Abstract
Context:
Pain is distressing for women with breast cancer. Pain medication may not provide full relief and can have negative side-effects. Cognitive-behavioral pain intervention protocols reduce pain severity and improve self-efficacy for pain management. These interventions’ impact on pain medication use is less clear. Intervention length and coping skills use might play a role in pain outcomes.
Objective:
Secondary analysis to examine differences in pain severity, pain medication use, pain self-efficacy, and coping skill use after 5- and 1-session cognitive-behavioral pain intervention protocols. Pain self-efficacy and coping skills use were assessed as mediators of intervention effects on pain and pain medication use.
Methods:
Women (N=327) with stage I-III breast cancer were enrolled in a randomized trial comparing individually-delivered, 5- and 1-session Pain Coping Skills Training (PCST). Pain severity, pain medication use, pain self-efficacy, and coping skills use were assessed pre-intervention and 5–8 weeks later (post-intervention).
Results:
Pain and pain medication use significantly decreased, while pain self-efficacy increased pre-post for women randomized to both conditions (p’s <.05). Five-session PCST participants demonstrated less pain (p=.03) and pain medication use (p=.04), and more pain self-efficacy (p=.02) and coping skills use (p=.04) at post-intervention compared to 1-session PCST participants. Pain self-efficacy mediated the relationship of intervention condition with pain and pain medication use.
Conclusion:
Both conditions led to improvements in pain, pain medication use, pain self-efficacy, and coping skills use, and 5-session PCST showed the greatest benefits. Brief cognitive-behavioral pain intervention improve pain outcomes, and pain self-efficacy may play a role in these effects.
Keywords: breast cancer, pain coping skills, pain severity, pain medication, pain self-efficacy, coping skills use
Introduction
Approximately 30–60% of women diagnosed with breast cancer experience pain [1, 2]. Breast cancer surgery and adjuvant therapies contribute to pain [3], and pain can persist after active treatment [2]. Medication is typically the first-line approach for breast cancer pain management [4, 5]. While medication is helpful, it does not always provide full relief and can be associated with significant side-effects (e.g., constipation, nausea) [6, 7]. There is growing interest in adjunctive, cognitive-behavioral interventions for breast cancer pain.
Guideline recommendations suggest that efficacious cognitive-behavioral pain management interventions, such as Pain Coping Skills Training (PCST), be integrated into cancer treatment [8–10]. PCST protocols teach patients cognitive-behavioral coping skills (e.g., progressive muscle relaxation, pleasant imagery, cognitive restructuring, activity pacing) to enhance their ability to cope with pain [11]. PCST has traditionally been delivered across 8–12, 1-hour sessions. While effective, such lengthy protocols are impractical in oncology settings where patients have minimal time and energy due to cancer treatment burden, and access to trained providers may be limited [12, 13]. Brief cognitive-behavioral pain management interventions reduce barriers to access and can improve outcomes (e.g., pain severity) [14–19]. In fact, a 2-hour in-person class in pain management skills resulted in a clinically significant improvement in pain severity that was non-inferior to an 8-session cognitive-behavioral pain management protocol [17]. Much of this research has been conducted in chronic pain samples (e.g., chronic low back pain); less work has examined the impact of different doses of brief cognitive-behavioral pain management interventions on pain and pain-related variables (e.g., pain medication use) in cancer patients [16, 19, 20].
There is also a need to understand mechanisms by which brief cognitive-behavioral pain management protocols may improve clinically relevant outcomes in cancer patients, such as pain severity and pain medication use. PCST aims to bolster patients’ self-efficacy for pain management, defined as one’s confidence in their ability to control pain without medication or other medical intervention [21]. PCST has been shown to increase pain self-efficacy in cancer samples, and increased pain self-efficacy is related to decreased pain severity [11, 22, 23]. Research has also demonstrated associations between greater pain coping skills use and improved outcomes (e.g., pain interference, quality of life, and treatment satisfaction) [24–26]. Enhancing women’s confidence in their ability to manage pain and increasing pain coping skills practice may also lead to reductions in pain medication use. In a study of women undergoing breast cancer surgery, participants who viewed an online 90-minute pain psychoeducation video and accessed a relaxation audiofile experienced increased odds of opioid cessation post-surgery compared to a health education control [20]. Still, research on the impact of PCST, in particular, on pain medication use among women deeper into breast cancer survivorship remains limited [27]. Likewise, closer examination of the mechanistic role that pain self-efficacy and pain coping skill use might play in the relationship between brief doses of PCST and critical pain variables (i.e., pain severity, pain medication use) in cancer patients is warranted.
We conducted a secondary analysis of data from a randomized trial designed to explore differences in intervention response between women with breast cancer and pain who received varying doses (i.e., 5- versus 1-session) of individually-delivered PCST [28]. The first aim of this analysis was to examine differences in pain severity, days with pain medication use, pain self-efficacy, and coping skill use for the entire sample, as well as between the 5- and 1-sesion PCST interventions. We hypothesized that overall pre- to post-intervention (i.e., 5–8 weeks after pre-intervention assessment) benefits would be found for pain severity, days with pain medication use, and pain self-efficacy. We also hypothesized that, at post-intervention, the 5-sesion PCST condition would demonstrate lower pain severity and days with pain medication use, and greater pain self-efficacy and coping skills use compared to the 1-session PCST condition. The second aim of this analysis was to explore the role of pain self-efficacy and coping skill use as unique mediators of the relationship between intervention condition and pain severity and days with pain medication use at post-intervention. We hypothesized that women assigned to 5-session PCST would report less pain severity and days with pain medication use at post-intervention, and that these effects would be mediated by more pain self-efficacy and more coping skills use.
