Abstract
Members of the forkhead box O1 (FOXO) family of transcription factors are expressed in granulosa cells during various stages of follicle development, and evidence from rodent and other model systems suggests that they may be involved in regulating follicular activation and oocyte maturation. In this report, we show that FOXO1, FOXO3, and FOXO4 are expressed in human luteinized mural granulosa cells, which may suggest that these transcription factors are also involved in human folliculogenesis and luteinization.
Female fertility is determined by the size of the primordial follicle pool formed during fetal life and by the rate of depletion of the pool after birth (1). In addition to reduced ovarian complement, early depletion of the follicular pool due to excess follicular activation and/or atresia can occur and results in infertility (2, 3). The events surrounding follicle activation, development of the preovulatory follicle, and follicular atresia are controlled both by ovarian factors, such as IGF-1 and estrogen, and by the gonadotropins LH and FSH (4).
The forkhead family of transcription factors is highly conserved in evolution, and its members are known to play critical roles in regulating cellular differentiation and proliferation in many systems (5). In the rodent ovary, members of the Foxo (forkhead box O1) subfamily, which includes Foxo1 (FKHR), Foxo3 (FKHRL1), and Foxo4 (AFX), have been shown to be expressed in granulosa cells during various stages of follicle development and are thought to play important roles in oocyte maturation, ovulation, and possibly luteinization (6–11). Specifically, Foxo1 has been shown to be negatively regulated by FSH and IGF-1 via the PI3-kinase pathway (12) and appears to mediate proliferation and differentiation of murine granulosa cells in response to FSH (11). Furthermore, studies using porcine granulosa cells have shown that Foxo1 is a regulator of cell cycle progression (7). Foxo3-null female mice have been shown to have abnormal ovarian follicular development with early degeneration of oocytes, resulting in age-dependent infertility (6). This appears to be due to early activation of primordial follicles in these mice, which leads to the early depletion of functional oocytes and the rapid onset of infertility (9, 10), a phenotype which resembles that of women with premature ovarian failure (POF). More recently, a transgenic mouse model with constitutively active expression of Foxo3 in oocytes was also shown to be infertile, resulting from retarded oocyte growth and follicular development (13). Furthermore, mutations that result in loss of activity of some of the forkhead transcription factors have been shown to be related to human infertility. Watkins et al. (14) recently identified potentially causal mutations in the FOXO3A and FOXO1A genes in women with POF, and mutations in the related FOXL2 forkhead transcription factor (15, 16) have also been linked to POF (17).
To date, studies of the forkhead transcription factors have largely involved the rodent ovary, and there have been no studies to determine whether these transcription factors are expressed in the human ovary. Therefore, the present study was undertaken to determine whether the FOXO family of transcription factors are expressed in human granulosa cells. Future studies will be necessary to determine their role in the human ovary.
To determine whether members of the FOXO family of forkhead transcription factors are expressed in the human ovary, we first tested for the presence of human FOXO1, FOXO3, and FOXO4 transcripts in commercially available human ovarian cDNA (Human Ovary PCR-Ready cDNA; Ambion, Austin, TX). Polymerase chain reaction (PCR) was used to identify transcripts of human FOXO1, FOXO3, and FOXO4, with β-actin as a control. Ovarian cDNA (2.5 ng) was used in a 50-μL PCR using the HotStar Taq DNA Polymerase Kit (Qiagen, Valencia, CA). The primers used to amplify Foxo cDNA fragments were as follows: FOXO1 (5′-cagccctggatcacagtttt-3′ and 5′-catccccttctccaagatca-3’); FOXO3 (5′-gcaagcacagagttggatga-3′ and 5′-caggtcgtccatgaggtttt-3′); and FOXO4 (5′-ttgaggccagagtctgaggt-3′ and 5′- aggatgctgcaaagagaagc-3′). The PCR cycling profile consisted of an initial denaturation step at 95°C for 15 min, followed by 35 cycles of 94°C for 60 s, 55°C for 60 s, and 72°C for 60 s. After PCR, the amplified products were subjected to electrophoresis on 1.5% agarose gels, which were then stained with ethidium bromide to visualize the anticipated fragments. As a control, β-actin was amplified in a similar fashion. Bands of the anticipated sizes were obtained, and a band for each gene was eluted, purified using a QIAquick gel extraction kit (Qiagen) and confirmed by sequencing.
