60.75 | 0.87 (0.25,3.03) | |
| >60.75 | 0.61 (0.16,2.34) | |
|
| ||
| Gender (M/F) | 0.85 (0.35,2.02)) | 0.70 |
|
| ||
| Race (Caucasian/African American) | 1 (0.29,3.46) | 0.99 |
|
| ||
| Hypertension (No/Yes) | 1.11 (0.43,2.87) | 0.83 |
|
| ||
| Involved serum free light chain isotype (K/L) | 0.68 (0.15,3.04) | 0.52 |
|
| ||
| MIDD subtype (HCDD/LCDD/LHCDD) | 0.33 | |
| LCDD | 0.38 (0.08,1.73) | |
| LHCDD | 0.91 (0.08,10.25) | |
|
| ||
| 0.75 | ||
| Involved FLC level (quartiles) | 1.13 (0.31,4.09) | |
| 29–105 | 1.89 (0.53,6.8) | |
| 105-240 | 1.04 (0.24,4.43) | |
| >240 | ||
|
| ||
| Involved/Uninvolved FLC ratio (quartiles) | 0.77 | |
| 12.78–66.66 | 1.48 (0.42,5.24) | |
| 66.66–203.58 | 1.86 (0.45,7.66) | |
| >203.58 | 1.02 (0.26,4.02) | |
|
| ||
| 0.51 | ||
| Serum M-protein level (quartiles) | 0.49 (0.13,1.82) | |
| 0.05–0.48 | 1.05 (0.34,3.24) | |
| >0.48 | ||
|
| ||
| Bone marrow plasmacytosis (quartiles) | 0.36 | |
| 12.5–20 | 1.47 (0.54,4.02) | |
| 20–29 | 1.43 (0.17,12.26) | |
| >29 | 0.47 (0.12,1.84) | |
|
| ||
| Serum albumin level (quartiles) | 0.42 | |
| 3.5–3.9 | 1.29 (0.36,4.65) | |
| 3.9–4.2 | 1.27 (0.41,3.99) | |
| >4.2 | 0.42 (0.1,1.74) | |
|
| ||
| Beta 2 microglobulin level (quartiles) | 0.04 | |
| 4.4–5.5 | 0.74 (0.23,2.43) | |
| 5.5–9.01 | 0.98 (0.27,3.6) | |
| >9.01 | 0.13 (0.03,0.66) | |
|
| ||
| Estimated GFR at diagnosis (quartiles) | 0.07 | |
| 10.925–23.2 | 4.7 (1.15,19.22) | |
| 23.2–42.1 | 3.13 (0.73,13.41) | |
| >42.1 | 4.98 (1.25,19.8) | |
|
| ||
| Dialysis prior to treatment (Yes/No) | 0.06 (0.01,0.47) | 0.0004 |
|
| ||
| 24-hr urine protein at diagnosis (quartiles) | 0.49 | |
| 469.25–2488.95 | 0.85 (0.24,2.93) | |
| 2488.95–6002.9 | 1.96 (0.59,6.5) | |
| >6002.9 | 1.5 (0.43,5.26) | |
|
| ||
| Hematuria at diagnosis (Yes/No) | 1.56 (0.51,4.81) | 0.43 |
|
| ||
| Bortezomib-based therapy (Yes/No) | 1.63 (0.61,4.34) | 0.32 |
|
| ||
| Lenalidomide-based therapy (Yes/No) | 0.9 (0.26,3.14) | 0.87 |
|
| ||
| ASCT (Yes/No)* | 0.83 (0.3,2.29) | 0.72 |
|
| ||
| Hematologic response (CR vs <CR)* | 0.62 (0.25,1.54) | 0.29 |
Positive HR is associated with increased probability of response
ASCT, autologous stem cell transplantation; FLC, free light chain; GFR, glomerular filtration rate; HCDD, heavy chain deposition disease; HR, hazard ratio; IQR, interquartile range; K/L, kappa/lambda; LCDD, light chain deposition disease; LHCDD, light and/or heavy chain deposition disease; MIDD; monoclonal immunoglobulin deposition disease; MGUS, monoclonal gammopathy of undetermined significance; MGRS, monoclonal gammopathy of renal significance.
Time-dependent covariate.
