Abstract
We evaluated how pain processing and situational pain catastrophizing differed between two music interventions (Unwind and favorite music) and a control condition (white noise). Healthy adults (n=70) completed quantitative sensory testing (QST) measuring pressure pain threshold (PPTh) and tolerance (PPTol), heat pain threshold (HPTh), offset analgesia (OA), temporal summation of pain (TSP), and conditioned pain modulation (CPM). Participants completed three QST rounds with the presence of white noise (control condition), a relaxing music app (Unwind), and their favorite music, which were presented in a randomized order. The Situational Pain Catastrophizing Scale was completed after each round. Friedman tests and post-hoc Wilcoxon signed-rank tests were used to compare pain processing and catastrophizing across the three conditions. Participants’ PPTh, PPTol, and HPTh were significantly higher during the favorite music condition compared to the other two conditions, indicating lower pain sensitivity when listening to favorite music. In contrast, OA was lower in the favorite music condition. Although TSP and CPM were induced by the QST paradigm, these did not differ across the three conditions. Situational pain catastrophizing was also significantly lower during the favorite music condition. Several measures of pain sensitivity and situational pain catastrophizing were lower when listening to favorite music compared to relaxing music or white noise. More research is necessary to determine the mechanism(s) by which music modulates pain processing.
Keywords: Pain Sensitivity, Music, Analgesia, Pain Catastrophizing, Quantitative Sensory Testing
INTRODUCTION
Music is a ubiquitous experience that transcends age, sex, race, and culture. With an increasing interest in non-opioid alternatives, music has gained attention as a potential adjunctive therapy to reduce pain.5 Previous studies suggest that music may reduce overall pain severity in individuals with chronic pain9, 27, 28, 39, 44 across diagnostic categories, including fibromyalgia,27,44 neuropathic, musculoskeletal and inflammatory pain,27 osteoarthritis,39 and during palliative care (predominantly cancer-related pain).28 Music may also reduce pain in different clinical settings, including in the emergency department9 and in the perioperative period.24, 36, 64 The impact of music on pain processing has been investigated in experimental settings using quantitative sensory testing (QST), which involves the application of well-defined and calibrated sensory stimuli to characterize psychophysical responses to pain.48 Individual differences in sensory processing discerned by QST have been associated with differences in clinical pain.58 Some studies have suggested that music alters pain processing, increasing pain thresholds and reducing temporal summation of pain.7, 22, 31, 35, 40
In addition to preliminary evidence of efficacy for reducing pain,38 music interventions have been characterized as low-risk, practical, and easy to implement. Prior studies have found that healthy adults and postsurgical patients report greater pain tolerance and less post-surgical pain when listening to music compared to white noise, which is often used as a control condition.4, 30, 53 A less explored question asks what type of music most effective, as musical taste differs, and different types of music evoke varying responses across individuals. Thus, whether an individual enjoys or feels connected to a particular piece of music, such as to their favorite music, may substantially impact the degree to which it reduces pain and negative psychological processes.31, 40, 64 Many studies investigating the impact of music on pain have used classical music.27, 39, 44, 64 Others have employed deconstructed simple beats and harmonies,18 a selection of relaxing soundtracks,7, 9 or a general selection of music genres for participants to choose from,11, 25 with variable effectiveness among individuals. At least one recent study has suggested the importance of choice and cognitive agency in particular.31
Similarly, music has been shown to modulate psychological factors associated with the experience of pain.23 A common negative psychological process associated with worsened pain is pain catastrophizing,2, 15, 16, 41, 47, 59 which occurs during or in anticipation of pain, and includes magnification, rumination, and feelings of helplessness in the face of pain. While catastrophizing is often assessed as a trait using the Pain Catastrophizing Scale (PCS),54 assessment during a procedure or in the setting of experimental pain testing can be accomplished using the briefer situational pain catastrophizing scale (SPCS). Notably, research has shown that greater situational pain catastrophizing is associated with lower pain thresholds and tolerance, as well as greater pain severity ratings among healthy adults.17 While one study did not observe a difference in situational catastrophizing between a control condition and relaxing music,7 it has yet to be explored in the context of favorite music.
The present study aimed to pragmatically investigate the impact of relaxing music versus participant-chosen favorite music on pain processing. Specifically, we investigated how nociceptive processing during quantitative sensory testing (QST) varied between two different types of music conditions (relaxing music on the Unwind app vs. participants’ personal selection of favorite music) and a control condition (white noise). We used a randomized block design, where each participant experienced all three conditions in a random order, and pain sensitivity was compared across the three conditions within the same participant. We also explored differences in negative catastrophic thinking about experimental pain stimuli (situational pain catastrophizing) across the three conditions.
METHODS
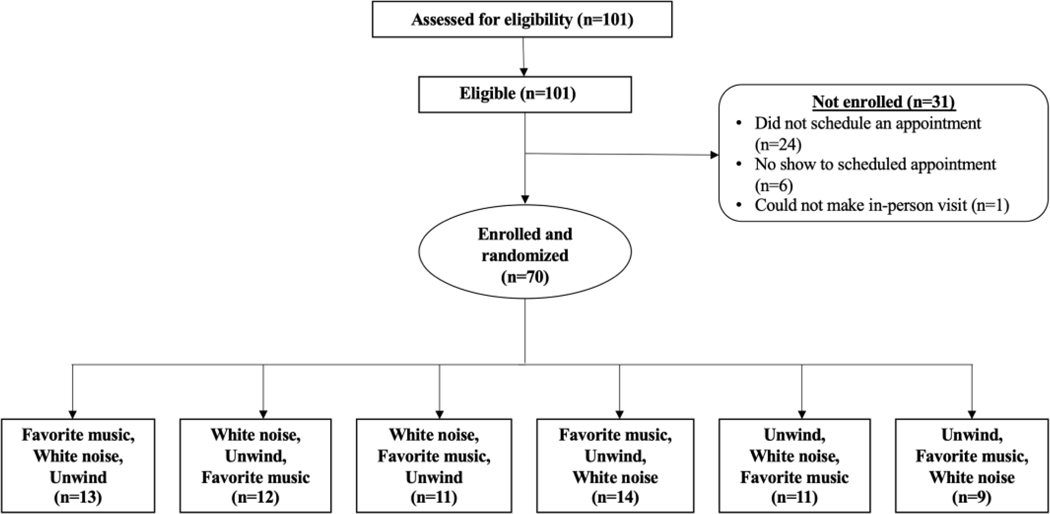
This observational study of healthy adults was approved by the Partners Human Research Committee/Institutional Review Board (2019P000824) and registered with ClinicalTrials.gov (NCT04087564). Participants were recruited from July 18, 2019-February 25, 2020 through Rally, a Partners Healthcare research website. All interested participants were screened via email to determine their eligibility. Inclusion criteria were: 1) ≥18 years of age, 2) English speaking, 3) no current chronic opioid use (prescription >30 days), and 4) no previous diagnosis of hearing loss, neuropathy, or chronic pain. Eligible participants were then scheduled for a study visit and were asked to provide a list of their seven favorite songs (Figure 1). Informed consent was obtained from each study participant. The study took approximately 1.5–2 hours to complete, and participants received a $40 Amazon gift card upon study completion.