We then tested whether FOXO1, FOXO3, and FOXO4 transcripts were present in human granulosa cells. We obtained luteinized mural granulosa cells from ten consecutive patients undergoing IVF for various reasons under an Institutional Review Board–approved protocol. The cumulusoocyte complexes were removed, and granulosa cells were obtained from the follicular aspirates. The granulosa cells were separated from red blood cells by a previously described protocol using Percoll gradient (18). Briefly, the follicle aspirates were centrifuged at 1800 rpm for 10 min, the supernatent was removed, and the cells were resuspended in 10 mL 1× phosphate-buffered saline (PBS) and spun at 1500 rpm for 10 min. The supernatant was aspirated, and the cells were resuspended in 4 mL of 1× PBS and layered on Percoll. After centrifugation at 2000 rpm for 30 min, the luteinized mural granulosa cells were washed in 1× PBS and spun at 1500 rpm for 10 min.
The cell pellet was then lysed in RLT buffer (Qiagen), and total RNA was extracted using the RNeasy Mini Kit (Qiagen) as described by the manufacturer. Reverse transcription of total RNA was then performed using the Omniscript Reverse Transcription Kit (Qiagen) as described in the manufacturer’s protocol. The PCR was used to identify the transcripts of human FOXO1, FOXO3, and FOXO4 as described above, again using β-actin as a control. Five microliters of the reverse transcription reaction was used in a 50-μL PCR using the HotStar Taq DNA Polymerase Kit (Qiagen). Positive control samples (human ovary cDNA [Ambion]) and negative control samples (sterile distilled water) were performed for each primer. The PCR primers and the PCR cycling profile were as described above. After PCR, the amplified products were subjected to electrophoresis on 1.5% agarose gels, which were then stained with ethidium bromide to visualize the anticipated fragments. As a control, β-actin was amplified in a similar fashion. A band for each gene was gel eluted, purified, and confirmed by sequencing as described above.
FOXO1 was found to be expressed in luteinized mural granulosa cells from all ten patients, as demonstrated by the 170-bp band in Figure 1a. The presence of a 186-bp band demonstrates the presence of FOXO3 in the luteinized mural granulosa cells of all patients (Fig. 1b). FOXO4, as demonstrated by the 173-bp band, was also present in luteinized mural granulosa cells of all patients, regardless of the cause of infertility (Fig. 1c).
Figure 1.
