Abstract
Background:
Routine long-term anticoagulation in pulmonary arterial hypertension (PAH) is controversial. To date, anticoagulation has been found to be beneficial or neutral in idiopathic disease (IPAH) and neutral-to-harmful in connective tissue disease (CTD-PAH). We sought to examine the association between anticoagulation and mortality, healthcare utilization, and quality of life (QoL) in PAH.
Methods:
The PHAR is a prospective registry of PAH patients referred to 58 pulmonary hypertension care centers in the United States. We compared patients who received anticoagulation during enrollment (questionnaire documented) to those who did not. Cox proportional hazard models were used for mortality, Poisson multivariate regression models for healthcare utilization and generalized estimating equations for QOL
Results:
Of 1175 patients included, 316 patients were treated with anticoagulation. IPAH/hereditary PAH (HPAH) comprised 46% of the cohort and CTD-PAH comprised 33%. After adjustment for demographics, clinical characteristics, site and disease severity, anticoagulation was not associated with mortality in the overall population (HR, 1.00; 95% CI, 0.72–1.36), IPAH/HPAH (HR, 1.19; 95% CI, 0.74–1.94), or CTD-PAH (HR 0.87; 95% CI, 0.53–1.42). Anticoagulation was associated with an increased rate of emergency department visits (IRR: 1.41), hospitalizations (IRR: 1.30), and hospital days (IRR 1.33). QOL measured by emPHasis-10 score was worse in patients receiving anticoagulation (mean difference 1.74; 95% CI 0.40–3.09).
Conclusions:
Anticoagulation is not associated with higher mortality, but is associated with increased healthcare utilization in the PHAR. PAH-specific QoL may be worse in patients receiving anticoagulation. The risks and benefits surrounding routine prescription of anticoagulation for PAH should be carefully considered.
Introduction
Early investigations of pulmonary arterial hypertension (PAH) demonstrated endothelial dysfunction and pulmonary vascular thrombosis in situ as key pathologic findings.1–3 Accordingly, single-center cohort studies examined the effect of anticoagulation in PAH with findings to suggest a mortality benefit, especially among patients with Idiopathic PAH (IPAH).1,4–8 More recent large-scale PAH registries have produced conflicting evidence. A COMPERA registry analysis suggested a mortality benefit with vitamin K antagonists (VKAs) in IPAH, but not in connective tissue disease associated pulmonary arterial hypertension (CTD-PAH).9 In contrast, analysis of the REVEAL registry did not find that anticoagulation affected survival in IPAH or CTD-PAH.10 Two subsequent meta-analyses examining anticoagulation in IPAH have produced discordant results with one study reporting a survival benefit and the other no effect.11,12 The sole meta-analysis examining anticoagulation in CTD-PAH found a trend toward increased mortality in systemic sclerosis associated PAH (SSc-PAH).11 Thus clinicians are left with conflicting data regarding the role and impact of anticoagulation in PAH.
Accordingly, the most recent society guidelines reflect the complexities of existing evidence. The European Society of Cardiology 2015 guidelines13 suggest anticoagulation in IPAH, hereditary PAH (HPAH), and PAH due to anorexigen use (Class IIb Level C), with case-by-case consideration in CTD-PAH (Class IIb Level C) while recommending against anticoagulation in HIV-related PAH and portopulmonary PAH. In contrast the 2019 CHEST guidelines do not carry a recommendation for or against anticoagulation.14 Without high quality evidence to clarify the association between anticoagulation and mortality in PAH, attention has shifted toward patient-specific decision making15 and the challenges of effective anticoagulation.16–18 However, there is little published data on the association between anticoagulation and non-mortality outcomes. The association between anticoagulation and healthcare utilization in PAH has never been examined. The association between anticoagulation and quality of life (QoL) has only been examined in the SSc-PAH population, finding that anticoagulation was associated with reduced QoL.19
This study was designed to augment risk/benefit considerations surrounding anticoagulation therapy in PAH using the Pulmonary Hypertension Association Registry (PHAR), a modern, multicenter, observational, US-based longitudinal registry of pulmonary hypertension patients. We sought to examine the association between anticoagulation use and mortality, healthcare utilization, and QoL.
Materials and Methods:
The Pulmonary Hypertension Association Registry (PHAR)
Characteristics of the PHAR, including rationale, criteria for enrollment, data collection procedures, and organizational structure have been described previously.20 The PHAR IRB approval is maintained at The University of Pennsylvania (Protocol Number 822830), which serves as single IRB. All patients provided informed consent for enrollment. Patients new to care at PHAR-associated pulmonary hypertension (PH) centers were non-consecutively enrolled beginning in 2015. The dataset analyzed in this study was locked on September 19, 2021. There were 58 pulmonary hypertension care centers in the U.S. represented at the time of analysis with 1772 total patients enrolled.
Baseline variables collected include demographics, socioeconomic data, clinical characteristics, symptom classification, and hemodynamics from right heart catheterization. Data on medical therapies, QoL, and healthcare utilization are collected at baseline and each follow up in approximately 6-month intervals. The two QoL metrics collected in the PHAR are the Medical Outcome Study Short Form-12 (SF-12)21 and the emPHasis-10 questionnaire.22 Healthcare utilization is self-reported at each follow-up and includes the number of emergency department (ED) visits and PH clinic visits since the last follow up, and hospitalizations with number of hospital days in the last 6 months. Comorbid conditions were not collected as part of the PHAR registry, nor was data on type of anticoagulant or VKA related monitoring. On average, there was less than 10% of missing data for the included variables
Study Population and Subgroups
This study included World Health Organization Group 1 PH patients that were at least 18 years old at enrollment with at least one follow up visit. Patients with PAH due to congenital heart disease and persistent PH of the newborn were excluded. The population was divided into comparator groups by anticoagulation use, defined as use of a VKA, heparin or direct oral anticoagulant (DOAC). Antiplatelet therapies such as aspirin or P2Y12 inhibitors were not considered anticoagulation. The primary predictor was anticoagulation treatment during registry enrollment (survey indication of active anticoagulation use at any visit, or anticoagulation within the last 6 months at any visit after baseline). For subgroup analyses, the IPAH and HPAH cohorts were grouped together to increase power based on hypothesized similarities in pathophysiology. The CTD-PAH sub-cohort included SSc-PAH.
Statistical Analysis
Categorical variables were compared using Chi-squared test or Fisher exact test. Continuous variables were reported as mean ± standard deviation or median with interquartile range and analyzed by t-test or Wilcoxon rank sum test. We employed Kaplan Meier curves and estimates of survival to describe survival with statistical testing by log-rank test and cox proportional hazard model. The proportional hazard assumption was tested for all Cox models by Shoenfeld residual testing. We found no evidence of non-proportionality. Healthcare utilization outcomes were reported as events per person-year and compared using Poisson regression modeling. We compared change in QoL over time between groups using generalized estimating equations clustered by participant. We performed an unadjusted and multivariate adjusted analysis for each model with 10-fold multiple imputation of missing covariates by chained equations. Rationale behind the variables selected for each of the adjusted models are described in detail in the supplementary materials. All analyses were conducted using STATA 17.0 (Stata Corp, College Station, TX). A p-value cutoff of 0.05 was considered statistically significant.