Methods
Participants
Women with breast cancer (N=327) were recruited from 2017–2020 [22]. Eligibility criteria included: 1) diagnosis of stage I-IIIC breast cancer in past two years; 2) self-reported worst pain severity in past week ≥5 out of 10 at screening; 3) ≥18 years of age; 4) life expectancy of ≥12 months. Exclusion criteria were: 1) cognitive impairment; 2) severe psychiatric disorder or condition (e.g., psychosis, suicidal intent) that would contraindicate safe participation; and/or 3) current or past (<6 months) engagement in Pain Coping Skills Training (PCST) for cancer pain. The parent study was a randomized trial of PCST approved by the Duke University Institutional Review Board (IRB #: Pro00070823) and registered on ClinicalTrials.gov (NCT02791646).
Procedures
Study staff reviewed electronic medical records to assess eligibility. Potentially eligible patients were mailed a recruitment letter, then contacted by study staff to complete a brief screening interview [28]. Eligible and interested patients provided informed consent, completed a pre-intervention assessment (A1), and then were randomly assigned (1:1 allocation) to 5- or 1-session PCST by a staff member with no participant contact. Five to eight weeks following randomization, participants completed a post-intervention assessment (A2). Both A1 and A2 assessments were completed via Qualtrics and consisted of self-report questionnaires measuring pain severity, pain medication use, pain self-efficacy, and coping skills use.
Intervention Conditions
Five-Session PCST consisted of five, individually-delivered, 60-minute weekly in-person intervention sessions. Sessions focused on pain education [29] and systematic training in cognitive-behavioral pain management skills using behavioral rehearsal modeling, guided practice, and feedback. Session 1 focused on training in progressive muscle relaxation and guided imagery. Subsequent sessions included training in activity-rest cycling, pleasant activity scheduling, brief applied relaxation (e.g., deep breathing), cognitive restructuring, and goal setting [28].
One-Session PCST consisted of one, individually-delivered, 60-minute in-person intervention session. Session content mirrored the first session in the 5-session PCST condition (i.e., progressive muscle relaxation, guided pleasant imagery) [28].
Participants in both conditions received 3 weekly caring text messages to prompt skills practice and provide encouragement (e.g., “Remember that your coping skills are available to you anytime and anywhere,” “Make time for doing relaxation so you can get the full benefit”).
Intervention Delivery and Fidelity
Study therapists were four PhD-level clinical psychologists and one advanced PhD clinical psychology student. Therapist training included a two-day workshop and a four-week certification in the manualized interventions. Therapists were certified for intervention delivery after demonstrating at least 90% (M=4.50/5.00) competence and adherence.
Treatment fidelity was assessed by a PhD-level clinical psychologist not otherwise involved in the study. Twenty percent of session recordings (N=108) were randomly reviewed; competence (M=4.71, SD=.28) and adherence (M=4.75, SD=0.44) were excellent.
Intervention procedures were adapted to telehealth delivery for the final portion of the study in response to the COVID-19 pandemic. Nineteen participants in the 5- (12.8%) and 1-session (7.7%) PCST interventions had 1 or more remote intervention sessions. There were no differences in response to PCST-Full or PCST-Brief based on delivery modality (χ2 = 0.03 and 0.23, respectively; ps>.05) [34].
Measures
Demographic and medical characteristics.
Participants’ demographic and medical characteristics were collected through self-report and electronic medical record review. At enrollment, information was collected regarding demographics (e.g., partner status, education, employment, income) and medical history (e.g., cancer stage, time since diagnosis, and surgeries and treatment received).
Pain Severity.
Pain severity was assessed with four items from the Brief Pain Inventory (BPI) [30]. Participants were asked to rate their pain at its worst, least, and average over the past week, as well as their current pain. Response options ranged from 0 (no pain) to 10 (worst pain imaginable). These four items were averaged to obtain a composite score at A1 and A2 (Cronbach’s α=.86 and α=.88, respectively), with higher scores indicating higher levels of pain severity. The BPI is recommended for use in all clinical trials assessing pain and is frequently used in cancer samples [22, 31, 32].
Pain Medication Use.
Pain medication use (e.g., over-the-counter, opioid, anti-neuropathic, corticosteroid, and anxiolytic medications) was indexed by self-reported number of days with any pain medication use in the past week (0–7 days).
Pain Self-Efficacy.
Self-efficacy for pain management was assessed with the five-item Chronic Pain Self-Efficacy Scale [33]. Participants were asked to rate their confidence in their ability to decrease pain, continue daily activities, keep pain from interfering with sleep, and make small-to-moderate and large reductions in pain using methods other than medication. Response options ranged from 10 (very uncertain) to 100 (very certain), with higher scores reflecting more pain self-efficacy. These five items were averaged to obtain a composite score at A1 and A2 (Cronbach’s α=.85 and α=.88, respectively). The Chronic Pain Self-Efficacy Scale has been widely used in cancer samples [16].
Coping Skills Use.
At A2, participants were asked how often they had practiced any PCST skill or idea since their last intervention session. Response options were: 0 (not at all), 1 (one time), 2 (a few days), 3 (several days), 4 (almost every day), and 5 (every day).
