Abstract
Research Question:
Has the acceptance of heritable genome-editing (HGE) and whole genome sequencing for preimplantation genetic testing (PGT-WGS) of human embryos changed after the onset of COVID-19 among infertility patients?
Design:
A written survey was conducted from April-June 2018 and July-December 2021 among patients at a university-affiliated infertility practice. The questionnaire ascertained the acceptance of HGE for specific therapeutic or genetic ‘enhancement’ indications and of PGT-WGS to prevent adult disease.
Results:
In 2021 and 2018, 172 patients (response rate: 90%) and 469 patients (response rate: 91%) completed the questionnaire, respectively. Overall, in 2021, significantly more participants reported a positive attitude towards HGE, for both therapeutic and enhancement indications. In 2021 vs. 2018, respondents were more likely to utilize HGE to have healthy children with their own gametes [85% vs. 77%), to reduce disease risk for adult-onset polygenic disorders (78% vs. 67%), to increase life expectancy (55% vs. 40%), intelligence (34% vs. 26%) and creativity (33% vs. 24%), respectively. Fifteen percent of the 2021 group reported a more positive attitude towards HGE due to COVID-19 and <1% a more negative attitude. In contrast, support for PGT-WGS was similar in 2021 and 2018.
Conclusions:
We observed a significantly increased acceptance of HGE, but not of PGT-WGS, after the onset of COVID-19. While the pandemic itself may have played a role in this change, the exact reasons remain unknown and warrant further investigation. This study also raises the question of whether the increased acceptability of HGE may indicate an increase in acceptability of emerging biomedical technologies in general.
Keywords: gene editing, COVID-19, CRISPR/Cas9, whole genome sequencing, pre-implantation genetic screening, human genome
Introduction
COVID-19 has re-exposed a growing skepticism towards science in society (Editorial, 2017; Pittinsky, 2015; Rutjens, 2018), but the ultimate effects of the pandemic on the acceptance of medical interventions remain unknown. These effects may be complex because, while the implementation of COVID-19 measures may have initially fueled such skepticism, not following scientific advice has resulted in deleterious consequences (e.g. severe disease) throughout the pandemic and may have increased acceptance (Rutjens, 2021). Little is known about whether COVID-19 has altered the acceptance of other emerging medical technologies, such as CRISPR/Cas9, in particular because this technology could be used to edit the human germline to alter susceptibility to novel viral pathogens (Adashi and Cohen, 2022). This potential was exploited prematurely in 2018 by a researcher in China who altered the CCR5 gene in human in-vitro fertilization (IVF) embryos with an eye toward conferring resistance to HIV (Cyranoski and Ledford, 2018). The delivery of seemingly healthy twins followed but the clinical use of CRISPR/Cas9 was widely condemned by the scientific community and the broader public (Lander et al., 2019). Regardless of this reaction, there is consensus that research exploring the potential of heritable genome editing (HGE) should continue under strict control (Baltimore et al., 2018). Organizations such as the International Society for Stem Cell Research call for participatory public engagement on its potential clinical use (International Society for Stem Cell Research (ISSCR), 2015).
A second emerging technology, preimplantation genetic testing (PGT) of IVF embryos using whole genome sequencing (PGT-WGS) (Winand et al., 2014), extends the scope of PGT from detecting specific monogenic diseases and aneuploidies to detecting genome-wide polygenic variants underlying complex diseases such as cancer, cardiovascular disease or diabetes (Navin et al., 2011; Xu et al., 2012). The current use of polygenic risk scores associated with preimplantation embryo testing already has given rise to some ethical challenges (such as exacerbating inequalities in society and devaluing certain traits) (Turley et al., 2021). Despite a likely much shorter timeframe for clinical implementation than HGE, PGT-WGS received much less public attention, and the acceptance of this technology remains unknown.