Figure 1. Study flow diagram.
Quantitative Sensory Testing of Pain
Each participant completed four rounds of Quantitative Sensory Testing (QST).7 For the first round (baseline), QST was completed in the absence of sound. Values for baseline testing are included in Supplemental Table S1 but were not otherwise used in the analysis. QST was then repeated for three more rounds, each with one of the three different conditions (white noise, Unwind, favorite music) playing during that round. To balance any potential order effects, the order of conditions was randomized (Figure 1). The duration of each QST round was approximately 20 minutes, followed by a 5-minute rest period.
Pressure pain threshold (PPTh) and tolerance (PPTol).
PPTh and PPTol were measured using a digital pressure algometer (Wagner FDX, Greenwich, CT, USA) with a flat round transducer (probe area, 0.785 cm). PPTh and PPTol were each assessed bilaterally at two locations, 1) the dorsal aspect of the proximal forearm (Forearm PPTh and Forearm PPTol) and 2) over the trapezius muscle at the upper back (Trapezius PPTh and Trapezius PPTol), as in previous studies.51, 52 Pressure was gradually increased (1 lb./sec), with a maximal exerted pressure of 20 lbs. Participants were asked to verbally indicate when the pressure first became painful (PPTh) and then again when they wanted the pressure to stop (i.e., no longer tolerable, PPTol). PPThs and PPTols were averaged across sides (left and right) at each site to compute four main pressure pain-related outcomes: 1) forearm PPTh and 2) forearm PPTol, 3) trapezius PPTh and 4) trapezius PPTol. PPTh and PPTol were assessed during each of the three conditions.
Heat pain threshold (HPTh) and offset analgesia (OA).
Contact heat stimuli were administered using a 1.6 X 1.6 cm square (2.56 cm2) contact thermode (Medoc Advanced Medical Systems, Ramat Yishai, Israel). The contact thermode was positioned on the skin on the volar aspect of the left forearm and affixed in place with a soft Velcro strap. The probe remained in place for the duration of both threshold and offset analgesia testing. The temperature of the probe started at a baseline temperature of 32°C and increased at a rate of 2°C/sec, with a maximum possible temperature of 50°C. Participants were instructed to press a button on a remote when thermal stimulation first became painful (HPTh). Subsequently, subjects were instructed to press a button when their pain intensity became a 5/10 rating in response to the thermal stimulation. During only the baseline testing session, the 5/10 threshold temperature was measured three times, and the average was then used to determine the starting temperature for the OA protocol used for that participant. The placement of the thermode was marked and replaced in the same location for each of the following conditions. For the subsequent three randomized order trials (white noise, Unwind, favorite music) the temperature probe was again placed on the forearm in the previously marked location and a single measurement of the HPTh and the 5/10 threshold were performed.
After HPTh and 5/10 threshold testing, the OA protocol began using the original temperature profile determined in the baseline round, with the same temperature profile used across all three conditions. OA was assessed once per condition (white noise, Unwind, favorite music) using methods adapted from previous descriptions.1, 26, 42, 45, 63 First, the thermode temperature was steadily increased from 32°C at a rate of 2°C/sec until the T1 plateau temperature was reached (T1=one degree less than the 5/10 rating). T1 plateau was held for five seconds, after which the temperature was increased 1°C to the T2 plateau (the previously indicated average 5/10 threshold temperature). T2 was held for five seconds, after which the temperature was decreased 1°C to the T3 plateau (same temperature as T1). T3 was held for five seconds, after which the thermode temperature steadily decreased to 32°C and was removed from the forearm. During the OA paradigm, participants were asked to rate their pain on a scale of 0 (no pain) to 10 (worst pain imaginable) approximately 2 seconds into each of the three temperature plateaus (T1, T2, T3). OA was calculated using the difference between T3 and T1 pain ratings.
Temporal summation of pain (TSP).
TSP was assessed using standardized weighted pinprick applicators (128mN, 256mN, and 512mN), similar to those described by Rolke et al.48 The pinpricks were applied to the dorsal aspect of the index finger between the first and second interphalangeal joints of each hand, and participants were asked to rate their pain on a scale from 0–10. The highest weighted probe that induced a pain score between 1–3/10 on single stimulation was then selected for repeated administration (temporal summation testing). After a break of at least 10 seconds after the single application, a train of 10 stimuli were applied at the same spot at a rate of 1 stimulation/sec. The subject again rated pain on a scale of 0–10 after the 5th and 10th stimulus. TSP was calculated using the difference between 10th stimulation pain score and the single stimulation pain score.
Conditioned pain modulation (CPM).
CPM is a phenomenon where one stimulus inhibits the perceived pain of another. A test stimulus is first applied alone and then in the presence of another painful conditioning stimulus. CPM is the difference in perceived pain from the test stimulus alone vs. in the presence of the conditioning stimulus.60 In the current study, PPTh measured over the trapezius served as the test stimulus, and cold pain applied to the hand on the contralateral side using immersion in a cold water bath was the conditioning stimulus, thus meeting the recommendation that the CPM paradigm employ both mechanical (i.e., pressure pain) and thermal (cold pain) stimuli.61
Participants’ PPTh over the left trapezius had been previously tested in isolation (see “Pressure Pain” above), which served as the baseline PPTh. In the CPM paradigm, participants were asked to briefly submerge their right hand up to their wrist in the cold-water bath (set at 6°C) for five seconds. After five seconds, the tester began assessing the participant’s PPTh (i.e., the conditioned PPTh). CPM was measured once per condition (white noise, Unwind, favorite music) and was calculated as shown in the following equation:46
Situational Pain Catastrophizing
After each QST session, subjects completed the 6-item Situational Pain Catastrophizing Scale (SPCS) [23] to assess participants’ negative, pain-related thinking that occurred in response to the experimental pain stimuli.54, 55 Participants were instructed to think about the most recent QST session and rate the statements (e.g., “I felt that I couldn’t stand it”) on a scale from 0 (not at all) to 4 (all the time). SPCS statements after each QST session were summed, with higher scores reflecting greater situational pain catastrophizing.
