Abstract
Aims:
To quantify the association between opioid agonist treatment (OAT) and overdose death by age group; test the hypothesis that across different age groups, opioid overdose mortality is lowest during OAT with buprenorphine compared with time out of treatment or OAT with methadone; and test associations between OAT and opioid overdose mortality in the presence of chronic circulatory, respiratory, liver, and kidney diseases.
Design:
Retrospective observational cohort study using linked administrative data.
Setting:
New South Wales, Australia.
Participants:
37,764 people prescribed OAT, 1 August 2002 and 31 December 2017.
Measurements:
OAT exposure, opioid overdose mortality, and key confounders were measured using linked population datasets on OAT entry and exit, hospitalisation, mental health care, incarceration, and mortality. ICD-10 codes were used to define opioid overdose mortality and chronic disease groups of interest.
Findings:
Relative to time out of treatment, time in OAT was associated with lower risk of opioid overdose death across all age groups and chronic diseases. Among people aged 50 years and older, there was weak evidence that buprenorphine may be associated with greater protection against opioid overdose death than methadone (generalised estimating equation [GEE] adjusted incident rate ratio (aIRR) 0.47; 95% confidence interval (CI) 0.21, 1.02; marginal structural models [MSM] aIRR 0.49; 95%CI 0.17, 1.41). Buprenorphine was associated with greater protection against overdose death than methadone for clients with circulatory (MSM aIRR 0.27; 95% CI 0.11, 0.67) or respiratory (MSM aIRR 0.26; 95% CI 0.07, 0.94) diseases, but not liver (MSM aIRR 0.59; 95% CI 0.14, 2.43) or kidney (MSM aIRR 1.16; 95% CI 0.31, 4.36) diseases.
Conclusions:
Opioid agonist treatment (OAT) appears to reduce mortality risk in people with opioid use disorder who are older or who have physical comorbidities. Opioid overdose mortality during OAT with buprenorphine appears to be lower and reduced in clients with circulatory and respiratory diseases compared with OAT with methadone.
Keywords: opioid agonist treatment, methadone, buprenorphine, overdose, older adults, comorbidity, multimorbidity
Introduction
Opioid agonist treatment (OAT) is associated with multiple beneficial outcomes for people with opioid dependence or opioid use disorder, notably reduced mortality across a range of causes (1). The strongest reductions in mortality are observed in relation to fatal overdose, with risk of death due to unintentional opioid overdose reduced by 69% during OAT relative to periods out of treatment (1). This protective association between OAT and overdose mortality is apparent for the two most commonly prescribed OAT medicines, methadone and buprenorphine (1,2), and persists in areas with high overdose mortality linked to illicit fentanyl and fentanyl analogues (3).
In a number of settings, the cohort of people seeking treatment for opioid use disorder is aging, with substantial increases in the proportion of clients aged in their 50s and above (4–7). The persistence of the protective effects of OAT on opioid overdose mortality in older clients is an important clinical question (8,9). Randomised controlled trials of OAT were undertaken with participant samples aged around 30 years (10), and are underpowered to examine mortality. Observational studies have provided considerable data on mortality during and after OAT, including important variations during treatment induction (2), but are yet to present data specific to older age groups on the risk of overdose death during OAT relative to that out of treatment. A recent meta-analysis of cohort studies reported that OAT was protective against overdose death in those aged 35 years and older (1), but was not able to give any indication of the relative risk specific to OAT clients approaching or in older adulthood. A study in primary care settings in England suggested that among those aged over 50 years, buprenorphine was more effective in reducing opioid overdose deaths than methadone, but did not compare time in and out of OAT for this age group, and was limited by small numbers of overdose deaths in older age groups (8).
Complicating interpretation of these findings and clinical decision-making for older adults in OAT is confounding due to the high prevalence of coexisting chronic diseases (11,12) which may increase overdose risk independently of age by compromising important physiological functions. Circulatory and respiratory diseases may increase the likelihood of death in an overdose event by increasing susceptibility to opioid induced hypoxia (13). Separately, liver and kidney diseases may interfere with metabolism and excretion of opioids and their metabolites as well as other drugs or medicines, potentially increasing overdose risk at previously tolerated opioid levels (14,15). Data on the risk of opioid overdose death in the presence of these chronic diseases, and implications for OAT provision, are sparse. A study of Scottish methadone clients identified a heightened risk of opioid overdose death in older clients in the presence of chronic diseases (respiratory, circulatory, and digestive diseases) (9), but did not account for time in and out treatment, and did not include a comparison to people treated with buprenorphine. Hickman et al. reported that for people with chronic disease comorbidities, opioid overdose deaths were less frequent during treatment with buprenorphine than methadone, but did not examine specific comorbidities (8). Given factors such as the lesser respiratory depressant effect of buprenorphine, the long half-life and risk of accumulation and toxicity with methadone in chronic liver disease, and the greater number of clinically meaningful drug interactions with methadone, it could be hypothesised that for some people with chronic conditions, there may be less clinical risk associated with buprenorphine compared to methadone (16–19).
To better support clinical management of older adults and those with chronic disease comorbidities receiving OAT, we aimed to quantify the association between OAT and overdose death by age group, and tested the hypothesis that across different age groups (and particularly in those aged 50 years and over), opioid overdose mortality rates would be lowest during OAT with buprenorphine compared to time out of treatment or OAT with methadone. We also tested associations between OAT and opioid overdose mortality rates in the presence of specific chronic diseases: circulatory disease, respiratory disease, liver disease, and kidney disease.
METHOD
Reporting of this study is in line with the REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) extension of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for reporting of observational studies (20,21). The statistical analysis plan was pre-registered with Open Science Framework and can be found at https://osf.io/z6cy9/.
Overview
The Opioid Agonist Treatment and Safety (OATS) Study is a retrospective cohort study using linked administrative data for all people prescribed OAT for the treatment of opioid dependence/opioid use disorder in the Australian state of New South Wales (NSW) between 1 August 2001 and 30 September 2018 (22,23). In NSW, OAT is prescribed in a range of settings including primary care (with dosing usually through community pharmacies), public and private specialist drug treatment clinics, and correctional facilities (24).
