Abstract
Background
Undifferentiated acute respiratory infections (ARIs) are a large and heterogeneous group of infections not clearly restricted to one specific part of the upper respiratory tract, which last for up to seven days. They are more common in pre‐school children in low‐income countries and are responsible for 75% of the total amount of prescribed antibiotics in high‐income countries. One possible rationale for prescribing antibiotics is the wish to prevent bacterial complications.
Objectives
To assess the effectiveness and safety of antibiotics in preventing bacterial complications in children aged two months to 59 months with undifferentiated ARIs.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 7), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1950 to August week 1, 2015) and EMBASE (1974 to August 2015).
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs comparing antibiotic prescriptions with placebo or no treatment in children aged two months to 59 months with an undifferentiated ARI for up to seven days.
Data collection and analysis
Two review authors independently assessed trial quality and extracted and analysed data using the standard Cochrane methodological procedures.
Main results
We identified four trials involving 1314 children. Three trials investigated the use of amoxicillin/clavulanic acid to prevent otitis and one investigated ampicillin to prevent pneumonia.
The use of amoxicillin/clavulanic acid compared to placebo to prevent otitis showed a risk ratio (RR) of 0.70 (95% confidence interval (CI) 0.45 to 1.11, three trials, 414 selected children, moderate‐quality evidence). Methods of random sequence generation and allocation concealment were not clearly stated in two trials. Performance, detection and reporting bias could not be ruled out in three trials.
Ampicillin compared to supportive care (continuation of breastfeeding, clearing of the nose and paracetamol for fever control) to prevent pneumonia showed a RR of 1.05 (95% CI 0.74 to 1.49, one trial, 889 selected children, moderate‐quality evidence). The trial was non‐blinded. Random sequence generation and allocation concealment methods were not clearly stated, so the possibility of reporting bias could not be ruled out.
Harm outcomes could not be analysed as they were expressed only in percentages.
We found no studies assessing mastoiditis, quinsy, abscess, meningitis, hospital admission or death.
Authors' conclusions
There is insufficient evidence for antibiotic use as a means of reducing the risk of otitis or pneumonia in children up to five years of age with undifferentiated ARIs. Further high‐quality research is needed to provide more definitive evidence of the effectiveness of antibiotics in this population.
Keywords: Child, Preschool; Humans; Acute Disease; Amoxicillin‐Potassium Clavulanate Combination; Amoxicillin‐Potassium Clavulanate Combination/therapeutic use; Ampicillin; Ampicillin/therapeutic use; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Otitis; Otitis/prevention & control; Pneumonia; Pneumonia/prevention & control; Randomized Controlled Trials as Topic; Respiratory Tract Infections; Respiratory Tract Infections/complications; Respiratory Tract Infections/drug therapy; Suppuration; Suppuration/drug therapy
Plain language summary
Antibiotics for common respiratory infections with unclear causes and undifferentiated symptoms in children up to five years of age
Review question Do antibiotics prevent more severe infections in children up to five years old with common upper acute respiratory infections (ARIs)?
Background Common upper ARIs are a large and varied groups of infections. They occur in any part of the upper respiratory system, last for up to seven days and have a wide variety of causes. They may lead to complications such as ear, throat and sinus infections. More common in pre‐school children, they are the most frequent reason for parents to seek medical assistance. Furthermore, they are responsible for 75% of the total prescribed antibiotics in high‐income countries. One possible rationale for prescribing antibiotics is the wish to prevent bacterial complications.
Methods This review focuses on the use of antibiotics to prevent clinical bacterial complications in children up to five years of age with common and undifferentiated ARIs. This is an update of a review previously published in 2014. The evidence is current to August 2015. In this update we retrieved 616 new studies, but none met our inclusion criteria.
Studies characteristics We included four trials (1314 children) in this review. Three trials (414 children, during seven days) investigated the use of an antibiotic (amoxicillin/clavulanic acid) to prevent otitis media. One trial (889 children, during two weeks) investigated the use of another antibiotic (ampicillin) to prevent pneumonia. Only one trial addressed harms. However, we could not analyse the data as it was expressed in percentages rather then absolute terms. No studies assessed other severe complications (mastoiditis, quinsy, abscess, meningitis), hospital admission or death.
Key results Current evidence does not provide support for the use of antibiotics to prevent otitis media and pneumonia in children up to five years of age with common upper ARIs.
Quality of the evidence In the trials treating otitis media, the quality of the evidence was moderate as the methods for avoiding bias were not clearly stated. Furthermore, in one trial a pharmaceutical company prepared the placebo syrup used in the trial.
In the study treating pneumonia, we classified the quality of the evidence as moderate, because the families previously knew if their children were receiving antibiotics or not. Furthermore, the methods for avoiding bias were not clearly stated by the trial authors.
Further high‐quality research is needed to provide more definitive evidence of the effectiveness of antibiotics in this population.
Summary of findings
for the main comparison.
| Amoxicillin/clavulanic acid compared with placebo for children with undifferentiated ARI to prevent complications (otitis) | ||||||
|
Patients: children up to 5 years of age with previous episodes of AOM and undifferentiated ARI Settings: 2 trials were conducted in private centres (1 multicentre study conducted in paediatric centres in France; 1 study in an otologic centre in Finland); 1 trial was conducted in the paediatric department of a hospital in Jordan Intervention: amoxicillin/clavulanic acid Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Amoxicillin/clavulanic acid | |||||
|
Otitis media Follow‐up: 7 to 12 days |
Medium risk population | RR 0.70 (0.45 to 1.11) | 414 (3) | ⊕⊕⊕⊝ moderate1 | — | |
| 18 per 100 | 13 per 100 (8 to 20) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AOM: acute otitis media; ARI: acute respiratory infection; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
*The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of the control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
1Random sequence generation and allocation concealment are unclear in two of the included trials. The method of blinding is unclear in all three trials. Nevertheless, the three trials were described as randomised and double‐blind. The studies were conducted on just two continents. Only one of the three studies was produced in the last 10 years.
2.
| Ampicillin compared with control for children with undifferentiated ARI to prevent complications (pneumonia) | ||||||
|
Patient or population: children under 5 years of age with undifferentiated ARI Settings: 1 trial conducted in a government health clinic in Indonesia Intervention: ampicillin Comparison: adjunctive therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Supportive care | Ampicillin | |||||
|
Pneumonia ‐ 12 to 58 months of age Follow‐up: 2 weeks |
Medium risk population | RR 1.10 (0.71 to 1.69) | 563 (1) | ⊕⊕⊕⊝ m oderate1 | — | |
| 12 per 100 | 13 per 100 (9 to 20) | |||||
|
Pneumonia ‐ 0 to 11 months of age Follow‐up: 2 weeks |
Medium risk population | RR 0.96 (0.52 to 1.76) | 326 (1) | ⊕⊕⊕⊝ m oderate1 | — | |
| 12 per 100 | 12 per 100 (6 to 21) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARI: acute respiratory infection; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
*The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of the control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
1Random sequence generation method and allocation concealment are unclear. There was no blinding. Data were extracted from only one included study, which was conducted more than 20 years ago.
Background
Description of the condition
Undifferentiated acute respiratory infections (ARIs) are a large and heterogeneous group of infections. They affect any area between the nose and the epiglottis and last for up to seven days (Schuetz 2012). Undifferentiated ARIs and acute infection‐related cough, with potentially suppurative infections, such as acute otitis media, acute sinusitis and acute pharyngitis, are the most common reasons that parents seek medical attention for their children (Akkerman 2005; Dowell 1998; WHO 2007). These infections are more common in pre‐school children, particularly those living in low‐income countries (Benguigui 2003; Emmelin 2007; Garenne 1992; Krishnan 2015; Rudan 2008). Even in high‐income countries, ARIs are worrisome (Akkerman 2005; Nascimento‐Carvalho 2006; Tan 2008; Zar 2014). According to Hay 2002, two out of three children younger than four in the UK visit their general practitioner (GP) at least once a year with an ARI. Up to three‐quarters of these present with a cough. This accounts for at least two million annual face‐to‐face consultations related to new episodes of cough, with up to half a million re‐consultations and many more episodes of respiratory illness not currently seen by GPs. Estimating the cost of GP time at GBP 7.30 per consultation and antibiotics at GBP 3 per prescription (assuming a 40% prescribing rate), the crude annual National Health Service (NHS) cost of treating cough in pre‐school children was estimated to be at least GBP 20 million (Hay 2002).
In Hay 2002, a study was cited that found the most common respiratory diagnoses in pre‐school children to be 'cold or sinusitis' (33%), 'bronchitis' (15%), 'tracheitis' (12%), 'pneumonia or bronchiolitis' (12%), 'pharyngitis' (10%), 'influenza' (9%), 'laryngitis or croup' (7%) and 'otitis media' (2%) (Hope‐Simpson 1973). According to another study cited in this review, there is large variation amongst doctors diagnosing and treating adults who present to primary care facilities with a respiratory tract illness (Howie 1971). This variation may be more apparent in pre‐school children because they present with fewer symptoms than older children and adults, they rarely expectorate sputum with their cough and it is not possible to establish the presence or absence of subjective symptoms, such as chest pain and sore throat (Margolis 1998). Precise diagnoses can often only be made retrospectively, therefore it has been recommended that research on acute problems in primary care settings be based on presenting symptoms or disease categories (Fahey 1998a; Fahey 1998b).
Description of the intervention
The immunological immaturity of young children translates into an enhanced susceptibility to many infections, with important health consequences as well as higher rates and longer duration of micro‐organism shedding (Posfay‐Barbe 2008). Early initiation of adequate antibiotic therapy is considered a cornerstone in the treatment of bacterial ARIs and is associated with improved clinical outcomes (Kumar 2009). However, overuse or prolonged use of antibiotics in patients with ARIs is associated with the increased resistance of common bacteria, high costs and adverse reactions (Chung 2007; Costelloe 2010; Little 2002).
Most childhood ARIs have a viral aetiology and are self limiting (Farha 2005; Jansen 2006; Kusel 2007), even though ARIs are responsible for 75% of prescribed antibiotics in high‐income countries (Jansen 2006). There is growing evidence from systematic reviews of acute tonsillitis/pharyngitis, otitis media, common cold and sinusitis that the benefits of antibiotics for symptom resolution are likely to be modest for most patients (Kenealy 2013; Spinks 2013; Venekamp 2015).
Concern about complications, diagnostic uncertainties and perceived parental expectations are some of the alleged reasons for this kind of prescribing, especially in young children (Del Mar 2012; Kumar 2003).
How the intervention might work
The decision to prescribe antibiotics is complex. Research, especially among adults, has suggested that low rates of antibiotic prescription in primary care settings might be associated with higher rates of infection (Little 2002). Therefore, reductions in prescription levels may lead to increases in mastoiditis (Sharland 2005), and increased hospital admissions for respiratory infections in the UK and the USA. In the USA, this increased level of hospital admissions was observed only among the elderly (Majeed 2004; Petersen 2007). These studies cannot determine whether adverse events occur less frequently in those people who receive antibiotics for minor infections than in those who do not. In addition, early initiation of adequate antibiotic therapy is considered the cornerstone in the treatment of bacterial ARIs and is associated with improved clinical outcomes (Kumar 2009).
