Abstract
Background:
Although psychiatric comorbidities are common among individuals at end of life, their impact on outcomes is poorly understood.
Methods:
We conducted a systematic literature review of six databases following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and aimed at assessing the relationship between psychiatric comorbidities and outcomes in palliative and end-of-life care. Six databases were included in our search. This review is registered on PROSPERO (CRD42022335922).
Results:
Our search generated 7,472 unique records. 88 full texts were reviewed for eligibility and 43 studies were included in the review. Clinically, psychiatric comorbidity was associated with poor quality of life, increased physical symptom burden, and low function. The impact of psychiatric comorbidity on health utilization varied, though many studies suggested that psychiatric comorbidity increased utilization of palliative care services. Quality of evidence was limited by lack of consistent approach to confounding variables as well as heterogeneity of the included studies.
Conclusion:
Psychiatric comorbidity is associated with significant differences in care utilization and clinical outcome among patients at end of life. In particular, patients with psychiatric comorbidity and serious illness are at high risk of poor quality of life and high symptom burden. Our finding that psychiatric comorbidity is associated with increased utilization of palliative care likely reflects the complexity and clinical needs of patients with serious illness and mental health needs. These data suggest that greater integration of mental health and palliative care services may enhance quality-of-life among patients at end of life.
Keywords: Psychiatry, mental health, depression, anxiety, end of life, palliative care
Introduction
Mental health comorbidities are common among individuals with serious illness and include both de novo conditions and exacerbations of pre-existing comorbidities.1 The etiology of mental health comorbidities of serious illness is multifactorial and includes the psychosocial stressors and/or neurobiological impact of serious illness. While epidemiologic data vary, about 40% of individuals with life-limiting serious illness experience clinically significant mood and anxiety symptoms.2–10 These data are reflected in studies across diverse serious illness settings, including oncology clinics,9,10 palliative care,2,5 and hospice.7 While the most well-studied mental health comorbidities among individuals with serious illness are anxiety and depression, there is growing recognition of the burden of other mental health comorbidities. Serious mental illness (psychotic disorders, bipolar affective disorder, severe personality disorders),11,12 trauma-related disorders,13,14 and substance use disorders15–17 may all be prevalent and negatively impact patients with serious illnesses.
Palliative care is recognized as the standard of care for managing symptoms, improving quality of life, aligning care with patients‘ goals and values, and providing psychosocial, spiritual, and existential elements of serious illness care. The prevalence of mental health comorbidities among individuals with serious illness is reflected in palliative care guidelines. Palliative care considers the psychiatric and psychological aspects of serious illness care as one of its core domains.18 Depression and anxiety may predict or prompt referral to palliative care providers among adults with serious illness and are among the most frequent concerns raised by patients during encounters with palliative care clinicians.5,19,20 Furthermore, mental health outcomes are frequently included in studies of palliative care interventions.21 Despite this, formalized mental health services are rarely integrated into palliative care.22 Further, patients with depression and anxiety receiving palliative care may be underdiagnosed and undertreated.23–25 Many palliative care clinicians feel unprepared to manage psychiatric comorbidities; in one survey of clinicians, 93% reported difficulties managing anxiety and only 33% felt they received adequate training in this area.8,26–28 These gaps impact palliative care research as well; in a review of 59 palliative care intervention studies, 70% did not provide any details about the psychological care component and only 25% used formal psychiatric scales.29
There are robust data from a range of medical settings and populations that mental health comorbidities negatively impact medical care and outcomes.6,30 The impact of mental health comorbidity across domains such as symptom burden, care utilization, survival, and quality-of-life has been demonstrated among individuals with diabetes,31,32 cardiovascular disease,33,34 cancer,35–37 and multiple other populations. However, there is a dearth of such data synthesized in palliative care which has inherent heterogeneity because it is transdiagnostic, delivered across settings, and ideally continued over the trajectory of serious illness. Understanding the association between mental health and outcomes in palliative care is important for a couple ofreasons. First, it enables palliative care clinicians to better tailor care to individual patients‘ needs through risk stratification and collaboration with mental health clinicians. Second, it provides a foundation for psychosocial palliative care research aimed at improving serious illness outcomes through interventions focused on the psychiatric and psychological aspects of palliative care.
In this paper, we present the results of a systematic literature review characterizing the associations between mental health comorbidity and clinical as well as health services outcomes among patients receiving palliative and end-of-life care. To the authors‘ knowledge, this is the first review to assess for associations between mental health comorbidity and health outcomes that is inclusive of a trans-diagnostic, trans-setting population of patients with serious illness receiving palliative and/or end-of-life care. Our hypotheses were that mental health comorbidity would be associated with worse clinical outcomes, higher health care utilization at end of life, and decreased access to and utilization of palliative care services relative to control groups.
Methods:
The review protocol was prospectively registered in PROSPERO (CRD42022335922), an international repository of prospective review protocols aimed at improving scientific transparency and reducing reporting bias.38 Our review was conducted and is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.39
Search strategy
We performed comprehensive searches to identify studies that addressed associations between mental health comorbidities and healthcare utilization, as well as patient-reported clinical outcomes in palliative and end-of-life care settings. Searches were run on March 7, 2022 in the six following databases: Ovid MEDLINE (ALL - 1946 to Present); Ovid EMBASE (1974 to present); CINAHL (EBSCO); and The Cochrane Library (Wiley); AgeLine (EBSCO); and PyscINFO (EBSCO). The search strategy, which was developed by a medical librarian (MD) in collaboration with KS and DS, included all appropriate controlled vocabulary and keywords for the concepts of “palliative/end-of-life care” and “mental illness.” The full search strategies for all databases are available in Supplement 1. To limit selection bias, there were no language, publication date, or article type restrictions on the search strategy.
Eligibility:
We used the following eligibility criteria for included studies:
Study design:
We included cohort, case-control, and cross-sectional studies, as well as secondary analyses of data from other study types analyzed as one of the above study types (e.g., observational data collected from a control group in an RCT).
Population:
We included studies that enrolled adults (as defined by study locations; either age ≥ 16 or 18) receiving palliative and/or end-of-life (defined as the last six months of life based on common definitions in the literature40) care.
Exposure:
We included as our exposure either diagnosis of mental health comorbidity identified by chart, clinical diagnosis, or validated scale. We also included studies that treated mental health symptoms as a continuous variable by virtue of using numeric scales (e.g., PHQ-9 for depression).
Control:
Studies included in this review either compared individuals with mental health disorders against those without mental health disorders or sought associations between mental health symptom severity and a given outcome.
Outcomes:
We included studies with clinical palliative care outcomes including symptom burden and quality of life. Health services outcomes including degree of health care utilization at the end of life and place of death were also ascertained. High-intensity end-of-life care was defined based on previously established criteria41–44, and included service use indicators such as intrahospital chemotherapy in the last 14 days of life, artificial nutrition, gastrostomy, tracheal intubation, cardiopulmonary resuscitation, mechanical ventilation, blood transfusion, surgery, imaging, or endoscopy in the last 31 days of life, at least one emergency department (ED) or intensive care unit (ICU) admission in the last 31 days of life, hospitalization in acute care unit during the 31 days preceding death, length in days of the last hospital stay, and death in ICU or ED. Palliative care was defined as care focused on improving the quality of life, reducing pain and other symptom burden, and providing psychosocial support to patients and their families, with the recognition that it can be provided in a variety of contexts such as ambulatory clinics, home-based programs, inpatient consultation services as well as inpatient palliative care units and dedicated hospice facilities.45,46
Setting:
We did not restrict inclusion by study setting.
Publication status and language:
We included full-text articles available in English and published in peer reviewed journals since 2000. The year 2000 was selected as a cutoff in order to identify studies showing associations in outcomes in contemporary care settings. Prior to 2000, hospital-based palliative care programs were unusual47 and we were concerned that end-of-life and palliative care experiences might be too different from contemporary care to make meaningful inferences.
Studies were excluded if the study population did not include terminally ill individuals (e.g., advanced cancer patients with prognosis of less than 6 months of life), patients receiving specialized palliative or end-of-life care, or decedents. Studies were also excluded if the comparator was psychological distress rather than a specific symptom or disorder (e.g., anxiety or depression), if the mental health diagnosis was limited to neurocognitive disorders or delirium, or if the study population was exclusively focused on pediatric patients.
Study selection
Retrieved studies were screened for inclusion using Covidence,48 a web-based literature review platform. Titles and abstracts were reviewed against the protocolized inclusion/exclusion criteria by two independent reviewers. KS voted on each title and abstract, with the second vote coming from DH, ME, SM or TF. Conflicts were resolved by a consensus method including the whole study team and led by DS, the senior investigator and a physician trained in both psychiatry and palliative medicine.