Sensitivity Analyses
We conducted additional analyses using the predictors of anticoagulation status at baseline visit and first follow up visit. Analysis by anticoagulation at first follow up was included to account for clinical changes related to PHCC expert evaluation. We completed a sensitivity analysis of incident cases alone to address the potential for immortal time bias. Subgroup and sensitivity analyses should be interpreted cautiously due to the limited power to detect statistically significant effects.
Detailed description of the study methodology is included in the Supplementary Materials.
Results:
Patient Characteristics
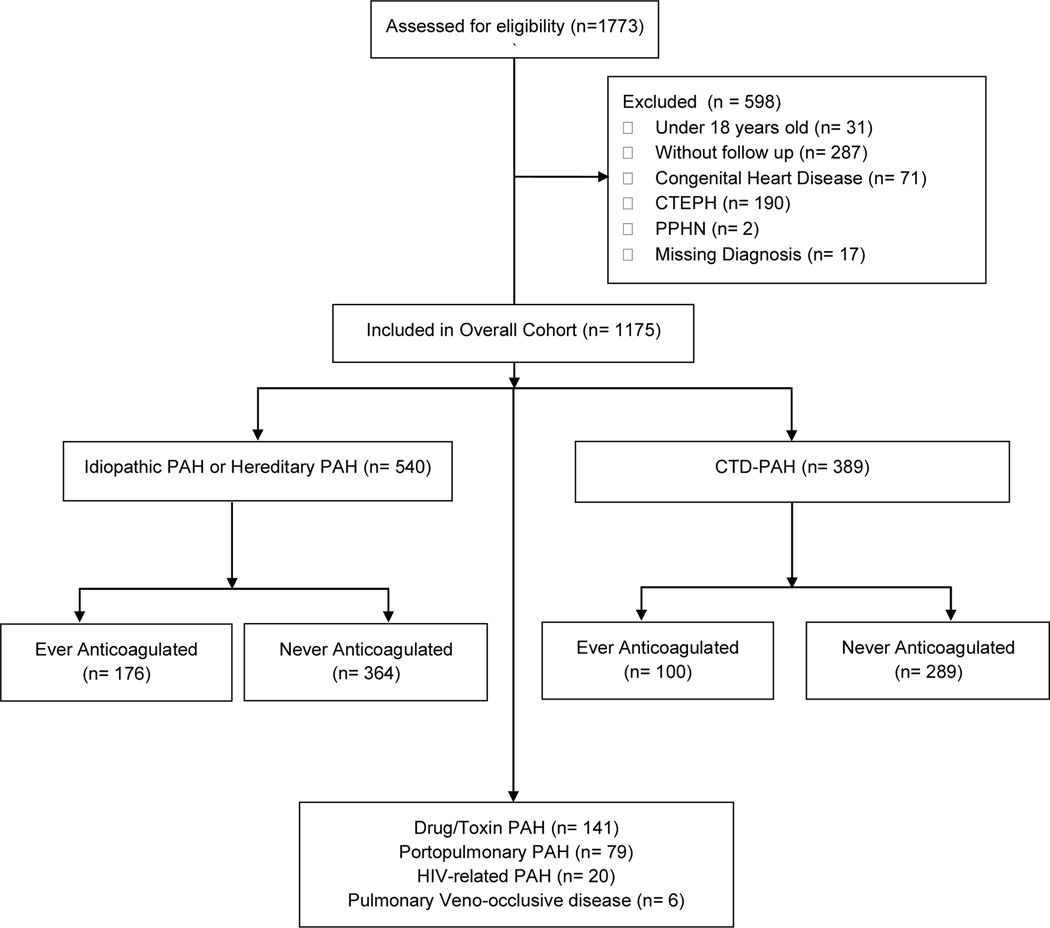
There were 1175 patients that met inclusion criteria, with 316 patients anticoagulated during enrollment (Figure 1). The median duration of follow up was 839 days. Of the 540 patients with IPAH/HPAH, 176 (32.6%) were treated with anticoagulation and of the 389 patients with CTD-PAH, 91 (25.7%) were treated with anticoagulation. There were 638 patients (54.3%) that were diagnosed with PAH within 6 months of enrollment and therefore considered incident cases.
Figure 1:
PHAR WHO Group 1 Pulmonary Hypertension CONSORT Diagram
In the overall cohort, patients who received anticoagulation were older, with a lower eGFR, more severe disease by World Health Organization functional class (WHO FC) and REVEAL 2 Lite risk score had a longer duration of follow up, and were more likely to be prescribed prostacyclin therapy, or supplemental oxygen (Table 1). On baseline invasive hemodynamics, anticoagulated patients had a higher pulmonary artery wedge pressure and lower right ventricular stroke work index (Table 2). Subgroup analysis cohorts are described in Tables S1–S4.
