Abstract
Background and Aims:
Colorectal cancer (CRC) screening guidelines include screening-colonoscopy and sequential high sensitivity fecal occult blood testing (HSgFOBT), with expectation of similar effectiveness based on the assumption of similar high adherence. However, adherence to screening-colonoscopy compared to sequential HSgFOBT has not been reported. In this randomized clinical trial, we assessed adherence and pathology findings for a single screening-colonoscopy versus sequential and non-sequential HSgFOBT.
Methods:
Participants aged 40-69 were enrolled in three centers, which represented different clinical settings. Participants were randomized into a single screening-colonoscopy arm versus sequential HSgFOBT arm comprised of 4-7 rounds. Initial adherence to screening-colonoscopy and sequential adherence to HSgFOBT, follow-up colonoscopy for positive HSgFOBT tests, crossover to colonoscopy, and detection of advanced neoplasia or large serrated lesions (ADN-SER) were measured.
Results:
3,523 participants were included in the trial with 1761 and 1762 participants randomized to the screening-colonoscopy and HSgFOBT arms, respectively. Adherence was 1473 (83.6%) for the screening-colonoscopy arm versus 1288 (73.1%) for the HSgFOBT arm after one round (RR=1.14, [95% CI 1.10-1.19] P≤0.001), but only 674 (38.3%) over four sequential HSgFOBT rounds (RR=2.19, [95% CI 2.05-2.33]). Overall adherence to any screening increased to 1558 (88.5%) in the screening-colonoscopy arm during the entire study period and 1493 in the HSgFOBT arm (84.7%) (RR=1.04, [95% CI 1.02-1.07]). 436 (24.7%) participants crossed over to screening-colonoscopy over the first four rounds. ADN-SER were detected in 121 (8.2%) of the 1473 participants in the colonoscopy arm who were adherent to protocol in the first 12 months of the study, whereas the detection of ADN-SER among those who were not sequentially adherent (N=709) to HSgFOBT was subpar (0.6%) (RR=14.72, [95% CI 5.46-39.67]) when compared to those who were sequentially adherent (3.3%) (N=647) (RR=2.52, [95% CI 1.61-3.98]) to HSgFOBT in the first four rounds. When including colonoscopies from HSgFOBT patients who were never positive yet crossed over (N=1483), 5.5% of ADN-SER were detected (RR=1.50, [95% CI 1.15-1.96]) in the first four rounds.
Conclusions:
Observed adherence to sequential rounds of HSgFOBT was suboptimal when compared to a single screening-colonoscopy. The detection of ADN-SER was inferior when non-sequential HSgFOBT adherence was compared to sequential adherence. However, the greatest number of ADN-SER was detected among those who crossed over to colonoscopy and opted to receive a colonoscopy. The effectiveness of a HSgFOBT screening program may be enhanced if crossover to screening-colonoscopy is permitted.
Keywords: crossover colonoscopy, screening guidelines, colorectal cancer screening, colonoscopy, fecal occult blood test
Lay summary:
Fecal-based CRC screening is effective when there is adherence to the program of sequential (annual) testing. In circumstances where the uptake of fecal-based tests is suboptimal, colonoscopy should be considered in order to obtain maximum benefit.
Introduction:
Colorectal cancer (CRC) is the second most common cause of cancer death in the United States (US), with approximately 151,030 new cases and 52,580 deaths estimated in the year 2022.1 Screening has a major impact on reducing the burden of CRC,2–6 and guidelines have been developed which recommend various methods, with the assumption of high adherence for all methods.7–9
Recommended CRC screening options in the US include every ten-year screening-colonoscopy and annual stool tests including the high-sensitivity guaiac-based fecal occult blood test (HSgFOBT), fecal immunochemical test (FIT) and multitarget stool-DNA tests. Screening-colonoscopy recommendations have been based on observational studies that demonstrate the ability to find and remove adenomas during colonoscopy, resulting in lower incidence and mortality of CRC compared to the general population.7, 9–16 Screening-colonoscopy is the primary screening test in some countries, particularly in the US.17 However it is invasive, requires considerable medical resources and cost, although is cost effective.18 The effectiveness of less sensitive annual or biennial guaiac-based fecal occult blood testing (gFOBT) in reducing CRC mortality compared to no screening has been demonstrated in randomized clinical trials (RCTs).19–21
It remains unclear whether patients are willing to adhere to annual HSgFOBT and how adherence impacts on the relative effectiveness of HSgFOBT screening versus screening-colonoscopy. Previous modeling studies have suggested that annual CRC screening with HSgFOBT or FIT provide life years gained (LYG) comparable to screening-colonoscopy, provided that adherence to a program of sequential fecal testing is high.11, 22 However, LYG will be reduced if adherence remains low.8, 11 Several studies have reported the adherence of patients offered multiple rounds of stool testing, including RCTs for gFOBT23–25 and organized programs of gFOBT and FIT.26, 27, 28 No RCT has directly compared the sequential adherent behavior of patients offered HSgFOBT versus screening-colonoscopy. In the future, such data will be available from the ongoing CONFIRM29 and COLONPREV30 trials. A preliminary report from the SCREESCO trial of a once-offered colonoscopy versus two rounds of offered FIT yielded higher participation rates in the FIT offered group, however longer-term studies within this consortium are needed to elucidate CRC incidence and mortality over time.31 A recent report from the NordICC trial did not compare fecal-based test to colonoscopy, however adherence to colonoscopy was suboptimal.32
The aim of this RCT was to track adherence and pathology findings over time in participants offered a single screening-colonoscopy versus sequential rounds of HSgFOBT. We also assessed the crossover rate from HSgFOBT to screening-colonoscopy and determined the effect of overall adherence and pathology findings.
Material and Methods:
Background on the National Colonoscopy Study
The National Colonoscopy Study (NCS) was an RCT comparing a single screening-colonoscopy to sequential annual rounds of HSgFOBT at three clinical centers in several areas of the country that have different healthcare delivery systems: The University of Minnesota partnership with MNGI Digestive Health (UMN, Minneapolis, MN), Kaiser-Permanente Washington, (KPWA, formerly Group Health Cooperative, Seattle, WA), and Louisiana State University Health Sciences Shreveport (LSUHS, Shreveport, LA). The coordinating center was at Memorial Sloan Kettering Cancer Center (MSK) (New York, NY) (Appendix Figure 1).
Study Setting
At UMN, participants were recruited from mailing lists of age-eligible people who volunteered to participate in cancer screening studies. UMN researchers (TRC) partnered with a large gastroenterology community practice serving the metropolitan areas of Minneapolis and St. Paul. This center represents fee-for-service and managed care settings with opportunistic screening. KPWA is an integrated healthcare delivery system in Washington State. Eligible subjects were recruited from the member database from a Seattle site of KPWA. LSUHS serves diverse communities in northern Louisiana. More than 50% of residents in northern Louisiana are Black and reside in a mixed urban and rural environment. These participants were recruited primarily from a wellness clinic for minority and underserved populations.
Study Design: Criteria for Enrollment
Participants enrolled were asymptomatic men and women aged 50-69, except for LSUHS, where enrollment ages were 40-69 due to the larger Black population and their reported increased risk of CRC.33, 34 Participants were interviewed by RCT coordinators and were excluded if they had a personal history of CRC, familial adenomatous polyposis, Lynch syndrome, or inflammatory bowel disease. Participants were also excluded if they reported a previous colonoscopy screening, or a flexible sigmoidoscopy within the last 5 years with findings, had serious medical comorbidities (e.g., myocardial infarction within the past year, congestive heart failure, an implanted defibrillator, chronic obstructive pulmonary disease, on anticoagulation) or were on current treatment for cancer other than non-melanoma skin cancer (Figure 1). Participants provided written informed consent prior to randomization. Accrual was from October 2004 to June 2008 with follow-up from 4 to 7 years with a newsletter in 2012 to thank participants in the study and state the close of the study.
Figure 1:
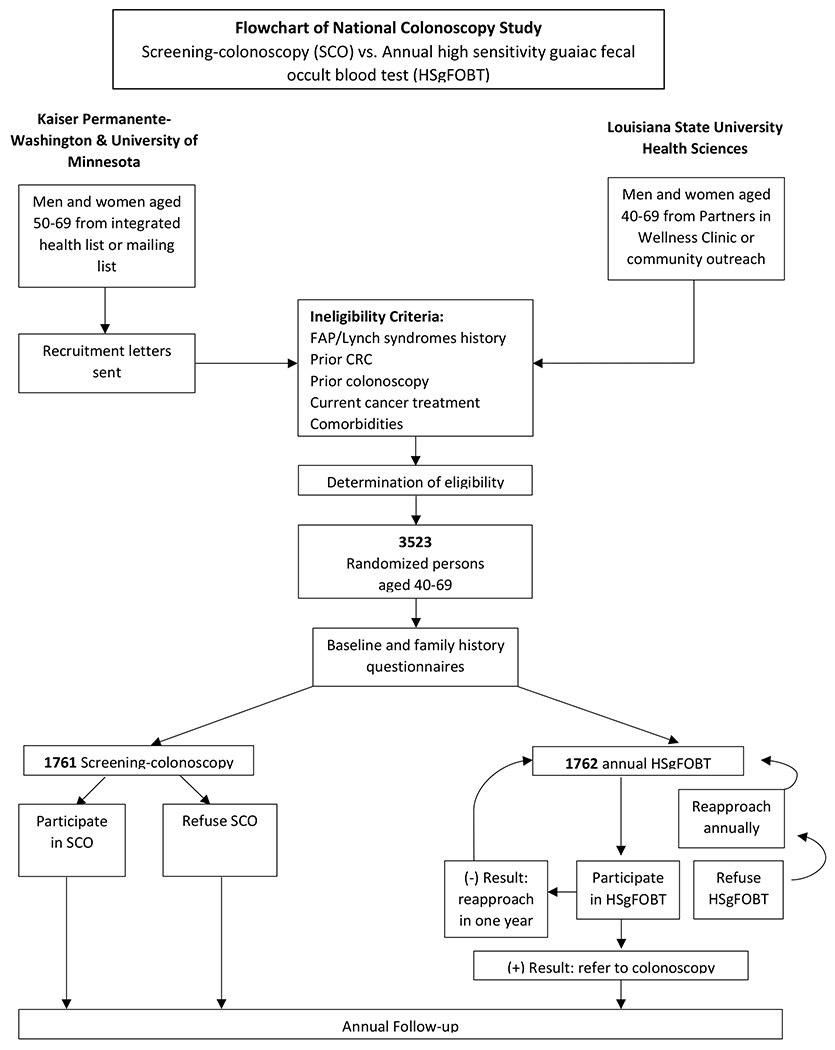
National Colonoscopy Study flowchart of participant eligibility
Screening colonoscopy (SCO), High sensitivity guaiac fecal occult blood test (HSgFOBT), familial adenomatous polyposis (FAP)
Study Design: Methodology
Eligible participants from UMN and KPWA received an introductory letter detailing information on CRC, study details, participant eligibility, and voluntary informed consent, while participants from LSUHS had face-to-face communication with staff at their wellness center. The informed consent provided equivalent detail on the procedure, benefits and harms of screening-colonoscopy and HSgFOBT. Participants understood they would be randomly assigned by a computer to their respective screening arm and would not have a choice in the assigned screening method. CRC screening, including insurance copays, was free of cost to all participants.
Willing participants were randomized into a one-time screening-colonoscopy at baseline or annual HSgFOBT. Participants who were randomized in the HSgFOBT arm required samples from three separate bowel movements each year. The participants in the HSgFOBT arm were offered 4 or more rounds of HSgFOBT. Those recruited early in the 3-year enrollment period were offered up to 5, 6, or 7 rounds. Randomization was 1:1 and used a permuted block method. Participants assigned to the screening-colonoscopy arm were contacted by the clinical center for scheduling. Those in the annual HSgFOBT arm were mailed HSgFOBT (Hemoccult SENSA®) kits with instructions.