There is an ongoing debate about the ethical and legal implications of HGE and PGT-WGS of human embryos. For example, there are concerns regarding equity in distributing its benefits and the inability to consent future generations that will inherit the altered DNA as well as the potential adverse effects (Bu, 2019; Cohen and Adashi, 2021; National Academy of Medicine, 2020). Thus, the attitudes towards these emerging technologies among potential recipients are a key factor in this debate. Because HGE and PGT-WGS require human embryos, patients undergoing IVF are likely to be first to encounter these technologies in a clinical setting. In addition, offspring from infertility treatments are at risk of infertility due to genetic factors contributing to infertility in many patients, providing a use case for HGE and PGT-WGS. A previous survey has shown that the majority of infertility patients support HGE to correct disease but not to enhance physical traits (McQueen et al., 2021). However, it is unclear how these attitudes have changed over time, in particular during the COVID-19 pandemic. In this study, we sought to determine whether the acceptance of HGE and PGT-WGS in individuals seeking infertility treatment changed after the onset of COVID-19.
Materials and Methods
We administered an anonymous, cross-sectional questionnaire before (April-June 2018) and after (July-December 2021) the outbreak of COVID-19 to patients attending an infertility clinic. The second study period included the interval from the onset of the Delta COVID-19 variant wave to the onset of the Omicron variant wave. Patients were approached by a physician and asked to complete a self-administered questionnaire (Supplemental Table 1) with fixed-choice questions. All patients, regardless of diagnosis, and their partners were eligible. At both time points, the questionnaire included questions regarding demographics; acceptance and self-assessed knowledge of HGE; and acceptance and knowledge of PGT-WGS. The 2021 questionnaire also included questions on the effect of COVID-19 and the live births from embryos altered using CRISPR/Cas9 in China in November 2018 (Supplemental Table 1). Specifically, one question noted that HGE could be used to confer resistance to viral illnesses in children and asked whether the pandemic changed their views on HGE. Another noted that HGE was used in China to confer resistance to HIV and asked whether this incident changed their views on HGE. The questionnaire was screened for directive phrasing and scientific jargon using feedback from a pilot group of patients. To reduce response bias, the questionnaire provided background information regarding HGE and PGT-WGS preceding the questions. Questions about risks of HGE were not included in the survey because these are unclear at present and remain an active area of research.
Statistical analyses
Data are presented as median (interquartile range, IQR) or proportion. Log-binomial regression was used to calculate risk ratios (RR) and adjusted risk ratios (aRR) with 95% confidence intervals (CI). We adjusted the models for variables that were appreciably different between the 2018 and 2021 surveys, which included gender, religion, knowledge of WGS, and knowledge of gene editing. All tests were two-sided and p values <0.05 were considered statistically significant. Data were analyzed using the Statistical Analysis Software 9.4 (SAS Institute Inc, Cary, NC).
Ethical approval
The questionnaire was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center (protocol 2018P000043).
Results
Characteristics of participants
Four hundred sixty-nine respondents (469/514, response rate: 91%, median age: 41[37-44] years) and 172 respondents (172/192, response rate: 90%, median age: 38[35-40] years) completed the 2018 and 2021 questionnaires, respectively. In both time periods, education levels were high, with a majority of patients having at least a college degree and some knowledge of HGE and WGS. Demographic details of both groups are listed in Table 1.
Table 1.
Characteristics of patients seeking infertility treatment who responded to a survey on the acceptance of whole genome sequencing and gene editing of human embryos, stratified by year of survey completion.
