Abstract
Background
Electroconvulsive therapy (ECT) is efficacious for treatment resistant depression. Treatment-induced cognitive impairment can adversely impact functional outcomes. Our pilot study linked the electric field to ictal theta power from a single suprathreshold treatment and ictal theta power to changes in phonemic fluency. In this study, we set out to replicate our findings and expand upon the utility of ictal theta power as a potential cognitive biomarker.
Methods
Twenty-seven subjects (nine male and eighteen female) received right unilateral ECT for treatment resistant depression. Pre-ECT magnetic resonance imaging and finite element modeling determined the 90th percentile maximum electric field in the brain (Ebrain). Two-lead electroencephalographs were digitally captured across the ECT course with the earliest suprathreshold treatment used to determine power spectral density. Clinical and cognitive outcomes were assessed pre-, mid-, and post-ECT. We assessed the relationship between Ebrain, ictal theta power, clinical outcome (Inventory of Depressive Symptomatology), and cognitive outcomes (phonemic and semantic fluency) with linear models.
Results
Ictal theta power in the Fp1 and Fp2 channels was associated with the electric field, antidepressant outcome, and phonemic and semantic fluency. The relationship between ictal theta power and phonemic fluency was strengthened in the longitudinal analysis. Ebrain was directly associated with phonemic and semantic fluency, but not antidepressant outcome.
Conclusion
Ictal theta power is a potential cognitive biomarker early on in ECT course to help guide parameter changes. Larger studies are needed to further assess ictal theta power’s role in predicting mood outcome and changes with ECT parameters.
Keywords: Electroconvulsive therapy, electric field, ictal theta power, biomarker, phonemic fluency, clinical outcomes
INTRODUCTION
Electroconvulsive therapy (ECT) is highly efficacious for treatment resistant depression. A generalized seizure is necessary, but not sufficient to induce ECT’s antidepressant effect (1). A multiplier of the electrical dosage required to reach the seizure threshold has been empirically derived and is responsible for ECT’s clinical efficacy (2). Seizure metrics as measured by electroencephalography (EEG) have been studied since the 1940s to identify markers of antidepressant and, less frequently, cognitive outcomes with varying results (3). While multiple ictal metrics have been associated with antidepressant improvement, their clinical application remains limited. Postictal suppression, early and mid-ictal amplitude, recruitment phase duration, coherence, and spectral delta power have been associated with ECT’s antidepressant effects (4-7). The largest and most rigorous of these studies reported that the relationship between EEG features and antidepressant response were too weak to guide stimulus dosing (8). Few studies have looked at ictal EEG measurements and cognitive outcomes in pulse wave ECT. These studies demonstrated an association with slow-wave amplitude and phonemic fluency or no association with cognitive outcomes (5, 9-11). Thus, clinical application of ictal EEG remains limited.
Modeling the ECT-induced electric field has quantified individual variability due to differences in neuroanatomy. The Ebrain is an estimate of the whole brain simulated measure of the peak electric field based on a single square wave pulse derived from anatomical MRI data and the amplitude delivered (12, 13). Recent work has shown regional electric fields to be associated with structural volume change in the hippocampus and amygdala in a dose-dependent fashion (14). In addition, stronger electric fields in the temporal lobes were associated with less optimal clinical outcomes in bitemporal ECT and impaired performance with phonemic fluency (15, 16). In a small sample of subjects (n=17), we have previously demonstrated the relationships between 1) Ebrain and ictal theta power (a frequency dependent measure of seizure power in the 4-8Hz band), and 2) ictal theta power and phonemic fluency (17) . The relationships of this exploratory analysis suggest that increased Ebrain was associated with increased ictal theta power, which was related to impaired phonemic fluency. Limitations of this investigation included the small sample size, the assessment of only one EEG acquisition per subject, and the variable time of EEG acquisition within each subject’s ECT course.
This study aimed to determine the relationships between ECT dosing, ictal theta power, and mood and cognitive outcomes with a larger, independent sample. We assessed longitudinal EEG changes over the ECT course with more acquisitions per subject. Based on our previous investigation, we focused on two-lead EEG acquisitions that were acquired during multiple suprathreshold treatments. We computed Ebrain and ictal theta power and compared them with mood and cognitive outcomes. We hypothesized that the electric field (Ebrain) would be associated with ictal theta power. We also hypothesized that ictal theta power acquired early in the ECT treatment would be associated with cognitive outcomes (phonemic fluency).
MATERIALS & METHODS
Subjects:
The University of New Mexico (UNM) Human Research Protections Office (HRPO) approved this investigation. All subjects were recruited through the UNM ECT service and signed procedural consent to the research protocol. The inclusion criteria included subjects diagnosed with major depressive disorder or bipolar type II with a current depressive episode, met indication for ECT, were right-handed, and age between 25-80 years. Subjects remained on antidepressant medications throughout the ECT course with minimal dose adjustments. Exclusion criteria included a neurological or neurodegenerative disorder, other psychiatric conditions, a drug or alcohol use disorder, contraindications to magnetic resonance imaging (MRI).
Electroconvulsive Therapy:
Subjects started with a RUL electrode placement. Amplitude was fixed at 800 milliamperes (mA). Twenty subjects were enrolled in an amplitude titration study and received fixed ECT at 1.0 ms pulse width (PW), 20 Hz frequency, 8 s train duration with a total charge of 256 mC (18). The remaining seven subjects used traditional pulse number titration with subsequent treatments at six times seizure threshold. If subjects failed to demonstrate an adequate antidepressant response, they received BT electrode placement using a traditional pulse number titration for the remainder of the series.