Music Conditions
All study sessions began with a baseline administration of QSTs, which occurred in silence. This round of testing served as a training session to introduce participants to the experimental paradigm, answer questions, and teach the protocol to ensure subsequent runs across the three conditions would be smoother and more uniform. Participants then completed three additional rounds of QST in the different conditions: 1) white noise, 2) Unwind, and 3) favorite music. The order of the conditions was randomized for each participant using the “Randomization” function in REDCap,29 and all participants experienced all conditions.
In the white noise condition, participants listened to a one-hour track titled “White noise” from Apple Music. As defined, white noise maintains the same intensity at all audible frequencies,33 dissimilar from the two music conditions, where frequency and intensity of sound elements vary. The Unwind smartphone-based app (Unwind; Bose Corporation, Boston, MA, USA) has five different instrumental tracks, all of which were created by a machine learning algorithm that combined elements of various human-composed instrumental soundtracks. These tracks were designed to be relaxing and have been previously employed in a study among patients presenting to the ED.7, 8, 19 Participants were presented with a list of tracks, of which they could listen to a short sample before selecting one of the five tracks to listen to during one round of QST. All tracks were approximately ten minutes in length, and thus these tracks were partially repeated during the course of the QST testing round. In the favorite music condition, participants listened to their self-selected favorite songs sent to study staff prior to visit. When asked to provide songs for the session, study staff told participants, “These [songs] do not have to be anything specific other than songs you enjoy listening to.” We requested that participants select seven songs to allow sufficient time to cover the QST session (pilot testing had indicated 20 minutes as longest possible duration), considering that most songs are at least three minutes in length, thus ensuring the completion of QST procedures without silence or repetition of songs. During the QST session, the seven selected songs were played in a randomized order using the shuffle feature on Apple Music. Depending on how long each session took to complete, all songs may not have been played. In all three conditions, a JBL Charge 3 speaker was used. Participants were asked to adjust the volume of the speaker to a comfortable level, such that they were still able to clearly hear and respond to instructions during testing.
Statistical analysis
Participants’ pain, situational catastrophizing, and demographic characteristics were summarized using frequencies, percentages, means, and standard deviations. Because the data were not normally distributed, non-parametric Friedman tests were used to assess differences in QST pain outcomes (PPTh and PPTol, HPTh and OA, TSP, and CPM) and situational catastrophizing across the three conditions (p≤.05). Post-hoc analyses with Wilcoxon signed- rank tests were conducted to make pair-wise comparisons among the three conditions when appropriate (p≤.05). Corrections for multiple comparisons were not conducted. For each pain variable, the effect size was calculated by dividing the test statistic by the square root of the number of observations.34 Spearman correlations were used to explore whether situational pain catastrophizing reported during each condition (white noise, Unwind, favorite music) was significantly associated with pain variables.
A power analysis was conducted to determine the minimum sample size to test our study aim. Results indicated that a required sample size to achieve 80% power for detecting an effect at a significance level of .05 was 64. Taking potential dropout into account, 70 participants were planned for recruitment. All data were analyzed using SPSS (v28).
RESULTS
Participant characteristics
Participant characteristics are summarized in Table 1. Screening identified 101 eligible participants, with some participants not scheduling a visit or otherwise declining participation (Figure 1). Ultimately, 70 participants presented for visit, provided informed consent, and completed all QST sessions and questionnaires. Participants were majority female (67%), had a college degree or higher (79%), and had an average age of 35 years (SD=18.3; range: 18–86). Participants self-identified as White (59%), Asian (23%), Black (6%), Hispanic/Latino (14%), and other (7%). Participant characteristics were similar across the six randomized groups (six possible orders of presentation of the three conditions), making it less likely that order of presentation could account for any observed effects.
Table 1.
Demographic characteristics
| Variables | M (SD) or N (%) |
|---|---|
|
| |
| Age (years) | 35.0 (18.3) |
| Gender | |
| Male | 23 (32.9%) |
| Female | 47 (67.1%) |
| Race | |
| White | 41 (58.6%) |
| Black | 4 (5.7%) |
| Asian | 16 (22.9%) |
| American Indian/Alaskan Native | 1 (1.4%) |
| Other | 5 (7.1%) |
| Prefer not to say | 1 (1.4%) |
| More than one race | 2 (2.9%) |
| Ethnicity | |
| Not Hispanic or Latino | 59 (84.3%) |
| Hispanic or Latino | 10 (14.3%) |
| Prefer not to say | 1 (1.4%) |
| Marital Status | |
| Single, never married | 46 (65.7%) |
| Married or in partnership | 13 (18.6%) |
| Separated or divorced | 5 (7.1%) |
| Together/living with partner | 5 (7.1%) |
| Widowed | 1 (1.4%) |
| Education | |
| High school/GED | 2 (2.9%) |
| Some college | 13 (18.6%) |
| Bachelor’s degree | 30 (42.9%) |
| Graduate/Professional degree | 25 (35.7%) |
Abbreviations: M=Mean; SD=Standard deviation; N=Number.
For the favorite music condition, 461 total tracks were compiled. To give a descriptive account of what type of music participants chose to listen to during the session, each music track was categorized based on genre. Chosen songs belonged to a variety of genres, including Pop (26%), Alternative/Indie (25%), R&B/Soul/Disco (13%), Country (9%), Hip-Hop/Rap (8%), Rock/Metal (7%), Electronic/Dance/EDM (5%), Folk (4%), Jazz (2%), and Classical (1%). Many participants had multiple genres represented among the seven songs in their playlist (see Supplemental Table S2 for a full list of favorite songs and genres).
Music and Pain Characteristics
Pressure Pain Threshold (PPTh) and Tolerance (PPTol)
Forearm pressure pain threshold (PPTh) was significantly different between the three conditions (χ2(2)=33.21, p<.001). Specifically, post-hoc pairwise group comparisons revealed that participants had a significantly higher forearm PPTh during the favorite music condition compared to both the white noise and Unwind conditions (Table 2). A similar pattern was found for forearm pressure pain tolerance (PPTol) (χ2(2)=34.40, p<.001), such that participants had a significantly higher PPTol during the favorite music condition compared to the white noise and Unwind conditions (Table 2). No significant difference was observed between the Unwind and white noise conditions for either forearm PPTh or PPTol.
Table 2.