Table 1:
Baseline demographics of PHAR by Anticoagulation Exposure
| Ever Anticoagulation (n=316) | No Anticoagulation (n=859) | p-value | |
|---|---|---|---|
| Age (years) (n=1171) | 59.1 ± 16.4 | 54.7 ± 15.6 | <0.001 |
|
| |||
| BMI (kg/m2))[n=1139) | 28.4 IQR [24.9–34.7] | 28.4 IQR [24.6–33.2] | 0.349 |
|
| |||
| Male Gender (n=1175) | 79 (25.0%) | 208 (24.2%) | 0.781 |
|
| |||
| Uninsured (n=1175) | 3 (1.0%) | 21 (2.4%) | 0.108 |
|
| |||
| Never Married (n=1160) | 52 (16.7%) | 159 (18.8%) | 0.415 |
|
| |||
| College Graduate (n=1167) | 93 (29.5%) | 298 (35.0%) | 0.080 |
|
| |||
| Unemployed (n=1175) | 65 (20.6%) | 201 (23.4%) | 0.304 |
|
| |||
| Ever Smoker (n=1169) | 146 (46.2%) | 403 (47.3%) | 0.751 |
|
| |||
| Current Alcohol Use (n=1169) | 103 (32.6%) | 282 (33.1%) | 0.881 |
|
| |||
| Meth Use (n=1169) | 39 (12.4%) | 143 (16.7%) | 0.068 |
|
| |||
| Follow Up Time (days) (n=1175) | 703 IQR [399–1073] | 591 IQR [310–922] | <0.001 |
|
| |||
| Incident Diagnosis of PAH (n=1171) | 163 (51.6%) | 475 (55.6%) | 0.226 |
|
| |||
| Primary Diagnosis (n=1175) | |||
| IPAH | 167 (52.8%) | 342 (39.8%) | |
| CTD PAH | 100 (31.6%) | 289 (33.6%) | |
| Drug/Toxin PAH | 27 (8.5%) | 114 (13.3%) | |
| Portopulmonary PAH | 6 (1.9%) | 73 (8.5%) | |
| Heritable PAH | 9 (2.8%) | 22 (2.6%) | |
| HIV-Related PAH | 5 (1.6%) | 15 (1.7%) | |
| Pulmonary Veno-occlusive disease | 2 (0.6%) | 4 (0.5%) | |
|
| |||
| Self-Identified Race/Ethnicity (n=1164) | 0.393 | ||
| Non-Hispanic White | 228 (72.4%) | 584 (68.8%) | |
| Hispanic/Latinx | 32 (10.2%) | 75 (8.8%) | |
| Black/African American | 37 (11.8%) | 121 (14.3%) | |
| Asian | 11 (3.5%) | 36 (4.2%) | |
| Other | 7 (2.2%) | 33 (3.9%) | |
|
| |||
| Taxable Income per year (n=931) | 0.262 | ||
| Less than $50,000 | 149 (60.1%) | 368 (53.9%) | |
| $50,000 to 100,000 | 53 (21.4%) | 179 (26.2%) | |
| $100,000 to $150,000 | 30 (12.1%) | 78 (11.4%) | |
| Greater than $150,000 | 16 (6.5%) | 58 (8.5%) | |
|
| |||
| US Census Region (n=1175) | 0.157 | ||
| Northeast | 61 (19.3%) | 166 (19.3%) | |
| Midwest | 51 (16.1%) | 165 (19.2%) | |
| South | 88 (27.9%) | 269 (31.3%) | |
| West | 107 (36.7%) | 235 (30.2%) | |
|
| |||
| Site Volume (n=1175) | 0.627 | ||
| First Quintile | 8 (2.5%) | 22 (2.6%) | |
| Second Quintile | 27 (8.5%) | 58 (6.8%) | |
| Third Quintile | 54 (17.1%) | 138 (16.1%) | |
| Fourth Quintile | 82 (26.0%) | 206 (24.0%) | |
| Fifth Quintile | 145 (45.9%) | 435 (50.6%) | |
|
| |||
| WHO Functional Class (n=1115) | 0.052 | ||
| Class 1 | 23 (7.8%) | 69 (8.4%) | |
| Class 2 | 84 (28.6%) | 291 (35.4%) | |
| Class 3 | 159 (54.1%) | 412 (50.2%) | |
| Class 4 | 28 (9.5%) | 49 (6.0%) | |
|
| |||
| REVEAL 2 Lite Category (n=1138) | 0.009 | ||
| Low | 88 (29.0%) | 324 (38.9%) | |
| Moderate | 92 (30.3%) | 219 (26.2%) | |
| High | 124 (40.8%) | 291 (34.9%) | |
|
| |||
| 6MWD (meters) (n=1008) | 314.3 ± 132.0 | 336.4 ± 131.2 | 0.019 |
|
| |||
| B-type Natriuetic Peptide (pg/mL) (n=645) | 182 IQR[69–473] | 109 IQR[42–332] | <0.001 |
|
| |||
| NT-proBNP (n=536) | 585 IQR[230–1886] | 539 IQR[193–1730] | 0.483 |
|
| |||
| eGFR (mL/min/1.73m2) (n=1145) | 73.7 ± 24.9 | 78.3 ± 28.0 | 0.011 |
|
| |||
| Therapy at enrollment (n=1173) | |||
| PDE5i | 235 (74.4%) | 674 (78.7%) | 0.119 |
| ERA | 170 (53.8%) | 483 (56.4%) | 0.433 |
| Prostacyclin (any) | 128 (40.5%) | 281 (32.8%) | 0.014 |
| Digoxin | 34 (10.8%) | 52 (6.1%) | 0.006 |
| On PAH-Specific Therapy | 275 (87.0%) | 755 (88.1%) | 0.618 |
| Dual Combination Therapy | 148 (46.8%) | 428 (49.9%) | 0.345 |
| Triple Therapy | 66 (20.9%) | 139 (16.3%) | 0.062 |
| Parenteral Prostacyclin | 92 (29.1%) | 194 (22.6%) | 0.022 |
| Intravenous Prostacyclin | 49 (15.5%) | 87 (10.2%) | 0.011 |
| Oxygen Supplementation | 161 (51.0%) | 322 (37.6%) | <0.001 |
Table 2:
Hemodynamics at Baseline Visit by Anticoagulation Exposure
| Ever Anticoagulation (n=316) |
No Anticoagulation (n=859) | p-value | |
|---|---|---|---|
| HR (beats per minute) [n = 812] | 78.5 ± 14.7 | 80.8 ± 16.2 | 0.065 |
| SBP Pressure (mm Hg) [n = 749] | 130.9 ± 24.2 | 128.2 ± 22.9 | 0.157 |
| DBP (mm Hg) [n = 745] | 77.8 ± 14.2 | 76.5 ± 13.8 | 0.243 |
| Pulse Pressure (mm Hg) [n = 1142] | 46.0 ± 14.6 | 46.2 ± 13.7 | 0.839 |
| RAP (mm Hg) [n = 1125] | 9 [5 – 14] | 9 [6 – 14] | 0.514 |
| Mean PAP (mm Hg) [n = 1146] | 48.2 ± 13.2 | 49.0 ± 13.4 | 0.333 |
| PAPi [n = 1098] | 4.8 [3.1 – 7.8] | 5.1 [3.4 – 8.4] | 0.234 |
| PVR (Woods Units) [n = 1040] | 8.8 [5.8 – 12.8] | 9.1 [6.2 – 12.7] | 0.379 |
| PAWP (mm Hg) [n = 1109] | 11 [8 – 15] | 10 [7 – 13] | 0.014 |
| RVSWI (g/m/beat/m2) [n = 748] | 12.8 [9.9 – 17.0] | 13.8 [10.4 – 17.9] | 0.019 |
| Cardiac Index (L/min/m2) [n = 1056] | 2.1 [1.7 – 2.6] | 2.2 [1.8 – 2.7] | 0.434 |
| Cardiac Output (L/min) [n = 1085] | 4.0 [3.2 – 5.0] | 4.1 [3.3 – 5.1] | 0.228 |
Anticoagulation use at the baseline visit decreased by year of entrance in the registry between 2015 and 2021 (Table 3). Of the 316 patients ever receiving anticoagulation, the median duration of anticoagulation use was 401 days (IQR 200–709) with 133 patients (42.1%) receiving anticoagulation for the full duration of study enrollment and 22 patients (7.0%) only receiving anticoagulation at their baseline visit. At baseline 206 patients were treated with anticoagulation, of whom 133 remained on anticoagulation throughout enrollment. At first follow up 204 patients reported anticoagulation. Final registry disposition was similar between anticoagulated patients and non-anticoagulated patients including proportions of patients that failed to follow up (5.4% vs 8.4%), transferred to another clinic (7.0% vs 7.1%), received lung transplantation (2.2% vs 1.8%), and withdrew from the registry (1.9% vs 2.1%). There were two patients (0.6%) in the anticoagulation cohort and two patients (0.2%) in the no anticoagulation cohort who died from bleeding.
