Abstract
Background
Attention deficit hyperactivity disorder (ADHD) is one of the most common psychiatric conditions affecting children and adolescents. Amphetamines are among the most commonly prescribed medications to manage ADHD. There are three main classes of amphetamines: dexamphetamine, lisdexamphetamine and mixed amphetamine salts, which can be further broken down into short‐ and long‐acting formulations. A systematic review assessing their efficacy and safety in this population has never been conducted.
Objectives
To assess the efficacy and safety of amphetamines for ADHD in children and adolescents.
Search methods
In August 2015 we searched CENTRAL, Ovid MEDLINE, Embase, PsycINFO, ProQuest Dissertation and Theses, and the Networked Digital Library of Theses and Dissertations. We also searched ClinicalTrials.gov, and checked the reference lists of relevant studies and reviews identified by the searches. No language or date restrictions were applied.
Selection criteria
Parallel‐group and cross‐over randomized controlled trials (RCTs) comparing amphetamine derivatives against placebo in a pediatric population (< 18 years) with ADHD.
Data collection and analysis
Two authors independently extracted data on participants, settings, interventions, methodology, and outcomes for each included study. For continuous outcomes, we calculated the standardized mean difference (SMD) and for dichotomous outcomes we calculated the risk ratio (RR). Where possible, we conducted meta‐analyses using a random‐effects model. We also performed a meta‐analysis of the most commonly reported adverse events in the primary studies.
Main results
We included 23 trials (8 parallel‐group and 15 cross‐over trials), with 2675 children aged three years to 17 years. All studies compared amphetamines to placebo. Study durations ranged from 14 days to 365 days, with the majority lasting less than six months. Most studies were conducted in the United States; three studies were conducted across Europe. We judged 11 included studies to be at a high risk of bias due to insufficient blinding methods, failing to account for dropouts and exclusions from the analysis, and failing to report on all outcomes defined a priori. We judged the remaining 12 studies to be at unclear risk of bias due to inadequate reporting.
Amphetamines improved total ADHD core symptom severity according to parent ratings (SMD ‐0.57; 95% confidence interval (CI) ‐0.86 to ‐0.27; 7 studies; 1247 children/adolescents; very low quality evidence), teacher ratings (SMD ‐0.55; 95% CI ‐0.83 to ‐0.27; 5 studies; 745 children/adolescents; low quality evidence), and clinician ratings (SMD ‐0.84; 95% CI ‐1.32 to ‐0.36; 3 studies; 813 children/adolescents; very low quality evidence). In addition, the proportion of responders as rated by the Clinical Global Impression ‐ Improvement (CGI‐I) scale was higher when children were taking amphetamines (RR 3.36; 95% CI 2.48 to 4.55; 9 studies; 2207 children/adolescents; very low quality evidence).
The most commonly reported adverse events included decreased appetite, insomnia/trouble sleeping, abdominal pain, nausea/vomiting, headaches, and anxiety. Amphetamines were associated with a higher proportion of participants experiencing decreased appetite (RR 6.31; 95% CI 2.58 to 15.46; 11 studies; 2467 children/adolescents), insomnia (RR 3.80; 95% CI 2.12 to 6.83; 10 studies; 2429 children/adolescents), and abdominal pain (RR 1.44; 95% CI 1.03 to 2.00; 10 studies; 2155 children/adolescents). In addition, the proportion of children who experienced at least one adverse event was higher in the amphetamine group (RR 1.30; 95% CI 1.18 to 1.44; 6 studies; 1742 children/adolescents; low quality evidence).
We performed subgroup analyses for amphetamine preparation (dexamphetamine, lisdexamphetamine, mixed amphetamine salts), amphetamine release formulation (long acting versus short acting), and funding source (industry versus non industry). Between‐group differences were observed for proportion of participants experiencing decreased appetite in both the amphetamine preparation (P < 0.00001) and amphetamine release formulation (P value = 0.008) subgroups, as well as for retention in the amphetamine release formulation subgroup (P value = 0.03).
Authors' conclusions
Most of the included studies were at high risk of bias and the overall quality of the evidence ranged from low to very low on most outcomes. Although amphetamines seem efficacious at reducing the core symptoms of ADHD in the short term, they were associated with a number of adverse events. This review found no evidence that supports any one amphetamine derivative over another, and does not reveal any differences between long‐acting and short‐acting amphetamine preparations. Future trials should be longer in duration (i.e. more than 12 months), include more psychosocial outcomes (e.g. quality of life and parent stress), and be transparently reported.
Keywords: Adolescent; Child; Child, Preschool; Humans; Amphetamines; Amphetamines/therapeutic use; Attention Deficit Disorder with Hyperactivity; Attention Deficit Disorder with Hyperactivity/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Amphetamines for attention deficit hyperactivity disorder in children and adolescents
Background Attention deficit hyperactivity disorder (ADHD) is a common problem affecting children and adolescents. ADHD is characterized by inattention (being easily distracted, unable to focus on one task), impulsivity (fidgety; constantly moving), and hyperactivity (impatient; acts without thinking). One of the most common treatments for managing ADHD is the drug class of amphetamines, which are a class of stimulant medications. They are thought to reduce the severity of symptoms associated with ADHD.
Review question
Do children and adolescents (under 18 years of age) diagnosed with ADHD benefit from treatment with amphetamines to reduce the core symptoms of ADHD, compared to other children and adolescents who receive no drug or a fake drug (placebo)?
Study characteristics As of August 2015, we identified 23 randomized controlled trials (RCTs: a type of scientific experiment in which people are randomly assigned to one of two or more treatments), which included 2675 children and adolescents between three years and 17 years of age. These studies compared amphetamines to placebo. Three different kinds of amphetamines were investigated: dexamphetamine, lisdexamphetamine and mixed amphetamine salts. The duration of the included studies ranged from 14 days to 365 days. The RCTs were conducted in the United States and Europe. Key results We found that amphetamines were effective at improving the core symptoms of ADHD in the short term, but that they were also linked to a higher risk of experiencing adverse events such as sleep problems, decreased appetite, and stomach pain. We found no evidence that one kind of amphetamine was better than another, and found no difference between amphetamines that act for longer periods of time versus those that act for shorter periods of time.
Quality of the evidence
The quality of the included studies was low to very low because of problems in their design and large differences between the studies. Well‐designed and clearly reported RCTs that are longer in duration are needed, so we may better understand the long‐term effects (both positive and negative) of amphetamines.
Summary of findings
for the main comparison.
| Amphetamines compared with placebo for attention deficit hyperactivity disorder in children and adolescents | ||||||
|
Patient or population: children or adolescents with ADHD Settings: Beligum, France, Germany, Hungary, Italy, Netherlands, Norway, Poland, Spain, Sweden, United Kingdom, United States Intervention: amphetamines (i.e. dexamphetamine, lisdexamphetamine, mixed amphetamine salts) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Amphetamine | |||||
| Total ADHD symptom score ‐ parent ratings (ADHD Rating Scale, Fourth Version; Conners' Rating Scale; Conners' Global Index; Conners' Abbreviated Symptom Questionnaire) Follow‐up: 7 to 49 days | ‐ | The mean total score in the intervention groups was 0.57 standard deviations lower (‐0.86 to ‐0.27) | SMD ‐0.57 (‐0.86 to ‐0.27) | 1247 (7) | ⊕⊝⊝⊝ Very low1,2,3 | Moderate effect** |
|
Total ADHD symptom score ‐ teacher ratings (ADHD Rating Scale, Fourth Version; Conners' Rating Scale; Conners' Global Index; Conners' Abbreviated Symptom Questionnaire) Follow‐up: 7 to 35 days |
‐ | The mean total score in the intervention groups was 0.55 standard deviations lower (‐0.83 to ‐0.27) | SMD ‐0.55 (‐0.83 to ‐0.27) | 745 (5) | ⊕⊕⊝⊝ Low1,2 | Moderate effect** |
| Total ADHD symptom score ‐ clinician ratings (ADHD Rating Scale, Fourth Version) Follow‐up: 7 to 28 days | ‐ | The mean total score in the intervention groups was 0.84 standard deviations lower (‐1.32 to ‐0.36) | SMD ‐0.84 (‐1.32 to ‐0.36) | 813 (3) | ⊕⊝⊝⊝ Very low1,2,3 | Large effect** |
| Proportion of responders (Clinical Global Impressions ‐ Improvement (CGI‐I) scale) | 187 per 1000 | 605 per 1000 |
RR 3.36 (2.48 to 4.55) |
2207 (9) | ⊝⊝⊝⊝ Very low1,2,3,4 | ‐ |
|
Academic performance (Permanent Product Measure of Performance; Wechsler Intelligence Scale for Children ‐ Revised; Barnell Lot, Ltd Math Test; Wide Range Achievement Test) Follow‐up: 7 to 21 days |
‐ | The mean score in the intervention groups was 0.51 standard deviations higher (0.31 to 0.70) | SMD 0.56 (0.39 to 0.73) | 826 (8) | ⊕⊕⊝⊝ Low1,2 | Moderate effect** |
| Retention: proportion of participants who completed the trial | 825 per 1000 | 864 per 1000 | RR 1.03 (0.97 to 1.10) | 2381 (11) | ⊕⊝⊝⊝ Very low1,2,3 | ‐ |
| Proportion of participants who experienced at least 1 adverse event | 366 per 1000 | 582 per 1000 |
RR 1.30 (1.18 to 1.44) |
1742 (6) | ⊕⊕⊝⊝ Low1,2 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**Magnitude of effect sizes have been defined according to Cohen 1988 (< 0.2 = small, 0.5 to 0.8 = moderate, > 0.8 = large) ADHD: Attention deficit hyperactivity disorder; CI: Confidence interval; GRADE: Grades of recommendation, assessment, development and evaluation; RR: Risk ratio; SMD: Standardized mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to the majority of studies included in this outcome having a high risk of bias. 2 Downgraded one level due to this outcome including comparisons of different amphetamine derivatives and release formulations. 3Downgraded one level due to presence of significant statistical heterogeneity (I² > 50%). 4Downgraded one level due to wide 95% CI indicating that the intervention effect for this outcome is highly variable.
Background
Description of the condition
Attention deficit hyperactivity disorder (ADHD) is one of the most common pediatric psychiatric conditions, affecting around 5% of children worldwide (Polanczyk 2007). ADHD is characterized by three core symptoms: inattention, impulsivity and hyperactivity, which are more frequently displayed than would be typical in children of the same age (APA 2000). The core symptoms are often presented to various degrees in different children, breaking ADHD down into three subtypes: the predominantly inattentive type, the predominantly hyperactive‐impulsive type, and the combined type (i.e. children displaying both inattention and hyperactivity) (APA 2000). The condition is often diagnosed at a young age, usually between the ages of three and six years (NIMH 2009). The potential for comorbidity is extremely high in this population and comorbidities are present in almost two‐thirds of pediatric ADHD cases, with the most common being oppositional defiant disorder (50%), conduct disorder (35%), anxiety disorder (33%), and depression (33%) (AHRQ 1999; Mayes 2009).
The symptoms of ADHD have been shown to permeate a child's performance across multiple settings, having long‐term effects on their academic performance and social development. Studies have also shown that children with ADHD are more likely to be irritable, impatient, and aggressive (NIH 2000). In addition, families who have children with ADHD often experience higher levels of parental stress and frustration, marital disruption, and social isolation (Edwards 1995). It has been estimated that 50% of childhood ADHD cases will persist into adolescence and adulthood (Biederman 1993), making it a chronic lifetime condition for many.
Description of the intervention
A wide variety of treatments have been used for the management of ADHD, including psychosocial interventions, dietary management, herbal and homeopathic remedies, and biofeedback. However, for the past few decades, the psychostimulant, methylphenidate, has been the first line of treatment (APA 2000), and has been found to be effective in 70% to 90% of school‐aged children (NIH 2000; Wigal 1999). Amphetamines are the second most frequently prescribed psychostimulant for pediatric ADHD, and are becoming an increasingly popular alternative for children who fail to respond to methylphenidate (Buck 2002). There are currently three different amphetamine preparations available, including: dexamphetamine (dextroamphetamine or d‐amphetamine sulfate), which comes in both short‐acting and long‐acting formulations; lisdexamphetamine, which is available as a long‐acting formulation (Vyvanase); and mixed amphetamine salts, which also comes in both short‐acting as well as long‐acting preparations (Buck 2002; The Medical Letter 2007).
How the intervention might work
Although the pathophysiology of ADHD is poorly understood, evidence has suggested that ADHD may be the result of insufficient production of norepinephrine and dopamine in the prefrontal cortex (Arnsten 2006). As such, the executive functions carried out by the prefrontal cortex are impaired, resulting in forgetfulness, distractibility, impulsivity, and inappropriate social behaviours (Anderson 1999). Others believe that the limbic system plays a major role in the pathophysiology of ADHD, and it is thought that hyperactivity and impulsivity result from abnormally low tonic dopamine activity within this region of the brain (Moore 2011). In either case, as a psychostimulant, amphetamines are thought to both promote marked neurotransmitter release into the synaptic cleft as well as disrupt normal reuptake of neurotransmitters, thereby increasing levels of norepinephrine and dopamine in these regions of the brain and affecting executive functioning (Arnsten 2006; Swanson 2007). A Cochrane Review of amphetamines for ADHD in adults found they improved short‐term symptom severity (Castells 2011).
Why it is important to do this review
Despite being one of the most thoroughly researched disorders in medicine, one of the major controversies regarding ADHD is the use of psychostimulants as a treatment option. While current evidence suggests that amphetamines may be beneficial for improving the core symptoms of ADHD, their effects on academic and social domains remain inconsistent and unclear (NIH 2000). Wide variations in the use and prescription of amphetamines across communities suggest that there is a lack of consensus among practitioners regarding which people with ADHD should be treated with amphetamines. Charach 2011 and Miller 1999 have conducted reviews assessing amphetamines for pediatric ADHD; however, the former focused only on long‐term effectiveness of amphetamines (i.e. > 12 months), while the latter is not only out of date, but also focused solely on the dexamphetamine preparation. It is imperative for healthcare providers, parents, and those diagnosed with ADHD to be aware of the most suitable treatment options available, and how they differ in terms of their efficacy and safety profiles. Our synthesis of all available, randomized controlled trials assessing the efficacy and safety of amphetamines for pediatric ADHD will provide evidence to better inform clinical practice and further research relating to ADHD management. While assessing amphetamines against other ADHD treatments, such as methylphenidate, psychotherapy and antidepressants is important, establishing whether amphetamines are superior to placebo is a necessary first step. Thus, this review will focus only on the amphetamine versus placebo comparison.
Objectives
To assess the efficacy and safety of amphetamines for ADHD in children and adolescents.
Methods
Criteria for considering studies for this review
Types of studies
Parallel‐group and cross‐over randomized controlled trials (RCTs).
Types of participants
Children and adolescents under 18 years of age and diagnosed with ADHD using specified diagnostic criteria such as the Diagnostic and Statistical Manual of Mental Disorders Third Edition (DSM‐III) (APA 1987), Fourth Edition (DSM‐IV) (APA 2000), or equivalent (note: since the fifth edition (DSM‐5) was released during the conduct of this review, studies utilizing this criteria are not included). We included trials that involved children/adolescents with some comorbid conditions (oppositional defiant disorder, conduct disorder, and anxiety). We excluded trials whose inclusion criteria included children/adolescents with psychiatric comorbidity that require highly specialized treatment programs (for example, autism, bipolar disorder, and psychosis).
Types of interventions
Intervention
Any oral form of amphetamine (i.e. amphetamine, dexamphetamine, lisdexamphetamine and mixed amphetamine salts), at any dose.
Control
Placebo.
Types of outcome measures
Primary outcomes
Change in core ADHD symptoms* (inattention, hyperactivity, impulsivity), as measured by a validated scale rated by children, parents, teachers, clinicians, or investigators such as Conners’ Parent Rating Scale ‐ Revised (CPRS‐R) (Conners 1998a), Conners’ Teacher Rating Scale ‐ Revised (CTRS‐R) (Conners 1998b), or the ADHD Rating Scale, Fourth Version (ADHD‐RS‐IV) (DuPaul 1998).
Secondary outcomes
Clinical improvement*, as measured by, for example, the Clinical Global Impression ‐ Improvement scale (CGI‐I) (Guy 1976).
Clinical severity, as measured by, for example, the Clinical Global Impression ‐ Severity scale (CGI‐S) (Guy 1976).
Academic performance*, as measured by any validated tool that purports to assess academic performance such as the Wechsler Intelligence Scale for Children (WISC) (Wechsler 1991).
Quality of life, as measured by a validated scale such as the Pediatric Quality of Life Inventory ‐ 32 (PedsQL‐32) (Varni 1998).
Retention: proportion of randomized participants who completed the trial*.
-
Adverse events (such as nausea, insomnia/sleep problems, and decreased appetite).
Proportion of adverse events.
Proportion of participants who experienced at least one adverse event*, as reported in the trials.
Proportion of participants who withdrew due to any adverse event.
Outcomes marked with an asterisk (*) were used to populate Table 1.
Time frames were denoted as short term (up to six months), medium term (between six and 12 months), and long term (over 12 months).
See Table 2 for further information.
1. Protocol decisions not used in this review.
| Types of outcome measures |
Primary outcomes Multiple perspectives (i.e. teacher, parent, clinician) are considered the gold standard when assessing the core symptoms of ADHD. As such, we will not favor one perspective over another. In the event that reports do not agree with one another, for example, teacher reports disagree with parent reports on the improvement of core symptoms, this may be quite telling about how a child’s environment impacts their ADHD given the varying demands between a school environment and home environment. This will be interpreted accordingly in the discussion. Secondary outcomes We will assess 'parental stress' as a secondary outcome. |
| Measures of treatment effect |
Dichotomous outcome data When a single study has utilized more than one measure to assess the same construct (e.g. ADHD core symptoms as assessed by teacher‐rated ADHD‐RS‐IV and teacher ratings of the Conners’ ADHD Rating Scale), treatment effects will be averaged across outcome measures in order to arrive at a single treatment effect for use in the meta‐analysis. Continuous outcome data For continuous outcomes, where the same rating scale has been used for all studies, we will calculate mean differences. |
| Unit of analysis issues |
Cross‐over trials For meta‐analyses that use that use a mean difference, we will compute standard deviations for the cross‐over trials taking into account correlation. If correlation coefficients are not available, we will impute them from other studies or use 0.5 as a conservative estimate (Follman 1992). For cross‐over trials where carry‐over is thought to be a problem, where no washout period is present, or when only data from the first period are available, we will analyze data from the first period only. Studies with multiple time points In studies where results are presented for several periods of follow‐up, we will analyze each outcome at each point in a separate meta‐analysis with other comparable studies taking measures at a similar time frame post‐randomization. Time frames will reflect short‐term (up to six months), medium‐term (between 6 months and 12 months), and long‐term (over 12 months) outcomes. |
| Assessment of reporting biases | For each primary meta‐analysis in which we have identified a sufficient number of studies (n ≥ 10) for inclusion, we will draw funnel plots in order to assess the possibility of publication bias. |
| Subgroup analysis and investigation of heterogeneity | We will conduct the following subgroup analyses.
|
| Sensitivity analysis | We will conduct the following sensitivity analyses.
|
ADHD: attention deficit hyperactivity disorder. ADHD‐RS‐IV: Attention Deficit Hyperactivity Rating Scale, Fourth Version.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases in May 2013, July 2014, and again on 12 August 2015.
Cochrane Central Register of Controlled Trials (CENTRAL; 2015 Issue 7; Ovid), which includes the Specialised Register of the Cochrane Developmental, Psychosocial and Learning Problems Group.
Ovid MEDLINE (1948 to Week 1, August 2015).
Embase (1974 to Week 1, August 2015; Ovid).
PsycINFO (1806 to Week 1, August 2015; Ovid).
ProQuest Dissertations and Theses (all available years).
Networked Digital Library of Theses and Dissertations (ndltd.org; all available years).
ClinicalTrials.gov (clinicaltrials.gov; all available years).
No language or date restrictions were applied.
Please see Appendix 1 to Appendix 7 for our search strategies.
Searching other resources
We inspected the reference lists of identified RCTs and review articles to identify additional publications.
Data collection and analysis
Selection of studies
Two review authors (SP and LS) independently screened all titles and abstracts retrieved from the search to identify those that appeared to meet the inclusion criteria. The same authors then obtained the full‐text articles of those studies and assessed their eligibility. Disagreements were resolved by SV.
Data extraction and management
Two review authors (SP and LS) independently extracted data related to study methods, participant characteristics, and outcomes by using a pre‐designed data collection form. Disagreements were resolved through discussion. SP entered all relevant data into Review Manager (RevMan 2014).
We emailed study authors up to three times (minimum one month wait between contact) to obtain missing or unclear data.
Assessment of risk of bias in included studies
Using the Cochrane 'Risk of bias' tool (Higgins 2011a), two review authors (SP and LS) independently assessed each included study as being at low risk, high risk, or unclear (uncertain) risk of bias for each of the seven domains explained in Appendix 8. Disagreements were resolved through discussion.
Measures of treatment effect
Dichotomous outcome data
We calculated the risk ratio (RR) and 95% confidence intervals (CIs) for dichotomous outcomes.
Continuous outcome data
For continuous outcomes, we used the Hedges’ method to calculate standardized mean differences (SMDs) with individual study weights calculated as the inverse of the variance, presented with 95% CIs (Hedges 1994). To ensure that all scales were pointing in the same direction, we multiplied the mean value of one set by ‐1 (Deeks 2011). We combined change scores and endpoint scores, however when both types of scores were available in the same study, priority was given to change scores since they adjust for any imbalances in baseline characteristics.
See Table 2 for further methods archived for future updates of this review.
Unit of analysis issues
Cross‐over trials
Since we calculated SMDs for all our continuous outcomes, we treated cross‐over studies as if they were parallel and computed a pooled standard deviation. Although this method does not account for the correlation in cross‐over studies, it prevented any overestimation of effect sizes, which is desirable when computing SMDs. Carry‐over was not reported in any of the cross‐over studies.
See Table 2 for further methods archived for future updates of this review.
Studies with multiple comparisons
For studies with more than two independent comparisons, such as amphetamine versus placebo versus psychotherapy, we excluded the psychotherapy arm. We handled studies with multiple and correlated interventions, for example, lisdexamphetamine versus mixed amphetamine salts versus placebo, or 10 mg dexamphetamine versus 20 mg of dexamphetamine versus placebo in the following way. For continuous outcomes of parallel‐group studies, we calculated means using the formulae described in Table 7.7.a of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). For dichotomous outcomes of parallel‐group studies, we summed the number of events across intervention arms. For continuous outcomes of cross‐over studies, we averaged both the means and the standard deviations of the relevant intervention arms across the groups. For dichotomous outcomes of cross‐over studies, we randomly dropped one arm and used the other in the meta‐analysis.