Pain and Catastrophizing Across Conditions
| Unwind (U) | White Noise (WN) | Favorite Music (FM) |
Effect Size
|
||||
|---|---|---|---|---|---|---|---|
| Variables | M (SD) | M (SD) | M (SD) | P Value | FM versus U | FM versus WN | U versus WN |
|
| |||||||
| QST | |||||||
| Forearm: pain pressure threshold*,† (lbs) | 6.69 (3.70) | 6.95 (3.78) | 7.88 (3.98) | <.001 | .43 | .39 | .11 |
| Forearm: pain pressure tolerance*,† (lbs) | 9.81 (4.65) | 10.08 (4.70) | 11.11 (4.72) | <.001 | .46 | .40 | .15 |
| Trapezius: pain pressure threshold* (lbs) | 10.68 (4.47) | 10.36 (4.49) | 11.13 (4.82) | .050 | .14 | .22 | .07 |
| Trapezius: pain pressure tolerance*,† (lbs) | 13.85 (4.63) | 13.70 (4.63) | 14.48 (4.57) | .011 | .28 | .27 | .02 |
| Heat pain threshold*,† (°C) | 41.12 (2.87) | 41.00 (3.30) | 41.81 (3.25) | .026 | .19 | .20 | .00 |
| Offset analgesia (T3-T1)*,† (change in NRS at T3-T1) | −1.20 (1.79) | −1.25 (1.82) | -0.60 (1.85) | .007 | .24 | .26 | .00 |
| Offset analgesia: time 1*,† (NRS) | 4.16 (.96) | 4.27 (1.89) | 3.45 (1.65) | <.001 | .28 | .36 | .03 |
| Offset analgesia: time 2*,† (NRS) | 5.58 (2.19) | 5.87 (2.12) | 5.09 (2.07) | <.001 | .24 | .35 | .14 |
| Offset analgesia: time 3 (NRS) | 2.96 (1.83) | 3.01 (2.06) | 2.85 (1.98) | .680 | .08 | .09 | .02 |
| Conditioned pain modulation (% change from PPTh) | 15.70% (24.62) | 13.53% (25.76) | 13.05% (20.37) | .453 | .03 | .09 | .04 |
| Pinprick temporal summation (10th-baseline NRS) | 2.53 (1.78) | 2.43 (1.82) | 2.32 (1.73) | .281 | .07 | .13 | .06 |
| Psychosocial | |||||||
| Situational pain catastrophizing*,† (range, 0–24) | 1.31 (2.30) | 1.61 (2.18) | 0.87 (1.85) | <.001 | .28 | .21 | .15 |
Abbreviations: QST, quantitative sensory tests; NRS, numerical rating scale; PPTh, pressure pain threshold.
Significant difference between the favorite music and white noise conditions (P < .05).
Significant difference between the favorite music and Unwind conditions (P < .05). Higher scores for threshold and tolerance reflect lower pain sensitivity. Lower scores on offset analgesia reflect lower pain scores given at the designated temperatures. Higher scores of CPM reflect greater modulation of pain (less sensitive), and higher scores on pinprick temporal summation reflect greater pain amplification.
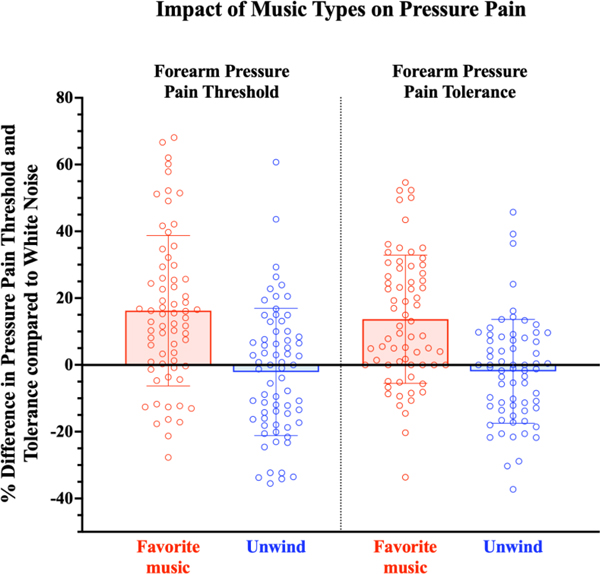
To gain further insight into how the exposure to music (favorite music or Unwind) vs. no music (white noise) played a role in pain processing, we explored the percent difference in pain processing between the favorite music vs. white noise conditions ((favorite music – white noise/white noise)*100), as well as the Unwind vs. white noise conditions ((Unwind– white noise/white noise)*100). Positive numbers indicated a decrease in pain sensitivity (higher PPTh or PPTol). There was some variation among individuals, but when expressing decreased sensitivity in the two music conditions (favorite music and Unwind) compared to the white noise condition, favorite music appeared to impact participants’ PPTh and PPTol more consistently than Unwind (Figure 2).
Figure 2. Percent difference in forearm pressure pain threshold (PPTh) and tolerance (PPTol) when listening to favorite music and relaxing music (Unwind) compared to white noise.
Participants reported a greater increase in PPTh and PPTol during the favorite music condition compared to white noise (PPTh mean change: 16% ± 23%; PPTol mean change: 14% ± 19%), indicating a decrease in pain sensitivity. Participants reported little change in PPTh during the Unwind condition compared to white noise (PPTh mean change: −2% ± 19%; PPTol mean change: −2% ± 16%). Percent difference in PPTh during favorite music compared to white noise was calculated using the formula ((favorite music-white noise)/white noise)*100. Percent difference in PPTh during the Unwind compared to white noise was calculated using the formula ((Unwind-white noise)/white noise)*100.
Similarly, there was a significant difference in trapezius PPTh (χ2(2)=6.01, p=0.050) and PPTol (χ2(2)=9.07, p=.011) across the three conditions, such that participants’ trapezius PPTh was higher during the favorite music condition compared to the white noise condition, and trapezius PPTol was higher during the favorite music condition compared to both the Unwind and white noise conditions (Table 2). There was not a significant difference in trapezius PPTh between the favorite music and Unwind condition. No significant differences in trapezius PPTh or PPTol were observed between the Unwind and white noise conditions.
Heat Pain Threshold (HPTh) and Offset Analgesia (OA)
We also observed a significant difference in heat pain threshold (HPTh) across the three conditions (χ2(2)=7.32, p=.026). Post-hoc analyses revealed that participants’ HPTh was significantly higher during the favorite music condition compared to the Unwind and white noise conditions (Table 2). No significant difference in HPTh between the Unwind and white noise conditions was observed.
