Abstract
Objective:
To describe the prevalence of PSA screening amongst transgender women. A transgender individual is someone whose gender identity differs from their birth sex or the societal norms of that assigned sex. There are no formal guidelines regarding PSA screening in transgender women, even though they retain prostatic tissue throughout the gender-affirming process, and there is a lack of existing data to adequately inform clinical practice.
Methods:
We identified a cohort of transgender women in the IBM MarketScan dataset using ICD codes. The patient’s eligibility for inclusion was determined on an annual basis for the years 2013-2019. For each year, we required continuous enrollment, 3 months of post-transgender diagnosis follow-up, and aged 40-80 without a prior diagnosis of prostate malignancy. This cohort was compared to cisgender men with similar eligibility criteria. The proportions of individuals undergoing PSA screening were compared using log-binomial regression.
Results:
A group of 2957 transgender women met the inclusion criteria. We saw significantly lower PSA screening rates among transgender individuals for ages 40-54 and 55-69, but higher rates within the age group 70-80 (p < 0.001 for all).
Conclusion:
This is the first study evaluating PSA screening rates for insured transgender women. While the rates for screening in transgender women over the age of 70 are higher, the overall rate of screening for all other age groups lags below the general population in this dataset. Further investigation is necessary to provide equitable care for the transgender community.
Keywords: transgender persons, prostate specific antigen, cancer screening, gender equity, preventative medicine
Introduction
In the United States, there are over 1.4 million individuals who identify as transgender and the number is growing.1 This diverse population comprises people whose gender identity (how one views one’s gender) or gender expression (the conveyance of gender to others) differs from the sex assigned at birth.2 This incongruence may lead patients to seek gender-affirming care, such as hormone treatment and gender-affirming surgery. Although transgender individuals have unique health concerns, consideration of gender identity is not reflected within national standards of medical practice, largely due to the absence of data that pertains specifically to the transgender population.3,4 Because of this, the medical treatment of transgender individuals may fall away from traditional gender-based guidelines of care, thus significantly contributing to healthcare disparities. A prime example of gender-based preventative care is PSA screening. Current PSA screening guidelines recommend beginning PSA testing in men aged 55-69. However, screening is recommended for men age 40 who are at high risk for the development of prostate cancer, such as those with a strong family history. Additionally, screening is not recommended in men over age 70, although men in this age group who are in excellent health can benefit from screening.24
For this study, we focused on transgender women: people assigned male sex at birth but who identify as women. We utilized the IBM® MarketScan® Research Databases to examine a particularly important area of medical practice: preventative screening. The MarketScan database is one of the largest convenience samples of the United States population that has employer-provided health insurance.5 In this study, we performed a review of the prevalence of prostate cancer screening, and associated demographic data, in patients who identify as transgender women. We hypothesized that the rate of PSA screening in the transgender female population would be significantly lower than the rate amongst cisgender males.
Methods
Data were obtained from medical claims in the IBM® MarketScan® Commercial and Medicare Supplemental Databases for the years 2013-2019. We included patients at all stages of transition. Transgender women were identified using standard ICD-9 and ICD-10 diagnosis codes chosen after a literature review regarding effectively identifying transgender patients via Medicare claims and electronic health data6–8 (Table S1). Individuals were included on an annual basis if they were aged 40-80 years, were continuously enrolled for that year, and had a transgender diagnosis prior to October 1 of that year to allow for a three-month follow-up period after the diagnosis. Individuals were excluded for all years following a diagnosis of prostate cancer or a prostatectomy, and were excluded in the year of their cancer diagnosis or prostatectomy if it occurred prior to the first recorded PSA screening within that year (Table S2 and S3). We included the range of 40 years to 80 years in order to catch those patients that may be included as outliers in the screening age population for the current guidelines (those aged 40-50 years and >70 years who are healthy). PSA screening was identified via claims with CPT codes 84152 and 84153 or HCPCS code G0103.
We assessed gender hormones used from the outpatient prescription claims, and defined hormone usage as having a prescription of estrogen plus at least one progesterone, anti-androgen, or GnRH agonist. Patients who have undergone orchiectomy as part of their gender affirming surgical care may only be on estrogen therapy.25 However, there were very few patients meeting these criteria in the MarketScan dataset and thus we decided to maintain the aforementioned definition of hormone usage (Tables S14, S15). Relevant medications were isolated using NDC codes (Table S5). Gender reassignment surgery was identified via CPT codes (Table S6).