Studies with multiple time points
We analysed studies separately according to their time frame. Time frames were denoted as short term (up to six months), medium term (between six and 12 months), and long term (over 12 months). All but one study (Gillberg 1997) were considered short term. Since Gillberg 1997 was the only medium‐term study, it was excluded from the meta‐analysis.
See Table 2 for further analyses archived for future updates of this review.
Dealing with missing data
We emailed study authors up to three times (with at least one month between contacts) to obtain missing data. For those studies that did not report outcomes using intention‐to‐treat analysis and for which missing data were unobtainable, we used the number of randomized participants as the denominator for dichotomous variables. For continuous outcomes, we used the sample size to calculate the mean and standard deviations in the study. For studies that did not report standard deviations, we calculated it from P values, CIs, or standard errors (as described in section 7.7.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b)). We did not use any imputations to deal with missing data.
Assessment of heterogeneity
We assessed statistical heterogeneity by examining the I² statistic (Higgins 2003), which quantifies the degree of heterogeneity in a meta‐analysis, and Chi² statistic (P value less than 0.10 as evidence of heterogeneity). We also reported Tau² estimates for each random‐effects meta‐analysis (Deeks 2011).
We explored heterogeneity by conducting a series of subgroup analyses (Subgroup analysis and investigation of heterogeneity), which were selected a priori and based on preliminary evidence from other studies (Castells 2011; Lundh 2012).
Assessment of reporting biases
See Table 2 for methods archived for future updates of this review.
Data synthesis
We synthesized the results in a meta‐analysis using the random‐effects model since studies were fairly heterogeneous in terms of their study design (inclusion of parallel‐group and cross‐over trials), intervention protocols, and study duration. We used the inverse variance method for continuous outcomes, and the Mantel‐Haenszel method for dichotomous outcomes.
Summary of findings
In Table 1, we present data on the following outcomes: total ADHD symptom score ‐ parent ratings, total ADHD symptom score ‐ teacher ratings, total ADHD symptom score ‐ clinician ratings, proportion of responders, academic performance, proportion of participants who completed the trial, and proportion of participants who experienced at least one adverse event. We presented continuous outcomes as SMDs and 95% CIs, and dichotomous outcomes as RRs and 95% CIs. Data regarding number of participants and studies were presented for each outcome. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE Working Group 2004) to determine the quality of the evidence, where evidence was downgraded if (1) the majority (> 50%) of included studies had a high risk of bias; (2) the outcome included comparisons of different amphetamine derivatives and release formulations; (3) the outcome had significant statistical heterogeneity (I² > 50%); and (4) the outcome had wide 95% CIs indicating that the intervention effect was highly variable.
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses.
Type of amphetamine: dextroamphetamine, lisdexamphetamine, or mixed amphetamine salts.
Type of amphetamine release formulation: long acting (extended release) or short acting (immediate release).
Funding source: with or without pharmaceutical industry funding. Since some studies failed to report their funding source, we grouped studies as 'industry funded', 'publicly funded', or 'not reported'.
We conducted subgroup analyses on the following outcomes, which had a sufficient number of studies (more than five), regardless of the degree of statistical heterogeneity present in the main analysis:
total score on core symptom ADHD scale ‐ parent ratings;
proportion of responders according to CGI‐I (Guy 1976);
academic performance;
retention: proportion of participants who completed the trial;
-
proportion of participants who dropped out/withdrew due to an adverse event;
proportion of participants experiencing decreased appetite;
proportion of participants experiencing insomnia;
proportion of participants experiencing abdominal pain; and
proportion of participants experiencing headaches.
We calculated a pooled effect size for each subgroup.
We were unable to conduct a subgroup analysis when all of the studies in a particular meta‐analysis belonged to only one strata of any subgroup.
See Table 2 for further analyses archived for future updates of this review.
Sensitivity analysis
We repeated our meta‐analyses using a fixed‐effect model.
See Table 2 for further analyses archived for future updates of this review.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
Figure 1 summarizes the flow of studies through the screening process. The electronic databases retrieved 7011 records while other sources yielded 198 records. After removing duplicates, we identified 5210 records for further consideration. After screening titles and available abstracts, we examined the full texts of 324 records, 34 met our inclusion criteria. From these, we identified 23 studies, four of which had multiple reports. These were: Borcherding 1990 (four reports), Coghill 2013 (three reports), Donnelly 1989 (two reports; one of which was the pilot, and the other was the full study), and Ramtvedt 2013 (two reports). In addition, we identified two ongoing clinical trials (Fanton 2009; NCT01711021); although recruitment has ended for both of these trials, none of the results have been published. We contacted the authors of both studies three times yielding no response. One non‐English language study is awaiting classification until we can ascertain if it was randomized and whether participants had a formal diagnosis of ADHD (Glos 1973). This information is unobtainable given our inability to contact the author. Another study also awaits classification as only the abstract has been published (Itil 1974). Information on whether treatments were randomized and whether participants had a formal diagnosis is needed. We contacted the authors three times yielding no response.
1.
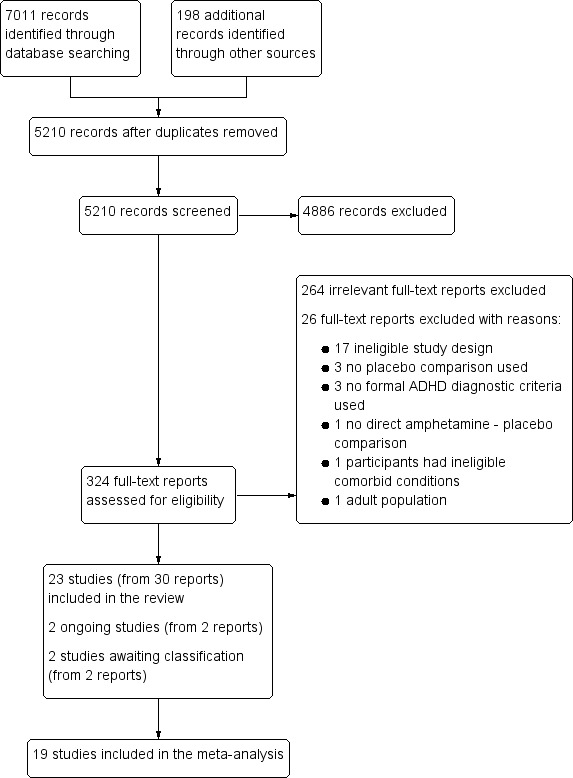
Study flow diagram
Included studies
Twenty‐three studies met the inclusion criteria: 15 studies were cross‐over trials (Barkley 2000; Biederman 2007a; Borcherding 1990; Childress 2015; Donnelly 1989; James 2001; Manos 1999; McCracken 2003; Nemzer 1986; Ramtvedt 2013; Sharp 1999; Shekim 1986; Short 2004; Swanson 1998a; Wigal 2009a), while eight studies were parallel‐group trials (Biederman 2002; Biederman 2007b; Coghill 2013; Findling 2011; Giblin 2011; Gillberg 1997; Pliszka 2000; Spencer 2006a).
Ten studies included a single comparison of an amphetamine derivative versus placebo (Borcherding 1990; Childress 2015; Coghill 2013; Donnelly 1989; Gillberg 1997; Nemzer 1986; Pliszka 2000; Ramtvedt 2013; Sharp 1999; Shekim 1986), and 11 studies compared more than one dose of an amphetamine derivative with placebo (Barkley 2000; Biederman 2002; Biederman 2007b; Findling 2011; Giblin 2011; Manos 1999; McCracken 2003; Short 2004; Spencer 2006a; Swanson 1998a; Wigal 2009a); two studies compared more than one amphetamine derivative, each at various doses, versus placebo (Biederman 2007a; James 2001).
Participants
In total, the 23 included studies recruited 2675 children and adolescents aged between three years and 17 years, 72% (n = 1925) of whom were boys; one study did not report the number of included boys and girls (Pliszka 2000).
Twenty‐two studies used various versions of the DSM criteria to confirm ADHD diagnosis in their participants, including criteria from the Third Edition (DSM‐III; four trials; n = 102; Borcherding 1990; Donnelly 1989; Nemzer 1986; Shekim 1986); Third Edition Revised (DSM‐III‐R; one trial; n = 62; Gillberg 1997); Fourth Edition (DSM‐IV; eight trials; n = 899; Barkley 2000; Biederman 2002; James 2001; Manos 1999; McCracken 2003; Sharp 1999; Short 2004; Swanson 1998a), and Fourth Edition, Text Revision (DSM‐IV‐TR; nine trials; n = 1553; Biederman 2007a; Biederman 2007b; Childress 2015; Coghill 2013; Findling 2011; Giblin 2011; Ramtvedt 2013; Spencer 2006a; Wigal 2009a). Pliszka 2000 (n = 59) diagnosed ADHD using the Diagnostic Interview Schedule for Children (Costello 1985).
Interventions
Twelve studies assessed mixed amphetamine salts (Barkley 2000; Biederman 2002; Biederman 2007a; Childress 2015; Gillberg 1997; James 2001; Manos 1999; McCracken 2003; Pliszka 2000; Short 2004; Spencer 2006a; Swanson 1998a); seven studies used dextroamphetamine (Borcherding 1990; Donnelly 1989; James 2001; Nemzer 1986; Ramtvedt 2013; Sharp 1999; Shekim 1986); and six studies looked at lisdexamphetamine (Biederman 2007a; Biederman 2007b; Coghill 2013; Findling 2011; Giblin 2011; Wigal 2009a). Two studies assessed two amphetamine derivatives (Biederman 2007a; James 2001).
Twelve studies randomized children and adolescents to set doses or dosing schedules (Barkley 2000; Biederman 2002; Biederman 2007b; Coghill 2013; Findling 2011; Giblin 2011; Manos 1999; McCracken 2003; Ramtvedt 2013; Short 2004; Spencer 2006a; Swanson 1998a). Seven studies used weight‐based dosing (Borcherding 1990; Donnelly 1989; James 2001; Nemzer 1986; Pliszka 2000; Sharp 1999; Shekim 1986), while six studies titrated children and adolescents to their optimal dose (Biederman 2007a; Childress 2015; Gillberg 1997; Pliszka 2000; Shekim 1986; Wigal 2009a). Two studies used both weight‐based dosing and titration (Pliszka 2000; Shekim 1986). The mean (range) doses investigated in the included studies were 34.22 mg/day (7.8 mg/day to 90 mg/day) for dextroamphetamine, 50.24 mg/day (30 mg/day to 70 mg/day) for lisdexamphetamine, and 19.86 mg/day (5 mg/day to 120 mg/day) for mixed amphetamine salts.
Duration
Study intervention length ranged from 14 days to 365 days, with a median of 28 days. Only one study was longer than 63 days (Gillberg 1997).
Location
Twenty studies were conducted in the United States (Barkley 2000; Biederman 2002; Biederman 2007a; Biederman 2007b; Borcherding 1990; Childress 2015; Donnelly 1989; Findling 2011; Giblin 2011; James 2001; Manos 1999; McCracken 2003; Nemzer 1986; Pliszka 2000; Sharp 1999; Shekim 1986; Short 2004; Spencer 2006a; Swanson 1998a; Wigal 2009a). One multicenter trial was conducted in 48 centers across 10 countries: Belgium, France, Germany, Hungary, Italy, Netherlands, Poland, Spain, Sweden, and the United Kingdom (Coghill 2013). The two remaining studies were conducted in Sweden (Gillberg 1997) and Norway (Ramtvedt 2013).
Outcomes
Details of all ADHD core symptom outcome measures used by study can be found in the Characteristics of included studies and Table 3. The most commonly used outcome tool for the primary outcome included the Conners' Rating Scales (Conners 1998a; Conners 1998b) and the ADHD‐RS‐IV (DuPaul 1998). For secondary outcomes, the most commonly utilized outcome tool for academic performance was the Permanent Product Measure of Performance (PERMP; Swanson 1998b). Only one study assessed quality of life (Findling 2011), and used the Youth Quality of Life ‐ Research Version questionnaire (YQOL‐R; Salum 2012)
2. ADHD core symptom outcome measures by study.
| Outcome | Outcome measure (respondent) | Studies | Measure used in meta‐analysis |
| Inattention | ADHD Rating Scale, Fourth Version (parent ratings) | Biederman 2007b | No (data presented in an unusable format) |
| ADHD Rating Scale, Fourth Version (clinician ratings) | Findling 2011 | Yes | |
| Spencer 2006a | Yes | ||
| Wigal 2009a | Yes | ||
| ADHD Rating Scale, Fourth Version (investigator/research personnel ratings) | Coghill 2013 | Yes | |
| Conners’ Rating Scale (parent ratings) | Borcherding 1990 | Yes | |
| Gillberg 1997 | No (only study that included long‐term data) | ||
| Conners’ Rating Scale (teacher ratings) | Gillberg 1997 | No (only study that included long‐term data) | |
| IOWA Conners’ Rating Scale | Pliszka 2000 | Yes | |
| SKAMP scale (teacher ratings) | Swanson 1998a | No (data not available) | |
| SKAMP scale (investigator/research personnel ratings) | Biederman 2007a | Yes | |
| McCracken 2003 | Yes | ||
| Hyperactivity/impulsivity | ADHD Rating Scale, Fourth Version (parent ratings) | Biederman 2007b | No (data presented in an unusable format) |
| ADHD Rating Scale, Fourth Version (clinician ratings) | Findling 2011 | Yes | |
| Spencer 2006a | Yes | ||
| Wigal 2009a | Yes | ||
| ADHD Rating Scale, Fourth Version (investigator/research personnel ratings) | Coghill 2013 | Yes | |
| Conners’ Rating Scale (parent ratings) | Gillberg 1997 | No (only study that included long‐term data) | |
| James 2001 | Yes | ||
| Conners’ Rating Scale (teacher ratings) | Gillberg 1997 | No (only study that included long‐term data) | |
| James 2001 | Yes | ||
| Total core symptom score | ADHD Rating Scale, Fourth Version (parent ratings) | Barkley 2000 | Yes |
| Biederman 2007b | Yes | ||
| ADHD Rating Scale, Fourth Version (teacher ratings) | Barkley 2000 | Yes | |
| ADHD Rating Scale, Fourth Version (clinician ratings) | Findling 2011 | Yes | |
| Spencer 2006a | Yes | ||
| Wigal 2009a | Yes | ||
| ADHD Rating Scale, Fourth Version (investigator/research personnel ratings) | Coghill 2013 | Yes | |
| Giblin 2011 | No (no data available) | ||
| Conners’ Rating Scale (parent ratings) | Biederman 2007b | No (data presented in an unusable format) | |
| Coghill 2013 | Yes | ||
| Giblin 2011 | No (data not available) | ||
| Gillberg 1997 | No (only study that included long‐term data) | ||
| Nemzer 1986 | Yes | ||
| Sharp 1999 | No (data not available) | ||
| Short 2004 | No (data presented in an unusable format) | ||
| Conners’ Rating Scale (teacher ratings) | Borcherding 1990 | No (no data available) | |
| Donnelly 1989 | Yes | ||
| Gillberg 1997 | No (only study that included long‐term data) | ||
| Nemzer 1986 | Yes | ||
| Sharp 1999 | No (data not available) | ||
| Short 2004 | No (data presented in an unusable format) | ||
| Conners’ Global Index (parent ratings) | Biederman 2002 | Yes | |
| Pliszka 2000 | Yes | ||
| Conners’ Global Index (teacher ratings) | Biederman 2002 | Yes | |
| Conners’ Abbreviated Symptom Questionnaire (parent ratings) | Manos 1999 | Yes | |
| Conners’ Abbreviated Symptom Questionnaire (teacher ratings) | Manos 1999 | Yes | |
| ADHD Questionnaire (developed within study) (parent ratings) | Ramtvedt 2013 | No (data presented in an unusable format) | |
| ADHD Questionnaire (developed within study) (teacher ratings) | Ramtvedt 2013 | No (data presented in an unusable format) | |
| SKAMP scale (investigator/research personnel ratings) | Childress 2015 | Yes |
ADHD: attention deficit hyperactivity disorder. IOWA: inattention/overactivity with aggression. SKAMP: Swanson, Kotkin, Agler, M‐Flynn and Pelham scale.
Excluded studies
We excluded a total of 290 studies. We excluded 264 clearly irrelevant reports and formally excluded 26 studies for the following reasons: 17 studies because they were not RCTs or used multiple cross‐over designs (this review only included single cross‐over RCTs); three studies because there was no placebo comparison, three studies because they did not use formal ADHD diagnostic criteria; one study because it had no direct amphetamine ‐ placebo comparison; one study because participants had ineligible comorbid conditions; and one study because it included adults.
See also Characteristics of excluded studies tables.
Risk of bias in included studies
A more in depth risk of bias assessment for each study can be found in Characteristics of included studies. In addition, Figure 2 provides a summary of this assessment.
2.
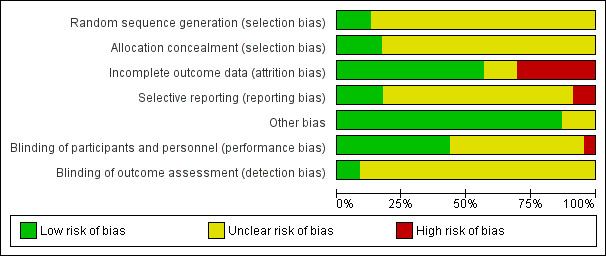
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Only three studies reported on how the random sequence was generated and were assessed as being at 'low' risk of bias on this domain (Biederman 2007b; Childress 2015; Findling 2011). We rated the other 20 studies as 'unclear' risk of bias as they did not adequately describe their methods of randomization.
Allocation concealment
Four studies described the methods used to conceal the allocation sequence and were rated as being at 'low' risk of bias on this domain (Biederman 2007a; Coghill 2013; Findling 2011; Manos 1999). The rest of the studies we assessed as 'unclear' risk of bias, as they did not sufficiently describe their methods of allocation concealment.
Blinding
Performance bias
Although blinding was intended in all of the studies, we assessed risk of bias by how authors described their amphetamine and placebo capsules and rated 10 studies as being at 'low' risk of bias on this domain (Biederman 2007b; Coghill 2013; James 2001; Manos 1999; Nemzer 1986; Pliszka 2000; Sharp 1999; Short 2004; Swanson 1998a; Wigal 2009a). We rated one study as being at 'high' risk of bias on this domain since they described their intervention and placebo as not being identical (Ramtvedt 2013). The other 12 studies we marked as being at 'unclear' risk of bias since they were not explicit about the similarities between the two interventions.
Detection bias
Only two studies explicitly stated that outcome assessors were blinded to interventions and therefore we judged them to be at 'low' risk of bias (Manos 1999; Short 2004). The other 21 studies we rated as 'unclear' risk of bias since they were not explicit about which parties were blinded to the intervention assignment.
Incomplete outcome data
We rated thirteen studies that adequately addressed dropouts and used appropriate statistical methods to compensate for dropouts as having a 'low' risk of bias on this domain (Biederman 2002; Biederman 2007a; Biederman 2007b; Childress 2015; Donnelly 1989; Findling 2011; Gillberg 1997; James 2001; McCracken 2003; Pliszka 2000; Sharp 1999; Spencer 2006a; Wigal 2009a). Seven studies failed to provide reasons for dropouts and failed to address any exclusions from their analyses, and therefore we rated them as having a 'high' risk of bias (Barkley 2000; Borcherding 1990; Coghill 2013; Ramtvedt 2013; Shekim 1986; Short 2004; Swanson 1998a). The three remaining studies did not discuss dropouts in their reports and we rated them as being at 'unclear' risk of bias (Giblin 2011; Manos 1999; Nemzer 1986).
Selective reporting
We assessed 17 studies as having 'unclear' risk of bias on this domain as the study protocols for most of them were not available, so we could not assess reporting bias (Barkley 2000; Biederman 2002; Biederman 2007b; Borcherding 1990; Donnelly 1989; Giblin 2011; Gillberg 1997; James 2001; Manos 1999; McCracken 2003; Nemzer 1986; Pliszka 2000; Sharp 1999; Shekim 1986; Short 2004; Swanson 1998a; Wigal 2009a). We rated four studies as having a 'low' risk of bias, as they appropriately reported on all outcomes defined in their protocols (Biederman 2007a; Childress 2015; Findling 2011; Spencer 2006a). Two studies we assessed as having a 'high' risk of bias since they failed to report on all outcomes mentioned in their registered protocols (Coghill 2013; Ramtvedt 2013).
Other potential sources of bias
We rated three studies as being at 'unclear' risk of bias on this domain since the validity of their primary outcome tools were not described (Borcherding 1990; Donnelly 1989; Ramtvedt 2013). The other 20 studies appeared to be free of other potential sources of bias and therefore we rated them as being at 'low' risk of bias on this domain.
Effects of interventions
See: Table 1
We included 19 studies in meta‐analyses, however, two of those studies had measured other outcomes that were relevant to this review, but were not reported in their results (Borcherding 1990; Swanson 1998a). Biederman 2007b had reported some of their results as bar graphs, which, when extracted using graphic digitizer software, gave implausible results and therefore was excluded from the meta‐analysis on those outcomes. Four studies were excluded from all meta‐analyses: Giblin 2011 had not reported data on any of the relevant outcomes in this review; Gillberg 1997 was the only medium‐term study and therefore could not be combined with the other short‐term studies; Ramtvedt 2013 had aggregated their parent‐ and teacher‐rated ADHD scores; and Short 2004 assessed amphetamines versus methylphenidate versus placebo and pooled the amphetamine and methylphenidate data in their results; we were unable to isolate the amphetamine versus placebo comparison.
Primary outcome
Change in core ADHD symptoms
We conducted a series of meta‐analyses for the primary outcome, change in core ADHD symptoms (inattention, hyperactivity, impulsivity), as measured by a validated scale rated by children, parents, teachers, clinicians, or investigators (Analysis 1.1 to Analysis 1.11).
1.1. Analysis.
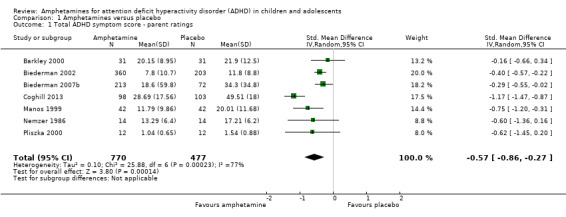
Comparison 1 Amphetamines versus placebo, Outcome 1 Total ADHD symptom score ‐ parent ratings.
1.11. Analysis.
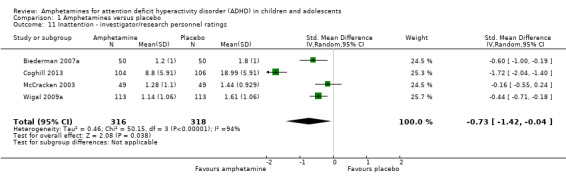
Comparison 1 Amphetamines versus placebo, Outcome 11 Inattention ‐ investigator/research personnel ratings.
