SUMMARY
The mammalian entorhinal cortex routes inputs from diverse sources into the hippocampus. This information is mixed and expressed in the activity of many specialized entorhinal cell types, which are considered indispensable for hippocampal function. However, functionally similar hippocampi exist even in non-mammals that lack an obvious entorhinal cortex, or generally any layered cortex. To address this dilemma, we mapped extrinsic hippocampal connections in chickadees, whose hippocampi are used for remembering numerous food caches. We found a well-delineated structure in these birds that is topologically similar to the entorhinal cortex and interfaces between the hippocampus and other pallial regions. Recordings of this structure revealed entorhinal-like activity, including border and multi-field grid-like cells. These cells were localized to the subregion predicted by anatomical mapping to match the dorsomedial entorhinal cortex. Our findings uncover an anatomical and physiological equivalence of vastly different brains, suggesting a fundamental nature of entorhinal-like computations for hippocampal function.
ETOC Blurb
Applegate et al. find that an area of the bird brain, DL/CDL, is similar to the mammalian entorhinal cortex. These regions have similar connectivity to the hippocampus and cortex, contain cells that have multi-field grid-like spatial firing patterns, and contain cells that respond to the animal’s head direction and running speed.
INTRODUCTION
A defining feature of episodic memories is the binding of different types of internal and external information.1,2 In the mammalian brain these streams of information are not conveyed directly to the hippocampus, a site of memory storage. Instead, signals first converge in the entorhinal cortex – a part of the hippocampal formation that in turn provides input to the hippocampus itself.3,4 Consistent with these convergent signals, entorhinal neurons exhibit a remarkable diversity of firing patterns that encode behaviorally-relevant variables like location, speed, head direction, environmental boundaries, sensory cues, objects, and rewards.5-12 Though the purpose of such an input region is unclear, theories of the entorhinal cortex have proposed its role in compressing information and encoding it in a format that may be optimal for memory storage.13,14 This type of a computation may be a general feature of memory systems.
A challenge to this idea is that many animals – in particular, non-mammals – are adept at hippocampal functions without having an apparent entorhinal cortex. Food-caching birds, for example, rely on the hippocampus to remember locations of many concealed food items in their environment.15,16 Recent work has demonstrated mammalian-like firing patterns, including place cells, in the avian hippocampus.17,18 Yet birds entirely lack a layered cerebral cortex, which in mammals includes an entorhinal region. Rather than propagating along a cortical sheet, signals in the non-mammalian forebrain are communicated between discrete pallial “nuclei”.19 These nuclei are only partially homologous to the mammalian cortex and contain neurons that differ from the cortex in their embryological origins, morphologies, and molecular signatures.20-23 It seems unlikely that the avian brain exactly replicates all details of the mammalian microcircuitry relevant for producing characteristic entorhinal activity patterns, like those of grid cells.24-27 This raises the possibility than entorhinal-like circuitry and firing patterns are not generally needed for hippocampal function.
Another possibility is that birds have an “interface” region between the hippocampus and the rest of the brain that performs similar functions to the entorhinal cortex. If such a region exists, it should share at least some features with the mammalian entorhinal cortex. A hypothetical avian entorhinal analog may or may not share an evolutionary precursor with the mammalian entorhinal cortex, and it could have substantially different cytoarchitecture and microcircuitry. However, this region would still perform computational functions similar to the mammalian entorhinal cortex.
The search for an entorhinal-like region is hampered by substantial uncertainty and disagreement about the structure of the avian hippocampal formation itself. Using different species, methods, and criteria, studies have proposed more than twenty ways of subdividing the hippocampal formation.28-33 The candidate entorhinal cortex has been placed in at least five different locations, ranging from the midline to the lateral wall of the brain.28-30,34,35 Neural recordings in non-mammals have so far been relatively scarce, and few have identified activity patterns uniquely characteristic of the entorhinal cortex.17,36,37 It is therefore unknown whether an entorhinal analog exists in the non-mammalian brain.
We set out to address this question using food-caching birds from the Paridae family, black-capped chickadees and tufted titmice.38,39 Recent work has identified a hippocampal subregion in these species that contains abundant place cells and is likely homologous to the dorsal hippocampus of rodents.17 This subregion, as well as the complementary subregion with fewer place cells, are well-defined starting points for the anatomical mapping of the circuit. We first wanted to understand the organization of inputs into the hippocampus. We then wanted to understand any physiological similarities between these inputs and the mammalian entorhinal cortex. Comparing phylogenetically distinct brains in this way could provide powerful insights into entorhinal function.
RESULTS
An input/output region of the avian hippocampus
Entorhinal cortex provides the largest input into the mammalian hippocampus and is a major target of hippocampal output.3 It also physically occupies the transitional zone between the hippocampus and the rest of the cortex.40 To search for a similar region in birds, we injected retrograde tracers into the hippocampus of black-capped chickadees (N=20 injections in 7 birds). Our tracing revealed three inputs from the avian homologue of the cerebral cortex (the pallium). We first focused on one of these input regions, which was immediately lateral to the hippocampus (Figures 1A-B). Hippocampus-projecting cells in this region formed a sharply defined boundary with the hippocampus. These cells were more densely packed than cells in the hippocampus itself, causing the boundary to coincide with a distinct cytoarchitectural transition. We decided to explore this region further because its position adjacent to the hippocampus was similar to the mammalian entorhinal cortex.
Figure 1. DL/CDL is a major input/output of the chickadee hippocampus.
(A) DAPI-labeled coronal slices of the chickadee brain at three locations. Location in mm anterior. Lateral boundaries of the hippocampus are indicated by arrows. The hippocampus is well-delineated cytoarchitecturally and has a lower cell density than the region lateral to it. Scale bars: 2 mm.
(B) Retrograde labeling of inputs into the hippocampus. Top: coronal slice outlines at three locations showing the hippocampus (light brown) and the region of interest (dotted rectangle). Middle: fluorescence within the region of interest following CTB injection into the hippocampus. Bottom: labeled cells annotated using a blob detection algorithm. At anterior locations, DL – located adjacent and lateral to the hippocampus – is the most prominent region labeled. In intermediate sections, the transition between DL and CDL is seen. At posterior locations, CDL extends laterally into a thin layer at the surface of the brain. Scale bars: 500 μm. From left to right, injections were at locations 3, 2, and 5 (Table S1).
(C) Simultaneous retrograde and anterograde tracing from the hippocampus, using injection of both CTB and a GFP-expressing virus. A two-dimensional color map (inset) was produced by linearly interpolating colors at the four corners, and is used to indicate the amounts of fluorescence produced by the two injections. Axonal fibers are anterogradely labeled by both CTB and GFP and therefore appear in magenta. Cell bodies are retrogradely labeled by CTB only and therefore appear in cyan. The two labels segregate within DL. Scale bar: 250 μm. Higher-magnification confocal images (right) show cell bodies of hippocampus-projecting neurons in dorsal DL and hippocampal axons with axonal terminals in ventral DL. Both injections were at location 2 (Table S1). Scale bar: 50 μm.
Relating this region to known anatomy was nontrivial due to discrepancies between published boundaries of the avian hippocampus. Studies relying on cell densities would place our region outside of the hippocampal formation.41,43,44 Other work, using anatomical tracing and forebrain landmarks like the parahippocampal sulcus, would consider it a part of the hippocampal formation.28,29,45,46 We decided to use the latter set of criteria, by which the bulk of our region corresponded to the dorsolateral division of the hippocampal formation (DL). DL has been previously proposed as one candidate for an entorhinal-like region in birds.28,46-48 The remainder of our region extended posterior-laterally into a very thin layer (Figure 1B). This portion matched the dorsolateral corticoid area (CDL), though it was exceptionally thin in chickadees compared to published species.33,45 There was no apparent cytoarchitectural division between DL and CDL.
We asked whether DL/CDL was also a target of hippocampal output. In the mammalian entorhinal cortex, efferent and afferent connections with the hippocampus are segregated between superficial and deep layers.4 We found that a similar pattern was present in the chickadee brain (Figure 1C). Retrogradely labeled somata were dorsal in DL and CDL, while labeled axonal fibers and terminals occupied a deeper part of these structures. We confirmed that these axons belonged to hippocampal neurons by also labeling them with a GFP-expressing virus (scAAV in Figure 1C) injected into the hippocampus (N=4 additional chickadees). Previous work has found similar dorsal/ventral divisions in DL, suggesting a conserved pattern across avian species.47-49 Like the entorhinal cortex, DL/CDL is therefore bidirectionally connected with the hippocampus and has a separation of hippocampal inputs and outputs.
Topographic organization of inputs into the hippocampus
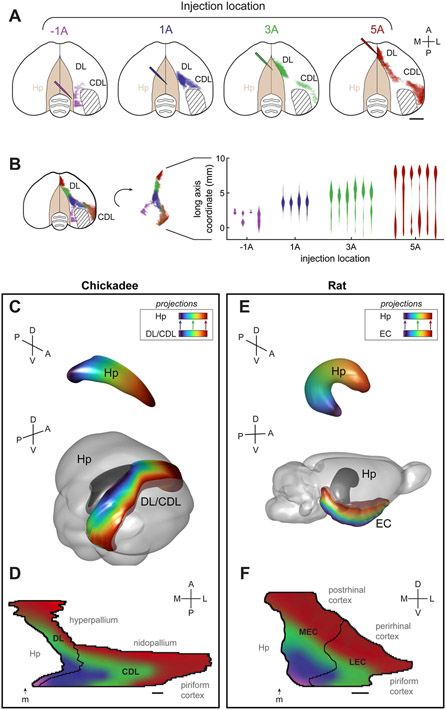
A hallmark of entorhinal anatomy is the topography of its connections with the hippocampus. Different “bands” of the entorhinal cortex project to different sections of the hippocampal long axis, likely contributing to functional differences along this axis.40,50 In birds, the apparent equivalent of the long axis is oriented primarily in the anterior-posterior direction.17,51-53 We therefore made injections of retrograde tracers in four sites along the anterior-posterior axis of the chickadee hippocampus. We constructed a 3D template model of the chickadee brain and registered all labeled cells to this model.
Retrograde labeling indeed revealed a topographic organization of projections to the hippocampus (Figure 2A-B). Each hippocampal injection labeled a long, continuous band of cells in DL/CDL. Bands projecting to different sections of the long axis were roughly parallel to one another, but curved in a complex way along the surface of the brain, without a consistent orientation relative to stereotaxic axes (Figure 2C). The band of cells projecting to posterior hippocampus included posterior DL and medial CDL. Progressively anterior parts of the hippocampus received inputs from the more anterior parts of DL and more lateral parts of CDL (p<0.001, linear regression for both).
Figure 2. DL/CDL inputs into the hippocampus are topographically organized.
(A) Dorsal views of the brain, showing the density of retrogradely-labeled cells in DL/CDL from each of the hippocampal injection locations. Each color is applied on a logarithmic scale and individually normalized to its peak fluorescence. Hashed patch in the 5A injection shows an area that was too close to an injection site, and where cells were not annotated. Diagonal hash indicates region the region of CDL that is predominantly a fiber tract and did not contain any labeled neurons. Injections at 1A, 3A, and 5A were performed in the same bird; injection at −1A is from a different bird. Posterior injections label cells in posterior DL and medial CDL. Progressively anterior injections label more anterior DL and more lateral CDL. Scale bar: 2 mm.
(B) Left: all retrograde labeling pooled from (A) and a cartoon showing the orientation of the DL/CDL long axis. Right: Violin plots showing the densities of retrogradely labelled cells along the DL/CDL long axis from every injection. Zero is the location of lambda on the DL/CDL long axis.
(C) Bottom: 3D model of a template chickadee brain showing the hippocampus and DL/CDL. Each part of DL/CDL is colored according to the main part of the hippocampus that it projects to, from posterior (blue) to intermediate (green) to anterior (red). Colors are smoothed using a gradient; gradient contours were drawn after registering data like those shown in (A) to the template brain. Top: 3D model of the hippocampus, colored from anterior to posterior using the same color map.
(D) Flattened map of DL/CDL formed by unfolding outlines of coronal slices in the chickadee brain. All brain regions bordered by DL/CDL are indicated. Arrow labeled “m” shows the location of the medial ridge, which was used to align outlines of different slices to each other. Map is colored using the same gradient as in (C). Scale bar: 1 mm.
(E) Model of the hippocampus and the entorhinal cortex in the rat, constructed using published data (see text). Color gradient is the same as in (C), but indicates location along the dorso-ventral (long) axis of the rat hippocampus.