Table 3:
Anticoagulation Status at Baseline Visit by Enrollment Year
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | p-value* | |
|---|---|---|---|---|---|---|---|---|
| All PAH | 28.6% | 21.4% | 18.2% | 16.6% | 16.1% | 15.5% | 21.5% | 0.165 |
| IPAH/HPAH | - | 34.1% | 25.3% | 23.2% | 19.6% | 18.4% | 20.0% | 0.024 |
| CTD-PAH | - | 14.0% | 13.4% | 13.3% | 13.7% | 14.5% | 27.8% | 0.140 |
For linear regression trend
Survival Analysis
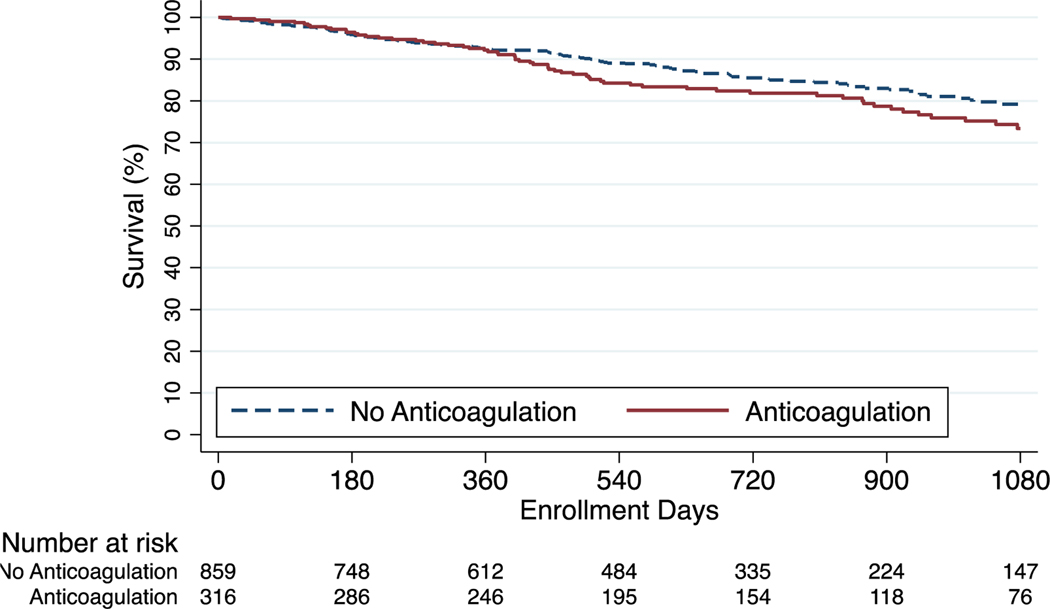
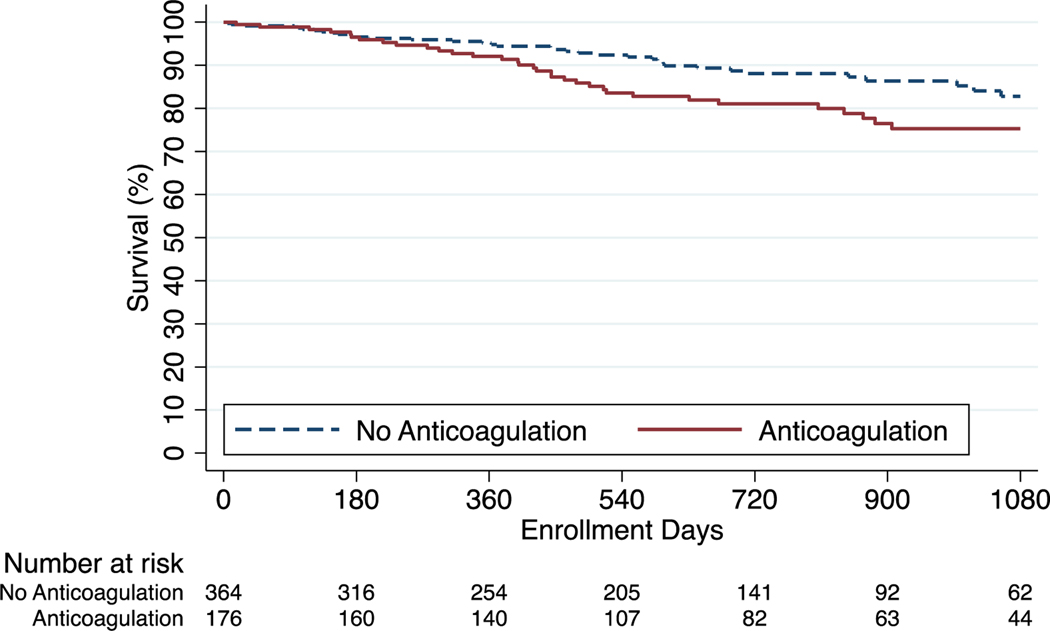
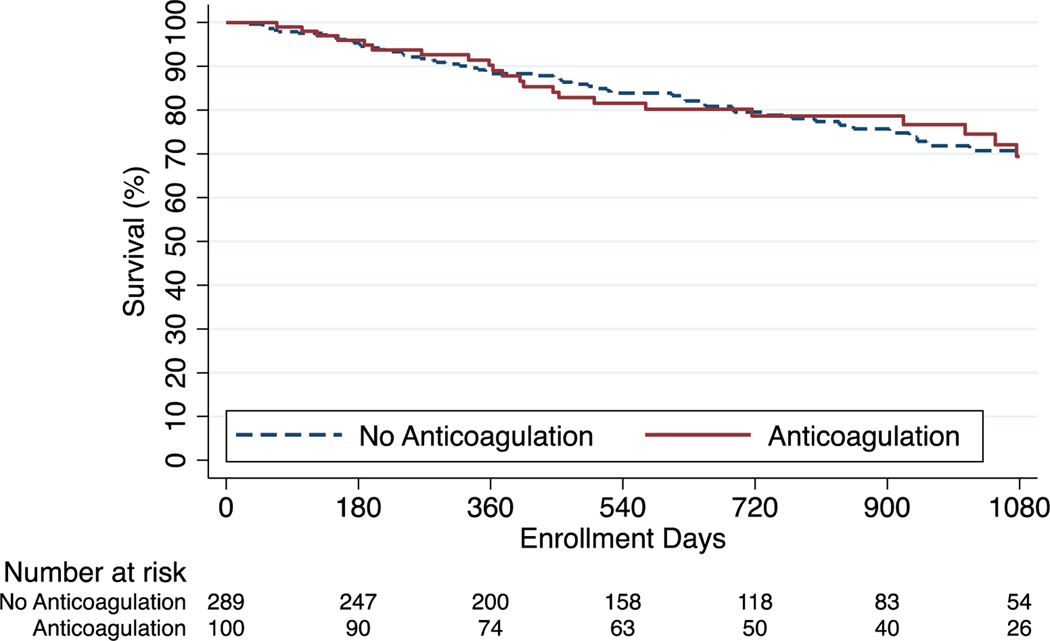
Kaplan Meier survival estimates at one, two, and three years were 91.8%, 81.9%, and 73.4% among anticoagulated patients and 92.3%, 85.5%, and 79.2% among those who did not receive anticoagulation (p=0.11). In cox regression analysis, anticoagulation was not associated with differences in survival in the unadjusted model (HR 1.29; 95% CI, 0.94–1.76) nor in the model adjusted for demographics, clinical characteristics, disease severity, and site volume (adjusted HR, 1.00; 95% CI, 0.72–1.36) (Figure 2). For the IPAH/HPAH cohort the survival estimates at one, two, and three years were 91.5%, 80.6%, and 74.8% among anticoagulated patients and 94.8%, 88.1%, and 82.8% among patients who did not receive anticoagulation (p=0.02). There was an association between anticoagulation and mortality in the unadjusted model (HR, 1.71; 95% CI, 1.07–2.74) that was no longer significant following adjustment (adjusted HR, 1.19; 95% CI, 0.74–1.94) (Figure 3). For the CTD-PAH cohort the survival estimates at one, two, and three years were 89.0%, 78.7%, and 69.4% in anticoagulated patients and 88.3%, 79.5%, and 70.7% in patients that did not receive anticoagulation (p=0.98) (Figure 4). Among CTD-PAH patients, anticoagulation was not associated with mortality in unadjusted (HR 1.01; 95% CI, 0.61–1.65) or adjusted analysis (adjusted HR 0.87; 95% CI, 0.53–1.42). The results were unchanged in the sensitivity analyses by anticoagulation definitions (Figure S1 and S2) and by incident cases (Figure S3).