Cognitive and Therapeutic Evaluations:
Subjects completed an extensive neurocognitive battery and depression symptom severity assessments pre-ECT (V1), mid-ECT (after treatment #6, V2), and post-ECT (V3) (19). The 30-item Inventory of Depressive Symptomatology-Clinician rated (IDS-C30) measure depression severity (20). Response was classified as a 50% or greater reduction from baseline IDS-C30 total score by the end of ECT course. Remission was a final IDS-C30 total score equal to or less than 12. The Delis Kaplan Executive Function System (DKEFS) Verbal Fluency test, which measured phonemic fluency (letter fluency (LF) and semantic fluency (category fluency (CF)), was used to measure cognitive impairment as these tests were found to be most sensitive to RUL ECT cognitive impairment in previous studies (21, 22). The DKEFS-VF LF and CF raw scores were converted into demographic-adjusted (age) scaled scores, which were used as the primary cognitive outcomes. The Montreal Cognitive Assessment (MoCA, version 7.1) and Test of Premorbid Function (TOPF) at V1 were used to screen for global cognitive function and premorbid intellectual function, respectively (23, 24).
EEG:
Standard 2-lead EEG monitoring system embedded in the Mecta Spectrum 5000Q® device captured ictal activity during suprathreshold treatments in the Fp1 (left-side of the forehead and contralateral side of treatment) and Fp2 channels (right-side of the forehead and ipsilateral side of treatment). The sampling rate was 140Hz and a band pass filter with cutoff frequencies of 1.4 Hz and 48 Hz was applied prior to exporting data. We examined the exported data and identified the beginning and end of the seizure. We used a continuous wavelet transform with a Morse wavelet family to calculate the average ictal power (μV2/Hz) in the different frequency bands. Due to the ictal activity that occurred right after a higher frequency stimulation, we found that time and frequency localization with this methodology was appropriate (25, 26). The majority of subjects (25/27 or 93%) had their earliest suprathreshold digital capture occur on treatment #2 or #3.
MRI:
T1 and T2 structural MRI were captured using a 3T Siemens scanner prior to ECT initiation. T1 parameters included the following: Repetition time (TR) = 2530 milliseconds (ms), echo time (TE) = 1.64, 3.5, 5.36, 7.22, 9.08 ms, Inversion time (TI) = 1200 ms, flip angle = 7.0 °, slices = 192, field of view = 256, matrix 256 × 256, voxel size = 1.0 × 1.0 × 1.0 millimeter (mm) and total acquisition time 6:03 (minutes: seconds).T2 parameters included the following: TR = 2530 ms, TE = 474 ms, flip angle = 120.0 °, slices = 192, field of view = 256, matrix 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm and total acquisition time = 7:05.
E-field Modeling:
Simulation of Non-Invasive Brain Stimulation (SimNIBS, version 3.2.3) for E-field modeling creates an anatomically realistic volume conductor model (13). The T1 and T2-weighted scans were segmented into tissues with a combination of FMRIB Software Library (FSL) (27) and Statistical Parametric Mapping 12 (SPM12) Computational Anatomy Toolbox (28, 29). SimNIBS then turns this segmentation into a tetrahedral head mesh using GMSH, a three-dimensional finite element (FE) mesh generator, with unique conductivity values for each tissue type. Electrodes are added to the head mesh in RUL placement. SimNIBS then uses a finite element solver to calculate electric fields corresponding to the stimulation. Simulations were performed using unit current and the resultant E-fields were then scaled to the assigned ECT current amplitudes (800 mA). Whole-brain E-field strength (V/m), Ebrain, was calculated at the 90th percentile of E-field magnitudes as an estimate of peak induced field strength, while avoiding the influence of tissue boundary effects.
Statistical Analysis:
Analyses focused on RUL treatments such that ECT endpoints coincided with the last RUL treatment (either V2 or V3, depending on antidepressant response). All data passed normality testing except for ictal powers in the upper bands (alpha, beta, and gamma) for the exploratory analysis. Linear regression models that compared the relationships between Ebrain, ictal power, and mood and cognitive outcomes were performed using R (version 4.2.1) (30-32). The cognitive outcomes (letter and category fluency) were adjusted for age using normative demographic-adjusted data. All linear and logistic regression models that investigated antidepressant outcomes as the dependent variable were adjusted for age and sex. Linear and logistic regression models that included cognitive outcomes as the dependent variable were adjusted by treatment number (“tx_number” in the models), sex, and premorbid intelligence (TOPF). Primary outcomes were assessed including the relationship between 1) Ebrain, mood and cognitive outcomes, the earliest suprathreshold treatment’s ictal theta power (4-8Hz), and seizure length, and 2) the earliest suprathreshold treatment’s ictal theta power relationship with cognitive and mood outcomes (see Table 2). We then generated dichotomous outcomes and Receiver Operator Characteristic (ROC) curves based on ictal theta power’s relationship to phonemic fluency to highlight the potential translation impact. Next, the average ictal theta power in Fp1 and Fp2 across sessions was taken for each subject and compared to Ebrain, cognitive and mood outcomes. Finally, an exploratory analysis between ictal delta (1.4-4 Hz), alpha (8-12Hz), beta (12-30Hz), and gamma (30-48Hz) powers’ relationship with Ebrain, cognitive and mood outcomes was performed. We used a Bonferroni correction for multiple comparisons across the four frequency bands during the exploratory analysis. Given that age is implicated as a risk factor for cognitive adverse effects, and is associated with higher seizure thresholds and weaker seizure expression, and is a covariate in our models, we have color-coded our data points by age (33, 34).