(F) Flattened map of the entorhinal cortex constructed as in (D), but using horizontal slices of the rat brain. Scale bar: 1 mm.
We sought to compare the topographic organization of hippocampal inputs between chickadees and mammals. We noted that hippocampus-projecting neurons in DL/CDL were near the brain surface (within ~0.5 mm) and did not have an appreciable topographic organization across depth. This allowed us to treat DL/CDL as a two-dimensional structure and construct its flattened map using classic cortical “unfolding” techniques that are usually not possible in the nucleated avian brain.40,54 Figure 2D shows a map constructed by unfolding outlines of annotated coronal slices of the chickadee brain.
Compared to its avian homolog, the rodent hippocampus appears to be rotated in stereotaxic coordinates by approximately a 90-degree “backward pitch” (Figure 2E). Because of this rotation, the anterior-posterior axis of the bird hippocampus is likely equivalent to the dorso-ventral axis in rodents.17,51-53 It occurred to us that the same transformation may extend to neighboring entorhinal-like regions. In rodents, the entorhinal cortex borders the hippocampus medially on the posterior surface of the brain, then extends to the lateral and ventral surfaces. In birds, DL/CDL also borders the hippocampus medially, but on the dorsal brain surface (Figure 2C). It then extends onto the lateral and posterior surfaces. To compare maps constructed from avian coronal slices, we therefore used horizontal slices of the rat brain, which are related to coronal slices by a 90-degree pitch rotation. Figure 2F shows such a map constructed from published data.40,55-57
Flattened maps of the chickadee DL/CDL and the rat entorhinal cortex were remarkably similar. Both regions bordered the hippocampus medially and cortical (pallial) structures laterally. In rats, cortical structures included the piriform cortex and neocortical areas (postrhinal and perirhinal cortices). In chickadees, structures in the same relative positions also included the piriform cortex, as well as hyperpallium and nidopallium. In both species, topographically organized bands of hippocampal projections were oriented orthogonally to the border with the hippocampus. Therefore DL/CDL and the entorhinal cortex were oriented similarly relative to other brain areas and had topologically equivalent organizations of hippocampal connections. Interestingly, the positions of medial and lateral entorhinal cortices (MEC and LEC) were similar to DL and CDL, suggesting a possible analogy of these regions.
Pathways for cortical inputs into the hippocampus
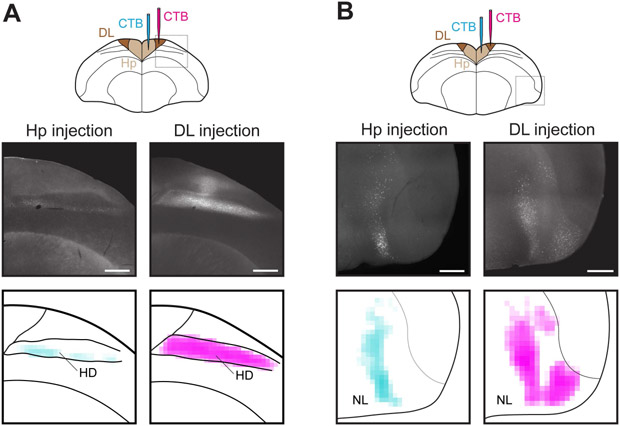
We asked what role DL/CDL played in relaying cortical inputs into the hippocampus. In mammals, many cortical regions project to the entorhinal cortex, but only a small subset of these project directly to the hippocampus.3 We sought to perform the same comparison in birds. We focused on DL, which is the larger part of the input structure in chickadees and could be targeted using conventional retrograde tracing techniques. Direct inputs into the hippocampus, besides DL/CDL, included two cortical structures, consistent with those described in other avian species.28,35,47 One of these was hyperpallium densocellulare (HD; Figure 3A). This region is primarily the target of the thalamofugal visual pathway, though it might also integrate other sensory modalities.58,59 The other input originated in the lateral part of the nidopallium (NL), including its frontal (NFL) and intermediate (NIL) portions (Figure 3B). This region is likely the target of the tectofugal visual pathway.60
Figure 3. DL/CDL is the main target of cortical pathways into the hippocampus.
(A) Top: outline of a coronal slice of the chickadee brain, showing injections of CTB into the hippocampus and into DL. Middle: examples of retrograde tracing in HD. Bottom: densities of retrogradely-labeled cells in HD in the same example images. Each color is applied on a logarithmic scale and normalized to the maximum across both plots. Whereas hippocampal injections label only the ventral part of HD, DL injections label the entire dorso-ventral extent of HD. Scale bar: 1 mm. Injections were at locations 2 and 6 (Table S1).
(B) Retrograde labeling in NL, shown as in (A). Injections were at locations 5 and 8 (Table S1).
See also Figure S1.
We next injected retrograde tracers into DL (N=13 injections in 9 birds). We found that cortical inputs into DL originated from the same two structures, HD and NL (Figures 3A-B). However, these inputs included additional portions of the structures that did not project directly to the hippocampus. In HD, hippocampus-projecting neurons were limited to the ventral portion of the region, whereas DL-projecting neurons occupied the entire dorso-ventral extent (Figure 3A). This resulted in a larger volume of HD projecting to DL than to the hippocampus (Figure S1A). In NL, the DL-projecting cells occupied an additional lateral portion that only sparsely projected to the hippocampus (Figure 3B). This also resulted in a larger DL-projecting volume of tissue, especially if only the lateral part of NL was considered (Figure S1B). Our results show that DL/CDL, like the entorhinal cortex, is the favored target of the cortical pathway into the hippocampal formation.
Spatial activity in DL
Our anatomical mapping led to clear predictions about neural activity in DL/CDL. In particular, the anterior portion of DL appeared to be equivalent to the dorsal MEC of rodents40 – the subregion renowned for spatially selective firing.5,61 We recorded DL neurons by adapting one-photon calcium imaging62 for freely-behaving tufted titmice – a species from the same family as chickadees that is larger and therefore more amenable to head-mounted microscopes (Figures 4A-B). As in chickadees, titmouse DL provided a topographic input to the hippocampus (Figure S2). We trained titmice to forage for scattered seeds in an environment comparably sized to those in which periodic firing patterns were observed in rodents (91x91 cm).5 Our approach was to first analyze DL activity for specific types of patterns that have been described in the dorsal MEC. Afterwards, we fit activity with a statistical model that allowed for complex types of selectivity.
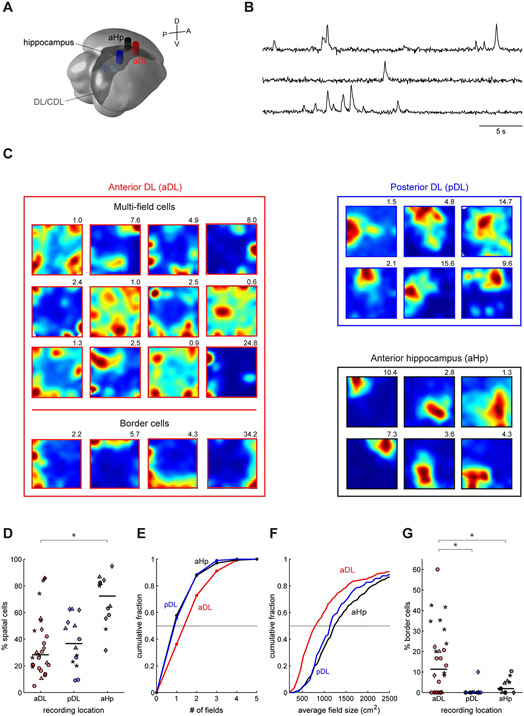
Figure 4. Cells in anterior DL exhibit multi-field patterns of spatial activity.
(A) Locations of three imaging coordinates in the titmouse brain. Each cylinder indicates the position and extent of the GRIN lens on the brain surface.
(B) Example calcium traces simultaneously recorded in anterior DL using a head-mounted one-photon microscope. Fluorescence is in arbitrary units extracted by the CNMF_E algorithm.94
(C) Examples of spatial maps from cells recorded in each of the three regions. For each cell, color map ranges from 0 (blue) to 99th percentile of the pixels (red). Maximum firing rate in calcium events per second is shown above each map. Most spatial cells in anterior DL exhibit multiple firing fields, whereas typical cells in posterior DL and the hippocampus exhibit one larger firing field. Examples are intentionally chosen to show multi-field cells we discovered in anterior DL and illustrate the main difference between the regions; an unbiased selection of firing patterns is shown in Figure S3.
(D) Fraction of cells identified that were significantly spatial in each of the recording sessions. Different symbols are used for different birds. Horizontal lines indicate medians. Spatial cells were more prevalent in the anterior hippocampus (p<0.001 Wilcoxon rank-sum test), but were abundant in all three recorded regions.
(E) Cumulative histograms of firing field size for spatial cells in each of the recorded regions.
(F) Cumulative histograms of the number of firing fields per spatial cell in each of the recorded regions.
(G) Fraction of spatial cells identified as border cells in each of the recording sessions. Different symbols are used for different birds. Border cells were more abundant in anterior DL than in posterior DL (p<0.001, Wilcoxon rank-sum test) and anterior hippocampus (p<0.05).
We recorded 1270 cells in anterior DL of four birds. A large fraction of the cells in this region were spatial (39±8% across birds, mean±SEM here and elsewhere, Figures 4C-D, S3). Most spatial cells displayed multi-field firing maps that were unlike those previously described in birds.17 Of the 405 spatial cells, 258 (64%) had more than one firing field, with many cells having three or more fields (Figure 4E). These cells superficially resembled grid cells, but their fields were not arranged in a periodic lattice (none of the cells passed the standard test for “gridness”).63 This irregular distribution of firing fields resembled MEC activity in three-dimensional tasks,64,65 even though our birds moved primarily in two dimensions during the experiment. Like in flying bats,65 firing fields of some cells were spaced more regularly than expected by chance – suggesting that some local organization was present in DL activity (Figure S4). By analogy with the study on bats, we refer to these cells as “grid-like” even though their firing fields do not conform to a grid. Multi-field cells fired throughout the entire environment, with some bias toward the corners of the arena (Figure S5).
Are multi-field firing patterns unique to DL, or do they result from our experimental parameters, like the use of a large environment?66-68 To test this, we recorded 463 cells in the anterior hippocampus in four additional titmice. As in prior work,17 the hippocampus in our recordings exhibited abundant spatial activity (69±3% of cells across birds, Figures 4C-D, S3). Although some of these cells had multiple fields, they had on average fewer fields than cells in DL (p<0.001 Chi-squared test, Figure 4E). In contrast to DL neurons, most hippocampal spatial cells had only one firing field (58%). Fields in the hippocampus were also larger than in DL (p<0.001 Wilcoxon rank-sum test, Figure 4F). Therefore, multi-field firing patterns are not ubiquitous in the hippocampal circuit, but may be more typical of DL.
Next, we asked whether spatial representations differed along the anterior-posterior axis of DL. MEC in rodents has a dorso-ventral organization that parallels its topographic connections with the hippocampus.5,40,69,70 Whereas dorsal MEC has finer spatial coding, with neurons exhibiting larger numbers of smaller firing fields, coding in ventral MEC is coarser. We recorded 274 neurons in the posterior part of DL in four additional titmice. Many of the neurons in this region were spatial (38±9% across birds, Figures 4C-D, S3), but exhibited larger firing fields than neurons in anterior DL (Figure 4F, p<0.001 Wilcoxon rank-sum test). Compared to anterior DL, these cells had fewer fields, with most cells having only one (p<0.001 Chi-squared test, Figure 4E). The anterior-posterior axis of DL is therefore organized functionally, much like its mammalian counterpart in MEC.
Another canonical feature of spatial activity in the mammalian MEC are border cells that fire near one or multiple boundaries of the environment.61 In anterior DL we also observed abundant cells with firing near the walls (Figure 4C). Eighty-three cells (21% of the spatial cells or 7% or all cells) were considered border cells by the standard analyses, which is similar to mammalian dorsal MEC (10-11% of cells).61,71 Of these cells, 41% had fields along multiple walls, also similar to mammals (about 1/3).61 Unlike in anterior DL, border cells were nearly absent in the anterior hippocampus (4% of the 313 spatial cells) and posterior DL (1% of the 104 spatial cells, Figure 4G). Border cells, like multi-field activity, may therefore also be a specialization of anterior DL.