Why it is important to do this review
Bacteria from different species commonly exchange genetic material, including 'resistance' genes. Antibiotics will affect pathogenic as well as nonpathogenic bacteria. Thus, it also creates the opportunity to transmit the genes related to bacterial resistance. The rate of antibiotic use is highest in children aged up to 59 months, most likely because they are considered prone to infections (McCaig 1995). The World Health Organization (WHO) has identified antibiotic resistance as one of the greatest threats to human health. In the European Union, about 25,000 patients die annually from multidrug‐resistant bacterial infections, which equates to healthcare costs and lost productivity of at least EUR 1.5 billion annually (Lancet 2009). Antimicrobial resistance can be attributed to indiscriminate or poor use of antibiotics. Individuals prescribed antibiotics in primary care settings for a respiratory infection might develop bacterial resistance, which can persist for up to 12 months. This increases population carriage of resistant organisms to first‐line antibiotics and the use of second‐line antibiotics (Costelloe 2010).
Clinical guidelines are supposed to help doctors prescribe antibiotics judiciously (Brink 2015; Nascimento‐Carvalho 2006; Tan 2008; van Balen 2004; WHO 2012), and might have a positive impact on antibiotic use. However, decisions to prescribe antibiotics may lead to inappropriate diagnoses in order to justify prescribing antibiotics (Thompson 2008). In the UK the National Institute for Health and Clinical Excellence (NICE) guidelines were developed to help GPs reduce unnecessary antibiotic prescriptions for ARIs (Tan 2008). Between 1996 and 2000, there was a marked decrease in antibiotic prescriptions in the UK. However, overall prescriptions are increasing again and associated with non‐specific upper respiratory tract infection diagnoses (Thompson 2009). This suggests that GPs may be avoiding using some diagnostic labels if formal guidelines suggest that antibiotic prescriptions are unnecessary (Thompson 2009). Concern from GPs and families regarding the danger of developing complications is probably an underlying cause of this diagnostic shift (Tan 2008). It must be emphasised that the success of these guidelines, which must always be supported by high‐quality evidence (Gjelstad 2013), will rely on the continuing education of prescribers and patients. Updating is also necessary when new information becomes available, particularly from randomised controlled trials (RCTs) and surveillance studies of local aetiology and antibiotic susceptibility patterns (Brink 2015). Meanwhile, RCTs generally do not have statistical power to examine the effects of interventions on rare outcomes and the patients included may not be representative of those seen in routine clinical practice.
The use of tests and laboratory markers has also been suggested to help physicians in this decision‐making process (Baron 2013; Doan 2014; Larsson 2005; Schuetz 2012). However, there is a lack of clear and definitive parameters to indicate using antibiotics. As a result, there is a pressing need to clarify whether prescribing antibiotics in children with undifferentiated ARIs and cough related to acute infections actually benefits the patients in everyday practice.
Objectives
To assess the effectiveness and safety of antibiotics in preventing bacterial complications in children aged two months to 59 months with undifferentiated ARIs.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCT) or quasi‐RCTs comparing antibiotic prescriptions with placebo or non‐treatment in children from two to 59 months of age with undifferentiated ARIs and cough related to acute infections.
Types of participants
Children from two to 59 months of age with undifferentiated ARIs and cough related to ARIs for up to seven days. Whenever data were available, we analysed the children by age subgroups to observe possible changes in the effectiveness of the intervention. Subgroups included those up to 12 months of age and from 12 months to 59 months. Had data been available, we would have separately analysed children with underlying immunodeficiencies or anatomical defects
Types of interventions
Antibiotics prescribed for undifferentiated ARIs and cough related to acute infections regardless of dosage and delivery system. We intended to compare antibiotics administered either alone or as an adjunctive therapy (antipyretics, bronchodilators, mucolytic agents, fluid supplements, etc.) to a placebo or no treatment.
Types of outcome measures
Primary outcomes
Cases of:
otitis media;
mastoiditis;
quinsy;
pneumonia;
abscess;
meningitis;
rates of admission to hospital;
death.
Secondary outcomes
Cases of antibiotic side effects, for example:
diarrhoea;
vomiting;
gastro‐intestinal symptoms;
rashes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 7), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1950 to August week 1, 2015) and EMBASE (1974 to August 2015).
We combined the search terms set out in Appendix 1 with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We used the same terms to search CENTRAL and adapted them to search EMBASE (Appendix 2). There were no language or publication restrictions.
Searching other resources
We searched ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) for completed and ongoing trials (latest search August 2015). We screened bibliographies of the trials in order to identify additional trials.
Data collection and analysis
Selection of studies
Two review authors (MG, MS) independently selected trials for inclusion. They screened the titles and abstracts for relevance, study design, types of participants, intervention and outcome measures. We used an eligibility form based on the selection criteria. We resolved disagreements by discussion. The review authors reviewed the full texts of the potentially relevant articles and independently assessed their eligibility. We also searched for full reports of all studies deemed relevant based on the abstract. Two review authors (MG, MS) independently assessed the selected studies. We listed the excluded studies and the reasons for exclusion. When essential information was not available, we excluded the trials from the review (Figure 1). Subsequently, we entered all search results into Review Manager 5.3 (RevMan 2014).
1.
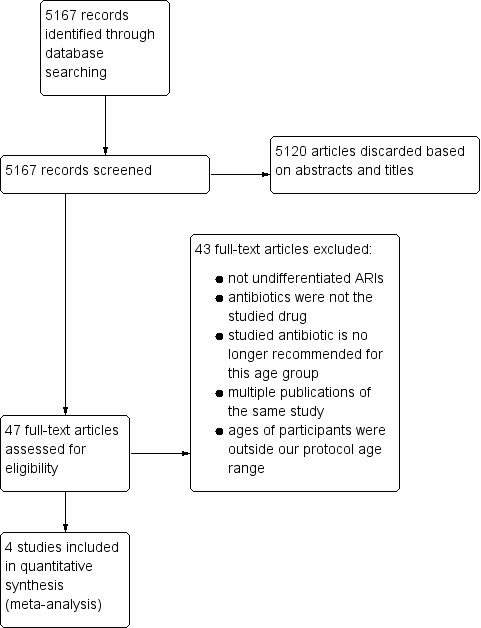
Study flow diagram.
Data extraction and management
Two review authors (MG, MS) independently extracted data from the full reports into an extraction form that was designed for this purpose. The review authors (MG, MS) cross‐checked data and resolved discrepancies through discussion. MG entered data into RevMan 2014, while MS validated the data.
Assessment of risk of bias in included studies
Two review authors (MG, MS) independently screened the methodological quality of the trials to be reported in the Results section. We resolved disagreement arising from different interpretations through consensus. We used the following criteria to assess the risk of bias (Higgins 2011). The risk of bias is presented graphically in Figure 2 and summarised in Figure 3.
2.
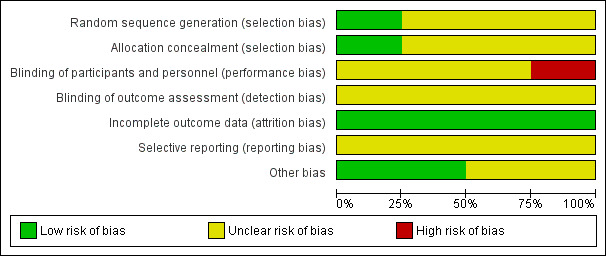
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
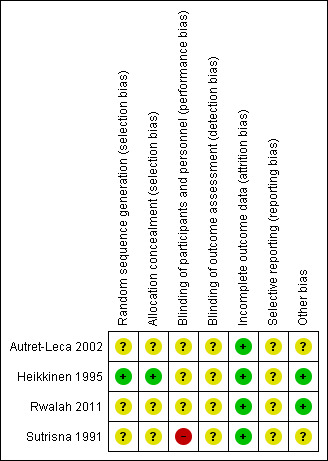
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Generation of the allocation sequence
Adequate (low risk of bias): if the allocation sequence was generated by a computer or random number table. We considered drawing of lots, tossing of a coin, shuffling of cards or throwing dice as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear (uncertain risk of bias): if the trial was described as randomised, but information about the sequence generation process to permit judgement of adequacy or inadequacy was not sufficient.
Inadequate (high risk of bias): if the allocation involved judgement or some method of non‐random categorisation of participants, such as: allocation by judgement of the clinician; allocation by preference of the participant; allocation based on the results of a laboratory test or a series of tests; allocation by availability of the intervention.
Allocation concealment
Adequate (low risk of bias): if participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
Unclear (uncertain risk of bias): if the trial was described as randomised, but the method used to conceal the allocation was not described.
Inadequate (high risk of bias): if the allocation sequence was known to the investigators who assigned participants.
Blinding (or masking)
Adequate (low risk of bias): if the trial was not blinded, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding; if there was blinding of participants and key study personnel ensured, and it is unlikely that the blinding could have been broken; either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
Unclear (uncertain risk of bias): if the trial was described as double‐blind, but the method of blinding was not described.
Inadequate (high risk of bias): if there was no blinding or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by lack of blinding; if blinding of key study participants and personnel was attempted, but it is likely that the blinding could have been broken; if either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Incomplete outcome data
Adequate (low risk of bias): if there were no missing outcome data; if reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); if missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups; if the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; if missing data have been imputed using appropriate methods.
Unclear (uncertain risk of bias): if the report gave the impression that there had been no drop‐outs or withdrawals, but this was not specifically stated.
Inadequate (high risk of bias): if the number or reasons for drop‐outs and withdrawals were not described. If the reason for missing outcome data was likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; if the proportion of missing outcomes compared with the observed event risk induced clinically relevant bias in the intervention effect estimate; if 'as‐treated' analysis was done with substantial departure of the intervention received from that assigned at randomisation.
Selective outcome reporting
Adequate (low risk of bias): if the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified.
Unclear (uncertain risk of bias): if the report gives insufficient information to permit judgement of adequacy or inadequacy. It is likely that the majority of studies will fall into this category.
Inadequate (high risk of bias): if not all of the study's pre‐specified primary outcomes have been reported; if one or more primary outcomes are reported using measurements, analysis methods or subsets of the data (for example, subscales) that were not pre‐specified; if one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); if one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; if the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Other potential threats to validity
Adequate (low risk of bias): the study appears to be free of other sources of bias.
Unclear (uncertain risk of bias): if there may be a risk of bias, but there is insufficient information to assess whether an important risk of bias exists; or insufficient rationale or evidence that an identified problem will introduce bias.