Analytic Strategy
Intent-to-treat analyses were conducted using SPSS (Version 27). Preliminary descriptive analyses included identification of outliers and examination of main study variable distributions for kurtosis, skewness, and assumptions of normality for multivariate data. All main study variables exhibited normal distributions, with kurtosis and skewness values within −2 and +2 [32]. Descriptive statistics were used to characterize pain severity, pain medication use, pain self-efficacy, and coping skill use. We reported mean pain severity scores at A1 and A2 elsewhere [34]; however, this is the first time we assessed within- and between-group change across timepoints. Paired sample t-tests were conducted to assess within-group differences from A1 to A2 within the full sample, as well as within 5- and 1-session PCST groups. Independent sample t-tests were used to assess between-group (5- versus 1-session PCST) differences at A2. Mediation analyses were conducted using Hayes PROCESS Macro (Version 2.16.2). Pain self-efficacy at A2 was specified as a continuous mediator of the relationship between intervention condition and pain severity at A2, and pain medication use at A2. Separately, coping skills use at A2 was specified as a continuous mediator of the relationship between intervention condition and pain severity at A2, and pain medication use at A2 [35, 36]. Intervention condition was categorized as 5-session PCST=0 versus 1-session PCST=1. Variables were determined to be mediators if the 95% confidence interval for the indirect effect did not contain zero [37]. Effect sizes were indexed by Cohen’s d: ≤.49=small, .50-.79=medium, ≥.80=large [38].
Results
Participant Characteristics
Across the entire sample, average age was 57.19 years old (SD=11.87). Approximately 62% of participants identified as Caucasian while 30% identified as Black/African-American. The majority of women endorsed non-Hispanic ethnicity (95.1%). Demographic characteristics by group condition are reported in Table 1.
Table 1.
Demographic Characteristics by Condition at A1
| 5-Session PCST (N=163) | 1-Session PCST (N=164) | |||
|---|---|---|---|---|
| N (%) | M (SD) | N (%) | M (SD) | |
| Age (years) | 56.93 (11.96) | 57.45 (1181) | ||
| Race | ||||
| White/Caucasian | 98 (60.1%) | 105 (64.0%) | ||
| Black/African American | 52 (31.9%) | 45 (27.4%) | ||
| Two or More Races | 4 (2.5%) | 5 (3.0%) | ||
| Asian | 5 (3.1%) | 4 (2.4%) | ||
| American Indian or Alaskan Native | 1 (0.6%) | 0 (0.0%) | ||
| Other | 1 (0.6%) | 2 (1.2%) | ||
| Not Reported/Declined | 2 (1.2%) | 3 (1.8%) | ||
| Ethnicity | ||||
| Non-Hispanic | 155 (95.1%) | 156 (95.1%) | ||
| Hispanic or Latino | 4 (2.5%) | 1 (0.6%) | ||
| Hispanic Mexican | 0 (0.0%) | 2 (1.2%) | ||
| Hispanic Cuban | 1 (0.6%) | 2 (1.2%) | ||
Mean time since diagnosis was approximately 10 months (SD=6.21). For most women (97.2%), this was their first breast cancer diagnosis, and 56% reported Stage I disease. Additional medical characteristics by group condition are reported in Table 2.
Table 2.
Medical Characteristics by Condition at A1
| Five-Session PCST (N= 163) | One-Session PCST (N= 164) | |||
|---|---|---|---|---|
| N(%) | M (SD) | N(%) | M (SD) | |
| Cancer diagnosis | ||||
| First/Initial | 155 (95.1%) | 162 (99.4%) | ||
| Recurrence | 8 (4.9%) | 1 (0.6%) | ||
| Months since diagnosis | 9.85 (5.91) | 10.37 (6.49) | ||
| Stage | ||||
| I | 95 (58.3%) | 88 (53.7%) | ||
| II | 52 (31.9%) | 61 (37.2%) | ||
| III | 16 (9.8%) | 15 (9.1%) | ||
| Breast Surgery History | ||||
| Mastectomy – unilateral | 36 (22.2%) | 18 (11.1%) | ||
| Mastectomy – bilateral | 28 (17.3%) | 37 (22.8%) | ||
| Breast Conserving Surgery | 94 (58.0%) | 91 (55.8%) | ||
| Lymph Node Removal | 145 (89.5%) | 141 (87.0%) | ||
| Reconstruction | 39 (24.1%) | 34 (21.0%) | ||
| Receipt of Chemotherapy | 12 (7.4%) | 15 (9.2%) | ||
| Receipt of radiation | 15 (9.2%) | 20 (12.3%) | ||
| Receipt of surgery | 10 (6.1%) | 12 (7.4%) | ||
| Receipt of endocrine therapy | 25 (15.4%) | 24 (14.8%) | ||
| Receipt of immunotherapy | 15 (9.3%) | 16 (9.9%) | ||
| Pain medication use | 103 (63.2%) | 109 (66.5%) | ||
| OTC | 87 (53.4%) | 90 (55.2%) | ||
| Opioid | 31 (19.0%) | 29 (17.8%) | ||
| Corticosteroid | 2(1.2%) | 6 (3.7%) | ||
| Antineuropathic | 16 (9.8%) | 14 (8.6%) | ||
| Anxiolytic | 19(11.7%) | 29 (17.8%) | ||
PCST = pain coping skills training; M = mean; SD = standard deviation.
Breast surgery history variables answered on Yes vs. No scale; Receipt of chemotherapy, radiation, surgery, endocrine therapy, and immunotherapy answered on Yes vs. No scale; Breast conserving surgery includes lumpectomy, quadrantectomy, partial mastectomy, segmental mastectomy; Receipt of chemotherapy, radiation, surgery, endocrine therapy, and immunotherapy is for week before baseline assessment. Pain medication use answered on Yes vs. No scale. OTC=over-the-counter.