Discussion
In this retrospective cohort study of patients with MIDD, 33 of 34 (97%) patients had a plasma cell dyscrasia, with 25 (74%) patients meeting the 2014 IMWG criteria for the diagnosis of symptomatic multiple myeloma, 3 (9%) SMM and 4 (12%) MGUS.30 One patient had a B-cell malignancy associated with an immunoglobulin paraprotein (chronic lymphocytic leukemia) and 1 patient had Waldenstrom macroglobulinemia in association with a plasma cell dyscrasia. Note that the high rate of associated multiple myeloma in this series probably reflects the referral bias of a patient population seen in a cancer center. Among the 28 patients whose hematologic data could be fully assessed, we observed a high rate of hematologic response (64% ≥ CR, 93% ≥ VGPR) as the best response to upfront treatment regimens. A high percentage of patients was treated with bortezomib-based regimens and melphalan-based autologous stem cell transplantation, consistent with prior reports showing these treatment modalities to be highly effective for the management of patients with MIDD.9,13–22
Given that the loss of renal function accounts for the main morbidity for patients with MIDD, we were interested in examining the renal responses to the treatments directed at the underlying neoplasm and analyzing the factors associated with renal response and survival. Despite increasing awareness of the contribution of monoclonal gammopathies to renal injury in patients with MIDD and MGRS in general, post-treatment renal outcomes in patients with MIDD have not been uniformly analyzed in the limited retrospective case series published5–12 and prospective randomized trials with renal response as the primary objective have not been performed. Furthermore, while assessment of hematologic response is standardized and reported according to the well-established IMWG criteria,24 there are no standard renal response criteria universally adopted for MGRS, or MIDD specifically.
The standardized amyloid response criteria, predominantly based on proteinuria and widely adopted by consensus and validated in the context of amyloidosis,27–29 are often used in MGRS, including MIDD, to assess renal response. However, renal dysfunction caused by MIDD is distinct from that caused by amyloid deposition, such that these criteria may have limited applicability. Compared with the amyloidosis population, among patients with MIDD, proteinuria is less common and reduced eGFR is most often the presenting clinical manifestation. Among 28 patients whose renal response was fully assessed, we report only 15 (54%) with significant proteinuria (>1 g/24 h) at baseline; renal response could not be assessed by the amyloid criteria for the other 13 patients, including 7 on dialysis at initial diagnosis. Likewise, IMWG renal response criteria for multiple myeloma,26 based on assessing the improvement in eGFR, is also not suitable for all patients with MIDD, some of whom present with proteinuria only and a fully preserved eGFR. Indeed, among the 28 patients in this cohort, 22 (79%), including the 7 patients on dialysis at baseline, had a decreased eGFR, while 6 (21%) presented with normal eGFR but significant proteinuria (> 1 g/24 h). Nine patients could be assessed by both sets of criteria, and concordant results were observed in all 9.
Overall, we demonstrated that each set of renal response assessment criteria is inadequate in MIDD when considered alone. Hence, we propose combining IMWG and amyloid renal response criteria to account for changes in both proteinuria and eGFR. Using this composite method in our cohort, we observed renal response, following therapy directed at the underlying clonal neoplasm, in a large proportion of patients with renal impairment from MIDD (22 of 28 patients; 79%), including 2 of 7 patients requiring hemodialysis at diagnosis who were able to discontinue dialysis.
Kastritis et al. have previously reported the ratio of 24 h proteinuria to eGFR as a sensitive marker of renal risk in patients with amyloidosis. This ratio accounts for changes in both proteinuria and eGFR.31 In their study, 24-hour proteinuria/eGFR ratio <30 mg/mL/min/1.73 m2 was associated with a 2-year progression to dialysis rate of 0% compared to 9% for a ratio of 31 to 99 and 35% for a ratio >100 (p<0.001). A reduction of this ratio by ≥25% or to <100 (if initially >100) at 3 months was associated with a 2-year progression to dialysis rate of 0% versus 24% for patients not meeting these criteria (p<0.001). While we were unable to confirm these findings due to our small cohort size, we observed that 2 patients who reached ESRD (not including those requiring hemodialysis upfront) had the highest 24 h proteinuria/eGFR ratio at diagnosis among all 28 patients. This observation should be confirmed and validated in large cohorts. It is worth noting that the 24 h proteinuria/eGFR ratio was used in Kastritis’s study as a prognostic factor rather than a tool for measuring renal response.