Data sources and cohort definition
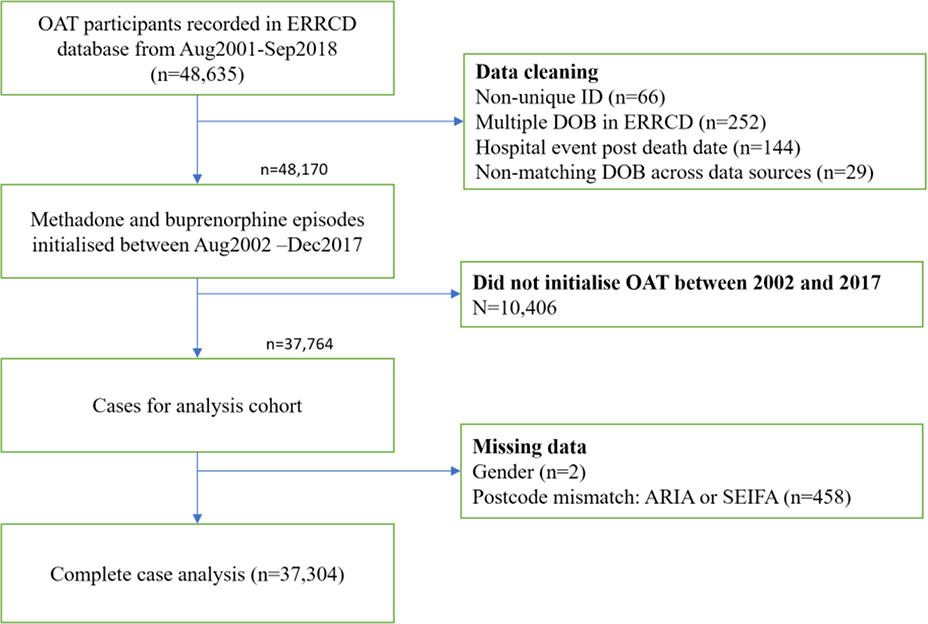
The dataset includes all OAT episodes, hospitalisations, ambulatory mental health contacts, contact with the criminal legal system and deaths, with probabilistic linkage to other state- and national databases (22,23). Details on each dataset and variables within them are provided in the supplementary materials. For the present analyses, the cohort was restricted to people initiating a treatment episode between 1 August 2002 and 31 December 2017. This start date was selected to permit a one-year lookback for participant hospitalisation and contact with the criminal legal system. The end date was selected as the criminal legal system data ended at 31 December 2017. A flowchart depicting the cohort definition process is shown in Figure 1.
Figure 1:
Cohort definition
Primary outcome
The primary outcome was fatal opioid overdose of unintentional or undetermined intent. We defined opioid overdose as an underlying cause of death of ICD-10 X42, X44, Y12, or Y14 in combination with a contributory cause of death of T40.0-T40.4 or T40.6, or an underlying cause of death of F11 or F19 with contributory causes of death from both of the above groups of codes (25,26).
Exposure
Deaths were defined as occurring in or out of OAT based on treatment episode dates, and we defined deaths in treatment as those occurring within one day of the end of an authority to prescribe OAT (25). We modelled opioid overdose mortality rates according to treatment exposure which had 3 categories: i) time prescribed methadone; ii) time prescribed buprenorphine; and iii) time out of OAT. The effect of treatment was estimated within age and chronic disease strata groups.
Time varying effect modifiers
Age
Age was categorised as <30, 30–39, 40–49, and 50+ years, calculated at the beginning of each follow-up year. 10-year age bands were pre-specified to avoid having small numbers of participants in older age groups.
Chronic disease groups
Diseases of the circulatory system, respiratory system, and chronic liver and kidney diseases were selected for analysis on the basis of plausible mechanisms that could increase the risk of overdose death in the presence of the specific comorbidity (13–15). Defining ICD-10 codes for each disease grouping are shown in Table 1. Chronic disease status was time-varying, with participants defined as having a chronic disease following a hospital admission with a diagnosis of circulatory disease, respiratory disease, liver disease, or kidney disease. Once a participant was identified as having a specific chronic disease, they were assumed to have this for the remainder of follow-up (27–31).
Table 1:
International Classification of Diseases – 10th revision diagnostic codes used to classify participants as having a chronic disease
| Circulatory disease | I00-I99 (Chapter IX Diseases of the circulatory system, excluding I85 oesophageal varices and I86.4 gastric varices); G45, G46 (selected episodic and paroxysmal disorders) |
| Respiratory disease | J00-J99 (Chapter X Diseases of the respiratory system); C34 (lung cancer); C45 (mesothelioma); A15, A16 (tuberculosis) |
| Liver disease | K70-K77 (Diseases of liver); C22 (liver cancer); R18 (ascites); I85 (oesophageal varices); I86.4 (gastric varices) |
| Kidney disease | N00-N19 (renal diseases and renal failure); C64 (kidney cancer) |
Covariates
Supplementary table 1 provides a summary of the confounders used in all models. Covariates identified in the literature and considered a priori to be important were sex, Indigenous status, and accessibility/remoteness. Residential remoteness (major cities vs regional/remote) was based on participants’ last known postcode of residence using the Accessibility/Remoteness Index of Australia Plus (ARIA+) 2016 (32). Postcodes were also used to define socio-economic disadvantage, based on the Socio-Economic Indexes for Areas produced by the Australian Bureau of Statistics (33).
When initiating someone onto treatment, their previous treatment experience may influence their new treatment plan. Hence, a categorical variable summarising previous treatment and duration was derived with the following levels: no prior OAT treatment, methadone for less than 29 days, buprenorphine less than 29 days, methadone for greater than 28 days, and buprenorphine for greater than 28 days.
Time-varying confounders
Calendar year was included and grouped into 4-year periods: 2002–2005, 2006–2009, 2010–2013 and 2014–2017. Substance use, psychosis and mood disorders have previously been identified as important factors with regard to treatment retention, along with the duration of previous treatment episodes and the tenure of the prescribing clinician (34). As such, time-varying comorbidities were derived from hospitalisations involving a diagnosis of substance use disorder, psychosis, mood disorder, or self-harm, and outpatient mental health visits, all within the last 12 months, as well as an indicator as to whether a non-fatal overdose was experienced during a prior treatment episode. Because criminal activity and incarceration were considered important for predicting treatment selection and retention, we derived indicators for recent (past 12 months) criminal charges and incarceration (34,35).