Inadequate (high risk of bias): if there is at least one important risk of bias, such as: a potential source of bias related to the specific study design used; or it was stopped early due to some data‐dependent process (including a formal‐stopping rule); or had extreme baseline imbalance; or has been claimed to have been fraudulent; or had some other problem.
Measures of treatment effect
Two review authors (MG, MS) analysed data using RevMan 2014. All the data were dichotomous. We used the risk ratio (RR) to measure treatment effectiveness.
Unit of analysis issues
All the included studies were parallel RCTs. The unit of analysis for each outcome was the individual research participant. We did not select any trial with a non‐standard design, such as cross‐over or cluster‐RCTs.
Dealing with missing data
When the missing data seemed to be unrelated to the actual values, we analysed the study according to the intention‐to‐treat (ITT) principle.
When the reasons for the missing data were unclear but seemed to be unrelated to clinical motives, we also conducted the analyses according to the ITT principle. We also conducted a sensitivity analysis, imagining a scenario in which there was the best possible response to the use of antibiotic to prevent the studied outcome. We should emphasise that it is unlikely that this hypothetical scenario occurs in clinical practice. It should be interpreted only as an estimate of the theoretical maximum benefit that could be obtained through the use of antibiotics. We addressed the potential impacts of the missing data on the findings of the review in the Discussion section (Higgins 2011).
Assessment of heterogeneity
We assessed the heterogeneity of the trials by visually inspecting the forest plots and by performing the Chi2 test (P value < 0.1 representing heterogeneity). We also used the I2 statistic to quantify inconsistencies among the trials. If the I2 had exceeded 50% and visual inspection of forest plots had supported these findings, we would have considered it to be indicative of substantial heterogeneity. Had this happened, we would have explored the underlying causes according to the limits permitted by the available data (Higgins 2011).
Assessment of reporting biases
It was not possible to examine the existence of publication bias graphically using a funnel plot (trial effect versus trial size) because we did not have more than five studies available for any one comparison.
Data synthesis
For similar types of studies we performed a meta‐analysis to calculate a weighted intervention effect across the trials. We used the fixed‐effect model for combination analysis. We expressed the results as RR with a 95% confidence interval (CI). Participants were analysed in their original randomised group using intention‐to‐treat (ITT) analysis. We also expressed the results as number needed to treat to benefit (NNTB) when appropriate. If considered appropriate, we pooled results of similar groups of trials. We used the fixed‐effect model and a 95% CI.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: otitis media and pneumonia. None of the included trials addressed our other outcomes, such as mastoiditis, quinsy, abscess, meningitis, admission to hospital, gastro‐intestinal symptoms or rashes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it relates to the studies that contribute data to the meta‐analyses for the pre‐specified outcomes (Atkins 2004). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade or upgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We investigated possible heterogeneity according to variations in the participants, interventions and outcomes. Whenever data were available, we analysed the children by age subgroups to observe possible changes in the effectiveness of the intervention. Subgroups included those up to 12 months of age and from 12 months to 59 months. We also intended to analyse separately those who attended day care and those who did not, as well as those with underlying immunodeficiencies or anatomical defects. However, no data were available for these comparisons.
We separately analysed subgroups that come from low‐income countries due to the high frequency of bacterial aetiology infections in children with ARIs. Finally, we analysed subgroups of the different available outcomes: otitis and pneumonia.
Sensitivity analysis
We carried out a subgroup analysis for subsets of high and low‐income countries due to the higher frequency of bacterial complications expected in children from low‐income regions (Analysis 1.2).
1.2. Analysis.
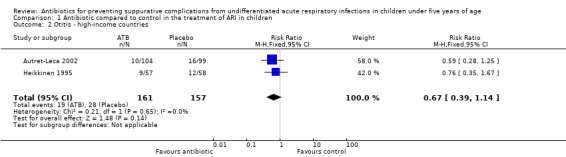
Comparison 1 Antibiotic compared to control in the treatment of ARI in children, Outcome 2 Otitis ‐ high‐income countries.
After reading the included articles we decided to conduct another analysis. However, we should emphasise that this was an exploratory analysis and was not previously included in the protocol. In one trial (Heikkinen 1995), 11 children failed to complete the study: seven in the intervention group and four in the control group. The reasons why the children did not complete the study are unclear. We simulated the best‐case scenario for the antibiotic effect, conducting an analysis taking into account the best results that could have been found with antibiotic treatment if these 11 children had completed the study. As such, we considered what the results would have been if the seven children who did not complete the study that had received treatment had not developed otitis. On the other hand, we also considered that the four patients in the control group that did not finish the study did develop the infection.
Results
Description of studies
Results of the search
This review was first published in 2014 (Alves Galvão 2014). At that time, we had retrieved 4551 records from the electronic searches. Out of these, 4507 studies were initially discarded based on their abstracts and titles. In this 2015 update, we retrieved 616 new studies and we initially discarded 613 studies based on their abstracts and titles. Overall, we retrieved a total of 5167 studies and discarded 5120 trials based on their abstracts and titles.
We assessed the remaining 47 trials in detail. Four trials were included and 43 did not meet the selection criteria (Figure 1). The four included trials are published trials and are described in the Characteristics of included studies table.
Included studies
The four included trials (Autret‐Leca 2002; Heikkinen 1995; Rwalah 2011; Sutrisna 1991), involving 1314 children, studied the use of antibiotics in the prevention of two complications of undifferentiated acute respiratory infections. Three of them dealt with the use of amoxicillin/clavulanic acid to prevent otitis in children with a previous history of this infection (Autret‐Leca 2002; Heikkinen 1995; Rwalah 2011). The remaining trial was about the use of ampicillin to prevent pneumonia (Sutrisna 1991).
The trials were conducted in Jordan (Rwalah 2011), France (Autret‐Leca 2002), Finland (Heikkinen 1995), and Indonesia (Sutrisna 1991), in private and public health services.
Excluded studies
We excluded 43 studies. The most common reasons for exclusion were as follows:
The trial was about specific infections of known bacterial aetiology, such as otitis, streptococcal tonsillo‐pharyngitis, pertussis, tuberculosis and other respiratory infections.
Antibiotics were not the studied drugs.
The studied antibiotic is no longer recommended to be used in children up to 59 months of age.
Ages of participants were outside our protocol age range.
Multiple publications of the same study.
The entire text could not be accessed.
Risk of bias in included studies
The overall risk of bias is included in the Characteristics of included studies table, presented graphically in Figure 2 and summarised in Figure 3.
Allocation
The four included trials were described as randomised (Autret‐Leca 2002; Heikkinen 1995; Rwalah 2011; Sutrisna 1991). Nevertheless, in three the information about the randomisation process was not sufficient to permit judgement of its adequacy and the methods used to conceal the allocation were not described (Autret‐Leca 2002; Rwalah 2011; Sutrisna 1991).
In one study the randomisation was computer‐generated (Heikkinen 1995). Participants and investigators enrolling participants were not aware of the assignment because the sequentially numbered treatment containers were of identical appearance.
Blinding
Three studies were described as double‐blind but the method of blinding was not clearly described. These trials studied the prevention of otitis (Autret‐Leca 2002; Heikkinen 1995; Rwalah 2011). The Sutrisna 1991 trial was a non‐blinded study about the prevention of pneumonia.
Incomplete outcome data
There were no missing participants in one study (Rwalah 2011).
In Heikkinen 1995, 11 of 115 randomised children failed to complete the study: seven in the intervention group and four in the control group. The reasons for this are unclear but they do not seem to be related to clinical motives. We analysed this study according to the ITT principle.
In Autret‐Leca 2002, five of 203 children failed to complete the study: one in the intervention group and four in the control group. We analysed this study according to the ITT principle, as presented by the trial authors.
In Sutrisna 1991, there were no missing data but 11 children were excluded due to secondary disorders (four in the treatment group and seven in the control group): they stopped taking ampicillin or paracetamol because they developed diarrhoea (four ampicillin, six control) or allergic reactions (one control). This was the only study considering the 'pneumonia' outcome. In the analysis process, we studied the sample according to the two age groups provided by the authors. Information about exclusions in each age group was not available and we analysed the trial per protocol, as was done by the trial authors.
Selective reporting
We did not identify any possible sources of reporting bias as the reports give insufficient information to reach any conclusion about adequacy.
Other potential sources of bias
We did not identify any other possible sources of bias.
Effects of interventions
Even though we intended to assess 12 outcomes, we could only assess two: antibiotic compared to control for undifferentiated acute respiratory infections (ARIs) in children younger than five, to prevent otitis and to prevent pneumonia.
Just one of the included trials addressed outcomes related to the adverse effects caused by antibiotics in children with undifferentiated ARI (Autret‐Leca 2002). However, this trial's data could not be analysed as the data were expressed in percentages rather than absolute terms.
Primary outcomes
1. Otitis media
Amoxicillin/clavulanic acid was compared to placebo in children with undifferentiated ARIs and previous episodes of otitis in three included trials with a total of 414 children (Autret‐Leca 2002; Heikkinen 1995; Rwalah 2011). The diagnosis of acute otitis media was based on an assessment of signs and symptoms as well as an otoscopic examination (bulging, opacity or lack of mobility of the tympanic membrane).
There was no statistical evidence of a protective effect of the antibiotic in the occurrence of otitis: risk ratio (RR) 0.70, 95% confidence interval (CI) 0.45 to 1.11 (Analysis 1.1; Table 1).
1.1. Analysis.
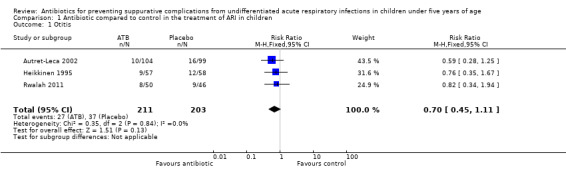
Comparison 1 Antibiotic compared to control in the treatment of ARI in children, Outcome 1 Otitis.
Considering the characteristics of some of the included studies, we decided to conduct other analyses, although we should emphasise they are only exploratory analyses.
We also separately analysed children from high and low‐income countries due to the higher frequency of bacterial complications expected in children from low‐income countries. The results were similar and compatible with both benefit and harm in the prevention of otitis: RR 0.67, 95% CI 0.39 to 1.14 in high‐income countries (Analysis 1.2) and RR 0.82, 95% CI 0.34 to 1.94 in low‐income regions (Rwalah 2011).
2. Mastoiditis
Not reported on.
3. Quinsy
Not reported on.
4. Pneumonia
Ampicillin was compared to supportive care (continuation of breastfeeding, clearing of the nose as needed and paracetamol for fever control) in children with undifferentiated ARIs in 889 participants in just one trial (Sutrisna 1991). The diagnosis of pneumonia was based on the World Health Organization (WHO) criteria for ARI management (WHO 2012). Although the research was conducted in a low‐income region where a bacterial aetiology of ARI could be expected to be more frequent, the intervention showed no effect: RR 1.05, 95% CI 0.74 to 1.49. We also presented the results separately in two age subgroups, as done by the trial authors. In the same way, a protective effect could not be demonstrated either when patients were analysed together or in two age groups (up to 11 months and from 12 to 58 months). In children up to 11 months of age the RR was 0.96 (95% CI 0.52 to 1.76). In children from 12 to 59 months of age the RR was 1.10 (95% CI 0.71 to 1.69) (Analysis 1.3; Table 2).