Demographic and clinical characteristics of the transgender cohort were summarized for all years using mean with standard deviation (SD), median interquartile range, or frequency with percentage as appropriate (Table S7). The rate of individuals receiving a PSA screening was calculated with 95% confidence intervals (CIs) using a binomial distribution separately for both the transgender and cisgender cohorts on a year-by-year basis, as well as the rate of those who received at least one PSA screening over any year in which they met inclusion criteria.
Log-binomial regression was used to estimate the rate ratio of PSA screening between transgender women versus cisgender men for each age group. This model included transgender status, year, age group, and the interaction between transgender status and age group. A second log-binomial regression was used to estimate the rate ratio of PSA screening between transgender women versus cisgender men for each year. An exploratory log-binomial regression was used to assess the association between PSA screening and the demographic and clinical characteristics. These characteristics included: year, age group, geographic region, urban versus rural residence classification, hormone use in that year, and gender-affirming surgery in that year or previous years. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC) and R version 3.4.3 (R Foundation for Statistical Computing, Vienne, Austria). Statistical significance was defined as p < 0.05, without accounting for multiple testing.
Results
There were 2,957 transgender women meeting inclusion criteria in at least one year, totaling 6,300 observations from non-distinct individuals over the period 2013-2019 (Figure S1). The mean age was 53.1 years (SD 8.3). Additionally, 14,278,025 cisgender men meeting identical inclusion criteria, except for the transgender diagnosis, were identified for a total of 31,830,617 observations from non-distinct individuals over the period 2013-2019. Patient demographics and clinical characteristics of the transgender cohort were shown in Supplementary Table S7, stratified by year of inclusion for all patients that met inclusion and exclusion criteria for that year.
The rates of PSA screening in transgender women and cisgender men were reported on a year-by-year basis in Table S8 and plotted in Figure 1. Additionally, the overall proportions of individuals who received at least one PSA screening during the year(s) in which they were included were reported in Table 1. Overall, the proportion of transgender women who received at least one PSA screening during the year(s) in which they were included was 32.6% (95% CI: 31.0% to 34.4%), whereas the proportion was 41.8% (95% CI: 41.76% to 41.81%) in cisgender men.
Figure 1.
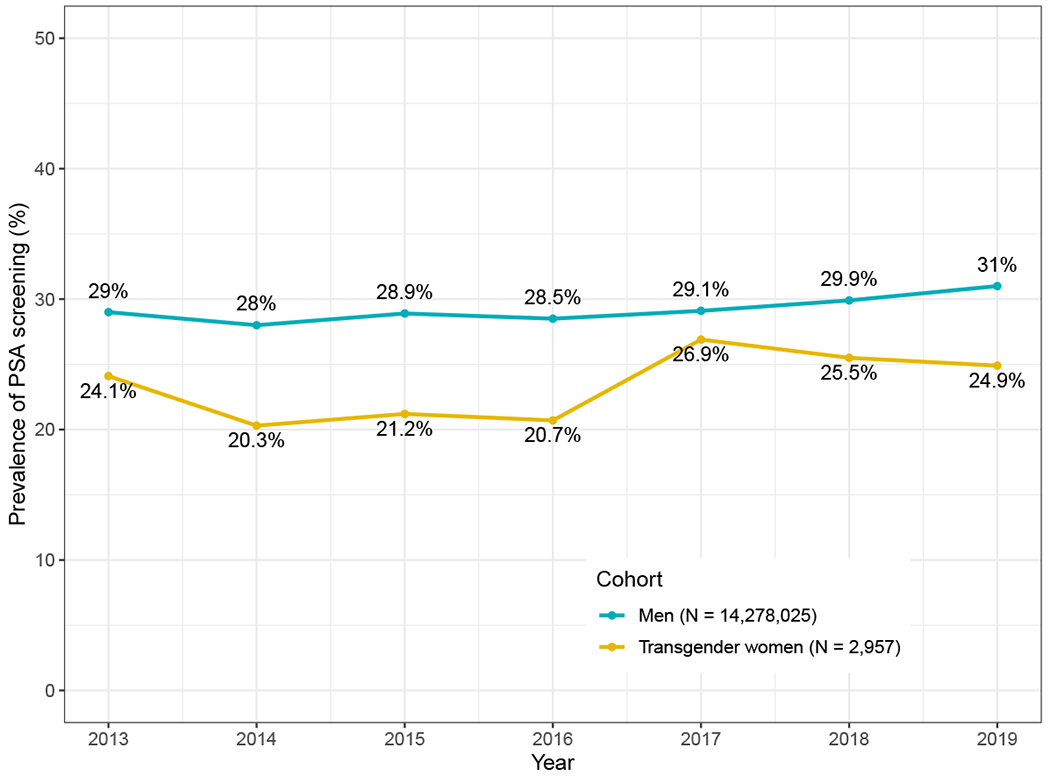
Rate of PSA screening by year among transgender women and cisgender men.