For all 11 outcomes, amphetamines were superior to placebo for reducing the core symptoms of ADHD.
Total ADHD symptom score ‐ parent ratings (SMD ‐0.57; 95% CI ‐0.86 to ‐0.27; Tau² = 0.10; I² = 77%; 7 studies; 1247 children/adolescents; Analysis 1.1).
Hyperactivity/impulsivity ‐ parent ratings (SMD ‐0.54; 95% CI ‐0.89 to ‐0.19; Tau² = 0.00; I² = 0%; 2 studies; 132 children/adolescents; Analysis 1.2).
Total ADHD symptom score ‐ teacher ratings (SMD ‐0.55; 95% CI ‐0.83 to ‐0.27; Tau² = 0.04; I² = 41%; 5 studies; 745 children/adolescents; Analysis 1.3).
Hyperactivity/impulsivity ‐ teacher ratings (SMD ‐1.13; 95% CI ‐1.63 to ‐0.62; 1 study; 70 children/adolescents; Analysis 1.4).
Inattention ‐ teacher ratings (SMD ‐1.43; 95% CI ‐2.35 to ‐0.52; 1 study; 24 children/adolescents; Analysis 1.5).
Total ADHD symptom score ‐ clinician ratings (SMD ‐0.84; 95% CI ‐1.32 to ‐0.36; Tau² = 0.16; I² = 88%; 3 studies; 813 children/adolescents; Analysis 1.6).
Hyperactivity/impulsivity ‐ clinician ratings (SMD ‐0.75; 95% CI ‐1.28 to ‐0.23; Tau² = 0.20; I² = 90%; 3 studies; 813 children/adolescents; Analysis 1.7).
Inattention ‐ clinician ratings (SMD ‐0.78; 95% CI ‐1.26 to ‐0.30; Tau² = 0.16; I² = 88%; 3 studies; 813 children/adolescents; Analysis 1.8).
Total ADHD symptom score ‐ investigator/research personnel ratings (SMD ‐1.15; 95% CI ‐1.87 to ‐0.44; Tau² = 0.37; I² = 94%; 3 studies; 630 children/adolescents; Analysis 1.9).
Hyperactivity/impulsivity ‐ investigator/research personnel ratings (SMD ‐1.46; 95% CI ‐ 1.83 to ‐1.08; Tau² = 0.03; I² = 41%; 2 studies; 280 children/adolescents; Analysis 1.10).
Inattention ‐ investigator/research personnel ratings (SMD ‐0.73; 95% CI ‐1.42 to ‐0.04; Tau² = 0.46; I² = 94%; 4 studies; 634 children/adolescents; Analysis 1.11).
1.2. Analysis.
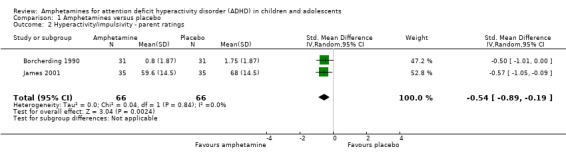
Comparison 1 Amphetamines versus placebo, Outcome 2 Hyperactivity/impulsivity ‐ parent ratings.
1.3. Analysis.
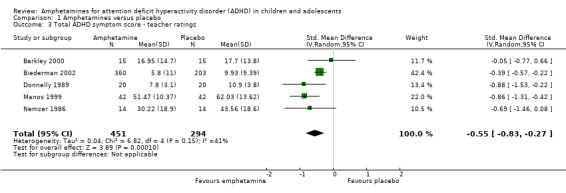
Comparison 1 Amphetamines versus placebo, Outcome 3 Total ADHD symptom score ‐ teacher ratings.
1.4. Analysis.
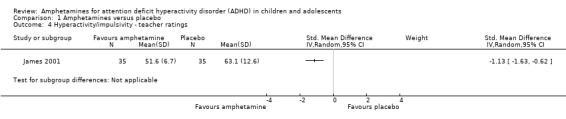
Comparison 1 Amphetamines versus placebo, Outcome 4 Hyperactivity/impulsivity ‐ teacher ratings.
1.5. Analysis.

Comparison 1 Amphetamines versus placebo, Outcome 5 Inattention ‐ teacher ratings.
1.6. Analysis.
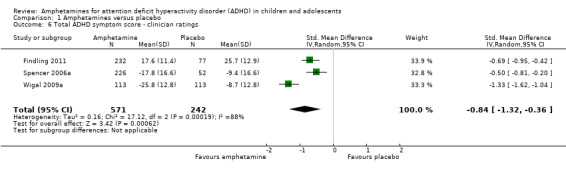
Comparison 1 Amphetamines versus placebo, Outcome 6 Total ADHD symptom score ‐ clinician ratings.
1.7. Analysis.
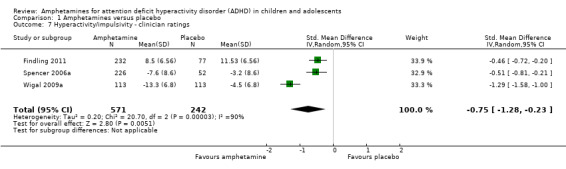
Comparison 1 Amphetamines versus placebo, Outcome 7 Hyperactivity/impulsivity ‐ clinician ratings.
1.8. Analysis.
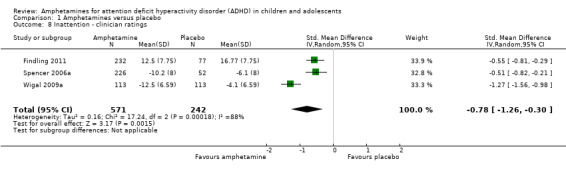
Comparison 1 Amphetamines versus placebo, Outcome 8 Inattention ‐ clinician ratings.
1.9. Analysis.
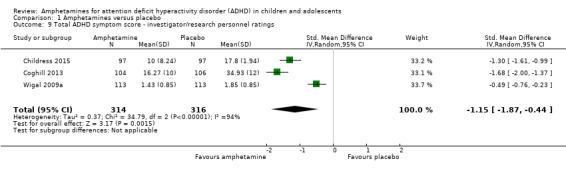
Comparison 1 Amphetamines versus placebo, Outcome 9 Total ADHD symptom score ‐ investigator/research personnel ratings.
1.10. Analysis.
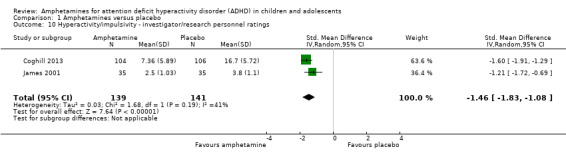
Comparison 1 Amphetamines versus placebo, Outcome 10 Hyperactivity/impulsivity ‐ investigator/research personnel ratings.
It is important to note, however, that the majority of these meta‐analyses included between one and three studies, and that Analysis 1.1, Analysis 1.6, Analysis 1.7, Analysis 1.8, Analysis 1.9, and Analysis 1.11 had considerable heterogeneity present with I² ranging from 77% to 94%. Only three outcomes included more than three studies: total ADHD symptom score ‐ parent ratings (seven studies; Analysis 1.1; Figure 3), total ADHD symptom score ‐ teacher ratings (five studies; Analysis 1.3; Figure 4), and total ADHD symptom score ‐ investigator/research personnel ratings (four studies; Analysis 1.11).
3.
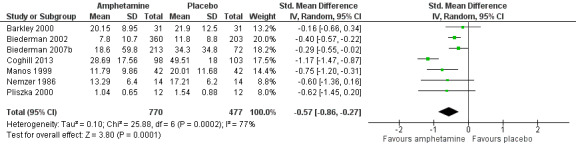
Forest plot of comparison: 1 Amphetamines versus placebo, outcome: 1.1 Total ADHD symptom score ‐ parent ratings.
4.
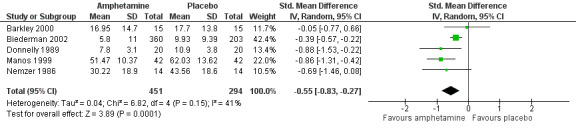
Forest plot of comparison: 1 Amphetamines versus placebo, outcome: 1.3 Total ADHD symptom score ‐ teacher ratings.
Secondary outcomes
We conducted meta‐analyses that compared amphetamines versus placebo on five of our six secondary outcomes (see Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.16).
1.12. Analysis.
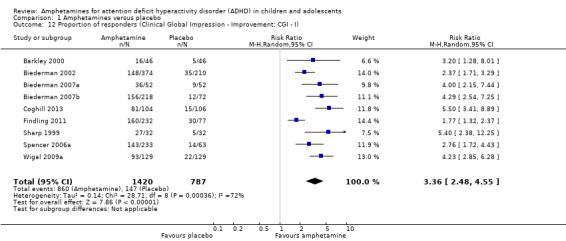
Comparison 1 Amphetamines versus placebo, Outcome 12 Proportion of responders (Clinical Global Impression ‐ Improvement; CGI ‐ I).
1.13. Analysis.
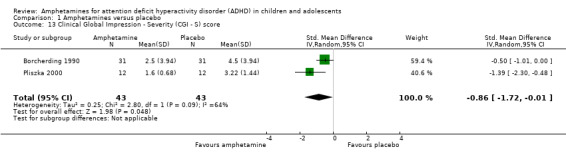
Comparison 1 Amphetamines versus placebo, Outcome 13 Clinical Global Impression ‐ Severity (CGI ‐ S) score.
1.14. Analysis.
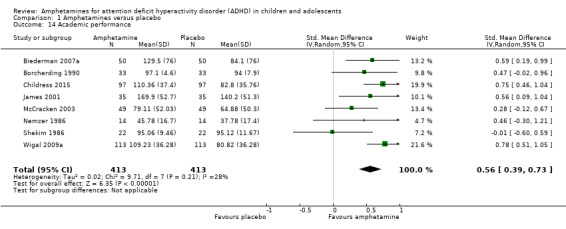
Comparison 1 Amphetamines versus placebo, Outcome 14 Academic performance.
1.15. Analysis.
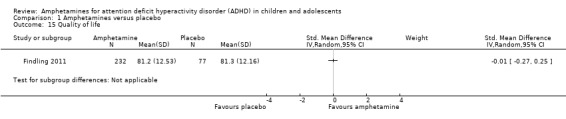
Comparison 1 Amphetamines versus placebo, Outcome 15 Quality of life.
1.16. Analysis.
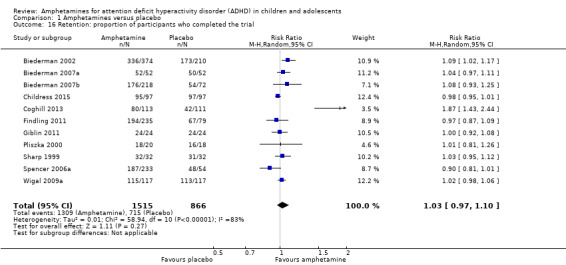
Comparison 1 Amphetamines versus placebo, Outcome 16 Retention: proportion of participants who completed the trial.
Clinical improvement
The proportion of responders was higher in the amphetamine group (RR 3.36; 95% CI 2.48 to 4.55; Tau² = 0.14; I² = 72%; 9 studies; 2207 children/adolescents; Analysis 1.12)
Clinical severity
We found evidence of a significant difference between the two groups on the CGI‐S (Guy 1976), in favour of amphetamine (SMD ‐0.86; 95% CI ‐1.72 to ‐0.01; Tau² = 0.25; I² = 64%; 2 studies; 86 children/adolescents; Analysis 1.13).
Academic performance
We found evidence that amphetamines may improve academic performance as compared to placebo (SMD 0.56; 95% CI 0.39 to 0.73; Tau² = 0.02; I² = 28%; 8 studies; 826 children/adolescents; Analysis 1.14).
Quality of life
As shown in the illustrative forest plot, the one study that provided data on quality of life (Findling 2011), found no difference between the two groups (SMD ‐0.01; 95% CI ‐0.27 to 0.25; 309 children/adolescents; see Analysis 1.15).
Retention
There was no difference between those given amphetamine and those given placebo for retention (RR 1.03; 95% CI 0.97 to 1.10; Tau² = 0.01; I² = 83%; 11 studies; 2381 children/adolescents; Analysis 1.16).
Adverse events
Proportion of adverse events
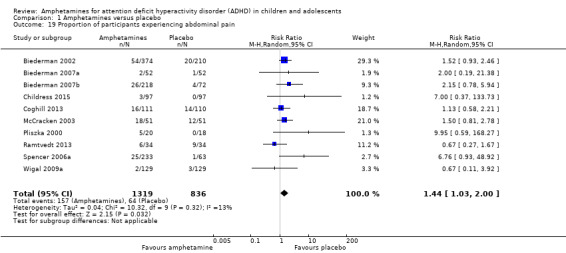
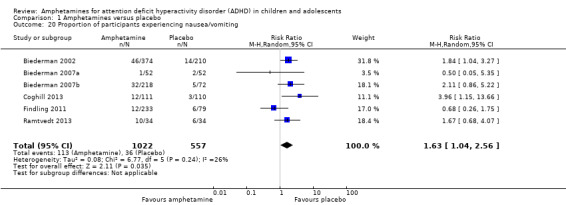
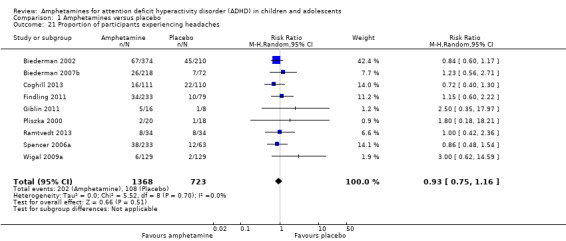
We performed a series of meta‐analyses of the most commonly reported adverse events. A higher proportion of children and adolescents in the amphetamine group as compared to placebo group experienced decreased appetite (RR 6.31; 95% CI 2.58 to 15.46; Tau² = 1.59; I² = 85%; 11 studies; 2467 children/adolescents; Analysis 1.17); insomnia/trouble sleeping (RR 3.80; 95% CI 2.12 to 6.83; Tau² = 0.42; I² = 59%; 10 studies; 2429 children/adolescents; Analysis 1.18); abdominal pain (RR 1.44; 95% CI 1.03 to 2.00; Tau² = 0.04; I² = 13%; 10 studies; 2155 children/adolescents; Analysis 1.19); and nausea/vomiting (RR 1.63; 95% CI 1.04 to 2.56; Tau² = 0.08; I² = 26%; 6 studies; 1579 children/adolescents; Analysis 1.20). There were no differences between the amphetamine and placebo groups in the proportion of children and adolescents who experienced headaches (RR 0.93; 95% CI 0.75 to 1.16; Tau² = 0.00; I² = 0%; 9 studies; 2091 children/adolescents; Analysis 1.21) and anxiety/nervousness (RR 1.22; 95% CI 0.78 to 1.93; Tau² = 0.09; I² = 32%; 5 studies; 1088 children/adolescents; Analysis 1.22).
1.17. Analysis.
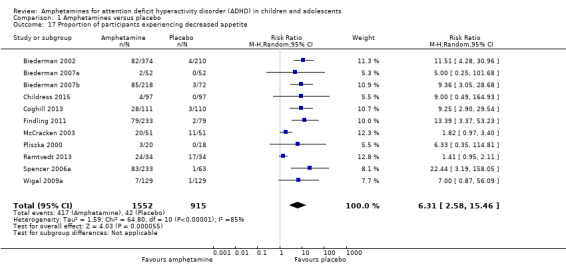
Comparison 1 Amphetamines versus placebo, Outcome 17 Proportion of participants experiencing decreased appetite.
1.18. Analysis.
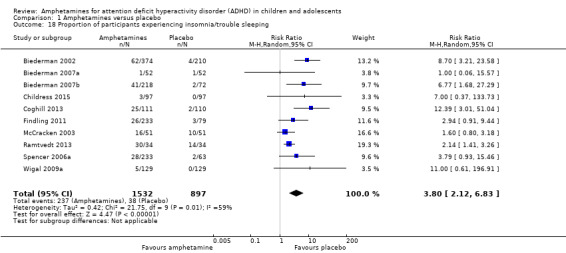
Comparison 1 Amphetamines versus placebo, Outcome 18 Proportion of participants experiencing insomnia/trouble sleeping.
1.19. Analysis.
Comparison 1 Amphetamines versus placebo, Outcome 19 Proportion of participants experiencing abdominal pain.
1.20. Analysis.
Comparison 1 Amphetamines versus placebo, Outcome 20 Proportion of participants experiencing nausea/vomiting.
1.21. Analysis.
Comparison 1 Amphetamines versus placebo, Outcome 21 Proportion of participants experiencing headaches.
1.22. Analysis.
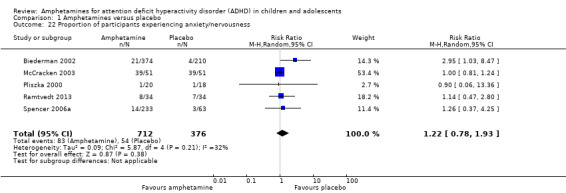
Comparison 1 Amphetamines versus placebo, Outcome 22 Proportion of participants experiencing anxiety/nervousness.
Proportion of participants who experienced at least one adverse event
The proportion of children and adolescents who experienced at least one adverse event was higher in the amphetamine group as compared to the placebo group (RR 1.30; 95% CI 1.18 to 1.44; Tau² = 0.00; I² = 1%; 6 studies; 1742 children/adolescents; see Analysis 1.23; Figure 5).
1.23. Analysis.
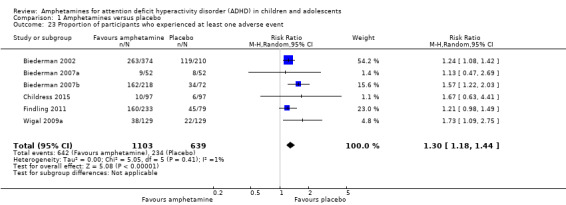
Comparison 1 Amphetamines versus placebo, Outcome 23 Proportion of participants who experienced at least one adverse event.
5.
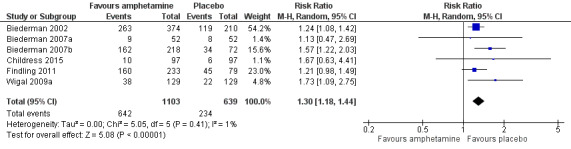
Forest plot of comparison: 1 Amphetamines versus placebo, outcome: 1.23 Proportion of participants who experienced at least one adverse event.
Proportion of participants who withdrew due to any adverse event
There was no difference between the amphetamine and placebo groups in the proportion of children and adolescents who withdrew due to an adverse event (RR 1.60; 95% CI 0.86 to 2.98; Tau² = 0.00; I² = 0%; 9 studies; 2160 children/adolescents; Analysis 1.24).
1.24. Analysis.
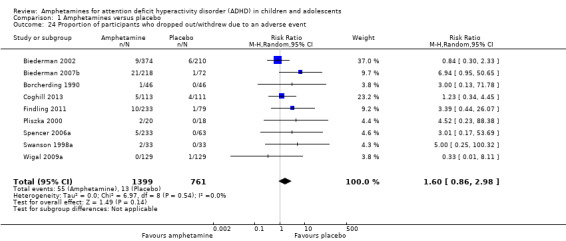
Comparison 1 Amphetamines versus placebo, Outcome 24 Proportion of participants who dropped out/withdrew due to an adverse event.
Subgroup analyses
Type of amphetamine
We conducted a series of subgroup analyses according to type of amphetamine (dexamphetamine, lisdexamphetamine, and mixed amphetamine salts). Mixed amphetamine salts were associated with improved parent ratings of total ADHD symptoms as compared to dexamphetamine and lisdexamphetamine, however, there appeared to be no between‐group differences (Chi² = 0.55, P value = 0.76; Analysis 2.1). Only one subgroup yielded between‐group differences (Chi² = 26.06, P < 0.00001; Analysis 2.6; Figure 6). There were no between‐group differences for any of the other outcomes.
2.1. Analysis.
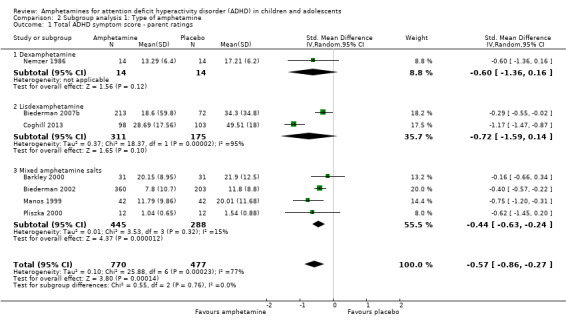
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 1 Total ADHD symptom score ‐ parent ratings.
2.6. Analysis.
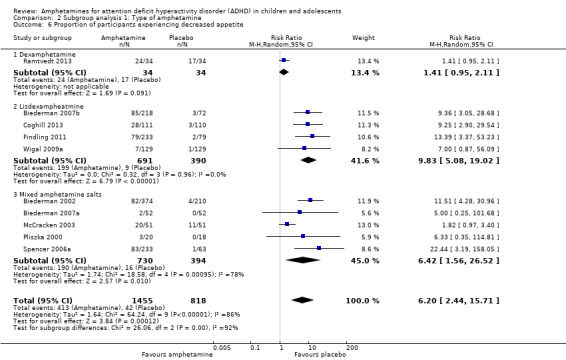
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 6 Proportion of participants experiencing decreased appetite.
6.
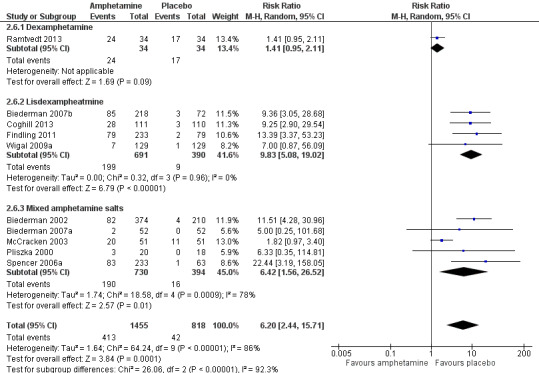
Forest plot of comparison: 2 Subgroup analysis 1: Type of amphetamine, outcome: 2.6 Proportion of participants experiencing decreased appetite.
Type of amphetamine release formulation
We conducted a subgroup analysis to explore the influence of long‐acting versus short‐acting amphetamine release formulations (Analysis 3.1 to Analysis 3.6). Both rentention: proportion of participants who completed the trial (Chi² = 4.50, P value = 0.03; Analysis 3.4) and proportion of participants experiencing decreased appetite (Chi² = 6.93, P value = 0.008; Analysis 3.5) yielded between‐group differences.