1.3. Analysis.
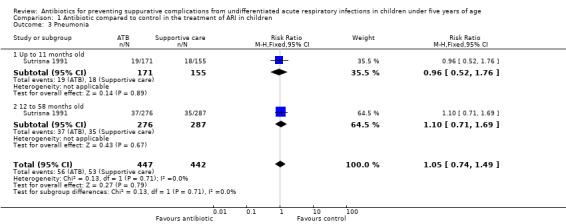
Comparison 1 Antibiotic compared to control in the treatment of ARI in children, Outcome 3 Pneumonia.
5. Abscess
Not reported on.
6. Meningitis
Not reported on.
7. Rates of admission to hospital
Not reported on.
8. Death
Not reported on.
Secondary outcomes
1. Diarrhoea
Not reported on.
2. Vomiting
Not reported on.
3. Gastro‐intestinal symptoms
Not reported on.
4. Rashes
Not reported on.
Discussion
Undifferentiated acute respiratory infections (ARIs) and infections such as otitis and pneumonia are common in children. Although less frequent, other infections such as mastoiditis and quinsy may be significant and even life‐threatening conditions, especially in children up to four years of age. These infections are also a major cause of concern amongst parents and policy makers due to their potential complications, healthcare costs, associated absenteeism from work (parents) and even mortality, especially in socially and economically disadvantaged populations. Moreover, all of these conditions may be under‐diagnosed due the smaller anatomy and difficulties of examining young children (Abdel‐Aziz 2010; Schraff 2001).
There are many trials on these topics in the medical literature. However, studies about the common clinical situation in which a physician must decide whether or not to prescribe an antibiotic to a patient presenting with an undifferentiated ARI, in order to avoid potentially life‐threatening complications, are less common. This observation is also supported by the contrast between the small number of studies that met the inclusion criteria for this review and the large number of records found in the initial search (i.e. four out of 5167 trials). We also highlight the fact that just one of the included trials addressed outcomes related to the adverse effects caused by antibiotics in children with undifferentiated ARI (Autret‐Leca 2002). However, the data from this study could not be analysed as they were expressed in percentages rather than absolute terms. The adverse effects of antibiotics should be considered an essential topic when determining the cost‐effectiveness of antibiotic prescription.
It has been established that the development of bacterial resistance and the need for new generations of antibiotics for outpatient treatment of infections are major causes of concern globally (Costelloe 2010; Gjelstad 2013). Furthermore, mortality caused by bacterial respiratory infections is more frequent in low‐income countries (Rudan 2004; Rudan 2008), where limited resources may impair the running of clinical trials. These facts may have contributed to the small number of trials meeting our inclusion criteria.
We included four trials in this review: three investigated the prevention of otitis and one investigated the prevention of pneumonia.
Otitis media is a common infection worldwide. For most children with mild otitis media in high‐income countries, an expectant observational approach seems to be justified (Venekamp 2015). However, there is concern over excessive antibiotic consumption in children in many high‐income countries (Arason 2010; Coco 2009; Kutty 2011). On the other hand, in low‐income countries where children have limited access to medical care, suppurative complications and hearing loss are more frequent (Klein 2000; Vergison 2010). In the studies of otitis in this review, although all patients had a previous history of this infection, it was not stated whether or not they were immunodeficient. Amoxicillin/clavulanic acid was the drug of choice. The results in the three trials were similar and did not provide evidence of a significant benefit of antibiotics in preventing otitis: risk ratio (RR) 0.70, 95% confidence interval (CI) 0.45 to 1.11 (Analysis 1.1). Although there was no statistical heterogeneity in the three trials, to take into account the characteristics of the studied samples in the selected studies, we conducted some exploratory analyses.
In one trial 11 children failed to complete the study: seven in the intervention group and four in the control group (Heikkinen 1995). The reasons why children did not complete the study are unclear. A question arose as to whether such drop‐outs could modify the results. We verified the best results that could be obtained in a hypothetical situation, conducting an analysis considering the results as if the seven children that did not complete the study who had received the antibiotic did not develop otitis, while incorporating the four in the control group as if they had developed the infection. In this case, a slight statistical protective effect of the antibiotic was displayed: RR 0.64, 95% CI 0.41 to 0.99. However, it should be emphasised that it would be necessary to treat 14 children with ARIs for a seven‐day period to prevent one case of otitis (number needed to treat to benefit (NNTB) 14, 95% CI 8 to 50).
We also separately analysed children from high (Finland and France) and low‐income countries (Jordan) due to the higher frequency of bacterial complications expected in children from low‐income regions. Otitis media was the only outcome that was reported in the trials conducted in both high and low‐income countries. The results in the two settings were similar and consistent with both benefit and harm (RR 0.67, 95% CI 0.39 to 1.14 in high‐income countries (Analysis 1.2) and RR 0.70, 95% CI 0.45 to 1.11 in low‐income countries Analysis 1.1)). This similarity may seem unexpected due to the higher frequency of bacterial complications in children from low‐income countries. However, the small number of studies and sample sizes for this outcome may have influenced the results obtained.
Pneumonia is a common infection in low‐income countries. Its annual incidence in these areas is estimated to be 150.7 million new cases, 11 to 20 million (7% to 13%) of which are severe enough to require hospital admission. An estimated nearly 1.2 million children younger than five years died in 2011 from pneumonia (Izadnegahdar 2013). It has been suggested that more than 95% of all episodes of clinical pneumonia in young children worldwide occur in low‐income countries (Rudan 2004).
We also analysed prevention of pneumonia in one study (Sutrisna 1991). Ampicillin was the antibiotic studied. Although the research was conducted in a low‐income region, where a bacterial aetiology could be expected to be more frequent, the intervention showed no effect. A protective effect could not be demonstrated when patients were analysed together, nor when they were analysed separately in two age groups (up to 11 months and from 12 to 58 months) (Analysis 1.3). The small number of selected trials for this important cause of childhood death in low‐income countries may reflect the limited resources of these regions for conducting clinical trials. It must be emphasised that the comparison is based on just one non‐blinded study and the data were analysed per protocol.
Both antibiotic resistance and side effects of antibiotics should be taken into account in further defining the risks and benefits of prescribing these drugs. However, we believe that if we only consider changes in bacterial resistance patterns, the essence of our observed results is unlikely to change in favour of the use of antibiotics to prevent the studied infections, as resistance to antibiotics seems to increase over time (Del Mar 2012).
Summary of main results
Four trials were included in this review. Three investigated the use of amoxicillin/clavulanic acid compared to placebo to prevent otitis and one investigated ampicillin compared to supportive care to prevent pneumonia.
Amoxicillin/clavulanic acid was compared to placebo to prevent otitis. The estimated reduction in the risk of otitis was compatible with both benefit and harm (RR 0.70, 95% CI 0.45 to 1.11, three trials, 414 selected children, moderate‐quality evidence) (Table 1). Methods of random sequence generation and allocation concealment were not clearly stated in two trials. Performance, detection and reporting bias could not be ruled out in three trials.
Ampicillin was compared to supportive care to prevent pneumonia. The estimated reduction in the risk of pneumonia was also compatible with both benefit and harm (RR 1.05, 95% CI 0.74 to 1.49) (Table 2). In the one trial looking at pneumonia parents were not blinded, but detection blinding was not clearly stated. Random sequence generation and allocation concealment methods were not clearly stated, and the possibility of reporting bias could not be ruled out.
Harm outcomes could not be analysed as they were expressed only in percentages.
We found no studies that assessed mastoiditis, quinsy, abscess, meningitis, hospital admission or death.
Further research is likely to have an important impact on the estimated effectiveness and may change the obtained results.
Overall completeness and applicability of evidence
The studies dealt with common clinical infections in clinical practice in an age group especially prone to severe secondary complications. Nevertheless, we believe that only limited evidence can be obtained from this review.
The selected trials were conducted on only two continents. Just one of them was produced in the last 10 years. It is possible that changes in the pattern of bacterial resistance and the immune status of paediatric populations has occurred over this period. Some of the possible causes of these changes over time are: inappropriate use of antibiotics, local epidemiological patterns and the implementation of new vaccines (Coco 2009; Dagan 2008). These facts may limit the external validity of the studies. Furthermore, there were no selected trials from the Americas or Africa, regions where ARIs and their bacterial complications are common. Two of the studies were conducted in private clinics in high‐income countries in Europe (318/414 participants) (Autret‐Leca 2002; Heikkinen 1995), where the burden of suppurative complications of undifferentiated ARI is not considered as important as in low‐income countries.
Quality of the evidence
We intended to study a common infection: undifferentiated ARI. The objective of our review, to assess the effectiveness and safety of antibiotics in preventing bacterial complications in children aged two to 59 months with undifferentiated ARIs, is a frequent dilemma in clinical practice. The use of antibiotics to prevent suppurative complications does not seem to be rare, whether or not this prescription is considered appropriate. Nevertheless, just four out of the 5167 trials identified by our searches met the inclusion criteria.
We classified the quality of the evidence obtained for the prevention of otitis media and pneumonia as moderate.
We downgraded the evidence for the prevention of otitis media due to some risks of bias. This infection cannot be considered rare but just three trials, including 414 children, met our inclusion criteria. The methods of random sequence generation and allocation concealment were not clearly stated in two trials (Autret‐Leca 2002; Rwalah 2011). Blinding of participants and personnel (performance bias) and outcome assessors (detection bias) was unclear in the three studies (Autret‐Leca 2002; Heikkinen 1995; Rwalah 2011). The reports give insufficient information to make a judgement about the occurrence of reporting bias in all the three studies (Autret‐Leca 2002; Heikkinen 1995; Rwalah 2011).
On the other hand, we think that we should clarify why we did not downgrade Autret‐Leca's study for the use of a placebo syrup prepared by a pharmaceutical company (Autret‐Leca 2002). As there was no statistical evidence of a protective effect of the studied antibiotic, we think that this fact did not influence the results.
We believe that further research, especially in other countries, may have an impact on the results.
We also downgraded the evidence for the prevention of pneumonia. We identified risks of bias in the trial studying pneumonia (Sutrisna 1991). This is also a common infection but we included just one single trial, including 889 children. This was a non‐blinded study for parents. The presence of detection bias was unclear. The methods of random sequence generation and allocation concealment were not clearly stated. Moreover, there is insufficient information to rule out reporting bias. The reduction in the risk of pneumonia was also compatible with both benefit and harm. Further research is likely to have an important impact on the estimated effectiveness and may change the obtained results.