Clinical research coordinators provided support to all study participants. Coordinators facilitated participants in scheduling their screening colonoscopy or their follow-up colonoscopy for those with a positive HSgFOBT, mailed the annual HSgFOBT kits, provided instructions, and were available to answer questions over the course of the study. Throughout the study, coordinators remained unbiased to participants’ preference to crossover to another screening arm and aided solely in the administrative support of each screening arm. The structure of the study design provided facilitation for adherence in both study arms. For the colonoscopy arm, scheduled participants were sent a colonoscopy appointment confirmation packet with detailed information, including maps, driving directions, a patient information brochure and bowel preparation instructions. Appointment reminder calls were timed to occur right before the required diet restrictions were to begin. If participants were concerned about travel costs, reimbursement was offered to overcome that barrier. The cost of bowel preparation prescription co-pays was also reimbursed to patients. For the HSgFOBT arm, Hemoccult II SENSA kits were sent on the anniversary of the participants’ randomization date. Coordinators were available to answer any questions regarding questions regarding completing test and to answer any misconceptions about HSgFOBT . For kits which were unreturned, a reminder letter was sent 4 weeks after the initial kit was sent, a second kit was sent 4 weeks afterwards, and a personalized phone call to the participant was made 10-12 weeks after the initial kit was mailed. Up to two replacement kits (three kits total) were provided to the participant. Subjects who had a negative HSgFOBT were notified within two weeks by mail. Participants who had a positive HSgFOBT were notified within two days by telephone and were scheduled a diagnostic colonoscopy at the same clinic.
All participants were asked to complete baseline and family history questionnaires (Table 1). Annual follow-up questionnaires pertaining to vital status and interim CRC screening were administered to ascertain any interval CRC findings. Medical records were requested from those reporting polyps or CRC. Participants in the screening-colonoscopy arm were contacted 30-days after their colonoscopy to determine if any adverse events had occurred. Blood samples were also requested among those who underwent colonoscopy. Whole blood, serum, and plasma were isolated, and aliquots were cryopreserved at −80°C at the MSK Molecular Epidemiology Laboratory (Orlow Lab) (Appendix Table 1). Figure 1 and Appendix Figure 2 depicts the flowchart of the study. Follow-up ended on the earliest of several possible events: CRC diagnosis, death, withdrawal, lost to follow-up, or study close date.
Table 1.
Lifestyle and Family History Characteristics of Study Cohort by Randomized Screening Assignment*
| Baseline variables | Overall N (%) | Participants randomized to screening-colonoscopy | Participants randomized to annual HSgFOBT |
|---|---|---|---|
|
| |||
| Registration | N=3523 | N=1761 | N=1762 |
| Average age (SD) | 55.4 (5.54) | 55.45 (5.50) | 55.39 (5.58) |
| Sex | |||
| Female | 1802 (51.2%) | 916 (52.0%) | 886 (50.3%) |
| Male | 1721 (48.9%) | 845 (48.0%) | 876 (49.7%) |
| Race | |||
| White | 2631 (74.7%) | 1355 (76.9%) | 1276 (72.4%) |
| Black | 565 (16.0%) | 276 (15.7%) | 286 (16.4%) |
| Other | 327 (9.3%) | 130 (7.4%) | 197 (11.2%) |
| Center location | |||
| Minnesota (UMN) | 2048 (58.1%) | 1024 (58.1%) | 1024 (58.1%) |
| Kaiser Permanente – Washington (KPWA) | 468 (13.3%) | 233 (13.2%) | 235 (13.3%) |
| Louisiana (LSUHS) | 1007 (28.6%) | 504 (28.6%) | 503 (28.6%) |
| Baseline Questionnaire† | N=3123 | N=1609 | N=1515 |
| Education | |||
| Graduate school graduate (master’s degree, doctorate) | 540 (17.3%) | 269 (16.8%) | 271 (18.0%) |
| College graduate | 792 (25.4%) | 433 (28.0%) | 359 (23.8%) |
| Associates degree or some college completed | 1234 (39.6%) | 625 (38.9%) | 609 (40.3%) |
| High school degree | 444 (14.6%) | 224 (14.0%) | 220 (14.6%) |
| Less than high school education | 105 (3.4%) | 54 (3.4%) | 51 (3.4%) |
| Body Mass Index | |||
| <18.5 (underweight) | 13 (0.4%) | 7 (0.4%) | 6 (0.4%) |
| 18.5-24.9 (normal) | 868 (28.0%) | 437 (27.4%) | 431 (27.8%) |
| 25-29.9 (overweight) | 1231 (39.7%) | 642 (40.2%) | 589 (39.2%) |
| ≥30 (obese) | 989 (31.9%) | 511 (32.0%) | 478 (31.8%) |
| Number of FDRs with CRC | |||
| 0 FDRs | 2816 (90.5%) | 1445 (89.9%) | 1371 (91.04%) |
| 1 FDR | 276 (8.9%) | 149 (9.2%) | 127 (8.4%) |
| 2 or more FDRs | 21 (0.7%) | 13 (0.9%) | 8 (0.5%) |
| Regular multivitamin use ‡ | |||
| Yes | 1680 (53.8%) | 884 (55.0%) | 796 (52.6%) |
| No | 1433 (46.0%) | 720 (44.8%) | 713 (47.1%) |
| Hormone replacement therapy use (females only) § | |||
| Yes | 590 (37.4%) | 303 (36.8%) | 287 (38.1%) |
| No | 979 (62.1%) | 517 (62.8%) | 462 (61.4%) |
| Aspirin use ‖ | |||
| Yes | 1060 (34.0%) | 551 (34.2%) | 509 (33.6%) |
| No | 2047 (65.5%) | 1051 (65.3%) | 996 (65.7%) |
| Smoking (100 cigarettes ever?) | |||
| Yes | 1560 (50.1%) | 804 (51.5%) | 756 (48.5%) |
| No | 1556 (49.9%) | 801 (51.5%) | 755 (48.5%) |
High sensitivity fecal occult blood test (HSgFOBT), First-degree relative (FDR) defined as parent or sibling with diagnosed with colorectal cancer, Colorectal cancer (CRC)
Asked among those willing to participate prior to obtaining informed consent. KPWA used electronic records, UMN was self-reported, and LSUHS face to face
88.6% of participants returned baseline questionnaire form
Do you currently take a multivitamin at least once a week on a regular basis?
Have you ever taken hormone replacement therapy for menopause for at least 6 months?
Have you been taking aspirin at least once a week for a year or more?
Participants mailed their completed HSgFOBT test kits to the MSK FOBT Central Laboratory for testing under supervision of MF. Two experienced laboratory technicians assessed positivity, defined as ≥1 of the HSgFOBT windows as positive. A study endoscopy credentialing committee approved endoscopy investigators for the study based on being well-trained and regularly performing high volume colonoscopies (>400 a year). Within each center, there was a primary endoscopist (JIA, ADF, PAJ) who performed the majority of the endoscopic procedures for the trial. Pathology was centrally reviewed by a gastrointestinal pathologist (MJO)35 who was blinded to the exam indication and study arm. Participants with advanced neoplasia were defined as those with tubulovillous or villous adenomas, high-grade dysplasia, an adenoma ≥1 cm in size, 3 or more tubular adenomas ≤ 1 cm, or CRC. Patients with non-advanced adenomas were those with only one or two tubular adenomas <1 cm. Serrated lesions were classified as hyperplastic polyp (goblet cell or microvesicular type), sessile serrated lesion, sessile serrated lesion with cytological dysplasia, and traditional serrated adenoma.36, 37 We used the classification from Rex36 and Gupta38 to designate serrated lesions that were large (≥1cm), multiple (≥ 3), or exhibiting dysplasia to permit a combined advanced neoplasia category that combined adenoma and serrated lesions (ADN-SER) and/or CRC as our main pathology outcome. Additional details on our classification are in Appendix Table 2.
Study Outcomes
Study outcomes for adherence included the baseline screening-colonoscopy as well as initial and sequential HSgFOBTs in the first 4 rounds. Within the screening-colonoscopy arm, we defined “per protocol” adherence as those who completed their initial screening-colonoscopy within 12 months of their randomization date. Each colonoscopy visit was defined as “an episode of care” which included the initial patient encounter and any subsequent visits needed to complete the colonoscopy. Participants who completed their screening-colonoscopy after 12 months of randomization were considered to be “any screening adherent.” The “per protocol” for HSgFOBT sequential adherence was defined either by completing four sequential HSgFOBT negative kits or having a positive HSgFOBT with the proper number of preceding negative HSgFOBT and diagnostic colonoscopy after the positive kit. Participants in the HSgFOBT arm who had either no prior HSgFOBT or prior negative HSgFOBT and crossed over to screening colonoscopy were considered non-adherent to protocol. Participants who were HSgFOBT-positive but did not complete a diagnostic-colonoscopy were also considered to be non-adherent. These patients were considered to be “any screening adherent” since they had at least some screening exposure throughout the study. Adherence by round and sequential adherence to HSgFOBT were also assessed. Those who were non-sequentially adherent to HSgFOBT, defined as those who were not sequentially adherent to the first four rounds for HSgFOBT but completed at least 1 but no more than 3 rounds, were examined as well. In further sensitivity analysis, we assessed adherence to multiple rounds of testing given adherence to the first round, as has been analyzed previously.24–26 Participant-level pathology findings, including ADN-SER, were from baseline (within 12 months of randomization date) and follow-up colonoscopy (>12 months post-randomization date) examinations in the colonoscopy arm. We also assessed the pathology outcomes from colonoscopies for follow-up of positive HSgFOBT from those who were sequentially and non-sequentially adherent, and from participant-initiated crossover to screening-colonoscopy in the HSgFOBT arm.
Statistical Analysis
We estimated the relative risk (RR) of adherence to screening-colonoscopy compared to sequential HSgFOBT to assess the risk of “per protocol adherence” and for “any screening adherence” analyses. We also estimated the RR of advanced neoplasia findings in the screening-colonoscopy arm compared to HSgFOBT arm. The study was designed to have 80% power at the two-sided alpha of 0.05 to detect a RR of 1.6 or greater for pathology findings of ADN-SER neoplasia in the screening-colonoscopy arm relative to 5% in the HSgFOBT arm with 1775 in each arm. Tests for between-arm differences, as well as between-site differences in terms of adherence, were based on Pearson chi-square tests.
Within the HSgFOBT arm, we used generalized estimating equations (GEE) to estimate the odds ratio (OR) of sequential adherence up to 7 rounds compared to baseline adherence, with adjustment for the 3 clinical centers, study entry year, sex, and age at randomization in the HSgFOBT arm. Analyses were conducted using SAS Software Version 9.4 (The SAS Institute, Cary, NC) and R version 4.2.2 with the tidyverse39 (v1.3.1) package.40
Study Oversight
The study was approved by the Institutional Review Boards at MSK, UMN, KPWA, and LSUHS. The MSK Data and Safety Monitoring Board reviewed the study annually during the accrual years. The authors had sole responsibility for study design, data collection, decision to submit the manuscript for publication, and drafting the manuscript. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS:
Accrual and Participant Characteristics
A total of 3523 participants were accrued over a three-year period with follow-up from 4 to 7 years (Appendix Table 3A). Among these participants, 1761 and 1762 were randomized to the screening-colonoscopy and annual HSgFOBT arm, respectively. The arms were balanced in terms of age, sex, smoking status, race, body mass index, and other characteristics (Table 1).
Adherence to Screening-Colonoscopy
Of 1761 participants randomized to screening-colonoscopy, 1473 (83.6%) completed the baseline examination. Adherence varied across the three clinical centers and was highest for UMN, with 89.4%, then KPWA with 77.7%, and lowest for LSUHS with 74.8% (P≤0.001) (Table 2a).