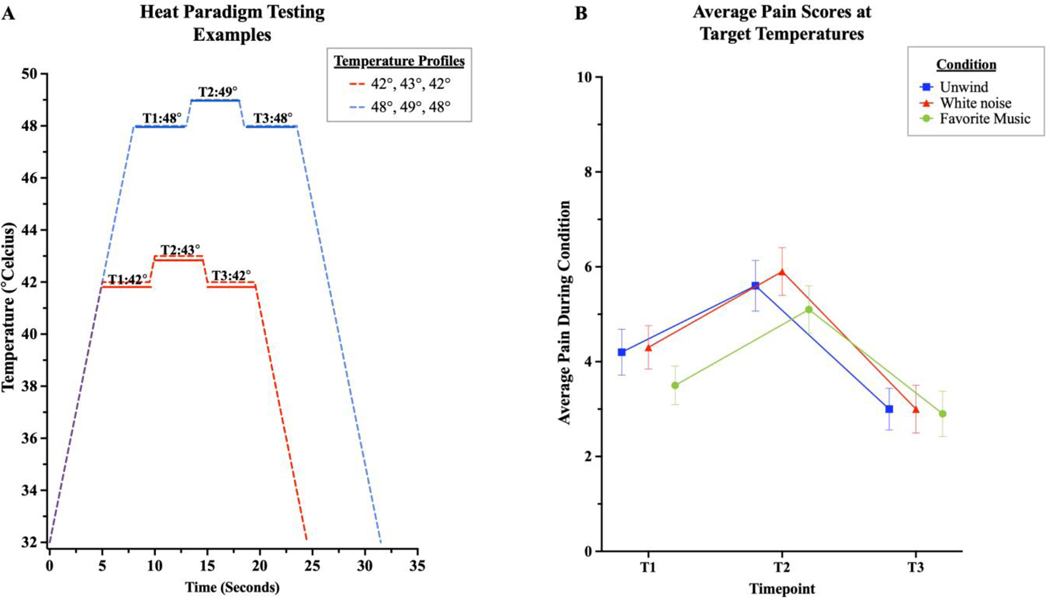
We employed an offset analgesia (OA) paradigm, where pain at a temperature either before (T1) or after (T3) a more painful stimulus (T2) is compared, with the time at T3 typically showing a disproportionately greater decrease in pain perception (offset analgesia=T3-T1).45 When analyzing pain ratings at these three timepoints, we observed that the initial heat pain ratings of participants’ at T1 were significantly different among the three conditions (χ2(2)=14.99, p<.001), with lower pain ratings during the favorite music condition compared to the Unwind and white noise conditions. However, pain ratings at T3 were not significantly different across conditions (p>.05). Correspondingly, there was a significant difference in OA (χ2(2)=10.02, p=.007) across the three conditions, such there was significantly less OA in the favorite music condition compared to the Unwind and white noise conditions. No significant difference in OA was observed between the Unwind and white noise conditions (Figure 3).
Figure 3. Offset analgesia (OA) paradigm.
A. The temperature profile used was tailored to each participant based on heat pain thresholds determined in the baseline run of QST, and identical temperature profile used across all three conditions. Example profiles are shown for participants with 5/10 heat pain thresholds closest to 43 and 49 on baseline testing. B. Average pain rating during the OA paradigm, shown across conditions. Pain ratings were significantly lower at T1 during the favorite music condition and were similar across the three conditions at T3. OA, calculated as difference in pain rating at T3-T1, was less during favorite music compared to the Unwind and white noise conditions.
Conditioned Pain Modulation (CPM)
Conditioned pain modulation (CPM) was determined by examining percentage change in trapezius PPTh (test stimulus) comparing baseline PPTh to the PPTh in the presence of cold pain in the contralateral hand (conditioning stimulus). An increase in the PPTh in the presence of the conditioning stimulus (cold water bath) indicates effective endogenous modulation of pain (i.e., pain inhibition). Table 2 highlights the mean percentage of change between the baseline and conditioned pain scores (calculated CPM), highlighting the fact that CPM was successfully induced by this QST protocol. However, there was no significant difference in CPM between the three conditions (χ2(2)=1.58, p>.05; Table 2).
Temporal Summation of Pain (TSP)
Temporal summation of pain (TSP) was successfully induced among participants by the QST protocol, with participants reporting more pain at the 10th stimulus compared to the single stimulus. However, TSP did not significantly differ across the three conditions (χ2(2)=2.54, p>.05; Table 2).
Music and Situational Pain Catastrophizing
There was a significant difference in situational pain catastrophizing across the three conditions (χ2(2)=14.31, p<.001). During the favorite music condition, participants rated their degree of catastrophic thinking about the pain they experienced in response to QST (SPCS score) significantly lower than catastrophizing during the Unwind and white noise conditions (Table 2). No significant difference in situational pain catastrophizing was observed between the Unwind and white noise conditions.
Situational Pain Catastrophizing and Pain Sensitivity
We explored whether situational pain catastrophizing was related to pain sensitivity within each of the three conditions to determine whether catastrophizing may importantly be associated with pain processing. Interestingly, within each condition (white noise, Unwind, favorite music) situational catastrophizing reported in that condition was not significantly related to any pain outcome in that condition (p>.05; see Supplemental Table S3).
DISCUSSION
This QST, lab-based study of healthy adults investigated the modulatory role of different types of music, compared to white noise, on several aspects of pain processing and situational pain catastrophizing. Individuals had a broad range of musical tastes, with their favorite songs including a wide range of genres and artists, suggesting that it might be difficult to choose the perfect “one-size-fits-all” music intervention (see Supplemental Table S2 for complete song list and categorization by genre). The variation in music choice between participants, as well as sometimes within an individual’s favorite playlist, precluded our ability to assess the relative efficacy of any particular type of music. However, participants’ PPTh, PPTol, and HPTh were significantly higher during the favorite music condition compared to both the Unwind and white noise conditions, indicating less pain sensitivity when listening to self-selected favorite music. Similarly, situational pain catastrophizing was significantly lower in the favorite music condition. Previous studies have shown variable analgesic benefits of music among participants.7, 22, 27, 31, 35, 38, 40 The current findings may add insight into the source of this variability, in that we observed a more profound response to one’s own personal favorite music than to a pre-selected relaxing music app with less choice and variety of music, or white noise. While it may be that some participants derived analgesic benefit in these conditions, favorite music resulted in an overall greater, more consistent effect on pain sensitivity, as well as negative pain-related thinking during pain testing.