3.1. Analysis.
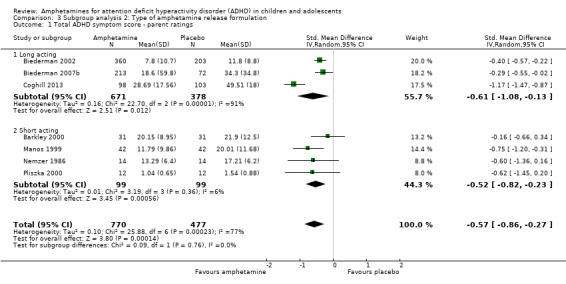
Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 1 Total ADHD symptom score ‐ parent ratings.
3.6. Analysis.
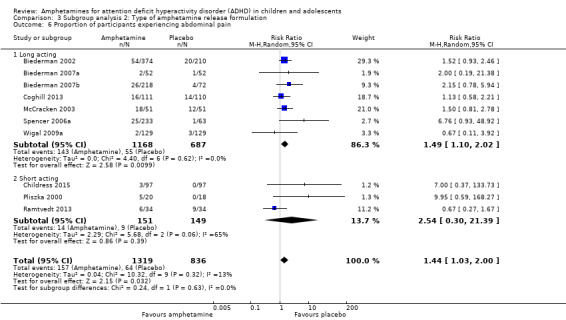
Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 6 Proportion of participants experiencing abdominal pain.
3.4. Analysis.
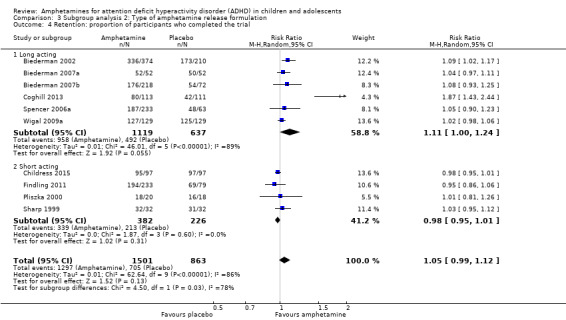
Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 4 Retention: proportion of participants who completed the trial.
3.5. Analysis.
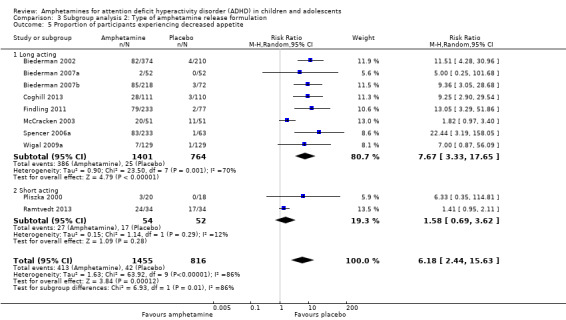
Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 5 Proportion of participants experiencing decreased appetite.
Funding source
We wanted to explore the influence of industry‐funded versus publicly‐funded sources (Analysis 4.1 to Analysis 4.3). Since five studies did not report their source of funding, we introduced another subgroup, 'not reported'. No between‐group differences were found.
4.1. Analysis.
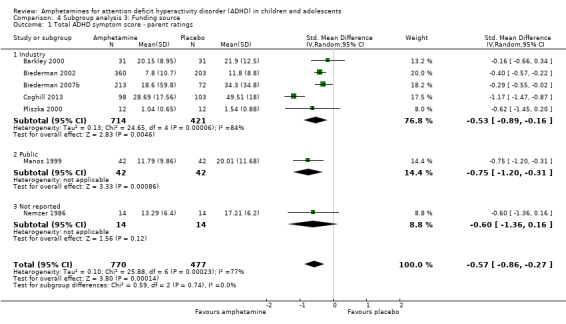
Comparison 4 Subgroup analysis 3: Funding source, Outcome 1 Total ADHD symptom score ‐ parent ratings.
4.3. Analysis.
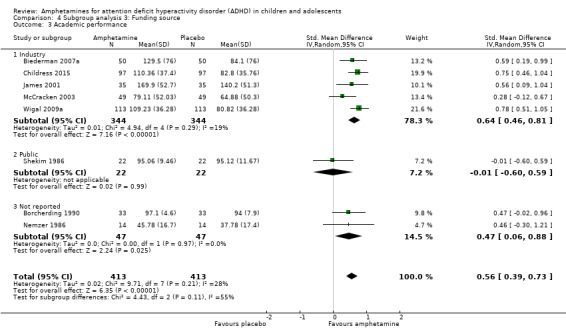
Comparison 4 Subgroup analysis 3: Funding source, Outcome 3 Academic performance.
Sensitivity analysis
We repeated the analyses by changing the statistical model from a random‐effects model to a fixed‐effect model. The results were similar (see Analysis 5.1 to Analysis 5.24).
5.1. Analysis.
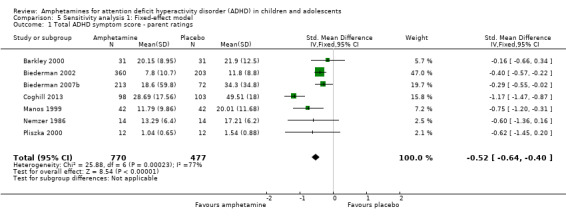
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 1 Total ADHD symptom score ‐ parent ratings.
5.24. Analysis.
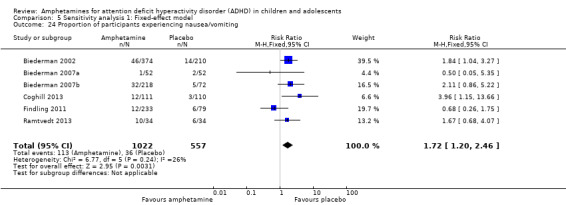
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 24 Proportion of participants experiencing nausea/vomiting.
Discussion
Summary of main results
We included 23 studies in this review, 19 of which we included in meta‐analyses. Overall, this review found that amphetamines were more efficacious than placebo for reducing ADHD core symptom severity in the short‐term, however, they did not influence retention in the trial and were associated with a number of adverse events. According to conventional cut‐offs (Cohen 1988), the largest effect sizes observed (i.e. an SMD > 0.8) were teacher ratings of hyperactivity/impulsivity, teacher ratings of inattention, and clinician ratings of total ADHD symptoms. The meta‐analyses with the most available data included parent and teacher ratings of total ADHD symptoms, both of which yielded low to moderate effect sizes.
The median study duration was only 28 days, therefore it was not possible to assess the long‐term efficacy and safety of amphetamines for pediatric ADHD. This is particularly problematic in a condition such as ADHD, which may require treatment for years.
There was a lot of variation in the amphetamine derivatives and release formulations utilized in the included studies. As such, we conducted subgroup analyses to explore their differences. Minimal between‐group differences were found, however, conclusions should not be drawn from these analyses as they are based on observational and not randomized comparisons. Furthermore, given that the majority of studies assessed mixed amphetamine salts and long‐acting release formulations, the subgroups assessing other amphetamine derivatives (i.e. lisdexamphetamine, dexamphetamine), and short‐acting release formulations, may not have been adequately powered to detect a true difference.
We performed a meta‐analysis of the most commonly reported adverse events in the primary studies. These included decreased appetite, insomnia/trouble sleeping, abdominal pain, nausea/vomiting, headaches and anxiety. Meta‐analysis revealed that most adverse events occurred more often in the amphetamine groups than in the placebo groups.
Overall completeness and applicability of evidence
This review focused only on the amphetamine versus placebo comparison. While it is important to assess amphetamines versus other active therapies, such as other stimulants, psychotherapy or antidepressants, we believe it is important to first establish whether or not amphetamines are superior to placebo. We were unable to assess the long‐term efficacy of amphetamines (i.e. beyond 12 months of use). The average duration of included studies was five weeks long, excluding one long‐term study that was 12 months long (Gillberg 1997). Short‐term trials are particularly problematic for chronic conditions, such as ADHD, as children will likely be on stimulant medications for much longer periods than have been studied. As mentioned earlier, adverse events occurred more frequently when children and adolescents were treated with amphetamines than when they were treated with placebo, however, it is important to point out the poor reporting around adverse events in the included studies. Some studies only reported on adverse events that were experienced by a certain percentage of children and adolescents, thereby potentially ignoring additional adverse events experienced at less than that fraction. Futhermore, many studies were unclear regarding their methods of collecting adverse events and whether they assessed the causality of these adverse events as it related to the interventions. Heterogeneity of terms used to describe adverse events was also a major hurdle when conducting this review, and limited our ability to appropriately synthesize the data. Finally, as with efficacy data, we were unable to assess the long‐term safety profile of amphetamines given the lack of long‐term trials.
Only one study explored the impact of amphetamines on children and adolescents' quality of life (Findling 2011). It found no effect, although this may be because the study was underpowered. This is a significant gap in the available evidence on the effects of amphetamines, and studies should include a focus on psychosocial factors such as parental stress or quality of life.
The external validity of our results was also limited by the inclusion/exclusion criteria of the included studies. Since we excluded studies that included children and adolescents with comorbidity other than conduct disorder, oppositional defiant disorder and anxiety, we cannot extrapolate the results of our review to patients with other commonly occurring comorbidity such as depression, and tic disorder.
Few trials reported on the ADHD subtype of their included children and adolescents. Therefore, we were unable to make any conclusions as regards the potential heterogeneity of effect of different formulations of amphetamines across different ADHD subtypes. Furthermore, since primary studies did not subgroup their results according to important prognostic factors, such as age and gender, we were unable to subgroup our meta‐analyses, which would have been particularly relevant for clinicians.
Although our review did assess the proportion of children and adolescents who dropped out due to an adverse event and found no differences between amphetamine and placebo groups (Analysis 1.24), it is worth noting that many studies in the meta‐analysis had zero events in the placebo group, resulting in potentially invalid results that overestimate variance. The high number of zero events may be attributable to a lack of power to detect these events given the much smaller sample sizes of the placebo groups (n = 900) as compared to the amphetamine groups (n = 1532).
Finally, it was difficult for us to accurately assess our results in a clinically meaningful way, since interpretation of scores is both sex‐ and age‐dependent.
Quality of the evidence
The overall quality of the evidence ranged from low to very low as assessed by the GRADE approach, indicating that there is room for improvement amongst the current evidence about the efficacy of amphetamines in managing ADHD in children and adolescents. The studies appear to have a multitude of methodological issues making it difficult to draw strong clinical conclusions. Moreover, most studies failed to report on how the random sequence was generated (90%), how allocation was concealed (85%), the methods used to blind participants and personnel (55%), and the methods used to blind outcome assessors (90%). As such, we were unable to determine whether it was a reporting problem or a methodological problem.
Potential biases in the review process
Limitations of our review include not being able to account for correlation in cross‐over studies given the formula for calculating SMDs, thereby yielding less precise effect sizes, which may result in overlooking any potential statistical heterogeneity between the studies. Furthermore, caution is warranted in the interpretation of our meta‐analyses of adverse events that contain rare events. Most methods of meta‐analysis, including the chosen Mantel‐Haenszel approach, perform poorly with rare events; they can yield misleading results, have very wide CIs or the statistical power can be too low to detect a difference.
Given the small number of studies included in the primary meta‐analyses (maximum of seven studies), we felt that a funnel plot would not provide meaningful information about potential publication bias, which may have led to an overestimation of treatment effects. Furthermore, the exclusion of one potentially eligible non‐English study may have also biased our findings. Egger 1997 found that non‐English studies tend to be negative, and so excluding them may have yielded an overestimation of treatment effects. On the other hand, other researchers have found that excluding trials reported in languages other than English do not significantly affect the results of a meta‐analysis (Moher 2003).
Another limitation arises from the subgroup comparisons. It must be noted that these analyses are based on observational and not randomized comparisons, and therefore should not be interpreted as conclusive evidence.
Another potential bias of our review is that one of the authors, Dr. Catherine J Nikles, has published a study on amphetamines for ADHD; however, two independent authors assessed the eligibility of this study, which was subsequently excluded due to the nature of the design (Nikles 2006).
Agreements and disagreements with other studies or reviews
We identified two previously conducted systematic reviews prior to conducting ours (Charach 2011; Miller 1999). Charach 2011 only assessed the long‐term (i.e. more than 12 months) efficacy and safety of amphetamines for pediatric ADHD, and included only one RCT that assessed amphetamines versus placebo, which was also included in our systematic review (Gillberg 1997). Miller 1999 also systematically assessed amphetamines for pediatric ADHD, however, reviewers only included studies that assessed the dexamphetamine derivative of amphetamines. Furthermore, this review was published in 1999, making it over 14 years old. As such, Miller 1999 included only three relevant RCTs in their review, which were also included in this review (Borcherding 1990; Donnelly 1989; Gillberg 1997). The majority of meta‐analyses conducted in the Miller 1999 review included RCTs that assessed any stimulant medication (methylphenidate or amphetamine) versus placebo. The one meta‐analysis that was restricted to the amphetamine versus placebo comparison showed that amphetamines improve total ADHD symptoms as rated by the Abbreviated Conners Teacher Rating Scale (ATRS; Conners 1990): MD ‐4.77; 95% CI ‐6.43 to ‐2.99. This is consistent with the results of our review for this outcome, which also showed improvement in this outcome in favour of amphetamine (SMD ‐0.55; 95% CI ‐0.83 to ‐0.27).
Authors' conclusions
Implications for practice.
Although the results of this review demonstrate that amphetamines improve the core symptoms of ADHD in children and adolescents in the short term, they are also associated with higher risk of experiencing adverse events. Given the lack of available evidence as regards the effects of amphetamines on ADHD‐subtypes, long‐term effectiveness and safety data, and the effects of amphetamines on psychosocial outcomes, it is difficult to extrapolate the results from this research to the clinical population. As such, when making treatment decisions, clinicians must use their own judgement in weighing the benefits with the safety profile of the intervention, accounting for the patient’s responsiveness to other stimulant medications (for example, methylphenidate), and integrating patient/family preferences and values when making treatment decisions.
This review does not provide evidence that supports any one amphetamine derivative over another, and does not reveal any differences between long‐acting and short‐acting formulations.
Implications for research.
Future RCTs should be longer in duration in order to explore the long‐term safety and efficacy of amphetamines. It would also be beneficial for future studies to subgroup their results based on important prognostic factors, such as age and gender, in order to yield more clinically relevant results. Furthermore, while it has been traditionally assumed that improvements in core ADHD symptoms result in improved overall functioning, including academic performance, peer relations and family functioning, there is a lack of data that supports these correlations. Initiatives such as COMET (Core Outcome Measures in Effectiveness Trials), which aims to develop standardized sets of research outcomes for various health conditions, will be pivotal to areas like ADHD in ensuring that future studies not only focus on symptom management as an outcome, but also on more global outcomes that focus on a child's overall functioning (Williamson 2012).
While there is evidence that shows industry funding has been associated with exaggerated treatment effects (Lundh 2012), the debate remains unresolved (Bero 2013; Sterne 2013). Future research should assess to what extent funding source, and author affiliation with the funding source, impact treatment effects, and recommendations on whether these factors should be included in the risk of bias assessment may be helpful.
Researchers should consult the Consolidated Standards of Reporting Trials (CONSORT) statement when designing their study and reporting their methods and results so that an appropriate risk of bias assessment can be made allowing for a more robust interpretation (Schulz 2010).
Acknowledgements
The authors wish to thank Soleil Surrette for her assistance in developing and reviewing the search strategy. We also want to thank Dr. Marek Varga for his help with translation.
We also wish to thank Geraldine McDonald (Co‐ordinating Editor), Joanne Wilson (Managing Editor), and Margaret Anderson (Trials Search Co‐ordinator) of the Cochrane Developmental, Psychosocial and Learning Problems Group for providing help and support.
We wish to thank those who peer reviewed this manuscript and providing their thoughtful feedback.
Appendices
Appendix 1. CENTRAL search strategy
1. exp Amphetamines/ 2. (amphetamine$ or dexamphetamine$ or methamphetamine$ or dextroamphetamine$ or lisdexamphetamine$ or vyvanase$ or Dexedrin3 or desoxyn$ or adderall$).mp. 3. Central Nervous System Stimulants/ 4. 1 or 2 or 3 5. exp Attention Deficit Disorder with Hyperactivity/ 6. Child Behavior Disorders/ 7. adhd.tw. 8. addh.tw. 9. adhs.tw. 10. "ad/hd".tw. 11. hyperactiv$.tw. 12. hyper‐activ$.tw. 13. overactiv$.tw. 14. over‐activ$.tw. 15. hyperkinesis/ 16. hyperkin$.tw. 17. hyper‐kin$.tw. 18. hkd.tw. 19. (minimal adj3 brain$ adj3 (damag$ or disorder$ or dysfunc$)).tw. 20. (attention$ adj3 (deficit$ or disorder$ or dysfunc$)).tw. 21. (behav$ adj3 (dysfunc$ or disorder$)).tw. 22. (impulsiv$ or inattentiv$ or inattention$).tw. 23. disruptiv$.tw. 24. or/5‐23 25. exp child/ 26. adolescent/ 27. (adoles$ or teen$ or youth$ or young people or young person$).tw. 28. (child$ or toddler$ or preschool$ or pre‐school or schoolchild$ or schoolgirl$ or schoolboy$ or girl$ or boy$).tw. 29. Pediatrics/ 30. p?ediatric$.tw. 31. or/25‐30 32. 4 and 24 and 31
Appendix 2. Ovid MEDLINE search strategy
1. exp Amphetamines 2. (amphetamine$ or dexamphetamine$ or methamphetamine$ or dextroamphetamine$ or lisdexamphetamine$ or vyvanase$ or Dexedrin3 or desoxyn$ or adderall$).mp. 3. Central Nervous System Stimulants/ 4. 1 or 2 or 3 5. exp Attention Deficit Disorder with Hyperactivity/ 6. Child Behavior Disorders/ 7. adhd.tw. 8. addh.tw. 9. adhs.tw 10. "ad/hd".tw. 11. hyperactiv$.tw. 12. hyper‐activ$.tw. 13. overactiv$.tw. 14. over‐activ$.tw. 15. hyperkinesis/ 16. hyperkin$.tw. 17. hyper‐kin$.tw. 18. hkd.tw. 19. (minimal adj3 brain$ adj3 (damag$ or disorder$ or dysfunc$)).tw. 20. (attention$ adj3 (deficit$ or disorder$ or dysfunc$)).tw. 21. (behav$ adj3 (dysfunc$ or disorder$)).tw. 22. (impulsiv$ or inattentiv$ or inattention$).tw. 23. disruptiv$.tw. 24. or/5‐23 25. exp child/ 26. adolescent/ 27. (adoles$ or teen$ or youth$ or young people or young person$).tw. 28. (child$ or toddler$ or preschool$ or pre‐school or schoolchild$ or schoolgirl$ or schoolboy$ or girl$ or boy$).tw. 29. Pediatrics/ 30. p?ediatric$.tw. 31. or/25‐30 32. 4 and 24 and 31 33. randomized controlled trial.pt. 34. controlled clinical trial.pt. 35. randomi#ed.ab. 36. placebo$.ab. 37. drug therapy.fs. 38. randomly.ab. 39. trial.ab. 40. groups.ab. 41. or/33‐40 42. exp animals/ not humans.sh. 43. 41 not 42 44. 32 and 43
Lines 33 to 43 are the Cochrane highly sensitive search strategy for identifying randomized trials in MEDLINE (Ovid version) (Lefebvre 2011).