We used prescriber preference for methadone or buprenorphine as a predictor of treatment selection, defined as a time varying confounder. The previous 5 client initiations at each time point that represented a change in a confounder status were used to determine whether a prescriber preferred methadone or buprenorphine. If there were 5 previous initiations, preference was given to the majority. If there were less than 5 and all were for the same treatment, preference was set as that treatment; otherwise preference was categorised as unknown.
Factors included in each of the models are discussed further below in the specific model sections and in supplementary table 2.
Statistical models
Analyses were completed using SAS V9.4 (SAS Institute, Cary, NC, USA). A complete case approach was used for analysis.
Descriptive statistics
Socio-demographic characteristics, comorbidities, and OAT exposure were summarised with frequencies and percentages.
Crude mortality rates
Crude mortality rates were estimated using standard person-time methods with 95% confidence intervals.
Generalised estimating equation model
Assessing treatment effectiveness in observational data can be complex as participants may change or cease medications over time for a variety of reasons. In addition to the need to control for confounding at baseline due to the lack of randomisation, time-dependent confounding may influence treatment changes over time and affect treatment group effectiveness comparisons. To address these issues, we used two approaches to modeling: firstly, generalised estimating equation (GEE) models, and secondly, marginal structural models (MSM).
A Poisson regression GEE model was fitted adjusting for sex, indigenous status, geographical remoteness, socio-economic disadvantage index and previous treatment duration. Time varying covariates included calendar year, age and recency measures for release from incarceration and co-morbid indicators for mental health, mood disorders, psychosis, substance use, self-harm and the chronic diseases groupings in Table 1. A working correlation structure was applied to provide robust parameter estimates.
Marginal structural model
Mortality incident rate ratios comparing the different treatment exposure periods were estimated using MSM with inverse probability weighting (36–38). The purpose of the inverse probability weighting is to adjust for the possibility of treatment bias introduced from the inability to randomise to treatment. We calculated inverse treatment propensity weights (IPTW) and a weight to adjust for bias introduced through censorship of observations (inverse probability censorship weights; IPCW). We followed the methodology for stabilised weights recommended by Hernàn (39) with the slight adaptation that weights were estimated for each new record in the dataset that represented a change in a time varying covariate value. In this longitudinal structure there were no structured visits in the design.
The MSM consisted of three models:
Model 1: Estimating a weighting for treatment selection at baseline and each time a confounder changed (time varying)
Model 2: Estimating a weighting for probability of not ceasing OAT each time a confounder changed (time varying)
Model 3: Fitting a weighted repeated measures model analysis using a GEE.
Full details of each of these models are provided in the supplementary materials.
Mortality rates by age and treatment exposure were estimated, as well as mortality rates in the presence of each the chronic diseases of interest. Each disease was modelled in a time varying manner and an effect modification analysis was used to examine the strength of association between the chronic comorbid diseases of interest and treatment exposure.
Additionally, the impact on mortality in people with none of the co-morbid diseases was estimated. This involved fitting a simplified model: the chronic disease effect modifiers were replaced by an indicator variable which was derived with value of 1 if none of the four diseases were present and 0 if any of the 4 diseases were present.
Post hoc analyses
Following the planned analyses, we plotted crude opioid overdose mortality rates, stratified by age group and chronic disease status (yes/no for each chronic disease). Findings from post hoc analyses should be considered exploratory.
Ethical approvals
Approval for this study was obtained from the NSW Population and Health Services Research Ethics Committee (2018/HRE0205) and the NSW Aboriginal Health and Medical Research Council Ethics Committee (1400/18).
RESULTS
The study population included 37,764 people and 286,948 person-years of observation (Table 2). Just under half of the person-years accumulated during OAT were time spent receiving methadone (121,926 person-years; 42.5%), and 49,095 person-years accumulated during OAT were time spent receiving buprenorphine (17.1%). More than one in five had a hospitalisation for a respiratory disease (n=8,681; 23.0%) and/or circulatory disease (n=7,871; 20.8%). Fewer people were hospitalised with either kidney disease (n=2,715; 7.2%) or liver disease (n=2,194; 5.8%). There were 1,075 opioid overdose deaths, for a crude mortality rate of 3.75 (95% confidence interval [CI] 3.53, 3.98) per 1,000 person-years (PY).
Table 2:
Participant and opioid agonist treatment episode characteristics
| Characteristic | N (%) | |
|---|---|---|
| Sex | 37,764 | |
| Men | 26,018 (68.9%) | |
| Women | 11,744 (31.1%) | |
| Unknown | 2 (<0.01%) | |
| Indigenous | 8,646 (22.9%) | |
| Age at cohort entry | ||
| <30 years | 16,613 (44.0%) | |
| 30–39 years | 13,013 (34.5%) | |
| 40–49 years | 6,507 (17.2%) | |
| >=50 years | 1,631 (4.3%) | |
| Incarceration history | 17,041 (45.1%) | |
| Geographical remoteness | ||
| Major Cities of NSW | 27,102 (71.8%) | |
| Regional/Remote NSW | 10,444 (27.7%) | |
| Unknown | 218 (0.6%) | |
| SEIFA | ||
| 1) Most Disadvantaged | 9,138 (24.2%) | |