Figure 2:
Kaplan-Meier Estimates of Survival - Overall Cohort
Figure 3:
Kaplan-Meier Estimates of Survival for IPAH/HPAH
Figure 4:
Kaplan-Meier Estimates of Survival CTD-PAH
Healthcare Utilization
In analysis adjusted for demographics, disease severity, PAH-targeted therapy, and site volume, anticoagulation was associated with increased ED visits in the overall cohort (adjusted IRR, 1.41; 95% CI, 1.28–1.56), IPAH/HPAH subgroup (adjusted IRR, 1.36; 95% CI, 1.17–1.59), and CTD-PAH subgroup (adjusted IRR, 1.71; 95% CI, 1.42–2.05). Similarly, there was an association between anticoagulation and increased hospital days for the overall cohort (adjusted IRR, 1.30; 95% CI, 1.23–1.37), IPAH/HPAH subgroup (adjusted IRR, 1.21; 95% CI, 1.10–1.32), and CTD-PAH subgroup (adjusted IRR, 2.13; 95% CI, 1.89–2.39), as well as an association between anticoagulation and increased hospitalizations for the overall cohort (adjusted IRR, 1.33; 95% CI, 1.14–1.55), IPAH/HPAH subgroup (adjusted IRR, 1.38; 95% CI, 1.09–1.75) and CTD-PAH subgroup (adjusted IRR, 1.91; 95% CI, 1.42–2.56). In the IPAH/HPAH subgroup there was no association between anticoagulation and number of PAH clinic visits (adjusted IRR, 0.97; 95% CI, 0.89–1.04). However there was an association with increased PAH clinic visits in the overall (adjusted IRR, 1.05; 95% CI, 1.00–1.11), and CTD-PAH cohorts (adjusted IRR, 1.12; 95% CI, 1.01–1.24). These results are displayed in Table 4.
Table 4:
Healthcare Utilization by Anticoagulation Exposure
| Ever Anticoagulation (events per person-year) | No Anticoagulation (events per person-year) | Incident Rate Ratio IRR (95% CI) | Adjusted IRR* IRR (95% CI) | |
|---|---|---|---|---|
| Overall n = 1020 | ||||
| ED Visits | 1.03 | 0.76 | 1.36 (1.23 – 1.49) | 1.41 (1.28 – 1.56) |
| Hospital Days | 3.39 | 2.71 | 1.25 (1.19 – 1.32) | 1.30 (1.23 – 1.37) |
| Hospitalizations | 0.44 | 0.34 | 1.30 (1.12 – 1.51) | 1.33 (1.14 – 1.55) |
| PAH Clinic Visits | 3.01 | 2.95 | 1.02 (0.97 – 1.08) | 1.05 (1.00 – 1.11) |
| IPAH/HPAH n = 479 | ||||
| ED Visits | 0.93 | 0.66 | 1.42 (1.23 – 1.64) | 1.36 (1.17 – 1.59) |
| Hospital Days | 2.63 | 2.10 | 1.25 (1.15 – 1.36) | 1.21 (1.10 – 1.32) |
| Hospitalizations | 0.41 | 0.27 | 1.52 (1.21 – 1.89) | 1.38 (1.09 – 1.75) |
| PAH Clinic Visits | 2.97 | 3.11 | 0.96 (0.89 – 1.03) | 0.97 (0.89 – 1.04) |
| CTD-PAH n = 337 | ||||
| ED Visits | 1.08 | 0.66 | 1.64 (1.38 – 1.94) | 1.71 (1.42 – 2.05) |
| Hospital Days | 4.26 | 2.81 | 1.51 (1.39 – 1.64) | 2.13 (1.89 – 2.39) |
| Hospitalizations | 0.46 | 0.33 | 1.42 (1.10 – 1.82) | 1.91 (1.42 – 2.56) |
| PAH Clinic Visits | 2.97 | 2.97 | 1.00 (0.91 – 1.10) | 1.12 (1.01 – 1.24) |
Adjusted for age, sex, bmi, WHO Functional Class, Race/Ethnicity, PAPi, PAH-specific, medication, and site volume
The association between anticoagulation and increased healthcare utilization remained consistent for the overall cohort and CTD-PAH subgroup in the sensitivity analyses by anticoagulation definition (tables S5 and S6). However, the interaction was less consistent for the IPAH/HPAH cohort. Baseline anticoagulation was associated with increased ED visits (adjusted IRR, 1.21; 95% CI, 1.01–1.44) but decreased hospital days (adjusted IRR, 0.85; 95% CI, 0.76–0.95) while anticoagulation at first follow up visit was not associated with ED visits and was associated with decreased hospital days (adjusted IRR, 0.82; 95% CI, 0.73–0.91). The sensitivity analyses by anticoagulation definition were not associated with hospitalizations or PAH clinic visits. The sensitivity analysis of incident cases (table S7) did not differ from the primary analysis
Health-Related QoL
Anticoagulation was associated with higher emPHasis-10 score (adjusted mean difference 1.74; 95% CI 0.40–3.09) representing worse PAH-specific QoL in the overall population. However, there was no association between anticoagulation and emPHasis-10 score in the IPAH/HPAH subgroup (adjusted mean difference 1.45; 95% CI −0.46–3.35) or CTD-PAH subgroup (adjusted mean difference 2.07; 95% −0.18–4.33). There was an association between anticoagulation and worse QoL as measured by the SF-12 mental component score (adjusted mean difference −1.49; 95% −2.70 - −0.28) in the overall population, but no other significant associations were appreciated in the subgroups examined for the SF-12 mental component score, nor for the SF-12 physical component score (Table 5). Anticoagulation was not clearly associated with QoL in the sensitivity analyses (Tables S8, S9, and S10).