Potential biases in the review process
In all the included studies the diagnosis of the infections was based on clinical signs and symptoms. We did not include additional tests to identify the aetiological agent of the infections as a selection criteria for the trials. Thus, it is reasonable to suppose that, in some cases, non‐bacterial otitis or pneumonia had been wrongly classified as a suppurative complication.
Agreements and disagreements with other studies or reviews
We could not find any other studies with the same objective as this review. However, the question of whether to use antibiotics to prevent infectious complications in children with ARIs has been recognised as a common problem in clinical practice.
Most of the systematic reviews in this area deal more specifically with the site of the infection (otitis, pharyngitis, sinusitis, pneumonia) (Altamimi 2012; Kabra 2013; Venekamp 2015), its aetiology (van Driel 2013), or the choice of immediate or delayed antibiotic use (Spurling 2013). It is not easy to compare this review with these given the differences in objectives and methodologies. The assessment of response to antibiotics in patients with cough and common cold in the Spurling 2013 review could be considered closer to the objectives of our review. Spurling found no benefit from the use of antibiotic therapy for such patients. Despite methodological differences, in our review we also could not find clinical benefits from the use of antibiotics in children with undifferentiated ARI for the prevention of pneumonia and otitis.
Authors' conclusions
Implications for practice.
There is inadequate evidence to determine the effectiveness of antibiotics in preventing the development of otitis and pneumonia in children up to five years of age with undifferentiated ARIs. Due to the small number of participants and only moderate quality of evidence, further high‐quality research is needed to provide more definitive evidence.
Implications for research.
Definitive conclusions may have been impaired by the small number of selected studies and the small number of participants. Further research is necessary to:
define the harms or benefits (or both) of prescribing antibiotics to children with undifferentiated ARI in regions where medical assistance is not easily obtained and where a bacterial aetiology is supposed to be more frequent;
verify whether possible changes in the characteristics of the aetiological agents, the populations and the antibiotics used in the last 10 years could change the present results;
increase knowledge about the phenomenon of antimicrobial resistance and the spread of this resistance in the community when antibiotics are given to this age group;
study the use of innovative markers or microbiological techniques to identify children that could benefit from antibiotic use;
provide more conclusive recommendations.
What's new
| Date | Event | Description |
|---|---|---|
| 5 August 2015 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 5 August 2015 | New search has been performed | This review was updated in August 2015. We retrieved 616 new studies but none met our selection criteria. We excluded three new trials (Gjelstad 2013; Higashi 2014; Ratjen 2013). |
Acknowledgements
We acknowledge Elizabeth Dooley from the Cochrane Acute Respiratory Infections (ARI) Group for helping us in all phases of the review process. We thank Anthony Harnden, Max Bulsara, Sam Ong and Yingfen Hsia for commenting on the draft protocol and Anne Lyddiatt, Imtiaz Jehan, Philipp Schuetz, Mark Jones and Matthew Thompson for commenting on the draft review. We thank Sarah Thorning, Trials Search Co‐ordinator from the ARI Group for her essential help with the search strategy. We would like to especially thank the kindness of Marta Roque, from the Iberoamerican Cochrane Centre, for her important help in the evaluation of the statistical aspects of this review. We also acknowledge Raimundo Santos and Clemax Sant'Anna for their kindness with the assessment and translation of some essential clinical trials. We thank Mell Siciliano who also helped us find the complete texts of some trials and Jonathan Haliburton for reviewing the English version of this text. Finally, we wish to thank Orla Ní Ógáin and Toby Lasserson, Editors at the Cochrane Editorial Unit, for their helpful comments and suggestions to improve our final draft.
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE (Ovid)
1 exp Respiratory Tract Infections/ 2 respiratory tract infection*.tw. 3 respiratory infection*.tw. 4 (urti or uri or ari).tw. 5 Nasopharyngitis/ 6 nasopharyngit*.tw. 7 exp Sinusitis/ 8 sinusit*.tw. 9 rhinitis/ 10 rhinit*.tw. 11 rhinosinusit*.tw. 12 Pharyngitis/ 13 pharyngit*.tw. 14 sore throat*.tw. 15 Tonsillitis/ 16 tonsillit*.tw. 17 exp Laryngitis/ 18 laryngit*.tw. 19 croup*.tw. 20 tracheitis/ 21 tracheit*.tw. 22 Epiglottitis/ 23 epiglottit*.tw. 24 Common Cold/ 25 common cold*.tw. 26 coryza.tw. 27 exp Otitis Media/ 28 otitis media.tw. 29 (AOM or OME).tw. 30 Cough/ 31 cough*.tw. 32 Influenza, Human/ 33 (influenza* or flu).tw. 34 exp Bronchitis/ 35 (bronchit* or bronchiolit*).tw. 36 exp Pneumonia/ 37 pneumon*.tw. 38 or/1‐37 39 exp Anti‐Bacterial Agents/ 40 antibiotic*.tw. 41 (cephalosporin* or cefadroxil* or cephalexin* or cephaloridine* or cephalothin* or cephapirin* or cefazolin* or cephradine* or cefaclor* or cefoxitin* or cefprozil* or cefuroxime* or cefdinir* or cefixime* or cefpodoxime* or ceftibuten or ceftriaxone* or cefotaxime*).tw. 42 (penicillin* or methicillin* or oxacillin* or ampicillin* or amoxicillin*).tw. 43 (macrolide* or erythromycin* or clarithromycin* or azithromycin* or dirithromycin* or roxithromycin* or troleandomycin*).tw. 44 (aminoglycoside* or amikacin* or gentamicin* or kanamycin* or neomycin* or tobramycin*).tw. 45 (cloramphenicol* or sulfonamide* or erythromycin* or trimethoprim*).tw. 46 or/39‐45 47 38 and 46 48 Infant/ 49 (infant* or infancy or baby* or babies).tw. 50 exp Child/ 51 (child* or schoolchild* or school age* or preschool* or kid or kids or toddler* or boy* or girl*).tw. 52 Pediatrics/ 53 (pediatric* or paediatric*).tw. 54 52 or 50 or 53 or 49 or 51 or 48 55 54 and 47
Appendix 2. Embase.com search strategy
1. 'respiratory tract infection'/exp 2. 'respiratory tract infection':ti,ab OR 'respiratory tract infections':ti,ab 3. 'respiratory infection':ti,ab OR 'respiratory infections':ti,ab OR urti:ti,ab OR uri:ti,ab OR ari:ti,ab 4. 'rhinopharyngitis'/exp 5. nasopharyngit*:ti,ab OR rhinopharyngit*:ti,ab 6. 'sinusitis'/exp 7. sinusit*:ti,ab 8. 'rhinitis'/de 9. rhinit*:ti,ab 10. 'rhinosinusitis'/exp 11. rhinosinusit*:ti,ab 12. 'pharyngitis'/exp 13. pharyngit*:ti,ab 14. 'sore throat'/exp 15. 'tonsillitis'/de 16. tonsillit*:ti,ab 17. 'laryngitis'/de 18. 'croup'/exp OR 'laryngotracheobronchitis'/exp 19. laryngit*:ti,ab OR croup*:ti,ab OR laryngotracheobronchit*:ti,ab 20. 'tracheitis'/exp 21. tracheit*:ti,ab 22. 'epiglottitis'/exp 23. epiglottit*:ti,ab 24. 'common cold'/exp 25. 'common cold':ti,ab OR 'common colds':ti,ab OR coryza*:ti,ab 26. 'otitis media'/exp 27. 'otitis media':ti,ab OR aom:ti,ab OR ome:ti,ab 28. 'coughing'/exp 29. cough*:ti,ab 30. 'influenza'/exp 31. flu*:ti,ab OR influenza*:ti,ab 32. 'bronchitis'/exp 33. bronchit*:ti,ab OR bronchiolit*:ti,ab 34. 'pneumonia'/exp 35. pneumon*:ti,ab 36. 'sore throat':ti,ab OR 'sore throats':ti,ab 37. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 O R #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 38. 'antibiotic agent'/exp 39. antibiotic*:ti,ab OR 'anti biotic':ti,ab OR 'antibiotics':ti,ab 40. (cephalosporin*:ti,ab OR cefadroxil*:ti,ab OR cephalexin*:ti,ab OR cephaloridine*:ti,ab OR cephalothin*:ti,ab OR cephapirin*:ti,ab OR cefazolin*:ti,abOR cephradine*:ti,ab OR cefaclor*:ti,ab OR cefoxitin*:ti,ab OR cefprozil*:ti,ab OR cefuroxime*:ti,ab OR cefdinir*:ti,ab OR cefixime*:ti,ab OR cefpodoxime*:ti,ab OR ceftibuten:ti,ab OR ceftriaxone*:ti,ab OR cefotaxime*:ti,ab) 41. (penicillin*:ti,ab OR methicillin*:ti,ab OR oxacillin*:ti,ab OR ampicillin*:ti,ab OR amoxicillin*:ti,ab) 42. (macrolide*:ti,ab OR erythromycin*:ti,ab OR clarithromycin*:ti,ab OR azithromycin*:ti,ab OR dirithromycin*:ti,ab OR roxithromycin*:ti,ab OR troleandomycin*:ti,ab) 43. (aminoglycoside*:ti,ab OR amikacin*:ti,ab OR gentamicin*:ti,ab OR kanamycin*:ti,ab OR neomycin*:ti,ab OR tobramycin*:ti,ab) 44. (cloramphenicol*:ti,ab OR sulfonamide*:ti,ab OR erythromycin*:ti,ab OR trimethoprim*:ti,ab) 45. #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 46. 'infant'/exp 47. (infant*:ti,ab OR infancy*:ti,ab OR baby*:ti,ab OR babies:ti,ab) 48. 'child'/exp 50. 'pediatrics'/exp 51. pediatric*:ti,ab OR paediatric*:ti,ab 52. (child*:ti,ab OR schoolchild*:ti,ab OR preschool*:ti,ab OR kid:ti,ab OR kids:ti,ab OR toddler*:ti,ab OR boy*:ti,ab OR girl*:ti,ab) OR 'school age':ti,ab OR 'school aged':ti,ab OR 'school ages':ti,ab 53. #46 OR #47 OR #48 OR #50 OR #51 OR #52 54. #37 AND #45 AND #53 55. 'crossover procedure'/exp 56. 'double blind procedure'/exp 57. 'randomized controlled trial'/exp 58. 'single blind procedure'/exp 59. (random*:ti,ab OR factorial*:ti,ab OR crossover*:ti,ab OR placebo*:ti,ab OR assign*:ti,ab OR allocat *:ti,ab OR volunteer*:ti,ab) OR 'double blind':ti,ab OR 'double blinded':ti,ab OR 'double blinding':ti,ab OR 'single blind':ti,ab OR 'single blinded':ti,ab OR 'single blinding':ti,ab 60. #55 OR #56 OR #57 OR #58 OR #59 61. #54 AND #60
Data and analyses
Comparison 1. Antibiotic compared to control in the treatment of ARI in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Otitis | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.45, 1.11] |
| 2 Otitis ‐ high‐income countries | 2 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.39, 1.14] |
| 3 Pneumonia | 1 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.74, 1.49] |
| 3.1 Up to 11 months old | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.52, 1.76] |
| 3.2 12 to 58 months old | 1 | 563 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.71, 1.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Autret‐Leca 2002.