| 2018 Questionnaire Respondents N=469 | 2021 Questionnaire Respondents N=172 | |
|---|---|---|
| Age (years) | 41 (37-44) | 38 (35-40) |
| Female | 297 (63) | 138 (80) |
| Self-reported ethnicity | ||
| Hispanic, Latino, or Spanish Origin | 25 (5) | 15 (9) |
| Self-reported race | ||
| American Indian/Alaska Native + Hawaii | 7 (2) | 1 (1) |
| Asian | 77 (16) | 28 (16) |
| Black/African American | 22 (5) | 4 (2) |
| White | 355 (76) | 132 (77) |
| No response | 8 (2) | 7 (4) |
| Education | ||
| Less than college | 83 (18) | 24 (14) |
| College graduate | 167 (36) | 73 (42) |
| Graduate school | 216 (46) | 73 (42) |
| No response | 3 (1) | 2 (1) |
| Political beliefs | ||
| Conservative | 51 (11) | 20 (12) |
| Moderate | 154 (33) | 56 (33) |
| Liberal | 214 (46) | 77 (45) |
| Other | 33 (7) | 18 (11) |
| No response | 17 (4) | 1 (1) |
| Religion | ||
| Agnostic or Atheist | 90 (19) | 22 (13) |
| Christian | 243 (52) | 91 (53) |
| Other (Buddhist, Hindu, Muslim, Jewish) | 93 (20) | 22 (13) |
| No affiliation | 21 (5) | 34 (20) |
| No response | 22 (5) | 3 (2) |
| Work in health care | 118 (25) | 57 (33) |
| Nulliparous (among female respondents) | 60 (20) | 19 (14) |
| History of miscarriage | 89 (56) | 30 (49) |
| Prior infertility treatment | 262 (56) | 95 (55) |
| In vitro fertilization (IVF) | 192 (73) | 75 (79) |
| Plan for preimplantation genetic testing | 199 (42) | 69 (40) |
| A lot or good amount of prior knowledge of gene editing | 53 (11) | 34 (20) |
| A lot or good amount of prior knowledge of whole genome sequencing | 41 (9) | 33 (19) |
Data presented as median (interquartile range) or n (%)
Effect of COVID-19 on attitudes towards HGE and PGT-WGS of human embryos
A majority of participants expressed support for HGE to treat or prevent human disease (Figure 1). Compared with 2018, in 2021 respondents were significantly more likely to report that they would utilize HGE to have healthy children with their own gametes, if not otherwise possible (85% vs 77%, aRR 1.10 (1.01-1.19)), and to reduce polygenic disease risk in adulthood (78% vs 67%, aRR 1.19 (1.07-1.33)). In contrast to the strong support for using HGE for disease prevention, in both time periods, support of this technology to enhance human abilities was relatively low (7% to 55%; Figure 1). However, respondents in 2021, compared to those in 2018, were more likely to support HGE to enhance life expectancy (55% vs 40%, aRR 1.77 (1.36-2.31)), intelligence (34% vs. 26%, aRR 1.37 (1.05-1.79)), and creativity (33% vs. 24%, aRR 1.50 (1.14-1.99)) and to alter physical characteristics of their children (10% vs. 7%, aRR 1.85 (0.98-3.5)). Support for correction of single-gene mutations using HGE was high and similar ((82% vs. 79%, aRR 1.05 (0.96-1.154)) in 2021 compared to 2018.
Figure 1. Comparison of the acceptance of whole genome sequencing and gene editing of human embryos in different scenarios between the pre- and post- COVID-19 groups of patients seeking infertility treatment.

Very high and high acceptance for the corresponding indications are colored in dark and light green, respectively; dark and light red represent very low and low acceptance, respectively. Log-binomial regression was used to calculate adjusted risk ratios and 95% confidence intervals. All models were adjusted for covariates that were notably different between the 2021 and 2018 respondents, including gender, religion, knowledge of whole genome sequencing, and knowledge of gene editing. significant.
Support for PGT-WGS to increase the chance of a live birth (aRR 0.97 (0.90-1.04)) and to reduce the risk of adult-onset disease (aRR 1.02 (0.93-1.12)) was universally high and similar in the 2021 and 2018 groups. The majority of respondents before (80%) and after the onset of COVID-19 (81%) would perform WGS even if their own risk of disease was revealed in the process (aRR 1.03 (0.94-1.13)) (Figure 1). Figure 2 shows a forest plot of crude and adjusted risk ratios for support of WGS and HGE from respondents in 2021 compared to respondents in 2018; RRs and CIs are specified in Supplemental Table 2.
Figure 2: Forest plot of crude and adjusted risk ratios for support of whole genome sequencing and human gene editing from respondents in 2021 compared to respondents in 2018.