Table 5:
Impact of Anticoagulation on QoL by Generalized Estimating Equation Modeling
| SF-12 Physical Component Score Mean Difference (95% CI) | SF-12 Mental Component Score Mean Difference (95% CI) | emPHasis-10 Mean Difference (95% CI) | ||
|---|---|---|---|---|
| Overall Cohort (n=1018) | Unadjusted | −1.95 (−3.20 – −0.70) | −1.03 (−2.26 – 0.21) | 2.23 (0.73 – 3.73) |
| Adjusted* | −0.92 (−2.04 – 0.20) | −1.49 (−2.70 – −0.28) | 1.74 (0.40 – 3.09) | |
| IPAH/HPAH (n=479) | Unadjusted | −1.34 (−3.11 – 0.44) | −0.70 (−2.39 – 0.99) | 1.35 (−0.81 – 3.52) |
| Adjusted* | −0.38 (−1.90 – 1.15) | −1.53 (−3.21 – 0.15) | 1.45 (−0.46 – 3.35) | |
| CTD-PAH (n=336) | Unadjusted | −2.44 (−4.62 – −0.25) | −1.46 (−3.47 – 0.56) | 2.76 (0.30 – 5.22) |
| Adjusted* | −1.32 (−3.32 – 0.69) | −1.92 (−3.92 – 0.07) | 2.07 (−0.18 – 4.33) |
Adjusted for age, BMI, sex, race/ethnicity, WHO functional class, PAH-targeted therapy, and site volume
Discussion:
In a large, multicenter, contemporary cohort, we found no association between mortality and anticoagulation treatment. Anticoagulation was consistently associated with increased healthcare utilization for the overall cohort, IPAH/HPAH subgroup, and the CTD-PAH subgroup in our primary analysis. These findings were durable on sensitivity analysis by anticoagulation definition for the overall cohort and CTD-PAH subgroup, but inconsistent for the IPAH/HPAH subgroup. This inconsistency suggests that there may not be a clear association between anticoagulation and healthcare utilization in the IPAH/HPAH subgroup, but may also be the result of multiple hypothesis testing. We found a weak association between anticoagulation and worsened QoL as measured by emPHasis10 score and SF-12 mental component score in the overall cohort. However, no association was detected between anticoagulation and QoL via the SF-12 physical component score, nor was any association noted between anticoagulation and QoL in the IPAH/HPAH or CTD-PAH subgroups. The primary analyses and sensitivity analysis altogether suggest against any meaningful association between anticoagulation and quality of life.
The analyses from the REVEAL and COMPERA registries remain the largest studies to examine the association between mortality and anticoagulation in PAH. Our findings oppose the morality benefit for IPAH demonstrated in the COMPERA registry analysis despite a similarly defined anticoagulation group. In contrast, our findings align with the REVEAL registry analysis, which matched new warfarin users to never users and found no association between mortality and anticoagulation in the IPAH cohort. Meta-analyses examining anticoagulation in IPAH have produced mixed results and are limited by publication bias, heterogeneity in study populations, shifts in medical therapy over time, and inclusion of patients with CTEPH in early observational studies.1,11,12,23 For patients with CTD-PAH, the mortality results in PHAR were concordant with the REVEAL and COMPERA analyses, in which anticoagulation was not associated with mortality in SSc-PAH.
Our results may differ from COMPERA while mirroring REVEAL in part due to the composition of each registry. In contrast to COMPERA, the PHAR cohort is younger, has less severe symptoms by WHO FC, is hemodynamically more severe, has proportionally fewer men and IPAH patients, and is prescribed more intensive PAH-targeted therapy. Compared to REVEAL, the PHAR cohort is similar in age, with a higher proportion of men, has similar severity of disease by WHO FC, and is prescribed more intensive PAH-targeted therapy. Of the PAH registries to assess mortality, the PHAR has the shortest duration of follow up and fewest mortality events.
The proportion of anticoagulated patients was lower in the PHAR compared to the COMPERA registry, with 34% of IPAH/HPAH patients and 26% of CTD-PAH patients, compared to 66% of IPAH patients and 50% of CTD-PAH patients in COMPERA. Within the PHAR, the proportion of patients receiving anticoagulation decreased over time suggesting a change in practice patterns related to COMPERA and REVEAL publications with subsequent weakening of guideline recommendations. Within the PHAR, patients who were prescribed anticoagulation were older than those patients who were not prescribed anticoagulation, with worse renal function, higher clinical risk (by WHO FC and Reveal Lite 2), higher rates of prostacyclin and supplemental oxygen prescription, worse right ventricular function, and higher PAWP. These differences might suggest that anticoagulation is more frequently prescribed to sicker patients, as has been noted in the COMPERA and REVEAL registries.9,10 Alternatively, this may reflect a higher incidence of comorbid conditions with an indication for anticoagulation (i.e arrhythmia or venous thromboembolic disease). Our finding of elevated PAWP in the anticoagulation cohort may suggest increased prevalence of left sided heart disease, which in turn may correlate with increased prevalence of atrial arrhythmias.24
The increased healthcare interactions associated with anticoagulation may be driven by bleeding complications and VKA titration.17 This also may be explained more severe disease among patients receiving anticoagulation, requiring care in the ED and hospital more frequently. No studies have examined the effect of long-term anticoagulation on disease progression by clinical (i.e WHO FC, six minute walk distance, REVEAL score), hemodynamic, or echocardiographic parameters. In contrast, the hazard for major bleeding is elevated for PAH patients on anticoagulation17 with one study noting a major bleeding rate of 5.4 per 100 patient-years in IPAH and 19 per 100 patient-years in CTD-PAH.25 Notably, the risk of bleeding appears to be higher in CTD-PAH compared to IPAH/HPAH,26 which may result from other disease manifestations (i.e gastrointestinal involvement of systemic sclerosis) or INR fluctuation related to medication interactions.