| 2nd Quintile | 7,098 (18.8%) | |
| 3rd Quintile | 9,864 (26.1%) | |
| 4th Quintile | 6,779 (18.0%) | |
| 5) Least Disadvantaged | 4,427 (11.7%) | |
| Unknown | 458 (1.2%) | |
| Hospitalisation | ||
| Circulatory disease | 7,871 (20.8%) | |
| Respiratory disease | 8,681 (23.0%) | |
| Liver disease | 2,194 (5.8%) | |
| Kidney disease | 2,715 (7.2%) | |
| >1 target disease | 5,468 (14.5%) | |
| Drug-related | 6,793 (18.0%) | |
| Non-drug mood disorder | 10,656 (28.2%) | |
| Self-harm/suicide attempt | 7,440 (19.7%) | |
| Psychiatric disorder | 4,291 (11.4%) | |
| Substance use disorder | 18,581 (49.2%) | |
| Treatment and Exposure | ||
| At least one methadone treatment episode | 27,471 (72.7%) | |
| At least one buprenorphine treatment episode | 24,087 (63.8%) | |
| At least one hospitalisation for a target chronic disease | 13,756 (36.4%) | |
| Treatment episodes | ||
| Methadone episodes | 60,688 (57.3%) | |
| Methadone person-years of observation | 121,927 (42.5%) | |
| Buprenorphine episodes | 55,535 (52.4%) | |
| Buprenorphine person-years of observation | 49,095 (17.1%) | |
| Person-years out of OAT | 115,927 (40.4%) | |
| Opioid overdose mortality rates per 1,000 PY (95% confidence interval) | ||
| Overall (n deaths = 1,075; PY= 286948) | 3.75 (3.53, 3.98) | |
| <30 years (n deaths = 172; PY = 67764) | 2.54 (2.18, 2.94) | |
| 30–39 years (n deaths = 429; PY = 112509) | 3.81 (3.47, 4.19) | |
| 40–49 years (n deaths = 304; PY = 74318) | 4.09 (3.65, 4.57) | |
| >=50 years (n deaths = 170; PY = 32357) | 5.25 (4.51, 6.09) | |
NSW: New South Wales. SEIFA: Socio-economic indexes for areas. PY: person-years
Mortality by age group
Opioid overdose mortality rates increased with age, from 2.54 (95% CI 2.18, 2.94) per 1,000 PY for those less than 30 years to 5.25 (05% CI 4.51, 6.09) per 1,000 person-years for those aged 50 years or greater (Table 1). Opioid overdose mortality rates during OAT increased with age: during buprenorphine, from 0.8 (95% CI 0.4, 1.6) per 1,000 PY in those <30 years to 1.8 (95% CI 1.0, 3.2) per 1,000 PY in those 50 years or older, and in methadone, from 1.4 (95% CI 1.0, 2.0) per 1,000 PY to 3.8 (95% CI 2.9, 4.9) per 1,000 PY (Table 3). Unadjusted models of associations between age group, OAT medicine, and overdose death are provided in the Supplementary Materials. In adjusted GEE and MSM models, opioid overdose mortality rates were lower during OAT with either methadone or buprenorphine, relative to periods of time out of treatment, for all age groups tested (Table 3). Contrary to our hypothesis, we found only weak evidence of a difference in opioid overdose mortality rates during OAT with buprenorphine compared to methadone. Wide confidence intervals included the null even among those aged 50 years and over, where we had expected to observe a difference (e.g. for those aged 50 years and over, GEE IRR 0.47; 95% CI 0.21, 1.02 and MSM IRR 0.49; 95% CI 0.17, 1.41) (Table 3).
Table 3:
Adjusted opioid overdose mortality incident rate ratios during time prescribed methadone or buprenorphine, relative to time out of OAT, and during time prescribed buprenorphine relative to time prescribed methadone, by age group
| Treatment | PY | N opioid overdose deaths | Crude mortality rate per 1,000 PY (95% CI) | GEE Adjusted IRR (95% CI) |
MSM Adjusted IRR (95% CI) |
|||
|---|---|---|---|---|---|---|---|---|
| Age group | Comparison of each treatment to time out of OAT | Comparison of buprenorphine to methadone | Comparison of each treatment to time out of OAT | Comparison of buprenorphine to methadone | ||||
| Out of OAT | 30,027 | 123 | 4.1 (3.4–4.9) | Ref. | Ref. | |||
| <30 years | Buprenorphine | 11,016 | 9 | 0.8 (0.4–1.6) | 0.20 (0.09–0.45) | 0.58 (0.24–1.38) | 0.18 (0.07–0.45) | 0.46 (0.17–1.26) |
| Methadone | 25,533 | 37 | 1.4 (1.0–2.0) | 0.34 (0.22–0.53) | Ref. | 0.39 (0.24–0.63) | Ref. | |
| Out of OAT | 45,140 | 321 | 7.1 (6.4–7.9) | Ref. | Ref. | |||
| 30–39 years | Buprenorphine | 18,229 | 23 | 1.3 (0.8–1.9) | 0.18 (0.11–0.29) | 0.80 (0.46–1.40) | 0.17 (0.10–0.31) | 0.61 (0.32–1.18) |
| Methadone | 47,208 | 77 | 1.6 (1.3–2.0) | 0.22 (0.16–0.30) | Ref. | 0.28 (0.20–0.39) | Ref. | |
| Out of OAT | 27,428 | 210 | 7.7 (6.7–8.8) | Ref. | Ref. | |||
| 40–49 years | Buprenorphine | 12,901 | 17 | 1.3 (0.8–2.1) | 0.17 (0.09–0.31) | 0.61 (0.32–1.17) | 0.20 (0.09–0.44) | 0.70 (0.30–1.64) |
| Methadone | 32,794 | 73 | 2.2 (1.8–2.8) | 0.28 (0.20–0.39) | Ref. | 0.28 (0.19–0.40) | Ref. | |
| Out of OAT | 10,800 | 102 | 9.4 (7.8–11.5) | Ref. | Ref. | |||
| 50+ years | Buprenorphine | 6,195 | 11 | 1.8 (1.0–3.2) | 0.18 (0.08–0.39) | 0.47 (0.21–1.02) | 0.22 (0.08–0.61) | 0.49 (0.17–1.41) |
| Methadone | 14,785 | 56 | 3.8 (2.9–4.9) | 0.39 (0.26–0.58) | Ref. | 0.45 (0.29–0.69) | Ref. | |
OAT = opioid agonist treatment. PY = person-years. GEE = generalized estimating equation. IRR = incident rate ratio. MSM = marginal structural model. Ref = reference category for IRR. GEE models are adjusted for year, sex, geographical remoteness, indigeneity, socio-economic disadvantage index, recent: incarceration, mental health ambulatory outpatient activity, previous OAT history, hospital admissions for mood and psychosis disorders, substance use and self-harm. MSM models are weight adjusted for treatment selection bias using: year^, sex, geographical remoteness, Indigenous status, socio-economic disadvantage index, recency of: criminal charges^, previous OAT history, most recent OAT^, hospital admissions for respiratory^, substance use^, previous NFOD on OAT, prescriber preference^ and censorship using: year^, sex, geographical remoteness, indigeneity, socio-economic disadvantage index, previous OAT history, treatment^ and treatment by year interaction^ and recency of: incarceration^, hospital admissions for mood^ and psychosis disorders^, substance use^ and mental health ambulatory outpatient activity^. ^=fitted at baseline and time varying.