Interestingly, among the patients with preserved baseline eGFR, who could only be assessed by amyloid criteria, all 6 (100%) had a renal response. In contrast, among the 13 patients who could only be assessed by IMWG criteria, only 7 (53%) had a renal response. Likewise, among the 15 patients who could be assessed by the amyloid criteria, 14 (93%) had a response while among the 22 patients who could be assessed by IMWG criteria, 15 (68%) had a response. This observation suggests that proteinuria is an early manifestation of the disease likely associated with reversible renal damage.
We also noted that the IMWG renal response criteria may lack granularity for assessing response in patients with mild-to-moderate renal insufficiency (eGFR 30–60 mL/min/1.73m2). These patients are classified together in these response criteria. For example, a patient whose eGFR improves from 31 to 59 mL/min/1.73m2 would be considered a non-responder according to the present IMWG criteria. We propose to segregate eGFR levels of 30–45 mL/min/1.73m2 and 45–60 mL/min/1.73m2 to enhance the resolution of the IMWG response criteria (Figure S2). In our proposed simplified model, a Renal MR denotes eGFR improvement by one category and a Renal PR by two categories, while a Renal CR denotes full renal recovery (eGFR >60 mL/min/1.73m2), regardless of the baseline eGFR.
In this series, risk factors associated with renal response and renal survival included baseline eGFR and β2 microglobulin, consistent with previous reports.9,12 These observations confirm that early diagnosis and treatment are crucial to salvaging renal function and reversing further glomerular filtration damage. The renal survival time curve also confirms prolonged renal survival in most patients, except for those losing renal function early in the course of disease, which may be explained by delayed diagnosis (Figure 1B). Hematologic response (CR vs. non-CR or ≥VGPR vs <VGPR) was not associated with renal response (p=0.66) in this cohort, in contrast with most previous reports.8,9,12 This discrepancy almost certainly results from the small number of patients who achieved <VGPR by hematologic criteria in this series, hindering our ability to detect such an association.
In summary, we observed in this retrospective study a high rate of hematologic response to upfront treatment regimens, which is likely attributable to the wide use of bortezomib-based regimens and ASCT. We also observed a large proportion of patients whose renal impairment from MIDD improved significantly after receiving therapy directed at the underlying clonal neoplasm, with 79% achieving a renal response, including 2 out of 7 patients on hemodialysis at diagnosis discontinuing hemodialysis after treatment. Most importantly, we highlight the disparity in the assessment of renal response encountered in the literature and show that IMWG and amyloid renal response criteria are both essential to adequately assess the renal response in MIDD. We thus emphasize the need to develop consensus renal response criteria for this disease, and our study informs the development of such criteria. Given the scarcity of outcome data in MIDD, especially in the era of novel anti-myeloma therapy, prospective studies using uniform renal response criteria are needed to optimize the management of these patients.
Supplementary Material
Acknowledgements:
The reported research was supported in part by the National Institutes of Health/National Cancer Institute (NIH/NCI) Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748). Editorial support in the preparation of the manuscript was provided by Hannah Rice, BA, ELS. K.M. is supported by a Multiple Myeloma Research Foundation Fellow Award, an American Society of Hematology Research Restart Award and a Royal Australasian College of Physicians Robert Maple-Brown Research Establishment Fellowship.