There was a weak association between anticoagulation and worsened QoL in this study. Although statistically significant, the absolute difference in emPHasis-10 score between groups was below the estimated minimally important difference of 6 points.27 The emPHasis-10 score may be a helpful marker of clinical status, and has been associated with six minute walk distance, WHO FC, and mortality.28 The lack of association between anticoagulation therapy and improved QoL may indirectly suggest against a net beneficial effect. Anticoagulation could exert a negative effect on QoL through increased bleeding events, frequency of lab draws, and lifestyle restrictions. One prior study examined the association between QoL and anticoagulation in PAH, finding that anticoagulation was associated with a worse SF-36 physical component score, but not associated with SF-36 mental score or scleroderma specific questionnaire in a cohort of 132 CTD-PAH patients, of whom 28.5% were anticoagulated.19 Conversely, in in our study we found an association between anticoagulation and worse SF-12 mental component score, but no association with physical component score. Discrepant SF survey results may be related to study population differences, rate of anticoagulation, and the use of SF-12 in the PHAR, a briefer instrument in comparison to the SF-36. The SF-12 and emPHasis-10 questionnaires may not be ideal instruments to evaluate QoL changes related to anticoagulation in PAH patients. Future studies may employ alternative validated instruments including PAH-SYMPACT,29 CAMPHOR,30 SF-36, or, Living with Pulmonary Hypertension questionnaire.31
Limitations
There are several limitations of this study worth noting. This was a retrospective analysis of an observational prospective registry with non-consecutive enrollment. The number of patients available for analysis from the PHAR registry is modest with relatively short follow up, although comparable to prior registry-based studies. The PHAR does not include information on comorbidities and indications for anticoagulation, which may affect outcomes, and may account for differences between the anticoagulated and non-anticoagulated groups. Differences between the anticoagulation and non-anticoagulated cohorts such as age and PAH treatments suggests an enrollment bias. Our study design carries risk for immortal time bias, being unable to establish a matched comparison of new anticoagulation starts due to power limitations. One would expect this bias to shift the analysis toward a benefit for anticoagulation and therefore strengthen our conclusions that anticoagulation either has no association with mortality or is potentially harmful. In addition, our findings on sensitivity analysis of incident cases supports the results of the primary analysis. The size of certain sub-populations examined in this study are small with a relatively small number of outcome events, therefore there is the potential for bias from over adjustment in our statistical modeling. Moreover, some associations found to be significant in primary analysis may not be supported by findings in subgroup or sensitivity analyses due to insufficient power. The PHAR does not include information on type of anticoagulant, exact stop-start dates, or the duration within the therapeutic window for warfarin. Healthcare outcomes and QoL are patient-reported in this study, and therefore carry inherent risk of bias. Bleeding events were not captured by the registry.
Strengths
This study provides novel insights into the association between anticoagulation and key non-mortality outcomes in PAH. The PHAR has a high level of completeness for demographic data, baseline invasive hemodynamics, QoL metrics, and healthcare utilization outcomes. The population spans centers across the United States and encompasses demographic diversity. The rates of PAH-targeted medication prescription in this study likely best reflect modern practice compared to prior cohorts.
Conclusions
Anticoagulation may not have a role as routine therapeutic intervention in PAH. Anticoagulation does not reduce mortality and is associated with increased healthcare utilization with a signal to suggest a neutral or negative effect on QoL. Although early cohorts of PAH patients previously reported clinical mortality benefit to anticoagulation with a plausible physiologic hypothesis to support the findings, modern clinical cohorts such as the PHAR have not borne out a clear benefit.
Supplementary Material
Acknowledgments:
The Pulmonary Hypertension Association Registry (PHAR) is supported by Pulmonary Hypertension Care Centers, Inc., a supporting organization of the Pulmonary Hypertension Association. The authors thank the other investigators, the staff, and particularly participants of the PHAR for their valuable contributions. A full list of participating PHAR sites and institutions can be found at www.PHAssociation.org/PHAR.
Financial Disclosure Statement:
N.A.K. reports consulting fees from United Therapeutics, Janssen, and Acceleron and serves on advisory board for United Therapeutics, Janssen, and Bayer. R.K. reports grant support from the Pulmonary Hypertension Association. T.T. reports grant support from United Therapeutics, Aria CV, Acceleron Pharma, and Tenax Therapeutics, consulting fees from Acceleron Pharma, Altavant science, United Therapeutics, and Actelion, and serves on advisory board for Acceleron Pharma, Altavant science, United Therapeutics, and Actelion. A.H. reports grant support from CMREF, NHLBI, and IMARA, consulting fees from Acceleron, GossamerBio, Jannsen, and United Therapeutics, and stock holdings in Tenax Therapeutics. D.G. reports grant support from Respira Therapeutics and Bayer Pharmaceuticals, and consulting fees from Bayer Pharmaceuticals. T.B. reports grant support from Bayer, Liquidia, and Johnson and Johnson, consulting fees from Bayer and Liquidia, and participates on the advisory board for MESA and PVDomicsx. M.C. reports consulting fees from Jannsen, Express Scripts Altavant, Gossamer, United Therapeutics, Aerovate, Acceleron, and Liquidia, expert testimony for Johnson and Johnson, and board trustee membership on the Pulmonary Hypertension Association E.H. reports consulting fees from Biotronik, and participation in data safety monitoring board for AADi Biosciences, SoniVie, and V-wave LTd. M.A.S. reports consulting fees from Acceleron and Bial, hemodynamic core lab work for Aadi, and serves on the steering committee for Janssen Pharm/Actelion. T.D. receives research funding from Acceleron, serves as a consultant for Janssen Pharm/Actelion, United Therapeutics, SCOPE-BIAL, Trio Health, and Aerovate and serves on the advisory committee/board for Janssen Pharm/Actelion, United Therapeutics, Liquidia, Altevant, and Trio Health.