Table 2a.
Adherence for Assigned Screening-Colonoscopy and HSgFOBT Arm Subjects and by Round of Screening
| Center | Screening-Colonoscopy Adherence (adherent/total) | HSgFOBT Adherence by Annual Year and Round | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Annual Round 1 | Annual Round 2 | Annual Round 3 | Annual Round 4 | Annual Round 5 | Annual Round 6 | Annual Round 7 | Odds Ratio* (95% CI) | ||
| UMN | 915/1024 (89.4%) | 798/1024 (77.9%) | 600/840 (71.4%) | 490/682 (71.8%) | 416/595 (69.9%) | 283/423 (66.9%) | 140/217 (64.5%) | 57/90 (63.3%) | 0.89 (0.85, 0.92) |
| KPWA | 181/233 (77.7%) | 180/235 (76.6%) | 151/214 (70.6%) | 146/200 (73.0%) | 136/180 (75.6%) | 52/83 (62.7%) | 34/52 (65.4%) | 8/15 (53.3%) | 0.93 (0.86,1.01) |
| LSUHS *** | 377/504 (74.8%) | 310/503 (61.6%) | 229/438 (52.3%) | 196/410 (47.8%) | 154/381 (40.4%) | 117/295 (39.7%) | 54/116 (46.6%) | 11/27 (40.7%) | 0.80 (0.76, 0.85) |
| Total | 1473/1761 (83.6%) ** | 1288/1762 (73.1%) ** | 980/1492 (65.7%) | 832/1292 (64.4%) | 706/1156 (61.1%) | 452/801 (56.4%) | 228/385 (59.2%) | 76/132 (57.6%) | 0.86 (0.83, 0.88) |
High sensitivity fecal occult blood test (HSgFOBT), 95% Confidence Intervals (95% CI)
Odds ratio defined over the 7-year study period from general estimating equations analysis adjusted for study entry year, age, sex, and center
The relative risk (RR) of screening-colonoscopy vs. HSgFOBT adherence in the first round was 1.18 (95% CI 1.14 −1.22)
Adherence for screening-colonoscopy at LSUHS was 83% for whites and 78% for Blacks (RR = 0.94, 95% CI: 0.86 – 1.02)34
Overall Adherence to HSgFOBT
Of the 1762 participants randomized to annual HSgFOBT, 1288 (73.1%) returned the baseline HSgFOBTs, and of the 7020 kits offered over the seven-year study period 4562 (64.9%) were completed. Within the first four rounds of the study, 674 participants were sequentially adherent (per-protocol) to HSgFOBT, whereas 709 participants were non-sequentially adherent to HSgFOBT. When considering those who were both sequentially and non-sequentially adherent to HSgFOBT, a total of 1383 participants had at least some screening exposure to HSgFOBT in these first 4 rounds. Among those who completed the first round of HSgFOBT, adherence ranged from 83.2% in the second round to 71.7% in the seventh round, suggesting that those who completed their first round of testing may have been more likely to complete subsequent tests (Appendix Table 4). Overall HSgFOBT annual adherence declined over the seven sequential rounds (OR= 0.86, [95% CI: 0.83, 0.88],) (Table 2a). Among the total completion rates for the HSgFOBT arm, 1389 (78.8%) completed at least one test, 1318 (74.8%) completed 25% of offered tests, 1217 (69.1%) completed 50% of offered tests, and 1066 (60.5%) completed greater than 75% of offered tests. Baseline adherence varied across the three centers (Table 2a).
Crossover to Colonoscopy From the HSgFOBT Arm
There were 530 (30.1%) HSgFOBT participants who elected to have a screening-colonoscopy outside of the study without having a positive HSgFOBT over the maximum 7 rounds. These “crossovers” included 426 individuals (24.2%) with prior negative HSgFOBT and 104 (5.9%) with no HSgFOBT. Crossover during the first four rounds was highest at UMN with 408 participants (39.8%), then KPWA with 62 (26.4%), and lowest for LSUHS with 28 (5.6%) (P≤0.001).
Comparison of Cumulative Adherence With Screening-Colonoscopy Versus Sequential Adherence to HSgFOBT In First 4 Rounds
The “per protocol” adherence to a program of four repeated HSgFOBT rounds in the 1762 individuals randomized to annual HSgFOBT was 674 (38.3%). Those considered adherent included 577 (32.7%) participants with 4 out of 4 negative HSgFOBTs and 98 (5.6%) with positive HSgFOBT by round 4. The screening-colonoscopy adherence of 83.6% was significantly higher than the 38.3% 4-round adherence for HSgFOBT (RR=2.19, [95% CI 2.05-2.33], P≤0.001) (Figure 2).
Figure 2:
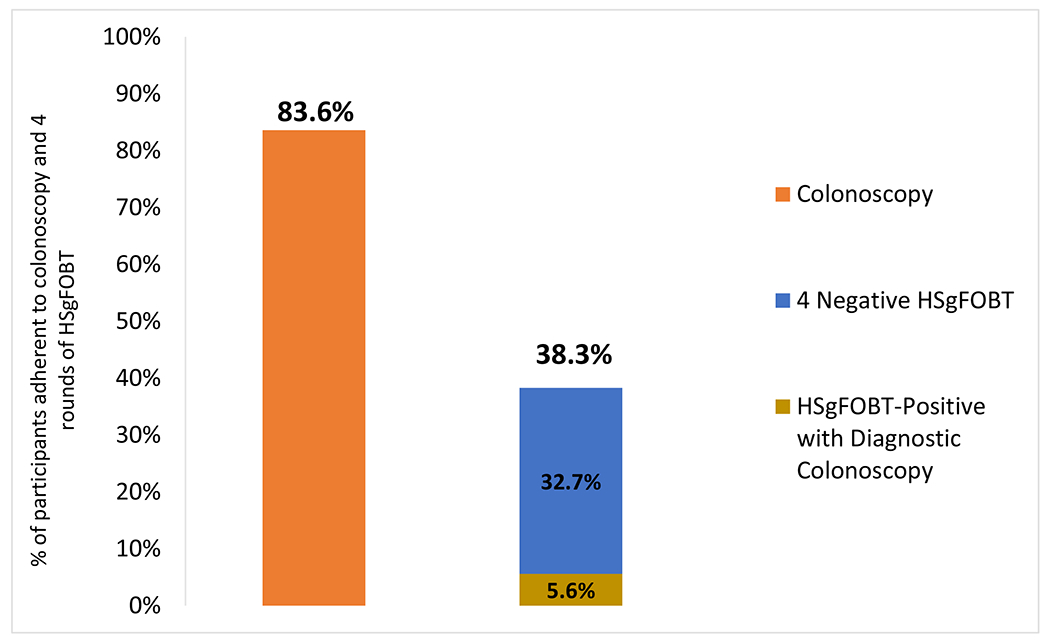
Adherence to screening-colonoscopy versus four rounds of screening for HSgFOBT (per protocol)
High sensitivity fecal occult blood test (HSgFOBT); Relative Risk (RR); 95% Confidence Intervals (95% CI)
*1473 participants completed per protocol initial screening-colonoscopies defined within <12 months after randomization
**92.2% of HSgFOBT positive patients completed their follow-up colonoscopy in the first four rounds
However, any screening adherence, defined as completion of at least one round of HSgFOBT or crossover to screening-colonoscopy in the HSgFOBT arm, or completion of any colonoscopy in the screening-colonoscopy arm, increased to 1483 (84.2%) and 1553 (88.2%) respectively (RR=1.05, [95% CI 1.02-1.08] P≤0.001) (Figure 3). Adherence rates did not differ by sex and age group within each study center (Appendix Figure 4).
Figure 3:
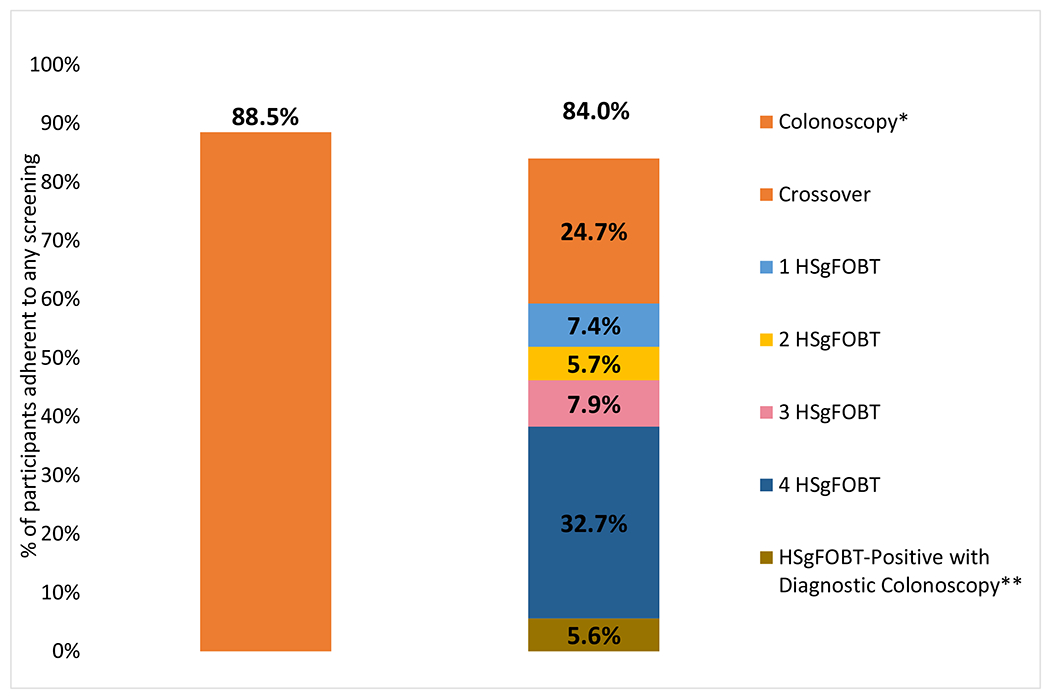
Adherence to screening-colonoscopy versus adherence to any screening over four screening rounds for HSgFOBT
High sensitivity fecal occult blood test (HSgFOBT)
Relative Risk (RR); 95% Confidence Intervals (95% CI)
*1473 participants completed initial screening-colonoscopies within <12 months after randomization and another 85 completed initial screening-colonoscopies > 12 months after randomization (N=1558)
** 92.2% of HSgFOBT positive patients completed their follow-up colonoscopy in the first four rounds
Diagnostic Colonoscopy Following Positive HSgFOBT
Among the 1288 participants completing the HSgFOBT at baseline (round 1), 51 (4.0%) had a positive HSgFOBT with slightly lower or stable rates for subsequent rounds (Table 2b). Over the course of the study (maximum of 7 rounds), 139 (7.9%) had a positive HSgFOBTs and were referred for diagnostic colonoscopy, with 127 (91.4%) completing diagnostic colonoscopy (Table 2b).
Table 2b.
HSgFOBT Positivity by 7 Rounds
| Positivity | Total * | Round 1 | Round 2 | Round 3 | Round 4 | Round 5 | Round 6 | Round 7 |
|---|---|---|---|---|---|---|---|---|
| Positivity by Round | 139 | 51/1288 (4.0%) | 28/980 (2.9%) | 23/832 (2.8%) | 14/706 (2.0%) | 11/452 (2.4%) | 9/228 (3.9%) | 3/76 (3.9%) |
High sensitivity fecal occult blood test (HSgFOBT)
127 participants (91.4%) completed follow-up colonoscopy directed from positive HSgFOBT
Pathology Findings in Screening-Colonoscopy and HSgFOBT Arms
In the “any screening adherence” analysis throughout the entire study, 399 (22.7%) of the 1761 participants in the screening-colonoscopy arm, and 215 (12.2%) of the 1762 within the HSgFOBT arm were found to have any adenomas or serrated lesions (RR: 1.86, [95% CI 1.59-2.16], P<0.001). ADN-SER (advanced neoplasia which also included serrated lesions) were detected in 135 (7.7%) of the 1761 participants in the screening-colonoscopy arm and 97 (5.5%) of the 1762 participants in the HSgFOBT arm (RR:1.39, [95% CI 1.08-1.79], P-value=0.01) (Figure 4). Pathology results by screening arm without the inclusion of serrated lesions were found to be slightly lower but comparable (Appendix Figure 3). Those in the HSgFOBT arm who crossed over to colonoscopy contributed 56 (68.0%) of the 82 advanced findings in HSgFOBT arm over the first four rounds/years.