Appendix 3. Embase search strategy
1. exp amphetamine/ 2. (amphetamine$ or dexamphetamine$ or methamphetamine$ or dextroamphetamine$ or lisdexamphetamine$ or vyvanase$ or Dexedrin3 or desoxyn$ or adderall$).mp. 3. central stimulant agent/ 4. 1 or 2 or 3 5. exp attention deficit disorder/ 6. behavior disorder/ 7. adhd.tw. 8. addh.tw. 9. adhs.tw. 10. "ad/hd".tw. 11. hyper‐activ$.tw. 12. hyperactiv$.tw. 13. overactiv$.tw. 14. over‐activ$.tw. 15. hyperkinesia/ 16. hyperkin$.tw. 17. hyper‐kin$.tw. 18. hkd.tw. 19. (minimal adj3 brain$ adj3 (damag$ or disorder$ or dysfunc$)).tw. 20. (attention$ adj3 (deficit$ or disorder$ or dysfunc$)).tw. 21. (behav$ adj3 (dysfunc$ or disorder$)).tw. 22. (impulsiv$ or inattentiv$ or inattention$).tw. 23. disruptiv$.tw. 24. or/5‐23 25. child/ 26. adolescent/ 27. (adoles$ or teen$ or youth$ or young people or young person$).tw. 28. (child$ or toddler$ or preschool$ or pre‐school or schoolchild$ or schoolgirl$ or schoolboy$ or girl$ or boy$).tw. 29. pediatrics/ 30. p?ediatric$.tw. 31. or/25‐30 32. 4 and 24 and 31 33. randomized controlled trial/ 34. controlled clinical trial/ 35. randomi#ed.ab. 36. placebo$.ab. 37. drug therapy/ 38. randomly.ab. 39. trial.ab. 40. single blind procedure/ 41. double blind procedure/ 42. or/33‐41 43. exp animal/ not human.sh. 44. 42 not 43
Appendix 4. PsycINFO search strategy
1. exp amphetamine/ 2. (amphetamine$ or dexamphetamine$ or methamphetamine$ or dextroamphetamine$ or lisdexamphetamine$ or vyvanase$ or Dexedrin3 or desoxyn$ or adderall$).mp. 3. exp attention deficit disorder/ 4. behavior disorder/ 5. adhd.tw. 6. addh.tw. 7. adhs.tw. 8. "ad/hd".tw. 9. hyper‐activ$.tw. 10. hyperactiv$.tw. 11. overactiv$.tw. 12. over‐activ$.tw. 13. hyperkin$.tw. 14. hyper‐kin$.tw. 15. hkd.tw. 16. (minimal adj3 brain$ adj3 (damag$ or disorder$ or dysfunc$)).tw. 17. (attention$ adj3 (deficit$ or disorder$ or dysfunc$)).tw. 18. (behav$ adj3 (dysfunc$ or disorder$)).tw. 19. (impulsiv$ or inattentiv$ or inattention$).tw. 20. disruptiv$.tw. 21. (adoles$ or teen$ or youth$ or young people or young person$).tw. 22. (child$ or toddler$ or preschool$ or pre‐school or schoolchild$ or schoolgirl$ or schoolboy$ or girl$ or boy$).tw. 23. pediatrics/ 24. p?ediatric$.tw. 25. randomi#ed.ab. 26. placebo$.ab. 27. drug therapy/ 28. randomly.ab. 29. trial.ab. 30. (doubl$ adj blind$).mp. 31. (singl$ adj blind$).mp. 32. 1 or 2 33. or/3‐20 34. or/21‐24 35. 32 and 33 and 34 36. or/25‐31 37. exp animals/ not humans.sh. 38. 36 not 37 39. 35 and 38
Appendix 5. Proquest Dissertations and Theses search strategy
Advanced search (attention deficit disorder OR attention deficit hyperactivity disorder OR hyperactivity) AND all(amphetamine OR adderall) AND all(children OR youth OR adolescent)
Appendix 6. Networked Digital Library of Theses and Dissertations search strategy
Attention Deficit Hyperactivity Disorder AND amphetamine AND (child OR youth OR adolescent)
Appendix 7. ClinicalTrials.gov search strategy
Advanced search Conditions: Attention Deficit Hyperactivity Disorder OR ADHD OR Attention Deficit Disorder OR ADD Interventions: amphetamine OR dexamphetamine OR methamphetamine OR dextroamphetamine OR lisdexamphetamine OR vyvanase OR dexedrine OR adderall Study results: All studies Age group: Child
Appendix 8. Risk of bias domains
| Domain | Description | Judgement |
| Random sequence generation | The method used to generate the allocation sequence is described in sufficient detail so as to assess whether it should have produced comparable groups. | What is the risk of selection bias due to inadequate generation of a randomized sequence? |
| Allocation concealment | The method used to conceal the allocation sequence is described in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | What is the risk of selection bias due to inadequate concealment of allocations prior to assignment? |
| Blinding of participants and personnel | The measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received and any information relating to whether the intended blinding was effective. | What is the risk of performance bias due to knowledge of the allocated interventions by participants and personnel during the study? |
| Blinding of outcome assessment | The measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received and any information relating to whether the intended blinding was effective. | What is the risk of detection bias due to knowledge of the allocated interventions by outcome assessors? |
| Incomplete outcome data | Assessment of the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. | What is the risk of attrition bias due to amount, nature, or handling of incomplete outcome data? |
| Selective outcome reporting | Attempts made to assess the possibility of selective outcome reporting by investigators. | What is the risk of reporting bias due to selective outcome reporting? |
| Other sources of bias | Assessment of other sources of bias in other domains not covered by the tool, including validity of outcome measures utilized. | What is the risk of bias due to problems not covered elsewhere in the table? |
Data and analyses
Comparison 1. Amphetamines versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total ADHD symptom score ‐ parent ratings | 7 | 1247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.86, ‐0.27] |
| 2 Hyperactivity/impulsivity ‐ parent ratings | 2 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.89, ‐0.19] |
| 3 Total ADHD symptom score ‐ teacher ratings | 5 | 745 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.83, ‐0.27] |
| 4 Hyperactivity/impulsivity ‐ teacher ratings | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Inattention ‐ teacher ratings | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Total ADHD symptom score ‐ clinician ratings | 3 | 813 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.32, ‐0.36] |
| 7 Hyperactivity/impulsivity ‐ clinician ratings | 3 | 813 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.28, ‐0.23] |
| 8 Inattention ‐ clinician ratings | 3 | 813 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.26, ‐0.30] |
| 9 Total ADHD symptom score ‐ investigator/research personnel ratings | 3 | 630 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.87, ‐0.44] |
| 10 Hyperactivity/impulsivity ‐ investigator/research personnel ratings | 2 | 280 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐1.83, ‐1.08] |
| 11 Inattention ‐ investigator/research personnel ratings | 4 | 634 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.42, ‐0.04] |
| 12 Proportion of responders (Clinical Global Impression ‐ Improvement; CGI ‐ I) | 9 | 2207 | Risk Ratio (M‐H, Random, 95% CI) | 3.36 [2.48, 4.55] |
| 13 Clinical Global Impression ‐ Severity (CGI ‐ S) score | 2 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.72, ‐0.01] |
| 14 Academic performance | 8 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.39, 0.73] |
| 15 Quality of life | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16 Retention: proportion of participants who completed the trial | 11 | 2381 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.97, 1.10] |
| 17 Proportion of participants experiencing decreased appetite | 11 | 2467 | Risk Ratio (M‐H, Random, 95% CI) | 6.31 [2.58, 15.46] |
| 18 Proportion of participants experiencing insomnia/trouble sleeping | 10 | 2429 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [2.12, 6.83] |
| 19 Proportion of participants experiencing abdominal pain | 10 | 2155 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.03, 2.00] |
| 20 Proportion of participants experiencing nausea/vomiting | 6 | 1579 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.04, 2.56] |
| 21 Proportion of participants experiencing headaches | 9 | 2091 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.16] |
| 22 Proportion of participants experiencing anxiety/nervousness | 5 | 1088 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.78, 1.93] |
| 23 Proportion of participants who experienced at least one adverse event | 6 | 1742 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.18, 1.44] |
| 24 Proportion of participants who dropped out/withdrew due to an adverse event | 9 | 2160 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.86, 2.98] |
Comparison 2. Subgroup analysis 1: Type of amphetamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total ADHD symptom score ‐ parent ratings | 7 | 1247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.86, ‐0.27] |
| 1.1 Dexamphetamine | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.36, 0.16] |
| 1.2 Lisdexamphetamine | 2 | 486 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.59, 0.14] |
| 1.3 Mixed amphetamine salts | 4 | 733 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.63, ‐0.24] |
| 2 Proportion of responders (CGI ‐ I) | 9 | 2205 | Risk Ratio (M‐H, Random, 95% CI) | 3.38 [2.51, 4.55] |
| 2.1 Dexamphetamine | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 5.4 [2.38, 12.25] |
| 2.2 Lisdexamphetamine | 4 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 3.62 [2.04, 6.41] |
| 2.3 Mixed amphetamine salts | 4 | 1076 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [2.14, 3.45] |
| 3 Academic performance | 8 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.39, 0.73] |
| 3.1 Dexamphetamine | 4 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.12, 0.67] |
| 3.2 Lisdexamphetamine | 1 | 226 | Std. Mean Difference (IV, Random, 95% CI) | 0.78 [0.51, 1.05] |
| 3.3 Mixed amphetamine salts | 3 | 392 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.29, 0.84] |
| 4 Retention: proportion of participants who completed the trial | 10 | 2364 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.12] |
| 4.1 Dexamphetamine | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.12] |
| 4.2 Lisdexamphetamine | 4 | 1084 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.92, 1.42] |
| 4.3 Mixed amphetamine salts | 5 | 1216 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 5 Proportion of participants who dropped out/withdrew due to an adverse event | 9 | 2161 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.86, 2.98] |
| 5.1 Dexamphetamine | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.78] |
| 5.2 Lisdexamphetamine | 4 | 1085 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.70, 5.91] |
| 5.3 Mixed amphetamine salts | 4 | 984 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.53, 3.06] |
| 6 Proportion of participants experiencing decreased appetite | 10 | 2273 | Risk Ratio (M‐H, Random, 95% CI) | 6.20 [2.44, 15.71] |
| 6.1 Dexamphetamine | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.95, 2.11] |
| 6.2 Lisdexampheatmine | 4 | 1081 | Risk Ratio (M‐H, Random, 95% CI) | 9.83 [5.08, 19.02] |
| 6.3 Mixed amphetamine salts | 5 | 1124 | Risk Ratio (M‐H, Random, 95% CI) | 6.42 [1.56, 26.52] |
| 7 Proportion of participants experiencing insomnia/trouble sleeping | 10 | 2429 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [2.12, 6.83] |
| 7.1 Dexamphetamine | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.41, 3.26] |
| 7.2 Lisdexamphetamine | 4 | 1081 | Risk Ratio (M‐H, Random, 95% CI) | 5.91 [2.84, 12.29] |
| 7.3 Mixed amphetamine salts | 5 | 1280 | Risk Ratio (M‐H, Random, 95% CI) | 3.34 [1.25, 8.96] |
| 8 Proportion of participants experiencing abdominal pain | 10 | 2155 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.03, 2.00] |
| 8.1 Dexamphetamine | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.27, 1.67] |
| 8.2 Lisdexamphetamine | 3 | 769 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.76, 2.19] |
| 8.3 Mixed amphetamine salts | 6 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.17, 2.45] |
| 9 Proportion of participants experiencing headaches | 9 | 2063 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.16] |
| 9.1 Dexamphetamine | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.42, 2.36] |
| 9.2 Lisdexamphetamine | 5 | 1077 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.73, 1.57] |
| 9.3 Mixed amphetamine salts | 3 | 918 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.14] |
| 10 Proportion of participants experiencing nausea/vomiting | 6 | 1579 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.04, 2.56] |
| 10.1 Dexamphetamine | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.68, 4.07] |
| 10.2 Lisdexamphetamine | 4 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.61, 3.61] |
| 10.3 Mixed amphetamine salts | 1 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.04, 3.27] |
2.2. Analysis.
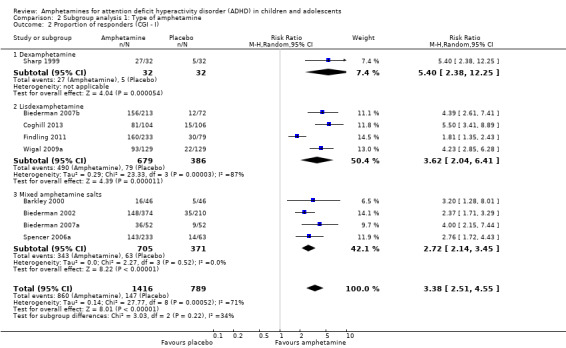
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 2 Proportion of responders (CGI ‐ I).
2.3. Analysis.
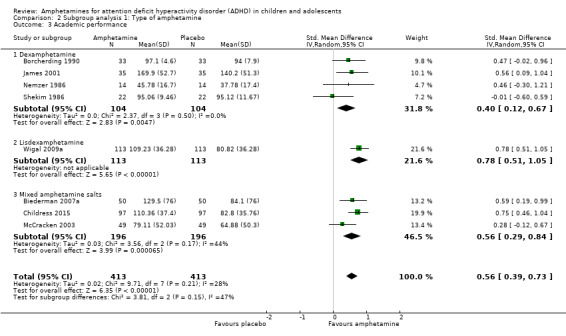
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 3 Academic performance.
2.4. Analysis.
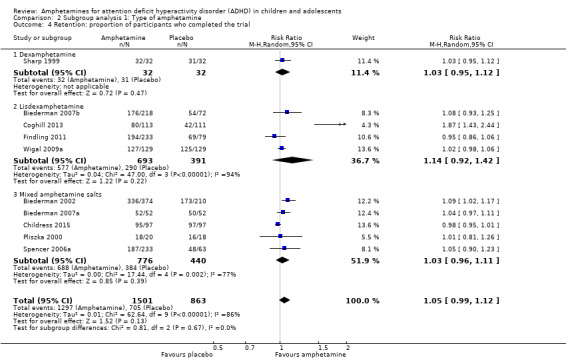
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 4 Retention: proportion of participants who completed the trial.
2.5. Analysis.
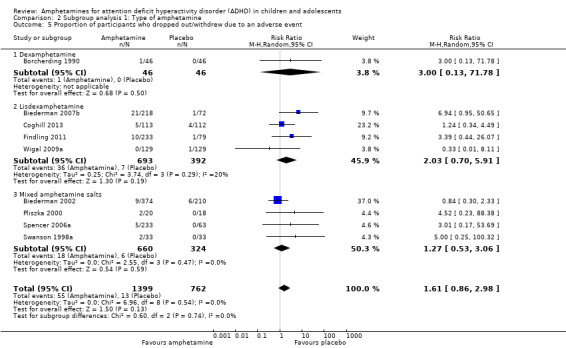
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 5 Proportion of participants who dropped out/withdrew due to an adverse event.
2.7. Analysis.
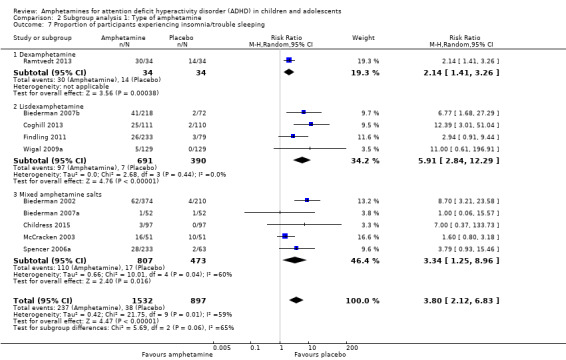
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 7 Proportion of participants experiencing insomnia/trouble sleeping.
2.8. Analysis.
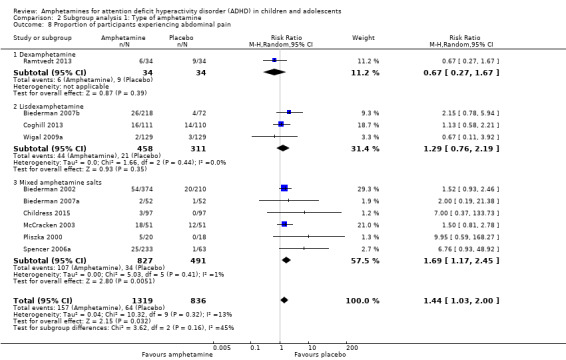
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 8 Proportion of participants experiencing abdominal pain.
2.9. Analysis.
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 9 Proportion of participants experiencing headaches.
2.10. Analysis.
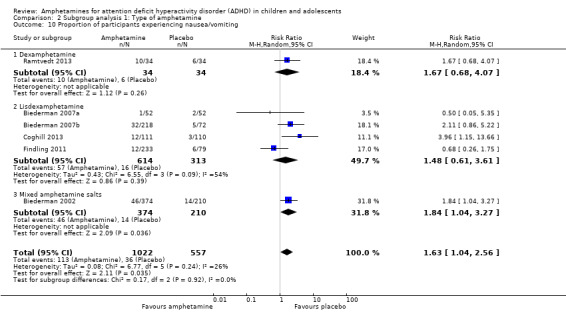
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 10 Proportion of participants experiencing nausea/vomiting.
Comparison 3. Subgroup analysis 2: Type of amphetamine release formulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total ADHD symptom score ‐ parent ratings | 7 | 1247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.86, ‐0.27] |
| 1.1 Long acting | 3 | 1049 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.08, ‐0.13] |
| 1.2 Short acting | 4 | 198 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.82, ‐0.23] |
| 2 Proportion of responders (CGI ‐ I) | 9 | 2105 | Risk Ratio (M‐H, Random, 95% CI) | 3.31 [2.44, 4.49] |
| 2.1 Long acting | 6 | 1662 | Risk Ratio (M‐H, Random, 95% CI) | 3.55 [2.63, 4.79] |
| 2.2 Short acting | 3 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [1.39, 6.02] |
| 3 Academic performance | 8 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.39, 0.73] |
| 3.1 Long acting | 4 | 494 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.36, 0.81] |
| 3.2 Short acting | 4 | 332 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.15, 0.81] |
| 4 Retention: proportion of participants who completed the trial | 10 | 2364 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.12] |
| 4.1 Long acting | 6 | 1756 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.24] |
| 4.2 Short acting | 4 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 5 Proportion of participants experiencing decreased appetite | 10 | 2271 | Risk Ratio (M‐H, Random, 95% CI) | 6.18 [2.44, 15.63] |
| 5.1 Long acting | 8 | 2165 | Risk Ratio (M‐H, Random, 95% CI) | 7.67 [3.33, 17.65] |
| 5.2 Short acting | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.69, 3.62] |
| 6 Proportion of participants experiencing abdominal pain | 10 | 2155 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.03, 2.00] |
| 6.1 Long acting | 7 | 1855 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.10, 2.02] |
| 6.2 Short acting | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.30, 21.39] |
3.2. Analysis.
Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 2 Proportion of responders (CGI ‐ I).
3.3. Analysis.
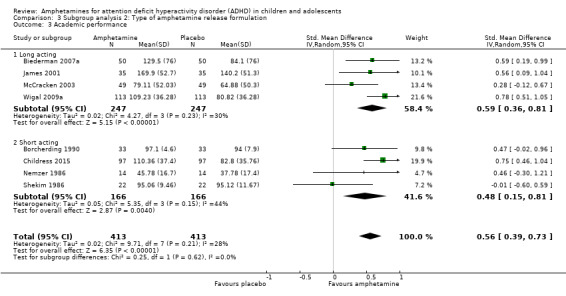
Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 3 Academic performance.
Comparison 4. Subgroup analysis 3: Funding source.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total ADHD symptom score ‐ parent ratings | 7 | 1247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.86, ‐0.27] |
| 1.1 Industry | 5 | 1135 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.89, ‐0.16] |
| 1.2 Public | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.20, ‐0.31] |
| 1.3 Not reported | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.36, 0.16] |
| 2 Proportion of responders (CGI ‐ I) | 9 | 2210 | Risk Ratio (M‐H, Random, 95% CI) | 3.37 [2.50, 4.53] |
| 2.1 Industry | 8 | 2146 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [2.39, 4.40] |
| 2.2 Not reported | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 5.4 [2.38, 12.25] |
| 3 Academic performance | 8 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.39, 0.73] |
| 3.1 Industry | 5 | 688 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.46, 0.81] |
| 3.2 Public | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.60, 0.59] |
| 3.3 Not reported | 2 | 94 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.06, 0.88] |
4.2. Analysis.
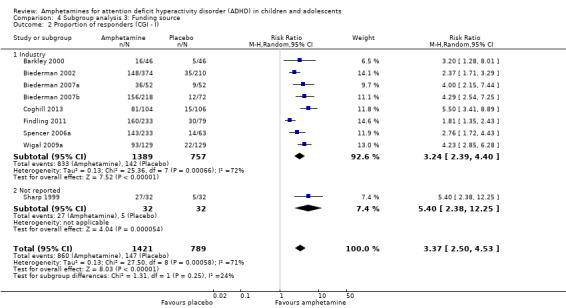
Comparison 4 Subgroup analysis 3: Funding source, Outcome 2 Proportion of responders (CGI ‐ I).
Comparison 5. Sensitivity analysis 1: Fixed‐effect model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total ADHD symptom score ‐ parent ratings | 7 | 1247 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.64, ‐0.40] |
| 2 Hyperactivity/impulsivity ‐ parent ratings | 2 | 132 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.89, ‐0.19] |
| 3 Total ADHD symptom score ‐ teacher ratings | 5 | 745 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.62, ‐0.32] |
| 4 Hyperactivity/impulsivity ‐ teacher ratings | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Inattention ‐ teacher ratings | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Total ADHD symptom score ‐ clinician ratings | 3 | 813 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.01, ‐0.68] |
| 7 Hyperactivity/impulsivity ‐ clinician ratings | 3 | 813 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐0.90, ‐0.58] |
| 8 Inattention ‐ clinician ratings | 3 | 813 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐0.94, ‐0.61] |
| 9 Total ADHD symptom score ‐ investigator/research personnel ratings | 3 | 630 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.08 [‐1.25, ‐0.91] |
| 10 Hyperactivity/impulsivity ‐ Investigator/research personnel ratings | 2 | 280 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐1.76, ‐1.23] |
| 11 Inattention ‐ investigator/research personnel ratings | 4 | 634 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐0.93, ‐0.60] |
| 12 Proportion of responders (CGI ‐ I) | 9 | 2207 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [2.68, 3.61] |
| 13 CGI ‐ S score | 2 | 86 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.15, ‐0.27] |
| 14 Quality of life | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15 Academic performance | 8 | 826 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.59 [0.45, 0.73] |
| 16 Retention: proportion of participants who completed the trial | 10 | 2364 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.04, 1.12] |
| 17 Proportion of participants who experienced at least one adverse event | 6 | 1742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.20, 1.47] |
| 18 Proportion of participants who dropped out/withdrew due to an adverse event | 9 | 2160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.08, 3.51] |
| 19 Proportion of participants experiencing decreased appetite | 11 | 2467 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.57 [4.03, 7.68] |
| 20 Proportion of participants experiencing insomnia/trouble sleeping | 10 | 2429 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.91 [2.82, 5.41] |
| 21 Proportion of participants experiencing abdominal pain | 10 | 2155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.18, 2.08] |
| 22 Proportion of participants experiencing headaches | 9 | 2091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 23 Proportion of participants experiencing anxiety/nervousness | 5 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.94, 1.56] |
| 24 Proportion of participants experiencing nausea/vomiting | 6 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.20, 2.46] |
5.2. Analysis.
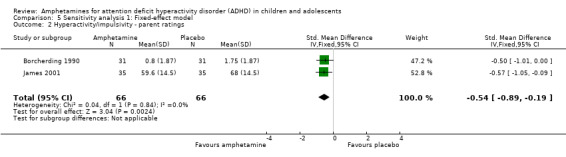
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 2 Hyperactivity/impulsivity ‐ parent ratings.
5.3. Analysis.
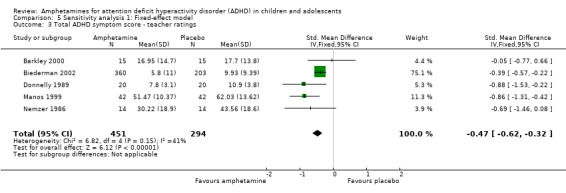
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 3 Total ADHD symptom score ‐ teacher ratings.
5.4. Analysis.
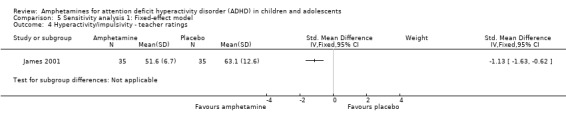
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 4 Hyperactivity/impulsivity ‐ teacher ratings.
5.5. Analysis.
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 5 Inattention ‐ teacher ratings.
5.6. Analysis.
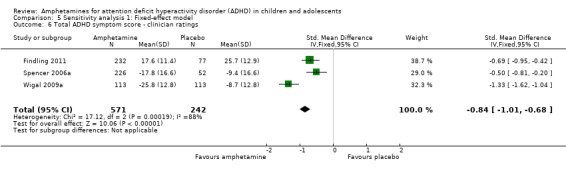
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 6 Total ADHD symptom score ‐ clinician ratings.
5.7. Analysis.
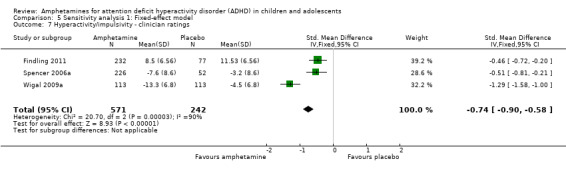
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 7 Hyperactivity/impulsivity ‐ clinician ratings.
5.8. Analysis.
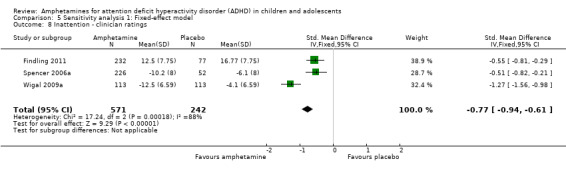
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 8 Inattention ‐ clinician ratings.
5.9. Analysis.
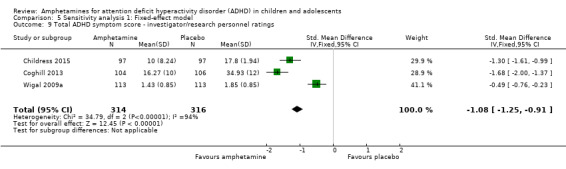
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 9 Total ADHD symptom score ‐ investigator/research personnel ratings.