All studies screened in for full-text review were evaluated by both the primary and senior investigators (KS and DS) and any discrepancies were resolved by consensus.
Data Extraction
Data extraction was conducted using a templated tool integrated into the Covidence platform (see supplement 2). Data from each article were extracted by two independent reviewers (KS, ME, DH, SM, and TF) and subsequently both data templates for each article were jointly reviewed by the study team to ensure consistency. Extracted data included: year(s) of data collection; study location; study design; data sources; sample inclusion and exclusion criteria, analytical sample size; sample characteristics; setting(s) of end-of-life care; psychiatric comorbidity studied; measures of psychiatric comorbidity; definitions of clinical or process of care outcomes; measures of outcomes used; main findings, including numerical values for correlation coefficients (r), Cohen‘s d (d), regression coefficients (β), adjusted or unadjusted odds ratios (aOR/OR), adjusted or unadjusted hazard ratios (aHR/HR) and statistical significance of reported results (p-value); and confounding variables accounted for in the analysis. With respect to identifying confounding variables, we included a priori confounders for our data extraction team including age, gender, race, ethnicity, disease status (e.g., stage), and comorbidity status. However, in recognition of the diverse study types we included and of the large number of potential confounders, we also gave study team members agency to identify and include other confounders identified in the studies. As all data were extracted by two study team members, we utilized consensus method about confounders. Study team members also used the study text to identify whether confounders were accounted for in statistical analytic strategies (which was treated as a binary yes/no) and this determination also underwent consensus discussion.
Critical appraisal
The quality of the evidence and the risk of bias was assessed for each retained study using The Joanna Briggs Institute Critical Appraisal tools for cross-sectional, case-controlled and cohort studies.49
Data Synthesis:
Each outcome of interest reported in included studies was integrated into the review. We distinguished those outcomes that achieved statistical significance (P<0.05) from those that did not in the narrative component of the review results. Those outcomes that could be aggregated based on overlap in construct and in statistical tool used for analysis (e.g., correlation scores between depression symptom severity and pain) are represented as a range.
We reported outcomes from across studies in two primary categories: clinical outcomes and health services outcomes. Within those categories, we reported outcomes in subcategories. For clinical outcomes, we reported quality-of-life and functional status (these were grouped together because functional status was a component of commonly used quality-of-life scales), symptom burden, and survival. For health services outcomes, we reported palliative care utilization (in which we included hospice and specialist-level palliative care across settings) and high-intensity care at the end of life (in which we included chemotherapy, acute & intensive care utilization, emergency department utilization, and procedures).
Results
Search results
The literature search generated 7,472 references after de-deduplication. Following title and abstract screening, 88 full texts were reviewed for eligibility and 43 studies were included in the final sample (see Figure 1). At the full text review stage, substantial inter-rated reliability was achieved (Cohen‘s kappa = 0.73 for full text review).
Figure 1:
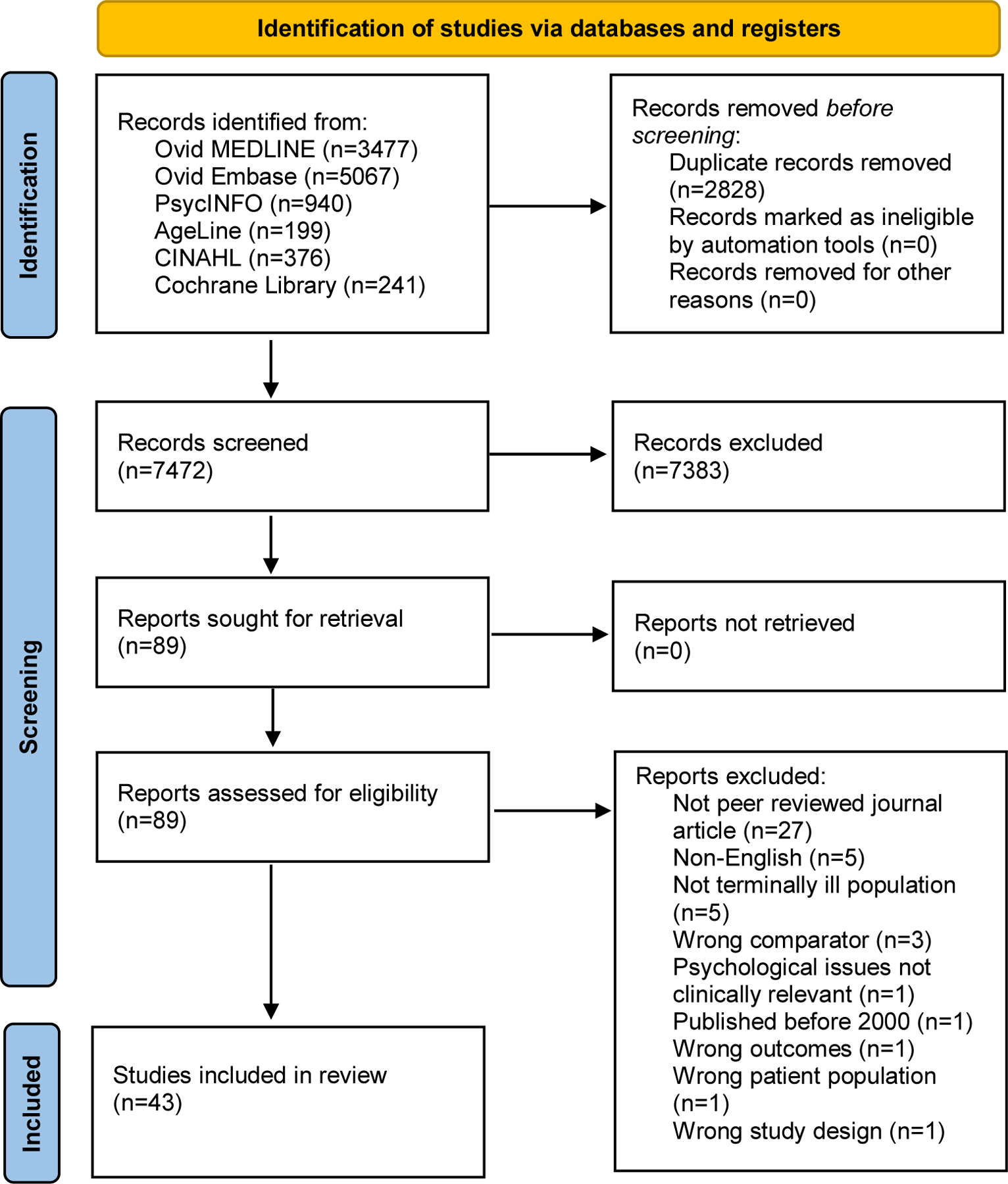
PRISMA Diagram.
Study characteristics
The 43 included studies were published between 2003 and 2021 and included 20 cohort studies, 15 cross-sectional studies, and eight secondary analyses of experimental data (see Table 1). Twenty-four studies included only clinical outcomes, 16 studies included only health services outcomes, and three studies reported both. In terms of geographic setting, 19 studies were conducted in Europe and 17 studies were conducted in North America, with the remaining studies coming from Australia, Brazil, India, Mexico, China and Taiwan. The size of the analytic samples varied greatly, ranging from 45 to 160,367 subjects. Fourteen of the included papers were retrospective cohort studies relying exclusively on decedent data,50–63 12 of which were described as nationwide or population-based and relied on large scale health databases.52,64,53–55,57–60,65,62,63
Table 1.