List of non-standard abbreviations
- PH
Pulmonary Hypertension
- PHAR
Pulmonary Hypertension Association Registry
- PAH
Pulmonary arterial hypertension
- CTD-PAH
Connective Tissue Disease associated pulmonary arterial hypertension
- IPAH
Idiopathic pulmonary arterial hypertension
- HPAH
Hereditary pulmonary arterial hypertension
- QoL
Quality of life
- RVSWI
Right ventricular stroke work index
- PAPi
Pulmonary Artery Pulsatility Index
- PAWP
Pulmonary Artery Wedge Pressure
- PDE5i
Phosphodiesterase type 5 inhibitor
- ERA
Endothelin receptor antagonist
- SSc-PAH
Systemic Sclerosis associated pulmonary arterial hypertension
- WHO FC
World Health Organization functional class
References
- 1.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: Natural history and the importance of thrombosis. Circulation. 1984;70(4):580–587. doi: 10.1161/01.CIR.70.4.580 [DOI] [PubMed] [Google Scholar]
- 2.Pietra GG, Edwards WD, Kay JM, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989;80(5):1198–1206. doi: 10.1161/01.CIR.80.5.1198 [DOI] [PubMed] [Google Scholar]
- 3.Lannan KL, Phipps RP, White RJ. Thrombosis, platelets, microparticles and PAH: More than a clot. Drug Discov Today. 2014;19(8):1230–1235. doi: 10.1016/j.drudis.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95(2):199–203. doi: 10.1016/j.amjcard.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 5.Frank H, Mlczoch J, Huber K, Schuster E, Gurtner HP, Kneussl M. The effect of anticoagulant therapy in primary and anorectic drug- induced pulmonary hypertension. Chest. 1997;112(3):714–721. doi: 10.1378/chest.112.3.714 [DOI] [PubMed] [Google Scholar]
- 6.Ogata M, Ohe M, Shirato K, Takishima T. Effects of a combination therapy of anticoagulant and vasodilator on the long-term prognosis of primary pulmonary hypertension. Jpn Circ J. 1993;57(1):63–69. doi: 10.1253/jcj.57.63 [DOI] [PubMed] [Google Scholar]
- 7.Rich S, Kaufmann E, Levy PS. The Effect of High Doses of Calcium-Channel Blockers on Survival in Primary Pulmonary Hypertension. N Engl J Med. 1992;327(2):76–81. doi: 10.1056/nejm199207093270203 [DOI] [PubMed] [Google Scholar]
- 8.Storstein O, Efskind L, Müller C, Rokseth R, Sander S. Primary Pulmonary Hypertension with Emphasis on its Etiology and Treatment. Acta Med Scand. 1966;179(2):197–212. doi: 10.1111/j.0954-6820.1966.tb05449.x [DOI] [PubMed] [Google Scholar]
- 9.Olsson KM, Delcroix M, Ghofrani HA, et al. Anticoagulation and survival in pulmonary arterial hypertension: Results from the comparative, prospective registry of newly initiated therapies for pulmonary hypertension (COMPERA). Circulation. 2014;129(1):57–65. doi: 10.1161/CIRCULATIONAHA.113.004526 [DOI] [PubMed] [Google Scholar]
- 10.Preston IR, Roberts KE, Miller DP, et al. Effect of warfarin treatment on survival of patients with pulmonary arterial hypertension (PAH) in the registry to evaluate early and Long-Term PAH Disease Management (REVEAL). Circulation. 2015;132(25):2403–2411. doi: 10.1161/CIRCULATIONAHA.115.018435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan MS, Usman MS, Siddiqi TJ, et al. Is Anticoagulation Beneficial in Pulmonary Arterial Hypertension? Circ Cardiovasc Qual Outcomes. 2018;11(9):e004757. doi: 10.1161/CIRCOUTCOMES.118.004757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P, Hu L, Yin Y, et al. Can anticoagulants improve the survival rate for patients with idiopathic pulmonary arterial hypertension? A systematic review and meta-analysis. Thromb Res. 2020;196:251–256. doi: 10.1016/j.thromres.2020.08.024 [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 14.Klinger JR, Elliott CG, Levine DJ, et al. Therapy for Pulmonary Arterial Hypertension in Adults: Update of the CHEST Guideline and Expert Panel Report. Chest. 2019;155(3):565–586. doi: 10.1016/j.chest.2018.11.030 [DOI] [PubMed] [Google Scholar]
- 15.Jose A, Eckman MH, Elwing JM. Anticoagulation in pulmonary arterial hypertension: a decision analysis. Pulm Circ. 2019;9(4). doi: 10.1177/2045894019895451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin AN, Johnson SG, Joline BR, Delate T, Witt DM. A descriptive evaluation of warfarin use in patients with pulmonary arterial hypertension. Thromb Res. 2014;133(5):790–794. doi: 10.1016/j.thromres.2014.02.029 [DOI] [PubMed] [Google Scholar]
- 17.Roldan T, Rios JJ, Villamañan E, Waxman AB. Complications associated with the use of oral anticoagulation in patients with pulmonary arterial hypertension from two referral centers. Pulm Circ. 2017;7(3):692–701. doi: 10.1177/2045893217721903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roldan T, Villamañán E, Rios JJ, Waxman AB. Assessment of the quality of anticoagulation management in patients with pulmonary arterial hypertension. Thromb Res. 2017;160:83–90. doi: 10.1016/j.thromres.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 19.Morrisroe K, Stevens W, Huq M, et al. Survival and quality of life in incident systemic sclerosis-related pulmonary arterial hypertension. Arthritis Res Ther. 2017;19(1). doi: 10.1186/s13075-017-1341-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray MP, Kawut SM. The Pulmonary Hypertension Association Registry: Rationale, Design, and Role in Quality Improvement. Adv Pulm Hypertens. 2018;16(4):185–188. doi: 10.21693/1933-088x-16.4.185 [DOI] [Google Scholar]
- 21.Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 22.Yorke J, Corris P, Gaine S, et al. EmPHasis-10: Development of a health-related quality of life measure in pulmonary hypertension. In: European Respiratory Journal. Vol 43. ; 2014:1106–1113. doi: 10.1183/09031936.00127113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frantz RP. Whither anticoagulation in pulmonary arterial hypertension? Conflicting Evidence REVEALed. Circulation. 2015;132(25). doi: 10.1161/CIRCULATIONAHA.115.019651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith B, Genuardi MV., Koczo A, et al. Atrial arrhythmias are associated with increased mortality in pulmonary arterial hypertension. Pulm Circ. 2018;8(3). doi: 10.1177/2045894018790316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henkens IR, Hazenoot T, Boonstra A, Huisman MV., Vonk-Noordegraaf A. Major bleeding with vitamin K antagonist anticoagulants in pulmonary hypertension. Eur Respir J. 2013;41(4):872–878. doi: 10.1183/09031936.00039212 [DOI] [PubMed] [Google Scholar]
- 26.Michel A, Gonzalez-Perez A, Saez M, Garcia Rodriguez LA. Risk of bleeding events among patients with systemic sclerosis and the general population in the UK: a large population-based cohort study. Clin Rheumatol. 2020;39(1):19–26. doi: 10.1007/S10067-019-04588-0 [DOI] [PubMed] [Google Scholar]
- 27.Borgese M, Badesch D, Bull T, et al. EmPHasis-10 as a measure of health-related quality of life in pulmonary arterial hypertension: data from PHAR. Eur Respir J. 2021;57(2). doi: 10.1183/13993003.00414-2020 [DOI] [PubMed] [Google Scholar]
- 28.Lewis RA, Armstrong I, Bergbaum C, et al. EmPHasis-10 health-related quality of life score predicts outcomes in patients with idiopathic and connective tissue disease-associated pulmonary arterial hypertension: results from a UK multicentre study. Eur Respir J. 2021;57(2). doi: 10.1183/13993003.00124-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin KM, Gomberg-Maitland M, Channick RN, et al. Psychometric Validation of the Pulmonary Arterial Hypertension-Symptoms and Impact (PAH-SYMPACT) Questionnaire: Results of the SYMPHONY Trial. Chest. 2018;154(4):848–861. doi: 10.1016/J.CHEST.2018.04.027 [DOI] [PubMed] [Google Scholar]
- 30.McKenna SP, Ratcliffe J, Meads DM, Brazier JE. Development and validation of a preference based measure derived from the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) for use in cost utility analyses. Health Qual Life Outcomes. 2008;6(1):1–8. doi: 10.1186/1477-7525-6-65/TABLES/8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonner N, Abetz L, Meunier J, Sikirica M, Mathai SC. Development and validation of the living with pulmonary hypertension questionnaire in pulmonary arterial hypertension patients. Health Qual Life Outcomes. 2013;11(1):1–16. doi: 10.1186/1477-7525-11-161/TABLES/9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.