At A1, average pain severity (M=4.04, SD=1.73) and pain self-efficacy (M=62.37, SD=20.85) were moderate within the entire sample. Across both 5- and 1-session PCST conditions at A1, women reported an average of approximately three days (SD=2.86) with pain medication use in the past week. At A2, approximately one-third of the entire sample reported coping skill use “almost every day” (n=108; 35.3%). Descriptive statistics for 5- and 1-session PCST on main study variables are reported in Table 3.
Table 3.
Change in Main Study Variables
| Entire Sample | 5-Session PCST | 1-Session PCST | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A1 M (SD) | A2 M (SD) | M Within-Group Diff | A1 M (SD) | A2 M (SD) | M Within- Group Diff | A1 M (SD) | A2 M (SD) | M Within- Group Diff | M Between-Group Diff | |
| Pain Severity | 4.04 (1.73) | 2.92 (1.76) | 1 10*** 95% CI (.91,1.29) | 4.03 (1.81) | 2.70 (1.66) | 1.33*** 95% CI (1.06,1.60) | 4.04 (1.65) | 3.13 (184) | 88*** 95% CI (.61,1.15) | −.44* 95% CI (−.83,−.05) |
| Pain Medication Use | 3.02 (2.86) | 2.31 (2.66) | 72*** 95% CI (.40,1.04) | 2.90 (2.86) | 1.99 (2.58) | 92*** 95% CI (.49,1.34) | 3.14 (2.86) | 2.61 (2.70) | .53* 95% CI (.06,1.00) | −.62* 95% CI (−1.21,−.02) |
| n (%) | n (%) | |||||||||
| OTC | 87 (53.4) | 55 (37.7) | 90 (55.2) | 71 (45.8) | ||||||
| Opioid | 31 (19.0) | 16 (110) | 29 (17.8) | 20 (12.9) | ||||||
| Corticosteroid | 2 (1.2) | 1 (0.7) | 6 (3.7) | 1 (0.6) | ||||||
| Anti-Epileptic | 16 (9.8) | 9 (6.2) | 14 (8.6) | 4 (2.6) | ||||||
| Anxiolytic | 19 (11.7) | 1 (0.7) | 29 (17.8) | 2 (1.3) | ||||||
| Pain Self-Efficacy | 62.37 (20.85) | 73.71 (19.92) | −11.38*** 95% CI (−13.83, −8.93) | 61.53 (21.28) | 76.44 (18.88) | −14 96*** 95% CI (−18.40, −11.52) | 63.21 (20.44) | 71.09 (20.59) | −7 91*** 95% CI (−11.35, − 4.47) | 5.35* 95% CI (−.90,9.80) |
| Coping Skills Use | - | 3.59 (1.07) | - | - | 3.72 (1.17) | - | - | 3.47 (0.96) | - | .25* 95% CI (.01,.49) |
| n (%) | n (%) | |||||||||
| Not at all | 3 (2.0) | 1 (0.6) | ||||||||
| One time | 3 (2.0) | 2 (1.3) | ||||||||
| A few days | 15 (10.0) | 19 (12.2) | ||||||||
| Several days | 36 (24.0) | 55 (35.3) | ||||||||
| Almost every day | 49 (32.7) | 59 (37.8) | ||||||||
| Every day | 44 (29.3) | 20 (12.8) | ||||||||
Note. PCST=Pain Coping Skills Training. M=mean; SD=standard deviation. Pain Medication Use answered on Yes vs. No scale. OTC=over-the-counter; M Within-Group Diff=difference from A1 to A2 on values of pain severity, pain medication use, and pain self-efficacy for entire sample, 5-Session PCST, and 1-Session PCST; M Between-Group Diff=difference on values of pain severity, pain medication use, pain self-efficacy, and coping skills use between 5-Session PCST and 1-Session PCST at A2; Coping Skills Use (mean and frequencies) was only assessed at A2.
p<.001;
**p<.01;
p<.05.
Within- and Between-Group Differences in Main Study Variables
In the full sample, pain severity (t(309)=11.32, p<.001, d=.64) and pain medication use (t(302)=4.47, p<.001, d=.26) significantly decreased, while pain self-efficacy significantly increased (t(304)=−9.13, p<.001, d=−.52) from A1 to A2. For women in both 5- and 1-session PCST, pain severity (5-session PCST: t(152)=9.68, p<.001, d=.78; 1-session PCST: t(156)=6.47, p<.001, d=.52) and pain medication use (5-session PCST: t(147)=4.27, p<.001, d=.35; 1-session PCST: t(154)=2.22, p=.03, d=.18) significantly decreased, and pain self-efficacy significantly increased (5-session PCST: t(149)=−8.60, p<.001, d=.70; 1-session PCST: t(154)=−4.54, p<.001, d=−.37) from A1 to A2.
Examining between groups at A2, pain severity (t(309)=−2.20, p=.03, d=−.25) and pain medication use (t(304)=−2.04, p=.04, d=−.23) were significantly lower in women randomized to 5-session PCST. Pain self-efficacy (t(304)=2.37, p=.02, d=.27) and coping skills use (t(304)=2.02, p=.04, d=.23) were significantly greater in women randomized to 5-session PCST. Frequencies for all types of pain medications decreased from A1 to A2 across both intervention conditions. There were no statistically significant differences on these variables between 5- and 1-Session PCST at A2 (ps>.05). Test statistics are shown in Table 3.