Basic characteristics of the included studies
| Study ID | Country | Total analytic sample | Study design | Psychiatric comorbidities studied | Measure of psychiatric comorbidity | Data on clinical outcomes | Data on healthcare utilization outcomes |
|---|---|---|---|---|---|---|---|
| Azevedo 2017 | Brazil | 115 | Cross-sectional study | Depression | Validated psychometric scale | ✓ | |
| Bickel 2020 | US | 5,341 | Secondary data analysis | PTSD | Chart diagnosis | ✓ | |
| Bryniarski 2021 | Poland | 134 | Cross-sectional study | Anxiety | Clinical diagnosis | ✓ | ✓ |
| Buzgova 2014 | Czech Republic | 93 | Cross-sectional study | Depression; anxiety | Validated psychometric scale | ✓ | |
| Buzgova 2015 | Czech Republic | 225 | Cross-sectional study | Depression; anxiety | Validated psychometric scale | ✓ | |
| Chan 2014 | China | 312 | Secondary data analysis | Depression | Validated psychometric scale |
✓ | |
| Chochinov 2012 | Canada | 15,770 | Cohort study | Schizophrenia | Chart diagnosis | ✓ | |
| Delgado-Guay 2009 | US | 216 | Secondary data analysis | Depression; anxiety | Validated psychometric scale | ✓ | |
| Fond 2019 | France | 12,373 | Cohort study | Schizophrenia | Chart diagnosis | ✓ | ✓ |
| Fond 2020 – 1 | France | 224,492 | Cohort study | Bipolar disorder | Chart diagnosis | ✓ | |
| Fond 2020 – 2 | France | 20,320 | Cohort study | Depression | Chart diagnosis | ✓ | |
| Fond 2021 | France | 38,612 | Cohort study | Depression, schizophrenia OR bipolar disorder | Chart diagnosis | ✓ | |
| Fujisawa 2015 | US | 125 | Secondary data analysis | Depression; anxiety | Validated psychometric scale | ✓ | |
| Ganzini 2010 | US | 256 | Cross-sectional study | Schizophrenia | Chart diagnosis | ✓ | |
| Grotmol 2017 | 8 EU countries | 563 | Cross-sectional study | Depression | Validated psychometric scale | ✓ | |
| Grotmol 2019 | 8 EU countries | 935 | Cross-sectional study | Depression | Validated psychometric scale | ✓ | |
| Henoch 2007 | Sweden | 105 | Cross-sectional study | Depression; anxiety | Validated psychometric scale | ✓ | |
| Hermann 2011 | US | 80 | Cohort study | Depression; anxiety | Validated psychometric scale | ✓ | |
| Huang 2018 | Taiwan | 9,555 | Cohort study | Schizophrenia | Chart diagnosis | ✓ | |
| Huang 2019 | US | 660 | Secondary data analysis | Depression | Validated psychometric scale | ✓ | |
| Irwin 2008 | US | 2,716 | Cross-sectional study | Anxiety | Chart diagnosis | ✓ | |
| Janssens 2019 | Belgium | 125 | Cross-sectional study | Depression; anxiety | Validated psychometric scale | ✓ | |
| Kashyap 2021 | US | 160,367 | Cohort study | Depression; anxiety; schizophrenia; bipolar disorder | Chart diagnosis | ✓ | |
| Lavin 2017 | US | 16,214 | Cohort study | Depression, schizophrenia OR anxiety | Chart diagnosis | ✓ | |
| Liu 2017 | China | 196 | Cohort study | Depression | Validated psychometric scale | ✓ | |
| Lloyd-Williams 2009 | UK | 87 | Cohort study | Depression | Validated psychometric scale | ✓ | |
| Lloyd-Williams 2014 | UK | 629 | Cohort study | Depression | Validated psychometric scale | ✓ | |
| Martens 2013 | Canada | 15,770 | Cohort study | Schizophrenia | Chart diagnosis | ✓ | |
| Masel 2016 | Austria | 68 | Cross-sectional study | Depression; anxiety | Validated psychometric scale | ✓ | |
| McDermott 2018 | US | 13,827 | Cohort study | Depression | Chart diagnosis | ✓ | |
| McMillan 2009 | US | 275 | Secondary data analysis | Depression | Validated psychometric scale | ✓ | |
| Mercadante 2019 | Italy | 314 | Secondary data analysis | Depression; anxiety | Validated psychometric scale | ✓ | |
| Meyer 2003 | UK | 45 | Cohort study | Depression | Validated psychometric scale | ✓ | |
| Mossman 2021 | US | 1,333 | Cohort study | Depression; anxiety | Chart diagnosis | ✓ | |
| Prescott 2017 | US | 529 | Secondary data analysis | Depression | Validated psychometric scale | ✓ | |
| Rodriguez-Mayoral 2020 | Mexico | 100 | Cohort study | Depression | Chart diagnosis | ✓ | |
| Smith 2003 | UK | 68 | Cross-sectional study | Depression; anxiety | Validated psychometric scale | ✓ | |
| Smitz 2006 | US | 109 | Cross-sectional study | Depression | Validated psychometric scale | ✓ | |
| Spencer 2010 | US | 635 | Cohort study | Anxiety | Chart diagnosis | ✓ | ✓ |
| Spilsbury 2018 | Australia | 63,508 | Cohort study | Schizophrenia | Chart diagnosis | ✓ | |
| Sudarisan 2019 | India | 234 | Cross-sectional study | Depression | Validated psychometric scale | ✓ | |
| Teunissen 2007 | The Netherlands | 79 | Cross-sectional study | Depression; anxiety | Validated psychometric scale | ✓ | |
| Viprey 2021 | France | 67,102 | Cohort study | Schizophrenia | Chart diagnosis | ✓ |
All of the samples included cancer patients. In 33 studies the whole sample consisted of advanced cancer patients,66–69,64,53,55,70,56,71–74,57,75–77,58,78–81,65,75,82,83,61,84–90,63 and in one study the whole sample consisted of cancer patients, but with heterogeneity in prognosis.54 Sixteen studies analyzed data exclusively from patients receiving palliative care or hospice care services.50,67,69,75,76,79–83,85–87,89,91,92
The most studied psychiatric comorbidities were depression in 31 reports and anxiety in 17 investigations. Twelve studies included both anxiety and depression but conducted subgroup analyses for each separately. Seven studies looked at schizophrenia. One study looked at post-traumatic stress disorder and one study looked at bipolar disorder in isolation. Three studies looked at more broadly defined categories of psychopathology – one study looked at “severe mental illness” defined as presence of either depression, schizophrenia or bipolar disorder,55 one study looked at “psychiatric illness” defined as presence of either depression, anxiety or schizophrenia59 and one study focused on “mental illness” defined as presence of depression, anxiety, schizophrenia, or bipolar disorder, but featured subgroup analyses for each of these distinct mental disorders.58
Included studies employed a variety of approaches to either assess or define psychiatric comorbidity. Eighteen studies relied on chart diagnoses and/or diagnostic codes (e.g., International Classification of Diseases codes). Studies that utilized ICD codes used either ICD-9 or -10 codes with older studies relying on ICD-9 codes and newer studies utilizing ICD-10 codes. Studies using data from a broader time perior (e.g., Chochinov‘s 2012 study utilizing records generated from 1995–200852) utilized both ICD-9 and -10 codes. Twenty-four studies utilized validated psychiatric symptom scales, with The Patient Health Questionnaire (PHQ),70–72,80,81 The Center for Epidemiologic Studies Depression Scale (CES-D),50,75,84,92 The Hospital Anxiety and Depression Scale,67–70,73,74,77,86,89,90 The Edmonton Symptom Assessment Scale,82,87 and The Edinburgh Depression Scale (EDS)79,80 being the most commonly used scales. One study relied on neuropsychiatric symptom assessment which was obtained through structured medical interviews performed at hospital admission.91
EOL clinical outcomes
Quality of life and functional status
Quality of life and functional status were assessed using The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire,50,67,68,71,73–75,77,79,89 which includes the Functional Scale, Symptom Scale and Global Health Status; The Karnofsky Performance Status Scale,81,85,88 The Hospice Quality of Life Index,74,75 The World Health Organization Quality of Life scale,89 Assessment of Quality of Life at the End of Life scale,73 and postmortem assessment completed by patients‘ primary caregivers.88
Thirteen studies found statistically significant inverse correlations between depressive symptoms and quality of life or functional status, including global quality of life, global health status, Karnofsky performance status, role, emotional, cognitive, social, and physical functioning. Seven studies found statistically significant inverse correlations between symptoms of anxiety and quality of life or functional status. Detailed findings are described in Table 2.
Table 2.
Summary of findings for quality of life and functional status measures
| Depression | Anxiety | |
|---|---|---|
| Global quality of life | r=−[0.29–0.68] 50,67,68,71,73–75,77,79,89 | r=−[0.23–0.49] 67,68,74,77,88 |
| Role functioning | r=−0.40; r=−0.54 67,68 No association 86 |
r=−0.31 67,68 No association 86 |
| Emotional functioning | r=−[0.35–0.59] 67,68,86 | r=−[0.31–0.79] 67,68,86 |
| Cognitive functioning | r=−[0.34–0.43] 67,68,86 | r=−[0.28–0.42] 67,68,86 |
| Social functioning | r=−0.31; r=−0.43 67,68 No association 86 |
r=−[0.34–0.38] 67,68,86 |
| Physical functioning | r=−[0.45–0.49]67,68 No association 86 |
r=−[0.30–0.36] 67,68 No association 86 |
| Global health status | r=−0.38; β=−2.1186 | r=−0.37; β=−1.75 86 |
| Poor Karnofsky performance status |
OR=5.4 85 No association 81 |
Average score: 61.22 vs 68.97 88 No association 81 |
Symptom burden
Symptom burden was assessed by The Edmonton Symptom Assessment Scale,69,72,78,82,85,87,90 Memorial Symptom Assessment Scale92 and The Numerical Rating Scale for pain assessment.81,91 Two studies relied solely on the patients‘ verbal report. 79,93
Sixteen studies found statistically significant associations between depression and symptom burden, including overall symptom burden or distress, number of symptoms endorsed, worse overall well-being, pain, lower energy, drowsiness or fatigue, gastrointestinal symptoms, dyspnea, poor appetite, insomnia, and additional symptoms of dizziness, dry mouth, numbness or tingling and sweats. Eight studies found statistically significant associations between anxiety and symptom burden, including overall symptom burden, worse overall well-being, pain, lower energy, drowsiness or fatigue, gastrointestinal symptoms, dyspnea, poor appetite and insomnia. Detailed findings are described in Table 3.