Footnotes
COI Statement: H.H. received consultancy fees and honoraria from Novartis; received research funding from Celgene and Takeda Pharmaceuticals; and served on the advisory committee for Takeda Pharmaceuticals. H.L. served on the advisory committee for Takeda Pharmaceuticals, Celgene, Janssen Pharmaceuticals, Sanofi, and Caelum Biosciences; received honorarium from Sanofi; and received research funding from Takeda Pharmaceuticals. N.K. received research funding from Amgen and Jansen Pharmaceuticals; and consulting fees from Clinical Care Options, Physician Education Resources, and Cancer Network. S.M. received funding from Allogene Therapeutics, Takeda Oncology, Juno Therapeutics, Bristol Myers Squibb, Janssen Oncology, and Fate Therapeutics; has served on an advisory panel for Legend Biotech, Evicore, Janssen Oncology, BioAscend; and has received honoraria from Plexus Communication, OncLive, and Physician Education Resource. D.J.V has received research funding from Genetech. M.H has received research funding from GSK, Amgen, Daiichi Sankyo, consulting fees from Curio Science LLC, Intellisphere LLC, BMS, and served on the advisory committee for GSK. A.L. has received consulting fees from Trillium Therapeutics, Pfizer, Iteos, Sanofi, Genmab, and Janssen; grant funding from Pfizer and Bristol Myers Squibb; payment for lectures and educational presentations from Janssen and COR2Ed; and royalties from Serametrix, Inc. U.A.S received grant funding from Celgene/BMS and Janssen and personal fees from MJH Life Sciences, Association of Community Cancer Centers, MashUpMD and Janssen Biotech outside the submitted work. E.A.J. is shareholder and Chief Medical Officer of Goldilocks Therapeutics, Inc. S.U. has received consulting/advisory fees, grant support and Speakers’ Bureau fees from Amgen, Takeda, Janssen, Sanofi and Bristol-Myers Squibb; consulting/advisory fees and grant support from Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Merck, Seattle Genetics, and Skyline Diagnostics; grant support and Speakers’ Bureau fees from Sanofi; grant support from Array BioPharma and Pharmacyclics; consulting/advisory fees from Karyopharm Therapeutics, Abbvie, Oncopeptides, Genentech, and Gilead. The remaining authors declare no competing financial interests that might benefit from this publication.
References
- 1.Sethi S, Fervenza FC, Rajkumar SV. Spectrum of manifestations of monoclonal gammopathy-associated renal lesions. Curr Opin Nephrol Hypertens. 2016;25(2):127–137. [DOI] [PubMed] [Google Scholar]
- 2.Leung N, Bridoux F, Batuman V, et al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019;15(1):45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaador K, Peeters H, Minnema MC, et al. Monoclonal gammopathy of renal significance (MGRS) histopathologic classification, diagnostic workup, and therapeutic options. Neth J Med. 2019;77(7):243–254. [PubMed] [Google Scholar]
- 4.Leung N, Bridoux F, Nasr SH. Monoclonal Gammopathy of Renal Significance. N Engl J Med. 2021;384(20):1931–1941. [DOI] [PubMed] [Google Scholar]
- 5.Lin J, Markowitz GS, Valeri AM, et al. Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J Am Soc Nephrol. 2001;12(7):1482–1492. [DOI] [PubMed] [Google Scholar]
- 6.Stratta P, Gravellone L, Cena T, et al. Renal outcome and monoclonal immunoglobulin deposition disease in 289 old patients with blood cell dyscrasias: a single center experience. Crit Rev Oncol Hematol. 2011;79(1):31–42. [DOI] [PubMed] [Google Scholar]
- 7.Nasr SH, Valeri AM, Cornell LD, et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7(2):231–239. [DOI] [PubMed] [Google Scholar]
- 8.Sayed RH, Wechalekar AD, Gilbertson JA, et al. Natural history and outcome of light chain deposition disease. Blood. 2015;126(26):2805–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kourelis TV, Nasr SH, Dispenzieri A, et al. Outcomes of patients with renal monoclonal immunoglobulin deposition disease. Am J Hematol. 2016;91(11):1123–1128. [DOI] [PubMed] [Google Scholar]
- 10.Li XM, Rui HC, Liang DD, et al. Clinicopathological characteristics and outcomes of light chain deposition disease: an analysis of 48 patients in a single Chinese center. Ann Hematol. 2016;95(6):901–909. [DOI] [PubMed] [Google Scholar]