Figure 4:
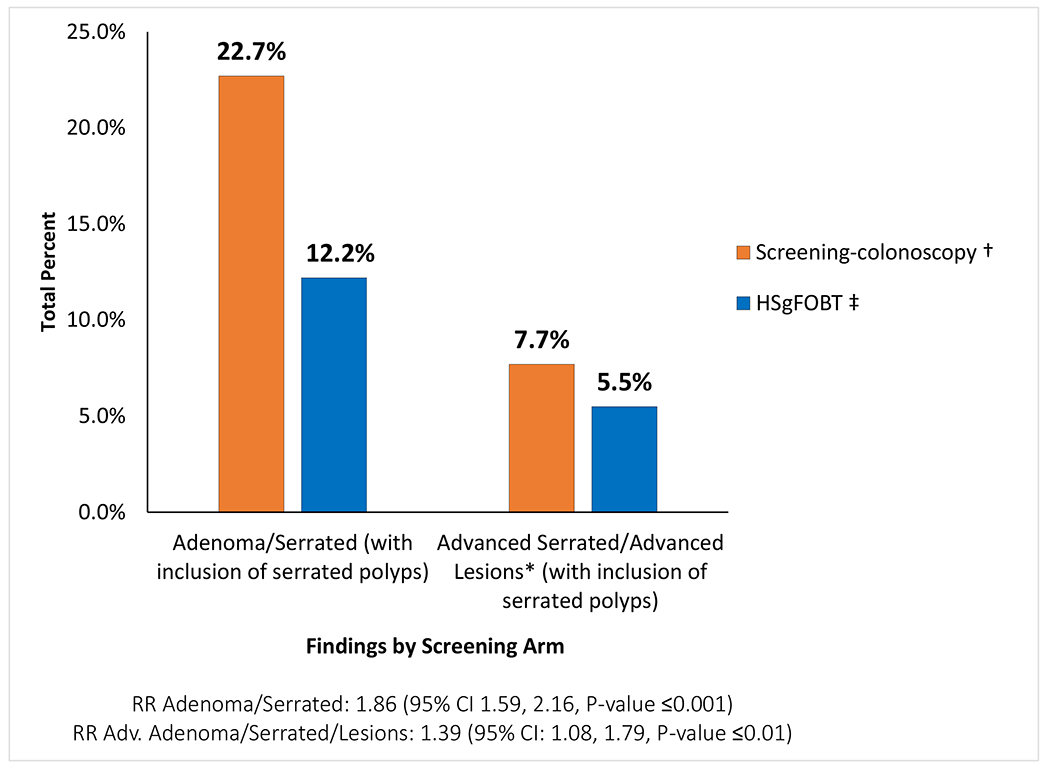
Pathology findings by screening arm with adenoma or serrated lesions over 4-7 years followed
High sensitivity fecal occult blood test (HSgFOBT); Relative Risk (RR); 95% Confidence Intervals (95% CI)
* Serrated polyp definition was based on Rex36 and Gupta38 definition on features of serrated lesions which are associated with advanced neoplasia. These features include proximal colon location of serrated lesions, increasing number and larger size of serrated lesions or traditional serrated adenomas histology (Appendix Table 2).
† Positive screening-colonoscopy pathology findings based on total 1761 participants randomized; 1558 participants with colonoscopies, of which 85 had initial colonoscopy after 12 months.
‡ HSgFOBT findings from follow-up colonoscopy after a positive HSgFOBT and from cross-over colonoscopy without a positive HSgFOBT based on 1762 participants randomized.
Among the 1473 participants who were per-protocol adherent in the screening-colonoscopy arm, 121 (8.2%) were found to have ADN-SER. Among the 674 participants who were sequentially adherent to the first four rounds of HSgFOBT, 22 (3.3%) had ADN-SER, which was significantly less than those adherent to colonoscopy (RR=2.52, [95% CI 1.61-3.93]) (Figure 5A). Out of the 709 participants who were non-sequentially adherent to the first four rounds, only 4 (0.6%) were found to have ADN-SER (RR=14.72, [95% CI 5.46-39.67]) (Figure 5B). Among the 1383 who had any screening exposure to HSgFOBT, 26 (1.9%) had ADN-SER. (RR=4.42 [95% CI 2.92-6.69]) (Figure 5C). When including colonoscopies from HSgFOBT patients who were never positive (i.e. crossed over), 5.5% of ADN-SER were detected (RR=1.50, [95% CI 1.15-1.96]) in the first four rounds, resulting in the greatest number of advanced lesions detected (Figure 5D). Among those with advanced findings, there were 16 participants with CRC: 6 in the screening-colonoscopy arm and 10 in the HSgFOBT arm. Additional information on the CRCs is in Appendix Table 5. Among those with a family history of CRC, 2 participants were found to have CRC and 21 were found to have advanced neoplasia (Appendix Table 6).
Figure 5:
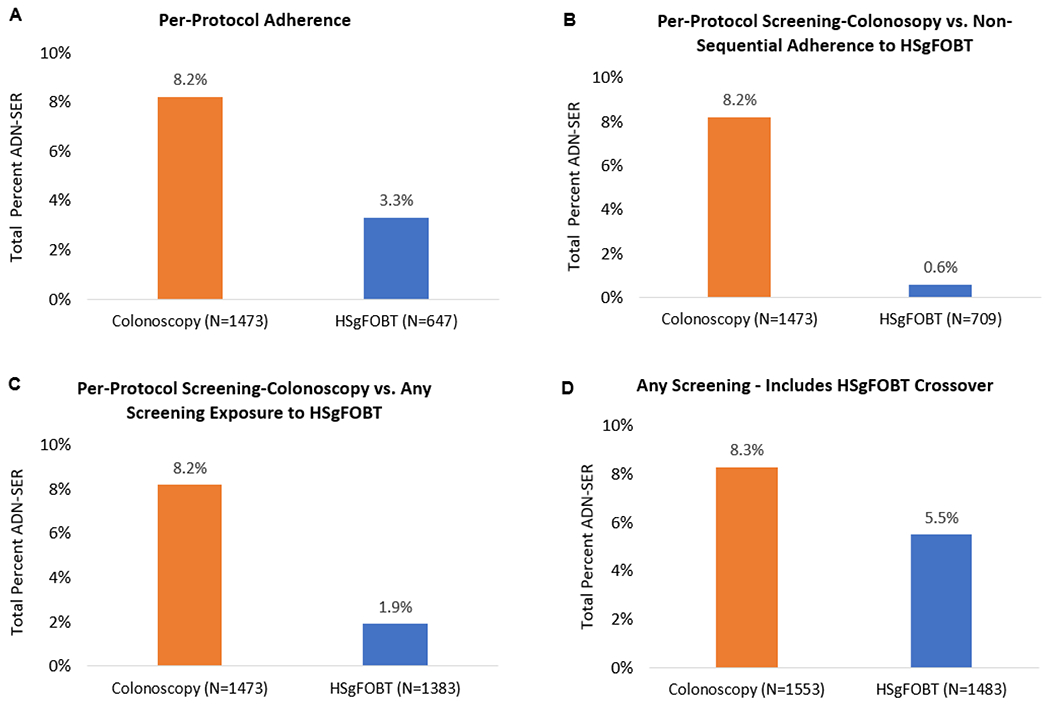
Colonoscopy and HSgFOBT pathology findings from adherence over the first 4 rounds
A) Per-protocol findings for screening-colonoscopy and HSgFOBT, defined as completion of screening-colonoscopy 12 months after randomization or [1] four sequential rounds of negative HSgFOBTs, or [2] participants who had a positive HSgFOBT with proper number of preceding negative and diagnostic colonoscopy after positive HSgFOBT
B) Per-protocol findings for screening-colonoscopy and individuals who completed at least 1 but no more than 3 rounds of HSgFOBTs
C) Per-protocol findings for screening-colonoscopy and individuals who completed at least 1 but no more than 3 rounds of HSgFOBTs combined with those who sequentially completed all four rounds of HSgFOBT
D) Includes all screening in the first four rounds defined as all screening-colonoscopies as well as all HSgFOBT including crossovers
Colonoscopy Quality and Adverse Events:
Within the screening-colonoscopy arm, 97.6% of colonoscopies reached the cecum and 98.0% were cleared of polyps and with adequate prep. Among patients who had HSgFOBT-positive diagnostic colonoscopies, 95.6% reached the cecum and 97.1% were cleared of polyps and with adequate prep. Minimal adverse events were reported throughout the course of the study, with only one patient reporting cramping after a colonoscopy with no acute distress.
Discussion:
Our RCT demonstrated that participant adherence to a single screening-colonoscopy (83.6%) is relatively comparable to a one-time HSgFOBT (73.1%) but considerably higher than adherence to sequential screening with HSgFOBT over 4 rounds (38.3%). The observed adherence in our RCT is lower than the assumed high adherence to sequential HSgFOBT in decision analyses41 used to inform screening guidelines.10, 11, 14, 22, 42 Thus, actual effectiveness of a HSgFOBT-based screening program will be less than estimated in the context of perfect screening to inform guidelines.14 Although the “per-protocol” adherence to sequential HSgFOBT was only 38.3%, another 21.2% of participants in the HSgFOBT arm completed partial rounds without crossing over to screening-colonoscopy, and 24.7% crossed over. Therefore, 84.2% of participants in the HSgFOBT arm had some screening by virtue of the crossover to colonoscopy or had completed partial HSgFOBT in the first four rounds. In all three clinical settings, adherence to screening-colonoscopy was higher than adherence to the first round of HSgFOBT and cumulative adherence to sequential HSgFOBT.
In the 2021 recommendations of the USPSTF, 9, 10 CRC screening is based on 100% adherence for the recommended strategies to inform population guidelines. However, the USPSTF also notes that research is clearly needed on differing levels of adherence with respect to various screening strategies. The goal of our trial was to assess the equivalence of adherence between two different CRC screening tests among those willing to be screened and the relative pathology detection of advanced neoplasia. Furthermore, our study was designed for best clinical practice since we provided facilitated access and free cost of screening for both arms for the study duration, centralized processing of HSgFOBT and pathology review, and experienced endoscopists.