Prior research has suggested that an individual’s music preference may be important to consider when exploring how music modulates pain processing, with certain songs evoking more or less enjoyment and effect, depending upon the individual.6, 31, 64 Evidence suggests that music may change both experimental and clinical pain compared to a control condition,13, 20, 27 including our previous work, which demonstrated increases in pain threshold and tolerance among individuals who listened to Unwind.5, 7 Previous research has shown that regardless of music type, participant choice has a significant greater analgesic effect than pre-selected music.21 The present work suggests that these effects appear more pronounced when individuals are allowed to choose their own preferred music.31 Although we did not directly manipulate the degree of choice, or isolate it as a factor, it is possible that the ability to choose and recognize one’s music during a painful, stressful experience may also provide a sense of control, which may be of particular importance when one is experiencing pain in a clinical context or situation which makes one feel powerless. Thus, when considering best how to employ music as an adjunctive strategy to manage clinical pain, it seems likely that preference may maximize music’s impact on pain threshold and tolerance. Music may also divert attention from painful stimuli by providing a more salient stimulus, in the form of memories, associations, and emotions that are evoked by their favorite song. Distraction and enhanced sensory engagement has previously been shown to effectively alter pain processing10, 50 and has frequently been postulated as a possible mechanism for music-induced reductions of pain.5, 10, 27, 28, 44, 49, 50, 56 Positive expectancy of an effect of music has been previously postulated as a possible mechanism,37 although the addition of positive expectancy to favorite music did not necessarily augment the analgesic efficacy of favorite music.32 In the current study, to gather information on participant-selected favorite music, we exposed participants to knowledge of the research design, and likely induced some expectations in participants.
Interestingly, we found evidence of favorite music’s impact on pain processing only for certain QST stimuli. Similar to previous reports, we observed reductions in PPTh and PPTol,7 as well as HPTh.49 Of note, we did not observe differences in the degree of CPM or TSP across the three conditions. It is possible that, although we observed effective CPM and TSP using our QST methodology, these effects were relatively modest (mean 15% increase of PPTh with CPM, two point mean increase in pain for TSP), which, combined with the high degree of variability among participants, may have made group differences more difficult to discern. Potentially, a ceiling effect could have limited discernment of music’s effect on CPM. If endogenous inhibitory capacity was maximally elicited by the conditioning stimulus in the CPM paradigm, it is possible that no additional inhibition by music was detectable. Alternatively, if music itself modulates central processing of pain, it may be replicative of the effect of these central pain modulatory processes.
We were also interested in assessing the impact of music on offset analgesia (OA), which consists of a disproportionate decrease in stimulus intensity after experience of a noxious stimulus.1, 26, 63 We observed effective OA, with a significantly lower pain rating at T3 compared to T1, and in this case we did observe group differences, with participants exhibiting less OA during the favorite music condition. One potential driver of this difference is the significantly lower starting point of pain at T1 in the favorite music condition, representing a floor effect (i.e., less overall potential for pain scores to be reduced at T3 compared to T1, resulting in overall less OA). Our OA protocol did not include a constant temperature control condition, in the interest of being cautious about total exposure and efficient timing to allow four rounds of testing (baseline + three conditions). Future research might probe whether a different impact of music on OA would be discernable if a condition-specific T1 temperature setting was used, or a constant temperature control was used, as prior research has indicated within- and across-stimulus measures may reflect different neural mechanisms.12, 62
Pain catastrophizing is a negative psychological process which has an established association with worse clinical pain.2, 3, 15, 16, 41, 59 Our findings agree with previous reports that sensory engagement through music or other means can reduce pain catastrophizing and negative affect.9, 10 Importantly, these effects may extend to situational pain catastrophizing (i.e., catastrophic thinking about pain during the procedure of pain testing), since participants reported lower SPCS scores during the favorite music condition. Similar to our previous study, we did not observe an impact of relaxing music (Unwind) on SPCS, compared to white noise.7 Although we did not observe a significant association between situational catastrophizing and pain sensitivity (see Supplemental Table S3), our design was not optimized to assess a mediational role of catastrophizing reduction in music’s analgesic efficacy. This impact of favorite music on catastrophizing during a painful procedure (QST) may bode well for the efficacious application of music as an adjunct analgesic during painful clinical procedures, and in the perioperative or other acute care settings, particularly among individuals who have relatively greater catastrophizing.9
Limitations
When interpreting these findings, it is important to consider several limitations of this study. First, participants were not blinded to the music conditions or the researchers’ interest in understanding the impact of music on pain. Second, while the current study aimed to compare the analgesic effect of two music conditions and a control condition, we did not investigate the mechanism(s) by which these effects were generated. For example, while participants were granted choice between five tracks during the Unwind app, and in the selection of their own self-described favorite music, choice and control were still limited to varying degrees. The playlist of favorite music was played in a random order during testing, removing agency in choice of the order of song selection, and due to time constraints, not all seven tracks were usually played during testing. We also did not ask participants to rate their enjoyment of the three conditions, precluding assessment of enjoyment as an important variable. Future studies designed to isolate music elements, expectation, distraction, choice, or psychosocial modulation are required to delineate the mechanism(s) of the analgesic effects of music. However, it seems likely that the mechanistic importance of any of these elements may be variably present in different individuals, just as music may be variably effective among individuals. Third, the duration of the beneficial impact of music on clinical pain and pain catastrophizing remains unknown, as these were brief and controlled applications of pain.
Finally, although ranging in age, study participants were predominantly highly educated, and White, limiting the generalizability of our findings. Future research examining the impact of music interventions on patients with acute procedural or chronic pain should include more diverse samples to increase generalizability and help determine whether this intervention could be used in practical clinical settings and to determine its relative efficacy among different individuals in different settings. Employing music interventions among chronic pain and surgical patients will allow assessment of the best and most efficacious application of music, as well as determine whether these findings generalize to clinical samples.
Future Directions
Previous studies have reported that music can reduce clinical pain,11, 22, 35, 36 is particularly beneficial as an adjunctive therapy in conjunction with pharmacological therapies for pain management, and may allow a decrease in opioid dose needed or reduce exposure to opioids. Indeed, prior research has demonstrated a decrease in opioid use with the addition of music.5, 14, 27, 43, 57 Additionally, music interventions are easy to implement and highly accessible, whereas other behavioral interventions are often expensive, require more effort, and may not be as accessible to everyone. Future studies might further probe the potential of music personalization through making use of the vast array of music platforms and listening modalities. Although the present findings show benefits in healthy adults, future studies in patients with different types of chronic pain may allow assessment of the potential clinical benefit and give further insight about which aspects of nociception and psychological processing may be modulated by different types of music. Similarly, longitudinal studies, involving daily, at home use of music may extend the initial evidence presented here.
CONCLUSION
An individual’s favorite music resulted in lower pain sensitivity compared to white noise and relaxing music (Unwind), most notably pressure pain threshold and tolerance and heat pain, as well as situational pain catastrophizing, in healthy volunteers. More research is necessary to explore whether the beneficial impact of preference of one’s own favorite music extends to clinical settings or patients with chronic pain, and what mechanism(s) may explain the effects observed by favorite music.