Mortality in the presence of specific chronic diseases
As with age groups, time in OAT was associated with a lower risk of opioid overdose death relative to time out of treatment for each of the analysed chronic disease groups (Table 4). Some disease-specific associations between specific OAT medicines and fatal opioid overdose were observed. Among participants with circulatory diseases, opioid overdose mortality rates were lower during OAT with buprenorphine relative to methadone in both GEE (IRR 0.37; 95% CI: 0.17, 0.78) and MSM (IRR 0.27; 95% CI: 0.11, 0.67) models. Among participants with respiratory diseases, mortality rates were lower during OAT with buprenorphine relative to methadone in the GEE and MSM models (0.38; 95% CI 0.15, 0.91 and 0.26; 95% CI 0.07, 0.94), respectively. This pattern was not observed in relation to liver or kidney diseases (Table 4).
Table 4:
Adjusted opioid overdose mortality incident rate ratios during time prescribed methadone or buprenorphine, relative to time out of OAT, and during time prescribed buprenorphine relative to time prescribed methadone, in people with and without evidence of chronic diseases
| Chronic disease | Treatment | PY | N opioid overdose deaths | Crude mortality rate per 1,000 PY (95% CI) | GEE Adjusted IRR (95% CI) |
MSM Adjusted IRR (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| Comparison of each treatment to time out of OAT | Comparison of buprenorphine to methadone | Comparison of each treatment to time out of OAT | Comparison of buprenorphine to methadone | |||||
| No evidence of target chronic diseases | Out of OAT | 87,386 | 424 | 4.9 (4.4–5.3) | Ref | Ref | ||
| Buprenorphine | 35,762 | 34 | 1.0 (0.7–1.3) | 0.19 (0.12–0.30) | 0.73 (0.46–1.18) | 0.21 (0.13–0.35) | 0.67 (0.38–1.17) | |
| Methadone | 87,342 | 113 | 1.3 (1.1–1.6) | 0.26 (0.20–0.34) | Ref | 0.32 (0.24–0.42) | Ref | |
| Circulatory disease | Out of OAT | 12,398 | 183 | 14.8 (12.8–17.1) | Ref | Ref | ||
| Buprenorphine | 6,213 | 12 | 1.9 (1.1–3.4) | 0.12 (0.06–0.24) | 0.37 (0.17–0.78) | 0.10 (0.04–0.24) | 0.27 (0.11–0.67) | |
| Methadone | 16,123 | 77 | 4.8 (3.8–6.0) | 0.32 (0.22–0.45) | Ref | 0.37 (0.25–0.55) | Ref | |
| Kidney disease | Out of OAT | 3,656 | 68 | 18.6 (14.7–23.6) | Ref | Ref | ||
| Buprenorphine | 1,794 | 8 | 4.5 (2.2–8.9) | 0.25 (0.10–0.59) | 0.87 (0.33–2.28) | 0.39 (0.12–1.35) | 1.16 (0.31–4.36) | |
| Methadone | 4,654 | 26 | 5.6 (3.8–8.2) | 0.28 (0.16–0.50) | Ref | 0.34 (0.18–0.66) | Ref | |
| Liver disease | Out of OAT | 3,110 | 46 | 14.8 (11.1–19.7) | Ref | Ref | ||
| Buprenorphine | 1,445 | 6 | 4.2 (1.9–9.2) | 0.29 (0.10–0.81) | 0.66 (0.22–1.97) | 0.32 (0.08–1.23) | 0.59 (0.14–2.43) | |
| Methadone | 3,811 | 25 | 6.6 (4.4–9.7) | 0.44 (0.24–0.81) | Ref | 0.54 (0.28–1.07) | Ref | |
| Respiratory disease | Out of OAT | 16,683 | 228 | 13.7 (12.0–15.6) | Ref | Ref | ||
| Buprenorphine | 7,824 | 13 | 1.7 (1.0–2.9) | 0.11 (0.05–0.26) | 0.38 (0.15–0.91) | 0.08 (0.02–0.29) | 0.26 (0.07–0.94) | |
| Methadone | 21,038 | 86 | 4.1 (3.3–5.0) | 0.29 (0.21–0.40) | Ref | 0.32 (0.22–0.46) | Ref | |
OAT = opioid agonist treatment. PY = person-years. GEE = generalized estimating equation. IRR = incident rate ratio. MSM = marginal structural model. Ref = reference category for IRR. GEE models are adjusted for year, sex, geographical remoteness, Indigenous status, socio-economic disadvantage index, recent: incarceration, mental health ambulatory outpatient activity, previous OAT history, hospital admissions for mood and psychosis disorders, substance use and self-harm. MSM models are weight adjusted for treatment selection bias using: year^, sex, geographical remoteness, indigeneity, socio-economic disadvantage index, recency of: criminal charges^, previous OAT history, most recent OAT^, hospital admissions for respiratory^, substance use^, previous NFOD on OAT, prescriber preference^ and censorship using: year^, sex, geographical remoteness, indigeneity, socio-economic disadvantage index, previous OAT history, treatment^ and treatment by year interaction^ and recency of: incarceration^, hospital admissions for mood^ and psychosis disorders^, substance use^ and mental health ambulatory outpatient activity^. ^=fitted at baseline and time varying.
Post-hoc analyses of age x disease interactions
In light of the findings of the planned analyses, we undertook exploratory, post-hoc analyses of opioid overdose mortality rates stratified by both age group and chronic disease status. Given the exploratory nature of these analyses and anticipated low statistical power, significance testing was not undertaken. Very wide confidence intervals were observed around the age-specific mortality rates in those with chronic diseases (Figure 2, Supplementary Table 5, Supplementary Figures 1, 2). Within each age group, comparing mortality rates during methadone and buprenorphine, minimal differences were observed in participants without circulatory or respiratory disease. For participants with circulatory or respiratory disease, the largest apparent disparity in overdose mortality rates was observed in those aged 50 years or over, with rates higher in methadone compared to buprenorphine (Figure 2). Similar analyses were undertaken for kidney and liver disease, with no clear age gradient observed (Supplementary Table 5, Supplementary Figure 1, 2).