5.10. Analysis.
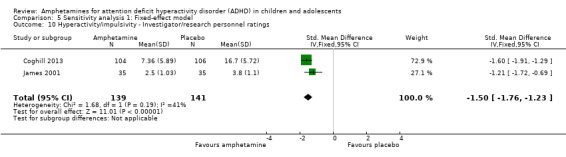
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 10 Hyperactivity/impulsivity ‐ Investigator/research personnel ratings.
5.11. Analysis.
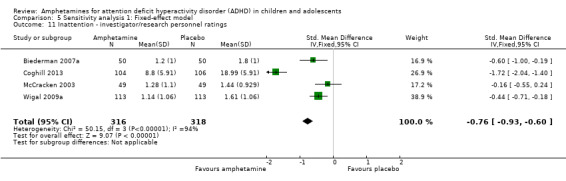
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 11 Inattention ‐ investigator/research personnel ratings.
5.12. Analysis.
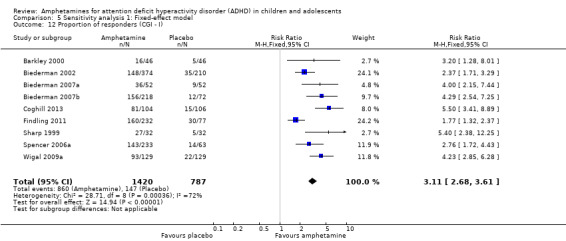
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 12 Proportion of responders (CGI ‐ I).
5.13. Analysis.
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 13 CGI ‐ S score.
5.14. Analysis.
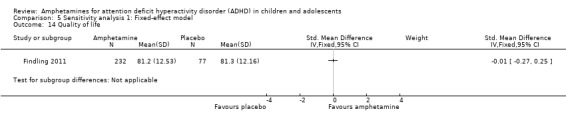
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 14 Quality of life.
5.15. Analysis.
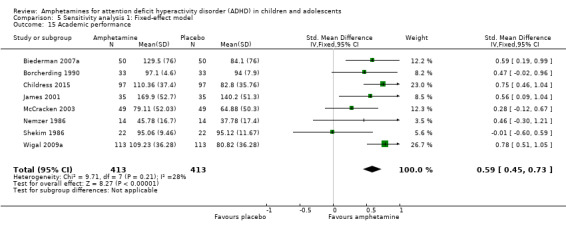
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 15 Academic performance.
5.16. Analysis.
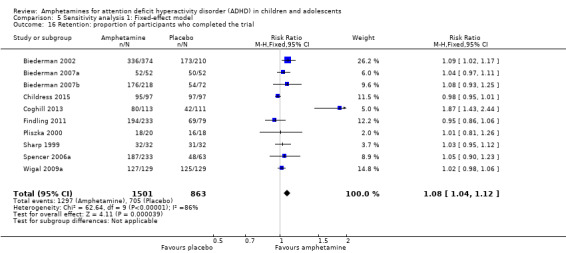
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 16 Retention: proportion of participants who completed the trial.
5.17. Analysis.
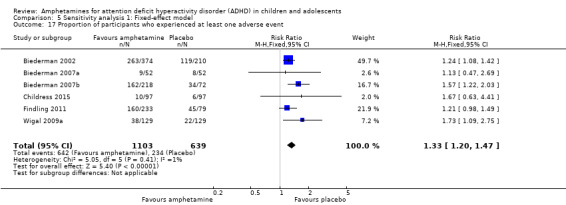
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 17 Proportion of participants who experienced at least one adverse event.
5.18. Analysis.
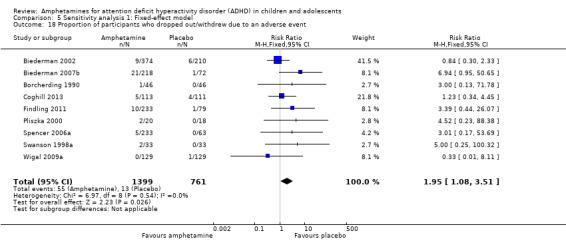
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 18 Proportion of participants who dropped out/withdrew due to an adverse event.
5.19. Analysis.
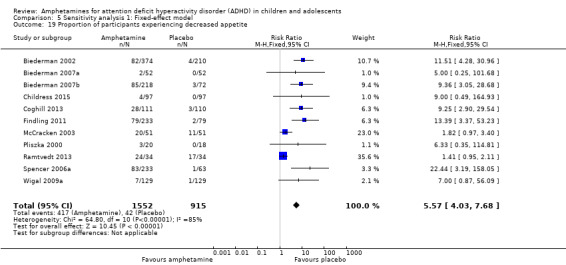
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 19 Proportion of participants experiencing decreased appetite.
5.20. Analysis.
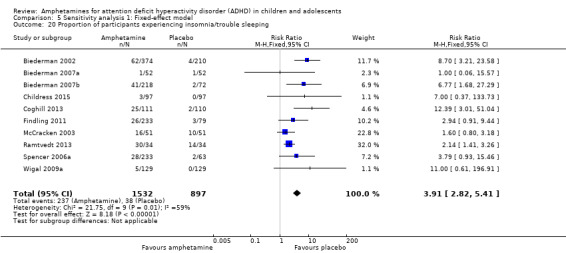
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 20 Proportion of participants experiencing insomnia/trouble sleeping.
5.21. Analysis.
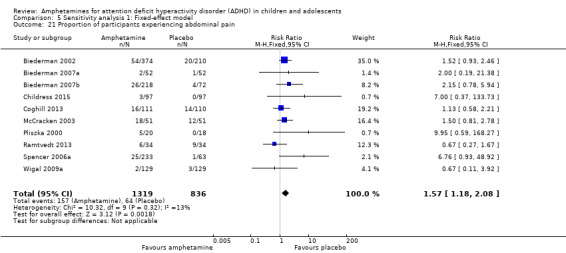
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 21 Proportion of participants experiencing abdominal pain.
5.22. Analysis.
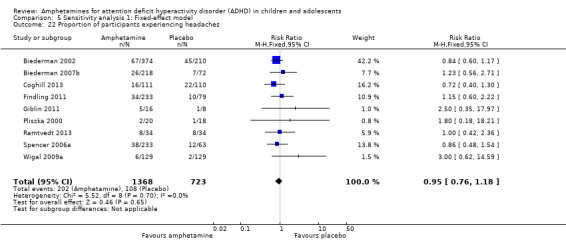
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 22 Proportion of participants experiencing headaches.
5.23. Analysis.
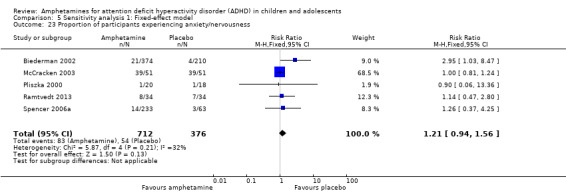
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 23 Proportion of participants experiencing anxiety/nervousness.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barkley 2000.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over trial
Country: United States Number of study sites: 1 Statistical methods: per protocol |
|
| Participants | Sample size: 46* children/adolescents with an ADHD diagnosis according to the DSM‐IV criteria Dropouts: NR Psychiatric comorbid conditions: NR Age range: 12 years to 17 years Mean age (SD): 14 (NR) years Sex: 30 (86%) males ADHD subtype: NR | |
| Interventions |
Five interventions (all 46 children/adolescents participated in each of the five interventions):
Duration: 35 days (5 x 7‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: not available Authors' affiliation: university Study funding: pharmaceutical industry *Clinical characteristics were only reported on children/adolescents who started the trial (n = 35) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only data for completers included in analysis. For one of the primary outcomes, only 37% of randomized children/adolescents included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not adequately described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Biederman 2002.
| Methods | Multi‐center, randomized, double‐blind, placebo‐controlled, parallel‐group trial
Country: United States
Number of study sites: 47 Statistical methods: modified ITT (last observation carried forward) |
|
| Participants | Sample size: 584* children/adolescents with an ADHD diagnosis according to DSM‐IV criteria Dropouts: 75 Psychiatric comorbid conditions: NR Age range: 6 years to 12 years Mean age (SD): 8.6 (1.7) years Sex: 434 (77%) males ADHD subtype: 28 (5%) hyperactive ‐ impulsive; 12 (2%) inattentive; 523 (93%) combined | |
| Interventions |
Four interventions (584 children/adolescents participated in one of four interventions):
Duration: 21 days |
|
| Outcomes |
Relevant outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: not available Authors' affiliations: university Study funding: pharmaceutical industry *Clinical characteristics are only provided on those children included in the primary efficacy analysis (n = 563) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Moderate attrition (13%). Reasons for attrition reported and 96% of randomized children/adolescents included in primary analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Biederman 2007a.
| Methods | Multi‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial Country: United States Number of study sites: 4 Statistical methods: modified ITT (all randomized children/adolescents who had at least one post‐randomization score on primary outcome measure) | |
| Participants | Sample size: 52 children/adolescents with an ADHD diagnosis according to the DSM‐IV‐TR criteria Dropouts: 2 Psychiatric comorbid conditions: NR Age range: 6 years to 12 years Mean age (SD): 9.1 (1.7) years Sex: 33 (64%) males ADHD subtypes: 52 (100%) combined | |
| Interventions |
Three interventions (all 52 children/adolescents participated in each of the three interventions):
Duration: 21 days (3 x 7‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes | ClinicalTrials.gov identifier: NCT00557011 Authors' affiliation: university and pharmaceutical industry Study funding: pharmaceutical industry *Mixed amphetamine salts ‐ extended release was randomly chosen to represent the amphetamine group in this study for binary outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Method of allocation concealment involved pre‐packaged, serially‐numbered drug kits, in which the next participant enrolled received the next available drug kit. Drug kits prepared by a third party |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although the primary analysis only included trial completers, attrition was low at 4% |
| Selective reporting (reporting bias) | Low risk | Data provided on all outcomes listed in the registered protocol. Study appears to be free of selective reporting |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Biederman 2007b.
| Methods | Multi‐center, randomized, double‐blind, placebo‐controlled, parallel‐group trial Country: United States Number of study sites: 40 Statistical methods: modified ITT (children/adolescents who had baseline and at least one post‐randomization primary efficacy measure, last observation carried forward) | |
| Participants | Sample size: 290 children/adolescents with an ADHD diagnosis according to the DSM‐IV‐TR criteria Dropouts: 60 Psychiatric comorbid conditions: NR Age range: 6 years to 12 years Mean age (SD): 9 (1.8) years Sex: 201 (69%) males ADHD subtype: 12 (4%) hyperactive ‐ impulsive; 278 (96%) combined | |
| Interventions |
Four interventions (290 children/adolescents participated in one of four interventions):
Duration: 28 days |
|
| Outcomes |
Relevant outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: not available Authors' affiliation: university Study funding: pharmaceutical industry *The authors reported their results of parent ratings of hyperactivity/impulsivity and inattention on the ADHD Rating Scale, Fourth Version, as well as total ADHD symptom scores on the Conners' Parent Rating Scale ‐ Revised as bar graphs. We sought to extract relevant data using graphic digitizer software but this gave implausible results. Consequently, this data could not be included in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by a computer program |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Moderate attrition (21%). However, 98% of randomized children/adolescents included in primary analysis. Reasons for dropouts provided |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo are described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Borcherding 1990.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial Country: United States Number of study sites: 1 Statistical methods: ITT (all randomized children/adolescents are included in the analysis, with any missing data imputed with the group mean value) | |
| Participants | Sample size: 46 children/adolescents with an ADHD diagnosis according to DSM‐III criteria Dropouts: NR Psychiatric comorbid conditions: oppositional defiant disorder, conduct disorder, reading developmental disorder, arithmetic disorder, dysthymic disorder Age range: 6 years to 12 years Mean age (SD): 8.6 (1.7) years Sex: 46 (100%) males ADHD subtype: NR | |
| Interventions |
Three interventions (all 46 children/adolescents participated in each of the three interventions):
Duration: 63 days (3 x 21‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: not available
Authors' affiliation: National Institute of Mental Health
Study funding: NR Outcomes were presented across four publications with varying sample sizes *Unpublished data on the ADHD symptoms as rated by the Conners' Teacher Rating Scale ‐ Short Form were sought on three separate occasions but were not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | This study has four associated publications, and all reports have varying numbers of participants. Upon communication with the corresponding author of these reports, it was confirmed that the numbers of participants vary in the four publications due to missing data and dropouts. Reasons for missing data not provided |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Unclear risk | No information on the validity of the primary outcome measure provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Childress 2015.
| Methods | Multi‐center, randomized, double‐blinded, placebo‐controlled, cross‐over trial Countries: United States Number of study sites: 7 Statistical methods: modified ITT (all randomized children/adolescents who took at least one dose of the study medication, and had at least one post‐randomization score on primary outcome measure) | |
| Participants | Sample size: 97 children/adolescents with an ADHD diagnosis according to DSM‐IV‐TR criteria Dropouts: 2 Psychiatric comorbid conditions: NR Age range: 6 years to 12 years Mean age (SD): 9.6 (1.86) years Sex: 59 (60.8%) males ADHD subtype: 0 (0%) hyperactive ‐ impulsive; 18 (18.6%) inattentive; 79 (81.4%) combined | |
| Interventions |
Two interventions (all 97 children/adolescents participated in both interventions):
Duration: 14 days (2 x 7‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
|
|
| Notes | ClinicalTrials.gov identifier: NCT01986062 Authors' affiliation: pharmaceutical industry Study funding: pharmaceutical industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated using an interactive web‐based response system |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very low attrition (2%) |
| Selective reporting (reporting bias) | Low risk | Data provided on all outcomes listed in the registered protocol. Study appears to be free of selective reporting |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Coghill 2013.
| Methods | Multi‐center, randomized, double‐blind, placebo‐controlled, parallel‐group trial Countries: Belgium, France, Germany, Hungary, Italy, Netherlands, Poland, Spain, Sweden, UK Number of study sites: 48 Statistical methods: modified ITT (last observation carried forward) | |
| Participants | Sample size: 336* children/adolescents with an ADHD diagnosis according to DSM‐IV‐TR criteria Dropouts: 140 Psychiatric comorbid conditions: ODD Age range: 6 years to 17 years Mean age (SD): 10.9 (2.8) Sex: 268 (81%) male ADHD subtype: 10 (3%) hyperactive‐impulsive; 53 (16%) inattentive; 268 (80.7%) combined; 1 (0.3%) NR | |
| Interventions |
Three interventions (336 children/adolescents participated in one of three interventions):
Duration: 49 days |
|
| Outcomes |
Relevant outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: NCT00763971
Authors' affiliation: university and pharmaceutical industry
Study funding: pharmaceutical industry
Outcomes were presented across three publications *Clinical characteristics were only reported on those who received at least one dose of the intervention to which they were randomized (n = 332) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Study utilized an interactive voice/web response system, which automatically generated a unique randomization number for each child/adolescent |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition (58%). 14 (4%) children/adolescents excluded from the efficacy analysis with no reasons provided |
| Selective reporting (reporting bias) | High risk | Outcomes listed in the registered protocol are not reported in any of the three publications |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo are described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment is not described |
Donnelly 1989.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial
Country: United States Number of study sites: 1 Statistical methods: ITT |
|
| Participants | Sample size: 20 children/adolescents with an ADHD diagnosis according to DSM‐III criteria Dropouts: NR Psychiatric comorbid conditions: oppositional defiant disorder, conduct disorder, mental learning disorder, language disorder Age range: NR Mean age (SD): 8 (2) years Sex: 20 (100%) males ADHD subtype: NR | |
| Interventions |
Three interventions (all 20 children/adolescents participated in each of the three interventions):
Duration: 63 days (3 x 21‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials identifier: not available Authors' affiliation: university and National Institute of Mental Health Study funding: NR Donnelly 1986 is a pilot study of Donnelly 1989 and therefore have overlapping data *Unpublished data on the Clinical Global Impression scale sought on three separate occasions but not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All recruited children/adolescents included in analyses. Only one dropout, with reasons provided |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Unclear risk | No information on the validity of the primary outcome measure provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Findling 2011.
| Methods | Multi‐center, randomized, double‐blind, placebo‐controlled, parallel‐group trial Country: United States Number of study sites: 45 Statistical methods: modified ITT (last observation carried forward) | |
| Participants | Sample size: 314 children/adolescents with an ADHD diagnosis according to DSM‐IV‐TR criteria Dropouts: 52 Psychiatric comorbid conditions: NR Age range: 13 years to 17 years Mean age (SD): 14.6 (1.31) years Sex: 249 (79%) males ADHD subtype: 203 (65%) combined | |
| Interventions |
Four interventions (312 children/adolescents participated in one of four interventions):
Duration: 28 days |
|
| Outcomes |
Relevant outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: NCT00735371 Authors' affiliation: university and pharmaceutical industry Study funding: pharmaceutical industry *Numbers are based on participants included in the safety analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by a web‐based computer system |
| Allocation concealment (selection bias) | Low risk | Allocation concealment ensured using the web‐based computer system and third party, which serially numbered treatment bottles for each participant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Moderate attrition (16%). However, 98% of randomized children/adolescents included in primary efficacy analysis. Reasons for dropouts provided |
| Selective reporting (reporting bias) | Low risk | Data provided on all outcomes listed in the registered protocol. Study appears to be free of selective reporting |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Giblin 2011.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, parallel‐group trial
Country: United States Number of study sites: 1 Statistical methods: modified ITT (all randomized children/adolescents who had both a baseline and a post‐randomization primary outcome assessment) |
|
| Participants | Sample size: 24 children/adolescents with an ADHD diagnosis according to DSM‐IV‐TR criteria Dropouts: 0 Psychiatric comorbid conditions: NR Age range: 6 years to 12 years Mean age (SD): 9.65 (2.2) years Sex: 10 (42%) males ADHD subtype: NR | |
| Interventions |
Four interventions (24 children/adolescents participated in one of four interventions):
Duration: 28 days |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: not available Authors' affiliation: private organization and pharmaceutical industry Study funding: pharmaceutical industry *Unpublished data sought on both outcome measures on three separate occasions but not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data not addressed; number of completers not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Gillberg 1997.
| Methods | Multi‐center, randomized, double‐blind, placebo‐controlled, parallel‐group trial Country: Sweden Number of sites: 4 Statistical methods: modified ITT (for inclusion into the analysis at least two measurements had to be available, with the last observation carried forward) | |
| Participants | Sample size: 62 children/adolescents with an ADHD diagnosis according to DSM‐III‐R criteria Dropouts: NR Psychiatric comorbid conditions: oppositional defiant disorder, conduct disorder, anxiety, autistic disorder, pervasive development disorder, motor tic disorder, Tourette syndrome, mild mental retardation Age range: 6 years to 11 years Mean age (SD): 9 (1.6) years Sex: 52 (84%) males ADHD subtype: NR | |
| Interventions |
Two interventions (62 children/adolescents participated in one of two interventions):
Duration: 365 days |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: not available Authors' affiliation: university and pharmaceutical industry Study funding: pharmaceutical industry and public funds |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Moderate attrition (14%). However, all dropouts occurred prior to randomization. Reasons for dropout provided. All randomized children/adolescents included in primary analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
James 2001.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial
Country: United States Number of study sites: 1 Statistical methods: per protocol |
|
| Participants | Sample size: 35 children/adolescents with an ADHD diagnosis according to DSM‐IV criteria Dropouts: NR Psychiatric comorbid conditions: oppositional defiant disorder, anxiety, enuresis, dysthymic disorder, learning disorder Age range: 6.9 years to 12.2 years Mean age (SD): 9.1 (1.5) years Sex: 21 (60%) males ADHD subtype: 35 (100%) combined | |
| Interventions |
4 interventions:*
Duration: 56 days (4 x 14‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrial.gov identifier: not available Authors' affiliation: National Insitute of Mental Health Study funding: NR *Doses were individualized and based on age, weight, prior medication experience and symptom severity (overall mean dose range: 7.8 mg/day to 12.8 mg/day) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (7%), and reasons provided. All dropouts occurred prior to randomization. All randomized children/adolescents completed the trial and included in primary analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Manos 1999.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial
Country: United States Number of study sites: 1 Statistical methods: unclear |
|
| Participants | Sample size: 84 children/adolescents with an ADHD diagnosis according to DSM‐IV criteria Dropouts: NR Psychiatric comorbid conditions: oppositional defiant disorder, anxiety, mood disorder, learning disability Age range: 5 years to 17 years Mean age (SD): 10.1 (NR) years Sex: 66 (79%) males ADHD subtypes: 38 (45%) inattentive; 46 (55%) combined | |
| Interventions |
Two conditions (84 children/adolescents participated in one of two conditions):
Children/adolescents received either the four mixed amphetamine salt OR methylphenidate conditions (determined by his or her physician) in a randomly assigned sequence Duration: 28 days (4 x 7‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrial.gov identifier: not available Authors' affiliations: university Study funding: public funds |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | A third party pharmacist prepared individually sealed bottles dated by week |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study did not describe if any children/adolescents dropped out from the trial |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo are described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinician, teacher and parent (outcome assessors) were blinded to treatment order |
McCracken 2003.
| Methods | Multi‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial Country: United States Number of study sites: 4 Statistical methods: modified ITT | |
| Participants | Sample size: 51 children/adolescents with an ADHD diagnosis according to DSM‐IV criteria Dropouts: 4 Psychiatric comorbid conditions: NR Age range: 6 years to 12 years Mean age (SD): 9.5 (1.9) years Sex: 44 (86%) males ADHD subtypes: 1 (2%) hyperactive ‐ impulsive; 50 (98%) combined | |
| Interventions |
Five interventions (all 51 children/adolescents participated in each of the five interventions):
Duration: 35 days (5 x 7‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes | ClinicalTrial.gov identifier: not available Authors' affiliation: university Study funding: pharmaceutical industry *We randomly chose mixed amphetamine salts (long acting), given at 20 mg/day, to represent the amphetamine group in this study for binary outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (4%) and reasons provided. All randomized children/adolescents included in primary analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Nemzer 1986.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial
Country: United States Number of study sites: 1 Statistical methods: NR |
|
| Participants |
Sample size: 14 children/adolescents with an ADHD diagnosis according to DSM‐III criteria Dropouts: NR Psychiatric comorbid conditions: NR Age range: 7 years to 12 years Mean age (SD): 9.36 (NR) years Sex: 11 (79%) males ADHD subtype: NR |
|
| Interventions |
Four interventions (all 14 children/adolescents participated in each of the four interventions):
Duration: 28 days (4 x 7‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: not available Authors' affiliation: university Study funding: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not discussed |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo are described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Pliszka 2000.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, parallel‐group trial
Country: United States Number of study sites: 1 Statistical methods: modified ITT (last observation carried forward) |
|
| Participants | Sample size: 59* children/adolescents with an ADHD diagnosis according to the Diagnostic Interview Schedule for Children Dropouts: 5 Psychiatric comorbid disorders: oppositional defiant disorder, conduct disorder, anxiety Age range: 6 years to 10 years Mean age (SD): 8.2 (1.6) years Sex: NR ADHD subtype: NR | |
| Interventions |
Three interventions (58 children/adolescents participated in one of three interventions):
Duration: 21 days |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: not available Author's affiliation: university and pharmaceutical industry Study funding: pharmaceutical industry *1 participant dropped out before the end of the study, and was not accounted for in the participant characteristic description (data only provided on 58 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (10%), and reasons provided. All randomized children/adolescents included in primary analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo are described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Ramtvedt 2013.