Supplementary Material
Highlights.
Impact of music on pain processing was assessed using quantitative sensory testing (QST).
Participants had lower pain sensitivity and less catastrophizing during their favorite music.
Patient-selected favorite music may be an effective and low-risk adjuvant analgesic.
PERSPECTIVE:
This article presents evidence that participant-chosen favorite music can alter several aspects of nociceptive processing, including catastrophic thinking about pain, compared to white noise or relaxing music. Employing an individual’s favorite music during episodic or procedural pain might represent a cost effective adjunctive analgesic strategy.
Acknowledgments
Disclosures:
This work was supported by the National Institutes of Health (NIH R35 GM128691-01).
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alter BJ, Aung MS, Strigo IA, Fields HL. Onset hyperalgesia and offset analgesia: Transient increases or decreases of noxious thermal stimulus intensity robustly modulate subsequent perceived pain intensity. PLoS One. 15:e0231124, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asmundson GJ, Katz J. Understanding the co- occurrence of anxiety disorders and chronic pain: state- of- the- art. Depress. Anxiety. 26:888–901, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch. Intern. Med. 163:2433–2445, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Basiński K, Zdun-Ryżewska A, Majkowicz M. The role of musical attributes in music-induced analgesia: a preliminary brief report. Front. Psychol 9:1761, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai PR, Carreiro S, Ranney ML, Karanam K, Ahtisaari M, Edwards R, Schreiber KL, Ben-Ghaly L, Erickson TB, Boyer EW Music as an adjunct to opioid-based analgesia. J. Med. Toxicol 13:249–254, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai PR, Gale JY, Patton ME, Schwartz E, Jambaulikar GD, Taylor SW, Edwards RR, Boyer EW, Schreiber KL. In Response: What Happens When Algorithmic Music Meets Pain Medicine. Pain Med. 21:3737–3738, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai PR, Gale JY, Patton ME, Schwartz E, Jambaulikar GD, Wade Taylor S, Edwards RR, Boyer EW, Schreiber KL. The impact of music on nociceptive processing. Pain Med. 21:3047–3054, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai PR, Schreiber KL, Taylor SW, Jambaulikar GD, Kikut A, Hasdianda MA, Boyer EW: The feasibility and acceptability of a smartphone-based music intervention for acute pain. In: Proceedings of the… Annual Hawaii International Conference on System Sciences. Annual Hawaii International Conference on System Sciences, NIH Public Access, 2019, pp. 3917. [PMC free article] [PubMed] [Google Scholar]
- 9.Chai PR, Schwartz E, Hasdianda MA, Azizoddin DR, Kikut A, Jambaulikar GD, Edwards RR, Boyer EW, Schreiber KL. A brief music app to address pain in the emergency department: Prospective study. J. Med. Internet Res 22:e18537, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colloca L, Raghuraman N, Wang Y, Akintola T, Brawn-Cinani B, Colloca G, Kier C, Varshney A, Murthi S. Virtual reality: physiological and behavioral mechanisms to increase individual pain tolerance limits. Pain. 161:2010, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danhauer SC, Vishnevsky T, Campbell CR, McCoy TP, Tooze JA, Kanipe KN, Arrington SA, Holland EK, Lynch MB, Hurd DD. Music for patients with hematological malignancies undergoing bone marrow biopsy: a randomized controlled study of anxiety, perceived pain, and patient satisfaction. J. Soc. Integr. Oncol 8:140, 2010 [PMC free article] [PubMed] [Google Scholar]
- 12.Derbyshire SW, Osborn J. Offset analgesia is mediated by activation in the region of the periaqueductal grey and rostral ventromedial medulla. Neuroimage. 47:1002–1006, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Dobek CE, Beynon ME, Bosma RL, Stroman PW. Music modulation of pain perception and pain-related activity in the brain, brain stem, and spinal cord: a functional magnetic resonance imaging study. The Journal of Pain. 15:1057–1068, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Ebneshahidi A, Mohseni M. The effect of patient-selected music on early postoperative pain, anxiety, and hemodynamic profile in cesarean section surgery. The journal of alternative and complementary medicine. 14:827–831, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nature Reviews Rheumatology. 7:216–224, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. The Journal of Pain. 17:T70–T92, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 140:135–144, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finlay KA, Wilson JA, Gaston P, Al-Dujaili EA, Power I. Post-operative pain management through audio-analgesia: Investigating musical constructs. Psychology of Music. 44:493–513, 2016 [Google Scholar]
- 19.Gale J, Chai P, Jambaulikar G, Patton M, Schwartz E, Boyer E, Edwards R, Schreiber K. (131) Impact of an Interactive Music App on Pain Threshold, Temporal Summation, and Conditioned Pain Modulation in Healthy Volunteers. The Journal of Pain. 20:S9, 2019 [Google Scholar]
- 20.Garza-Villarreal EA, Jiang Z, Vuust P, Alcauter S, Vase L, Pasaye EH, Cavazos-Rodriguez R, Brattico E, Jensen TS, Barrios FA. Music reduces pain and increases resting state fMRI BOLD signal amplitude in the left angular gyrus in fibromyalgia patients. Front. Psychol 6:1051, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garza-Villarreal EA, Pando V, Vuust P, Parsons C. Music-induced analgesia in chronic pain conditions: a systematic review and meta-analysis. BioRxiv.105148, 2017 [PubMed] [Google Scholar]
- 22.Garza-Villarreal EA, Wilson AD, Vase L, Brattico E, Barrios FA, Jensen TS, Romero-Romo JI, Vuust P. Music reduces pain and increases functional mobility in fibromyalgia. Front. Psychol 5:90, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatchel RJ: Clinical essentials of pain management, American Psychological Association, 2005. [Google Scholar]
- 24.Ginsberg J, Raghunathan K, Bassi G, Ulloa L. Review of Perioperative Music Medicine: Mechanisms of Pain and Stress Reduction Around Surgery. Frontiers in Medicine. 9:821022, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Good M, Anderson GC, Ahn S, Cong X, Stanton- Hicks M. Relaxation and music reduce pain following intestinal surgery. Res. Nurs. Health 28:240–251, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Grill JD, Coghill RC. Transient analgesia evoked by noxious stimulus offset. J. Neurophysiol 87:2205–2208, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Guétin S, Giniès P, Siou DKA, Picot M-C, Pommié C, Guldner E, Gosp A-M, Ostyn K, Coudeyre E, Touchon J. The effects of music intervention in the management of chronic pain: a single-blind, randomized, controlled trial. The Clinical journal of pain. 28:329–337, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Gutgsell KJ, Schluchter M, Margevicius S, DeGolia PA, McLaughlin B, Harris M, Mecklenburg J, Wiencek C. Music therapy reduces pain in palliative care patients: a randomized controlled trial. J. Pain Symptom Manage 45:822–831, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform 42:377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hole J, Hirsch M, Ball E, Meads C. Music as an aid for postoperative recovery in adults: a systematic review and meta-analysis. The Lancet. 