Figure 2:
Crude opioid overdose mortality rates, stratified by age group and circulatory disease (panel A) and respiratory disease (panel B).
DISCUSSION
OAT with methadone or buprenorphine was associated with reduced risk of opioid overdose death, relative to time out of OAT, across the lifespan. We confirm that opioid overdose risk increases with age in and out of OAT. We found only weak evidence of a difference in opioid overdose mortality rates during OAT with buprenorphine relative to methadone in people aged 50 years and over, with IRRs well below 1 but wide confidence intervals, particularly in the MSM model, complicating interpretation. Lower opioid overdose risk was observed during OAT with buprenorphine compared to methadone among participants with circulatory and respiratory diseases. This may be linked to buprenorphine’s limited effect on respiratory depression, with a ‘ceiling effect’ at higher doses, in contrast to the linear relationship between methadone dose and respiratory depression (40). Our post-hoc analyses, while exploratory, suggest that there may be interactions between age and chronic disease; these findings need replication via planned analyses in other datasets with sufficient numbers of participants across different age groups, with and without chronic diseases.
Other evidence and implications
There is limited published clinical guidance for the prescribing of OAT for older adults or people with comorbid health problems. Some national clinical guidelines highlight the importance of careful assessment and regular review of older adults in OAT (41,42), and there is one set of guidelines that recommends buprenorphine as the first line treatment for older adults in light of fewer adverse drug interactions relative to methadone (although not in reference to evidence on treatment outcomes in older adults) (43). We are not aware of any clinical guidelines that specifically highlight treatment strategies for OAT clients with chronic diseases. Our findings suggest that particular comorbidities maybe more important than age per se in determining opioid overdose risk during OAT; specifically, respiratory and circulatory diseases. This extends and contrasts with findings from the UK (8,9). The reduction in mortality risk associated with buprenorphine relative to methadone in people aged 50 years or older is less and weaker than observed in UK primary care patients (8), and needs further replication and synthesis of results. Our findings also suggest that the type of comorbidity (such as circulatory and respiratory problems) may be of importance, rather than the number of comorbidities as shown previously (8). Replication studies in other settings are needed to disentangle the effects of age and physical morbidity on overdose risk during OAT, along with analyses that assess the impacts of sex, gender, race, ethnicity and other social determinants of health on the associations identified here. Replication in a range of settings will support generalizability and help to clarify and strengthen clinical recommendations.
Many people with opioid use disorder have poor access to primary health care, and chronic diseases are likely to be underdiagnosed. Furthermore, adherence to treatment in the event of diagnosis may be difficult for multiple reasons, including ongoing substance use, housing instability, and mental health problems. Regular screening and assessment for the presence and severity of cardiovascular and respiratory diseases should be considered, particularly in clients who are mid-life or older age. Although there is no widespread consensus on what age is considered “older” with this population, premature aging as a result of chronic substance use and exposure to multiple social disadvantages (44) suggests that screening should begin at an earlier age than would be considered in the general population. In specific contexts, there may be sub-populations of people in OAT with an elevated risk of chronic disease, such as Indigenous people or racialized populations. Such groups may need enhanced chronic disease screening; for example, beginning at an earlier age or more frequently than otherwise.
These findings, and others relating to potential medication interactions (43), suggest that buprenorphine may be preferred to methadone as a first line treatment when prescribing OAT for patients with significant circulatory and respiratory comorbidities. In some cases, however, clients with these coexisting conditions will have been prescribed methadone for a number of years, and may be reluctant to change to buprenorphine, or will be entering treatment with a preference for methadone. An important opportunity presents for a discussion with the client on the relative risks and benefits of each medicine, as an unwanted change in prescribing may be destabilising or precipitate treatment exit, thereby increasing overdose risk to a greater extent than if they were to remain on methadone. Recent advances in clinical protocols for transfer from methadone to buprenorphine can assist this process (45). If, after an informed discussion there is a client preference to continue with methadone treatment, other strategies to reduce risks are warranted, including client and carer education about overdose signs and risks, including interactions between opioids and alcohol; naloxone provision; and review of concomitantly prescribed medicines that may contribute to respiratory depression, such as benzodiazepines, gabapentinoids, and sedating antidepressants and antipsychotics.
Limitations
This study used a large linked administrative health dataset with analyses that attempted to adjust for non-random treatment assignment and a range of confounders. However, administrative data were lacking in detailed clinical information. For example, chronic disease status was defined using hospitalisation data. These likely reflect more serious clinical presentations and under-estimate true prevalence (46). Age may still therefore be somewhat of a proxy for physical comorbidities, which may affect the observed associations. Uncertainty in chronic disease ascertainment may also arise as a result of varying coding practices. The MSM models presented can provide consistent estimates of causal effects when all confounders have been accounted for and the correct models have been specified (47,48). In this analysis, key variables that we were unable to measure or control were adequacy of OAT dose, severity of opioid dependence, and use of other central nervous system depressants; if these factors differed by OAT type, it may explain greater risk observed in those treated with methadone.
Previous studies have used prescriber preference as an instrument to adjust for confounding bias when estimating a treatment effect (49). However, due to the way in which OAT is regulated in Australia, not all prescribers are permitted to prescribe both methadone and buprenorphine. As such, the assumption that the instrument can detect unmeasured confounders fails. Therefore, rather than undertake an instrumental variable analysis, we incorporated prescriber preference into the marginal structural model. The decision to base prescriber preference on the previous five new prescriptions was based on a consideration of relevant studies (49–51), but is largely subjective given the absence of consensus in the literature.
Conclusion
OAT reduces opioid overdose mortality risk for older clients relative to time out of treatment. In OAT clients with circulatory or respiratory illnesses, buprenorphine may be preferred as a first line treatment over methadone for reducing opioid overdose risk. However, other factors, including client preferences and current medications, remain important in determining appropriate treatment plans.