Table 3.
Summary of findings for symptom burden measures
| Depression | Anxiety | |
|---|---|---|
| Overall symptom burden or distress | r=[0.49–0.68]; d=1.08 72,82,87,92 | d=1.35 82 |
| Number of symptoms endorsed | r=0.45 92 | no data available |
| Overall well-being | r=−[0.44–0.73] 69,77,82,85,87 | r=−[0.21–0.44] 69,77,82 |
| Intensity of specific physical symptoms: | ||
| Pain | r=[0.19–0.51] 67,69,77,78,82,85,87,92,93 No association79,90,92 |
r=[0.41–0.46] 67,69,77,82,91 No association87,90 |
| Lower energy, drowsiness or fatigue | r=[0.25–0.49] 67,69,77–79,82,85–87,90,92 No association78,81,82,90,92 |
r=[0.25–0.48] 67,69,82,86,90 No association90 |
| Gastrointestinal symptoms | r=[0.29–0.32] 67–69,77–79,85,91 No association86,87,97 |
r=[0.35–0.36] 67–69,77,86 No association90 |
| Dyspnea | r=[0.24–0.44] 67,69,77,78,85,92 No association79,87,90,92 |
r=0.21 69,77,82 No association90 |
| Poor appetite | r=[0.35–0.46] 67,69,77,82,84,85 No association87 |
r=0.24 67,82 |
| Insomnia | r=0.20 77,85,90 No association82,97 |
r=0.38 77,82,90 |
| Other symptoms | r=[0.21–0.34] 92 | no data available |
Survival
No studies examined the relationship between anxiety and survival, but four studies found an association between depression and shorter survival among patients with advanced cancer.79,80,84,85 One study from the United Kingdom found that a one-point increase in the EDS score at baseline increased risk of death over the 12-month follow-up period by 7%.79 In another study conducted by the same research team, patients with a PHQ < 9 at baseline survived 3 weeks longer than those with a PHQ > 9 (indicating moderate or severe depression), and the adjusted Cox proportional hazards regression model estimated risk of death to be 1.38 times higher among patients with PHQ > 9.80 A secondary analysis of 2 US-based randomized controlled trials of early palliative care interventions indicated that higher baseline CES-D scores were significantly associated with greater mortality risk (HR= 1.042) 84. Finally, major depressive disorder was associated with lower probability of 1-year survival in Latin American palliative care patients.85
Healthcare utilization at the EOL
Palliative care
Associations between palliative care and mental health comorbidities are described in Table 4.
Table 4.
Summary of findings for palliative care use outcomes. “Severe mental illness” is defined by the authors of the cited paper as the presence of either depression, schizophrenia or bipolar disorder. NS: No significant difference.
| Depression | Anxiety | Schizophrenia | Bipolar Disorder | Severe mental illness | |
|---|---|---|---|---|---|
| Odds of palliative care (PC) use | Any PC use in last 6 months of life: OR=1.3461
-In last month: aOR=1.8253 -In last 3 days: aOR=2.2353 Hospice enrollment: SHR=1.1965 |
PC use in the last 6 months of life: OR = 1.9561 Emergency PC admission: OR=5.12991 Death at hospice: NS88 |
Any PC in the 2 years prior to death: 6.57% schizophrenia vs 17.40% control 52 Admissions to PC units: -In the last month of life: aOR=1.27, 1.6163,64 -In the last 3 days of life: aOR=1.44, 2.5263,64 PC consultation in the last month of life: aOR=0.5957 Use of community-based specialist PC in the last month of life (limited to decedents with chronic illness): 11.9% schizophrenia vs. 24.7% control62 |
PC use in the last month of life: aOR=1.4954 -In the last 3 days of life: aOR=2.1454 |
PC use in the last month of life: aOR=1.3255 |
| Duration of palliative care (PC) use | Duration of PC follow-up: 28 days depression vs. 19 days control53 Duration of hospice care:75 days depression vs. 38.97 days control76 Duration of inpatient hospice care: 19 days depression vs. 9 days control76 Likelihood of 90+ days of hospice stay: aOR=1.2965 |
Duration of PC follow-up: OR=1.04491 (duration not provided) | Duration of PC follow-up: 9–36% longer with schizophrenia63,64 Duration of hospice care: 107 schizophrenia vs 63 days control56 |
Duration of PC follow: 29 days bipolar vs. 19 days control54 | Duration of PC follow-up: 45% longer55 |
Four studies investigated the relationship between depression and palliative care use, and all found an association between depression and increased palliative care utilization, with the difference between patients with depression and healthy controls becoming more pronounced closer to death.53,59,65,76 Patients with depression had greater odds of hospice enrollment and greater odds of using palliative care services, as well as longer period of hospice care and longer palliative care follow-up before death.65,76
Three studies investigated the relationship between anxiety and palliative care use.61,88,91 Anxiety was associated with a higher likelihood of having a palliative care encounter in the last six months of life and with longer duration of palliative care treatment, but was not associated with an increased odds of dying under hospice care.61,88 A study from Poland found that among patients receiving palliative care, those with anxiety at the time of a hospital admission were five times more likely to have emergency admission to a palliative care unit.91
Six studies investigated the relationship between schizophrenia and palliative care use.52,56,57,62–64 Overall, a diagnosis of schizophrenia was associated with increased utilization of palliative care services. Patients with schizophrenia were more likely to access palliative care in the last two years of life, more likely to be admitted to a palliative care unit in the last month of life, and in the last three days of life, had longer average hospice stays and longer periods of palliative care follow-ups before death. However, a study from Taiwan showed schizophrenia diagnosis was not associated with any significant differences in the use of palliative care, hospice ward care, or hospice home care in the last month of life, but was associated with lower odds of using palliative care consultation services in the last month of life.57
Only one study investigated the relationship between bipolar disorder and palliative care use.54 Patients with bipolar disorder were more likely to access palliative care services at the end of life and had longer palliative care follow-up before death than controls without bipolar disorder.
Similarly, a study looking at severe mental illness defined as the presence of either depression, schizophrenia or bipolar disorder found that among women who died from breast cancer, women with severe mental illness were more likely to access palliative care in the last month of life and had longer palliative care follow-up period than controls without severe mental illness.55
High-intensity care
Associations between high-intensity end-of-life care and mental health comorbidities are described in Table 5.
Table 5.