Table 2.
Primary Outcomes
| Ebrain Linear Models | Results |
|---|---|
| Letter Fluency ~ Ebrain + tx_number + Sex + TOPF | R2 = 0.13, (ß = −0.10, t22 = −2.73, p = 0.013* |
| Category Fluency ~ Ebrain + tx_number + Sex + TOPF | R2 = 0.58, (ß = −0.16, t22 = −5.41, p = 2.3e-05* |
| Depression Score ~ Ebrain + Age + Sex | R2 = -0.13, (ß = −0.0004, t22 = −0.122, p = 0.90 |
| Ictal Theta Power Linear Models (4-8Hz) | Earliest Suprathreshold Treatment | Suprathreshold Treatment Averages |
|---|---|---|
| Fp1 ~ Ebrain + Age + Sex | R2 = 0.32, (ß = 1.002, t23 = 2.87, p = 0.009* | R2 = 0.63, (ß = 0.54, t23 = 2.52, p = 0.02* |
| Fp2 ~ Ebrain + Age + Sex | R2 = 0.38, (ß = 1.13, t23 = 3.44, p = 0.002* | R2 = 0.67, (ß = 0.47, t23 = 2.41, p = 0.02* |
| Letter Fluency ~ Fp1 + TOPF + Sex + tx_number | R2 = 0.07, (ß = −0.045, t21 = −2.37, p = 0.03* | R2 = 0.35, (ß = −0.07, t21 = −4.08, p = 0.0005* |
| Letter Fluency ~ Fp2 + TOPF + Sex + tx_number | R2 = 0.17, (ß = −0.05, t21 = −2.94, p = 0.008* | R2 = 0.45, (ß = −0.08, t21 = −4.87, p = 8.2e-05* |
| Category Fluency ~ Fp1 + TOPF + Sex + tx_number | R2 = 0.19, (ß = −0.05, t21 = −2.30, p = 0.03* | R2 = 0.24, (ß = −0.06, t21 = −2.67, p = 0.01* |
| Category Fluency ~ Fp2 + TOPF + Sex + tx_number | R2 = 0.22, (ß = −0.06, t21 = −2.50, p = 0.02* | R2 = 0.21, (ß = −0.06, t21 = −2.48, p = 0.02* |
| Depression Score ~ Fp1 + Age + Sex | R2 = 0.04, (ß = 0.003, t23 = 2.03, p = 0.05* | R2 = 0.02, (ß = 0.005, t23 = 1.83, p = 0.08 |
| Depression Score ~ Fp2 + Age + Sex | R2 = 0.14, (ß = 0.004, t23 = 2.64, p = 0.01* | R2 = 0.15, (ß = 0.008, t23 = 2.72, p = 0.01* |
signify p < 0.05
RESULTS
Clinical and Demographic Characteristics:
Demographics, clinical, and cognitive data are summarized in Table 1. Total enrollment included 29 subjects with 2 subjects lost to follow-up. One subject was excluded from the cognitive analyses for missing data. The average age for the subjects (n = 27) was 56.5 years with a standard deviation of 12.7. Nine of the 27 subjects were male. Nineteen of the 27 subjects remained in RUL ECT throughout the ECT course (see Table 1). Concurrent pharmacotherapy included antidepressants (n = 26), antidepressant augmentation with second antidepressant, antipsychotic or lamotrigine (n = 14), or benzodiazepine (n = 7).
Table 1.
Demographic and Clinical Characteristics
| Clinical and Demographic Characteristics | N=27 |
|---|---|
| Age | 56.5 (12.7) |
| Sex: male/female | 9/18 |
| TOPF (n=26) | 113 (11.0) |
| Baseline Montreal Cognitive Assessment | 24.3 (4.1) |
| Ebrain, mean (SD) | 122.5 (18.5) |
| Baseline Inventory of Depression Scale | 48.3 (9.2) |
| RUL ECT Change in Inventory of Depressive Symptomatology | 43.7% (29.1%) |
| Change in Letter Fluency | −2.1 (3.5) |
| Change in Category Fluency | −2.6 (3.9) |
| Seizure Length | 44.4 (21.0) |
| Fp1 ictal theta power | 86.0 (37.6) |
| Fp2 ictal theta power | 77.5 (37.0) |
| Fp1 ictal theta power intrasubject average | 83.2 (29.7) |
| Fp2 ictal theta power intrasubject average | 76.2 (28.5) |
| RUL only/bitemporal switch | 19/8 |
| RUL Responder | 10/19 |
| RUL Remitter | 7/19 |
| RUL suprathreshold treatment number captured by EEG | 2.4 (0.7) |
| Total RUL treatment numbers received | 6.2 (2.0) |
| RUL only Responder and Remitter Rate (n=19) | 89.47% |
| Overall Responder and Remitter Rate (Including bitemporal switchers) | 70.37% |
Format is mean (SD), '/' to indicate proportion, or percentage
Longitudinal and Frequency Analysis:
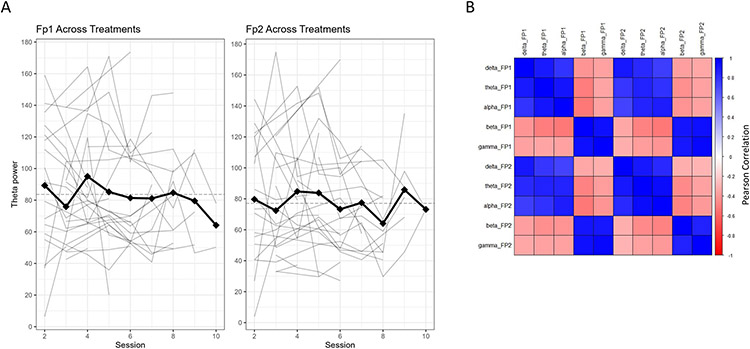
116 RUL suprathreshold treatments were captured across 27 subjects. The mean paired difference of standard deviations of Fp1 and Fp2 within each subject was significant with a 1-sample 95% bootstrap CI of (−0.12, −0.01). A negative Phi1 (AR(1) parameter) mean indicates a negative autocorrelation, meaning the next value is negatively correlated with the previous value. The observations zigzag around a flat trajectory instead of having a smooth upward or downward trajectory (see Figure 1A). Fp1 and Fp2 ictal powers in the same frequency bands were closely correlated with each other. Low frequency bands (delta, theta, and alpha) were negatively correlated with higher frequency bands (beta and gamma) (see Figure 1B).