| Methods | Multi‐center, randomized, placebo‐controlled, cross‐over trial Country: Norway Number of study sites: 4 Statistical methods: unclear | |
| Participants | Sample size: 36* children/adolescents with an ADHD diagnosis according to DMS‐IV‐TR criteria Dropouts: 0 Psychiatric comorbid disorders: oppositional defiant disorder, anxiety/depression, learning disability Age range: 9 years to 14 years Mean age (SD): 11.4 (1.4) years Sex: 29 (81%) males ADHD subtype: 10 (28%) inattentive; 1 (3%) hyperactive ‐ impulsive; 25 (69%) combined | |
| Interventions |
Three interventions (all 36 children/adolescents participated in each of the three interventions):
Duration: 42 days (3 x 14‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: NCT01220440
Authors' affiliation: university and hospital
Study funding: public funds
Outcomes were presented across two publications *Data only provided on those children/adolescents who completed the trial; no information provided regarding number of children/adolescents randomized **Data is presented as an aggregate score of parent and teacher ratings and therefore could not be incorporated into the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Primary analysis only included a subset of completers without any reason provided. No information on non‐completers provided |
| Selective reporting (reporting bias) | High risk | Although the registered protocol stated that they would collect adverse events using the Side‐Effects Rating Scale, this was not reported in the published manuscript |
| Other bias | Unclear risk | No information on the validity of the primary outcome measure provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions and placebo were not identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Sharp 1999.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial
Country: United States Number of study sites: 1 Statistical methods: ITT (missing data were imputed using group means) |
|
| Participants | Sample size: 32* children/adolescents with an ADHD diagnosis according to DSM‐IV criteria Dropouts: 1 Psychiatric comorbid disorders: oppositional defiant disorder, conduct disorder, depression, separation anxiety, specific phobias, tic disorder, enuresis, reading disorder Age range: 6.2 years to 12.7 years Mean age (SD): 8.9 (1.7) years Sex: 0 (0%) males ADHD subtype: 32 (100%) combined | |
| Interventions |
Three interventions (all 32 children/adolescents participated in each of the three interventions):
Duration: 63 days (3 x 21‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
|
|
| Notes |
ClinicalTrial.gov identifier: not available Authors' affiliation: university and National Institute for Mental Health Study funding: NR *Clinical characteristics were presented on 42 children/adolescents, 10 of whom participated in a separate pilot program **Unpublished data on the ADHD core symptoms sought on three separate occasions but not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Moderate attrition (14%), with reasons provided |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo are described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Shekim 1986.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial
Country: United States Number of study sites: 1 Statistical methods: NR |
|
| Participants | Sample size: 22 children/adolescents with an ADHD diagnosis according to DSM‐III criteria Dropouts: NR Psychiatric comorbid disorders: 0 Age range: 6 years to 12 years Mean age (SD): 9.75 (2.08) years Sex: 22 (100%) males ADHD subtype: NR | |
| Interventions |
Two interventions (all 22 children/adolescents participated in both interventions):
Duration: 28 days (2 x 14‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials.gov identifier: not available Authors' affiliation: university Study funding: public funds |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for exclusions from the analysis not provided. Methods of analysis not described. Number of individuals included in the analyses not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Short 2004.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial
Country: United States Number of study sites: 1 Statistical methods: per protocol |
|
| Participants | Sample size: 34* children/adolescents with an ADHD diagnosis according to DSM‐IV criteria Dropouts: NR Psychiatric comorbid conditions: NR Age range: 3 years to 5.9 years Mean age (SD): 5.3 (NR) years Sex: 24 (85%) males ADHD subtype: 5 (17%) inattentive; 23 (83%) hyperactive ‐ impulsive or combined | |
| Interventions |
Two conditions (28 children/adolescents participated in one of two conditions)*:
Amphetamine or methylphenidate determined by a physician Duration: 28 days (4 x 7‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
|
|
| Notes |
ClinicalTrial.gov identifier: not available Authors' affiliations: university Study funding: public funds *Clinical characteristics only presented on children/adolescents included in analysis (n = 28) **The authors did not separate the two active interventions (amphetamine and methylphenidate) in their analysis. We contacted the authors on three occasions to obtain the data on amphetamines only, but we received no response, and therefore outcomes could not be included in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not sufficiently described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for attrition not described. In addition, six participants were dropped from the analysis, and their last data point was not carried forward |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to order of trial interventions |
Spencer 2006a.
| Methods | Multi‐center, randomized, double‐blind, placebo‐controlled, parallel‐group trial Country: United States Number of study sites: NR Statistical methods: modified ITT (those with at least one post‐baseline primary efficacy assessment) | |
| Participants | Sample size: 287* children/adolescents with an ADHD diagnosis according to DSM‐IV‐TR criteria Dropouts: 52 Psychiatric comorbid conditions: NR Age range: 13 years to 16 years Mean age (SD): 14.2 (1.2) years Sex: 182 (66%) males ADHD subtypes: 114 (41%) inattentive; 7 (2.5%) hyperactive‐impulsive; 157 (56.5%) combined | |
| Interventions |
Five interventions (287 children/adolescents participated in one of five interventions):
Duration: 28 days (4 x 7‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials.gov Identifier: NCT00507065 Authors' affiliation: university and pharmaceutical industry Study funding: pharmaceutical industry *Clinical characteristics only provided on the study's ITT population (n = 278) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (10%), and reasons provided. Only 3% of children/adolescents excluded from analysis due to no post‐baseline primary efficacy assessment |
| Selective reporting (reporting bias) | Low risk | Data provided on all outcomes listed in the registered protocol. Study appears to be free of selective reporting |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Swanson 1998a.
| Methods | Single‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial
Country: United States Number of study sites: 1 Statistical methods: unclear |
|
| Participants | Sample size: 33 children/adolescents with an ADHD diagnosis according to DSM‐IV criteria Dropouts: 3 Psychiatric comorbid conditions: NR Age range: 7 years to 14 years Mean age (SD): 10.58 (1.81) years Sex: 26 (79%) males ADHD subtypes: NR | |
| Interventions |
Six interventions (all 33 children/adolescents participated in each of the six interventions):
Duration: 49 days (6 x 7‐day treatment periods defined by the six medication conditions above, plus an extra seven‐day period to provide an opportunity to make‐up any missed weeks) |
|
| Outcomes |
Relevant outcomes:
Other outcomes:
|
|
| Notes |
ClinicalTrials identifier: not available Authors' affiliation: university and pharmaceutical industry Study funding: pharmaceutical industry *Unpublished data on outcomes sought on three separate occasions but not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Low attrition (8%), but reasons for dropout not provided, and only 88% of children/adolescents contributed to primary analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and the possibility of reporting bias could not be assessed |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
Wigal 2009a.
| Methods | Multi‐center, randomized, double‐blind, placebo‐controlled, cross‐over trial Country: United States Number of study sites: 7 Statistical methods: modified ITT (children/adolescents who received at least one dose of study medication, with at least one post‐randomization measurement of the primary efficacy variable ‐ last observation carried forward) | |
| Participants | Sample size: 117* children/adolescents with an ADHD diagnosis according to DSM‐IV‐TR criteria Dropouts: 6 Psychiatric comorbid disorders: NR Age range: 6 years to 12 years Mean age (SD): 10.1 (1.5) years Sex: 98 (76%) males ADHD subtypes: NR | |
| Interventions |
Two interventions (all 117 children/adolescents participated in both interventions):
Duration: 14 days (2 x 7‐day treatment periods) |
|
| Outcomes |
Relevant outcomes:
|
|
| Notes |
ClinicalTrials.gov Identifier: NCT00500149 Authors' affiliation: university and pharmaceutical industry Study funding: pharmaceutical industry *Clinical characteristics provided on 129 children/adolescents first enrolled into an open‐label dose‐optimisation phase. Of these, 117 children/adolescents randomized to the double‐blind phase |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (9%), and reasons provided; 97% of randomized children/adolescents included in primary analysis. Four individuals not included due to no post‐baseline efficacy measure |
| Selective reporting (reporting bias) | Unclear risk | Data provided on all outcomes listed in the registered protocol. However, also reported on additional outcomes not listed in protocol |
| Other bias | Low risk | Study appears to be free of other biases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention and placebo described as identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described |
ADHD: attention deficit hyperactivity disorder. DSM‐III: Diagnostic and Statistical Manual of Mental Disorders, Third Edition. DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised. DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. ITT: intention‐to‐treat. NR: not reported.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akhondzadeh 2003 | No placebo comparison assessed |
| Alexandris 1968 | ADHD diagnosis not confirmed using formal diagnostic criteria |
| Arnold 2011 | No direct amphetamine ‐ placebo comparison |
| Biederman 2006 | Not a randomized controlled trial |
| Biederman 2008 | Not a randomized controlled trial |
| Boellner 2010 | Not a randomized controlled trial |
| Brown 2010 | Not a randomized controlled trial |
| Denhoff 1971 | ADHD diagnosis not confirmed using formal diagnostic criteria |
| Donner 2007 | Not a randomized controlled trial |
| Efron 1997 | No placebo comparison assessed |
| Findling 2009 | Not a randomized controlled trial |
| Greenhill 2003 | No placebo comparison assessed |
| Kamien 1998 | Study design was a series of multiple, cross‐over, randomized controlled trials; our review included only single, cross‐over, randomized controlled trials |
| Lopez 2008 | Not a randomized controlled trial |
| McGough 2005 | Not a randomized controlled trial |
| Najib 2009 | Not a randomized controlled trial |
| Nikles 2006 | Study design was a series of multiple, cross‐over, randomized controlled trials; our review included only single, cross‐over, randomized controlled trials |
| Quintana 2007 | Not a randomized controlled trial |
| Scheffer 2005 | Included children/adolescents who had comorbid bipolar disorder and were treated with mood stabilizers (divalproex sodium) |
| Sleator 1974 | Not a randomized controlled trial |
| Spencer 2005 | Not a randomized controlled trial |
| Spencer 2006b | ADHD diagnosis not confirmed using formal diagnostic criteria |
| Turgay 2010 | Not a randomized controlled trial |
| Wigal 2009b | Not a randomized controlled trial |
| Wigal 2010a | Not a randomized controlled trial |
| Wigal 2010b | Study participants were adults with ADHD |
ADHD: attention deficit hyperactivity disorder.
Characteristics of studies awaiting assessment [ordered by study ID]
Glos 1973.
| Methods | Double‐blind, controlled trial* |
| Participants | Sample size: 20 hyperactive children** Mean age: 9.4 years |
| Interventions | Amphetamine and placebo |
| Outcomes | |
| Notes | *Information on whether or not the trial was randomized is necessary for inclusion into this review **Information on whether participants were diagnosed with ADHD and according to formal diagnostic criteria is necessary for inclusion into this review. Since we were unable to contact the author due to lack of contact information, we are unable to classify this study as either included or excluded |
Itil 1974.
| Methods | Double‐blind, three‐way study design |
| Participants | Children with hyperactivity |
| Interventions | Thioridazine, dextroamphetamine and placebo |
| Outcomes | Global behavior evaluation |
| Notes | Only abstract available. Detailed outcome data not in abstract. Unsure:
Author has been contacted three times but no response received as yet |
ADHD: attention deficit hyperactivity disorder.
Characteristics of ongoing studies [ordered by study ID]
Fanton 2009.
| Trial name or title | Effectiveness of an extended‐release stimulant medication in treating preschool children with attention deficit hyperactivity disorder |
| Methods | Six‐week, placebo‐controlled, cross‐over trial |
| Participants | Children aged 3 years to 6 years with ADHD |
| Interventions | Mixed amphetamine salts (long acting) and placebo |
| Outcomes |
Primary
Secondary
|
| Starting date | June 2008 |
| Contact information | Dr. John Fanton, Baystate Medical Center |
| Notes | Clinicaltrials.gov identifier: NCT00712699. States that recruitment period ended in August 2010. However, no results have been published as yet. Currently, only abstract available |
NCT01711021.
| Trial name or title | A randomized, double‐blind, placebo‐controlled, cross‐over, laboratory, classroom study to evaluate the safety and efficacy of d‐amphetamine transdermal drug delivery system (d‐ATS) compared to placebo in children and adolescents with ADHD |
| Methods | Two‐week, double‐blind, randomized cross‐over trial |
| Participants | Children and adolescents aged 6 years to 17 years with ADHD |
| Interventions | D‐Amphetamine Transdermal System and placebo patch |
| Outcomes |
Primary
|
| Starting date | October 2012 |
| Contact information | Dr. James Waxmonsky (affiliation not stated) |
| Notes | ClinicalTrials.gov identifier: NCT01711021. States that recruitment period ended in March 2013. However, no results have been published as yet. No abstract available |
ADHD: attention deficit hyperactivity disorder.
Differences between protocol and review
Please refer to Table 2 for a summary of protocol decisions not used in this review.
Methods. Data collection and analysis. Assessment of risk of bias in included studies.
In our protocol, we had included funding source in the risk of bias assessment. However, given the ongoing debate of the influence of funding source on treatment effect estimates, we removed this from the assessment. Instead, we explored the influence of industry on treatment effects by performing a subgroup analysis on funding source (industry versus publicly funded versus not reported).
Methods. Data collection and analysis. Unit of analysis issues.
Studies with multiple comparisons
For dichotomous outcomes of cross‐over studies, we randomly dropped one arm and used the other in the meta‐analysis.
Methods. Data collection and analysis. Assessment of heterogeneity
We added that we assessed statistical heterogeneity by examining "Chi² (P value less than 0.10 as evidence of heterogeneity)", and "Tau² estimates for each random‐effects meta‐analysis".
Methods. Data collection and analysis. 'Summary of findings'.
We added a description of what was included in the 'Summary of findings' table beneath the subsection on 'Data synthesis'.
Methods. Data collection and analysis. Subgroup analysis and investigation of heterogeneity.
We had planned to perform a subgroup analysis based on type of questionnaire used (teacher, parent, clinician, investigator). However, given the importance of each of these informants individually in the overall assessment of ADHD, these were separated in our primary analysis.
Methods. Data collection and analysis. Sensitivity analysis.
We did not conduct a sensitivity analysis according to study design (parallel‐group versus cross‐over trial) as planned, since all included cross‐over studies were treated as if they were parallel‐group studies.
Contributions of authors
SP and SV conceived this review and share overall responsibility for this review. SP led the design and ongoing co‐ordination of this review with oversight from LS, LH, LU, BV, CJN, and SV. SP developed the additional search strategies and carried out the searches for this review. SP and BV developed the analysis plan. SP retrieved the papers for this review. SP and LS independently assessed the retrieved papers against the eligibility criteria for this review. SP and LS independently appraised the risk of bias in the papers for this review. SP and LS independently extracted the data from the papers for this review. SP wrote to authors of included studies for additional information for this review. SP managed the data for this review, including entering data into RevMan and analysing the data under the guidance of BV. SP interpreted the data for this review with input from all authors. SP wrote the review. All authors critically read and edited the review.
Sources of support
Internal sources
None, Other.
External sources
-
Alberta Innovates ‐ Health Solutions, Canada.
Salary support for Dr. Sunita Vohra
Declarations of interest
Salima Punja ‐ none known. Larissa Shamseer ‐ none known. Lisa Hartling ‐ none known. Liana Urichuk ‐ received salary support from the Addiction & Mental Health Program of Alberta Health Services ‐ Edmonton Zone during the course of this review. Dr Urichuk also received a grant for a research project entitled "Neurofeedback for children with Attention Deficit Hyperactivity Disorder" from the Sick Kids Hospital Foundation and John and Lotte Hecht Memorial Foundation. Ben Vandermeer ‐ none known. Catherine Jane Nikles ‐ published a paper in the area of amphetamines for ADHD (Nikles 2006). The study was excluded due to the nature of the design. Dr Nikles was not involved in assessing the eligibility of this study, which was performed by two independent authors. Sunita Vohra ‐ is the recipient of an Alberta Innovates ‐ Health Scholar salary award. Dr Vohra's institution receives funds from Alberta Innovates ‐ Health Solutions and protects 75% of her time for research. Other funders include Health Canada, Canadian Institutes of Health Research, Women & Children’s Health Research Institute and National Health, Medical Research Council (Australia) and SERIN‐ETD Acupuncture Research Fund. SERIN is a commercial entity who provided acupuncture needles for a trial. The population is not pediatric ADHD. None of these companies pose a conflict of interest as regards this review.