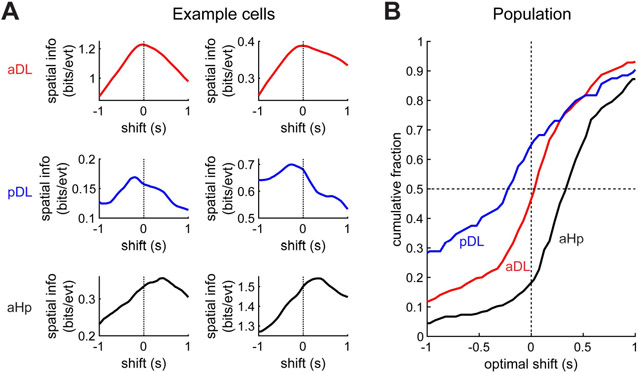
Finally, we examined the temporal characteristics of these spatial neurons. In all species tested, including birds, hippocampal is most strongly correlated to the animal’s position in the future.17,72-74 In MEC, however, activity is less prospective, with latencies closer to zero.75,76 We measured spatial information encoded by each cell’s activity at various lags relative to the behavioral trajectory and determined the lag at which spatial information was maximized (Figure 5A). As in previous studies, most spatial cells in the hippocampus had a positive (prospective) lag with an average of 350±40 ms (p<0.001 t-test, N=313 cells, Figure 5B). In contrast, the lag was close to zero in DL, and even significantly negative (retrospective) in posterior DL (−40±40 ms and −400±100 ms in anterior and posterior DL, N=405 and 104 cells, p<0.001 comparison to the hippocampus in both cases). These analyses show that hippocampal inputs from MEC in mammals and from DL in birds are not only spatially similar, but have analogous temporal characteristics.
Figure 5. DL, like the entorhinal cortex, is less prospective than the hippocampus.
(A) Examples of six cells in the three recorded titmouse brain regions. For each cell, spatial information is quantified at different temporal shifts of spikes relative to the behavioral trajectory. The optimal shift tends to the positive (prospective) for the hippocampus, close to zero for anterior DL, and retrospective (negative) for posterior DL.
(B) Cumulative histograms of the optimal shifts for cells in each of the recorded regions.
Representation of other navigational variables in DL
Mammalian MEC encodes an array of navigational variables other than position – notably head direction and speed.6,8 We found both of these variables represented by titmouse DL neurons. In anterior DL, 34±5% of cells across four birds had significant head direction tuning (Figures 6A-B), and 40±9% of cells had significant speed tuning (Figures 6C-D). Speed cells included those with positive and those with negative correlations between speed and firing rate, though the former were more common (77%, p<0.001 Chi-squared test). Consistent with recordings along the dorso-ventral axis of MEC,77 we found head direction and speed cells in both anterior and posterior DL (Figures 6B,D). As in previous studies, these types of cells were also found in the hippocampus (Figures 6B,D).
Figure 6. DL represents a variety of navigational variables other than location.
(A) Examples of head direction cells in anterior DL, showing two cells with narrow tuning (top) and two cells with broader tuning (bottom). Each plot shows firing rate as a function of head direction in polar coordinates. Peak firing rate in calcium events per second is indicated for each cell.
(B) Fraction of cells identified as head direction cells in each of the recording sessions. Different symbols are used for different birds. Horizontal lines indicate medians. Head direction cells are found in all three regions, with higher abundance in the hippocampus (p<0.001 Wilcoxon rank-sum test).
(C) Examples of speed cells in anterior DL, showing three cells with positive speed tuning and one with negative speed tuning.
(D) Fraction of cells identified as speed cells, plotted as in (B). Speed cells were found in all three regions.
(E) Example 3 min trajectory of the titmouse overlaid on a 3D schematic of the recorded arena. Behavioral data points are recorded every 50 ms. Trajectory indicates periods of relative immobility separated by fast, ballistic “dashes”.
(F) Example recording of the bird’s speed, showing four dashes.
(G) Examples of cells in anterior DL with modulation by time relative to the dash. Zero indicates dash onset. Each trace is an average across all dashes recorded in the session.
(H) Fraction of cells identified as dash-modulated cells, plotted as in (B). Dash-modulated cells were found in all three regions, but were more abundant in anterior DL compared to the anterior hippocampus (p<0.05 Wilcoxon rank-sum test).
(I) Activity of all dash-modulated cells in anterior DL, sorted according to the time of the peak firing rate. Each cell’s activity is normalized from 2.5th (blue) to 97.5th (red) percentile of its firing rates in the plotted window. Firing of all cells is concentrated around 0.5-1 s relative to the dash, but forms a sequence spanning the entire event.
Avian behavior offers an additional opportunity to study how entorhinal-like activity represents the animal’s movement. Titmice, like most small passerine birds, move on the ground by hopping rather than walking. In a foraging task, they approach a food target via a ballistic sequence of hops (“a dash”) lasting about 1 s (Figures 6E-F). We found that activity of many cells in anterior DL was modulated by these dashes (30±8% across birds, Figures 6G-H). Much of the activity was concentrated between 0.5 and 1.5 s after dash onset, roughly corresponding to the time birds were approaching the target. However, different cells had different latencies, forming a sequence of activity that spanned the entire dash (p < 0.001 shuffle test, Figure 6I). This activity could not be explained entirely by speed tuning: for example, cells that fired at the end of the dash did not generally fire during similar speeds at the beginning of the dash (p<0.001, paired t-test). We also found some dash tuning in the posterior DL and the anterior hippocampus, but it was weaker and involved fewer cells than in the anterior DL (Figure 6H). Altogether, our results show that DL represents a remarkable diversity of behaviorally-relevant variables in a spatial task.
Mixed selectivity of DL neurons
How are the different encoded variables organized across the DL population? For each pair of variables we considered (location, head direction, speed, and time relative to a dash), we found DL cells representing both variables, only one variable, or neither (Figure 7A). Across the population, there was no evidence of distinct classes of cells encoding individual variables. These results suggest that DL might recapitulate an important property of the entorhinal cortex and mammalian cortex in general – the mixed selectivity of cells to task-related parameters.6,78-80
Figure 7. DL exhibits mixed representation of navigational variables.
(A) Representation of pairs of variables by anterior DL neurons. For each pair of variables (location, head direction, speed, and time relative to dash), each cell’s selectivity to those variables is shown. For each pair, there are cells representing neither variable, representing only one variable, or representing both.
(B) Examples of two cells identified by the linear-nonlinear-Poisson model as being modulated by all four analyzed variables. Conventional tuning curves are plotted, as in Figures 4 and 6.
(C) Venn diagram of anterior DL cells, classified according to what subset of the four variables they were modulated by. The diagram classifies 1002 cells that were considered by the model to be modulated by at least one variable; an additional 268 cells were not modulated by any variable.
(D) Examples of head direction tuning curves showing multiple peaks. These cells were not detected as head direction cells by standard analysis, but were identified as head direction-modulated by the model.
(E) Examples of non-motonotic speed tuning identified by the model.
(F) Examples of complex modulation by time relative to dashes identified by the model.
See also Figure S6.
So far, we have focused on searching for specific, hand-picked types of neurons inspired by the mammalian literature. We next sought to use a less biased approach to characterize DL activity. First of all, we wanted to account for complex tuning curves – e.g., tuning of a cell to multiple head directions. Second of all, we wanted to account for possible conjunctive tuning of individual cells to multiple variables. Finally, we wanted to account for arbitrary correlations between these variables. For example, due to biases in an animal’s trajectory, a place-selective cell might appear to be tuned to head direction. We implemented a linear-nonlinear-Poisson model based on one developed for rodent MEC.79 This model fits each cell’s activity as a linear combination of responses to multiple behavioral variables. The tuning curve for each variable is allowed to be an arbitrarily complex shape.
The model detected 79% of the 1270 cells in anterior DL as selective to at least one of the behavioral variables. The remaining minority of cells were best fit by a constant firing rate fit to the entire behavioral session (but, of course, could be selective for some variables we did not measure). Many cells were best fit by a combination of two, three, or four variables, with every possible subset of these variables present in the recorded population (Figure 7B-C). In anterior DL, 40±10% of these neurons were selective for multiple variables, which is similar to the fraction in dorsal MEC (37%).79 In some cases, tuning curves exhibited complex shapes: for example, some cells had non-monotonic tuning for speed, while others had multi-peaked modulation by head direction and dashes (Figures 7D-F). Similar types of complex tuning and mixed selectivity were also found in the posterior DL and the hippocampus (Figure S6). Overall, our results demonstrate highly complex, mixed representations of navigational variables in the hippocampal system of food-caching birds. Much like in the mammalian MEC, these multiplexed representations are already present in DL – the major input into the hippocampus.
DISCUSSION
We have identified a brain region (DL/CDL) in food-caching birds that has many anatomical and physiological similarities to the mammalian entorhinal cortex. DL, the medial part of this region, is one of several previous candidates that had been proposed as an avian entorhinal analog.28-33 Anatomically, this region is located at the interface between the hippocampus and the rest of the cortex, and appears to be the main route of information in and out of the hippocampus. Like the entorhinal cortex, it is topographically connected with the long axis of the hippocampus and parallels the hippocampal organization of activity along this axis. Physiologically, DL contains most cell types identified in rodents during foraging tasks – including multi-field cells, border cells, head direction cells, speed cells, and neurons with complex conjunctive representations. These similarities are present in spite of birds being highly divergent from mammals in brain development and architecture, most notably lacking a layered cortex. An avian structure with so many characteristic features of the entorhinal cortex was therefore unexpected, and emphasizes the fundamental role these features likely play in hippocampal computation.
In addition to analogous spatial representations in the mammalian MEC and avian DL, we found an interesting similarity in the timing of these signals. Whereas the hippocampi of both mammals and birds are predictive of future location, their inputs from both MEC and DL are much less predictive.17,72-76 Converting incoming signals into a prediction of the future may therefore be a function of the hippocampus itself, and this function might be conserved across vertebrates. This computational role of the hippocampus is consistent with theoretical work on predictive representations.13
In addition to the similarities, there were also notable physiological and anatomical differences between mammalian and avian circuits. Physiologically, there were some differences in the fractions of different cells types. Both DL and the hippocampus had more speed cells than their mammalian counterparts (~30% vs. 15%).8,81 The avian hippocampus also had a large fraction of head direction cells (65%). Some of these differences are difficult to interpret because the mammalian hippocampal formation is heterogeneous. The fraction of head direction cells we observed in birds might be unexpected compared to the rodent CA1 (~25%) but is in line with the presubiculum (50-60%).82-84 The same situation is true in the anterior DL, where head direction cells were about 35% of the population. This is low compared to the expected fraction in layers III and V of MEC (60-70%), but high compared to layer II (about 0%).6,77 Understanding which of the differences between species are genuine will require a more detailed mapping between mammalian and avian circuits.
Another major difference between DL and the mammalian entorhinal cortex is the lack of cells with regularly arranged grid firing fields. The fields we observed also tended to be larger and more widely spaced than those seen in mammals.70 The firing in DL instead resembled irregular patterns reported in MEC during 3D navigation.64,65 One of these studies has proposed that switching between regular and irregular arrangements of fields can be achieved by changing a single “temperature” parameter in a spatial system.65 Perhaps avian DL is in a parameter regime that produces irregular arrangements of fields even during 2D navigation. Alternatively, some models have suggested that field arrangement can be influenced by the statistics of movement in the environment,13 which is also different between hopping birds and walking mammals. Besides the lack of periodicity, the cells we recorded in birds generally had a larger spacing of fields than the cells found in mammals. Overall, our results add to the growing body of evidence that, even though multi-field firing patterns are fundamental to the entorhinal cortex, their regularity is not a requirement for the function of this region.
Several previous studies have presented evidence linking DL to the entorhinal cortex. For instance, anatomical tracing and electrical stimulation in pigeons have suggested a general pattern of information flow in the lateral-to-medial direction within the hippocampal formation.35,47,48 Some authors have proposed that the lateral parts of the circuit are therefore analogous to the entorhinal cortex.28,46,47 However, a sharp delineation of input/output subregions in the hippocampal formation has not been found in other species so far, and might be unique to chickadees. The entire hippocampal circuit is larger in chickadees and other food-caching birds, so it is possible that a specialization of distinct subregions has accompanied this evolutionary enlargement. Such specialization might be a common phenomenon across other enlarged neural systems, such as the visual system in primates and the song system in songbirds.88,89
Unlike DL, CDL – the lateral part of the region we identified – has not been generally proposed as an entorhinal analog. According to our anatomical analysis, CDL seems to occupy the same position as the rodent LEC (Figure 2). In rodents, LEC borders the piriform cortex and is the primary source of olfactory inputs into the hippocampus,90,91 though it also processes other sensory modalities.92,93 CDL in birds is also adjacent to the piriform cortex and in other species receives inputs from the olfactory system.45,94 This role might explain its exceedingly small size in chickadees, which barely use smell and have one of the smallest olfactory bulbs among vertebrates.95 It remains to be seen whether CDL has functional similarities to LEC. Neural recordings of this region might be more practical in other avian species.