The 83.6% adherence to screening-colonoscopy in our RCT with facilitated access is consistent with the 88.9% adherence to screening-colonoscopy observed in a prior screening trial at the same clinical centers (Appendix Table 3).43 Our study was also conducted in the US where there is more familiarity with colonoscopy than in other countries.44 A population-based RCT from Spain (COLONPREV) reported screening-colonoscopy uptake at 24.6%,30 and in another European RCT (NordICC), screening-colonoscopy uptake ranged from 29% to 60% across the participating countries.45 A recent randomized health services study from Poland reported favorable participation when subjects were offered a sequential (invitation to primary colonoscopy and invitation to FIT for non-responders) or active choice (invitation offering a choice of colonoscopy or FIT) compared to invitation for screening-colonoscopy alone. However, adherence was still low, ranging from 18 to 26%.46 By contrast, adherence was 90.5% in an observational study of patients referred for screening colonoscopy in NYC using patient facilitation 47 and 93.1% for women in a study based in US military hospitals.48 In a Dallas RCT,49, 50 in a safety-net healthcare system that compared FIT, colonoscopy, or usual care, screening completion remained low at 38% for the colonoscopy outreach arm, 28% for the FIT outreach arm, and 11% for the usual care group.51
Adherence to stool-based studies should be evaluated separately for initial uptake and sequential adherence. In our study, adherence to the initial round of adherence for HSgFOBT was 73.1%, but adherence to all 4 rounds was 38.3%. The range of adherence reported for gFOBT has been as low as 14.1%52 to 38%20 with one study of volunteers that reported 75%.19 Our sequential adherence over 4 rounds with HSgFOBT is comparable to the sequential adherence reported for FIT of 43%27 to 48%.23 Although the initial uptake of FIT was higher than HSgFOBT, the sequential uptake is comparable.49, 50, 53–55
It should be noted that we observed the lowest HSgFOBT adherence rates at LSUHS. While it was outside the scope of our study to investigate the behavioral reasons of adherence and drop-off rates over time, adherence to screening-colonoscopy at LSUHS were numerically similar to that of KPWA. Moreover, a prior report within this trial reported comparable adherence rates in the screening-colonoscopy arm between Blacks and whites at LSUHS.33 These differences between screening-colonoscopy and HSgFOBT rates may suggest that a one-time screening-colonoscopy may be better suited in community health settings as opposed to longer term fecal-based screening tests over time.
Participant facilitation plays an important role in adherence. The structure of our study design was to optimize adherence to the study assignment of both arms. A recent study from the NordICC pragmatic trial reported suboptimal differences in CRC mortality among those invited for colonoscopy compared to those in the usual care group. However when these findings were analyzed among those who were actually screened (i.e. per-protocol), a 50% and 30% reduction in mortality and incidence was observed among those who participated colonoscopy versus the usual-care group, thus pointing to the importance of screening adherence to reduce CRC incidence and mortality.32 A cluster RCT in San Francisco that used patient navigation 38% adherence to screening-colonoscopy, 67% adherence to FOBT, and 69% adherence when given choice of either test.56 In the same randomized “choice” trial, these services were provided only in the first round of screening, but subsequent rounds without facilitation resulted in lower adherence rates – 38% for colonoscopy, 14% for FOBT, and 42% for those offered a choice.57 By contrast, the COLONPREV study30 and CONFIRM trial29 used facilitation reminders for annual/biennial FOBT. A study in Delaware reported colonoscopy screening uptake rates from 57% to 74% with use of facilitation to coordinate screening and care.58 Adherence to follow-up colonoscopy for FOBT or FIT positive patients reported in other studies was 58-78% without facilitation ,24–26, 59 compared to the 91.4% our study, thus pointing to the importance of this facilitation to promote adherence. In our study, we had a dedicated staff of coordinators who provided equal support in both the screening-colonoscopy and HSgFOBT arms. Patient navigation has been shown have a profound impact in improving cancer screening rates within vulnerable health populations.60 A previous report has shown that when compared to participants who received patient navigation vs. those who did not, those who received navigation were more likely to complete colonoscopy exams, have adequate prep, and have fewer missed appointments.61 Such navigation can make a critical difference in decreasing CRC incidence and mortality and provide significant population-level public health benefits.
There have been limited observations of crossover between colonoscopy and FOBT because few studies include both modalities. In our study, the overall crossover rate from HSgFOBT to screening-colonoscopy was 30.1%. Like adherence, crossover to the alternative offered test may depend on many factors, including the familiarity with and availability of various screening methods and availability of such methods. In a Kaiser-Permanente study,26, 62 the rate of crossover from FIT to screening-colonoscopy was 25% which is similar to the crossover rate of 24.7% in the first four rounds of our study. In a RCT in Spain (COLONPREV), a country where colonoscopy is less available and not as publicized, more people crossed over to FIT from colonoscopy (23.2%) than the reverse (1.2%).30 Our study demonstrated the influence of healthcare settings on crossover rate, which was considerably higher for the opportunistic setting (UMN, 39.8%) than for the integrated health care setting (KPWA, 26.4%) or the minority serving center LSUHS, where the crossover was the lowest (5.6%).
A strength of our study is the multi-center setting, which consisted of several geographic locations, and healthcare settings; these factors provided outcomes which are more generalizable compared to those reported from a single institution, cohort, or health study. In addition, participants in both arms had equivalent facilitation, resulting in high rates of completion of screening and diagnostic colonoscopy. All colonoscopies were completed by a small number of attending endoscopists credentialed by an NCS committee, all HSgFOBTs were returned to the same central laboratory for tracking and testing, and all pathology was prospectively reviewed by the study pathologist blinded to the patients’ clinical status and study arm. Furthermore, our participants provided informed consent about the study, suggesting a baseline willingness to adhere. An important aspect of study design is whether individual participants have agreed to enter a screening study prior to accrual, and whether there was informed consent with knowledge of all intervention arms prior to randomization.29 Our RCT had informed consent to participate in CRC screening, suggesting a baseline willingness to be screened.
This study has several limitations. We used a HSgFOBT rather than FIT.10, 53 FIT has largely replaced HSgFOBT in most clinical settings,63 much due to the convenience of fewer stool samples and no dietary restrictions.64 These differences have primarily affected the initial uptake, with sequential adherence being comparable.65 A recent review has suggested that FIT performance may be superior to that of gFOBT since FIT is a more direct measure of human hemoglobin.66 However, comparisons of FIT to gFOBT have been with an older, less sensitive guaiac-based test.65 Prior RCTs have shown that gFOBT is effective in reducing mortality for CRC.19–21, 67, 68 Although FIT is now widely used in the US, it was not the standard stool test at the initiation of our study. The HSgFOBT used in this study was the same guaiac test recommended by the USPSTF at the time of our study as having comparable LYG to that of colonoscopy.11, 22 At present, HSgFOBT continues to be used and recommended in the US.42, 69
As with any RCT, healthy volunteer bias is possible with informed consent as it may sample from a study population consisting of health aware individuals. In contrast to pragmatic-based studies such as the NordICC trial,32 our study population consisted of individuals who signed up for a screening study, indicating a willingness to accept screening with either method. The patients who were recruited to our study may have been healthier or more motivated to receive cancer screening. However, our multi-center center study consisted of individuals from differing backgrounds, therefore our estimates reflect a probable estimate of compliance within this population. Moreover, CRC screening for each arm were provided at no cost for the participants. Up until recently,70 the burden of copays for polyps found during routine colonoscopy or diagnostic colonoscopy following a positive fecal-based test have posed barriers in receiving subsequent CRC care. Reasons for lack of follow-up have varied from fear of colonoscopy and breakdown in subsequent communication71 to insurance-related challenges in receiving follow-up exams,72 all of which were assuaged by our study design and patient facilitation. A recent American Gastroenterology Association statement from Lieberman et al supports the notion that the full cost of screening should be covered by payers without cost sharing, which has the potential to ameliorate barriers to screening and socioeconomic inequities in CRC outcomes.73 The free cost of screening within both arms of our study may have facilitated adherence, as it allowed participants to bypass these barriers. Finally, by design we had a small number of endoscopists who performed the colonoscopies in our study, all of whom were well-experienced and vetted by our credentialing committee to ensure best clinical practice. However, due to the limited number of endoscopists, this may have affected the generalizability of our results, both in terms of the number of adenomas detected and overall patient outcomes
The data generated by NCS are novel in that they were based on a randomized design that compared a single screening-colonoscopy to multiple rounds of HSgFOBT with equivalent facilitation in each arm throughout the study, and in three different clinical and geographical settings. We demonstrated that those who are motivated for CRC screening by enrolling in an RCT are more likely to adhere to a single screening-colonoscopy than to multiple rounds of annual HSgFOBT, even with equal facilitation in both arms. In addition, a substantial percentage of participants assigned to HSgFOBT subsequently opted for screening-colonoscopy. Considering the participants who crossed over to screening-colonoscopy and participants in partial rounds, the percent with any screening exposure/coverage was equivalent in the two groups. However, while adherence to some degree of screening was equivalent, it is not likely to be equivalent in potential effectiveness given that it was not full adherence. Screening-colonoscopy may be acceptable in an opportunistic screening environment, whereas stool testing requires an organized screening program that can facilitate annual testing and adherence with follow-up colonoscopy.8, 74 It has been stated that in a real-world setting patient facilitation may be costly to institutions and therefore not always feasible to implement. However, economic evaluation of patient facilitation has illustrated to be beneficial in completing colonoscopies, as well as yield a net financial benefit for providers.75, 76 This was exemplified in our own study, with 88.5% adherence to screening-colonoscopy and 91.4% completion of diagnostic colonoscopy following a positive HSgFOBT test. This consideration may have substantial downstream effects on the overall outcomes of CRC incidence and mortality in the greater population.
Based on our study, in cases where sequential stool-based screening is suboptimal and colonoscopy is accessible within the population, offering the option of crossover to screening-colonoscopy may increase screening efficacy. Moreover, when compared to participants who were non-sequentially and even sequentially adherent to HSgFOBT, the greatest number of advanced lesions were detected among those who crossed over to colonoscopy. Guidelines that recommend HSgFOBT should emphasize adherence to sequential HSgFOBT rather than a single test uptake and should stress the need for organized systems that facilitate high sequential adherence to maximize the impact in CRC incidence and mortality.11, 77
What You Need to Know:
Background and context:
Guidelines recommend colorectal cancer (CRC) screening by several different fecal-based tests or colonoscopy, with the assumption of equivalent adherence, which assume equivalent effectiveness.
New findings:
Facilitated adherence to full sequential rounds of HSgFOBT was 38.3% compared to 83.6% for colonoscopy. Any CRC screening in HSgFOBT participants improved to 84.0% with crossover colonoscopy or partial sequential in those who deviated from the protocol. Adherence among those who completed any colonoscopy in the colonoscopy arm was 88.5%.
Limitations:
Randomized men and women among those willing to be screened, which may result in healthy volunteer bias. The study was not powered for CRC incidence and mortality reduction.
Clinical Research Relevance:
Adherence must be emphasized when screening is recommended. Colonoscopy availability in HSgFOBT screening programs may increase the effectiveness of screening.
Basic Research Relevance:
Poor sequential adherence to a CRC screening program of stool-based test, such as HSgFOBT, will reduce its effectiveness.
Acknowledgments:
The authors would like to thank Gavin Watt, Deborah Engelhard, Mindy Geisser, and Jill Cordes from University of Minnesota; Lisa Ross from Kaiser Permanente-Washington; Lorraine Post, Martha Lennard, and Georgia Morgan from Louisiana State University Health Sciences, and Javier Cotignola, Sarah Yoo, Pampa Roy, Brian Clas, and Diana Tommasi, from the Memorial Sloan Kettering Orlow Lab for their skilled technical assistance. We also thank the men and women who took part in this study and the NCS staff.
Grant Support:
This study was primarily supported by a grant from the National Cancer Institute (R01-CA079572) (Winawer and Zauber). This study was also supported by grants U01-CA199335 and U01-CA253913 from the National Cancer Institute (NCI) as part of the Cancer Intervention and Surveillance Modeling Network (CISNET) (Zauber, Lansdorp-Vogelaar, Rutter, Knudsen, Meester, Hahn). Additional funding was obtained to partially support the study from the Cantor Colon Cancer Fund (Winawer), the Tavel-Reznik Fund (Winawer), and the NIH/NCI Cancer Center Support Grant P30 CA008748 (Zauber, Winawer). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Abbreviations:
- ADN-SER
combined of advanced adenomas and serrated lesions with neoplastic progression
- CRC
colorectal cancer
- FIT
fecal immunochemical test
- HSgFOBT
high sensitivity guaiac based fecal occult blood test
- RCT
randomized clinical trial
- SSL
sessile serrated lesions
Appendix
Appendix Figure 1.
Site Locations for National Colonoscopy Study
Appendix Figure 2.