386:1659–1671, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Howlin C, Rooney B. Cognitive agency in music interventions: Increased perceived control of music predicts increased pain tolerance. European Journal of Pain. 25:1712–1722, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Hsieh C, Kong J, Kirsch I, Edwards RR, Jensen KB, Kaptchuk TJ, Gollub RL. Well-loved music robustly relieves pain: A randomized, controlled trial. PLoS One. 9:e107390, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inayama E, Yamada Y, Kishida M, Kitamura M, Nishino T, Ota K, Takahashi K, Shintani A, Ikenoue T. Effect of music in reducing pain during hemodialysis access cannulation: A crossover randomized controlled trial. Clin. J. Am. Soc. Nephrol 17:1337–1345, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larner AJ. Effect size (Cohen’s d) of cognitive screening instruments examined in pragmatic diagnostic accuracy studies. Dement. Geriatr. Cogn. Dis. Extra 4:236–241, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linnemann A, Kappert MB, Fischer S, Doerr JM, Strahler J, Nater UM The effects of music listening on pain and stress in the daily life of patients with fibromyalgia syndrome. Front. Hum. Neurosci 9:434, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Petrini MA. Effects of music therapy on pain, anxiety, and vital signs in patients after thoracic surgery. Complement. Ther. Med 23:714–718, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Lunde SJ, Vuust P, Garza-Villarreal EA, Vase L. Music-induced analgesia: How does music relieve pain? Pain. 160:989–993, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Matsota P, Christodoulopoulou T, Smyrnioti ME, Pandazi A, Kanellopoulos I, Koursoumi E, Karamanis P, Kostopanagiotou G. Music’s use for anesthesia and analgesia. The journal of alternative and complementary medicine. 19:298–307, 2013 [DOI] [PubMed] [Google Scholar]
- 39.McCaffrey R, Freeman E. Effect of music on chronic osteoarthritis pain in older people. J. Adv. Nurs 44:517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Mitchell LA, MacDonald RA. An experimental investigation of the effects of preferred and relaxing music listening on pain perception. J. Music Ther 43:295–316, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Nicholas MK, Linton SJ, Watson PJ, Main CJ, Group DotFW. Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: a reappraisal. Phys. Ther 91:737–753, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. The Journal of the American Society of Anesthesiologists. 115:1063–1071, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Nilsson U, Unosson M, Rawal N. Stress reduction and analgesia in patients exposed to calming music postoperatively: a randomized controlled trial. Eur. J. Anaesthesiol 22:96–102, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Onieva-Zafra MD, Castro-Sánchez AM, Matarán-Peñarrocha GA, Moreno-Lorenzo C. Effect of music as nursing intervention for people diagnosed with fibromyalgia. Pain Manag. Nurs 14:e39–e46, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Oudejans LC, Smit JM, van Velzen M, Dahan A, Niesters M. The influence of offset analgesia on the onset and offset of pain in patients with fibromyalgia. Pain. 156:2521–2527, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Overstreet DS, Michl AN, Penn TM, Rumble DD, Aroke EN, Sims AM, King AL, Hasan FN, Quinn TL, Long DL. Temporal summation of mechanical pain prospectively predicts movement-evoked pain severity in adults with chronic low back pain. BMC Musculoskelet. Disord 22:1–13, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev. Neurother 9:745–758, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rolke R, Baron R, Maier Ca, Tölle T, Treede R-D, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür I Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 123:231–243, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain. 134:140–147, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Schreiber KL, Campbell C, Martel MO, Greenbaum S, Wasan AD, Borsook D, Jamison RN, Edwards RR. Distraction analgesia in chronic pain patients: the impact of catastrophizing. Anesthesiology. 121:1292–1301, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Schreiber KL, Martel MO, Shnol H, Shaffer JR, Greco C, Viray N, Taylor LN, McLaughlin M, Brufsky A, Ahrendt G. Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. PAIN®. 154:660–668, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schreiber KL, Zinboonyahgoon N, Flowers KM, Hruschak V, Fields KG, Patton ME, Schwartz E, Azizoddin D, Soens M, King T. Prediction of persistent pain severity and impact 12 months after breast surgery using comprehensive preoperative assessment of biopsychosocial pain modulators. Ann. Surg. Oncol 28:5015–5038, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simcock XC, Yoon RS, Chalmers P, Geller JA, Kiernan HA, Macaulay W. Intraoperative Music Reduces Perceived Pain After Total Knee Arthroplasty–A Blinded, Prospective, Randomized, Placebo-Controlled Clinical Trial. The journal of knee surgery. 21:275–278, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol. Assess 7:524, 1995 [Google Scholar]
- 55.Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 96:319–324, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Verhoeven K, Christopher E, Dimitri MVR, Valéry L, Geert C. Distraction from pain and executive functioning: an experimental investigation of the role of inhibition, task switching and working memory. European Journal of Pain. 15:866–873, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Tang H, Guo Q, Liu J, Liu X, Luo J, Yang W. Effects of intravenous patient-controlled sufentanil analgesia and music therapy on pain and hemodynamics after surgery for lung cancer: a randomized parallel study. The Journal of alternative and complementary medicine. 21:667–672, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. The Journal of the American Society of Anesthesiologists. 100:115–119, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Wideman TH, Sullivan MJ. Development of a cumulative psychosocial factor index for problematic recovery following work-related musculoskeletal injuries. Phys. Ther 92:58–68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. European Journal of Pain-Kidlington. 14:339, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Yarnitsky D, Bouhassira D, Drewes A, Fillingim R, Granot M, Hansson P, Landau R, Marchand S, Matre D, Nilsen K. Recommendations on practice of conditioned pain modulation (CPM) testing. European journal of pain. 19:805–806, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J. Neurosci 29:10264–10271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yelle MD, Rogers JM, Coghill RC. Offset analgesia: a temporal contrast mechanism for nociceptive information. Pain. 134:174–186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeo JK, Cho DY, Oh MM, Park SS, Park MG. Listening to music during cystoscopy decreases anxiety, pain, and dissatisfaction in patients: a pilot randomized controlled trial. J. Endourol 27:459–462, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.