What accounts for the presence of an entorhinal-like region in birds? There are at least three explanations, which are not mutually exclusive. One possibility is that DL/CDL and the entorhinal cortex are derived from the same evolutionary precursor and have conserved some of the same ancestral functions. Our topological analysis places DL/CDL in the same location as the entorhinal cortex relative to other brain regions – at the lateral terminus of the hippocampal transverse axis. This axis shares multiple gene expression signatures between mammals and non-mammals,21,96 suggesting that DL/CDL and the entorhinal cortex might also be transcriptionally similar. It is conceivable that even partially conserved cells types and connectivity are sufficient to account for the entorhinal-like activity patterns we observed. Of course, there are also likely to be significant transcriptional differences between DL/CDL and the entorhinal cortex. DL/CDL might also combine the genetic features of multiple adjacent mammalian regions – much like a region recently described in amphibians that combines entorhinal and subicular cell types.22 Detailed transcriptomics data in birds will hopefully shed light on these possibilities.
A second possibility is that entorhinal-like activity in birds is the result of convergent evolution. A large body of theoretical work has identified the benefits of entorhinal-like activity patterns in a spatial memory system. For example, multi-field representations with different field spacings across cells are a particularly efficient way to encode spatial location by neural activity.14,68,97 Activity of head direction cells and border cells is thought to be indispensable for the spatial computations in the circuit.6,61,98 Work across brain regions has also shown how complex forms of mixed selectivity, like the ones observed in DL/CDL and the entorhinal cortex, can increase the computational capacity and flexibility of a neural network.6,78,79,99 It is possible that evolutionary pressure has forced even unrelated neural circuits to produce similar entorhinal-like patterns of activity.
The third possibility is that some entorhinal-like patterns of activity arise naturally within the hippocampal circuit. For instance, recent work has shown that grid cells can appear in neural networks that are simply trained to perform certain spatial navigation tasks.101,102 This process might not require highly specific circuitry, but could occur in an arbitrary recurrent network exposed to appropriate behavioral demands. Other work has shown that grid-like firing can result from dimensionality reduction operations on the activity of place cells.13 These types of operations could be implemented naturally by a network in which a smaller number of neurons receive feedback from a larger population of hippocampal neurons103 – a general architecture that appears to characterize both the avian DL/CDL and the mammalian entorhinal cortex.
Studies in mammals have focused on the roles of specific cell types in generating entorhinal activity. They have also begun to characterize in detail the microcircuit organization, connectivity, and plasticity rules within this brain area. Although some of these details may be similar between birds and mammals, many are likely to be quite different. The discovery of an entorhinal-like region in birds allows for a potentially powerful comparative approach to study each of these features. It may indicate which features of entorhinal anatomy and physiology are fundamental to entorhinal function, and which ones are specializations unique to particular species of animals.
STAR METHODS
Resource Availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dmitriy Aronov (da2006@columbia.edu).
Materials availability
This study did not generate new unique reagents. All biological materials other than birds are commercially available.
Data and code availability
The following data will be located on Dryad upon publication: For the retrograde tracing, we annotate the stereotaxic location of all labeled cells and report the injection location (Table S1), assigned brain region, bird identity, and fluorophore of CTB used. For the calcium imaging experiments, we include annotations of all sessions. For every timepoint of the trial, we include the animal’s location, head direction, and the calcium activity of each ROI detected with CNMF_E.106 The DOI for these data sets are listed in the key resources table. Microscopy data reported in this paper will be shared by the lead contact upon request.
All custom code used in analysis will be located on Dryad upon publication.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| scAAV9-CBh-GFP | UNC Vector Core | N/A |
| AAV9-pAAV.CAG.GCaMP6f.WPRE.SV40 | Chen et al.104 | Addgene plasmid # 100836 |
| Chemicals, peptides, and recombinant proteins | ||
| Cholera Toxin Subunit B, Alexa Fluor 488 conjugate | Fisher Scientific | C34775 |
| Cholera Toxin Subunit B, Alexa Fluor 555 conjugate | Fisher Scientific | C34776 |
| Cholera Toxin Subunit B, Alexa Fluor 647 conjugate | Fisher Scientific | C34778 |
| 4',6-diamidino-2-phenylindole, dihydrochloride (DAPI) | Fischer Scientific | PI62247 |
| Paraformaldehyde | Electron Microscopy Sciences | 15710 |
| Critical commercial assays | ||
| nVista System | Inscopix | N/A |
| Miqus motion capture cameras | Qualysis | N/A |
| Deposited data | ||
| Retrograde tracing | This paper | DOI upon publication |
| Calcium imaging | This paper | DOI upon publication |
| Experimental models: Organisms/strains | ||
| Poecile atricapillus (Black-capped chickadee) | Wild caught (NY state) | N/A |
| Baeolophus bicolor (Tufted titmouse) | Wild caught (NY state) | N/A |
| Software and algorithms | ||
| ImageJ | NIH, open source | N/A |
| MATLAB 2020a | Mathworks | https://www.mathworks.com |
| BONSAI | Lopes et al., 2015105 | https://bonsai-rx.org |
| Arduino | Open-source | https://www.arduino.cc/en/software |
| Illustrator CC 2021 | Adobe | https://www.adobe.com/products/illustrator.html |
Experimental model and subject details
All animal procedures were approved by the Columbia University Institutional Animal Care and Use Committee and carried out in accordance with the US National Institutes of Health guidelines. The subjects were 20 black-capped chickadees (Poecile atricapillus) and 14 tufted titmice (Baeolophus bicolor) collected from multiple sites in New York State using Federal and State scientific collection licenses. Subjects were at least 4 months old at the time of the experiment, but age was not determined more precisely.
Chickadees were chosen for the anatomical experiments because it was easier to collect them in the numbers needed for these experiments. Titmice were chosen for the calcium imaging experiments because they are about 2x larger than chickadees and therefore better suited to handle the weight of the calcium imaging camera and cable.
Neither chickadees nor titmice are visibly sexually dimorphic. All surgical and behavioral procedures were performed blindly to sex, and sex was determined only post-mortem after the experiments. Of the chickadees, 16 (4 female, 11 male) were used for retrograde tracing, 4 (all male) were used for anterograde tracing, and one (male) was used to create a 3D rendering of the brain. 12 titmice (7 female, 5 male) were used for awake behaving calcium imaging experiments. Three additional titmice (all female) were used for the anatomical tracing, to verify results obtained in chickadees.
Prior to experiments, all birds were housed in an aviary in groups of 1-3, on a ‘winter’ light cycle (9 h: 15 h light:dark). Birds were given an ad-libitum supply of a small-bird diet (either Small Bird Maintenance Diet, 0001452, Mazuri or Organic High Potency Fine Bird foods, HPF, Harrison’s Bird Foods). Upon transfer to the lab for experiments, birds had primary flight feathers trimmed and were individually housed.
Methods details
Surgery
For all surgical procedures, birds were anesthetized with 1-2% isoflurane in oxygen. Feathers were removed from the surgical site, and the skin was treated with Betadine. Birds were then placed into a stereotaxic apparatus for the duration of the surgery. The head was rotated to an angle appropriate for each experiment (see below), measured as the angle between the groove in the bone at the base of the upper beak mandible and the horizontal table surface. Throughout the surgery, saline was injected subcutaneously to prevent dehydration (~0.4 mL every 30-60 min). After the surgery, an analgesic (buprenorphine, 0.05 mg/kg) was injected intraperitoneally.
3D brain reconstruction
To facilitate surgical planning and visualize the 3D arrangement of brain structures, we constructed a template chickadee brain. The template included boundaries of the hippocampus, DL/CDL, and the brain surface. In one bird, stiff wire (Malin Co., 0.006” music wire) was inserted horizontally from the lateral wall of the brain toward the midline to create two visible tracts. The bird was immediately perfused (see below), and following tissue fixation, the brain was sliced sagittally. Slices were annotated (Adobe Illustrator) and aligned using custom MATLAB code, with the two lesions used for alignment.
Anatomical tracing experiments
Injection coordinates for different brain regions are given in Table S1. For the hippocampus, coordinates were chosen to span the long axis of the hippocampus. For DL, coordinates were chosen based on the approximate centers of areas retrogradely labeled by hippocampal injections. These topographically matched coordinates in DL were anterior to the hippocampal coordinates: hippocampal locations 2, 4 and 5 (1A, 3A, and 5A) corresponded to DL locations 6-8 (2A, 3.5A, and 5.5A). For retrograde tracing, we used cholera toxin subunit B (CTB in 1X PBS, 0.4% weight/volume) fluorescently conjugated to Alexa Fluor in one of three different wavelengths (488 nm, 555 nm, and 647 nm; C34775, C34776, C34778, respectively, Invitrogen). We made injections in 1-3 tracing locations per animal, using different wavelengths and sometimes different hemispheres for different locations in individual birds. For the anterograde tracing experiment, scAAV9-CBh-GFP virus (UNC viral core) was injected in the posterior hippocampus.
Injections were made using Nanoject II (Drummond Scientific) and a pulled glass pipette. Immediately after the pipette was lowered into the brain, the brain surface around the pipette was sealed with a silicone adhesive (Kwikcast, World Precision Instruments). This prevented the surface from drying out and injected liquid from leaking out of the brain. For retrograde tracing, 2 injections of 50 nL of CTB were made over the course of 5 min at each location. For anterograde tracing, one 385 nL injection of the virus was made over the course of 7 min. In both cases, the pipette was left in place for an additional 5 min after the injections. For all anatomy experiments, the animal was perfused one week following surgery.
Verification of anatomical results in tufted titmice
To verify that the recording coordinates for the calcium imaging experiments were appropriate, we injected retrograde tracer into the titmouse hippocampus. We chose locations in the posterior hippocampus (N=2, location 9, Table S1) and in the anterior hippocampus (N=2, location 10, Table S1). These locations were chosen because they had been characterized in prior work to have very different degrees of spatial selectivity,17 and were both portions of the titmouse hippocampus adjacent to the dorsal brain surface. For each injection, we found the corresponding portion of DL with dense retrograde labeling. These positions were selected as our DL recoding locations for the imaging experiments (locations 11-12, Table S1). As in chickadees, these positions were anterior to our injection coordinates.
Surgeries for calcium imaging
For calcium imaging, all injections were made in titmice at a 65° head angle. At the beginning of the surgery, dexamethasone (2 mg/kg) was injected intraperitoneally to prevent inflammation around the implant. All recordings were conducted in the right hemisphere. To anchor the implant, 6 partial craniotomies were made through the top layer of the skull, surrounding the recording location. Insect pins were inserted to connect pairs of these craniotomies to each other. These insect pins and the craniotomies were then covered with light-cured dental cement (D69-0047, Pearson Dental), fully surrounding the recording location. A full craniotomy and durotomy of ~1.4 mm diameter was then made over the recording location.
For all experiments, viral injections were used to induce GCaMP6f expression (AAV9-CAG-GCaMP6f-WPRE-SV40, 100836-AAV9, Addgene). For the hippocampal recordings, an injection of 880 nL of the virus was made at over the course of 11 min, and then the pipette was left for an additional 25 min. For the DL recordings, a grid of 12 injections spaced 0.2 mm apart was made into DL coordinates. Each DL injection contained 58 nL administered over 3.5 min, and the pipette was left in the brain for an additional 3 min. Clear silicone elastomer (Kwiksil, World Precision Instruments) was used to seal the brain surface during all virus injections.
Following the injections, for the hippocampal recordings, a 1 mm diameter GRIN lens (1050-004595, Inscopix) was lowered 0.3 mm into the brain, and the surrounding exposed brain surface was covered with the clear elastomer. Inserting the lens into the brain prevented the regrowth of the dura, and the 1 mm diameter lens was small enough to avoid damaging the sagittal sinus. The lens was then secured to the outer anchor points using light-cured dental cement.
For the DL recordings, a thin layer of the clear elastomer was placed on the brain. An optical window (3 mm round cover glass #0, Warner Instruments) was then placed on the silicone and secured to the surrounding bone using dental cement (C&B Metabond Quick Adhesive, S380 Parkell). Another thin layer of the elastomer was placed on top of the optical window, and a GRIN lens was then secured over the optical window using dental cement attachment to the bone. An optical window was used to avoid damaging the surface of the relatively thin DL structure. Because DL was far from the midline and the sagittal sinus, we were able to remove a big enough area of the dura to prevent regrowth for the duration of the experiment.