- 11.Mohan M, Buros A, Mathur P, et al. Clinical characteristics and prognostic factors in multiple myeloma patients with light chain deposition disease. Am J Hematol. 2017;92(8):739–745. [DOI] [PubMed] [Google Scholar]
- 12.Joly F, Cohen C, Javaugue V, et al. Randall-type monoclonal immunoglobulin deposition disease: novel insights from a nationwide cohort study. Blood. 2019;133(6):576–587. [DOI] [PubMed] [Google Scholar]
- 13.Tovar N, Cibeira MT, Rosinol L, et al. Bortezomib/dexamethasone followed by autologous stem cell transplantation as front line treatment for light-chain deposition disease. Eur J Haematol. 2012;89(4):340–344. [DOI] [PubMed] [Google Scholar]
- 14.Kastritis E, Migkou M, Gavriatopoulou M, Zirogiannis P, Hadjikonstantinou V, Dimopoulos MA. Treatment of light chain deposition disease with bortezomib and dexamethasone. Haematologica. 2009;94(2):300–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jimenez-Zepeda VH, Trudel S, Winter A, Reece DE, Chen C, Kukreti V. Autologous stem cell transplant for light chain deposition disease: incorporating bortezomib to the induction therapy. Am J Hematol. 2012;87(8):822–823. [DOI] [PubMed] [Google Scholar]
- 16.Ziogas DC, Kastritis E, Terpos E, et al. Hematologic and renal improvement of monoclonal immunoglobulin deposition disease after treatment with bortezomib-based regimens. Leuk Lymphoma. 2017;58(8):1832–1839. [DOI] [PubMed] [Google Scholar]
- 17.Royer B, Arnulf B, Martinez F, et al. High dose chemotherapy in light chain or light and heavy chain deposition disease. Kidney Int. 2004;65(2):642–648. [DOI] [PubMed] [Google Scholar]
- 18.Weichman K, Dember LM, Prokaeva T, et al. Clinical and molecular characteristics of patients with non-amyloid light chain deposition disorders, and outcome following treatment with high-dose melphalan and autologous stem cell transplantation. Bone Marrow Transplant. 2006;38(5):339–343. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz EC, Gertz MA, Fervenza FC, et al. Long-term outcome of autologous stem cell transplantation in light chain deposition disease. Nephrol Dial Transplant. 2008;23(6):2052–2057. [DOI] [PubMed] [Google Scholar]
- 20.Hassoun H, Flombaum C, D’Agati VD, et al. High-dose melphalan and auto-SCT in patients with monoclonal Ig deposition disease. Bone Marrow Transplant. 2008;42(6):405–412. [DOI] [PubMed] [Google Scholar]
- 21.Brioli A, Zamagni E, Pasquali S, et al. Long-term follow-up after autologous stem cell transplantation for light- and heavy-chain deposition disease. Bone Marrow Transplant. 2012;47(9):1248–1249. [DOI] [PubMed] [Google Scholar]
- 22.Batalini F, Econimo L, Quillen K, et al. High-Dose Melphalan and Stem Cell Transplantation in Patients on Dialysis Due to Immunoglobulin Light-Chain Amyloidosis and Monoclonal Immunoglobulin Deposition Disease. Biol Blood Marrow Transplant. 2018;24(1):127–132. [DOI] [PubMed] [Google Scholar]
- 23.Kastritis E, Theodorakakou F, Roussou M, et al. Daratumumab-based therapy for patients with monoclonal gammopathy of renal significance. Br J Haematol. 2021;193(1):113–118. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. [DOI] [PubMed] [Google Scholar]
- 25.Kumar SK, Callander NS, Adekola K, et al. Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18(12):1685–1717. [DOI] [PubMed] [Google Scholar]
- 26.Dimopoulos MA, Sonneveld P, Leung N, et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. J Clin Oncol. 2016;34(13):1544–1557. [DOI] [PubMed] [Google Scholar]
- 27.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79(4):319–328. [DOI] [PubMed] [Google Scholar]
- 28.Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124(15):2325–2332. [DOI] [PubMed] [Google Scholar]
- 29.Jain A, Ramasamy K. Time to Redefine Risk-Stratification and Response Criteria in Immunoglobulin Light Chain Amyloidosis? Clin Lymphoma Myeloma Leuk. 2020;20(10):e769–e776. [DOI] [PubMed] [Google Scholar]
- 30.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–548. [DOI] [PubMed] [Google Scholar]
- 31.Kastritis E, Gavriatopoulou M, Roussou M, et al. Renal outcomes in patients with AL amyloidosis: Prognostic factors, renal response and the impact of therapy. Am J Hematol. 2017;92(7):632–639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