Mediation Models
Pain self-efficacy mediated the relationship between intervention condition and pain severity at A2 (Indirect Effect: B=.18, 95% CI [.04, .35]), such that women assigned to 5-session PCST reported lower pain severity at A2 via higher pain self-efficacy at A2. In this model, the direct effect between intervention and pain severity at A2 was not significant. Similarly, pain self-efficacy mediated the relationship between intervention condition and pain medication use at A2 (Indirect Effect: B=.20, 95% CI [.03, .38]), such that women assigned to 5-session PCST reported lower pain medication use at A2 via higher pain self-efficacy at A2. In this model, the direct effect between intervention and pain medication use was not significant. Unstandardized and standardized model parameter estimates are shown in Figures 1a and 1b.
Figure 1.
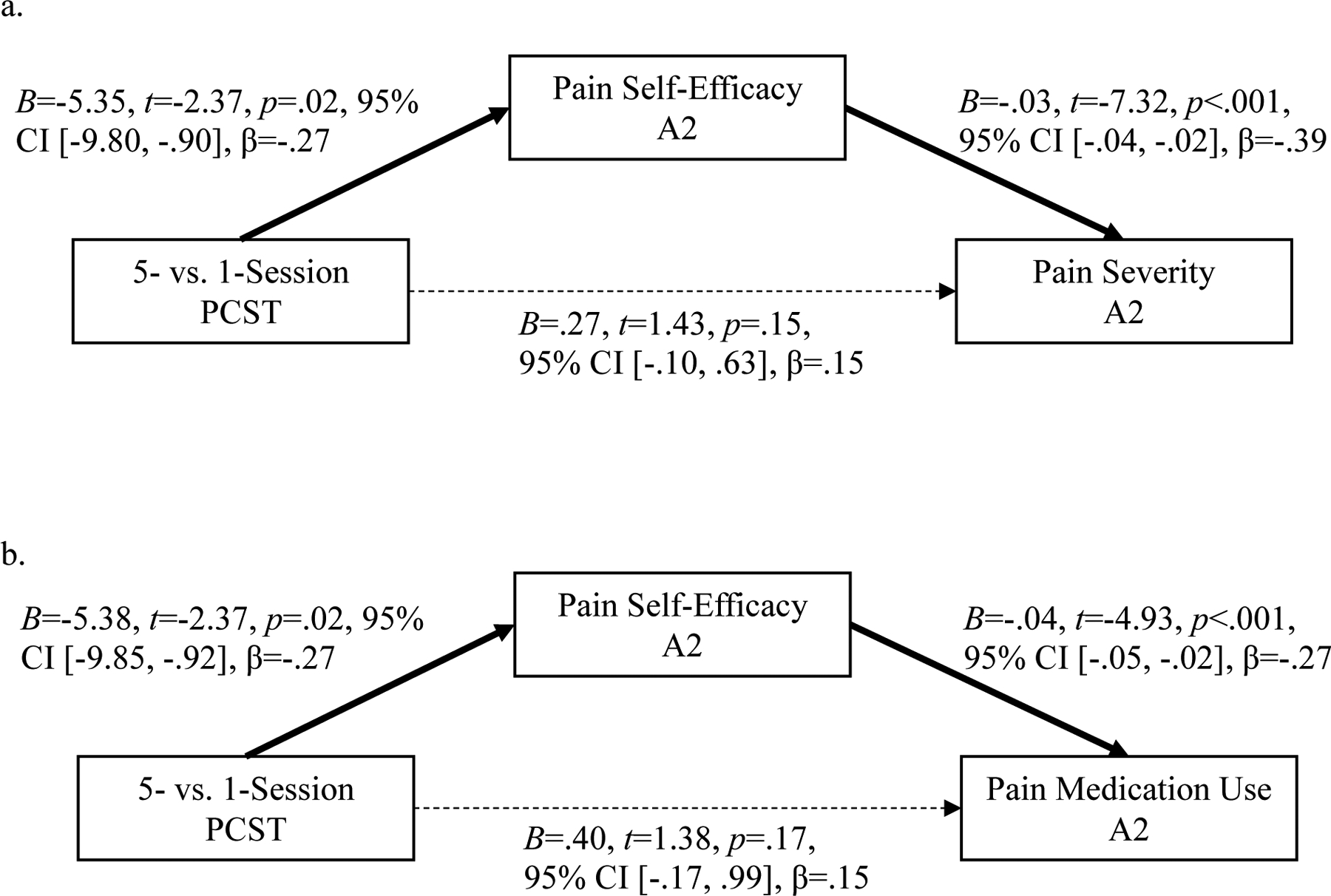
Pain Self-Efficacy as Mediator
Note. 5-Session PCST=0 vs. 1-Session PCST=1; Solid line indicates a significant path; Dotted line indicates a non-significant path.
Coping skills use did not mediate the relationship between intervention condition and pain severity at A2 (Indirect Effect: B=−.02, 95% CI [−.08, .04]). Assignment to 5-session PCST predicted significantly more coping skills use (B=−.25, p=.04) and less pain severity at A2 (B=.47, p=.02), but coping skills use did not significantly relate to pain severity (B=.08, p=.41). Likewise, coping skills use did not mediate the relationship between intervention and pain medication use at A2 (Indirect Effect: B=−.01, 95% CI [−.10, .07]). Assignment to 5-session PCST predicted significantly more coping skills use (B=−.24, p=.05) and less pain medication use at A2 (B=.61, p=.05), but coping skills use was not significantly associated to pain medication use (B=.04, p=.79). Unstandardized and standardized model parameter estimates are shown in Figures 2a and 2b.
Figure 2.

Coping Skills Use as Mediator
Note. 5-Session PCST=0 vs. 1-Session PCST=1; Solid line indicates a significant path; Dotted line indicates a non-significant path.