Fig. 1.
Ictal power characteristics. A The longitudinal analysis of ictal theta power in Fp1 and Fp2 EEG channels versus ECT session. Ictal theta power in the Fp2 channel (ipsilateral to treatment) is higher than ictal theta power in the Fp1 channel (contralateral to treatment). The dark line signifies the mean of ictal theta power across all subjects at each session. B A correlation matrix of ictal powers across frequency bands shows Fp1 and Fp2 are highly correlated within frequency bands.
Ebrain and Clinical Outcomes:
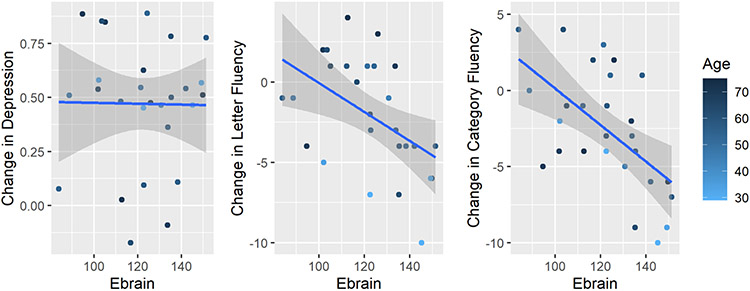
Ebrain was not associated with antidepressant outcomes (ß = −0.0004, t(3,23) = −0.12, p = 0.90), but was associated with phonemic (ß = −0.10, t(2,22) = −2.73, p = 0.01) and category fluency (ß = −0.16, t(2,22) = −5.41, p < 0.0001) (see Table 2 and Figure 2).
Fig. 2.
Ebrain (V/m) mood and cognitive outcomes. The y-axis is the fractional reduction of the IDS-C score from baseline (pre-ECT). A higher fraction indicates greater improvement. The change in IDS-C has no association with electric field strength (t22 = −0.12, p = 0.90). Phonemic fluency worsen as the electric field increases in strength (t22 = −2.73, p = 0.013). Semantic fluency worsen as the electric field increases in strength (t22 = −5.41, p = 2.3e-05).
Ebrain and Ictal Power:
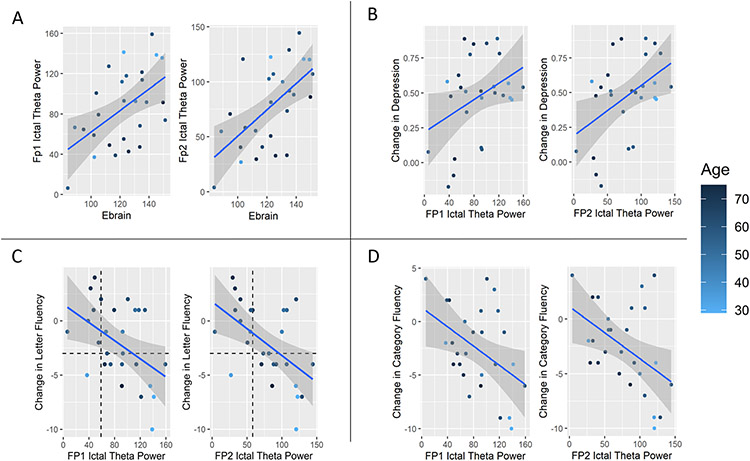
Ebrain was associated with ictal theta power in an early suprathreshold treatment in the Fp1 and Fp2 channels (ß = 1.002, t(3,23) = 2.87, p = 0.009; ß = 1.13, t(3,23) = 3.44, p = 0.002) and longitudinally (ß = 0.63, t(3,23) = 2.52, p = 0.02; ß = 0.67, t(3,23) = 2.41, p = 0.02) (see Table 2 and Figure 3A). An exploratory analysis of other frequency bands showed that only ictal alpha power in the Fp1 and Fp2 channels were associated with Ebrain (ß = 1.38, t(3,23) = 3.08, p = 0.005; ß = 1.21, t(3,23) = 3.67, p = 0.001) (see Table 3). A single outlier was noted in the upper bands of alpha, beta, and gamma. Removing this outlier did not change our primary outcomes, but it did normalize ictal alpha power results and strengthen correlations across all models into significance in the alpha band (p < 0.0125) except for Fp1 ictal alpha power and the antidepressant outcome (p = 0.02). The other bands did not have any changes. In addition, beta and gamma bands were noted to be right-skewed. All tests were rerun with a natural log formation of the data in these bands with no significant changes in results.