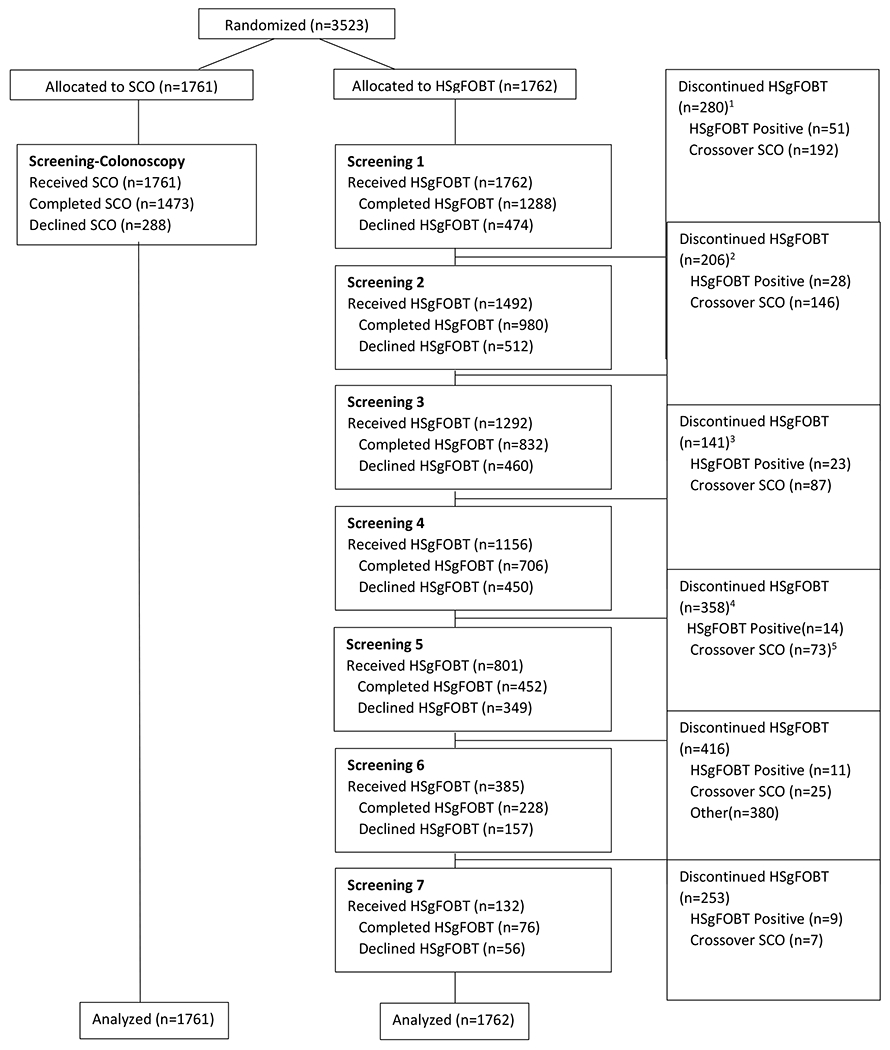
National Colonoscopy Study flowchart by screening round
Screening colonoscopy (SCO), High sensitivity guaiac fecal occult blood test (HSgFOBT)
1, 2, 3, 4: Had a colonoscopy and later went on to participate in more screening in some form (i.e. did not discontinue forever), n = 10, 6, 5, 2, respectively
5: 1 patient crossed over in round 4. They did not receive an FOBT kit in round 4.
Appendix Figure 3.
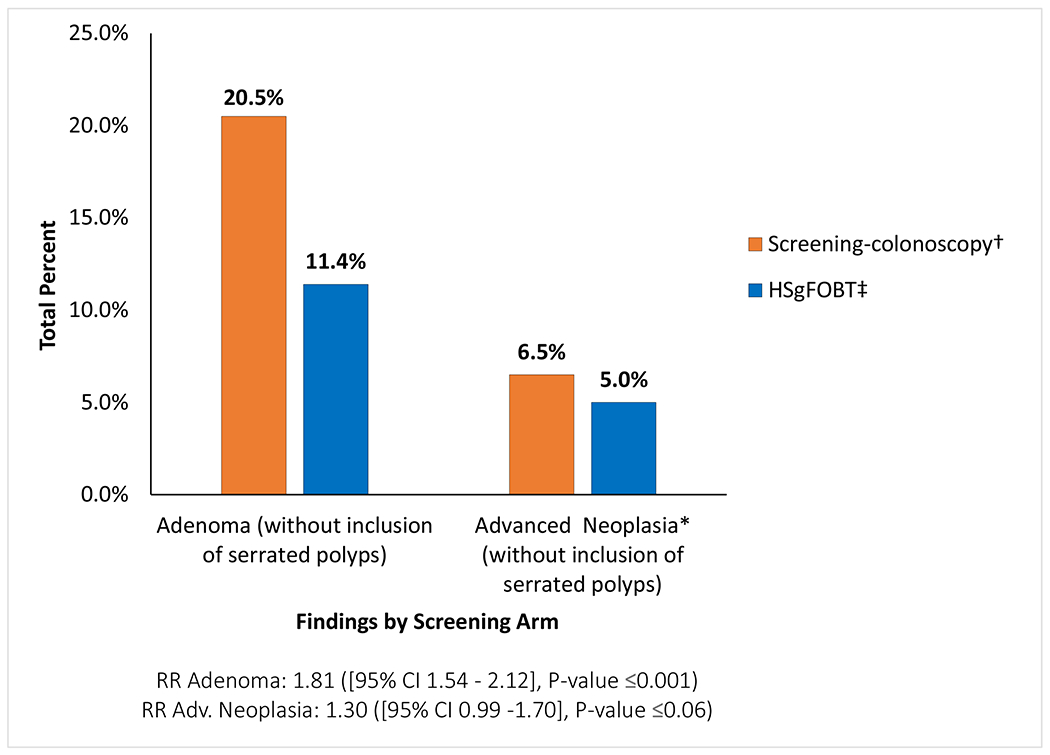
Pathology findings by screening arm for adenomas and advanced neoplasia* without inclusion of serrated polyps
High sensitivity fecal occult blood test (HSgFOBT); Relative Risk (RR); 95% Confidence Intervals (95% CI)
* Advanced neoplasia defined as lesions with any villous component, high grade dysplasia, or 1 cm or larger, or 3 or more tubular adenomas <1 cm. Colorectal cancer is also included in this category
† Screening-colonoscopy pathology findings based on total 1761 participants randomized; 1558 participants with colonoscopies, of which 85 had initial colonoscopy after 12 months.
97.6% of screening-colonoscopy arm participants reached the cecum and 98.0% of colonoscopies were cleared. 95.6% of HSgFOBT-positive colonoscopies participants performed reached the cecum and 97.1% of colonoscopies were cleared.
‡ HSgFOBT findings from follow-up colonoscopy after a positive HSgFOBT and from cross-over colonoscopy without a positive HSgFOBT based on 1762 participants randomized
Appendix Figure 4a:
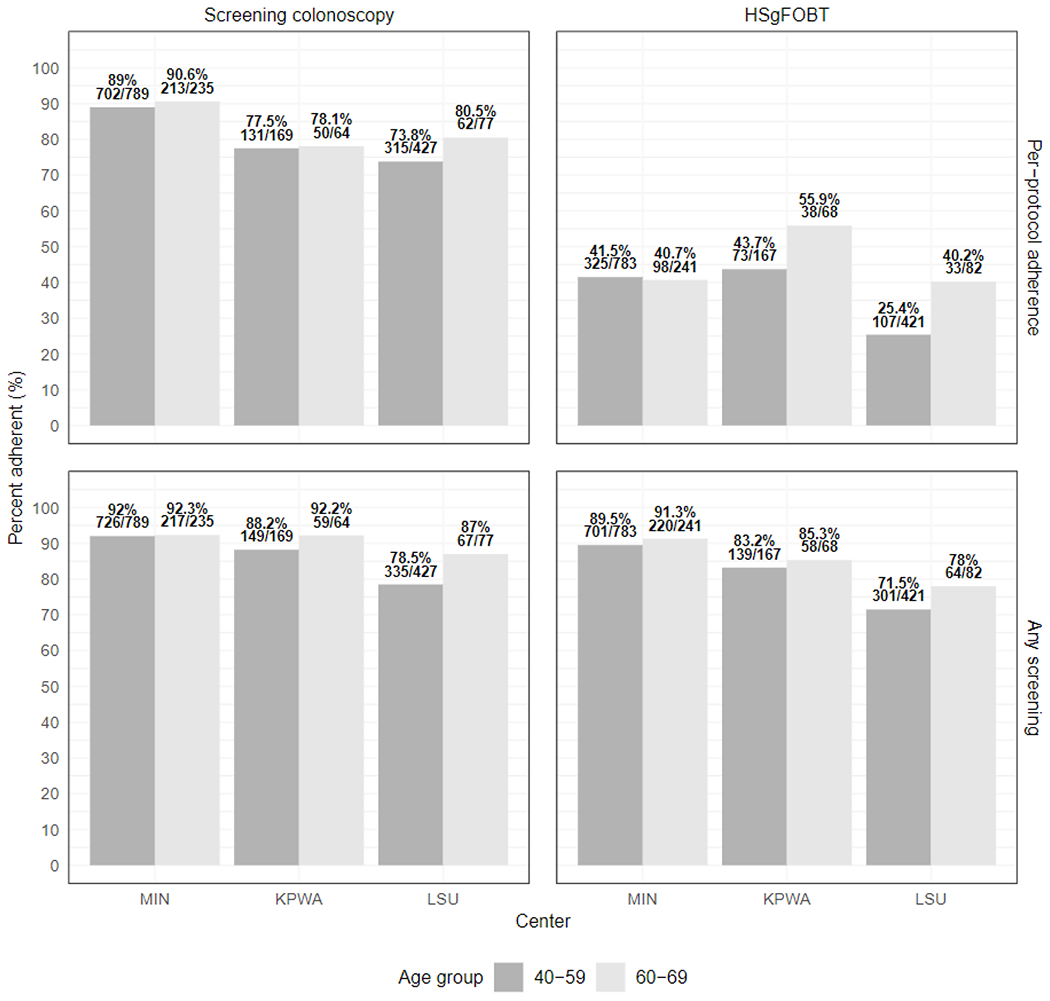
Adherence proportions by age groups by per-protocol adherence and any adherence
High sensitivity fecal occult blood test (HSgFOBT)
University of Minnesota (MIN), Kaiser-Permanente Washington State (KPWA), Louisiana State University (LSU)
Appendix Figure 4b:
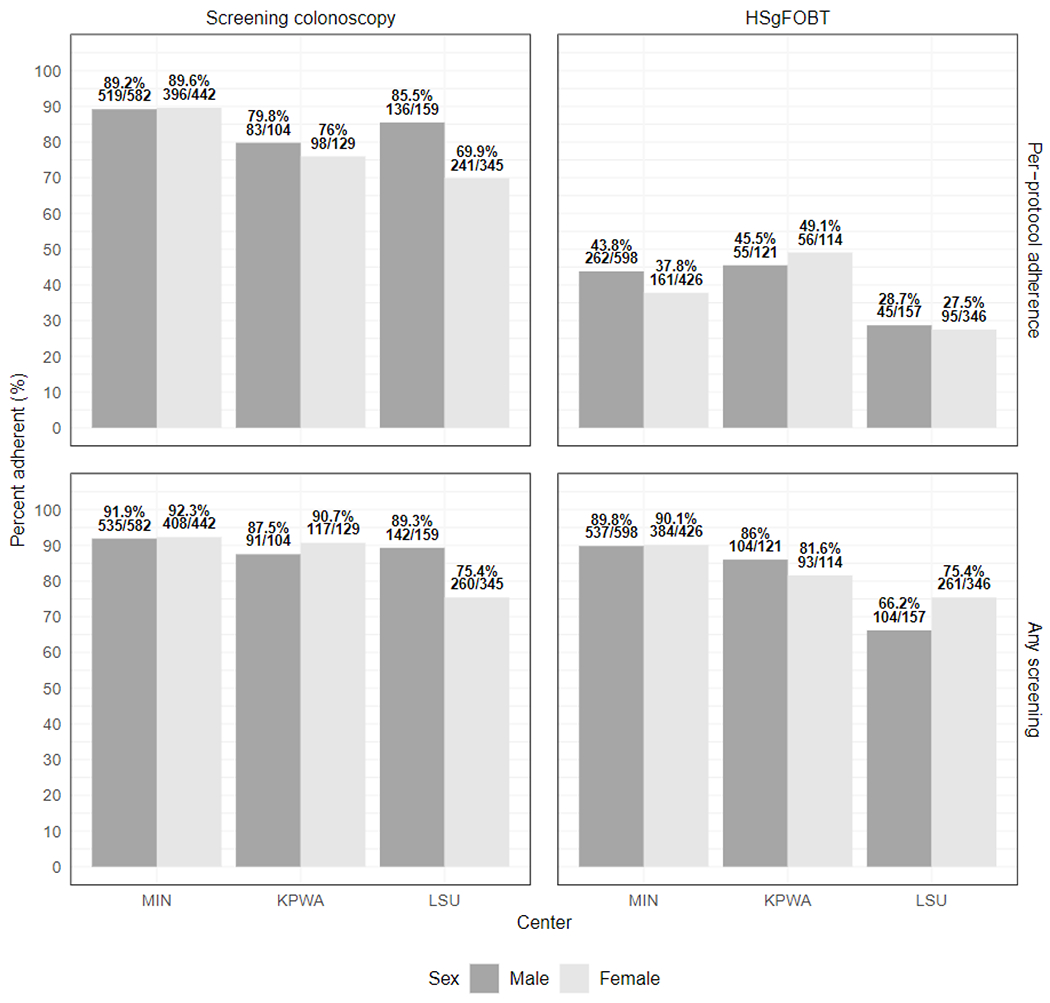
Adherence proportions by sex by per-protocol adherence and any adherence
High sensitivity fecal occult blood test (HSgFOBT)
University of Minnesota (MIN), Kaiser-Permanente Washington State (KPWA), Louisiana State University (LSU)
Appendix Table 1.