Discussion
This was a secondary analysis of data from a randomized trial of an individually-delivered cognitive-behavioral pain management protocol for women with breast cancer and at least moderate pain [28]. We aimed to examine pain severity, pain medication use, pain self-efficacy, and coping skills use for the entire sample, as well as by intervention condition (i.e., 5-versus 1-session PCST). We also explored pain self-efficacy and coping skills use as unique mediators of the relationship between condition and pain severity, and pain medication use at post-intervention.
We observed significant improvements in pain severity, pain medication use, and pain self-efficacy from pre- to post-intervention in the entire sample. Similar trends were observed within 5- and 1-session PCST conditions. These findings support hypotheses and suggest that pain and critical pain-related variables (i.e., pain medication use, pain self-efficacy) improve over the course of both 5- and 1-session PCST interventions. Strong results across both groups may be related to robust engagement, with 77% (n=125) of participants attending all sessions of the 5-session protocol and 99% of participants (n=162) attending the single session of the 1-session protocol.
Notably, women assigned to 5-session PCST reported significantly less pain severity and pain medication use, and greater pain self-efficacy and coping skills use at post-intervention compared to women who received 1-session PCST, suggesting that 5 sessions of PCST leads to larger benefits on these variables. The parent study found that average pain severity was reduced by approximately 28.5% from pre- to post-assessment in 5-session PCST, while women in 1-session PCST experienced a 14.8% reduction in pain across timepoints [34]. The current analysis showed that average pain medication use decreased by a full day in the 5-session PCST group, and half a day in the 1-session PCST group. Although there were no significant differences on types of pain medications used across intervention conditions, use of all pain medications decreased in frequency from A1 and A2. Notably, opioid use decreased by 8% and 4.9% in 5- and 1-Session PCST, respectively. Women in 5-sesion PCST showed an increase in pain self-efficacy of more than 10 points, while pain self-efficacy for women in 1-session PCST increased by approximately 8 points. These are clinically relevant improvements, and add to the body of literature investigating the effect of abbreviated forms of PCST on pain and pain-related variables (e.g., pain medication use, pain self-efficacy) for cancer patients [16, 19, 20, 22].
Greater intervention dose may explain why women randomized to 5-session PCST demonstrated larger improvements in pain severity, pain medication use, and pain self-efficacy. While women in both conditions were taught progressive muscle relaxation and guided pleasant imagery, women in the 5-session protocol received instruction in additional cognitive-behavioral pain coping skills (e.g., pleasant activity planning, activity-rest cycling, cognitive restructuring). Given this, it is not surprising that women randomized to 5-session PCST reported slightly more coping skills use at post-intervention, as they had a larger repertoire of skills. Moreover, women in 5-session PCST likely benefitted from stronger therapeutic alliance and ongoing social support across additional intervention sessions; this may have also contributed to intervention differences.
However, coping skills use for women in 1-session PCST was also quite high, and women in the 1-session condition also experienced significant improvements in pain severity, pain medication use, and pain self-efficacy from pre- to post-intervention. These data extend prior work on the benefits of abbreviated cognitive-behavioral pain management protocols to women with breast cancer and moderate pain [14–20]. While 5-session PCST may offer greater benefit due to the larger intervention dose and broader suite of skills taught, single-session protocols are a viable option for cancer patients experiencing pain, particularly when resources (i.e., patient, system) are limited. Future research should explore whether there are specific subgroups of women with breast cancer for which the single-session PCST protocol is sufficient and preferred.
These findings differ from research in a chronic pain sample, in which a single-session, 2-hour protocol was non-inferior to an 8-session cognitive-behavioral pain management intervention [17]. It is possible that the group differences we observed are related to the sample and inclusion criteria (i.e., women with at least moderate pain). A worst pain score of ≥ 5 is high compared to inclusion criteria for other pain trials and may have generated a sample for whom PCST was especially relevant. It is possible that a single 60-minute session of PCST is insufficient for women reporting at least moderate pain; for these individuals, training in additional skills (e.g., cognitive restructuring) or an increased dose of brief training (i.e., a longer one-session protocol) may be necessary [17]. Future work should aim to identify the optimal combination of skills for particular patient populations. This question might be answered with a multiphase optimization strategy trial (MOST), wherein ideal components of PCST can be efficiently identified and tested.
In line with our second hypothesis, we found that increased pain self-efficacy mediated the relationship between intervention condition and decreased pain severity, as well as decreased pain medication use. These findings are a novel addition to the literature on brief cognitive-behavioral pain management interventions, as most research on pain self-efficacy has focused on longer, 6–8 session protocols compared to a usual care control [22, 23]. Our findings also highlight the effect of pain self-efficacy on pain severity specifically. Much existing research has assessed the impact of self-efficacy for symptom management on health-related quality of life or symptom distress [39, 40]. To our knowledge, no other study has explored the mediating role of pain self-efficacy on pain medication use for women with breast cancer. Women who participate in 5-session PCST may feel more confident in their ability to manage pain on their own, and subsequently use less pain medication. Thus, increased pain self-efficacy may influence both the pain experience (i.e., pain severity) and pain-related behaviors (e.g., medication use). Many patients are interested in minimizing pain medication use due to fear of addiction and stigma surrounding opioids [6]. Increasing pain self-efficacy via PCST might be a critical mechanistic pathway to reduce pain medication use in cancer samples.