In both hippocampus and DL surgeries, a microendoscope camera (nVista, Inscopix) was used to position a magnetic baseplate. The position was chosen such that the camera was centered on the lens and focused just below the brain surface. The baseplate was secured to the bone using light-cured dental cement and then painted black (Black onyx, OPI Nail Lacquer).
Behavioral experiments
Behavioral arena
Random foraging experiments were conducted in an enclosed square arena (91 x 91 x 127 cm). All surfaces of the arena were black. One wall of the arena opened to serve as a door, and another wall had a single white cue card positioned at its center. The floor was made of rubber (5812T33, McMaster-Carr). Four feeders were mounted at the center of each quadrant of the arena and dropped small pieces of sunflower seeds at irregular intervals (2.5 mg every 2-10 s). Because of the bounce of the floor, the four feeders produced a nearly uniform distribution of seeds on the arena surface.
Four infrared cameras for behavioral tracking (400300W, Qualysis) and one color camera for video monitoring (400200VC, Qualysis) were secured roughly in the corners and the center of the ceiling, respectively. During all sessions, white noise was played over a speaker to mask inadvertent room noises. Sessions lasted ~1.5 hours, and each bird was run once per day.
Behavioral habituation
All behavioral experiments were performed on titmice. Prior to surgery, birds were habituated to food deprivation and handling similarly to previous work.107 They were first given a 4-day period of acclimation and habituation to daily handling. After this period, they were gradually habituated to food deprivation by not being given food for some amount of time at the beginning of the lights-on period each day. This food deprivation period was gradually increased from 2 h to 4 h over the course of 2 weeks. Birds were weighed daily at the end of the food deprivation period, and the length of this period was increased only if the weight was stable (fluctuations less than 0.3 g) for 3 days.
Surgery was performed on animals that had habituated to food deprivation. Following surgery animals received ad-libitum food for at least one week. They were then gradually re-habituated to food deprivation. Once birds were again maintaining a stable weight, they were introduced to the arena (see below) for daily behavioral sessions. Each session began at the end of the food deprivation period and lasted 1.5 h. After the session, birds were given ad-libitum food in their home cages for the rest of the day. This period (typically 3 days) continued until coverage of the arena was consistently greater than 98%, measured as the fraction of “pixels” covered by the animal in 1.5 h, if the arena is divided into a 40x40 grid of pixels.
Once birds were exhibiting sufficient coverage of the arena, they were habituated to the weight of the microscope and the microscope cable. Birds were first fit with a leg-loop harness,108 that remained permanently on the bird and whose purpose was to minimize the forces applied by the cable to the bird’s head. The harness consisted of a 3D-printed plastic attachment for the cable (21 x 9.5 x 6.5 mm) pressed against the bird’s back and two loops of elastic string (4 cm for each leg, Outus Elastic Cord, B06XJNNGMC, Amazon) that were hooked onto the bird’s thighs. Birds were first run in the behavioral arena with only the harness for 1-2 days to ensure they maintained coverage of the arena. For the remainder of the sessions, the Inscopix microscope was snapped into the magnetic headplate, and the cable at a point ~12 cm from the microscope was attached to the harness. To further minimize the forces exerted by the cable, strands of a thin elastic string (1 mm Flat Electric Crystal Stretch String, JY001162W, Amazon) were tied to the cable to provide a low spring-constant force opposing gravity. Birds were recorded on random foraging sessions until they had completed 3-4 sessions with 98% coverage of the arena. In two early pilot birds, more sessions were acquired.
Histology and histological imaging
Birds used for hippocampal recordings had an imprint of the GRIN lens on the surface of the brain, which could be used to verify recording coordinates. These birds were perfused after the completion of all the behavioral sessions. In birds used for DL recordings, we used an additional procedure to mark the recording location. Prior to perfusion birds were anesthetized with 1-2% isoflurane and placed into the stereotaxic apparatus. Coordinates of the lens relative to fiducial points on the skulls were obtained, and the entire implant was then removed. A pipette was lowered 0.5 mm into the brain at the center of where the lens had been placed, and 15 μL of DiI solution (5% wt/vol in DMSO, D3911, Invitrogen) was injected to mark the location. The animal was then immediately perfused.
For all perfusions, animals were administered ketamine and xylazine (10 mg/kg and 4 mg/kg respectively), then transcardially perfused with 1X PBS followed by 4% paraformaldehyde in PBS. The brains were extracted and stored in 4% paraformaldehyde in PBS for 2 days. They were then sectioned coronally into 100 μm-thick slices. Slices were stained with fluorescent DAPI (300 nM in 1X PBS, D1306, Invitrogen) and mounted in Vectashield mounting medium (H-1400-10, Vector Laboratories). Slices were imaged using a AZ100 Multizoom Slide Scanner (Nikon) using filters for DAPI and all injected fluorophores. For the images in Figure 1C, additional images were taken using a laser scanning A1R confocal Microscope (Nikon).
Quantification and statistical analysis
All analysis was performed using custom code in MATLAB, except where specified otherwise. All code will be available for download following publication.
Quantification of labeled cells in anatomical tracing
To detect retrogradely labeled cells in histological slices, we used a standard Difference-of-Gaussians algorithm for blob detection. We used a series of five sigma values, logarithmically spaced from 2.5 to 12.5 μm – corresponding to blob sizes of 5 to 25 μm. The slice image was smoothed with Gaussians of each of these sigma values. Successive pairs of smoothed images were subtracted from one another to compute four difference-of-Gaussians, and these differences were stacked into a 3-dimensional matrix. Local maxima exceeding 0.02 in this matrix were detected as the centers of the blobs. If two detected blobs were overlapping, the one with the lower value was eliminated.
Slices were then annotated manually to assign detected cells to regions of interest (DL/CDL, HD, and NL). The boundaries of these brain regions were determined by comparing the DAPI-stained images to published atlases from other species.29,31,33 Cells were then aligned to stereotaxic coordinates by aligning slices to the reference chickadee brain (described above). To quantify the volume of a particular labeled brain region, detected cells were binned into cubic voxels with 0.1 mm side length, and the resulting volume was smoothed with the MATLAB function imgaussfilt3 (sigma 0.05 mm). Continuous voxels containing more than 5 cells each were considered part of the labeled region.
Topography of DL/CDL input
To quantify the topography of DL and CDL, we first defined the long axis of DL/CDL by pooling all retrogradely labeled cells in this region from all injections and fitting a line to their coordinates in the horizontal plane. The coordinate of each cell along the long axis could then be defined by projecting its location onto this line. We defined DL as all cells medial to 3.5L and CDL as all cells lateral to 3.5L. We then performed linear regression to measure the relationship of the long-axis coordinate of cells in DL and CDL to the injection location in the hippocampus.
Construction of 2D unfolded maps
Unfolded maps of the chickadee brain were constructed using coronal slices from one of the chickadees that had injections of the retrograde tracer in three fluorophore wavelengths at different anterior-posterior positions in the hippocampus. We adapted a standard method for creating unfolded cortical maps.40,54 For each slice, we marked the “medial ridge” – the dorsal-most point on the midline of the brain at which the right and left hemispheres of the forebrain contacted one another. At posterior locations (where cerebellum was present), the two forebrain hemispheres no longer contacted each other dorsally; however, a dorsal ridge continuous with the marked anterior locations was still evident and could be marked. In addition to the medial ridge, we marked the extent of retrogradely labeled cells in DL/CDL, using their approximate projections onto the brain surface. If multiple fluorophore wavelengths were present in the slice, we also marked approximate boundaries between groups of cells labeled by the different wavelengths. Finally, we marked (if present in the slice) the approximate boundary of DL and CDL on the brain surface.
All marked points in a slice were connected by tracing the outline of the brain surface. This outline was then unfolded to create a line segment with all of the marked points located at different positions on the segment. Segments obtained from different coronal slices were aligned using the medial ridge to create the 2D map. The boundaries of DL/CDL with other brain regions were determined using a published atlas of the chicken brain.29
To construct unfolded maps of the rat brain, we used horizontal sections from a published atlas.56 The surface of the entorhinal cortex was traced using the digital version of the atlas. Boundaries of MEC and LEC were also marked using this atlas. We defined the medial ridge as a “kink” evident in horizontal slices, at which the primarily-posterior wall of the cortex made a sharp turn in the anterior direction and became a primarily-medial wall. Outlines of the horizontal slices were unfolded and aligned using the medial ridge, as in chickadees. The topography of projections to the hippocampus was deduced from a published study.40
Behavioral analysis
To track the bird’s position in the behavioral arena, a spherical marker covered in infrared-reflective tape (3M Scotchlite 7610 Reflective tape, MKR-TSPE-2, B&L Engineering) was connected to the top of the Inscopix microscope. This marker was tracked using four infrared cameras recording at 300 fps (see above). The 2D position of this marker projected onto the floor of the arena and was used as the position of the animal for all analyses. Location was downsampled to 20 fps to align with the rate of image acquisition for calcium data.
To determine head direction we trained a deep neural network109,110 to identify the tip of the bird’s beak and the base of its neck, and drew a vector from the neck to the beak. Head direction was considered to be the angle of this vector’s projection onto the floor. The direction of the wall with the door to the arena was assigned to be the zero-degree head direction.
The animal's speed was determined by calculating instantaneous speed and smoothing with a 1 s square window.
Calcium imaging
Imaging data were collected at 20 fps. Neuronal traces were extracted from raw fluorescence movies using a constrained non-negative matrix factorization algorithm intended for 1-photon calcium imaging data (CNMF_E).62,106 We used a multi-scale approach111 to extract stable fluorescent traces from long videos (~1.5 h in our case). Before applying CNMF_E to the raw videos, we applied a motion correction algorithm.112 The vast majority of data contained no motion above a 1 pixel RMS shift, and any sessions that exceeded 2 pixels RMS of shift were eliminated from the analysis.
The multi-scale CNMF_E approach was run in three steps. First, data were temporally downsampled by a factor of 20. Cell footprints were found in the downsampled movie using the standard CNMF_E algorithm. These footprints were then used to extract temporal traces on segments of the non-downsampled data. Finally, the raw traces were deconvolved to detect the time and amplitude of each calcium event. To eliminate some infrequent imaging artifacts, any calcium events with an amplitude greater than 1.5 times larger than the 99th percentile of all calcium events for that cell were eliminated from all analyses. For all firing rate calculations in the paper, events were weighed by their amplitude.
Analysis of spatial activity
For all spatial analyses, periods where the bird was still (speed less than 5 cm/s for more than 5 s) were eliminated. Spatial firing maps were constructed by first dividing the 91x91 cm environment into 40x40 bins. For each neuron, the number of calcium events and the animal’s occupancy in each bin were calculated. The resulting two matrices were smoothed using a 13x13 Hamming window. Firing rate maps were constructed by dividing the smoothed firing rate map by the smoother occupancy and scaled to convert the units of each bin into events/s. Any bin with less than 0.1 s of occupancy after smoothing was replaced by NaN. Because only sessions with at least 98% coverage of the arena were included in this study (see above), only a small minority of pixels were excluded.
To detect spatially significant cells, two metrics from previous work were used: spatial information and spatial stability.17,113 Spatial information / was defined using the equation:
| Equation 1 |
Where is the index of the spatial bin, is the average neural firing in bin , is the average firing rate of the neuron, and is the probability of the animal being in the bin during the session.
To compute spatial stability, we divided the session into non-overlapping 5-min segments. These segments were randomly assigned to one of two groups, such that both groups contained the same number of segments. For each group, the spatial firing map was computed, and then the correlation between the maps of each group was determined. This procedure was repeated 10 times, and then the average correlation was found.
To determine the significance of spatial information and spatial stability, we shuffled the data by circularly shifting the calcium event data by timepoints relative to the behavior. For each shuffle, a random value of was chosen, where is the total number of behavioral timepoints. The same procedures as above were used to compute spatial information and spatial stability of the shuffled data, and the shuffling procedure was repeated 500 times. Values were considered significantly high if they exceeded 99% of the respective shuffled distributions. A cell was considered “spatial” if both its spatial information and its spatial stability were significant. For calculating the normalized spatial information (Figure 7), the mean and standard deviation of the shuffled distribution were used to compute a z-score.