Participation in the biorepository by study arm
| Randomization Arm | |||
|---|---|---|---|
| Blood Sample* | Screening-Colonoscopy | HSgFOBT** | Total |
| Blood Sample | 1376 (78.1%) | 105 (6.0%) | 1481 |
| No Blood Sample | 385 (21.9%) | 1660 (94.0%) | 1945 |
| Total | 1761 | 1762 | 3423 |
High sensitivity fecal occult blood test (HSgFOBT)
The biospecimens continue being monitored both on-site and remotely 24x7
Only participants who had a positive HSgFOBT test were eligible for blood draw at their follow-up colonoscopy
Appendix Table 2a.
Classification of serrated lesions** for neoplastic potential in the screening-colonoscopy and HSgFOBT arm
| Serrated Lesions | ||||||
|---|---|---|---|---|---|---|
| Histology | Size | Number of polyps | Location | Classification | Total Findings Per Participant in Screening-Colonoscopy Arm | Total Findings Per Participant in HSgFOBT Arm |
| HP (microvesicular or goblet cell) | <10 mm | Any number | Rectosigmoid | Hyperplastic | 188 | 72 |
| HP (microvesicular or goblet cell) | ≤ 5 mm | ≤ 3 | Proximal to sigmoid | Hyperplastic | 42 | 20 |
| HP (microvesicular or goblet cell) | Any | ≥ 4 | Proximal to sigmoid | Non-advanced (neoplasia) | 0 | 1 |
| HP (microvesicular or goblet cell) | >5 mm | ≤ 1 | Proximal to sigmoid | Non-advanced (neoplasia) | 5 | 4 |
| SSL or TSA | < 10 mm | < 3 | Any | Non-advanced (neoplasia) | 39 | 21 |
| SSL or TSA | < 10 mm | ≥ 3 | Any | Advanced (neoplasia)* | 0 | 0 |
| SSL or TSA | ≥ 10 mm | 1 | Any | Advanced (neoplasia)* | 14 | 6 |
| HP (microvesicular or goblet cell)*** | ≥ 10 mm | Any | Rectosigmoid | Advanced (neoplasia)* | 7 | 2 |
| SSL | ≥ 10 mm | ≥ 2 | Any | Advanced (neoplasia)* | 3 | 1 |
| SSL with dysplasia | Any | Any | Any | Advanced (neoplasia)* | 2 | 2 |
High sensitivity fecal occult blood test (HSgFOBT), Hyperplastic polyps (HP), Sessile serrated lesion (SSL),Traditional serrated adenoma (TSA)
Advanced neoplasia classification includes colorectal cancer
Serrated lesions classifications were based on definitions from Rex and Gupta
HP (microvesicular or goblet cell) ≥ 10 mm in rectosigmoid (any number of polyps) were based on classification from Gupta1
Appendix Table 2b.
Classification of adenomas for neoplastic potential in the screening-colonoscopy and HSgFOBT arm
| Adenomas | ||||||
|---|---|---|---|---|---|---|
| Histology | Size | Number of polyps | Location | Reference | Total Findings Per Participant in Screening-Colonoscopy Arm | Total Findings Per Participant in HSgFOBT Arm |
| Tubular | < 10 mm | 1 or 2 | Any | Non-advanced (neoplasia) | 293 | 143 |
| Tubular | < 10 mm | ≥ 3 | Any | Advanced (neoplasia)* | 31 | 23 |
| Tubular | ≥ 10 mm | ≥1 | Any | Advanced (neoplasia)* | 73 | 56 |
| Tubulovillous, villous, or high-grade dysplasia | Any | >1 | Any | Advanced (neoplasia)* | 24 | 28 |
High sensitivity fecal occult blood test (HSgFOBT), Hyperplastic polyps (HP), Sessile serrated lesion (SSL),Traditional serrated adenoma (TSA)
Advanced neoplasia classification includes colorectal cancer
Appendix Table 3a.
Comparison Between NCS Study 1 and Study 2
| Study 1 – Screening-Colonoscopy vs. Usual Care | Study 2 – SCO vs. Sequential HSgFOBT Adherence | |
|---|---|---|
| Accrual | 2000-2002 | 2004-2008 |
| Follow-up | 2002 | 2012 |
| Adherence to baseline colonoscopy | 88.9% (622/700) | 83.6% (1473/1761) |
High sensitivity fecal occult blood test (HSgFOBT)
Appendix Table 3b.
Comparison of Colonoscopy Baseline Pathology Findings Between Study 1 and Study 2
| Screening-Colonoscopy Baseline Findings | ||||
|---|---|---|---|---|
| Study 1 (N): | (%) | Study 2 (N):** | (%): | |
| Adenoma | 112 | 16.0% | 332 | 19.6% |
| Advanced neoplasia* | 37 | 5.3% | 100*** | 5.7% |
| Non-advanced adenoma | 75 | 10.7% | 232 | 13.2% |
| Hyperplastic/Other | 118 | 16.9% | 282 | 16.0% |
| No polyps | 386 | 55.1% | 860 | 48.8% |
| Non-adherent | 78 | 11.1% | 287 | 16.3% |
Advanced neoplasia defined as lesions with any villous component, high grade dysplasia, or 1 cm or larger, or 3 or more tubular adenomas <1 cm. Colorectal cancer is also included in this category. Advanced neoplasia category does not include 3 or more small tubular adenomas in Study 1 but is included in Study 2.
These results do not include the serrated polyp findings and are from the baseline colonoscopies
One participant was found to have colorectal cancer shortly after randomization and diagnosed with colorectal cancer shortly before scheduled screening-colonoscopy
Appendix Table 4.
Adherence among participants who completed the first round of HSgFOBT*
| Round 1 | Round 2 | Round 3 | Round 4 | Round 5** | Round 6** | Round 7** | |
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total HSgFOBT completed | 1288/1762 (73.1%) | 912/1096 (83.2%) | 780/946 (82.5%) | 668/842 (79.3%) | 421/571 (73.7%) | 211/287 (73.5%) | 71/99 (71.7%) |
High sensitivity fecal occult blood test (HSgFOBT)
Participants eligible for HSgFOBT by completing the first round of the study
Participants who joined the study early in the study period were eligible for 5 or more later rounds
Appendix Table 5. Colorectal cancers overview:
There were 16 confirmed CRCs, 10 in the HSgFOBT arm and 6 in the colonoscopy arm. Of the 10 CRCs in the FOBT arm, 6 were found through a HSgFOBT directed colonoscopy while 3 were discovered through an off-study colonoscopy. 1 subject was symptomatic and sought medical care for the symptoms prior to attempting to complete the HSgFOBT slides. 3 of the 6 CRCs in the colonoscopy arm were discovered in the baseline colonoscopy. 2 CRCs were found in a surveillance colonoscopy. One CRC was an incidental finding after a ruptured appendix in the colonoscopy arm. The annual study update questionnaires are used to ascertain any interval CRC’s.
a. Cancers found by year and study arm.
| Screening-Colonoscopy Arm | HSgFOBT Arm | |||||
|---|---|---|---|---|---|---|
| Study Year | Baseline Colonoscopy | Follow-up Colonoscopy | Other | HSgFOBT + Directed Colonoscopy | Crossover Colonoscopy | Other |
| Year 0 | 3 | 0 | 1* | 1 | 1 | 1† |
| Year 1 | 0 | 0 | 0 | 2 | 2 | 0 |
| Year 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Year 3 | 0 | 1 | 0 | 1 | 0 | 0 |
| Year 4 | 0 | 0 | 0 | 1 | 0 | 0 |
| Year 5 | 0 | 1 | 0 | 1 | 0 | 0 |
| Total | 3 | 2 | 1 | 6 | 3 | 1 |
High sensitivity fecal occult blood test (HSgFOBT)
CRC found through hospital surgery/laparoscopy for raptured appendix
CRC found through barium enema
Appendix Table 5b.
Colorectal cancers stage found by study arm
| Screening-Colonoscopy | HSgFOBT | |
|---|---|---|
| Stage I | 3 | 5 |
| Stage II | 0 | 2 |
| Stage III | 3 | 3 |
| Stage IV | 0 | 0 |
| Total | 6 | 10 |
High sensitivity fecal occult blood test (HSgFOBT)
Appendix Table 5c.
Colorectal cancer locations and number of cancers.
| Screening-Colonoscopy | HSgFOBT | |
|---|---|---|
| Location: | ||
| Rectum | 1 | 2 |
| Sigmoid-Colon | 3 | 5 |
| Descending Colon | 1 | 0 |
| Splenic Flexure | 0 | 1 |
| Ascending Colon | 0 | 1 |
| Cecum | 1 | 1 |
| Total Number of Colorectal Cancer | 6 | 10 |
High sensitivity fecal occult blood test (HSgFOBT)
Appendix Table 6.
Pathology outcomes of participants with one or more first-degree relatives with colorectal cancer
| No FDRs N (%) | 1 FDR N (%) | 2 or more FDRs N (%) | All patients | |
|---|---|---|---|---|
| Sample Size | 2816 (90.5%) | 276 (8.9%) | 21 (0.7%) | 3523 |
| Randomization Arm | ||||
| Screening-colonoscopy | 1445 (51.3%) | 149 (54.0%) | 13 (61.9%) | 1761 |
| HSgFOBT | 1371 (48.7%) | 127 (46.0%) | 8 (38.1%) | 1762 |
| Pathology Findings | ||||
| Cancer* | 13 (0.5%) | 2 (0.7%) | 0 | 16 (0.5%) |
| Advanced Neoplasia | 189 (6.7%) | 21 (7.6%) | 0 | 232 (6.63%) |
First-degree relative (FDR)
One additional participant who had CRC did not disclose family history questionnaire
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Reinier Meester was appointed Director of Health Economics and Outcomes Research at Freenome in May 2022, after this study was completed. Dr. Robin Mendelsohn was a speaker for WebMD and Medscape. All other authors declare no conflicts of interest.