Summary of findings for high intensity care utilization outcomes
| Depression | Anxiety | Schizophrenia | Bipolar Disorder | PTSD | Aggregated psychiatric illness | ||
|---|---|---|---|---|---|---|---|
| Blood transfusion | ↑ | ||||||
| ⇔ | no difference63 | no difference54 | no difference55 | ||||
| ↓ | aOR =0.8253 | aOR=0.7264 | |||||
| Surgery | ↑ | ||||||
| ⇔ | |||||||
| ↓ | aOR=0.8253 | aOR=0.7164;aOR=0.7363 | aOR=0.8654 | aOR=0.8355 | |||
| Imagining | ↑ | ||||||
| ⇔ | |||||||
| ↓ | aOR=0.6953 | OR=[0.37–0.80]57,63,64 | aOR=0.7754 | aOR=0.8855 | |||
| Artificial nutrition | ↑ | aOR=1.3753 | OR = 1.4157 | aOR=1.3154 | |||
| ⇔ | no difference88 | no difference64 | no difference51 | ||||
| ↓ | aOR = 0.6263 | ||||||
| Mechanical ventilation | ↑ | OR = 1.1557 | |||||
| ⇔ | no difference53 | no difference88 | no difference63,64 | no difference54 | no difference55 | ||
| ↓ | |||||||
| Cardiopulmonary resuscitation | ↑ | OR = 1.3457 | |||||
| ⇔ | no difference53 | no difference88 | no difference64 | no difference54 | |||
| ↓ | |||||||
| Chemotherapy | ↑ | 70 | OR= 1.4261 | ||||
| ⇔ | no difference61,65,70 | no difference 70 | |||||
| ↓ | aOR=0.7053 | OR=[0.53–0.60]57,63,64; 56 | aOR=0.7854 | aOR=0.7055 | |||
| Odds or frequency of ED or ICU use | ↑ | aOR=1.2658;OR=1.4061 | HR=1.2062; OR = 1.2157 | aOR=1.1258 | RR=1.1051 | OR=1.6459; RR=1.3859 | |
| ⇔ | no difference53,58 | no difference 88 | no difference 58,63,64 | no difference 54 | |||
| ↓ | aOR=0.9053 | aOR=0.7865 | OR=[0.41–0.85]55,59 | ||||
| Odds or frequency of other acute or inpatient care | ↑ | OR = 1.8561 | aOR=1.4163 | RR=1.0951 | |||
| ⇔ | no difference 61 | ||||||
| ↓ | aOR=0.9053 | HR=0.3062; aRR=0.7352; aOR=0.7364; RR=0.7360; aOR=0.7465; aRR=0.7952 | aOR=0.7954 | OR=0.5959 | |||
| Length of acute or inpatient care, including ED or ICU | ↑ | β=1.2453 ; 76 | 22%63; 20%64 longer | 22% longer54 | |||
| ⇔ | no difference 57 | no difference51 | |||||
| ↓ | aRR=0.8052; RR=0.7960 | RR=0.8859 | |||||
| In-hospital death, including death in ED or ICU | ↑ | ||||||
| ⇔ | no difference 56 | no difference 54 | |||||
| ↓ | aOR=0.8153 | OR=[0.65–0.75]63–65; 60 | OR =0.6759 | ||||
Six studies investigated the relationship between depression and high intensity care.53,58,61,65,70,76 Patients with depression had lower odds of receiving blood transfusion, surgery and imaging, but higher odds of undergoing artificial nutrition in the last month of life.53 No statistically significant differences were found for undergoing mechanical ventilation or cardiopulmonary resuscitation in the last month of life.53 Data on administration of chemotherapy at the end of life were mixed.53,61,65,70 Patients with depression had slightly lower odds of repeated admissions to acute care units in the last month of life53 but once admitted, their average length of stay was longer.53,76 Patients with depression were less likely to die in the ICU or ED, 53 but no conclusion could be drawn on the likelihood of ED visits or ICU admissions in the last month of life.53,61
Four studies investigated the relationship between anxiety and high intensity care.58,61,70,88 As with depression, data on administration of chemotherapy at the end of life were mixed.61,70 No associations were found between anxiety and resuscitation, mechanical ventilation, or artificial nutrition.88 Patients with anxiety had greater odds of iterative ED visits58 as well as inpatient hospitalizations61 in the last month of life. No clear pattern has been identified for anxiety and ICU use.61,88
Nine studies investigated the relationship between schizophrenia and high intensity end-of-life care.52,56–58,60,62–65 Individuals with schizophrenia were less likely to initiate and undergo chemotherapy in the last month or 14 days of life,56,57,63,64 and less likely to undergo surgery63,64 or imaging57,63,64 in the last month of life. No clear pattern could be identified for mechanical ventilation,57,63,64 blood transfusion,63,64, cardiopulmonary resuscitation,57,64 and artificial nutrition63,64 in the last month of life. Data on the likelihood of receiving care in the ICU or ED context were mixed. 57,58,63–65 However, individuals with schizophrenia were less likely to receive other forms of inpatient care at the end of life,62–65 are hospitalized less frequently,52,60 and are ultimately less likely to die in ICU, ED or in the broader hospital setting. 60,63–65 The relationship between schizophrenia and length of inpatient stay remains unclear. 52,60,63
Two studies investigated the relationship between bipolar disorder and high-intensity end-of-life care.54,58 Patients with bipolar disorder had lower odds of undergoing surgery, imaging, and chemotherapy, but higher odds of receiving artificial nutrition in the last month of life.54 No statistically significant differences were found for mechanical ventilation, cardiopulmonary resuscitation, or blood transfusion in the last month of life.54 Although patients with bipolar disorder were less likely to be admitted to an acute care unit, they stayed longer if admitted.54 Patients with bipolar disorder were more likely to have recurrent ED visits in the last month of life,58 but no statistically significant differences were found for the odds of death in ED/ICU setting or the odds of ED and ICU admission in the last month of life.54
One study investigated the relationship between post-traumatic stress disorder (PTSD) and high-intensity end-of-life care.51 In a population of veterans receiving care in the Veterans Administration, PTSD diagnosis was associated with more ER visits and more hospital admissions in the last 12 months of life. No significant differences were found for the mean length of inpatient stay or odds of artificial nutrition at the point of death.
In a study aggregating patients with “preexisting psychiatric illness” defined as presence of either depression, anxiety or schizophrenia, psychiatric illness was associated with lower odds of acute care hospitalizations or ICU care in the last month of life. Patients with preexisting psychiatric illness spent fewer days in the ICU in the last month of life and were less likely to die in the hospital, but were more likely to have an ED visit in the last month of life.59 Another study looking at “severe mental illness” defined as the presence of either depression, schizophrenia or bipolar disorder found that among women who died from breast cancer, women with severe mental illness were less likely to receive chemotherapy in the last 14 days of life, less likely to undergo surgery and imaging or endoscopy studies in the last month of life, as well as less likely to have an ED visit or be admitted to ICU in the last month of life.55 No significant association was found between severe mental illness and mechanical ventilation or blood transfusion in the last 31 days of life.55
Quality of studies
The quality of studies was variable. Included studies met between 100% and less than 40% of the quality and bias criteria delineated by The Joanna Briggs Institute Critical Appraisal tools (see tables 6–8). Regardless of the type of data analysis used (i.e., cohort, cross-sectional, case-controlled), all studies used appropriate statistical analysis and measured outcomes in a valid and reliable way. Lack of appropriate identification of confounding factors and lack of strategies to deal with confounding factors were the most common gaps in study quality.
Table 6:
Quality assessment of cohort studies
| COHORT ANALYSIS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Citation | 1. Were the two groups similar and recruited from the same population? | 2. Were the exposures measured similarly to assign people to both exposed and unexposed groups? | 3. Was the exposure measured in a valid and reliable way? | 4. Were confounding factors identified? | 5. Were strategies to deal with confounding factors stated? | 6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | 7. Were the outcomes measured in a valid and reliable way? | 8. Was the follow up time reported and sufficient to be long enough for outcomes to occur? | 9. Was follow up complete, and if Not, were the reasons to loss to follow up described and explored? | 10. Were strategies to address incomplete follow up utilized? | 11. Was appropriate statistical analysis used? |
| Bickel 2020 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Unclear | Unclear | Yes |
| Chochinov 2012 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| Fond 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| Fond 2020 – 1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| Fond 2020 – 2 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| Fond 2021 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| Hermann 2011 | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Unclear | Yes |
| Huang 2018 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| Kashyap 2021 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| Lavin 2017 | Yes | Yes | No | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| Liu 2017 | Yes | Yes | Yes | No | No | Unclear | Yes | N/A | N/A | N/A | Yes |
| Lloyd-Williams 2009 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Lloyd-Williams 2014 | Yes | es | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Martens 2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| McDermott 2018 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| Mercadante 2019 | Yes | Yes | Yes | Yes | Yes | No | Yes | Unclear | Unclear | Unclear | Yes |
| Meyer 2003 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Unclear | Unclear | Yes |
| Mossman 2021 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | N/A | N/A | N/A | Yes |
| Prescott 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes |
| Rodriguez-Mayoral 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes |
| Spencer 2010 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Unclear | Unclear | Yes |
| Spilsbury 2018 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | Yes |
| Viprey 2021 | Yes | Yes | Yes | Yes | Yes | N/A | Yes | N/A | N/A | N/A | Yes |
Table 8:
Quality assessment of case-control studies
| CASE-CONTROL ANALYSIS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Citation | 1. Were the groups comparable other than the presence of disease in cases or the absence of disease in controls? | 2. Were cases and controls matched appropriately? | 3. Were the same criteria used for identification of cases and controls? | 4. Was exposure measured in a standard, valid and reliable way? | 5. Was exposure measured in the same way for cases and control s? | 6. Were confounding factors identified? | 7. Were strategies to deal with confounding factors stated? | 8. Were outcomes assessed in a standard, valid and reliable way for cases and control s? | 9. Was the exposure period of interest long enough to be meaningful? | 10. Was appropriate statistical analysis used? |
| Fujisawa 2015 | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
Discussion
This systematic review assesses the impact of mental health comorbidity on serious illness outcomes at both the clinical and health services levels, provides descriptive results about the study populations and methods used to assess mental health disorders, and provides an appraisal of the quality of the existing literature. Even in the setting of heterogeneity in study design, measurement methods for mental health comorbidity, and outcomes, our results demonstrate that mental health comorbidity negatively impacts a broad range of outcomes relevant to palliative care. Individuals with mental health comorbidity experience poorer quality of life, lower levels of function, and greater physical symptom burden. However, these associations are currently limited to depression and anxiety because of a lack of studies reporting on the association between other mental health symptoms and diagnoses (e.g., psychotic disorders, post-traumatic stress disorder, bipolar affective disorder) and clinical outcomes. There is greater diversity of mental health comorbidities in health services-oriented studies; we identified studies examining both common mental health comorbidities (anxiety, depression) and serious mental illness (chronic mental illnesses which have a persistent impact on function such as psychotic disorders).