Table 1:
Yearly PSA screening rates of transgender women and men in Marketscan research databases with 95% confidence intervals
| Year | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Any Year | |
|---|---|---|---|---|---|---|---|---|---|
| Transgender women | Number of individuals | 428 | 723 | 697 | 888 | 1,164 | 1,299 | 1,101 | 2,957 |
| Number with PSA screening | 103 | 147 | 148 | 184 | 313 | 331 | 274 | 965 | |
| PSA screening rate (95% CI) | 24.07% (20.15, 28.46) | 20.33% (17.49, 23.49) | 21.23% (18.23, 24.50) | 20.72% (18.13, 23.57) | 26.89% (24.38, 29.55) | 25.48% (23.15, 27.96) | 24.89% (22.38, 27.57) | 32.63% (30.95, 34.36) | |
| Cisgender Men | Number of individuals | 7,933,148 | 8,040,389 | 5,045,691 | 4,865,809 | 4,357,417 | 4,276,782 | 3,311,381 | 14,278,025 |
| Number with PSA screening | 2,297,488 | 2,253,265 | 1,460,275 | 1,387,619 | 1,269,893 | 1,278,918 | 1,027,316 | 5,966,527 | |
| PSA screening rate (95% CI) | 28.96% (28.93, 28.99) | 28.02% (27.99, 28.06) | 28.94% (28.90, 28.98) | 28.52% (28.48, 28.56) | 29.14% (29.10, 29.19) | 29.9% (29.86, 29.95) | 31.02% (30.97, 31.07) | 41.79% (41.76, 41.81) |
Legend:
The proportion of transgender women and cisgender men receiving a PSA screening was calculated on both a year-by-year basis and for the overall proportion who received at least 1 PSA screening during the year(s) in which they were included (which could range from 1 to 7 years if inclusion criteria were met consistently).
Comparison by Age Group
The rate of PSA screening was compared between transgender women and cisgender men using log-binomial regression for each age group (Table 2). Adjusting for year, the rate of PSA screening among the transgender cohort was 26% (95% CI: 20%, 31%) lower than the cisgender male cohort of 40-54 years old, 17% (95% CI: 13%, 22%) lower than the cisgender male cohort of 55-69 years old, and 88% (95% CI: 60%, 121%) higher than the cisgender male cohort of 70-80 years old (Figure 2).
Table 2:
PSA screening rate ratio by age, log-binomial regression adjusting for year
| Age | Rate Ratio of PSA screening between transgender women and men (95% CI) | P-Value |
|---|---|---|
| 40-54 | 0.74 (0.69, 0.80) | <0.001 |
| 55-69 | 0.83 (0.78, 0.87) | <0.001 |
| 70-80 | 1.88 (1.60, 2.21) | <0.001 |
Legend:
A comparison of the rate of PSA screening by age between transgender women and cisgender men. These rate ratios are reported using the cisgender male cohort as the reference group. A ratio <1 indicates a lower rate of PSA screening among the transgender cohort and a ratio > 1 indicates a higher rate of PSA screening compared to the reference group.
Figure 2.