| Methods | RCT | |
| Participants | Children at risk of AOM Age range: 3 months to 3 years of age | |
| Interventions | Amoxicillin/clavulanic acid or placebo | |
| Outcomes | Otitis | |
| Notes | Children at risk of AOM | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but information about the randomisation process was insufficient to permit judgement of adequacy or inadequacy |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial was described as double‐blind, but the method of blinding was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial was described as double‐blind, but the method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data are balanced in numbers across the groups |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement of adequacy or inadequacy |
| Other bias | Unclear risk | A pharmaceutical company prepared the placebo syrup used in the trial |
Heikkinen 1995.
| Methods | RCT | |
| Participants | Children at risk of AOM Age range: 1 to 4 years of age | |
| Interventions | Amoxicillin/clavulanic acid or placebo | |
| Outcomes | Otitis | |
| Notes | Children at risk of AOM | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence was described as randomised and generated by a computer |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment because of sequentially numbered drug containers of identical appearance |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial was described as double‐blind but the method of blinding was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial was described as double‐blind but the method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data are unclear but do not seem to be related to clinical motives |
| Selective reporting (reporting bias) | Unclear risk | The report gives insufficient information to permit judgement of adequacy or inadequacy |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Rwalah 2011.
| Methods | RCT | |
| Participants | Children at risk of AOM Age range: up to 4 years of age | |
| Interventions | Amoxicillin/clavulanic acid 40 mg/kg/day or placebo | |
| Outcomes | Otitis | |
| Notes | Children at risk of AOM | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but there was insufficient information about the sequence generation process to permit judgment of adequacy or inadequacy |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial was described as double‐blind but the method of blinding was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial was described as double‐blind but the method of blinding was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing participants |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement of adequacy or inadequacy |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Sutrisna 1991.
| Methods | RCT | |
| Participants | Children up to 5 years of age | |
| Interventions | Ampicillin: 25 to 30 mg/kg per dose, every 6 hours, during 5 days and supportive care or just supportive care | |
| Outcomes | Pneumonia | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised, but there was insufficient information about the sequence generation process to permit judgement of adequacy or inadequacy |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised, but the method used to conceal the allocation was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. 11 children were excluded because of secondary disorders during the study |
| Selective reporting (reporting bias) | Unclear risk | There is insufficient information to permit judgement of adequacy or inadequacy |
| Other bias | Unclear risk | The trial appears to be free of other sources of bias |
AOM: acute otitis media RCT: randomised clinical trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ackerman 1968 | Tetracycline was one of the studied antibiotics; nowadays it is not recommended for the age range in our protocol |
| Arroll 2008 | Review |
| Betend 1972 | Trial is not about undifferentiated acute respiratory infections |
| Bloomfield 2004 | Trial conducted in hospitalised children with respiratory syncytial virus infection |
| Bovier‐Lapierre 1971 | Trial is not about undifferentiated acute respiratory infections |
| Carr 1973 | There is no placebo or non‐treatment control group in this trial |
| Castello 1981 | Not a RCT |
| De Mattia 1986 | Comparison between 2 antibiotics. Ages of participants are not clearly stated |
| De Sario 1986 | Study is not about undifferentiated respiratory infections |
| Easton 2010 | Not a RCT |
| Gjelstad 2013 | It is an article about medical education |
| Gordon 1974 | Data about the effects of antibiotic compared to control in relieving symptoms, preventing complications or causing adverse events were not provided |
| Gronås 1971 | Trial is a comparison of 2 antibiotics for respiratory infections probably caused by Haemophilus influenzae |
| Guerrier 1972 | Ages of participants were outside our protocol age range |
| Gupta 1977 | Comparison among 3 antibiotics; there is no placebo or non‐treatment control group |
| Hardy 1956 | Data for participants in our protocol age range were not available |
| He 2006 | The entire text could not be accessed |
| Henness 1982 | Study is a comparison among antibiotics; there is no placebo or non‐treatment control group |
| Higashi 2014 | Ages of participants were outside our protocol age range |
| Kundu 1976 | Ages of participants were outside our protocol age range |
| Lake 1971 | Ages of participants were outside our protocol age range |
| Lexomboon 1971 | Data about the effects of antibiotics compared to control in relieving symptoms, preventing complications and causing adverse events in children included in our protocol age range were not available |
| Limson 1972 | Ages of participants were outside our protocol age range |
| Lines 1973 | Trial is a comparison between 2 antibiotics |
| Little 1997 | Ages of participants were outside our protocol age range |
| Maeda 1999 | Data for children included specifically in our protocol age range were not available |
| Masbernard 1979 | Ages of participants were outside our protocol age range |
| McGuinness 1986 | Study is a comparison among antibiotics; no placebo or non‐treatment control group |
| Newell 1970 | Trial is not about undifferentiated acute respiratory tract infection |
| Oggero 1985 | Trial is a comparison between 2 antibiotics |
| Pan 2000 | Ages of participants were outside our protocol age range |
| Price 1975 | Trial is not about undifferentiated acute respiratory tract infections |
| Ratjen 2013 | An article about cystic fibrosis |
| Reinert 1991 | Data for children up to 5 years of age were not available |
| Rentsch 1980 | Not a RCT. Ages of participants were outside our protocol age range |
| Roper 1970 | Ages of participants were outside our protocol age range |
| Taylor 1977 | Trial was not about undifferentiated respiratory infections. Laboratory tests were used to exclude children with infections of bacterial aetiology |
| Todd 1981 | There are insufficient data for the sample age range and for the outcomes that are the subject of this review |
| Varricchio 2006 | In this trial 2 antibiotics are compared; there was no non‐treatment or placebo control group |
| Varricchio 2008 | Trial about culture‐proven acute bacterial infection |
| Windorfer 1980 | Comparison between 2 antibiotics; no placebo or non‐treatment control group |
| Zwart 2003 | No subgroup analyses for our protocol age range |
| Zwart 2004 | Ages of participants were outside our protocol age range |
RCT: randomised controlled trial
Differences between protocol and review
After reading the included articles we decided to conduct another analysis. In one trial (Heikkinen 1995), 11 children failed to complete the study: seven in the intervention group and four in the control group. The reasons why the children did not complete the study are unclear. We simulated the best‐case scenario for the antibiotic effect, conducting an analysis taking into account the best results that could have been found with antibiotic treatment if these 11 children had completed the study. As such, we considered what the results would have been if the seven children who did not complete the study that had received treatment had not developed otitis. On the other hand, we also considered that the four patients in the control group that did not finish the study did develop the infection.
Furthermore, some of the specific outcomes that we intended to study could not be analysed because they were not reported in the studies (mastoiditis, quinsy, abscess, meningitis, admission to hospital and death).
Contributions of authors
Márcia G Alves Galvão (MG), Marilene AR Crispino Santos (MS) and Antonio JL Alves da Cunha (AC) were responsible for the conception of this protocol. They also approved this document for publication. MG and MS selected trials and extracted data. AC was appointed as an arbitrator to resolve disagreements between MG and MS on the selection of the trials. MG drafted the review. MS supervised the day‐to‐day work. MS and AC commented on the review critically for its content.
Sources of support
Internal sources
The authors declare that no funding was received for this systematic review, Other.
External sources
The authors declare that no funding was received for this systematic review, Other.
Declarations of interest
Márcia G Alves Galvão: none known. Marilene Augusta Rocha Crispino Santos: none known. Antonio JL Alves da Cunha: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Autret‐Leca 2002 {published data only}
- Autret‐Leca E, Giraudeau B, Ployet MJ, Jonville‐Bera A. Amoxicillin/clavulanic acid is ineffective at preventing otitis media in children with presumed viral upper respiratory infection: a randomized, double‐blind equivalence, placebo‐controlled trial. British Journal of Clinical Pharmacology 2002;54(6):652‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heikkinen 1995 {published data only}
- Heikkinen T, Ruuskanen O, Ziegler T, Waris M, Puhakka H. Short‐term use of amoxicillin‐clavulanate during upper respiratory tract infection for prevention of acute otitis media. Journal of Pediatrics 1995;126(2):313‐6. [DOI] [PubMed] [Google Scholar]
Rwalah 2011 {published data only}
- Rwalah MM, Rashdan HAA. Short term antibiotics in children with upper respiratory tract infection. Rawal Medical Journal 2011;36(2):133‐6. [0303‐5212] [Google Scholar]
Sutrisna 1991 {published data only}
- Sutrisna B, Frerichs RR, Reingold AL. Randomised, controlled trial of effectiveness of ampicillin in mild acute respiratory infections in Indonesian children. Lancet 1991;338(8765):471‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ackerman 1968 {published data only}
- Ackerman BD. Treatment of undifferentiated respiratory infections in infants. Clinical Pediatrics 1968;7(7):391‐5. [DOI] [PubMed] [Google Scholar]
Arroll 2008 {published data only}
- Arroll B, Kenealy T, Falloon K. Are antibiotics indicated as an initial treatment for patients with acute upper respiratory tract infections? A review. New Zealand Medical Journal 2008;121(1284):64‐70. [PubMed] [Google Scholar]
Betend 1972 {published data only}
- Betend B, Goddon R, David L. Therapeutic trial in a children's hospital of a new orally administered form of cephalexin. Lyon Medical 1972;228(14):205‐8. [PubMed] [Google Scholar]
Bloomfield 2004 {published data only}
- Bloomfield P, Dalton D, Karleka A, Kesson A, Duncan G, Isaacs D. Bacteraemia and antibiotic use in respiratory syncytial virus infections. Archives of Disease in Childhood 2004;89(4):363‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bovier‐Lapierre 1971 {published data only}
- Bovier‐Lapierre M, David M, Lauras B. Trial of use of a new oral antibiotic: cephalexin in granules in 59 children. Lyon Medical 1971;226(20):895‐900. [PubMed] [Google Scholar]
Carr 1973 {published data only}
- Carr WG. Clindamycin palmitate hydrochloride: a clinical trial in a pediatric office practice. Current Therapeutic Research, Clinical & Experimental 1973;15(9):630‐40. [PubMed] [Google Scholar]
Castello 1981 {published data only}
- Castello D, Sacchetti L, Brero P, Poncini L. Results of a clinical trial of the use of a methoxyamine (cefuroxime) in respiratory tract infections in children. Minerva Pediatrica 1981;33(17):881‐6. [PubMed] [Google Scholar]
De Mattia 1986 {published data only}
- Mattia D, Santoro A, Grumo AR, Santoro N. Xibornol in inflammatory involvement of the upper airways in childhood. A comparative clinical investigation. Minerva Pediatrica 1986;38(21):1017‐24. [PubMed] [Google Scholar]
De Sario 1986 {published data only}