Studies focused on clinical outcomes (such as symptom burden and quality of life) versus health services outcomes (such as health care utilization) generally differed in several aspects. Most studies examining clinical outcomes were cross-sectional studies that treated mental health comorbidity as a continuous symptom variable (e.g., severity of depressive symptoms), rather than a dichotomous diagnostic variable (e.g., presence or absence of a major depression diagnosis). In contrast, health services studies were predominantly large, population-based cohort studies that relied on national hospital databases or insurance claims data sets and used specific mental disorder diagnostic codes to define the cohorts.
Our review has several notable strengths. To our knowledge, it is the first systematic review to evaluate associations between transdiagnostic mental health comorbidity and a range of serious illness outcomes at both the clinical and health services level. We were able to identify studies of patients receiving palliative or end-of-life care across a range of countries and clinical settings and were expansive in our outcome measures. Even in the setting of this breadth, we maintained a high degree of methodologic rigor including using prospective protocol registration and following PRISMA guidelines.
Weaknesses of our review include the exclusion of grey literature, conference abstracts, and non-English literature, introducing publication and selection bias. Because of heterogeneity in study design, mental health comorbidity measures, and outcome measures, we were unable to conduct aggregate analysis of the data. Included studies were also variable in quality, particularly with respect to their approach to confounding variables. Of note, few studies controlled for the potential confounder of multiple mental health comorbidities and studies did not generally differentiate between pre-existing mental health comorbidity versus new onset comorbidity in the setting of serious illness. Furthermore, our inclusion of data from multiple countries was complicated by differences in care models and nomenclature across countries and by the overrepresentation of high-income countries and by overrepresentation of cancer patients, which is a common challenge in palliative care research because of its longstanding close connection to oncology. Finally, identifying associations between psychiatric symptoms or disorders and other outcomes is contingent on being able to effectively measure mental health status. Accurate psychiatric diagnosis can be challenging among individuals with life-limiting medical illnesses. While many common screenings such as the PHQ-9 and the Hospital Anxiety and Depression Scale have been validated among patients with serious illness, other screening tools may underperform relative to other forms of assessment.3,94,95 Furthermore, clinical psychiatric diagnosis may be particularly challenging among patients with serious illness and may be confounded by overlapping symptoms (e.g., anergia, anorexia, and insomnia in depression).28 These complexities may influence the outcomes of included studies and present an additional potential confounder.
Our a priori hypothesis was that mental health comorbidity would be associated with poorer clinical outcomes and increased use of low-value, high-intensity care at the end of life. Interestingly, studies demonstrated that mental health diagnoses were associated with increased utilization of palliative care services. This was contradictory to our hypothesis that mental health diagnosis would confer decreased access to or utilization of palliative care services. One possible reason for this finding is that individuals with serious illness and mental health comorbidity may be perceived as more complex by medical subspecialists and may also experience more significant symptoms and poorer quality of life, all of which may drive palliative care referral. This reformulated hypothesis is consistent with data demonstrating that mental health comorbidity may predict palliative care referral,19,61 that mental health concerns are among the most frequently reported concerns by patients to palliative care clinicians,5 and that palliative care itself operationally identifies psychiatric and psychological care in the context of serious illness as one of its core domains.18
Our a priori hypotheses also did not bear out with respect to high intensity end-of-life care. Our data did not show an association between psychiatric comorbidity and high-intensity end-of-life care. Additional study may help determine whether decreased use of aggressive and/or disease-focused end-of-life care are related to stigma against individuals with mental illness (particularly serious mental illness),96 differential care choices among patients living with mental illness, or another cause.
Our findings have implications for both research and clinical practice. There is a pressing need for more sophisticated epidemiologic research and for intervention research focused on mental health comorbidity among individuals with serious medical illness. As noted, studies included in our analytic sample were heavily skewed towards depression and anxiety and varied widely in their management of confounders and their methods of diagnosis or screening. There is a particular need for further research on the prevalence and impact of potentially significant but understudied mental health comorbidities in patients with serious medical illness including trauma-related disorders, panic disorder, sleep disorders, and eating disorders. There is also a need for greater consensus about valid psychiatric diagnosis in patients with serious medical illness, including systematized research and clinical methods for differentiating normative and pathological states. Few studies in our analytic sample were able to control for the possibility of multiple mental health comorbidities or for the diagnostic blurring that may occur when a patient with a related disorder screens positive for another disorder (e.g, a patient with PTSD may screen positive on a PHQ-9 due to related symptoms such as affective disturbance and insomnia). As such, while many studies accounted for common general confounders such as age, gender, and medical status, further psychiatric palliative care research will depend on more extensive and targeted assessment, a broader range of included disorders, and management of unique psychiatric-palliative care confounders such as de novo versus pre-existing illness, prior mental healthcare exposure, and attitudes/stigma about mental health. Furthermore, there are only very limited data on the causality between mental health comorbidity and the associated outcomes included in this review. Research focused on downstream effects of mental health interventions on symptom burden, quality of life, functional status, and health care utilization patterns may help establish causality and guide further intervention development. Epidemiologic research must be linked to a greater investment in intervention studies in the psychological and psychiatric aspects of palliative care. Such study should include both development and assessment of interventions themselves, but also a focus on understanding what approaches may minimize stigma and increase acceptability of mental health interventions for patients with serious illness more broadly. Currently, most existing mental health intervention research—both pharmacologic and behavioral—has not been conducted in individuals with serious medical illness. In addition, mental health components of palliative care interventions are poorly described and measured.29
Clinically, our findings reinforce that mental health comorbidity is impactful in serious illness. Even without a fully realized understanding of causality between symptom clusters, mental health comorbidity—in addition to conferring its own suffering—is part of a constellation of poor function, low quality of life, and high symptoms. Importantly, patients with serious illness and mental health comorbidity access palliative care at higher rates than controls. Palliative care clinicians are well positioned to become stewards of mental health for individuals with serious mental illness—a role reinforced by the inclusion of a domain on psychological and psychiatric aspects of care in Clinical Practice Guidelines for Quality Palliative Care.18 However, there are a number of existing barriers to addressing mental health comorbidity effectively. Diagnosis and management of psychiatric comorbidities in the palliative care setting is extremely complex.28 Clinicians may face uncertainty about the applicability of existing screening tools to patients with serious illness and there may be equipoise between normative and pathological reactions. Furthermore, patients who accept medical interventions such as pain management may reject treatments perceived to be psychological in nature. More robust evidence around the diagnosis and management of psychiatric comorbidities may help clinicians feel empowered to make accurate diagnoses and effective interventions. Furthermore, buttressing the expertise of palliative care social workers with doctoral-level mental health clinicians who have specialized training in serious illness may help expand both the clinical workforce and the evidence base for psychiatric and psychological treatment of palliative care patients.
In summary, we conducted a systematic review of associations between mental health comorbidity and clinical and health services outcomes in palliative and end-of-life care. As we expected, mental health comorbidity was consistently associated with a range of negative clinical outcomes, including increased pain and symptom burden, decreased quality of life, and loss of function. Although mental health comorbidity was associated with differential health care utilization, some of the patterns, including increased utilization of palliative care services, were surprising to us. Our study indicates the need for further research on mental health comorbidity in palliative and end-of-life care given the links between mental health and many of the core aims of palliative care. Furthermore, many of the studies included in the review were situated in high-income countries, oncologic settings, and specifically focused on depression and anxiety suggesting the need for more globally oriented research inclusive of non-oncologic palliative care patients and of a wider range of mental health comorbidities.
Supplementary Material
Table 7:
Quality assessment of cross-sectional studies
| CROSS-SECTIONAL ANALYSIS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Citation | 1. Were the criteria for inclusion in the sample clearly defined? | 2. Were the study subjects and the setting described in detail? | 3. Was the exposure measured in a valid and reliable way? | 4. Were objective, standard criteria used for measurement of the condition? | 5. Were confounding factors identified? | 6. Were strategies to deal with confounding factors stated? | 7. Were the outcomes measured in a valid and reliable way? | 8. Was appropriate statistical analysis used? |
| Azevedo 2017 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Bryniarski 2021 | No | No | No | No | No | Yes | Yes | Yes |
| Buzgova 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Buzgova 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Chan 2014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Delgado-Guay 2009 | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Ganzini 2010 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Grotmol 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Grotmol 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| HeNoch 2007 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Huang 2019 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Irwin 2008 | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Janssens 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Masel 2016 | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| McMillan 2009 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Smith 2003 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Smitz 2006 | Yes | Yes | No | Yes | No | No | Yes | Yes |
| Sudarisan 2019 | No | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Teunissen 2007 | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
Key Message:
Palliative care clinicians should be aware that common psychiatric symptoms and/or disorders are impactful across a range of serious illness and end-of-life outcomes.