Features of spatial cells
To detect the firing fields of a cell, its firing rate map was normalized to values between 0 and 1, using the 5th and 95th percentiles of all the values in the rate map. A field was defined as a continuous region larger than 200 cm2, in which all normalized values exceeded 0.5. This procedure was used to determine the number of fields, as well as their sizes.
To determine border selectivity, we adopted a published border score.61 In this procedure, was defined as the maximum fraction of any wall contacted by a single field. It was 0 if no fields touched any walls and 1 at least one of the walls was fully covered by a field. A mean firing distance was computed by averaging, across all pixels that belonged to any field, the distances to the nearest wall, weighed by the firing rates. This value was 0 if all firing was at the exact center of the environment and 1 if all firing was on the edges of the environment. The border score was then defined as:
| Equation 2 |
To determine if a border score was significant, shuffled values were computed as in the “Analysis of spatial activity” above. A neuron was classified as a border cell if it was significantly spatial, and its border score exceeded that of 99% of the shuffles.
To examine the optimal temporal shift of neurons, behavior was shifted between −2 s and +2 s relative to neural data in 50 ms increments. For each temporal shift, spatial information was recalculated as above. We determined the temporal shift at which the spatial information was maximal.
To attempt detecting grid cells, we measured the gridness score63 of each cell. First, we computed the unbiased autocorrelation of the cell’s rate map.5 We then defined the radius of the central peak as the distance closest to the center at which autocorrelation was either negative or exhibited a local minimum. We then considered a set of annulus-shaped samples of the autocorrelation map, where the inner radius of each annulus was the radius of the central peak, and the outer radius was varied in steps of 1 bin from a minimum of 4 bins more than the radius of the central peak to 4 bins less than the width of the environment. We then calculated the Pearson correlation of each annulus with its rotated versions at 60° and 120° (group 1) and then 30°, 90°, and 150° (group 2). The difference between the minimum of the group-1 correlation values and the maximum of the group-2 correlation values was computed, and the gridness score was defined as the maximum difference across all annulus samples. Cells whose gridness score exceeded 99% of the reshuffled samples (“Analysis of spatial activity” above) were defined as grid cells.
Analysis of head direction tuning and speed tuning
To detect head direction cells, we used a method based on those previously published6. We first divided the animal’s head direction into 9° bins. For each of these 40 bins, we defined a vector whose amplitude was the average firing rate in the bin, and whose direction was the midpoint of the bin. As in previous studies, we computed the mean vector length (MVL) as the length of the vector average of these 40 vectors. We then shuffled the data as in the “Analysis of spatial activity” above. A cell was classified as a head direction cell if its MVL exceeded 99% of the values computed on the shuffled data.
To detect speed cells, we also adopted a previously used metric.8 The speed score was defined as the Pearson correlation coefficient between the animal’s speed and the firing rate computed in 50-ms bins. A neuron’s speed score was considered significant if it was either below the 0.5th percentile or above 99.5th percentile of values from shuffled data, computed as above. This procedure detected both positively and negatively tuned cells. For displaying in figures, speed tuning curves were binned between 0 and 20 cm/s in 0.5 cm/s bins and smoothed with a 2.5 cm/s window.
Analysis of dash modulation
A dash onset was defined as a positive speed threshold crossings of 15 cm/s that was not preceded by another positive threshold crossing in less than 1 s. We measured peristimulus time histograms (PSTHs) aligned to dash onsets, with the firing rate smoothed by a 250-ms rolling window. Neurons modulated by dashes tended to have a peak in firing rate between 0.5 s and 1.5 s after the dash onset. We therefore quantified the strength and timing of dash modulation by detecting the maximum and the minimum of the PSTH between 0 and 2 seconds. We defined a dash score as:
| Equation 3 |
Where and are the firing rates at the maximum and the minimum, respectively. To determine the significance of this peak, we constructed shuffled data as in the “Analysis of spatial activity” above. Shuffling was repeated 500 times, and a cell was considered dash-modulated if the peak of its PSTH exceeded 99% of the peaks from shuffled data.
We then tested whether dash modulation could be entirely explained by speed modulation by asking whether firing rates were identical between time points at the beginning and the end of the dash when the bird was moving at the same speed. For each dash cell we found the maximum value of the PSTH at the end of a dash. We then determined the animal’s average speed during that peak in firing and found the time at the beginning of the dash when the animal was moving at the same speed. We determined the value of that PSTH at this time, and used a paired t-test to compare values at the beginning and the end of the dash.
To determine whether peaks in PSTHs formed a sequence (Figure 6I), we asked whether the timing of these peaks was consistent across dashes. For each dash-modulated neuron, we randomly sorted all dashes into two equal-sized groups. We computed PSTHs separately for each group (PSTH 1 and 2), and detected the peaks of both PSTHs. For each neuron, we then computed the absolute difference in the peak timing of PSTH 1 and PSTH 2, and found the median absolute differences across neurons. We asked whether this median difference was smaller when PSTH 1 was paired with PSTH 2 from a randomly selected neuron instead of PSTH 2 from the same neuron. This procedure was repeated 1000 times, and the p value was determined as a fraction of these comparisons in which the within-neuron difference was greater than the across-neuron difference.
Model of multiplexed representations
Our model was largely adapted from an existing linear-nonlinear-Poisson model of entorhinal activity.79 To begin, the animal’s behavioral state was evaluated in 50-ms bins, synchronized with the 20 Hz acquisition of imaging data. In each bin, the animal’s position, head direction, speed, and time since last dash were averaged. These variables were discretized into positional bins, head direction bins, speed bins, and dash-time bins. As in prior analyses, any time bins where the animal’s speed was less than 5 cm/s for more than 5 s were eliminated from the model fit.
The animal’s position in the 91x91 cm environment was divided evenly into 5x5 bins (p = 25). For head direction, the 360° range of angles was divided into 18 identical bins (h = 18). The bird’s speed was discretized into 10 identical bins between 0 cm/s and 20 cm/s (s = 10). Time points that exceeded 20 cm/s were assigned to the last bin. Time since the most recent dash was assigned to 30 bins with logarithmically spaced edges between 0 and 35 s (d = 30). Any time points that were more than 35 s since the last dash were assigned to the last bin.
The animal’s state was represented by 4 sparse matrices , , , and , where each matrix had , , , and columns respectively and T rows, where T is the number of time bins in the session. For each row, these matrices had a value of 0 in all columns except the column corresponding to the value of the behavioral variable in that time bin.
Sixteen models were considered, each including some subset of the four behavioral variables (place, head direction, speed, dash modulation). The models are listed in Table S2. For each model, the matrices corresponding to the included variables were horizontally concatenated to form a matrix .
Each column of corresponded to a particular bin of a particular behavioral variable. We wanted to estimate the contribution of that bin to a neuron’s firing. Therefore, we defined a vertical vector of parameters , whose number of rows was equal to the number of columns in .
For a particular vector , the estimated firing rate of the neuron was:
| Equation 4 |
In the original model, a Poisson distribution was used to compute, in each time bin, the probability of observing a certain number of spikes. In our calcium imaging data, the number of calcium events was not always an integer, since events were weighed by their amplitudes. We therefore used a Gamma distribution instead of the Poisson distribution. Here, is the mean of the distribution and the scale parameter is set to 1 such that the variance of the distribution () is equal to the mean ().
We defined as the number of calcium events observed in time bin . From the Gamma distribution, the likelihood of observing this number is given by
| Equation 5 |
We then sought to maximize the log-likelihood across all time bins considered in the analysis. Taking the logarithm of Equation 5 and summing across all time bins, we get
| Equation 6 |
Here, is a small value to prevent taking logarithms of 0, and is a smoothness parameter defined as:
| Equation 7 |
For all (, ) corresponding to adjacent bins (e.g. spatial bins that share a physical border in the environment or head direction bins that are adjacent on the circle). We then computed the optimal set of parameters by minimizing using MATLAB function fminunc.
Model comparisons
To fit a model to a particular neuron’s activity, the behavioral session was divided into 5 time windows, which were identical in length after some of the time bins were excluded by speed thresholding. Each window was further subdivided into 10 identical sub-windows. All of the 50-ms time bins were then sorted into 10 groups, where the group included all bins that were within the sub-window of each of the 5 windows. For each of the 10 groups, we left that group out of the data, and fit the model to the remaining 9 groups to estimate parameters . We then then computed the likelihood (Equation 6) of observing data in the left-out group given these parameters. This procedure provided us with 10 likelihood values, corresponding to each of the left-out groups. To compare any two models, we computed the average difference between these values for the two models.
To select the most appropriate model for each neuron, we first fit all models (Model #0-Model #15, Table S2) to the data using the above procedure. We determined which of the one-feature models #1-#4 was the best performing model. We then asked if that model better that Model #0, which fits a single parameter to all the firing rate bins. If it was, we then found the best-performing two-feature model (Models #5-10) and compared it to the best one-feature model. We repeated this procedure for the three-feature models (Model #11-14) and the four-feature model (Model #15).
Supplementary Material
HIGHLIGHTS.