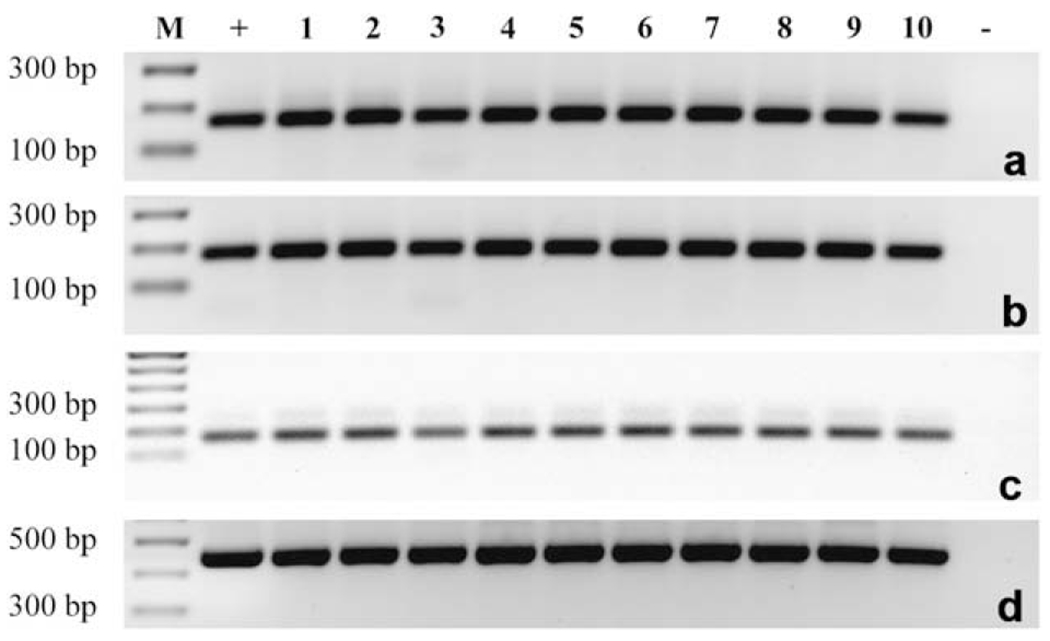
Expression of FOXO1, FOXO3, and FOXO4 in human luteinized mural granulosa cells. Total RNA isolated from human granulosa cells taken from ten different patients were reverse-transcribed and polymerase chain reaction (PCR) amplified with gene-specific primer pairs. Human luteinized mural granulosa cells from all ten patients were positive for the 170-bp product of FOXO1 (a), the 186-bp product of FOXO3 (b), and the 173-bp product of FOXO4 (c), as well as for β-actin (d). M = molecular weight marker; + = positive control; − = negative control.
This is the first time that members of the FOXO subclass of forkhead transcription factors, specifically FOXO1, FOXO3 and FOXO4, have been shown to be expressed in human luteinized mural granulosa cells. Previously, these transcription factors have been shown to be expressed in rodent and porcine granulosa cells, where they are thought to play important roles in normal follicular development, maturation, ovulation, and possibly luteinization (6–11). Foxo1 is selectively expressed in granulosa cells of growing follicles, where it is differentially regulated by hormones, including FSH and LH. Activation of Foxo family members has been shown to block cellular proliferation by inducing expression of genes such as p27kip and Fas ligand, which cause cell cycle arrest and apoptosis (19–21). Studies by Park et al. (11) demonstrate that proliferation and differentiation of granulosa cells in response to FSH plus activin requires removal of Foxo1-dependent repression of the cyclin D2 promoter. In addition to transcriptional regulation of Foxo1, FSH and IGF-1 also stimulate rapid PI3K-dependent phosphorylation of Foxo1 (12). Phosphorylation of Foxo1 results in its exclusion from the nucleus, providing additional control of its transcriptional activity (8). Thus, Foxo1 appears to be a tightly-controlled key regulator of granulosa cell proliferation and differentiation.
Loss of Foxo3 transcriptional activity in Foxo3a−/− female mice leads to global follicular activation, resulting in oocyte death, premature depletion of the follicular pool, and secondary infertility, which suggests that Foxo3 normally functions as a repressor of follicular activation (6, 9). Foxo3 and Foxo4 are expressed in luteal cells (12), which may suggest additional roles for these transcription factors in follicular differentiation.
We have demonstrated expression of three FOXO family members, FOXO1, FOXO3, and FOXO4 in human granulosa cells. Evidence from studies in rodents and other model systems, together with the identification of mutations in the human FOXO1 and FOXO3 genes in women with POF (14), suggest that the FOXO class of transcription factors may also play important roles in human folliculogenesis and luteinization. Future directions will include the quantification of the expression of these transcription factors and correlation with diagnosis of infertility, specifically relating to ovarian function, such as advancing age, diminished ovarian reserve, and anovulation. In addition, correlation of FOXO gene expression levels with clinical parameters, including ovarian response, E2 levels, and eggs retrieved, may provide further insights into the roles of these genes in human folliculogenesis.