Supplementary Material
Acknowledgements:
SL is supported by a Fonds de recherché du Québec – Santé Research Scholar grant (296569). SN is supported by an Australian National Health and Medical Research Council (NHRMC) Career Development Fellowship (1163961). LD is supported by an NHMRC Senior Principal Research Fellowship. MH acknowledges funding from NIHR Health Protection Research Unit in Behavioural Science and Evaluation, NIHR School of Public Health Research, NIHR Biomedical Research Centre, and NIHR Senior Investigator Award.
Record linkage was conducted by the NSW Ministry of Health and the Centre for Health Record Linkage. The Cause of Death Unit Record File (COD URF) was provided by the Australian Coordinating Registry for the COD URF on behalf of the NSW Registry of Births Deaths and Marriages, NSW Coroner and the National Coronial Information System. Incarceration data from the Reoffending Database was provided by the NSW Bureau of Crime Statistics and Research. The authors wish to acknowledge all data custodians for providing access to the datasets used in this study
Primary funding:
National Institutes of Health R01 DA144740
Footnotes
Declarations of interests: In the past three years, LD has received investigator-initiated untied educational grants for studies of opioid medications in Australia from Indivior and Seqirus. SN has received investigator-initiated untied educational grants from Seqirus, and is a named investigator on an implementation trial funded by Indivior. SL has previously received an untied educational grant from Indivior.
References
- 1.Santo T Jr, Clark B, Hickman M, Grebely J, Campbell G, Sordo L, et al. Association of Opioid Agonist Treatment With All-Cause Mortality and Specific Causes of Death Among People With Opioid Dependence: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2021. Sep 1;78(9):979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimber J, Larney S, Hickman M, Randall D, Degenhardt L. Mortality risk of opioid substitution therapy with methadone versus buprenorphine: A retrospective cohort study. Lancet Psychiatry. 2015;2:901–8. [DOI] [PubMed] [Google Scholar]
- 3.Pearce LA, Min JE, Piske M, Zhou H, Homayra F, Slaunwhite A, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020. Mar 31;368:m772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EMCDDA. Treatment and care for older drug users. Lisbon: European Monitoring Centre for Drugs and Drug Addiction; 2010. [Google Scholar]
- 5.Lynch A, Arndt S, Acion L. Late- and Typical-Onset Heroin Use Among Older Adults Seeking Treatment for Opioid Use Disorder. Am J Geriatr Psychiatry. 2020;((Lynch, Arndt) Department of Psychiatry (AL, SA), University of Iowa, Iowa City, IA, United States(Acion) Instituto de Calculo, Universidad de Buenos Aires-CONICET (LA), Argentina). [DOI] [PubMed] [Google Scholar]
- 6.Huhn AS, Strain EC, Tompkins DA, Dunn KE. A hidden aspect of the U.S. opioid crisis: Rise in first-time treatment admissions for older adults with opioid use disorder. Drug Alcohol Depend. 2018;193:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han B, Polydorou S, Ferris R, Blaum CS, Ross S, McNeely J. Demographic Trends of Adults in New York City Opioid Treatment Programs--An Aging Population. Subst Use Misuse. 2015;50(13):1660–7. [DOI] [PubMed] [Google Scholar]
- 8.Hickman M, Steer C, Tilling K, Lim AG, Marsden J, Millar T, et al. The impact of buprenorphine and methadone on mortality: a primary care cohort study in the United Kingdom. Addiction. 2018;113(8):1461–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao L, Robertson JR, Bird SM. Non drug-related and opioid-specific causes of 3262 deaths in Scotland’s methadone-prescription clients, 2009–2015. Drug Alcohol Depend. 2019. Apr 1;197:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev [Internet]. 2014. [cited 2021 Dec 9];(2). Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002207.pub4/full
- 11.Rosen D, Smith ML, Reynolds CF. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488–97. [DOI] [PubMed] [Google Scholar]
- 12.Han BH, Cotton BP, Polydorou S, Sherman SE, Ferris R, Arcila-Mesa M, et al. Geriatric Conditions Among Middle-aged and Older Adults on Methadone Maintenance Treatment: A Pilot Study. J Addict Med. 2022. Feb;16(1):110–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le TT, Park S, Choi M, Wijesinha M, Khokhar B, Simoni-Wastila L. Respiratory events associated with concomitant opioid and sedative use among Medicare beneficiaries with chronic obstructive pulmonary disease. BMJ Open Respir Res. 2020. Mar 1;7(1):e000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davison SN. Clinical Pharmacology Considerations in Pain Management in Patients with Advanced Kidney Failure. Clin J Am Soc Nephrol. 2019. Jun 7;14(6):917–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakoski M, Goyal P, Spencer-Safier M, Weissman J, Mohr G, Volk M. Pain management in patients with cirrhosis. Clin Liver Dis. 2018;11(6):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCance-Katz EF, Sullivan L, Nallani S. Drug Interactions of Clinical Importance among the Opioids, Methadone and Buprenorphine, and other Frequently Prescribed Medications: A Review. Am J Addict. 2010;19(1):4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coe MA, Lofwall MR, Walsh SL. Buprenorphine Pharmacology Review: Update on Transmucosal and Long-Acting Formulations. J Addict Med. 2019;13(2):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994. May;55(5):569–80. [DOI] [PubMed] [Google Scholar]
- 19.Modesto-Lowe V, Brooks D, Petry N. Methadone deaths: risk factors in pain and addicted populations. J Gen Intern Med. 2010. Apr;25(4):305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLOS Med. 2015. Oct 6;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. PLOS Med. 2007. Oct 16;4(10):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larney S, Jones N, Fiellin DA, Nielsen S, Hickman M, Dobbins T, et al. Data Resource Profile: The Opioid Agonist Treatment and Safety (OATS) Study, New South Wales, Australia. Int J Epidemiol. 2020. Dec 1;49(6):1774–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larney S, Hickman M, Fiellin DA, Dobbins T, Nielsen S, Jones NR, et al. Using routinely collected data to understand and predict adverse outcomes in opioid agonist treatment: Protocol for the Opioid Agonist Treatment Safety (OATS) Study. BMJ Open. 2018;8:e025204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Australian Institute of Health and Welfare. National Opioid Pharmacotherapy Statistics Annual Data Collection [Internet]. 2022. [cited 2022 Jun 21]. Available from: https://www.aihw.gov.au/reports/alcohol-other-drug-treatment-services/national-opioid-pharmacotherapy-statistics/data
