Abstract
Although several immunomodulatory drugs are available for multiple sclerosis, most present significant side effects over long-term use. Therefore, delineation of nontoxic drugs for MS is an important area of research. The β-hydroxy β-methylbutyrate (HMB) is accessible in local GNC stores as a muscle-building supplement in human. This study underlines the importance of HMB in suppressing clinical symptoms of experimental autoimmune encephalomyelitis (EAE) in mice, an animal model of MS. Dose-dependent study shows that oral HMB at a dose of 1 mg/kg body wt/d or higher significantly suppresses clinical symptoms of EAE in mice. Accordingly, orally-administered HMB attenuated perivascular cuffing, preserved the integrity of blood-brain barrier and blood-spinal cord barrier, inhibited inflammation, maintained the expression of myelin genes, and blocked demyelination in the spinal cord of EAE mice. From the immunomodulatory side, HMB protected regulatory T cells, and suppressed Th1 and Th17 biasness. Using PPARα−/− and PPARβ−/− mice, we observed that HMB required PPARβ, but not PPARα, to exhibit immunomodulation and suppress EAE. Interestingly, HMB reduced the production of nitric oxide via PPARβ to protect Tregs. These results describe a novel anti-autoimmune property of HMB that may be beneficial in the treatment of MS and other autoimmune disorders.
Keywords: EAE, MS, HMB, PPAR, Tregs, Nitric oxide
Introduction
Multiple sclerosis (MS) is an autoimmune, inflammatory and degenerative disease of the CNS that leads to debilitating symptoms (1-5). Current treatments for MS include Tysabri, fingolimod and different forms of interferon-β (IFN-β) (6-8). However, these drugs have been shown to exhibit reduced effectiveness and severe toxic effects over chronic use. For example, Tysabri treatment can cause lung infection, breathing problems, chest pain, wheezing, urinary tract infection, vaginitis, nausea, vomiting, and liver damage. Most importantly, Tysabri also increases the chance of getting progressive multifocal encephalopathy, a severe brain infection, which may ultimately cause disability and death. Fingolimod (Gilenya) is known to cause blurred vision, eye pain and blind spot in the center of vision as well as headache, stomach pain, diarrhea, etc. Similarly, IFN-β also exhibits a number of side effects including flu-like symptoms, menstrual disorders in females, reduction in neutrophil and white blood cell count, upsurge in aspartate aminotransferase and alanine aminotransferase levels, and development of neutralizing antibodies to IFN-β (9-11). In addition, there are some immunosuppressive drugs for MS may have deleterious effects on fertility, pregnancy outcomes and the unborn child (12). Therefore, describing a new, safe and effective therapeutic option for MS is an important area of research.
The β-hydroxy β-methylbutyrate (HMB), available in local GNC stores, is a muscle-building supplement in human. It is known to increase exercise-induced gains in muscle size and muscle strength and improve exercise performance. Most importantly, even after long-term use, HMB does not exhibit any side effect. Here, we delineate that HMB treatment was capable of reducing the disease process of EAE in mice. HMB enriched Tregs and suppressed Th1 and Th17 biasness. Interestingly, HMB required PPARβ, but not PPARα, to upregulate Tregs and suppress EAE. Furthermore, we found that HMB attenuated the expression of iNOS and the production of nitric oxide via PPARβ to protect and/or upregulate Tregs. Therefore, HMB may be of therapeutic importance in MS and other autoimmune disorders.
Materials and methods
Reagents:
L-glutamine and β-mercaptoethanol were obtained from Invitrogen. Fetal bovine serum (FBS) and RPMI 1640 were from Mediatech. Heat-killed M. tuberculosis (H37RA) was purchased from Difco Labs. Incomplete Freund’s adjuvant (IFA) was obtained from Calbiochem. MOG35-55, solvent blue 38, cresyl violet acetate, and lithium carbonate were purchased from Sigma.
Induction of EAE:
C57BL/6 mice (8-10 week old male) were immunized s.c. with 100 μg of MOG35-55 and 60 μg M. tuberculosis in IFA. as described (13-15). Mice also received two doses of pertussis toxin (150 ng/mouse) on 0 and 2 dpi (13, 16). Animal maintenance and experimental protocols were approved by the Rush University Medical Center. Animals were observed daily for clinical symptoms as described (14, 17-21). Experimental animals were scored by a masked investigator, as follows: 0, no clinical disease; 0.5, piloerection; 1, tail weakness; 1.5, tail paralysis; 2, hind limb weakness; 3, hind limb paralysis; 3.5, forelimb weakness; 4, forelimb paralysis; 5, moribund or death.
Treatment with HMB:
HMB was solubilized in 0.1% methyl cellulose, and EAE mice were treated with HMB once daily at different doses in a volume of 50 μl via gavage (13, 15) starting from 10 day post-immunization (dpi), the onset of the disease. Therefore, in some experiments, EAE mice were treated with 50 μl 0.1% methyl cellulose via gavage.
Typically, any animal experiment is justified with 99% confidence interval that generates p = 0.99 and (1-p) = (1-0.99) =0.01; ε is the margin of error = 0.05. Based on these value, the resultant sample size is:
Therefore, six mice (n=6) were used in each group.