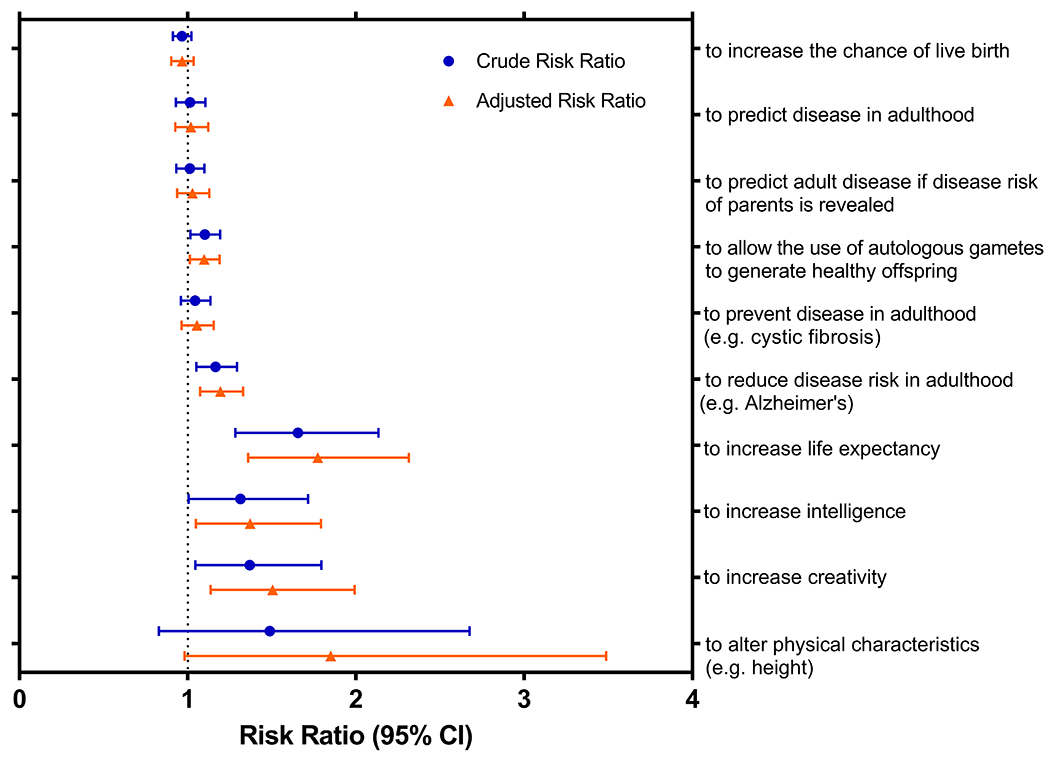
We used log-binomial regression to calculate crude and adjusted risk ratios (RR) and 95% confidence intervals (CI). All models were adjusted for covariates that were notably different between the 2018 and 2021 respondents, including gender, religion, knowledge of whole genome sequencing, and knowledge of gene editing.
Factors affecting patient attitudes
When the 2021 group was specifically asked how the COVID-19 pandemic had changed their views on HGE, 15% of respondents reported a more positive attitude and <1% a more negative attitude. In contrast, the HGE of human embryos in China in 2018 only had a small overall effect with 8% reporting a more positive and 5% a more negative attitude (Figure 3).
Figure 3.

Effect of the COVID-19 pandemic and the 2019 human germline editing incident in China on the acceptance of gene editing of human embryos.
Subgroups analysis
For disease prevention and to increase the chance of a live birth, respondents with conservative political views were less likely than those with moderate or liberal political views to support WGS and HGE (Supplemental Figure 1). However, most respondents supported the use of PGT-WGS and HGE for disease correction, irrespective of political views. Religious beliefs and educational background affected respondents’ attitudes to a lesser extent. For enhancement, acceptance was low across political views, religious beliefs and educational backgrounds. Prior knowledge of these technologies did not significantly alter patient attitudes (Supplemental Figure 2).