- 25.Jones NR, Hickman M, Nielsen S, Larney S, Dobbins T, Ali R, et al. The impact of opioid agonist treatment on fatal and non-fatal drug overdose among people with a history of opioid dependence in NSW, Australia, 2001–2018: Findings from the OATS retrospective linkage study. Drug Alcohol Depend. 2022. Apr 15;236:109464. [DOI] [PubMed] [Google Scholar]
- 26.Santo T, Bharat C, Colledge-Frisby S, Chrzanowska A, Man N, Moran L, et al. Mortality among people with substance use disorders: A toolkit for classifying major causes of death. Sydney: National Drug and Alcohol Research Centre, UNSW Sydney; 2022. [Google Scholar]
- 27.KDIGO. Chapter 1: Definition and classification of CKD. Kidney Int Suppl. 2013. Jan 1;3(1):19–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021. Nov;18(11):785–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008. Jan 1;31(1):204–12. [DOI] [PubMed] [Google Scholar]
- 30.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol. 2006. Jan 1;44(1):217–31. [DOI] [PubMed] [Google Scholar]
- 31.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85. [DOI] [PubMed] [Google Scholar]
- 32.Hugo Centre for Population and Migration Studies. Accessibility/Remoteness Index of Australia [Internet]. no date [cited 2022 Jun 21]. Available from: https://able.adelaide.edu.au/hugo-centre/services/aria
- 33.Australian Bureau of Statistics. Socio-Economic Indexes for Areas (SEIFA 2016) [Internet]. [cited 2022 Jun 21]. Available from: https://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa
- 34.Bharat C, Larney S, Barbieri S, Dobbins T, Jones NR, Hickman M, et al. The effect of person, treatment and prescriber characteristics on retention in opioid agonist treatment: a 15-year retrospective cohort study. Addiction. 2021;116(11):3139–52. [DOI] [PubMed] [Google Scholar]
- 35.Burns L, Gisev N, Larney S, Dobbins T, Gibson A, Kimber J, et al. A longitudinal comparison of buprenorphine and methadone treatment for opioid dependence in New South Wales, Australia. Addiction. 2015;110:646–55. [DOI] [PubMed] [Google Scholar]
- 36.Robins JM. Correction for non-compliance in equivalence trials. Stat Med. 1998. Feb 15;17(3):269–302; discussion 387–389. [DOI] [PubMed] [Google Scholar]
- 37.Robins JM, Hernán MÁ, Brumback B. Marginal Structural Models and Causal Inference in Epidemiology. Epidemiology. 2000. Sep;11(5):550–60. [DOI] [PubMed] [Google Scholar]
- 38.Robins JM, Greenland S, Hu FC. Estimation of the Causal Effect of a Time-Varying Exposure on the Marginal Mean of a Repeated Binary Outcome. J Am Stat Assoc. 1999. Sep 1;94(447):687–700. [Google Scholar]
- 39.Hernán MA, Brumback BA, Robins JM. Estimating the causal effect of zidovudine on CD4 count with a marginal structural model for repeated measures. Stat Med. 2002;21(12):1689–709. [DOI] [PubMed] [Google Scholar]
- 40.Mégarbane B, Hreiche R, Pirnay S, Marie N, Baud FJ. Does High-Dose Buprenorphine Cause Respiratory Depression? Toxicol Rev. 2006. Jun 1;25(2):79–85. [DOI] [PubMed] [Google Scholar]
- 41.Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group. Drug misuse and dependence: UK guidelines on clinical management. London: Department of Health; 2017. [Google Scholar]
- 42.Gowing L, Ali R, Dunlop A, Farrell M, Lintzeris N. National guidelines for medication-assisted treatment of opioid dependence. Canberra: Commonwealth Department of Health; 2014. [Google Scholar]
- 43.Canadian Coalition for Seniors’ Mental Health. Canadian Guidelines on Opioid Use Disorder Among Older Adults [Internet]. Toronto: Canadian Coalition on Seniors’ Mental Health; 2019. [cited 2022 Mar 1]. Available from: https://ccsmh.ca/wp-content/uploads/2019/11/Canadian_Guidelines_Opioid_Use_Disorder_ENG.pdf [Google Scholar]
- 44.Zolopa C, Høj SB, Minoyan N, Bruneau J, Makarenko I, Larney S. Ageing and older people who use illicit opioids, cocaine or methamphetamine: a scoping review and literature map. Addiction [Internet]. 2022. [cited 2022 Feb 14];n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/add.15813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lintzeris N, Mankabady B, Rojas-Fernandez C, Amick H. Strategies for Transfer From Methadone to Buprenorphine for Treatment of Opioid Use Disorders and Associated Outcomes: A Systematic Review. J Addict Med. 2022. Apr;16(2):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lujic S, Simpson JM, Zwar N, Hosseinzadeh H, Jorm L. Multimorbidity in Australia: Comparing estimates derived using administrative data sources and survey data. PLOS ONE. 2017. Aug 29;12(8):e0183817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend. 2014. May 1;138:118–23. [DOI] [PubMed] [Google Scholar]
- 48.Lintzeris N, Nielsen S. Benzodiazepines, methadone and buprenorphine: interactions and clinical management. Am J Addict. 2010. Feb;19(1):59–72. [DOI] [PubMed] [Google Scholar]
- 49.Brookhart MA, Wang PS, Solomon DH, Schneeweiss S. Evaluating Short-Term Drug Effects Using a Physician-Specific Prescribing Preference as an Instrumental Variable. Epidemiology. 2006. May;17(3):268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uddin MdJ, Groenwold RHH, de Boer A, Afonso ASM, Primatesta P, Becker C, et al. Evaluating different physician’s prescribing preference based instrumental variables in two primary care databases: a study of inhaled long-acting beta2-agonist use and the risk of myocardial infarction. Pharmacoepidemiol Drug Saf. 2016;25(S1):132–41. [DOI] [PubMed] [Google Scholar]
- 51.Homayra F, Hongdilokkul N, Piske M, Pearce LA, Zhou H, Min JE, et al. Determinants of selection into buprenorphine/naloxone among people initiating opioid agonist treatment in British Columbia. Drug Alcohol Depend. 2020. Feb 1;207:107798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.