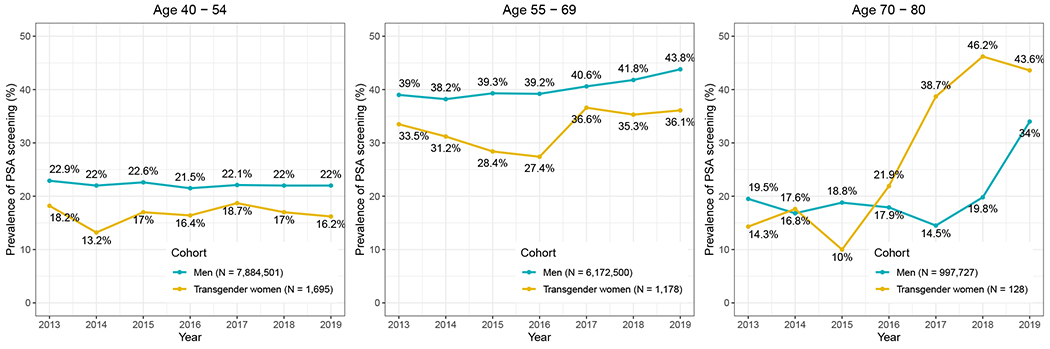
Annual rate of PSA screening, stratified by age group, among transgender women and cisgender men.
Comparison by Year
The rate of PSA screening was compared between transgender women and cisgender men using log-binomial regression for each year (Supplementary Table S8). The transgender cohort has a statistically significantly lower rate of PSA screening than cisgender men in all years except for 2017. Overall, there was a significant difference in the rate ratios for different years (p = 0.02), however, there was not a monotonic increasing or decreasing pattern. Specifically, there were no statistically significant differences in the rate ratios in consecutive years from 2013-2016 and 2017-2019, but we did see a statistically significant decrease in the magnitude of the rate ratios from 2016 to 2017.
Demographic Exploration
The results of the log-binomial regression, which explored the association of various clinical and demographic characteristics with PSA screening of the transgender cohort, are shown in Supplementary Table S9. There was no statistically significant difference in the rate of screening in transgender females between any of the years when compared to 2013. However, the year variable overall was statistically significant with a p-value of 0.021, due to the increased screening rate in this population seen from 2016 to 2017 (p = 0.007). We observed that older age and hormone use were associated with increased PSA screening rates, whereas North Central/Northeast/West regionality and gender-affirming surgery were associated with decreased PSA screening rates. Roughly 7% of the cohort did not have prescription information available in MarketScan. We repeated the analysis by excluding those individuals and the results were consistent (Table S11).
Discussion
In this novel study, we found that transgender women undergo PSA testing at a substantially lower rate than cisgender men within the context of uncertainty regarding the potential benefit of early prostate cancer detection. These findings suggest a need to build evidence for prostate cancer guidelines that support equity in medical care for transgender women. There is limited healthcare research for the transgender community, especially regarding preventative medicine outside of the realm of sexual health.9 There is a dearth of large-scale prospective studies specifically analyzing the incidence of various malignancies within the transgender population,10 but health equity necessitates creating a body of knowledge to inform best practices.11, 12
While we do observe lower yearly PSA screening rates in transgender women compared to cisgender men within the MarketScan dataset, once the patients are stratified into age groups, the gap in screening narrows with each decade of age, revealing two important patterns in the data. First, transgender women over the age of 70 received PSA screenings at a higher rate than cisgender men after the year 2016. The reason behind this finding is not readily apparent. Individuals over the age of 70 are outside the American Urological Association’s recommended guidelines for receiving prostate cancer screening, unless the patient has a life expectancy greater than 10-15 years, at which point the benefits of screening outweigh the risks.24 However, the mortality rate among the transgender population is twice that of the general population, as transwomen are at an increased risk of death due to cardiovascular disease, HIV-related illness, and suicide.13 Because all patients in MarketScan are privately insured, our cohort may have a perceived higher life expectancy due to the ability to receive preventative medicine to avoid the top causes of mortality seen in the general transgender population thus introducing a bias to this population to be considered.14
A second trend observed in the dataset is an increasing rate of PSA screening for transgender women over time. This increase may be attributed to a combination of insurance reform and a rising awareness of the transgender population. The transgender community has faced discrimination in numerous areas of society, but the provision of healthcare has been a historically difficult struggle. In 2014, the CDC released the results of their Behavioral Risk Factor Surveillance System survey (BRFSS), which showed a two-fold increase in the number of people identifying as transgender in the United States compared to a survey conducted in 2011.15, 16 Later that year, a ban on Medicare coverage of gender assignment surgery was repealed, allowing transgender individuals to submit gender-affirming procedures for coverage.17, 18 With the passage of the Affordable Care Act in 2010, more attention has been paid to providing general healthcare to transgender individuals, including preventative and mental health care.17 The increasing rates of PSA screening among transwomen observed in MarketScan may therefore reflect these evolving changes.