Fig. 3.
Ictal theta power at an early suprathreshold treatment outcome. A Ictal theta power versus Ebrain. As the electric field increases, the seizure strength increases (Fp1: t23 = 2.87, p = 0.009; Fp2: t23 = 3.44, p = 0.002). B Change in Depression versus Ictal Theta Power. As the seizure strength increases, depressive symptoms decrease (Fp1: t23 = 2.45, p = 0.05; Fp2: t23 = 2.64, p = 0.01). C Change in Letter Fluency versus ictal theta power. As the seizure strength increases, phonemic fluency worsens (Fp1: t21 = −2.37, p = 0.03; Fp2: t21 = −2.94, p = 0.008). The horizontal dotted line indicates cognitive impairment, while the dotted vertical line is the logistic regression threshold. D Change in Category Fluency versus ictal theta power. As the seizure strength increases, semantic fluency worsens (Fp1: t21 = −2.30, p = 0.03; Fp2: t21 = −2.50, p = 0.02).
Table 3:
Exploratory Analysis of Ictal Delta, Alpha, Beta, and Gamma Power
| Ictal Power Linear Models | Frequency Band: delta (1.4-4Hz) | Frequency Band: Alpha (8-13Hz) |
|---|---|---|
| Fp1 Ictal Power~ Ebrain + Age + Sex | R2 = 0.25, ß = 1.72, t23 = 2.50, p= 0.02) | R2 = 0.27, ß = 1.38, t23 = 3.08, p = 0.005* |
| Fp2 Ictal Power ~ Ebrain + Age + Sex | R2 = 0.28, ß = 1.76, t23 = 2.62, p= 0.02) | R2 = 0.35, ß = 1.21, t23 = 3.67, p = 0.001* |
| Letter Fluency ~ Fp1 + TOPF + Sex + tx_number | R2 = −0.01, ß = −0.02, t21 = −1.82, p= 0.08) | R2 = 0.005, ß = −0.03, t21 = −1.94, p = 0.07 |
| Letter Fluency ~ Fp2 + TOPF + Sex + tx_number | R2 = 0.02, ß = −0.02, t21 = −2.06, p= 0.05) | R2 = 0.09, ß = −0.05, t21 = −2.46, p = 0.02 |
| Category Fluency ~ Fp1 + TOPF + Sex + tx_number | R2 = 0.13, ß = −2.67, t21 = −1.91, p= 0.07) | R2 = 0.12, ß = −0.03, t21 = −1.78, p = 0.09 |
| Category Fluency ~ Fp2 + TOPF + Sex + tx_number | R2 = 0.13, ß = −2.67, t21 = −1.90, p= 0.07) | R2 = 0.15, ß = −0.04, t21 = −2.05, p = 0.05 |
| Depression Score ~ Fp1 + Age + Sex | R2 = −0.007, ß = 0.001, t23 = 1.65, p= 0.10) | R2 = −0.05, ß = 0.001, t23 = 1.24, p = 0.23 |
| Depression Score ~ Fp2 + Age + Sex | R2 = 0.03, ß = 0.002, t23 = 1.92, p= 0.07) | R2 = 0.005, ß = 0.003, t23 = 1.74, p = 0.10 |
| Ictal Power Linear Models | Frequency Band: Beta (13-30Hz) | Frequency Band: Gamma(30-48Hz) |
| Fp1 Ictal Power~ Ebrain + Age + Sex | R2 = −0.03, ß = −0.009, t23 = −0.10, p = 0.35 | R2 = 0.02, ß = −0.48, t23 = −1.90, p = 0.07 |
| Fp2 Ictal Power~ Ebrain + Age + Sex | R2 = −0.02, ß = −0.002, t23 = −0.20, p = 0.84 | R2 = 0.06, ß = −5.54, t23 = −2.06, p = 0.05 |
| Letter Fluency ~ Fp1 + TOPF + Sex + tx_number | R2 = −0.14, ß = 0.72, t21 = 0.75, p = 0.46 | R2 = −0.12 ß = 0.003, t21 = 1.03, p = 0.32 |
| Letter Fluency ~ Fp2 + TOPF + Sex + tx_number | R2 = −0.16, ß = 0.50, t21 = 0.60, p = 0.56 | R2 = −0.16, ß = 0.002, t21 = 0.56, p = 0.58 |
| Category Fluency ~ Fp1 + TOPF + Sex + tx_number | R2 = 0.11, ß = −2.52, t21 = −1.73, p = 0.09 | R2 = 0.04, ß = 0.003, t21 = 1.08, p = 0.29 |
| Category Fluency ~ Fp2 + TOPF + Sex + tx_number | R2 = 0.12, ß = −2.57, t21 = −1.80, p = 0.08 | R2 = 0.03, ß = 0.003, t21 = 1.01, p = 0.33 |
| Depression Score ~ Fp1 + Age + Sex | R2 = −0.10, ß = 0.05, t23 = −0.70, p = 0.49 | R2 = −0.01, ß = 0.0004, t23 = −1.62, p = 0.12 |
| Depression Score ~ Fp2 + Age + Sex | R2 = −0.08, ß = 0.07, t23 = −0.95, p = 0.35 | R2 = 0.03, ß = −0.0004, t23 = −1.95, p = 0.06 |
signify p < 0.0125 (Bonferroni Correction)
Ictal Power and Clinical Outcomes:
Ictal theta power in an early suprathreshold treatment in the Fp1 and Fp2 channels was associated with antidepressant (ß = 0.003, t(3,23) = 2.03, p = 0.05; ß = 0.004, t(3,23) = 2.64, p = 0.01), phonemic (ß = −0.045, t(4,21) = −2.37, p= 0.03; ß = −0.05, t(4,21) = −2.94, p = 0.008), and category fluency (ß = −0.05, t(4,21) = −2.30, p = 0.03; ß = −0.06, t(4,21) = −2.50, p = 0.02) (see Table 2 and Figures 3B, C, and D). A longitudinal analysis of ictal theta power found an association with antidepressant outcomes in the Fp2 channel only (ß = 0.008, t(3,23) = 2.72, p = 0.01), while the phonemic fluency association was strengthened in both channels (Fp1: ß = −0.07, t(4,21) = −4.08, p = 0.0005; Fp2: ß = −0.08, t(4,21) = −4.87, p < 0.0001) and category fluency’s association was similar to ictal theta power in the earliest suprathreshold treatment (Fp1: ß = −0.06, t(4,21) = −2.67, p = 0.01; Fp2: ß = −0.06, t(4,21) = −2.48, p = 0.02) (see Table 3). In the exploratory analysis, ictal delta, alpha, beta, and gamma band had no noted associations (see Table 3 and the additional testing subsection).