Registration: Registered at ClinicalTrials.gov (NCT00102011).
Approval:
The study was approved by the Institution Review Board of Memorial Sloan Kettering Cancer Center, University of Minnesota, Kaiser Permanente-Washington, and Louisiana State University Health Sciences.
Data Transparency Statement: Summary-level, deidentified data, data forms, and analytic methods may be available upon request and contingent on collaboration with corresponding author, Dr. Ann G. Zauber.
References:
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33. [DOI] [PubMed] [Google Scholar]
- 4.Atkin W, Wooldrage K, Parkin DM, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017;389:1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst 2011;103:1310–22. [DOI] [PubMed] [Google Scholar]
- 7.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. Jama 2016;315:2564–2575. [DOI] [PubMed] [Google Scholar]
- 8.Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;153:307–323. [DOI] [PubMed] [Google Scholar]
- 9.USPSTF. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:1965–1977. [DOI] [PubMed] [Google Scholar]
- 10.Knudsen AB, Rutter CM, Peterse EFP, et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA 2021;325:1998–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, et al. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2008;149:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2016;315:2576–94. [DOI] [PubMed] [Google Scholar]
- 13.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. Jama 2016;315:2595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JS, Perdue LA, Henrikson NB, et al. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama 2021;325:1978–1997. [DOI] [PubMed] [Google Scholar]
- 16.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977–81. [DOI] [PubMed] [Google Scholar]
- 17.Fedewa SA, Goodman M, Flanders WD, et al. Elimination of cost-sharing and receipt of screening for colorectal and breast cancer. Cancer 2015;121:3272–80. [DOI] [PubMed] [Google Scholar]
- 18.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev 2011;33:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993;328:1365–71. [DOI] [PubMed] [Google Scholar]
- 20.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472–7. [DOI] [PubMed] [Google Scholar]
- 21.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467–71. [DOI] [PubMed] [Google Scholar]
- 22.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2008;149:627–37. [DOI] [PubMed] [Google Scholar]
- 23.Crotta S, Segnan N, Paganin S, et al. High rate of advanced adenoma detection in 4 rounds of colorectal cancer screening with the fecal immunochemical test. Clin Gastroenterol Hepatol 2012;10:633–8. [DOI] [PubMed] [Google Scholar]
- 24.Steele RJ, Kostourou I, McClements P, et al. Effect of repeated invitations on uptake of colorectal cancer screening using faecal occult blood testing: analysis of prevalence and incidence screening. Bmj 2010;341:c5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steele RJ, McClements PL, Libby G, et al. Results from the first three rounds of the Scottish demonstration pilot of FOBT screening for colorectal cancer. Gut 2009;58:530–5. [DOI] [PubMed] [Google Scholar]
- 26.Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Annals of internal medicine 2016;164:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osborne JM, Wilson C, Duncan A, et al. Patterns of participation over four rounds of annual fecal immunochemical test-based screening for colorectal cancer: what predicts rescreening? BMC Public Health 2017;18:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabeneck L, Tinmouth JM, Paszat LF, et al. Ontario’s ColonCancerCheck: results from canada’s first province-wide colorectal cancer screening program. Cancer Epidemiol Biomarkers Prev 2014;23:508–15. [DOI] [PubMed] [Google Scholar]
- 29.Dominitz JA, Robertson DJ, Ahnen DJ, et al. Colonoscopy vs. Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer (CONFIRM): Rationale for Study Design. Am J Gastroenterol 2017;112:1736–1746. [DOI] [PubMed] [Google Scholar]
- 30.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012;366:697–706. [DOI] [PubMed] [Google Scholar]
- 31.Forsberg A, Westerberg M, Metcalfe C, et al. Once-only colonoscopy or two rounds of faecal immunochemical testing 2 years apart for colorectal cancer screening (SCREESCO): preliminary report of a randomised controlled trial. Lancet Gastroenterol Hepatol 2022;7:513–521. [DOI] [PubMed] [Google Scholar]
- 32.Bretthauer M, Løberg M, Wieszczy P, et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med 2022. [DOI] [PubMed] [Google Scholar]
- 33.Mendelsohn RB, Winawer SJ, Jammula A, et al. Adenoma Prevalence in Blacks and Whites Having Equal Adherence To Screening Colonoscopy: The National Colonoscopy Study. Clinical gastroenterology hepatology 2017;15:1469–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Brien MJ, Zhao Q, Yang S. Colorectal serrated pathway cancers and precursors. Histopathology 2015;66:49–65. [DOI] [PubMed] [Google Scholar]
- 36.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315–29; quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2020;115:415–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Team RC. R: A Language and Environment for Statistical Computing, 2019. [Google Scholar]
- 40.Wickman H, Averick M, Bryan J. Welcome to the Tidyverse: Journal of Open Source Software, 2019. [Google Scholar]
- 41.Meester RGS, Peterse EFP, Knudsen AB, et al. Optimizing colorectal cancer screening by race and sex: Microsimulation analysis II to inform the American Cancer Society colorectal cancer screening guideline. Cancer 2018;124:2974–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf AM, Fontham ET, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA: a cancer journal for clinicians 2018;68:250–281. [DOI] [PubMed] [Google Scholar]
- 43.Shaukat A, Church TR, Shanley R, et al. Development and validation of a clinical score for predicting risk of adenoma at screening colonoscopy. Cancer Epidemiol Biomarkers Prev 2015;24:913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper CP, Gelb CA. Opportunities to Expand Colorectal Cancer Screening Participation. Journal of women’s health (2002) 2016;25:990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bretthauer M, Kaminski MF, Løberg M, et al. Population-Based Colonoscopy Screening for Colorectal Cancer: A Randomized Clinical Trial. JAMA Intern Med 2016;176:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilonis ND, Bugajski M, Wieszczy P, et al. Participation in Competing Strategies for Colorectal Cancer Screening: A Randomized Health Services Study (PICCOLINO Study). Gastroenterology 2021;160:1097–1105. [DOI] [PubMed] [Google Scholar]
- 47.Lebwohl B, Neugut Al, Stavsky E, et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol 2011;45:e47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med 2005;352:2061–8. [DOI] [PubMed] [Google Scholar]
- 49.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med 2013;173:1725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer 2016;122:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singal AG, Gupta S, Skinner CS, et al. Effect of Colonoscopy Outreach vs Fecal Immunochemical Test Outreach on Colorectal Cancer Screening Completion: A Randomized Clinical Trial. JAMA 2017;318:806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gellad ZF, Stechuchak KM, Fisher DA, et al. Longitudinal adherence to fecal occult blood testing impacts colorectal cancer screening quality. Am J Gastroenterol 2011;106:1125–34. [DOI] [PubMed] [Google Scholar]
- 53.Hol L, van Leerdam ME, van Ballegooijen M, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. GUT 2010;59:62–8. [DOI] [PubMed] [Google Scholar]
- 54.van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008;135:82–90. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman RM, Steel S, Yee EF, et al. Colorectal cancer screening adherence is higher with fecal immunochemical tests than guaiac-based fecal occult blood tests: a randomized, controlled trial. Prev Med 2010;50:297–9. [DOI] [PubMed] [Google Scholar]
- 56.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang PS, Wheat CL, Abhat A, et al. Adherence to Competing Strategies for Colorectal Cancer Screening Over 3 Years. Am J Gastroenterol 2016;111:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grubbs SS, Polite BN, Carney J Jr., et al. Eliminating racial disparities in colorectal cancer in the real world: it took a village. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013;31:1928–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Issaka RB, Singh MH, Oshima SM, et al. Inadequate Utilization of Diagnostic Colonoscopy Following Abnormal FIT Results in an Integrated Safety-Net System. Am J Gastroenterol 2017;112:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson HD, Cantor A, Wagner J, et al. Effectiveness of Patient Navigation to Increase Cancer Screening in Populations Adversely Affected by Health Disparities: a Meta-analysis. J Gen Intern Med 2020;35:3026–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rice K, Gressard L, DeGroff A, et al. Increasing colonoscopy screening in disparate populations: Results from an evaluation of patient navigation in the New Hampshire Colorectal Cancer Screening Program. Cancer 2017;123:3356–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levin TR, Corley DA, Jensen CD, et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large, Community-based Population. Gastroenterology 2018;155(5):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sokoro A, Shafer LA, Darr M, et al. Utility of fecal immunochemical test vs guaiac fecal occult blood test for assessment of gastrointestinal bleed in hospitalized patients. Clin Chim Acta 2020;500:202–207. [DOI] [PubMed] [Google Scholar]
- 64.Akram A, Juang D, Bustamante R, et al. Replacing the Guaiac Fecal Occult Blood Test With the Fecal Immunochemical Test Increases Proportion of Individuals Screened in a Large Healthcare Setting. Clin Gastroenterol Hepatol 2017;15:1265–1270.e1. [DOI] [PubMed] [Google Scholar]
- 65.Hassan C, Giorgi Rossi P, Camilloni L, et al. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Aliment Pharmacol Ther 2012;36:929–40. [DOI] [PubMed] [Google Scholar]
- 66.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;152:1217–1237.e3. [DOI] [PubMed] [Google Scholar]
- 67.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603–7. [DOI] [PubMed] [Google Scholar]
- 68.Kewenter J, Brevinge H, Engaras B, et al. Results of screening, rescreening, and follow-up in a prospective randomized study for detection of colorectal cancer by fecal occult blood testing. Results for 68,308 subjects. Scand J Gastroenterol 1994;29:468–73. [DOI] [PubMed] [Google Scholar]
- 69.Lauby-Secretan B, Vilahur N, Bianchini F, et al. The IARC Perspective on Colorectal Cancer Screening. N Engl J Med 2018;378:1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.What you need to know about proposed changes to Medicare payment policies: American Gastroenterological Association; 2022. [Google Scholar]
- 71.Llovet D, Serenity M, Conn LG, et al. Reasons For Lack of Follow-up Colonoscopy Among Persons With A Positive Fecal Occult Blood Test Result: A Qualitative Study. Official journal of the American College of Gastroenterology | ACG 2018;113:1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jetelina KK, Yudkin JS, Miller S, et al. Patient-Reported Barriers to Completing a Diagnostic Colonoscopy Following Abnormal Fecal Immunochemical Test Among Uninsured Patients. Journal of General Internal Medicine 2019;34:1730–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lieberman D, Ladabaum U, Brill JV, et al. Reducing the Burden of Colorectal Cancer: AGA Position Statements. Gastroenterology 2022;163:520–526. [DOI] [PubMed] [Google Scholar]
- 74.Gini A, Selby K. Fecal Immunochemical Tests: The Right Colorectal Cancer Screening Test for the Average-Risk Population? Clin Gastroenterol Hepatol 2022. [DOI] [PubMed] [Google Scholar]
- 75.Elkin EB, Shapiro E, Snow JG, et al. The economic impact of a patient navigator program to increase screening colonoscopy. Cancer 2012;118:5982–8. [DOI] [PubMed] [Google Scholar]
- 76.Rice K, Sharma K, Li C, et al. Cost-effectiveness of a patient navigation intervention to increase colonoscopy screening among low-income adults in New Hampshire. Cancer 2019;125:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winawer SJ, Fischer SE, Levin B. Evidence-Based, Reality-Driven Colorectal Cancer Screening Guidelines: The Critical Relationship of Adherence to Effectiveness. Jama 2016;315:2065–6. [DOI] [PubMed] [Google Scholar]