Interestingly, coping skills use did not mediate the relationship of intervention group with pain severity and pain medication use. Thus, in this sample, enhancing pain self-efficacy appears to be a mechanism by which PCST leads to change in important pain outcomes, while increased coping skills use is not. This finding is highly relevant to the development and deployment of cognitive-behavioral pain coping interventions. Findings suggest that improving pain self-efficacy, or a patient’s belief in their ability to manage pain on their own, may be more important than frequency of coping skills use [41]. As such, in-session behavioral rehearsal, modeling, and guided practice should be prioritized to help cancer patients build mastery over skills and bolster pain self-efficacy.
Our study is strengthened by its large sample size and design, which allowed us to compare 5- and 1-session PCST. We focused on several clinically relevant variables (e.g., pain severity, pain medication use, pain self-efficacy, coping skills use) which, to our knowledge, have not yet been explored together in a sample of women with breast cancer and moderate-to-severe pain. Understanding how these variables relate to each other and differ based on PCST dose represents a novel contribution to the cancer pain literature. Findings underscore the benefits of brief pain coping skills protocols and demonstrate the key role of pain self-efficacy in driving intervention effects on pain and pain medication use.
Limitations should be noted. The specified mediator and outcome variables were assessed at the same timepoint. Future research should investigate these pathways longitudinally to more rigorously confirm mediation. Medication use was self-reported by participants and thus susceptible to recall bias, particularly as data quality of 7-day opioid use tend to be poor [42]. Additionally, data on daily frequency and dosage of medication was not collected. This information could provide a more nuanced understanding of changes in pain medication use. Coping skills use was also self-reported on a categorical scale. Future research might consider additional, objective measures of coping skills use (e.g., ecological momentary assessment, minutes of engagement with skills audio recordings or didactic videos on study-specific mobile apps).
Presented results have meaningful clinical implications. The 5-session PCST group demonstrated less pain severity and pain medication use, and more pain self-efficacy and coping skills use at post-intervention compared to the 1-session PCST group. Yet, women randomized to 1-session PCST still showed improvements, suggesting a single session of pain coping skills training may be an appropriate option for women with breast cancer and pain who are unable or unwilling to attend more than one intervention session. Second, pain self-efficacy emerged as a mediator of intervention effects on pain severity and pain medication use. Researchers and clinicians alike should ensure that cognitive-behavioral pain management protocols enhance participant confidence in their ability to manage pain on their own, as this may drive reductions in pain severity and pain medication use.
Key Message:
Brief cognitive-behavioral pain interventions reduce pain and pain medication use, and increase pain self-efficacy and coping skills use. Improvements are larger for 5- versus 1-session protocols. Enhanced pain self-efficacy may underpin intervention effects on pain and pain medication use, highlighting this as a critical mechanism for improving pain outcomes.
Funding:
This work was supported by the National Institutes of Health [NIH/NCI R01CA202779].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have no relevant financial or non-financial interests to disclose.
References
- 1.Ilhan E, et al. , The prevalence of neuropathic pain is high after treatment for breast cancer: a systematic review. Pain, 2017. 158(11): p. 2082–2091. [DOI] [PubMed] [Google Scholar]
- 2.Wang K, et al. , Prevalence of pain in patients with breast cancer post-treatment: A systematic review. Breast, 2018. 42: p. 113–127. [DOI] [PubMed] [Google Scholar]
- 3.Runowicz CD, et al. , American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol, 2016. 34(6): p. 611–35. [DOI] [PubMed] [Google Scholar]
- 4.Satija A, et al. , Breast cancer pain management - a review of current & novel therapies. Indian J Med Res, 2014. 139(2): p. 216–25. [PMC free article] [PubMed] [Google Scholar]
- 5.Portenoy RK, Mehta Z, and Ahmed E, Cancer pain management: General principles and risk management for patients receiving opioids. UpToDate, ed. Abrahm J. Waltham, MA: UpToDate. [Google Scholar]
- 6.Bulls HW, et al. , Cancer and Opioids: Patient Experiences With Stigma (COPES)-A Pilot Study. J Pain Symptom Manage, 2019. 57(4): p. 816–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dworkin RH, et al. , Considerations for extrapolating evidence of acute and chronic pain analgesic efficacy. Pain, 2011. 152(8): p. 1705–1708. [DOI] [PubMed] [Google Scholar]
- 8.Integration of behavioral and relaxation approaches into the treatment of chronic pain and insomnia. NIH Technology Assessment Panel on Integration of Behavioral and Relaxation Approaches into the Treatment of Chronic Pain and Insomnia. JAMA, 1996. 276(4): p. 313–318. [DOI] [PubMed] [Google Scholar]
- 9.Swarm RA, et al. , Adult Cancer Pain, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw, 2019. 17(8): p. 977–1007. [DOI] [PubMed] [Google Scholar]
- 10.Gordon DB, et al. , American pain society recommendations for improving the quality of acute and cancer pain management: American Pain Society Quality of Care Task Force. Arch Intern Med, 2005. 165(14): p. 1574–80. [DOI] [PubMed] [Google Scholar]
- 11.Syrjala KL, et al. , Psychological and behavioral approaches to cancer pain management. J Clin Oncol, 2014. 32(16): p. 1703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casellas-Grau A, Font A, and Vives J, Positive psychology interventions in breast cancer. A systematic review. Psychooncology, 2014. 23(1): p. 9–19. [DOI] [PubMed] [Google Scholar]