Funding:
This work was supported by the Weill Cornell Geriatrics and Palliative Medicine Summer Scholars (GPS) in Aging Research Program and by the National Institute on Aging [grant number T32AG049666].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None of the authors have any conflicts of interest.
References:
- 1.Cheung S et al. A Model to Improve Behavioral Health Integration into Serious Illness Care. J. Pain Symptom Manage 58, 503–514.e1 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Austin P, Wiley S, McEvoy PM & Archer L Depression and anxiety in palliative care inpatients compared with those receiving palliative care at home. Palliat. Support. Care 9, 393–400 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Rayner L, Price A, Hotopf M & Higginson IJ Expert opinion on detecting and treating depression in palliative care: A Delphi study. BMC Palliat. Care 10, 10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sewtz C et al. Longitudinal observation of anxiety and depression among palliative care cancer patients. Ann. Palliat. Med 10, 3836–3846 (2021). [DOI] [PubMed] [Google Scholar]
- 5.van Oorschot B et al. Anxiety, depression and psychosocial needs are the most frequent concerns reported by patients: preliminary results of a comparative explorative analysis of two hospital-based palliative care teams in Germany and Japan. J. Neural Transm. Vienna Austria 1996 127, 1481–1489 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell AJ, Vahabzadeh A & Magruder K Screening for distress and depression in cancer settings: 10 lessons from 40 years of primary-care research. Psychooncology 20, 572–584 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Kozlov E et al. Prevalence, Severity, and Correlates of Symptoms of Anxiety and Depression at the Very End of Life. J. Pain Symptom Manage 58, 80–85 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkin N, Vickerstaff V & Candy B ‗Worried to death‘: the assessment and management of anxiety in patients with advanced life-limiting disease, a national survey of palliative medicine physicians. BMC Palliat. Care 16, 69 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obispo-Portero B et al. Anxiety and depression in patients with advanced cancer during the COVID-19 pandemic. Support. Care Cancer 30, 3363–3370 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naser AY et al. Depression and Anxiety in Patients With Cancer: A Cross-Sectional Study. Front. Psychol 12, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shalev D, Fields L & Shapiro PA End-of-Life Care in Individuals With Serious Mental Illness. Psychosomatics 61, 428–435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards D et al. End of life care for people with severe mental illness: Mixed methods systematic review and thematic synthesis (the MENLOC study). Palliat. Med 2692163211037480 (2021) doi: 10.1177/02692163211037480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glick DM, Cook JM, Moye J & Kaiser AP Assessment and Treatment Considerations for Post Traumatic Stress Disorder at End of Life. Am. J. Hosp. Palliat. Care 35, 1133–1139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bickel KE, Kennedy R, Levy C, Burgio KL & Bailey FA The Relationship of Post-traumatic Stress Disorder to End-of-life Care Received by Dying Veterans: a Secondary Data Analysis. J. Gen. Intern. Med 35, 505–513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moryl N & Malhotra VT A Case for Palliative Care and Addiction Specialists Collaboration and Joint Research. JAMA Netw. Open 4, e2143436 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Ebenau A et al. Palliative care for people with substance use disorder and multiple problems: a qualitative study on experiences of patients and proxies. BMC Palliat. Care 18, 56 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magoon C & Shalev D Toward holistic care: Including substance use in mental health-palliative care integration. Palliat. Support. Care 20, 453–454 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrell BR, Twaddle ML, Melnick A & Meier DE National Consensus Project Clinical Practice Guidelines for Quality Palliative Care Guidelines, 4th Edition. J. Palliat. Med 21, 1684–1689 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Sasahara T et al. Assessment of reasons for referral and activities of hospital palliative care teams using a standard format: a multicenter 1000 case description. J. Pain Symptom Manage 47, 579–587.e6 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Mossman B et al. Anxiety, depression, and end-of-life care utilization in adults with metastatic cancer. Psychooncology 30, 1876–1883 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Bouça-Machado R et al. Clinical trials in palliative care: a systematic review of their methodological characteristics and of the quality of their reporting. BMC Palliat. Care 16, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pincus HA et al. Mental Health and Serious Illness Care (2018).
- 23.Kozlov E, Eghan C, Moran S, Herr K & Reid MC Palliative Care Providers‘ Practices Surrounding Psychological Distress Screening and Treatment: A National Survey. Am. J. Hosp. Palliat. Care 35, 938–944 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed J Depressive symptoms in the last year of life: early screening and varied treatment pathways needed. Evid. Based Nurs 24, 68–68 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Hayes JE, Hart B & Phillips J Specialist palliative care nurses‘ management of the needs of patients with depression. Int. J. Palliat. Nurs 23, 298–305 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Shalev D et al. Bridging the Behavioral Health Gap in Serious Illness Care: Challenges and Strategies for Workforce Development. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 28, 448–462 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Patterson KR, Croom AR, Teverovsky EG & Arnold R Current state of psychiatric involvement on palliative care consult services: results of a national survey. J. Pain Symptom Manage 47, 1019–1027 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Lee W et al. Caring for depression in the dying is complex and challenging – survey of palliative physicians. BMC Palliat. Care 21, 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozlov E, Niknejad B & Reid MC Palliative Care Gaps in Providing Psychological Treatment: A Review of the Current State of Research in Multidisciplinary Palliative Care. Am. J. Hosp. Palliat. Med 35, 505–510 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mental Disorders and Medical Comorbidity. RWJF https://www.rwjf.org/en/library/research/2011/02/mental-disorders-and-medical-comorbidity.html (2011). [PubMed]
- 31.Katon W et al. Depression and diabetes: a potentially lethal combination. J. Gen. Intern. Med 23, 1571–1575 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams LH et al. Depression and incident lower limb amputations in veterans with diabetes. J. Diabetes Complications 25, 175–182 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albert NM et al. Depression and clinical outcomes in heart failure: an OPTIMIZE-HF analysis. Am. J. Med 122, 366–373 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Celano CM, Villegas AC, Albanese AM, Gaggin HK & Huffman JC Depression and Anxiety in Heart Failure: a Review. Harv. Rev. Psychiatry 26, 175–184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown LF, Kroenke K, Theobald DE, Wu J & Tu W The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology 19, 734–741 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham R, Sarfati D, Stanley J, Peterson D & Collings S Cancer survival in the context of mental illness: a national cohort study. Gen. Hosp. Psychiatry 37, 501–506 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Bamonti PM, Moye J & Naik AD Pain is associated with continuing depression in cancer survivors. Psychol. Health Med 1–14 (2018) doi: 10.1080/13548506.2018.1476723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page MJ, Shamseer L & Tricco AC Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst. Rev 7, 32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Page MJ et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ n71 (2021) doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hui D et al. Concepts and Definitions for “Actively Dying,” “End of Life,” “Terminally Ill,” “Terminal Care,” and “Transition of Care”: A Systematic Review. J. Pain Symptom Manage 47, 77–89 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Earle CC et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int. J. Qual. Health Care J. Int. Soc. Qual. Health Care 17, 505–509 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Earle CC et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 21, 1133–1138 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Earle CC et al. Trends in the aggressiveness of cancer care near the end of life. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 22, 315–321 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Earle CC et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 26, 3860–3866 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hui D & Bruera E Models of Palliative Care Delivery for Patients With Cancer. J. Clin. Oncol 38, 852–865 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiencek C & Coyne P Palliative care delivery models. Semin. Oncol. Nurs 30, 227–233 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Hughes MT & Smith TJ The growth of palliative care in the United States. Annu. Rev. Public Health 35, 459–475 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Covidence - Better systematic review management. Covidence https://www.covidence.org/.
- 49.Critical Appraisal Tools | JBI. https://jbi.global/critical-appraisal-tools.