Ictal Power and Dichotomous Outcomes (+/− responder; +/− cognitive impairment):
A logistic regression determined that ictal theta power was not associated with responder/remitter criteria at the end of RUL ECT (Fp1: ß = 0.33, t(3,23) = 1.53, p = 0.14; Fp2: ß = 0.23, t(3,23) = 1.23, p = 0.23). Given phonemic fluency’s reliable association with ictal theta power at an early suprathreshold treatment and the potential translational impact, a logistic regression model was used to define cognitive impairment as a loss of three or more scaled-score points in DKEFS LF (See horizontal dotted line Figures 3C). The association between cognitive impairment and ictal theta power at an early suprathreshold treatment was significant (Fp1: ß = 0.006, t(4,21) = 2.07, p = 0.05; Fp2: ß = 0.007, t(4,21) = 2.77, p = 0.01) with the threshold for cognitive safety for Fp1 and Fp2 being 64.8 and 60.0 μV2/Hz, respectively (see vertical lines in figure 3C). Receiving Operator Characteristic (ROC) curves were generated for ictal theta power and cognitive safety with an area under the curve (AUC) of 0.77 for both channels. In the longitudinal analysis, the association between cognitive impairment and ictal theta power in Fp1 and Fp2 channels was similar (ß = 0.007, t(4,21) = 2.34, p = 0.03; ß = 0.008, t(4,21) = 2.59, p = 0.02) with thresholds being calculated at 56.9 and 64.4 μV2/Hz and an AUC of 0.81 and 0.82, respectively.
Ebrain, Ictal Theta Power, and Seizure Length:
Adjusting for age and sex, Ebrain was not related to seizure length (ß = −0.18, t(3,23) = 1.07, p = 0.30). Ictal theta power in the Fp2 only was related to seizure length (Fp1: ß = −0.22, t(3,23) = 1.84, p = 0.08; Fp2: ß = 0.27, t(3,23) = 2.46, p = 0.02).
DISCUSSION
Using MRI data and 2-lead EEG, we calculated electric field strength (Ebrain) and ictal theta power in an early suprathreshold treatment in 27 adult subjects with depression who were treated with ECT. We investigated the association between Ebrain, ictal theta power (4–8 Hz), and mood and cognitive outcomes based on our pilot study results (17). We found that the Ebrain was associated with ictal theta power and cognitive outcomes but not antidepressant outcomes. Ictal theta power was associated with both Ebrain and phonemic fluency. In addition, ictal theta power was associated with category fluency and antidepressant outcomes. In an exploratory analysis in other frequency bands (delta, alpha, beta, and gamma), using the Bonferonni correction for multiple comparisons, we found that ictal alpha power was associated with Ebrain only. A longitudinal analysis found ictal theta power ipsilateral to treatment slightly higher than the contralateral side. Fp1 and Fp2 ictal power tended to be tightly correlated within the same frequency band, while lower frequencies (1.4-13Hz) were tightly correlated to each other and negatively correlated to higher frequency bands (13-48Hz). Ictal theta power ipsilateral to treatment was correlated with seizure length. Our results replicated our earlier findings that ictal theta power is associated with the electric field and cognitive outcomes in a larger study with a broader age range and more consistency with capturing suprathreshold treatments.
Ictal theta power based on 2-lead EEG collection should be considered as a measure of seizure activity emanating through the prefrontal cortex and may reflect the strength of the seizure at that location. The spreading seizure hypothesis suggests that the seizure itself may generate theta-alpha activity (TAA) as it propagates across the frontal cortex (35). The hippocampus may also contribute to ictal theta power given that the hippocampus is a powerful theta wave generator, has been linked to ECT cognitive and mood outcomes, and our pilot project showed an increase in ictal theta power as a whole brain phenomenon (16, 17, 36, 37). However, caution is warranted in interpreting neuronal activity in the subcortical region from the Fp1 and Fp2 electrodes given their distance from the area (38).