- Sario PN, Bianco R, Marco A, Gavioli F, Orecchia L, Salvadori G, et al. Miocamycin in pediatrics. Results of multicenter study in Piedmont and Valle d'Aosta. Annali dell'Ospedale Maria Vittoria di Torino 1986;29:317‐28. [PubMed] [Google Scholar]
Easton 2010 {published data only}
- Easton G, Saxena S. Antibiotic prescribing for upper respiratory tract infections in children: how can we improve?. London Journal of Primary Care 2010;3:37‐41. [1757‐1472] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gjelstad 2013 {published data only (unpublished sought but not used)}
- Gjelstad S, Høye S, Straand J, Brekke M, Dalen I, Lindbæk M. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing (Rx‐PAD) study). BMJ 2013;347:f4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 1974 {published data only}
- Gordon M, Lovell S, Dugdale AE. The value of antibiotics in minor respiratory illness in children. A controlled trial. Medical Journal of Australia 1974;1(9):304‐6. [PubMed] [Google Scholar]
Gronås 1971 {published data only}
- Gronås HE, Hovig B. Azidocillin and ampicillin in the treatment of upper respiratory tract infections. Nordisk Medicin 1971;86(27):822‐6. [PubMed] [Google Scholar]
Guerrier 1972 {published data only}
- Guerrier Y. Upper respiratory tract infections in otorhinolaryngology. Annales d Oto‐Laryngologie et de Chirurgie Cervico‐Faciale 1972;89(1):73‐8. [PubMed] [Google Scholar]
Gupta 1977 {published data only}
- Gupta S, Bhushan V, Srivastava G. Therapeutic trial of chloramphenicol, eskaycillin (ampicillin) and co‐trimoxazole in upper respiratory tract infections of childhood. Indian Pediatrics 1977;14(5):391‐3. [PubMed] [Google Scholar]
Hardy 1956 {published data only}
- Hardy L, Traisman H. Antibiotics and chemotherapeutic agents in the treatment of uncomplicated respiratory infections in children; a controlled study. Journal of Pediatrics 1956;48(2):146‐56. [DOI] [PubMed] [Google Scholar]
He 2006 {published data only}
- He BB, Chen F. Clinical efficacy of cefixime in the treatment of children with respiratory tract infection. Chinese Journal of Antibiotics 2006;31(9):571‐2, 575. [Google Scholar]
Henness 1982 {published data only}
- Henness DM. A clinical experience with cefadroxil in upper respiratory tract infection. Journal of Antimicrobial Chemotherapy 1982;10(Suppl B):125‐35. [DOI] [PubMed] [Google Scholar]
Higashi 2014 {published data only}
- Higashi F, Kubo H, Yasuda H, Nukiwa T, Yamaya M. Additional treatment with clarithromycin reduces fever duration in patients with influenza. Respiratory Investigation 2014;52:302‐9. [DOI] [PubMed] [Google Scholar]
Kundu 1976 {published data only}
- Kundu SC. Therapeutic usefulness of combination therapy with oral penicillin G and trisulfapyrimidine in the treatment of acute upper respiratory tract infections. Journal of the Indian Medical Association 1976;67(11):252‐2. [PubMed] [Google Scholar]
Lake 1971 {published data only}
- Lake B. Ampicillin in acute infections of general practice. A controlled trial. Medical Journal of Australia 1971;1(12):636‐40. [PubMed] [Google Scholar]
Lexomboon 1971 {published data only}
- Lexomboon U, Duangmani C, Kusalasai V, Sunakorn P, Olson LC, Noyes HE. Evaluation of orally administered antibiotics for treatment of upper respiratory infections in Thai children. Journal of Pediatrics 1971;78(5):772‐8. [DOI] [PubMed] [Google Scholar]
Limson 1972 {published data only}
- Limson BM, Siasoco RE, Dial FP. A new cephalosporin derivative, cephradine (TM), in the treatment of acute infective diseases. Current Therapeutic Research, Clinical & Experimental 1972;14(3):101‐6. [PubMed] [Google Scholar]
Lines 1973 {published data only}
- Lines DR, Vimpani GV, Pearson CC. The use of 7‐chlorolincomycin in the treatment of childhood respiratory disease. Medical Journal of Australia 1973;1(9):439‐41. [DOI] [PubMed] [Google Scholar]
Little 1997 {published data only}
- Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics.[see comment]. BMJ 1997;315(7104):350‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maeda 1999 {published data only}
- Maeda S, Yamada Y, Nakamura H, Maeda T. Efficacy of antibiotics against influenza‐like illness in an influenza epidemic. Pediatrics International 1999;41(3):274‐6. [DOI] [PubMed] [Google Scholar]
Masbernard 1979 {published data only}
- Masbernard A, Salord JC. Therapy with cefaclor: analysis of infections in 189 French patients. Postgraduate Medical Journal 1979;55(Suppl 4):72‐6. [PubMed] [Google Scholar]
McGuinness 1986 {published data only}
- McGuinness BW, Cooper J, Kent PL. Trimethoprim versus amoxycillin for upper respiratory tract infection. Practitioner 1986;230(1420):905‐8. [PubMed] [Google Scholar]
Newell 1970 {published data only}
- Newell AC. Clinical trial of a new antibiotic. Medical Journal of Australia 1970;2(7):321‐6. [DOI] [PubMed] [Google Scholar]
Oggero 1985 {published data only}
- Oggero R, Pugliaro G, Galvagno G, Frigerio M. Antibacterial efficacy of a new chemotherapeutic agent in acute infections of the upper respiratory tract in childhood. Controlled clinical trial of xibornol and josamycin. Minerva Pediatrica 1985;37(10):419‐24. [PubMed] [Google Scholar]
Pan 2000 {published data only}
- Pan Q, Ornstein S, Gross AJ, Hueston WJ, Jenkins RG, Mainous AG, et al. Antibiotics and return visits for respiratory illness: a comparison of pooled versus hierarchical statistical methods. American Journal of the Medical Sciences 2000;319(6):360‐5. [DOI] [PubMed] [Google Scholar]
Price 1975 {published data only}
- Price JD, Harding JW. Amoxycillin in the treatment of respiratory tract infection in children. British Journal of Clinical Practice 1975;29(8):203‐6. [PubMed] [Google Scholar]
Ratjen 2013 {published data only}
- Ratjen F. Antibiotic therapy: what have we learned from recent trials?. Pediatric Pulmonology 2013;48:150‐1. [Google Scholar]
Reinert 1991 {published data only}
- Reinert P, Narcy P, Paliwoda A, Rouffiac E. Evaluation of tixocortol pivalate‐neomycin combination versus ++a placebo excipient in acute rhinopharyngitis in children. Annales de Pediatrie 1991;38(7):503‐8. [PubMed] [Google Scholar]
Rentsch 1980 {published data only}
- Rentsch M. The treatment with minocyciline (Minocin) in daily pediatric practice (author's transl). Schweizerische Rundschau fur Medizin Praxis 1980;69(47):1756‐9. [PubMed] [Google Scholar]
Roper 1970 {published data only}
- Roper DJ, Harding JW. General practice trial of flucloxacillin in common respiratory tract infections. British Journal of Clinical Practice 1970;24(10):419‐21. [PubMed] [Google Scholar]
Taylor 1977 {published data only}
- Taylor B, Abbott GD, Kerr MM, Fergusson DM. Amoxycillin and co trimoxazole in presumed viral respiratory infections of childhood: placebo controlled trial. British Medical Journal 1977;2(6086):552‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Todd 1981 {published data only}
- Todd J, Todd N, Damato J, Todd W. Diagnosis and treatment of purulent nasopharyngitis ‐ a double‐blind, two‐drug evaluation. Pediatric Research 1981;15:623. [DOI] [PubMed] [Google Scholar]
Varricchio 2006 {published data only}
- Varricchio A, Tricarico D, Lucia A, Utili R, Tripodi MF, Giudice M, et al. Inhaled tobramycin in children with acute bacterial rhinopharyngitis. International Journal of Immunopathology & Pharmacology 2006;19(1):131‐40. [PubMed] [Google Scholar]
Varricchio 2008 {published data only}
- Varricchio A, Capasso M, Gioacchino M, Ciprandi G. Inhaled thiamphenicol and acetylcysteine in children with acute bacterial rhinopharyngitis. International Journal of Immunopathology & Pharmacology 2008;21(3):625‐9. [DOI] [PubMed] [Google Scholar]
Windorfer 1980 {published data only}
- Windorfer A, Maier Lenz H, Bauer P, Alterthum K. Cefadroxil in infections of the upper respiratory tract in pediatrics. Infection 1980;8(Suppl 5):610‐3. [Google Scholar]
Zwart 2003 {published data only}
- Zwart S, Rovers MM, Melker Ruut A, Hoes AW. Penicillin for acute sore throat in children: randomised, double blind trial.[see comment]. BMJ 2003;327(7427):1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwart 2004 {published data only}
- Zwart S, Rovers MM, Melker RA, Lindbaek M. Penicillin V did not shorten symptoms in children with sore throat. Evidence‐Based Medicine 2004;9:77. [Google Scholar]
Additional references
Abdel‐Aziz 2010
- Abdel‐Aziz M, El‐Hoshy H. Acute mastoiditis: a one year study in the pediatric hospital of Cairo university. BMC Ear, Nose and Throat Disorders 2010;10:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akkerman 2005
- Akkerman AE, Kuyvenhoven MM, Wouden JC, Verheij TJ. Determinants of antibiotic overprescribing in respiratory tract infections in general practice. Journal of Antimicrobial Chemotherapy 2005;56:930‐6. [DOI] [PubMed] [Google Scholar]
Altamimi 2012
- Altamimi S, Khalil A, Khalaiwi KA, Milner RA, Pusic MV, Al Othman MA. Short‐term late‐generation antibiotics versus longer term penicillin for acute streptococcal pharyngitis in children. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD004872.pub3] [DOI] [PubMed] [Google Scholar]
Arason 2010
- Arason VA, Sigurdsson JA. The problems of antibiotic overuse. Scandinavian Journal of Primary Health Care 2010; Vol. 28:65‐6. [DOI] [PMC free article] [PubMed]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baron 2013
- Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson RB Jr, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clinical Infectious Diseases 2013;57:485‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benguigui 2003
- Benguigui Y. Acute respiratory infections control in the context of the IMCI strategy in the Americas. Revista Brasileira de Saúde Materno Infantil 2003;3(1):25‐6. [Google Scholar]
Brink 2015
- Brink AJ, Cotton M, Feldman C, Finlayson H, Friedman R, Green R, et al. Updated recommendations for the management of upper respiratory tract infections in South Africa. South African Medical Journal 2015;105:344‐52. [DOI] [PubMed] [Google Scholar]
Chung 2007
- Chung A, Perera R, Brueggemann AB, Elamin AE, Harnden A, Mayon‐White R, et al. Effect of antibiotic prescribing on antibiotic resistance in individual children in primary care: prospective cohort study. BMJ 2007;335:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coco 2009
- Coco AS, Horst MA, Gambler AS. Trends in broad‐spectrum antibiotic prescribing for children with acute otitis media in the United States, 1998–2004. BMC Pediatrics 2009;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costelloe 2010
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta‐analysis. BMJ 2012;18(340):2096. [DOI] [PubMed] [Google Scholar]
Dagan 2008
- Dagan R, Klugman KP. Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infectious Diseases 2008;8(12):785‐95. [DOI] [PubMed] [Google Scholar]
Del Mar 2012
- Mar C, Glasziou P, Lowe JB, van Driel ML, Hoffmann T, Beller E. Addressing antibiotic resistance ‐ focusing on acute respiratory infections in primary care. Australian Family Physician 2012;41(11):839‐40. [PubMed] [Google Scholar]
Doan 2014
- Doan Q, Enarson P, Kissoon N, Klassen TP, Johnson DW. Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006452.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowell 1998
- Dowell SF, Marcy SM, Phillips WR, Gerber MA, Schwartz B. Principles of judicious use of antimicrobial agents for pediatric upper respiratory tract infections. Pediatrics 1998;101:163‐5. [Google Scholar]
Emmelin 2007
- Emmelin A, Wall S. Indoor air pollution: a poverty‐related cause of mortality among the children of the world. Chest 2007;132:1615‐23. [DOI] [PubMed] [Google Scholar]
Fahey 1998a
- Fahey T, Stocks N, Thomas T. Quantitative systematic review of randomised controlled trials comparing antibiotic with placebo for acute cough in adults. BMJ 1998;316:906‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fahey 1998b
- Fahey T, Stocks N, Thomas T. Systematic review of the treatment of upper respiratory tract infection. Archives of Disease in Childhood 1998;79:225‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farha 2005
- Farha T, Thomson AH. The burden of pneumonia in children in the developed world. Paediatric Respiratory Reviews 2005;6(2):76‐82. [DOI] [PubMed] [Google Scholar]
Garenne 1992
- Garenne FE, Ronsmans C, Campbell H. The magnitude of mortality from acute respiratory infections in children under 5 years in developing countries. World Health Statistics Quarterly 1992;45:180‐91. [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool (www.guidelinedevelopment.org). Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Hay 2002
- Hay AD, Wilson AD. The natural history of acute cough in children aged 0 to 4 years in primary care: a systematic review. British Journal of General Practice 2002;52:401‐9. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester, UK: John Wiley & Sons, Ltd.