New
References
References to studies included in this review
Barkley 2000 {published data only}
- Barkley RA, Connor DF, Kwasnik D. Challenges to determining adolescent medication response in an outpatient clinical setting: comparing Adderall and methylphenidate for ADHD. Journal of Attention Disorders 2000;4(2):102‐13. [DOI: 10.1177/108705470000400204] [DOI] [Google Scholar]
Biederman 2002 {published data only}
- Biederman J, Lopez FA, Boellner SW, Chandler MC. A randomized, double‐blind, placebo‐controlled, parallel‐group study of SLI381 (Adderall XR) in children with attention‐deficit/hyperactivity disorder. Pediatrics 2002;110(2):258‐66. [DOI] [PubMed] [Google Scholar]
Biederman 2007a {published data only}
- Biederman J, Boellner SW, Childress A, Lopez FA, Krishnan S, Zhang Y. Lisdexamfetamine dimesylate and mixed amphetamine salts extended‐release in children with ADHD: a double‐blind, placebo‐controlled, crossover analogue classroom study. Biological Psychiatry 2007;62(9):970‐6. [PUBMED: 17631866] [DOI] [PubMed] [Google Scholar]
Biederman 2007b {published data only (unpublished sought but not used)}
- Biederman J, Krishnan S, Zhang Y, McGough JJ, Findling RL. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP‐104) in children with attention‐deficit/hyperactivity disorder: a phase III, multicenter, randomized, double‐blind, forced‐dose, parallel‐group study. Clinical Therapeutics 2007;29(3):450‐63. [PUBMED: 17577466] [DOI] [PubMed] [Google Scholar]
Borcherding 1990 {published data only (unpublished sought but not used)}
- Borcherding BG, Keysor CS, Rapoport JL, Elia J, Amass J. Motor/vocal tics and compulsive behaviors on stimulant drugs: is there a common vulnerability?. Psychiatry Research 1990;33(1):83‐94. [PUBMED: 2217661] [DOI] [PubMed] [Google Scholar]
- Elia J, Borcherding BG, Potter WZ, Mefford IN, Rapoport JL, Keysor CS. Stimulant drug treatment of hyperactivity: biochemical correlates. Clinical Pharmacaology and Therapeutics 1990;48(1):57‐66. [DOI: 10.1038/clpt.1990.118] [DOI] [PubMed] [Google Scholar]
- Elia J, Borcherding BG, Rapoport JL, Keysor CS. Methylphenidate and dextroamphetamine treatments of hyperactivity: are there true nonresponders?. Psychiatry Research 1991;36(2):141‐55. [PUBMED: 2017529] [DOI] [PubMed] [Google Scholar]
- Elia J, Welsh PA, Gullotta CS, Rapoport JL. Classroom academic performance: improvement with both methylphenidate and dextroamphetamine in ADHD boys. Journal of Child Psychology and Psychiatry 1993;34(5):785‐804. [DOI: 10.1111/j.1469-7610.1993.tb01071.x] [DOI] [PubMed] [Google Scholar]
Childress 2015 {published data only}
- Childress AC, Brams M, Culter AJ, Kollins SH, Northcutt J, Padilla A, et al. The efficacy and safety of evekeo, racemic amphetamine sulfate, for treatment of attention‐deficit/hyperactivity disorder symptoms: a multicenter, dose‐optimized, double‐blind, randomized, placebo‐controlled crossover laboratory classroom study. Journal of Child and Adolescent Psychopharmacology 2015;25(5):402‐14. [DOI: 10.1089/cap.2014.0176] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coghill 2013 {published data only}
- Banachewski T, Soutullo C, Lecendreux M, Johnson M, Zuddas A, Hodgkins P, et al. Health‐related quality of life and functional outcomes from a randomized, controlled study of lisdexamfetamine dimesylate in children and adolescents with attention deficit hyperactivity disorder. CNS Drugs 2013;27(10):829‐40. [DOI: 10.1007/s40263-013-0095-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology 2013;23(10):1208‐18. [DOI: 10.1016/j.euroneuro.2012.11.012] [DOI] [PubMed] [Google Scholar]
- Coghill DR, Banaschewski T, Lecendreux M, Zuddas A, Dittmann RW, Otero IH, et al. Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention‐deficit/hyperactivity disorder: results from a randomized, controlled trial. European Child and Adolescent Psychiatry 2014;23(2):61‐8. [PUBMED: 23708466] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donnelly 1989 {published data only (unpublished sought but not used)}
- Donnelly M, Rapoport JL, Ismond DR. Fenfluramine treatment of childhood attention deficit disorder with hyperactivity: a preliminary report. Psychopharmacology Bulletin 1986;22(1):152‐4. [PubMed] [Google Scholar]
- Donnelly M, Rapoport JL, Potter WZ, Oliver J, Keysor CS, Murphy DL. Fenfluramine and dextroamphetamine treatment of childhood hyperactivity: clinical and biochemical findings. Archives of General Psychiatry 1989;46(3):205‐12. [DOI: 10.1001/archpsyc.1989.01810030011002] [DOI] [PubMed] [Google Scholar]
Findling 2011 {published data only}
- Findling RL, Childress AC, Cutler AJ, Gasior M, Hamdani M, Ferreira‐Cornwell MC, et al. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(4):395‐405. [PUBMED: 21421179] [DOI] [PubMed] [Google Scholar]
Giblin 2011 {published data only (unpublished sought but not used)}
- Giblin GM, Strobel AL. Effect of lisdexamphetamine dimesylate on sleep in children with ADHD. Journal of Attention Disorders 2011;15(6):491‐8. [DOI: 10.1177/1087054710371195] [DOI] [PubMed] [Google Scholar]
Gillberg 1997 {published data only}
- Gillberg C, Melander H, Knorring AL, Janols LO, Thernlund G, Hagglof B, et al. Long‐term stimulant treatment of children with attention‐deficit hyperactivity disorder symptoms: a randomized, double‐blind, placebo‐controlled trial. Archives of General Psychiatry 1997;54(9):857‐64. [PUBMED: 9294377] [DOI] [PubMed] [Google Scholar]
James 2001 {published data only}
- James RS, Sharp WS, Bastain TM, Lee PP, Walter JM, Czarnolewski M, et al. Double‐blind, placebo‐controlled study of single‐dose amphetamine formulations in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(11):1268‐76. [PUBMED: 11699800] [DOI] [PubMed] [Google Scholar]
Manos 1999 {published data only}
- Manos MJ, Short EJ, Findling RL. Differential effectiveness of methylphenidate and Adderall in school‐age youths with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(7):813‐9. [PUBMED: 10405498] [DOI] [PubMed] [Google Scholar]
McCracken 2003 {published data only}
- McCracken JT, Biederman J, Greenhill LL, Swanson JM, McGough JJ, Spencer TJ, et al. Analog classroom assessment of a once‐daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(6):673‐83. [PUBMED: 12921475] [DOI] [PubMed] [Google Scholar]
Nemzer 1986 {published data only}
- Nemzer ED, Arnold E, Votolato NA, McConnell H. Amino acid supplementation as therapy for attention deficit disorder. Journal of the American Academy of Child Psychiatry 1986;25(4):509‐13. [DOI] [PubMed] [Google Scholar]
Pliszka 2000 {published data only}
- Pliszka SR, Browne RG, Olvera RL, Wynne SK. A double‐blind, placebo‐controlled study of Adderall and methylphenidate in the treatment of attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(5):619‐26. [DOI: 10.1097/00004583-200005000-00016] [DOI] [PubMed] [Google Scholar]
Ramtvedt 2013 {published data only}
- Ramtvedt BE, Aabech HS, Sundet K. Minimizing adverse events while maintaining clinical improvement in a pediatric attention‐deficit/hyperactivity disorder crossover trial with dextroamphetamine and methylphenidate. Journal of Child and Adolescent Psychopharmacology 2014;24(3):130‐9. [DOI: 10.1089/cap.2013.0114] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramtvedt BE, Røinås E, Aabech HS, Sundet KS. Clinical gains from including both dextroamphetamine and methylphenidate in stimulant trials. Journal of Child and Adolescent Psychopharmacology 2013;23(9):597‐604. [DOI: 10.1089/cap.2012.0085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharp 1999 {published data only (unpublished sought but not used)}
- Sharp WS, Walter JM, Marsh WL, Ritchie GF, Hamburger SD, Castellanos FX. ADHD in girls: clinical comparability of a research sample. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(1):40‐7. [PUBMED: 9893415] [DOI] [PubMed] [Google Scholar]
Shekim 1986 {published data only}
- Shekim WO, Bylund DB, Alexson J, Glaser RD, Jones SB, Hodges K, et al. Platelet MAO and measures of attention and impulsivity in boys with attention deficit disorder and hyperactivity. Psychiatry Research 1986;18(2):179‐88. [DOI: 10.1016/0165-1781(86)90029-6] [DOI] [PubMed] [Google Scholar]
Short 2004 {published data only}
- Short EJ, Manos MJ, Findling RL, Schubel EA. A prospective study of stimulant response in preschool children: insights from ROC analyses. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(3):251‐9. [DOI: 10.1097/00004583-200403000-00005] [DOI] [PubMed] [Google Scholar]
Spencer 2006a {published data only}
- Spencer TJ, Wilens TE, Biederman J, Weisler RH, Read SC, Pratt R. Efficacy and safety of mixed amphetamine salts extended release (Adderall XR) in the management of attention‐deficit/hyperactivity disorder in adolescent patients: a 4‐week, randomized, double‐blind, placebo‐controlled, parallel‐group study. Clinical Therapeutics 2006;28(2):266‐79. [DOI: 10.1016/j.clinthera.2006.02.011] [DOI] [PubMed] [Google Scholar]
Swanson 1998a {published data only (unpublished sought but not used)}
- Swanson JM, Wigal S, Greenhill LL, Browne R, Waslik B, Lerner M, et al. Analog classroom assessment of Adderall (R) in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37(5):519‐26. [PubMed] [Google Scholar]
Wigal 2009a {published data only}
- Wigal SB, Kollins SH, Childress AC, Squires L, for the 311 Study Group. A 13‐hour laboratory school study of lisdexamfetamine dimesylate in school‐aged children with attention‐deficit/hyperactivity disorder. Child and Adolescent Psychiatry and Mental Health 2009;3(1):17. [DOI: 10.1186/1753-2000-3-17] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akhondzadeh 2003 {published data only}
- Akhondzadeh S, Tavakolian R, Davari‐Ashtiani R, Arabgol F, Amini H. Selegiline in the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2003;27(5):841‐5. [DOI: 10.1016/S0278-5846(03)00117-9] [DOI] [PubMed] [Google Scholar]
Alexandris 1968 {published data only}
- Alexandris A, Lundell FW. Effect of thioridazine, amphetamine and placebo on the hyperkinetic syndrome and cognitive area in mentally deficient children. Canadian Medical Association Journal 1968;98(2):92‐6. [PMC free article] [PubMed] [Google Scholar]
Arnold 2011 {published data only}
- Arnold LE, Disilvestro RA, Bozzolo D, Bozzolo H, Crowl L, Fernandez S, et al. Zinc for attention‐deficit/hyperactivity disorder: placebo‐controlled double‐blind pilot trial alone and combined with amphetamine. Journal of Child and Adolescent Psychopharmacology 2011;21(1):1‐19. [PUBMED: 21309695] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biederman 2006 {published data only}
- Biederman J, Wigal SB, Spencer TJ, McGough JJ, Mays DA. A post hoc subgroup analysis of an 18‐day randomized controlled trial comparing the tolerability and efficacy of mixed amphetamine salts extended release and atomoxetine in school‐age girls with attention‐deficit/hyperactivity disorder. Clinical Therapeutics 2006;28(2):280‐93. [DOI: 10.1016/j.clinthera.2006.02.008] [DOI] [PubMed] [Google Scholar]
Biederman 2008 {published data only}
- Biederman J, Seidman LJ, Petty CR, Fried R, Doyle AE, Cohen DR, et al. Effects of stimulant medication on neuropsychological functioning in young adults with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2008;69(7):1150‐6. [DOI] [PubMed] [Google Scholar]
Boellner 2010 {published data only}
- Boellner SW, Stark JG, Krishnan S, Zhang Y. Pharmacokinetics of lisdexamfetamine dimesylate and its active metabolite, d‐amphetamine, with increasing oral doses of lisdexamfetamine dimesylate in children with attention‐deficit/hyperactivity disorder: a single‐dose, randomized, open‐label, crossover study. Clinical Therapeutics 2010;32(2):252‐64. [PUBMED: 20206783] [DOI] [PubMed] [Google Scholar]
Brown 2010 {published data only}
- Brown TE, Landgraf JM. Improvements in executive function correlate with enhanced performance and functioning and health‐related quality of life: evidence from 2 large, double‐blind, randomized, placebo‐controlled trials in ADHD. Postgraduate Medicine 2010;122(5):42‐51. [PUBMED: 20861587] [DOI] [PubMed] [Google Scholar]
Denhoff 1971 {published data only}
- Denhoff E, Davids A, Hawkins R. Effects of dextroamphetamine on hyperkinetic children: a controlled double blind study. Journal of Learning Disabilities 1971;4(9):491‐8. [DOI: 10.1177/002221947100400906] [DOI] [Google Scholar]
Donner 2007 {published data only}
- Donner R, Michaels MA, Ambrosini PJ. Cardiovascular effects of mixed amphetamine salts extended release in the treatment of school‐aged children with attention‐deficit/hyperactivity disorder. Biological Psychiatry 2007;61(5):706‐12. [DOI: 10.1016/j.biopsych.2006.05.002] [DOI] [PubMed] [Google Scholar]
Efron 1997 {published data only}
- Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double‐blind, crossover trial. Pediatrics 1997;100(4):662‐6. [PUBMED: 9310521] [DOI] [PubMed] [Google Scholar]
Findling 2009 {published data only}
- Findling RL, Ginsberg LD, Jain R, Gao J. Effectiveness, safety, and tolerability of lisdexamfetamine dimesylate in children with attention‐deficit/hyperactivity disorder: an open‐label, dose‐optimization study. Journal of Child and Adolescent Psychopharmacology 2009;19(6):649‐62. [PUBMED: 20035583] [DOI] [PubMed] [Google Scholar]
Greenhill 2003 {published data only}
- Greenhill LL, Swanson JM, Steinhoff K, Fried J, Posner K, Lerner M, et al. A pharmacokinetic/pharmacodynamic study comparing a single morning dose of Adderall to twice‐daily dosing in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(10):1234‐41. [PUBMED: 14560174] [DOI] [PubMed] [Google Scholar]
Kamien 1998 {published data only}
- Kamien M. The use of an N‐of‐1 randomised clinical trial in resolving therapeutic doubt. The case of a patient with an 'attention disorder'. Australian Family Physician 1998;27 Suppl 2:S103‐5. [PubMed] [Google Scholar]
Lopez 2008 {published data only}
- Lopez FA, Ginsberg LD, Arnold V. Effect of lisdexamfetamine dimesylate on parent‐rated measures in children aged 6 to 12 years with attention‐deficit/hyperactivity disorder: a secondary analysis. Postgraduate Medicine 2008;120(3):89‐102. [DOI: 10.3810/pgm.2008.09.1910] [DOI] [PubMed] [Google Scholar]
McGough 2005 {published data only}
- McGough JJ, Biederman J, Wigal SB, Lopez FA, McCracken JT, Spencer T, et al. Long‐term tolerability and effectiveness of once‐daily mixed amphetamine salts (Adderall XR) in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(6):530‐8. [DOI: 10.1097/01.chi.0000157550.94702.a2] [DOI] [PubMed] [Google Scholar]
Najib 2009 {published data only}
- Najib J. The efficacy and safety profile of lisdexamfetamine dimesylate, a prodrug of d‐amphetamine, for the treatment of attention‐deficit/hyperactivity disorder in children and adults. Clinical Therapeutics 2009;31(1):142‐76. [DOI: 10.1016/j.clinthera.2009.01.015] [DOI] [PubMed] [Google Scholar]
Nikles 2006 {published data only}
- Nikles CJ, Mitchell GK, Mar CB, Clavarino A, McNairn N. An n‐of‐1 trial service in clinical practice: testing the effectiveness of stimulants for attention‐deficit/hyperactivity disorder. Pediatrics 2006;117(6):2040‐6. [DOI] [PubMed] [Google Scholar]
Quintana 2007 {published data only}
- Quintana H, Cherlin EA, Duesenberg DA, Bangs ME, Ramsey JL, Feldman PD, et al. Transition from methylphenidate or amphetamine to atomoxetine in children and adolescents with attention‐deficit/hyperactivity disorder ‐ a preliminary tolerability and efficacy study. Clinical Therapeutics 2007;29(6):1168‐77. [DOI: 10.1016/j.clinthera.2007.06.017] [DOI] [PubMed] [Google Scholar]
Scheffer 2005 {published data only}
- Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo‐controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. American Journal of Psychiatry 2005;162(1):58‐64. [PUBMED: 15625202] [DOI] [PubMed] [Google Scholar]
Sleator 1974 {published data only}
- Sleator EK, Neumann A, Sprague RL. Hyperactive children: a continuous long‐term placebo‐controlled follow‐up. JAMA 1974;229(3):316‐7. [DOI: 10.1001/jama.1974.03230410040022.] [DOI] [PubMed] [Google Scholar]
Spencer 2005 {published data only}
- Spencer TJ, Biederman J, Wilens TE. Efficacy and tolerability of long‐term, open‐label, mixed amphetamine salts extended release in adolescents with ADHD. CNS Spectrums 2005;10 Suppl 15:14‐21. [DOI: 10.1017/S1092852900014103] [DOI] [Google Scholar]
Spencer 2006b {published data only}
- Spencer TJ, Abikoff HB, Connor DF, Biederman J, Pliszka SR, Boellner S, et al. Efficacy and safety of mixed amphetamine salts extended release (Adderall XR) in the management of oppositional defiant disorder with or without comorbid attention‐deficit/hyperactivity disorder in school‐aged children and adolescents: a 4‐week, multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled, forced‐dose‐escalation study. Clinical Therapeutics 2006;28(3):402‐18. [DOI: 10.1016/j.clinthera.2006.03.006] [DOI] [PubMed] [Google Scholar]
Turgay 2010 {published data only}
- Turgay A, Ginsberg L, Sarkis E, Jain R, Adeyi B, Gao J, et al. Executive function deficits in children with attention‐deficit/hyperactivity disorder and improvement with lisdexamfetamine dimesylate in an open‐label study. Journal of Child and Adolescent Psychopharmacology 2010;20(6):503‐11. [DOI: 10.1089/cap.2009.0110] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wigal 2009b {published data only}
- Wigal SB, Kollins SH, Childress AC, Squires L, for the 311 Study Group. A 13‐hour laboratory school study of lisdexamfetamine dimesylate in school‐aged children with attention‐deficit/hyperactivity disorder. Child and Adolescent Psychiatry and Mental Health 2009;3(1):17. [DOI: 10.1186/1753-2000-3-17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wigal 2010a {published data only}
- Wigal SB, Kollins SH, Childress AC, Adeyi B. Efficacy and tolerability of lisdexamfetamine dimesylate in children with attention‐deficit/hyperactivity disorder: sex and age effects and effect size across the day. Child and Adolescent Psychiatry and Mental Health 2010;4:32. [DOI: 10.1186/1753-2000-4-32] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wigal 2010b {published data only}
- Wigal T, Brams M, Gasior M, Gao J, Squires L, Giblin J, et al. Randomized, double‐blind, placebo‐controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention‐deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design. Behavioral and Brain Functions 2010;6:34. [DOI: 10.1186/1744-9081-6-34] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Glos 1973 {published data only}
- Glos J. Amphetamines in the treatment of hyperactive and unstable children. Ceskoslovenska Pediatrie 1973;28(10):559‐60. [PubMed] [Google Scholar]
Itil 1974 {published data only}
- Itil TM, Simeon J. Proceedings: computerized EEG in the prediction of outcome of drug treatment in hyperactive childhood behavior disorders. Psychopharmacology Bulletin 1974;10(4):36. [PUBMED: 4610619] [PubMed] [Google Scholar]
References to ongoing studies
Fanton 2009 {published data only}
- Fanton J, Waslick B, Harvey E. The 49th Annual National Institute of Mental Health (NIMH) New Clinical Drug Evaluation Unit (NCDEU) meeting Hollywood, Florida, June 29‐July 2, 2009: posters most relevant to child and adolescent psychopharmacology. Journal of Child and Adolescent Psychopharmacology 2009; Vol. 19, issue 6:786. [DOI] [PubMed]
NCT01711021 {published data only}
- NCT01711021. A randomized, double‐blind, placebo‐controlled, crossover, laboratory classroom study to evaluate the safety and efficacy of d‐amphetamine transdermal drug delivery system (d‐ATS) compared to placebo in children and adolescents with ADHD. http://clinicaltrials.gov/ct2/show/record/NCT01711021 (accessed 11 February 2014).
Additional references
AHRQ 1999
- Agency for Health Care Policy and Research. Diagnosis of attention‐deficit/hyperactivity disorder. Summary: technical review: number 3. www.ahrq.gov/clinic/epcsums/adhdsutr.htm (accessed 1 May 2012).
Anderson 1999
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Natural Neuroscience 1999;2(11):1032‐7. [DOI] [PubMed] [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III‐R). 3rd Edition. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR). 4th Edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
Arnsten 2006
- Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology 2006;31(11):2376‐83. [DOI] [PubMed] [Google Scholar]
Bero 2013
- Bero LA. Why the Cochrane risk of bias tool should include funding source as a standard item [editorial]. Cochrane Database of Systematic Reviews 2013, issue 12:1. [DOI: 10.1002/14651858.ED000075] [DOI] [PMC free article] [PubMed]
Biederman 1993
- Biederman J, Faraone SV, Spencer T, Wilens T, Norman D, Lapey KA, et al. Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. American Journal of Psychiatry 1993;150(12):1792‐8. [DOI] [PubMed] [Google Scholar]
Buck 2002
- Buck ML. Amphetamines in the treatment of attention‐deficit/hyperactivity disorder. Pediatric Pharmacotherapy 2002;8(3):1‐3. [Google Scholar]
Castells 2011
- Castells X, Ramos‐Quiroga JA, Bosch R, Nogueira M, Casas M. Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD007813.pub2] [DOI] [PubMed] [Google Scholar]
Charach 2011
- Charach A, Dashti B, Carson P, Booker L, Lim CG, Lillie E, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at‐risk preschoolers; long‐term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Agency for Healthcare Research and Quality 2011. [PubMed]
Cohen 1988
- Cohen J. Statistical Power Analysis in Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates Inc, 1988. [Google Scholar]
Conners 1990
- Conners CK. Conners’ Abbreviated Symptom Questionnaire: Parent Version, Teacher Version Manual. Toronto: Multi‐Health Systems, 1990. [Google Scholar]
Conners 1998a
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS‐R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):257‐68. [DOI] [PubMed] [Google Scholar]
Conners 1998b
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS‐R): factor structure, reliability and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):279‐91. [DOI] [PubMed] [Google Scholar]
Costello 1985
- Costello EJ, Edelbrock CS, Costello AJ. Validity of the NIMH Diagnostic Interview Schedule for Children: a comparison between psychiatric and pediatric referrals. Journal of Abnormal Child Psychology 1985;13(4):579‐95. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DuPaul 1998
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale—IV: Checklists, Norms, and Clinical Interpretation. New York: The Guilford Press, 1998:186‐201. [Google Scholar]
Edwards 1995
- Edwards L, Salant V, Howard VF, Brougher J, McLaughlin TF. Effectiveness of self‐management on attentional behavior and reading comprehension for children with attention deficit disorder. Child and Family Behavior Therapy 1995;17(2):1‐17. [DOI: 10.1300/J019v17n02_01] [DOI] [Google Scholar]
Egger 1997
- Egger M, Zellwenger‐ Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomized controlled trials published in English and German. Lancet 1997;350(9074):329‐9. [DOI: 10.1016/S0140-6736(97)02419-7] [DOI] [PubMed] [Google Scholar]
Follman 1992
- Follman D, Elliott P, Suh II, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI: 10.1016/0895-4356(92)90054-Q] [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Education and debate: grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐9. [DOI: 10.1136/bmj.328.7454.1490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guy 1976
- Busner J, Targum SD. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health, 1976. [Google Scholar]
Hedges 1994
- Hedges LV. Statistical considerations. In: Cooper H, Hedges LV (editors). The Handbook of Research Synthesis. New York (NY): Russell Sage Foundation 1994.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Mayes 2009
- Mayes SD, Calhoun SL, Bixler EO, Vgontzas AN, Mahr F, Hillwig‐Garcia J, et al. ADHD subtypes and comorbid anxiety, depression and oppositional‐defiant disorder: differences in sleep problems. Journal of Pediatric Psychology 2009;34(33):328‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1999
- Miller A, Lee S, Raina P, Klassen A, Zupancic J, Olsen L. A review of therapies for attention‐deficit/hyperactivity disorder. www.cadth.ca/en/products/health‐technology‐assessment/publication/20 (accessed 11 February 2014).
Moher 2003
- Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomized trials published in languages other than English in systematic reviews. Health Technology Assessment 2003;7(41):1‐90. [DOI] [PubMed] [Google Scholar]
Moore 2011
- Moore EA. Ritalin, Adderall and other treatments for ADHD. The Amphetamine Debate: The Use of Adderall, Ritalin and Related Drugs for Behavior Modification, Neuroenhancement, and Anti‐Aging Purposes. Jefferson, North Carolina: McFarland and Company, Inc, 2011:80‐107. [Google Scholar]
NIH 2000
- National Institute of Health. National Institutes of Health consensus development conference statement: diagnosis and treatment of attention‐deficit/hyperactivity disorder (ADHD). Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(2):182‐97. [DOI] [PubMed] [Google Scholar]
NIMH 2009
- National Institute for Mental Health. Attention Deficit Hyperactivity Disorder (ADHD). www.nimh.nih.gov/health/publications/attention‐deficit‐hyperactivity‐disorder/complete‐index.shtml (accessed 1 May 2012).
Polanczyk 2007
- Polanczyk G, Silva de Lima M, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metagression analysis. American Journal of Psychiatry 2007;164(6):942‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salum 2012
- Salum GA, Patrick DL, Isolan LR, Manfro GG, Fleck MP. Youth Quality of Life Instrument‐Research version (YQOL‐R): psychometric properties in a community sample [Youth Quality of Life Instrument‐Research version (YQOL‐R): propriedades psicométricas em uma amostra comunitária]. Journal of Pediatrics 2012;88(5):443‐8. [PUBMED: 22850664] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. Research Methods & Reporting: CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. BMJ 2010;340:c332. [DOI: 10.1136/bmj.c332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2013
- Sterne JAC. Why the Cochrane risk of bias tool should not include funding source as a standard item [editorial]. Cochrane Database of Systematic Reviews 2013, issue 12:1. [DOI: 10.1002/14651858.ED000076] [DOI] [PMC free article] [PubMed]
Swanson 1998b
- Swanson J, Wigal S, Greenhill L, Browne R, Waslick B, Lerner M, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacoly Bulletin 1998;34(1):55‐60. [PUBMED: 9564199] [PubMed] [Google Scholar]
Swanson 2007
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. Etiologic subtypes of attention‐deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychology Reviews 2007;17(1):39‐59. [PUBMED: 17318414] [DOI] [PubMed] [Google Scholar]
The Medical Letter 2007
- The Medical Letter Online. Lisdexamfetamine dimesylate (Vyvanase) for ADHD. The Medical Letter on Drugs and Therapeutics 2007;49(1265):58‐9. [PubMed] [Google Scholar]
Varni 1998
- Varni JW, Katz ER, Seid M, Quiggins DJ, Friedman‐Bender A. The Pediatric Quality of Life Inventory‐32 (PedsQL‐32): reliability and validity. Cancer 1998;82(6):1184‐96. [PUBMED: 9506367] [DOI] [PubMed] [Google Scholar]
Wechsler 1991
- Wechsler D. Wechsler Intelligence Scale for Children. San Antonio, TX: The Psychological Corporation, 1991. [Google Scholar]
Wigal 1999
- Wigal T, Swanson JM, Regino R, Lerner MA, Soliman I, Steinoff K, et al. Stimulant medications for the treatment of ADHD: efficacy and limitations. Mental Retardation and Development Disabilities Research Reviews 1999;5(3):215‐24. [Google Scholar]
Williamson 2012
- Williamson PR, Clarke M. The COMET (Core Outcome Measures in Effectiveness Trials) Initiative: its role in improving Cochrane Reviews [editorial]. Cochrane Database of Systematic Reviews 2012, issue 4. [DOI: 10.1002/14651858.ED000041] [DOI] [PMC free article] [PubMed]
References to other published versions of this review
Punja 2012
- Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles CJ, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009996] [DOI] [PMC free article] [PubMed] [Google Scholar]