The electric field and seizure may have differential effects on cognitive domains. Semantic fluency is more strongly related to Ebrain, and phonemic fluency to ictal theta power, especially in the longitudinal analysis. Neuroanatomical differences in semantic and phonemic fluency are well-documented, with semantic fluency depending more on left posterior middle and inferior temporal gyri and parahippocampal gyri, while phonemic fluency is more dependent on the anterior part of the left middle and inferior frontal gyri with a more extensive frontoparietal and perisylvian white matter network (39, 40). An increased electric field strength during ECT treatment in deep brain structures and in the posterior temporal region may explain Ebrain’s stronger association with semantic fluency. The spreading seizure hypothesis may explain the ictal theta power’s strong association with seizure strength and the seizure’s subsequent impact on phonemic fluency, given phonemic fluency’s reliance on frontal cortex neurocircuitry.
We note several limitations to our study to assist with interpretation. While this study was larger than our pilot study, the sample is still considered modest and replication in larger studies is warranted before implementing ictal theta power as a cognitive safety marker. Many limitations to electric field modeling have been raised in the literature, such as it only describes the spatial extent of direct electrical stimulation, does not account for pulse width, frequency, and train duration, and does not account for anisotropy related effects from white matter or white matter disease (41). Our study was insufficiently powered to examine differences in ECT parameters and medication classes. In addition, the relationship between the spatial distribution of the electric field and the spatial topography of the induced seizure has yet been determined. Finally, our treatment algorithm for switching electrode placement hinges on the patient meeting remission/remittance criteria based on the IDS-C30 at the midpoint. As such, treatment number could not be included as a covariate on models that contained mood outcomes.
In conclusion, ictal theta power based on the standard of care 2-lead EEG that is obtained during ECT is easy to collect and could have a significant translational impact as a cognitive safety biomarker. Ictal theta power in an early suprathreshold treatment is associated with the induced electric field and phonemic fluency outcomes. In addition, ictal theta power may also be associated with semantic fluency and antidepressant outcomes. Ictal theta power along with Ebrain could possibly serve as both a safety and response biomarker early in the ECT course to help guide individualized dosing strategies to maximize efficacy and safety. Larger studies are needed to further assess the translational impact of ictal theta power on cognitive outcomes and the impact on ECT dosing.
Acknowledgments:
This study was supported by the National Institute of General Medical Sciences of the National Institutes of Health under the Award Number P30GM122734, grant S10OD025313, and the Office of The Director, National Institutes of Health, MH125126. Z.-D. Deng is supported by the National Institute of Mental Health Intramural Research Program (ZIAMH002955). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. McClintock is a Consultant to Pearson Assessment, and receives royalties from Guilford Press, Inc. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Verghese A (2000): Is a grandmal seizure necessary and sufficient for the efficacy of electro convulsive therapy? Indian J Psychiatry. 42:57–59. [PMC free article] [PubMed] [Google Scholar]
- 2.Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. (2000): A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 57:425–434. [DOI] [PubMed] [Google Scholar]
- 3.Francis-Taylor R, Ophel G, Martin D, Loo C (2020): The ictal EEG in ECT: A systematic review of the relationships between ictal features, ECT technique, seizure threshold and outcomes. Brain Stimul. 13:1644–1654. [DOI] [PubMed] [Google Scholar]
- 4.Kimball JN, Rosenquist PB, Dunn A, McCall WV (2009): Prediction of antidepressant response in both 2.25xthreshold RUL and fixed high dose RUL ECT. J Affect Disord. 112:85–91. [DOI] [PubMed] [Google Scholar]
- 5.Perera TD, Luber B, Nobler MS, Prudic J, Anderson C, Sackeim HA (2004): Seizure expression during electroconvulsive therapy: relationships with clinical outcome and cognitive side effects. Neuropsychopharmacology. 29:813–825. [DOI] [PubMed] [Google Scholar]
- 6.Luber B, Nobler MS, Moeller JR, Katzman GP, Prudic J, Devanand DP, et al. (2000): Quantitative EEG during seizures induced by electroconvulsive therapy: relations to treatment modality and clinical features. II. Topographic analyses. J ECT. 16:229–243. [DOI] [PubMed] [Google Scholar]
- 7.Sackeim HA, Luber B, Katzman GP, Moeller JR, Prudic J, Devanand DP, et al. (1996): The effects of electroconvulsive therapy on quantitative electroencephalograms. Relationship to clinical outcome. Arch Gen Psychiatry. 53:814–824. [DOI] [PubMed] [Google Scholar]
- 8.Nobler MS, Luber B, Moeller JR, Katzman GP, Prudic J, Devanand DP, et al. (2000): Quantitative EEG during seizures induced by electroconvulsive therapy: relations to treatment modality and clinical features. I. Global analyses. J ECT. 16:211–228. [DOI] [PubMed] [Google Scholar]
- 9.Azuma H, Fujita A, Otsuki K, Nakano Y, Kamao T, Nakamura C, et al. (2007): Ictal electroencephalographic correlates of posttreatment neuropsychological changes in electroconvulsive therapy: a hypothesis-generation study. J ECT. 23:163–168. [DOI] [PubMed] [Google Scholar]
- 10.Krystal AD, Weiner RD, Dean MD, Lindahl VH, Tramontozzi LA 3rd, Falcone G, et al. (2003): Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J Neuropsychiatry Clin Neurosci. 15:27–34. [DOI] [PubMed] [Google Scholar]