Our cohort had a hormone use rate of 25.2%, which is low compared to the general population of transgender females.19 The reason for the low hormone use rates in our cohort is likely two-fold. The previously published rates of hormone use in the overall transgender population included individuals of all age ranges, while our study focused on patients aged 40 and up. Because younger transgender patients are more likely to seek gender-affirming hormone treatment,20 the low rates seen in MarketScan may be the result of cohort differences in an older population. Secondly, obtaining adequate coverage of gender-affirming hormones from insurance providers is difficult, and many individuals may have their claims rejected.19 Consequently, it has been reported that nearly 10% of transgender individuals use nonprescription medications for hormone replacement therapy in lieu of those provided from a licensed healthcare provider,21,17 and this subset was not captured in our MarketScan population.
The results of this study have implications for future policy regarding screening recommendations for prostate cancer in transgender women. It is critical to generate holistic research within the area of transgender medicine to address the health care needs of this population. In addition to quantitative data, key personnel interviews with primary care physicians would generate qualitative information regarding the current real-time practice of preventative healthcare in transgender individuals. This would provide unique insights into clinical decision-making and help delineate potential mutable causes of disparity in screening. Similar studies have been performed for cervical cancer screening in transgender men,22, 23 and opening this conversation with providers will foster discussion and encourage efforts in education.
These findings should be interpreted in the context of certain limitations. MarketScan is not meant to record information primarily for a transgender population. However, it contains several variables that can be tangentially utilized to identify individuals belonging to the population of interest. For example, information about gender, prescription hormone medications, and gender-affirming surgical options can be assessed in MarketScan. Furthermore, our study findings rely heavily on the identification of transgender patients through diagnosis codes in medical claims. Thus, the accuracy of the findings presented in this study depends on the completeness and correctness of the original coding in this secondary data set. In addition, the MarketScan databases do not contain information on race, education level, ethnicity, ZIP code-level geographic data, or annual income. We cannot report definitive observations regarding the relationship between PSA screening and social determinants of health as outlined above. Finally, compared to cisgender counterparts, transgender individuals are less likely to have privately insured healthcare or any type of health insurance15,20 which may limit our study’s generalizability. This is a particular concern in the study because of the introduction of definitive confounders and biases such as employment bias and insurance bias. It’s well established that those patients who have insurance may be significantly different than other transgender patients without insurance because of their access to preventative care.14 Future studies will require granular data sources, such as electronic health records, that are inclusive of individuals from all payer types including federal and state government programs and private coverage plans along with healthcare organizations.
Conclusion
For privately insured patients identified in the MarketScan datasets, we see a lower PSA screening rate among transgender women compared to cisgender men. The health care needs of the transgender population lack key evidence in many areas. Because little is known about the prevalence of prostate cancer in transgender women, this finding highlights the necessity for future studies regarding the pathogenesis, prevention, and detection of prostate malignancy in this population, regardless of their stage of gender transition. Further efforts to address the healthcare concerns of individuals within the transgender community are paramount to diminishing the disparities they currently face.
Supplementary Material
Acknowledgement:
The Duke Biostatistics, Epidemiology, and Research Design Core’s support was made possible by the CTSA Grant (UL1TR002553) from the National Center for Advancing Translational Sciences (NCATS) of the NIH and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not represent the official views of NCATS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Flores A: How many adults identify as transgender in the United States. Edited by U. S. o. L. Williams Institite, p.1–5, 2016. Available at https://williamsinstitute.law.ucla.edu/wp-content/uploads/Trans-Adults-US-Aug-2016.pdf. Accessed March 02, 2021.
- 2.Deebel NA, Morin JP, Autorino R et al. : Prostate Cancer in Transgender Women: Incidence, Etiopathogenesis, and Management Challenges. Urology, 110: 166–171, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Rosser BRS, Hunt SL, Capistrant BD et al. : Understanding Prostate Cancer in Gay, Bisexual, and Other Men Who Have Sex with Men and Transgender Women: A Review of the Literature. Curr Sex Health Rep, 11: 430–441, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joint R, Chen ZE, Cameron S: Breast and reproductive cancers in the transgender population: a systematic review. BJOG, 125: 1505–1512, 2018. [DOI] [PubMed] [Google Scholar]
- 5.Health IW: The IBM MarketScan Research Databases for Life Sciences Researchers: Data Brochure, p. 3–14, 2019. Available at https://www.ibm.com/downloads/cas/OWZWJ0QO. Accessed December 18, 2021.