Discussion
The present study demonstrates that patients seeking infertility treatment supported the use of HGE to preserve human health, while the enhancement of human traits with HGE was less acceptable. These findings are consistent with previous surveys of the general public (Cary Funk, 2016; Funk C, 2018; Hendriks et al., 2018; Kobayashi et al., 2022; Scheufele et al., 2017; Whitman, January 2018), and with health care providers proving to be generally more reluctant (Armsby et al., 2019). However, we found a significantly higher acceptance of HGE for therapeutic and in particular ‘enhancement’ indications in post-COVID-19 respondents. Stratification by political and religious views, as well as educational background, while revealing some statistically significant differences, showed surprisingly high overall acceptance for therapeutic HGE across different backgrounds. In addition, prior knowledge of the technologies did not affect acceptance of HGE. This may be due to the absence of widespread politicization and religious debate around these technologies at present, resulting in respondents making judgements based on their intrinsic ethical system and not political or religious doctrine. Interestingly, the acceptance of PGT-WGS did not significantly increase in the wake of the pandemic, indicating a specific effect on HGE.
What drove this change in attitudes towards HGE? It is likely that several factors, in addition to the mere passage of time between the two questionnaires, contributed to this change. First, the pandemic itself may have contributed because the proportion of respondents specifically reporting a more positive view of HGE as a result of COVID-19 outweighed those with a more negative view (Fig. 2). Second, the possibility of protecting children from novel pathogens using HGE may have driven attitudes as well (Kim and Lee, 2022; Li et al., 2022), particularly in participants who experienced disruption of their medical care due to the pandemic, such as patients seeking infertility treatment (Schirmer et al., 2021). Third, the successful implementation of a novel mRNA vaccine technology and the rollout of effective COVID-19 vaccines may have resulted in a general increase in trust in medical intervention and technologies specifically in our study population, despite mistrust in other segments of the U.S. population. This is consistent with our observation that the acceptance of genetic ‘enhancement’ using HGE also was significantly increased in 2021. Fourth, pandemic-related behavioral changes, including prolonged time off work, the increased time available to follow the media and medical research and the absence of negative events surrounding HGE during this time may have contributed as well (Salon et al., 2021). However, it is also possible that factors unrelated to the pandemic contributed to the observed change. For example, participants in 2018 may have been more skeptical of institutions in general or the acceptance of technological interventions in embryos may have been unusually low in the 2018 group, potentially due to the political climate in the US at that time. The clinical implementation of polygenic risk scoring of human embryos (PGT-P) (Treff et al., 2019a; Treff et al., 2019b) recently received considerable media attention (Pagnaer et al., 2021) and may have resulted in an increased acceptance of technologies targeting human embryos. In this case, one may expect the acceptance of PGT-WGS, a technology extending conventional PGT of human embryos, to be significantly increased as well. However, we did not observe this in our study.
We observed similarly high acceptance rates for PGT-WGS in the 2021 and 2019 groups of participants. Enthusiasm for PGT-WGS and screening for complex traits in general may have been somewhat dampened by the negative media coverage of the recent implementation of screening for intelligence using PGT-P (Pagnaer et al., 2021). Questions also remain about the quality of single-cell datasets, the relation between variants and clinical phenotypes (Moorthie et al., 2022; Winand et al., 2014) and potential undesired effects of choosing certain genetic variants over others. However, there is a wide array of potential use cases for PGT-WGS and the advancing understanding of the human genome may enhance its clinical utility.
The strengths of this study include the high response rates and a study population comprised of stakeholders who will most likely be the first to encounter these emerging technologies in a clinical setting. In addition, to our knowledge, this is the first study analyzing the acceptance of HGE and PGT-WGS in any population after the onset of the COVID-19 pandemic. Limitations include the restriction of our study population to a group of highly educated, predominantly Caucasian patients seeking fertility treatment in New England, which potentially limits the generalizability of the results to other study populations. The results also could be affected by volunteer bias, though this would be minimal given the response rates of 90% and 91%. In addition, it is possible that the recent decision by the US Supreme Court to reverse Roe v Wade may result in a change in the perception of the status of human embryos among sections of the US population.