- 11.Moulier V, Guehl J, Eveque-Mourroux E, Quesada P, Rotharmel M (2022): A Retrospective Study of Postictal Suppression during Electroconvulsive Therapy. J Clin Med. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Datta A, Bikson M, Parra LC (2019): Realistic volumetric-approach to simulate transcranial electric stimulation-ROAST-a fully automated open-source pipeline. J Neural Eng. 16:056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thielscher A, Antunes A, Saturnino GB (2015): Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? Annu Int Conf IEEE Eng Med Biol Soc. 2015:222–225. [DOI] [PubMed] [Google Scholar]
- 14.Argyelan M, Oltedal L, Deng ZD, Wade B, Bikson M, Joanlanne A, et al. (2019): Electric field causes volumetric changes in the human brain. Elife. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fridgeirsson EA, Deng ZD, Denys D, van Waarde JA, van Wingen GA (2021): Electric field strength induced by electroconvulsive therapy is associated with clinical outcome. Neuroimage Clin. 30:102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng ZD, Argyelan M, Miller J, Quinn DK, Lloyd M, Jones TR, et al. (2022): Electroconvulsive therapy, electric field, neuroplasticity, and clinical outcomes. Mol Psychiatry. 27:1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller J, Jones T, Upston J, Deng ZD, McClintock SM, Ryman S, et al. (2022): Ictal Theta Power as an Electroconvulsive Therapy Safety Biomarker: A Pilot Study. J ECT. 38:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbott CC, Deng Z-D, Argyelan M, Ryman S, McClintock SM (2021): Prelminary Results for ECT Amplitude-Determined Seizure Titration to Improve Clinical Outcomes. American College of Neuropsychopharmacology. San Juan, Puerto Rico: Neuropsychopharmacology, pp 519–553. [Google Scholar]
- 19.McClintock SM, Choi J, Deng ZD, Appelbaum LG, Krystal AD, Lisanby SH (2014): Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy. J ECT. 30:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, et al. (2004): The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 34:73–82. [DOI] [PubMed] [Google Scholar]
- 21.Delis DC, Kramer JH, Kaplan E, Holdnack J (2004): Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 10:301–303. [DOI] [PubMed] [Google Scholar]
- 22.Abbott CC, Quinn D, Miller J, Ye E, Iqbal S, Lloyd M, et al. (2021): Electroconvulsive Therapy Pulse Amplitude and Clinical Outcomes. Am J Geriatr Psychiatry. 29:166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. (2005): The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 53:695–699. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D (2009): Test of Premorbid Functioning. The Psychological Corporation, San Antonion, TX. [Google Scholar]
- 25.Azuma H, Ogawa H, Suzuki E, Akechi T (2020): Intraclass correlations of seizure duration by wavelet transform, sample entropy, and visual determination in electroconvulsive therapy. Neuropsychopharmacol Rep. 40:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faust O, Acharya UR, Adeli H, Adeli A (2015): Wavelet-based EEG processing for computer-aided seizure detection and epilepsy diagnosis. Seizure. 26:56–64. [DOI] [PubMed] [Google Scholar]
- 27.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23 Suppl 1:S208–219. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen JD, Madsen KH, Puonti O, Siebner HR, Bauer C, Madsen CG, et al. (2018): Automatic skull segmentation from MR images for realistic volume conductor models of the head: Assessment of the state-of-the-art. Neuroimage. 174:587–598. [DOI] [PubMed] [Google Scholar]
- 29.Friston KJ (2007): Statistical Parametric Mapping: The analysis of funtional brain images. Amsterdam: Elsevier/Academic Press. [Google Scholar]
- 30.Team R (2020): RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. [Google Scholar]
- 31.Team RC (2020): R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 32.Wickham H (2016): ggplot2: Elegant Graphics for Data Analysis. Verlag New York: Springer. [Google Scholar]
- 33.de Arriba-Arnau A, Dalmau Llitjos A, Soria V, Savino S, Salvat-Pujol N, Curto J, et al. (2021): Factors Predicting Ictal Quality in Bilateral Electroconvulsive Therapy Sessions. Brain Sci. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirov GG, Owen L, Ballard H, Leighton A, Hannigan K, Llewellyn D, et al. (2016): Evaluation of cumulative cognitive deficits from electroconvulsive therapy. Br J Psychiatry. 208:266–270. [DOI] [PubMed] [Google Scholar]
- 35.Sip V, Scholly J, Guye M, Bartolomei F, Jirsa V (2021): Evidence for spreading seizure as a cause of theta-alpha activity electrographic pattern in stereo-EEG seizure recordings. PLoS Comput Biol. 17:e1008731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunez A, Buno W (2021): The Theta Rhythm of the Hippocampus: From Neuronal and Circuit Mechanisms to Behavior. Front Cell Neurosci. 15:649262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson ST, Sanacora G, Bloch MH (2017): Hippocampal volume changes following electroconvulsive therapy: a systematic review and meta-analysis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huels ER, Kafashan M, Hickman LB, Ching S, Lin N, Lenze EJ, et al. (2023): Central-positive complexes in ECT-induced seizures: Possible evidence for thalamocortical mechanisms. Clin Neurophysiol. 146:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biesbroek JM, Lim JS, Weaver NA, Arikan G, Kang Y, Kim BJ, et al. (2021): Anatomy of phonemic and semantic fluency: A lesion and disconnectome study in 1231 stroke patients. Cortex. 143:148–163. [DOI] [PubMed] [Google Scholar]
- 40.Vonk JMJ, Rizvi B, Lao PJ, Budge M, Manly JJ, Mayeux R, et al. (2019): Letter and Category Fluency Performance Correlates with Distinct Patterns of Cortical Thickness in Older Adults. Cereb Cortex. 29:2694–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sartorius A (2022): Electric field distribution models in ECT research. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]