Acknowledgments
Supported by a grant from the NIH (R01HD047603) (to M.D.P.) and a grant from the Helping Hands of Los Angeles Inc. (to M.D.P.).
Footnotes
M.P. is Medical Editor of the babycenter.com website sponsored by Johnson and Johnson and is Editor of ASRM News. D.T. is currently employed by Celera. F.-T.K. has nothing to disclose. P.Z. has nothing to disclose. S.K. has nothing to disclose. A.K. has nothing to disclose.
REFERENCES
- 1.Erickson GF. Role of growth factors in ovary organogenesis. J Soc Gynecol Investig 2001;8:S13–6. [DOI] [PubMed] [Google Scholar]
- 2.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol 1986;67:604–6. [PubMed] [Google Scholar]
- 3.Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod 2003;18:199–206. [DOI] [PubMed] [Google Scholar]
- 4.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 2002;296:2178–80. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann OJ, Sowden JC, Carlsson P, Jordan T, Bhattacharya SS. FOX’S in development and disease. Trends Genet 2003;19:339–44. [DOI] [PubMed] [Google Scholar]
- 6.Hosaka T, Biggs WH, Tieu D third, Boyer AD, Varki NM, Cavenee WK, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A 2004;101:2975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham MA, Zhu Q, Hammond JM. FoxO1a can alter cell cycle progression by regulating the nuclear localization of p27kip in granulosa cells. Mol Endocrinol 2004;18:1756–67. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham MA, Zhu Q, Unterman TG, Hammond JM. Follicle-stimulating hormone promotes nuclear exclusion of the forkhead transcription factor FoxO1a via phosphatidylinositol 3-kinase in porcine granulosa cells. Endocrinology 2003;144:5585–94. [DOI] [PubMed] [Google Scholar]
- 9.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003;301:215–8. [DOI] [PubMed] [Google Scholar]
- 10.Brenkman AB, Burgering BM. Foxo3a eggs on fertility and aging. Trends Mol Med 2003;9:464–7. [DOI] [PubMed] [Google Scholar]
- 11.Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, et al. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem 2005;280:9135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol 2002;16:580–99. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, et al. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development 2007;134:199–209. [DOI] [PubMed] [Google Scholar]
- 14.Watkins WJ, Umbers AJ, Woad KJ, Harris SE, Winship IM, Gersak K, et al. Mutational screening of Foxo3a and Foxo1a in women with premature ovarian failure. Fertil Steril 2006;86:1518–21. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura K, Kumagai J, Sudo S, Chun SY, Pisarska M, Morita H, et al. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci U S A 2004;101:7323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, et al. The murine winged-helix transcription factor FOXL2 is required for granulosa cell differentiation and ovary maintenance. Development 2004;131:933–42. [DOI] [PubMed] [Google Scholar]
- 17.Harris SE, Chand AL, Winship IM, Gersak K, Aittomaki K, Shelling AN. Identification of novel mutations in FOXL2 associated with premature ovarian failure. Mol Hum Reprod 2002;8:729–33. [DOI] [PubMed] [Google Scholar]
- 18.Yap OW, Chandrasekher YA, Giudice LC. Growth factor regulation of insulin-like growth factor binding protein secretion by cultured human granulosa-luteal cells. Fertil Steril 1998;70:535–40. [DOI] [PubMed] [Google Scholar]
- 19.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 1999;96:857–68. [DOI] [PubMed] [Google Scholar]
- 20.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 2000;404:782–7. [DOI] [PubMed] [Google Scholar]
- 21.Collado M, Medema RH, Garcia-Cao I, Dubuisson ML, Barradas M, Glassford J, et al. Inhibition of the phosphoinositide 3-kinase pathway induces a senescence-like arrest mediated by p27Kip1. J Biol Chem 2000;275:21960–8. [DOI] [PubMed] [Google Scholar]