- 50.Azevedo C, Pessalacia JDR, Mata L. R. F. da, Zoboli ELCP & Pereira M. da G. Interface between social support, quality of life and depression in users eligible for palliative care. Rev. Esc. Enferm. U P 51, e03245 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Bickel KE, Kennedy R, Levy C, Burgio KL & Bailey FA The Relationship of Post-traumatic Stress Disorder to End-of-life Care Received by Dying Veterans: a Secondary Data Analysis. J. Gen. Intern. Med 35, 505–513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chochinov HM, Martens PJ, Prior HJ & Kredentser MS Comparative health care use patterns of people with schizophrenia near the end of life: a population-based study in Manitoba, Canada. Schizophr. Res 141, 241–246 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Fond G et al. Recurrent major depressive disorder‘s impact on end-of-life care of cancer: A nationwide study. J. Affect. Disord 263, 326–335 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Fond G et al. End-of-Life Care Among Patients With Bipolar Disorder and Cancer: A Nationwide Cohort Study. Psychosom. Med 82, 722–732 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Fond G et al. End of life breast cancer care in women with severe mental illnesses. Sci. Rep 11, 10167 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganzini L, Socherman R, Duckart J & Shores M End-of-life care for veterans with schizophrenia and cancer. Psychiatr. Serv. Wash. DC 61, 725–728 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Huang H-K, Wang Y-W, Hsieh J-G & Hsieh C-J Disparity of end-of-life care in cancer patients with and without schizophrenia: A nationwide population-based cohort study. Schizophr. Res 195, 434–440 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Kashyap M, Harris JP, Chang DT & Pollom EL Impact of mental illness on end-of-life emergency department use in elderly patients with gastrointestinal malignancies. Cancer Med 10, 2035–2044 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavin K et al. Effect of Psychiatric Illness on Acute Care Utilization at End of Life From Serious Medical Illness. J. Pain Symptom Manage 54, 176–185.e1 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Martens PJ, Chochinov HM & Prior HJ Where and how people with schizophrenia die: a population-based, matched cohort study in Manitoba, Canada. J. Clin. Psychiatry 74, e551–557 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Mossman B et al. Anxiety, depression, and end-of-life care utilization in adults with metastatic cancer. Psychooncology 30, 1876–1883 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Spilsbury K, Rosenwax L, Brameld K, Kelly B & Arendts G Morbidity burden and community-based palliative care are associated with rates of hospital use by people with schizophrenia in the last year of life: A population-based matched cohort study. PloS One 13, e0208220 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viprey M et al. Palliative and high-intensity end-of-life care in schizophrenia patients with lung cancer: results from a French national population-based study. Eur. Arch. Psychiatry Clin. Neurosci 271, 1571–1578 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Fond G et al. End-of-life care among patients with schizophrenia and cancer: a population-based cohort study from the French national hospital database. Lancet Public Health 4, e583–e591 (2019). [DOI] [PubMed] [Google Scholar]
- 65.McDermott CL, Bansal A, Ramsey SD, Lyman GH & Sullivan SD Depression and Health Care Utilization at End of Life Among Older Adults With Advanced Non-Small-Cell Lung Cancer. J. Pain Symptom Manage 56, 699–708.e1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bryniarski P, Bryniarska M, Jezioro M, Andrysiak D & Filipczak-Bryniarska I Factors connected with anxiety and other neuropsychiatric symptoms in advanced gastric cancer. Acta Neuropsychiatr 34, 10–14 (2022). [DOI] [PubMed] [Google Scholar]
- 67.Bužgová R, Hajnová E, Sikorová L & Jarošová D Association between unmet needs and quality of life in hospitalised cancer patients no longer receiving anti-cancer treatment. Eur. J. Cancer Care (Engl.) 23, 685–694 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Bužgová R, Jarošová D & Hajnová E Assessing anxiety and depression with respect to the quality of life in cancer inpatients receiving palliative care. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc 19, 667–672 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Delgado-Guay M, Parsons HA, Li Z, Palmer JL & Bruera E Symptom distress in advanced cancer patients with anxiety and depression in the palliative care setting. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 17, 573–579 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Fujisawa D et al. Psychological factors at early stage of treatment as predictors of receiving chemotherapy at the end of life. Psychooncology 24, 1731–1737 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Grotmol KS et al. Depression-A Major Contributor to Poor Quality of Life in Patients With Advanced Cancer. J. Pain Symptom Manage 54, 889–897 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Grotmol KS et al. Patients with advanced cancer and depression report a significantly higher symptom burden than non-depressed patients. Palliat. Support. Care 17, 143–149 (2019). [DOI] [PubMed] [Google Scholar]
- 73.Henoch I, Bergman B, Gustafsson M, Gaston-Johansson F & Danielson E The impact of symptoms, coping capacity, and social support on quality of life experience over time in patients with lung cancer. J. Pain Symptom Manage 34, 370–379 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Hermann CP & Looney SW Determinants of quality of life in patients near the end of life: a longitudinal perspective. Oncol. Nurs. Forum 38, 23–31 (2011). [DOI] [PubMed] [Google Scholar]
- 75.Huang L-T & McMillan SC Mutual Effects of Depression on Quality of Life in Patients and Family Caregivers. Oncol. Nurs. Forum 46, 208–216 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Irwin SA et al. Psychiatric issues in palliative care: recognition of depression in patients enrolled in hospice care. J. Palliat. Med 11, 158–163 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Janssens A et al. Prognostic Understanding and Quality of Life in Patients With Advanced Lung Cancer: A Multicenter Study. Clin. Lung Cancer 20, e369–e375 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Liu Y et al. Symptom Frequencies and Intensities in Hospitalized Patients With Advanced Cancer Having Depressive Disorder. Am. J. Hosp. Palliat. Care 34, 456–460 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Lloyd-Williams M, Shiels C, Taylor F & Dennis M Depression--an independent predictor of early death in patients with advanced cancer. J. Affect. Disord 113, 127–132 (2009). [DOI] [PubMed] [Google Scholar]
- 80.Lloyd-Williams M, Payne S, Reeve J & Dona RK Thoughts of self-harm and depression as prognostic factors in palliative care patients. J. Affect. Disord 166, 324–329 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Masel EK et al. Psyche at the end of life: Psychiatric symptoms are prevalent in patients admitted to a palliative care unit. Palliat. Support. Care 14, 250–258 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Mercadante S, Adile C, Ferrera P, Cortegiani A & Casuccio A Symptom hyper-expression in advanced cancer patients with anxiety and depression admitted to an acute supportive/palliative care unit. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 27, 3081–3088 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Meyer HAM, Sinnott C & Seed PT Depressive symptoms in advanced cancer. Part 2. Depression over time; the role of the palliative care professional. Palliat. Med 17, 604–607 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Prescott AT et al. The role of a palliative care intervention in moderating the relationship between depression and survival among individuals with advanced cancer. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc 36, 1140–1146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodríguez-Mayoral O et al. Depressive disorder and clinical factors: Impact on survival in palliative care cancer patients. Gen. Hosp. Psychiatry 64, 133–135 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Smith EM, Gomm SA & Dickens CM Assessing the independent contribution to quality of life from anxiety and depression in patients with advanced cancer. Palliat. Med 17, 509–513 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Smitz LL & Woods AB Prevalence, Severity, and Correlates of Depressive Symptoms on Admission to Inpatient Hospice. J. Hosp. Palliat. Nurs 8, 86–91 (2006). [Google Scholar]
- 88.Spencer R, Nilsson M, Wright A, Pirl W & Prigerson H Anxiety disorders in advanced cancer patients: correlates and predictors of end-of-life outcomes. Cancer 116, 1810–1819 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sudarisan SSP, Abraham B & George C Prevalence, correlates of depression, and its impact on quality of life of cancer patients attending a palliative care setting in South India. Psychooncology 28, 1308–1313 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Teunissen SCCM, de Graeff A, Voest EE & de Haes JCJM Are anxiety and depressed mood related to physical symptom burden? A study in hospitalized advanced cancer patients. Palliat. Med 21, 341–346 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Bryniarski P, Bryniarska M, Jezioro M, Andrysiak D & Filipczak-Bryniarska I Factors connected with anxiety and other neuropsychiatric symptoms in advanced gastric cancer. Acta Neuropsychiatr 1–21 (2021) doi: 10.1017/neu.2021.27. [DOI] [PubMed] [Google Scholar]
- 92.McMillan SC & Rivera HRJ The Relationship Between Depressive Symptoms and Symptom Distress in Patients With Cancer Newly Admitted to Hospice Home Care. J. Hosp. Palliat. Nurs 11, 41–51 (2009). [Google Scholar]
- 93.Chan WCH, Kwan CW, Chi I & Chong AML The impact of loneliness on the relationship between depression and pain of Hong Kong Chinese terminally ill patients. J. Palliat. Med 17, 527–532 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Mitchell AJ, Meader N & Symonds P Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. J. Affect. Disord 126, 335–348 (2010). [DOI] [PubMed] [Google Scholar]
- 95.Chilcot J et al. The factor structure of the PHQ-9 in palliative care. J. Psychosom. Res 75, 60–64 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Irwin KE et al. Predictors of Disruptions in Breast Cancer Care for Individuals with Schizophrenia. The Oncologist 22, 1374–1382 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buzgova R, Hajnova E, Sikorova L & Jarosova D Association between unmet needs and quality of life in hospitalised cancer patients no longer receiving anti-cancer treatment. Eur. J. Cancer Care (Engl.) 23, 685–694 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