- 6.Blosnich JR, Cashy J, Gordon AJ et al. : Using clinician text notes in electronic medical record data to validate transgender-related diagnosis codes. J Am Med Inform Assoc, 25: 905–908, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proctor K, Haffer SC, Ewald E et al. : Identifying the Transgender Population in the Medicare Program. Transgend Health, 1: 250–265, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roblin D, Barzilay J, Tolsma D et al. : A novel method for estimating transgender status using electronic medical records. Ann Epidemiol, 26: 198–203, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edmiston EK, Donald CA, Sattler AR et al. : Opportunities and Gaps in Primary Care Preventative Health Services for Transgender Patients: A Systemic Review. Transgend Health, 1: 216–230, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun H, Nash R, Tangpricha V et al. : Cancer in Transgender People: Evidence and Methodological Considerations. Epidemiol Rev, 39: 93–107, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford J, Reisner SL, Honnold JA et al. : Experiences of transgender-related discrimination and implications for health: results from the Virginia Transgender Health Initiative Study. Am J Public Health, 103: 1820–1829, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kachen A, Pharr JR: Health Care Access and Utilization by Transgender Populations: A United States Transgender Survey Study. Transgend Health, 5: 141–148, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Blok CJ, Wiepjes CM, van Velzen DM et al. : Mortality trends over five decades in adult transgender people receiving hormone treatment: a report from the Amsterdam cohort of gender dysphoria. Lancet Diabetes Endocrinol, 9: 663–670, 2021. [DOI] [PubMed] [Google Scholar]
- 14.Dickey LM, Budge SL, Katz-Wise SL et al. : Health disparities in the transgender community: Exploring differences in insurance coverage. Psychology of Sexual Orientation and Gender Diversity, 3: 275–282, 2016. [Google Scholar]
- 15.Baker KE: Findings From the Behavioral Risk Factor Surveillance System on Health-Related Quality of Life Among US Transgender Adults, 2014-2017. JAMA Intern Med, 179: 1141–1144, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meerwijk EL, Sevelius JM: Transgender Population Size in the United States: a Meta-Regression of Population-Based Probability Samples. American Journal of Public Health, 107: e1–e8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroumsa D: The state of transgender health care: policy, law, and medical frameworks. Am J Public Health, 104: e31–e38, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stryker S: Transgender history : the roots of today’s revolution, Second edition. Revised edition. ed. New York, NY: Seal Press, p.190–195, 2017. [Google Scholar]
- 19.James SE, Herman JL, Rankin S, Keisling M, Mottet L, & Anafi M : The Report of the 2015 U.S. Transgender Survey. Edited by N. C. f. T. Equality, p.18–231, 2016. Available at https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf. Accessed March 02, 2022.
- 20.Grant JM ML, Tanis J, Herman JL, Harrison J, Keisling M: National transgender discrimination survey report on health and health care. Natl Cent Transgender Equal Natl Gay Lesbian Task Force, p. 1–23, 2010. [Google Scholar]
- 21.de Haan G, Santos GM, Arayasirikul S et al. : Non-Prescribed Hormone Use and Barriers to Care for Transgender Women in San Francisco. LGBT Health, 2: 313–323, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Gatos KC: A Literature Review of Cervical Cancer Screening in Transgender Men. Nursing for Women’s Health, 22: 52–62, 2018. [DOI] [PubMed] [Google Scholar]
- 23.Dhillon N, Oliffe JL, Kelly MT et al. : Bridging Barriers to Cervical Cancer Screening in Transgender Men: A Scoping Review. Am J Mens Health, 2020. May-Jun; 14(3):1–10, 1557988320925691. doi: 10.1177/1557988320925691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman E, Radix AE, Bouman WP, Brown GR, de Vries ALC, Deutsch MB, Ettner R, Fraser L, Goodman M, Green J, Hancock AB, Johnson TW, Karasic DH, Knudson GA, Leibowitz SF, Meyer-Bahlburg HFL, Monstrey SJ, Motmans J, Nahata L, … Arcelus J (2022). Standards of Care for the Health of Transgender and Gender Diverse People, Version 8. International Journal of Transgender Health, 23(S1): S1–S260. doi: 10.1080/26895269.2022.2100644 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