Hope‐Simpson 1973
- Hope‐Simpson RE, Miller DL. The definition of acute respiratory illnesses in general practice. Postgraduate Medical Journal 1973;49:763‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howie 1971
- Howie JG, Richardson IM, Gill G, Durno D. Respiratory illness and antibiotic use in general practice. Journal of the Royal College of General Practitioners 1971;21:657‐63. [PMC free article] [PubMed] [Google Scholar]
Izadnegahdar 2013
- Izadnegahdar R, Cohen AL, Klugman KP, Qazi SA. Childhood pneumonia in developing countries. Lancet Respiratory Medicine 2013;7:574‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jansen 2006
- Jansen A, Sanders E, Schilder A, Hoes A, Jong V, Hak E. Primary care management of respiratory tract infections in Dutch preschool children. Scandinavian Journal of Primary Health Care 2006;24(4):231‐6. [DOI] [PubMed] [Google Scholar]
Kabra 2013
- Kabra SK, Lodha R, Pandey RM. Antibiotics for community‐acquired pneumonia in children. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD004874.pub3] [DOI] [PubMed] [Google Scholar]
Kenealy 2013
- Kenealy T, Arroll B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD000247.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2000
- Klein JO. The burden of otitis media. Vaccine 2000;19(Suppl 1):2‐8. [DOI] [PubMed] [Google Scholar]
Krishnan 2015
- Krishnan A, Amarchand R, Gupta V, Lafond KE, Suliankatchi RA, Saha S, et al. Epidemiology of acute respiratory infections in children ‐ preliminary results of a cohort in a rural north Indian community. BMC Infectious Diseases 2015;15:462. [DOI: 10.1186/s12879-015-1188-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2003
- Kumar S, Little P, Britten N. Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. BMJ 2003;326:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2009
- Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009;136:1237‐48. [DOI] [PubMed] [Google Scholar]
Kusel 2007
- Kusel MM, Klerk N, Holt PG, Landau LI, Sly PD. Occurrence and management of acute respiratory illnesses in early childhood. Journal of Paediatrics and Child Health 2007;43:139‐46. [DOI] [PubMed] [Google Scholar]
Kutty 2011
- Kutty N. Treating children without antibiotics in primary healthcare. Oman Medical Journal 2011;26:303‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lancet 2009
- Editorial. Urgently needed: new antibiotics. Lancet 2009;374:1868. [DOI] [PubMed] [Google Scholar]
Larsson 2005
- Larsson M, Falkenberg T, Dardashti A, Ekman T, Törnquist S, Kim Chuc N, et al. Overprescribing of antibiotics to children in rural Vietnam. Scandinavian Journal of Infectious Diseases 2005;37:442‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Little 2002
- Little P, Watson L, Morgan S, Williamson I. Antibiotic prescribing and admissions with major suppurative complications of respiratory tract infections: a data linkage study. British Journal of General Practice 2002;52:187‐93. [PMC free article] [PubMed] [Google Scholar]
Majeed 2004
- Majeed A, Williams S, Jarman B, Aylin P. Prescribing of antibiotics and admissions for respiratory tract infections in England. BMJ 2004;329:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Margolis 1998
- Margolis P, Gadomski A. Does this infant have pneumonia?. JAMA 1998;279:308‐13. [DOI] [PubMed] [Google Scholar]
McCaig 1995
- McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office‐based physicians in the United States. JAMA 1995;273:214‐9. [PubMed] [Google Scholar]
Nascimento‐Carvalho 2006
- Nascimento‐Carvalho CM. Outpatient antibiotic therapy as a predisposing factor for bacterial resistance: a rational approach to airway infections [Antibioticoterapia ambulatorial como fator de indução da resistência bacteriana: uma abordagem racional para as infecções de vias aéreas]. Jornal de Pediatria 2006;Suppl 82:146‐52. [DOI] [PubMed] [Google Scholar]
Petersen 2007
- Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward A. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ 2007;335:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Posfay‐Barbe 2008
- Posfay‐Barbe K, Zeer D, Pittet D. Infection control in paediatrics. Lancet Infectious Diseases 2008;8(1):19‐31. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rudan 2004
- Rudan I, Tomaskovic L, Boschi‐Pinto C, Campbell H on behalf of WHO Child Health Epidemiology Reference Group. Global estimate of incidence of clinical pneumonia among children under five years of age. Bulletin of the World Health Organization 2004;82:12. [PMC free article] [PubMed] [Google Scholar]
Rudan 2008
- Rudan I, Boschi‐Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization 2008;86:408‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schraff 2001
- Schraff S, McGinn JD, Derkay CS. Peritonsillar abscess in children: a 10‐year review of diagnosis and management. International Journal of Pediatric Otorhinolaryngology 2001;57:213‐8. [DOI] [PubMed] [Google Scholar]
Schuetz 2012
- Schuetz P, Müller B, Christ‐Crain M, Stolz D, Tamm M, Bouadma L, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD007498.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharland 2005
- Sharland M, Kendall H, Yeates D, Randall A, Hughes G, Glasziou P, et al. Antibiotic prescribing in general practice and hospital admissions for peritonsillar abscess, mastoiditis, and rheumatic fever in children: time trend analysis. BMJ 2005;331:328‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinks 2013
- Spinks A, Glasziou PP, Mar CB. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD000023.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spurling 2013
- Spurling GK, Del Mar CB, Dooley L, Foxlee R, Farley R. Delayed antibiotics for respiratory infections. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD004417.pub4] [DOI] [PubMed] [Google Scholar]
Tan 2008
- Tan T, Little P, Stokes T, on behalf of the Guideline Development Group. Antibiotic prescribing for self limiting respiratory tract infections in primary care: summary of NICE guidance. BMJ 2008;337:a437. [DOI] [PubMed] [Google Scholar]
Thompson 2008
- Thompson PL, Gilbert RE, Long PF, Saxena S, Sharland M, Wong IC. Has UK guidance affected general practitioner antibiotic prescribing for otitis media in children?. Journal of Public Health 2008;30:479‐86. [DOI] [PubMed] [Google Scholar]
Thompson 2009
- Thompson PL, Spyridis N, Sharland M, Gilbert RE, Saxena S, Long PF, et al. Changes in clinical indications for community antibiotic prescribing for children in the UK from 1996‐2006: will the new NICE prescribing guidance on upper respiratory tract infection be ignored?. Archives of Disease in Childhood 2009;94(5):337‐40. [DOI] [PubMed] [Google Scholar]
van Balen 2004
- Balen JA, Verheij TJ, Pijnenborg L, Assendelft WJ, Nederlands Huisartsen Genootschap. Summary of the practice guideline 'Acute cough' from the Dutch College of General Practitioners. Nederlands Tijdschrift voor Geneeskunde 2004;148:725‐8. [PubMed] [Google Scholar]
van Driel 2013
- Driel ML, Sutter AIM, Keber N, Habraken H, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD004406.pub2] [DOI] [PubMed] [Google Scholar]
Venekamp 2015
- Venekamp RP, Sanders SL, Glasziou PP, Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD000219.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vergison 2010
- Vergison A, Dagan R, Arguedas A, Bonhoeffer J, Cohen R, Dhooge I, et al. Otitis media and its consequences: beyond the earache. Lancet 2010;10:195–203. [DOI] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. International Classification of Diseases (ICD). http://www.who.int/classifications/icd/en/ 2007 (accessed 20 November 2007).
WHO 2012
- World Health Organization. Recommendations for management of common childhood conditions. Evidence for technical update of pocket book recommendations. World Health Organization, 2012. [PubMed] [Google Scholar]
Zar 2014
- Zar HJ, Ferkol TW. The global burden of respiratory disease‐impact on child health. Pediatric Pulmonology 2014;49:430‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Alves Galvão 2009
- Alves Galvão MG, Rocha Crispino Santos MA, Alves da Cunha AJ. Antibiotics for preventing suppurative complications from undifferentiated acute respiratory infections in children under five years of age. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007880] [DOI] [Google Scholar]
Alves Galvão 2014
- Alves Galvão MG, Rocha Crispino Santos MA, Alves da Cunha AJ. Antibiotics for preventing suppurative complications from undifferentiated acute respiratory infections in children under five years of age. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD007880.pub2] [DOI] [PubMed] [Google Scholar]