Histological analysis:
Histological analysis was performed in spinal cord sections of EAE mice as described by us (13, 20, 21). Briefly, mice were anesthetized and perfused with PBS (pH 7.4) and then with 4% (w/v) paraformaldehyde solution in PBS followed by dissection of cerebellum and whole spinal cord from each mouse. The tissues were further fixed and then divided into two halves: one-half was used for histology analysis whereas the other half for myelin staining as described earlier (13, 17, 20, 21). For histological analysis, routine histology was performed to obtain perivascular cuffing and morphological details of spinal cord and cerebellar tissues. Paraformaldehyde-fixed tissues were embedded in paraffin, and serial sections (4 μm) cut. Sections were stained with conventional H&E staining method. Digital images were collected under bright-field setting using an x40 objective. Slides were assessed in a blinded fashion for inflammation by three examiners in different anatomical compartments (meninges and parenchyma). Inflammation was scored using the following scale as described: for meninges and parenchyma: 0, no infiltrating cells; 1, few infiltrating cells; 2, numerous infiltrating cells; and 3, widespread infiltration. For vessels: 0, no cuffed vessel; 1, one or two cuffed vessels per section; 2, three to five cuffed vessels per section and 3, more than five cuffed vessels per section. At least six serial sections of each spinal cord and cerebellar tissues from each of five mice per group were scored.
Assessment of blood-brain barrier (BBB) and blood-spinal cord barrier (BSB) permeability:
It was performed as described before (13, 20-22). Briefly, mice received 200 μl of 20 μM Alexa 680-SE-NIR dye (Invitrogen) via tail vain. After 2 h, mice were scanned in Odyssey (ODY-0854, Licor-Inc) infrared scanner at 700- and 800-nm channels followed by perfusion with 4% paraformaldehyde. Spinal cord and different regions of brain were scanned in Odyssey infrared scanner. The red background came from 800nm filter, whereas the green signal was from Alexa 680 dye at 700 nm channel. The density of the Alexa 680 signal was quantified with the help of Quantity One, version 4.6.2 software using the volume contour tool analysis module.
Staining for myelin:
Serial longitudinal sections of paraformaldehyde-fixed spinal cords were stained with Luxol fast blue for myelin as described earlier (13, 17, 21). Slides were assessed in a blinded fashion for demyelination by three examiners using the following scale: 0, normal white matter; 1, rare foci; 2, a few areas of demyelination; and 3, large areas of demyelination. At least six serial sections of each spinal cord and cerebellum from each of five mice per group were scored and statistically analyzed by ANOVA.
Semi-quantitative RT-PCR and real-time PCR analyses:
Total RNA was isolated from spinal cord by using RNeasy mini kit (Qiagen) following manufacturer’s protocol. To remove any contaminating genomic DNA, total RNA was digested with DNase. Semi-quantitative RT-PCR was carried out as described earlier (13, 23, 24) using a RT-PCR kit from Clontech. Briefly, 1 μg of total RNA was reverse transcribed using oligo(dT)12-18 as primer and MMLV reverse transcriptase (Clontech) in a 20 μl reaction mixture. The resulting cDNA was appropriately-diluted, and diluted cDNA was amplified using Titanium Taq DNA polymerase and primers.
iNOS:
Sense: 5'-CCCTTCCGAAGTTTCTGGCAGCAGC-3'
Antisense: 5'-GGCTGTCAGAGCCTCGTGGCTTTGG-3'
IL-1β:
Sense: 5'-CTCCATGAGCTTTGTACAAGG-3'
Antisense: 5'-TGCTGATGTACCAGTTGGGG-3'
MOG:
Sense: 5'-CCTCTCCCTTCTCCTCCTTC-3'
Antisense: 5'-AGAGTCAGCACACCGGGGTT-3'
PLP:
Sense: 5'-CTTTGCTTCCCTGGTGGCCA-3'
Antisense: 5'-TGTTGGCCTCTGGAACCCCT-3'
GAPDH:
Sense: 5'-GGTGAAGGTCGGTGTGAACG3'
Antisense: 5'-TTGGCTCCACCCTTCAAGTG-3'
Amplified products were electrophoresed on a 1.8% agarose gels and visualized by ethidium bromide staining.
Real-time PCR was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems) as described earlier (13, 23, 24). The mRNA expressions of respective genes were normalized to the level of GAPDH mRNA. Data were processed by the ABI Sequence Detection System 1.6 software and analyzed by ANOVA.
Flow cytometry:
Single-cell suspensions isolated from mouse spleen were stained with Zombie Aqua™ Fixable Viability Kit (Biolegend) according to the manufacturer’s instructions as described before (14, 25). Cells were washed with FACS buffer (ThermoFisher) and stained with CD3-Brilliant Violet 605, CD4-FITC and CD8-APC-Cy7 (Biolegend) for extracellular stains. For intracellular staining, cells were stained according to manufacturer’s instructions using the eBioscience™ Foxp3/Transcription Factor Staining Buffer set (ThermoFisher). Cells were then stained with anti-IL-17-APC, anti-IFNγ-PE, anti-GM-CSF-PE-Cy7 (Biolegend), and anti-Foxp3-APC (ThermoFisher). Multicolor flow cytometric analyses were performed using the LSRFortessa analyzer (BD Biosciences) and analyzed using the FlowJo Software (v10).