DL/CDL is a major input into the hippocampus of food-caching birds
DL/CDL has similar anatomical organization to the mammalian entorhinal cortex
DL contains entorhinal-like spatial cells, including multi-field grid-like cells
These data suggest that DL is an avian entorhinal analog
ACKNOWLEDGEMENTS
We thank Stephanie Hale, Selmaan Chettih, Felix Moll, Michael Long, and Luke Hammond for technical assistance; the Black Rock Forest Consortium, Timothy Green, and Jennifer Scribner, for field site help; Selmaan Chettih, Isabel Low, and Hannah Payne for comments on the manuscript. Imaging was performed with support from the Zuckerman Institute's Cellular Imaging platform. This work was supported by the Beckman Foundation Young Investigator Award, the New York Stem Cell Foundation — Robertson Neuroscience Investigator Award, NIH Director’s New Innovator Award (DP2-AG071918), NIH training grant (T32 EY013933, to MCA), NSF Graduate Research Fellowship Program (to MCA), Simons Society of Fellows (to ELM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Tulving E. (1972). Episodic and semantic memory. In Organization of memory, Tulving E and Donaldson W, eds., pp. 381–403. 10.1017/S0140525X00047257. [DOI] [Google Scholar]
- 2.Eichenbaum H. (2006). Remembering: Functional organization of the declarative memory system. Curr. Biol 16, 643–645. 10.1016/j.cub.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Burwell RD, and Amaral DG (1998). Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices. J. Comp. Neurol 205, 179–205. [DOI] [PubMed] [Google Scholar]
- 4.Witter MP, Doan TP, Jacobsen B, Nilssen ES, and Ohara S (2017). Architecture of the entorhinal cortex: A review of entorhinal anatomy in rodents with some comparative notes. Front. Syst. Neurosci 11, 1–12. 10.3389/fnsys.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hafting T, Fyhn M, Molden S, Moser MB, and Moser EI (2005). Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 6.Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, and Moser EI (2006). Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762. 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- 7.Brun VH, Solstad T, Kjelstrup KB, Fyhn M, Witter MP, Moser EI, and Moser MB (2008). Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus 18, 1200–1212. 10.1002/hipo.20504. [DOI] [PubMed] [Google Scholar]
- 8.Kropff E, Carmichael JE, Moser MB, and Moser EI (2015). Speed cells in the medial entorhinal cortex. Nature 523, 419–424. 10.1038/nature14622. [DOI] [PubMed] [Google Scholar]
- 9.Kinkhabwala AA, Gu Y, Aronov D, and Tank DW (2020). Visual cue-related activity of cells in the medial entorhinal cortex during navigation in virtual reality. Elife 9, 1–24. 10.7554/eLife.43140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler WN, Hardcastle K, and Giocomo LM (2019). Remembered reward locations restructure entorhinal spatial maps. Science 363, 1447–1452. 10.1126/science.aav5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boccara CN, Nardin M, Stella F, Neill JO, and Csicsvari J (2019). The entorhinal cognitive map is attracted to goals. Science 363, 1443–1447. [DOI] [PubMed] [Google Scholar]
- 12.Høydal ØA, Skytøen ER, Andersson SO, Moser MB, and Moser EI (2019). Object-vector coding in the medial entorhinal cortex. Nature 568, 400–404. 10.1038/s41586-019-1077-7. [DOI] [PubMed] [Google Scholar]
- 13.Stachenfeld KL, Botvinick MM, and Gershman SJ (2017). Hippocampus as predictive map. Nat. Neurosci 28, 391–397. 10.1017/CBO9781107415324.004. [DOI] [PubMed] [Google Scholar]
- 14.Mathis A, Herz AVM, and Stemmler M (2012). Optimal population codes for space: Grid cells outperform place cells. Neural Comput. 24, 2280–2317. 10.1162/NECO_a_00319. [DOI] [PubMed] [Google Scholar]
- 15.Krushinskaya N. (1966). Some complex forms of feeding behavior of nutcracker Nucifraga caryocatactes, after removal of old cortex. J. Evol. Biochem. Physiol, 563–568. [Google Scholar]
- 16.Sherry DF, and Vaccarino AL (1989). Hippocampus and memory for food caches in black-capped chickadees. Behav. Neurosci 103, 308–318. 10.1037/0735-7044.103.2.308. [DOI] [Google Scholar]
- 17.Payne HL, Lynch GF, and Aronov D (2021). Neural representations of space in the hippocampus of a food-caching bird. Science 373, 343–348. 10.1126/science.abg2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal A, Sarel A, Derdikman D, Ulanovsky N, and Gutfreund Y (2021). Spatial coding in the hippocampus of flying owls. bioRxiv, 2021.10.24.465553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, et al. (2004). Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol 473, 377–414. 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colquitt BM, Merullo DP, Konopka G, Roberts TF, and Brainard MS (2021). Cellular transcriptomics reveals evolutionary identities of songbird vocal circuits. Science 371. 10.1126/science.abd9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tosches MA, Yamawaki TM, Naumann RK, Jacobi AA, Tushev G, and Laurent G (2018). Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360, 881–888. 10.1126/science.aar4237. [DOI] [PubMed] [Google Scholar]
- 22.Hain D, Gallego-Flores T, Klinkmann M, Macias A, Ciirdaeva E, Arends A, Thum C, Tushev G, Kretschmer F, Tosches MA, et al. (2022). Molecular diversity and evolution of neuron types in the amniote brain. Science 377. 10.1126/science.abp8202. [DOI] [PubMed] [Google Scholar]
- 23.Tömböl T, Davies DC, Németh A, Sebestény T, and Alpár A (2000). A comparative Golgi study of chicken (Gallus domesticus) and homing pigeon (Columba livia) hippocampus. Anat. Embryol. (Berl) 201, 85–101. 10.1007/PL00008235. [DOI] [PubMed] [Google Scholar]
- 24.Couey JJ, Witoelar A, Zhang SJ, Zheng K, Ye J, Dunn B, Czajkowski R, Moser MB, Moser EI, Roudi Y, et al. (2013). Recurrent inhibitory circuitry as a mechanism for grid formation. Nat. Neurosci 16, 318–324. 10.1038/nn.3310. [DOI] [PubMed] [Google Scholar]
- 25.Zutshi I, Fu ML, Lilascharoen V, Leutgeb JK, Lim BK, and Leutgeb S (2018). Recurrent circuits within medial entorhinal cortex superficial layers support grid cell firing. Nat. Commun 9. 10.1038/s41467-018-06104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burak Y, and Fiete IR (2009). Accurate path integration in continuous attractor network models of grid cells. PLoS Comput. Biol 5. 10.1371/journal.pcbi.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buetfering C, Allen K, and Monyer H (2014). Parvalbumin interneurons provide grid cell-driven recurrent inhibition in the medial entorhinal cortex. Nat. Neurosci 17, 710–718. 10.1038/nn.3696. [DOI] [PubMed] [Google Scholar]
- 28.Atoji Y, and Wild JM (2006). Anatomy of the avian hippocampal formation. Rev. Neurosci 5, 3–16. [DOI] [PubMed] [Google Scholar]
- 29.Puelles L, Martinez-de-la-Torre M, Martinez S, Watson C, and Paxinos G (2019). The chick brain in stereotaxic coordinates and alternate stains 2nd ed. (Academic Press; ). [Google Scholar]
- 30.Erichsen JT, Bingman VP, and Krebs JR (1991). The distribution of neuropeptides in the dorsomedial telencephalon of the pigeon (Columba livia): A basis for regional subdivisions. J. Comp. Neurol 314, 478–492. 10.1002/cne.903140306. [DOI] [PubMed] [Google Scholar]
- 31.Stokes TM, Leonard CM, and Nottebohm F (1974). The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J. Comp. Neurol 156, 337–374. 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- 32.Székely AD, and Krebs JR (1996). Efferent connectivity of the hippocampal formation of the zebra finch (Taenopygia guttata): An anterograde pathway tracing study using Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol 368, 198–214. . [DOI] [PubMed] [Google Scholar]
- 33.Karten HJ, and Hodos W (1967). A stereotaxic atlas of the brain of the pigeon: Columba livia (Johns Hopkins Press; ). [Google Scholar]
- 34.Atoji Y, Wild JM, Yamamoto Y, and Suzuki Y (2002). Intratelencephalic connections of the hippocampus in pigeons (Columba livia). J. Comp. Neurol 447, 177–199. 10.1002/cne.10239. [DOI] [PubMed] [Google Scholar]
- 35.Casini G, Bingman VP, and Bagnoli P (1986). Connections of the pigeon dorsomedial forebrain studied with WGA-HRP and 3H-proline. J. Comp. Neurol 245, 454–470. 10.1002/cne.902450403. [DOI] [PubMed] [Google Scholar]
- 36.Vinepinsky E, Cohen L, Perchik S, Ben-Shahar O, Donchin O, and Segev R (2020). Representation of edges, head direction, and swimming kinematics in the brain of freely-navigating fish. Sci. Rep 10, 1–16. 10.1038/s41598-020-71217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Yishay E, Krivoruchko K, Ron S, Ulanovsky N, Derdikman D, and Gutfreund Y (2021). Directional tuning in the hippocampal formation of birds. Curr. Biol 31, 2592–2602.e4. 10.1016/j.cub.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Sherry DF (1984). Food storage by black-capped chickadees: memory for the location and content of caches. Anim. Behav 32, 451–464. [Google Scholar]
- 39.Pravosudov VV, and Grubb TC (1997). Management of fat reserves and food caches in tufted titmice (Parus bicolor) in relation to unpredictable food supply. Behav. Ecol 8, 332–339. 10.1093/beheco/8.3.332. [DOI] [Google Scholar]
- 40.Dolorfo CL, and Amaral DG (1998). Entorhinal cortex of the rat: Topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J. Comp. Neurol 398, 25–48. . [DOI] [PubMed] [Google Scholar]
- 41.Sherry DF, Vaccarino AL, Buckenham K, and Herz RS (1989). The hippocampal complex of food-storing birds. Brain Behav Evol 34, 308–317. 10.1159/000116516. [DOI] [PubMed] [Google Scholar]
- 42.Kahn MC, Siegel JJ, Jechura TJ, and Bingman VP (2008). Response properties of avian hippocampal formation cells in an environment with unstable goal locations. Behav. Brain Res 191, 153–163. 10.1016/j.bbr.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 43.Barnea A, and Nottebohm F (1994). Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl. Acad. Sci. U. S. A 91, 11217–11221. 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freas CA, Roth TC, Ladage LD, and Pravosudov VV (2013). Hippocampal neuron soma size is associated with population differences in winter climate severity in food-caching chickadees. Funct. Ecol 27, 1341–1349. 10.1111/1365-2435.12125. [DOI] [Google Scholar]
- 45.Atoji Y, and Wild JM (2005). Afferent and efferent connections of the dorsolateral corticoid area and a comparison with connections of the temporo-parieto-occipital area in the pigeon (Columba livia). J. Comp. Neurol 485, 165–182. 10.1002/cne.20490. [DOI] [PubMed] [Google Scholar]
- 46.Kahn MC, Hough GE, Ten Eyck GR, and Bingman VP (2003). Internal connectivity of the homing pigeon (Columba livia) hippocampal formation: An anterograde and retrograde tracer study. J. Comp. Neurol 459, 127–141. 10.1002/cne.10601. [DOI] [PubMed] [Google Scholar]
- 47.Atoji Y, and Wild JM (2004). Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J. Comp. Neurol 475, 426–461. 10.1002/cne.20186. [DOI] [PubMed] [Google Scholar]
- 48.Hough GE, Pang KCH, and Bingman VP (2002). Intrahippocampal connections in the pigeon (Columba livia) as revealed by stimulation evoked field potentials. J. Comp. Neurol 452, 297–309. 10.1002/cne.10409. [DOI] [PubMed] [Google Scholar]
- 49.Heyers D, Musielak I, Haase K, Herold C, Bolte P, Güntürkün O, and Mouritsen H (2022). Morphology, biochemistry and connectivity of Cluster N and the hippocampal formation in a migratory bird. Brain Struct. Funct 227, 2731–2749. 10.1007/s00429-022-02566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strange BA, Witter MP, Lein ES, and Moser EI (2014). Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci 15, 655–669. 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 51.Smulders TV (2017). The avian hippocampal formation and the stress response. Brain Behav. Evol 46, 1. [DOI] [PubMed] [Google Scholar]
- 52.Herold C, Schlömer P, Mafoppa-Fomat I, Mehlhorn J, Amunts K, and Axer M (2019). The hippocampus of birds in a view of evolutionary connectomics. Cortex 118, 165–187. 10.1016/j.cortex.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 53.Damphousse CC, Miller N, and Marrone DF (2022). Functional dissociation along the rostrocaudal axis of Japanese quail hippocampus. PLoS One 17, e0277414. 10.1371/journal.pone.0277414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Essen DC, and Maunsell JHR (1980). Two-dimensional maps of the cerebral cortex. J. Comp. Neurol 191, 255–281. 10.1002/cne.901910208. [DOI] [PubMed] [Google Scholar]
- 55.Calabrese E, Badea A, Watson C, and Johnson GA (2013). A quantitative magnetic resonance histology atlas of postnatal rat brain development with regional estimates of growth and variability. Neuroimage 71, 196–206. 10.1016/j.neuroimage.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paxinos G, and Watson C (2013). The rat brain in stereotaxic coordinates 7th ed. (Academic Press; ). [DOI] [PubMed] [Google Scholar]
- 57.Barrière DA, Magalhães R, Novais A, Marques P, Selingue E, Geffroy F, Marques F, Cerqueira J, Sousa JC, Boumezbeur F, et al. (2019). The SIGMA rat brain templates and atlases for multimodal MRI data analysis and visualization. Nat. Commun 10, 1–13. 10.1038/s41467-019-13575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karten HJ, Hodos W, Nauta WJ, and Revzin AM (1973). Neural connections of the “visual wulst” of the avian telencephalon. Experimental studies in the piegon (Columba livia) and owl (Speotyto cunicularia). J. Comp. Neurol 150, 253–278. 10.1002/cne.901500303. [DOI] [PubMed] [Google Scholar]
- 59.Atoji Y, Sarkar S, and Wild JM (2017). Differential projections of the densocellular and intermediate parts of the hyperpallium in the pigeon (Columba livia). J. Comp. Neurol 526, 146–165. 10.1002/cne.24328. [DOI] [PubMed] [Google Scholar]