- 13.Greer JA, et al. , Randomized Trial of a Tailored Cognitive-Behavioral Therapy Mobile Application for Anxiety in Patients with Incurable Cancer. Oncologist, 2019. 24(8): p. 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litt MD, Shafer DM, and Kreutzer DL, Brief cognitive-behavioral treatment for TMD pain: long-term outcomes and moderators of treatment. Pain, 2010. 151(1): p. 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner JA, Mancl L, and Aaron LA, Short- and long-term efficacy of brief cognitive-behavioral therapy for patients with chronic temporomandibular disorder pain: a randomized, controlled trial. Pain, 2006. 121(3): p. 181–194. [DOI] [PubMed] [Google Scholar]
- 16.Kelleher SA, et al. , Feasibility, engagement, and acceptability of a behavioral pain management intervention for colorectal cancer survivors with pain and psychological distress: data from a pilot randomized controlled trial. Support Care Cancer, 2021. [DOI] [PubMed] [Google Scholar]
- 17.Darnall BD, et al. , Comparison of a Single-Session Pain Management Skills Intervention With a Single-Session Health Education Intervention and 8 Sessions of Cognitive Behavioral Therapy in Adults With Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Netw Open, 2021. 4(8): p. e2113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziadni MS, et al. , Efficacy of a Single-Session “Empowered Relief” Zoom-Delivered Group Intervention for Chronic Pain: Randomized Controlled Trial Conducted During the COVID-19 Pandemic. J Med Internet Res, 2021. 23(9): p. e29672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadlandsmyth K, et al. , A single-session acceptance and commitment therapy intervention among women undergoing surgery for breast cancer: A randomized pilot trial to reduce persistent postsurgical pain. Psychooncology, 2019. 28(11): p. 2210–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darnall BD, et al. , “My Surgical Success”: Effect of a Digital Behavioral Pain Medicine Intervention on Time to Opioid Cessation After Breast Cancer Surgery-A Pilot Randomized Controlled Clinical Trial. Pain Med, 2019. 20(11): p. 2228–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somers TJ, Wren AA, and Shelby RA, The context of pain in arthritis: self-efficacy for managing pain and other symptoms. Curr Pain Headache Rep, 2012. 16(6): p. 502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somers TJ, et al. , A Pilot Study of a Mobile Health Pain Coping Skills Training Protocol for Patients With Persistent Cancer Pain. J Pain Symptom Manage, 2015. 50(4): p. 553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rini C, et al. , Automated Internet-based pain coping skills training to manage osteoarthritis pain: a randomized controlled trial. Pain, 2015. 156(5): p. 837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winger JG, et al. , Coping Skills Practice and Symptom Change: A Secondary Analysis of a Pilot Telephone Symptom Management Intervention for Lung Cancer Patients and Their Family Caregivers. J Pain Symptom Manage, 2018. 55(5): p. 1341–1349e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoni MH, et al. , How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol, 2006. 74(6): p. 1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerns RD, et al. , Can we improve cognitive-behavioral therapy for chronic back pain treatment engagement and adherence? A controlled trial of tailored versus standard therapy. Health Psychol, 2014. 33(9): p. 938–47. [DOI] [PubMed] [Google Scholar]
- 27.Broderick JE, et al. , Nurse practitioners can effectively deliver pain coping skills training to osteoarthritis patients with chronic pain: A randomized, controlled trial. Pain, 2014. 155(9): p. 1743–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelleher SA, et al. , Optimizing delivery of a behavioral pain intervention in cancer patients using a sequential multiple assignment randomized trial SMART. Contemp Clin Trials, 2017. 57: p. 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melzack R and Wall PD, Pain mechanisms: a new theory. Science, 1965. 150(3699): p. 971–9. [DOI] [PubMed] [Google Scholar]
- 30.Cleeland CS and Ryan KM, Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore, 1994. 23(2): p. 129–38. [PubMed] [Google Scholar]
- 31.Dworkin RH, et al. , Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain, 2008. 9(2): p. 105–21. [DOI] [PubMed] [Google Scholar]
- 32.Turk DC, et al. , Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain, 2003. 106(3): p. 337–345. [DOI] [PubMed] [Google Scholar]
- 33.Anderson KO, et al. , Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain, 1995. 63(1): p. 77–83. [DOI] [PubMed] [Google Scholar]
- 34.Somers TJ, et al. (in press). Behavioral cancer pain intervention dosing: Results of a sequential multiple assignment randomized trial. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norman G, Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theory Pract, 2010. 15(5): p. 625–32. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan GM and Artino AR Jr., Analyzing and interpreting data from likert-type scales. J Grad Med Educ, 2013. 5(4): p. 541–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes AF, Introduction to mediation, moderation, and conditional process analysis. 2013, New York, NY: The Guilford Press. [Google Scholar]
- 38.Cohen J, A power primer. Psychol Bull, 1992. 112(1): p. 155–9. [DOI] [PubMed] [Google Scholar]
- 39.Baik SH, et al. , Cancer-Relevant Self-Efficacy Is Related to Better Health-Related Quality of Life and Lower Cancer-Specific Distress and Symptom Burden Among Latina Breast Cancer Survivors. Int J Behav Med, 2020. 27(4): p. 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White LL, et al. , Self-Efficacy for Management of Symptoms and Symptom Distress in Adults With Cancer: An Integrative Review. Oncol Nurs Forum, 2019. 46(1): p. 113–128. [DOI] [PubMed] [Google Scholar]
- 41.Hyland KA, et al. , Fatigue Perpetuating Factors as Mediators of Change in a Cognitive Behavioral Intervention for Targeted Therapy-Related Fatigue in Chronic Myeloid Leukemia: A Pilot Study. Ann Behav Med, 2022. 56(2): p. 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Alili M, et al. , A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol, 2016. 82(1): p. 268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]