Future Directions
While there is some indication that the change in attitudes towards HGE is related to the pandemic itself, the reasons remain to be elucidated and need further investigation. In this context, it also will be important to elicit other potential (unknown) factors that might influence societal and individual attitudes but tend to be overlooked by policy makers. Qualitative interviews with stakeholder groups may provide other candidates for possible contributing factors, which could then be examined in future survey studies. Future studies should also seek to identify the specific diseases that comprise the most acceptable targets for HGE, examine how potential undesired effects such as CRISPR/Cas9 off-site targeting, may affect the acceptability of individual use cases and whether the increased acceptance of HGE indicates a wider shift in public perception of emerging medical technologies in general, at least in parts of the population.
Supplementary Material
Key Message.
A survey conducted among patients attending an infertility center reveals a significantly increased acceptance of heritable genome editing after the COVID-19 pandemic and highlights questions deserving future research.
Acknowledgments
We acknowledge the Boston IVF center for its assistance during the project. WN is supported by a K08 career development grant from NICHD (1K08HD098556-01A1). This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Funding
This study was supported by a K08 grant from NICHD (1K08HD098556-01A1) and a National Institutes of Health UL1 Award from the National Center for Advancing Translational Sciences (UL1 TR002541) through Harvard Catalyst. The authors have no conflict of interest.
Biography

Dr Werner Neuhausser is a specialist in Reproductive Endocrinology and Infertility at Beth Israel Deaconess Medical Center and Harvard Medical School. His main research interests are human embryology and germ cell development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
None reported
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- Adashi EY, and Cohen IG (2022). CRISPR immunity: a case study for justified somatic genetic modification? J Med Ethics 48, 83–85. [DOI] [PubMed] [Google Scholar]
- Armsby AJ, Bombard Y, Garrison NA, Halpern-Felsher BL, and Ormond KE (2019). Attitudes of Members of Genetics Professional Societies Toward Human Gene Editing. CRISPR J 2, 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D, Charo A, Daley G, Doudna J, Kato K, Kim J, Lovell-Badge R, Merchant J, Nath I, Pei D, et al. (2018). Statement by the Organizing Committee of the Second International Summit on Human Genome Editing. 2nd International Summit on Human Genome Editing. [Google Scholar]
- Bu Q (2019). Reassess the Law and Ethics of Heritable Genome Editing Interventions: Lessons for China and the World. Issues Law Med 34, 115–146. [PubMed] [Google Scholar]
- Cary Funk BK, Elizabeth Podrebarac Sciupac (2016). U.S. Public Wary of Biomedical Technologies to ‘Enhance’ Human Abilities. Pew Research Center. [Google Scholar]
- Cohen IG, and Adashi EY (2021). Legal and Ethical Issues in the Report Heritable Human Genome Editing. Hastings Cent Rep 51, 8–12. [DOI] [PubMed] [Google Scholar]
- Cyranoski D, and Ledford H (2018). Genome-edited baby claim provokes international outcry. Nature 563, 607–608. [DOI] [PubMed] [Google Scholar]
- Editorial. (2017). Beware the anti-science label. Nature 545, 133–134. [DOI] [PubMed] [Google Scholar]
- Funk C HM (2018). Public views of gene editing for babies depend on how it would be used. Pew Research Center. [Google Scholar]
- Hendriks S, Giesbertz NAA, Bredenoord AL, and Repping S (2018). Reasons for being in favour of or against genome modification: a survey of the Dutch general public. Hum Reprod Open 2018, hoy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Society for Stem Cell Research (ISSCR) (2015). Statement on Human Germline Genome Modification.