- 60.Husband SA, and Shimizu T (1999). Efferent projections of the ectostriatum in the pigeon (Columba livia). J. Comp. Neurol 406, 329–345. [PubMed] [Google Scholar]
- 61.Solstad T, Boccara CN, Kropff E, Moser MB, and Moser EI (2008). Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868. 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- 62.Mackevicius EL, Gu S, Denisenko NI, and Fee MS (2022). Self-organization of songbird neural sequences during social isolation. bioRxiv, 2022.02.18.480996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, and Moser M-B (2010). Development of the spatial representation system in the rat. Science 328, 1576–1580. 10.1515/9781474452618-010. [DOI] [PubMed] [Google Scholar]
- 64.Grieves RM, Jedidi-Ayoub S, Mishchanchuk K, Liu A, Renaudineau S, Duvelle É, and Jeffery KJ (2021). Irregular distribution of grid cell firing fields in rats exploring a 3D volumetric space. Nat. Neurosci 24, 1567–1573. 10.1038/s41593-021-00907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ginosar G, Aljadeff J, Burak Y, Sompolinsky H, Las L, and Ulanovsky N (2021). Locally ordered representation of 3D space in the entorhinal cortex. Nature 596, 404–409. 10.1038/s41586-021-03783-x. [DOI] [PubMed] [Google Scholar]
- 66.Park EH, Dvorak D, and Fenton AA (2011). Ensemble place codes in hippocampus: CA1, CA3, and dentate gyrus place cells have multiple place fields in large environments. PLoS One 6, 1–9. 10.1371/journal.pone.0022349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rich PD, Liaw HP, and Lee AK (2014). Large environments reveal the statistical structure governing hippocampal representations. Science 345, 814–817. 10.1126/science.1255635. [DOI] [PubMed] [Google Scholar]
- 68.Eliav T, Maimon SR, Aljadeff J, Tsodyks M, Ginosar G, Las L, and Ulanovsky N (2021). Multiscale representation of very large environments in the hippocampus of flying bats. Science 372. 10.1126/science.abg4020. [DOI] [PubMed] [Google Scholar]
- 69.Fyhn M, Molden S, Witter MP, Moser EI, and Moser MB (2004). Spatial representation in the entorhinal cortex. Science 305, 1258–1264. 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- 70.Stensola H, Stensola T, Solstad T, Frøland K, Moser MB, and Moser EI (2012). The entorhinal grid map is discretized. Nature 492, 72–78. 10.1038/nature11649. [DOI] [PubMed] [Google Scholar]
- 71.Bjerknes TL, Moser EI, and Moser MB (2014). Representation of geometric borders in the developing rat. Neuron 82, 71–78. 10.1016/j.neuron.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 72.Muller RU, and Kubie JL (1989). The firing of hippocampal place cells predicts the future position of freely moving rats. J. Neurosci 9, 4101–4110. 10.1523/jneurosci.09-12-04101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mehta MR, and Wilson MA (2000). From hippocampus to V1: Effect of LTP on spatio-temporal dynamics of receptive fields. Neurocomputing 32-33, 905–911. 10.1016/S0925-2312(00)00259-9. [DOI] [Google Scholar]
- 74.Dotson NM, and Yartsev MM (2021). Nonlocal spatiotemporal representation in the hippocampus of freely flying bats. Science 373, 242–247. 10.1126/science.abg1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Wijngaarden JBG, Babl SS, and Ito HT (2020). Entorhinal-retrosplenial circuits for allocentric-egocentric transformation of boundary coding. Elife 9, 1–25. 10.7554/eLife.59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell MG, Attinger A, Ocko SA, Ganguli S, and Giocomo LM (2021). Distance-tuned neurons drive specialized path integration calculations in medial entorhinal cortex. Cell Rep. 36, 109669. 10.1016/j.celrep.2021.109669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giocomo LM, Stensola T, Bonnevie T, Van Cauter T, Moser MB, and Moser EI (2014). Topography of head direction cells in medial entorhinal cortex. Curr. Biol 24, 252–262. 10.1016/j.cub.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Rigotti M, Barak O, Warden MR, Wang XJ, Daw ND, Miller EK, and Fusi S (2013). The importance of mixed selectivity in complex cognitive tasks. Nature 497, 585–590. 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hardcastle K, Maheswaranathan N, Ganguli S, and Giocomo LM (2017). A multiplexed, heterogeneous, and adaptive code for navigation in medial entorhinal cortex. Neuron 94, 375–387.e7. 10.1016/j.neuron.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tseng SY, Chettih SN, Arlt C, Barroso-Luque R, and Harvey CD (2022). Shared and specialized coding across posterior cortical areas for dynamic navigation decisions. Neuron 110, 2484–2502.e16. 10.1016/j.neuron.2022.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Góis ZHTD, and Tort ABL (2018). Characterizing speed cells in the rat hippocampus. Cell Rep. 25, 1872–1884.e4. 10.1016/j.celrep.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 82.Taube JS, Muller RU, and Ranck JB (1990). Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci 10, 420–435. 10.1523/jneurosci.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Acharya L, Aghajan ZM, Vuong C, Moore JJ, Mehta MR, Acharya L, Aghajan ZM, Vuong C, Moore JJ, and Mehta MR (2016). Causal Influence of Visual Cues on Hippocampal Article Causal Influence of Visual Cues on Hippocampal Directional Selectivity. Cell 164, 197–207. 10.1016/j.cell.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 84.Simonnet J, and Fricker D (2018). Cellular components and circuitry of the presubiculum and its functional role in the head direction system. Cell Tissue Res. 373, 541–556. 10.1007/s00441-018-2841-y. [DOI] [PubMed] [Google Scholar]
- 85.Medina L, and Abellán A (2009). Development and evolution of the pallium. Semin. Cell Dev. Biol 20, 698–711. 10.1016/j.semcdb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 86.Medina L, and Reiner A (2000). Do birds possess homlogues of mammalian primary visual, somatosensory and motor cortices? Trends Neurosci. 23, 1–12. [DOI] [PubMed] [Google Scholar]
- 87.Shimizu T, and Bowers AN (1999). Visual circuits of the avian telencephalon: evolutionary implications. Behav. Brain Res 98, 183–191. [DOI] [PubMed] [Google Scholar]
- 88.Nottebohm F, Stokes TM, and Leonard CM (1976). Central control of song in the canary, Serinus canarius. J. Comp. Neurol 165, 457–486. 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 89.Van Essen DC, and Felleman DJ (1991). Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47. [DOI] [PubMed] [Google Scholar]
- 90.Haberly LB, and Price JL (1978). Association and commissural fiber systems of the olfactory cortex of the rat II. Systems originating in the olfactory peduncle. J. Comp. Neurol 181, 781–807. 10.1002/cne.901810407. [DOI] [PubMed] [Google Scholar]
- 91.Kosel KC, Van Hoesen GW, and West JR (1981). Olfactory bulb projections to the parahippocampal area of the rat. J. Comp. Neurol 198, 467–482. 10.1002/cne.901980307. [DOI] [PubMed] [Google Scholar]
- 92.Mathiasen ML, Hansen L, and Witter MP (2015). Insular projections to the parahippocampal region in the rat. J. Comp. Neurol 523, 1379–1398. 10.1002/cne.23742. [DOI] [PubMed] [Google Scholar]
- 93.Neunuebel JP, Yoganarasimha D, Rao G, and Knierim JJ (2013). Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J. Neurosci 33, 9246–9258. 10.1523/JNEUROSCI.0946-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bingman VP, Casini G, Nocjar C, and Jones TJ (1994). Connections of the piriform cortex in homing pigeons (Columba livia) studied with fast blue and WGA-HRP. Brain. Behav. Evol 43, 206–218. 10.1159/000113635. [DOI] [PubMed] [Google Scholar]
- 95.Bang BG, and Cobb S (1968). The size of the olfactory bulb in 108 species of birds. Auk 85, 55–61. 10.2307/4083624. [DOI] [Google Scholar]
- 96.Abelián A, Desfilis E, and Medina L (2014). Combinatorial expression of Lef1, Lhx2, Lhx5, Lhx9, Lmo3, Lmo4, and Prox1 helps to identify comparable subdivisions in the developing hippocampal formation of mouse and chicken. Front. Neuroanat 8, 59. 10.3389/fnana.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solstad T, Moser EI, and Einevoll GT (2006). From grid cells to place cells: a mathematical model. Hippocampus 1031, 1026–1031. 10.1002/hipo. [DOI] [PubMed] [Google Scholar]
- 98.Poulter S, Hartley T, and Lever C (2018). The neurobiology of mammalian navigation. Curr. Biol 28, R1023–R1042. 10.1016/j.cub.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 99.Fusi S, Miller EK, and Rigotti M (2016). Why neurons mix: High dimensionality for higher cognition. Curr. Opin. Neurobiol 37, 66–74. 10.1016/j.conb.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 100.Pravosudov VV (1985). Search for and storage of food by Parus cinctus lapponicus and Parus montanus borealis (Paridae). Zool. Zhurnal (Journal Zool. 64, 1036–1043. [Google Scholar]
- 101.Cueva CJ, and Wei XX (2018). Emergence of grid-like representations by training recurrent neural networks to perform spatial localization. 6th Int. Conf. Learn. Represent. ICLR 2018 - Conf. Track Proc., 1–19. [Google Scholar]
- 102.Sorscher B, Mel GC, Ocko SA, Giocomo LM, and Ganguli S (2022). A unified theory for the computational and mechanistic origins of grid cells. Neuron, 1–17. 10.1016/j.neuron.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 103.Dordek Y, Soudry D, Meir R, and Derdikman D (2016). Extracting grid cell characteristics from place cell inputs using non-negative principal component analysis. Elife 5, 1–36. 10.7554/eLife.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr R a, Orger MB, Jayaraman V, et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lopes G, Bonacchi N, Frazão J, Neto JP, Atallah BV, Soares S, Moreira L, Matias S, Itskov PM, Correia PA, et al. (2015). Bonsai: an event-based framework for processing and controlling data streams. Front. Neuroinform 9, 1–14. 10.3389/fninf.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou P, Resendez SL, Rodriguez-Romaguera J, Jimenez JC, Neufeld SQ, Giovannucci A, Friedrich J, Pnevmatikakis EA, Stuber GD, Hen R, et al. (2018). Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. Elife 7, 1–37. 10.7554/eLife.28728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Applegate MC, and Aronov D (2022). Flexible use of memory by food-caching birds. Elife 11, 1–28. 10.7554/eLife.70600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mott R, Herrod A, Hodgson JC, and Clarke RH (2015). An evaluation of the use of predicted harness spans for correctly fitting leg-loop harnesses in seabird research. Waterbirds 38, 420–424. 10.1675/063.038.0406. [DOI] [Google Scholar]
- 109.Mathis A, Mamidanna P, Abe T, Cury KM, Murthy VN, Mathis MW, and Bethge M (2018). Markerless tracking of user-defined features with deep learning. 1–14. [DOI] [PubMed] [Google Scholar]
- 110.Nath T, Mathis A, Chen AC, Patel A, Bethge M, and Mathis MW (2019). Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc 14, 2152–2176. 10.1038/s41596-019-0176-0. [DOI] [PubMed] [Google Scholar]
- 111.Friedrich J, Yang W, Soudry D, Mu Y, Ahrens MB, Yuste R, Peterka DS, and Paninski L (2017). Multi-scale approaches for high-speed imaging and analysis of large neural populations. PLoS Comput. Biol 13, e1005685. 10.1371/journal.pcbi.1005685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guizar-Sicairos M, Thurman ST, and Fienup JR (2008). Efficient subpixel image registration algorithms. Opt. Lett 33, 156. 10.1364/ol.33.000156. [DOI] [PubMed] [Google Scholar]
- 113.Skaggs WE, and McNaughton B (1993). An information theoretic approach to deciphering the hippcampal code. In Advances in Neural Information Processing Systems 5, pp. 1030–1038. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following data will be located on Dryad upon publication: For the retrograde tracing, we annotate the stereotaxic location of all labeled cells and report the injection location (Table S1), assigned brain region, bird identity, and fluorophore of CTB used. For the calcium imaging experiments, we include annotations of all sessions. For every timepoint of the trial, we include the animal’s location, head direction, and the calcium activity of each ROI detected with CNMF_E.106 The DOI for these data sets are listed in the key resources table. Microscopy data reported in this paper will be shared by the lead contact upon request.
All custom code used in analysis will be located on Dryad upon publication.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| scAAV9-CBh-GFP | UNC Vector Core | N/A |
| AAV9-pAAV.CAG.GCaMP6f.WPRE.SV40 | Chen et al.104 | Addgene plasmid # 100836 |
| Chemicals, peptides, and recombinant proteins | ||
| Cholera Toxin Subunit B, Alexa Fluor 488 conjugate | Fisher Scientific | C34775 |
| Cholera Toxin Subunit B, Alexa Fluor 555 conjugate | Fisher Scientific | C34776 |
| Cholera Toxin Subunit B, Alexa Fluor 647 conjugate | Fisher Scientific | C34778 |
| 4',6-diamidino-2-phenylindole, dihydrochloride (DAPI) | Fischer Scientific | PI62247 |
| Paraformaldehyde | Electron Microscopy Sciences | 15710 |
| Critical commercial assays | ||
| nVista System | Inscopix | N/A |
| Miqus motion capture cameras | Qualysis | N/A |
| Deposited data | ||
| Retrograde tracing | This paper | DOI upon publication |
| Calcium imaging | This paper | DOI upon publication |
| Experimental models: Organisms/strains | ||
| Poecile atricapillus (Black-capped chickadee) | Wild caught (NY state) | N/A |
| Baeolophus bicolor (Tufted titmouse) | Wild caught (NY state) | N/A |
| Software and algorithms | ||
| ImageJ | NIH, open source | N/A |
| MATLAB 2020a | Mathworks | https://www.mathworks.com |
| BONSAI | Lopes et al., 2015105 | https://bonsai-rx.org |
| Arduino | Open-source | https://www.arduino.cc/en/software |
| Illustrator CC 2021 | Adobe | https://www.adobe.com/products/illustrator.html |