- Kim TH, and Lee SW (2022). Therapeutic Application of Genome Editing Technologies in Viral Diseases. Int J Mol Sci 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Miyoshi T, Kobayashi T, Hayakawa I, Urayama KY, Uchiyama M, Muto K, Takeuchi Y, Taira M, Sago H, et al. (2022). Public attitudes in the clinical application of genome editing on human embryos in Japan: a cross-sectional survey across multiple stakeholders. J Hum Genet. [DOI] [PubMed] [Google Scholar]
- Lander ES, Baylis F, Zhang F, Charpentier E, Berg P, Bourgain C, Friedrich B, Joung JK, Li J, Liu D, et al. (2019). Adopt a moratorium on heritable genome editing. Nature 567, 165–168. [DOI] [PubMed] [Google Scholar]
- Li S, Holguin L, and Burnett JC (2022). CRISPR-Cas9-mediated gene disruption of HIV-1 coreceptors confers broad resistance to infection in human T cells and humanized mice. Mol Ther Methods Clin Dev 24, 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen DB, Warren CM, Xiao AH, Shulman LP, and Jain T (2021). Disparities among infertility patients regarding genetic carrier screening, sex selection, and gene editing. J Assist Reprod Genet 38, 2319–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthie S, Hall A, Babb de Villiers C, Janus J, Brigden T, Blackburn L, and Kroese M (2022). How can we address the uncertainties regarding the potential clinical utility of polygenic score-based tests? Per Med. [DOI] [PubMed] [Google Scholar]
- National Academy of Medicine, N.A.o.S., and the Royal Society. (2020). Heritable Human Genome Editing. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Navin N, Kendall J, Troge J, Andrews P, Rodgers L, Mclndoo J, Cook K, Stepansky A, Levy D, Esposito D, et al. (2011). Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnaer T, Siermann M, Borry P, and Tsuiko O. (2021). Polygenic risk scoring of human embryos: a qualitative study of media coverage. BMC Med Ethics 22, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittinsky TL (2015). America’s crisis of faith in science. Science 348, 511–512. [DOI] [PubMed] [Google Scholar]
- Rutjens BT, Heine SJ, Sutton RM, van Harreveld F (2018). Attitudes towards science. In Olson JM. (Ed.). Advances in experimental social psychology, Academic Press; 57, 125–165. [Google Scholar]
- Rutjens BT, van der Linden S, & van der Lee R (2021). Science skepticism in times of COVID-19. Group Processes & Intergroup Relations, 24(2), 276–283. [Google Scholar]
- Salon D, Conway MW, Capasso da Silva D, Chauhan RS, Derrible S, Mohammadian AK, Khoeini S, Parker N, Mirtich L, Shamshiripour A, et al. (2021). The potential stickiness of pandemic-induced behavior changes in the United States. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufele DA, Xenos MA, Howell EL, Rose KM, Brossard D, and Hardy BW (2017). U.S. attitudes on human genome editing. Science 357, 553–554. [DOI] [PubMed] [Google Scholar]
- Schirmer AD, Kawwass JF, and Adashi EY (2021). Fertility care amidst the COVID19 pandemic: the American experience. J Ovarian Res 14, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treff NR, Eccles J, Lello L, Bechor E, Hsu J, Plunkett K, Zimmerman R, Rana B, Samoilenko A, Hsu S, et al. (2019a). Utility and First Clinical Application of Screening Embryos for Polygenic Disease Risk Reduction. Front Endocrinol (Lausanne) 10, 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treff NR, Zimmerman R, Bechor E, Hsu J, Rana B, Jensen J, Li J, Samoilenko A, Mowrey W, Van Alstine J, et al. (2019b). Validation of concurrent preimplantation genetic testing for polygenic and monogenic disorders, structural rearrangements, and whole and segmental chromosome aneuploidy with a single universal platform. Eur J Med Genet 62, 103647. [DOI] [PubMed] [Google Scholar]
- Turley P, Meyer MN, Wang N, Cesarini D, Hammonds E, Martin AR, Neale BM, Rehm HL, Wilkins-Haug L, Benjamin DJ, et al. (2021). Problems with Using Polygenic Scores to Select Embryos. N Engl J Med 385, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman D (January 2018). U.S. Public Opinion and Interest on Human Enhancements Technology. Washington, DC: AARP Research. [Google Scholar]
- Winand R, Hens K, Dondorp W, de Wert G, Moreau Y, Vermeesch JR, Liebaers I, and Aerts J (2014). In vitro screening of embryos by whole-genome sequencing: now, in the future or never? Hum Reprod 29, 842–851. [DOI] [PubMed] [Google Scholar]
- Xu X, Hou Y, Yin X, Bao L, Tang A, Song L, Li F, Tsang S, Wu K, Wu H, et al. (2012). Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell 148, 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