Statistical analysis:
Levels of significance for comparison between two groups were determined by one-sided two-sample Mann–Whitney rank-sum test and the Student t test distribution. Analyses were performed by GraphPad Prism 7.02 software. Wherever required, repeated measures one-way ANOVA was employed. Pair-wise comparisons with Bonferroni correction were performed to find out if there was any significant difference detected from ANOVA.
RESULTS
Oral HMB inhibits the disease process of EAE in mice:
HMB is a commonly-used body-building drug and here, we examined if oral administration of HMB attenuated the clinical symptoms of EAE in mice. EAE was induced in male C57/BL6 mice by MOG35-55 immunization. Mice were treated daily with different doses of HMB via gavage from the 10 days post-immunization or dpi (the onset of acute phase). As obvious from Figure 1A, HMB dose-dependently inhibited clinical symptoms of EAE and this inhibition was significant even with the lowest dose of HMB (1 mg/kg body wt/d) tested [adjusted p = 0.01 for EAE vs EAE+HMB (1 mg/kg body wt/d) by Dunnett’s multiple comparison analysis]. However, greater inhibition of EAE [adjusted p = 0.0001 for EAE vs EAE+HMB (2 mg/kg body wt/d) and EAE vs EAE+HMB (5 mg/kg body wt/d) by Dunnett’s multiple comparison analysis] was observed at higher doses of HMB (Fig. 1A).
Figure 1. Treatment of EAE by oral administration of HMB.
A) MOG-induced active EAE mice were treated with different doses of HMB daily via gavage starting from 10 dpi (the onset of acute phase). Clinical scores were recorded until 34 dpi. Data are expressed as the mean ± SEM of six mice per group. **p < 0.01 & ***p < 0.001. After 2 weeks of treatment, EAE mice were also analyzed for footprint. On the walking track, we applied white paper strips and obtained the footprints of mice of different groups [B, control; C, EAE; D, EAE+HMB (5 mg/kg/d); E, EAE+Vehicle] on paper using black ink. A total of 30-40 steps for each group were determined. Four different footprint measurements (F, stride length; G, print length; H, toe spread; I, sway length) were calculated in centimeters from the recorded prints of mice. Six mice (n=6 per group) were used in two independent experiments. **p < 0.01; ***p < 0.001.
Oral administration of HMB led to preservation of the integrity of blood-brain barrier (BBB) and blood-spinal cord barrier (BSB) in EAE mice:
One of the characteristics of active MS and EAE is the breakdown of BBB and BSB in a section of the brain and spinal cord, respectively (26-28). This loss of integrity ultimately allows different blood molecules and toxins to enter into the CNS. Therefore, here, we examined whether HMB treatment restored the integrity of BBB and BSB in EAE mice. Infrared signals were not visible on areas over the brain and the spinal cord in control HBSS-injected mice (Fig. 2A). In contrast, we detected some infrared signals on areas over the brain and the spinal cord of EAE, and vehicle-treated EAE mice (Fig. 2A), indicating likely breakdown of BBB and BSB. However, treatment with HMB, but not vehicle, led to the decrease in infrared signals over brain and spinal cord of EAE mice (Fig. 2A). To confirm these results further, the spinal cord and different parts of the brain were analyzed for infrared signals in an Odyssey infrared scanner. Consistent to live mice scanning signals, more infrared dye was noticeable in spinal cord (Fig. 2B), frontal cortex (Fig. 2C), midbrain (Fig. 2D), and cerebellum ( Fig, 2E ) of EAE mice as compared to control mice. Scanning of infrared signals also indicated significant increase in infrared dye in spinal cord (Fig. 2F) and different parts (frontal cortex, midbrain, and cerebellum) of the brain (Fig. 2G) of EAE mice in comparison to control mice. However, oral treatment with HMB, but not vehicle, markedly reduced the entry of infrared dye into the spinal cord and different parts of the brain of EAE mice (Fig. 2A-G).
Figure 2. Effect of oral HMB on the integrity of BBB and BSB and inflammatory infiltration into the CNS of EAE mice.
MOG-induced active EAE mice were treated with HMB (5 mg/kg body wt/d) via gavage starting from 10 dpi. Control and different groups of EAE mice on 20 dpi (n=4 in each group) received 200 μl of 20 μM Alexa 680-SE-NIR dye (Life Technologies) via tail vein. A) After 2 h, mice were scanned in Odyssey (ODY-0854, Licor-Inc) infrared scanner at 700- and 800-nm channels. Mice were perfused with 4% paraformaldehyde. Spinal cord (B), frontal cortex (C), midbrain (D) and cerebellum (E) were scanned in Odyssey infrared scanner. The red background came from 800nm filter, whereas the green signal was from Alexa 680 dye at 700 nm channel. The density of the Alexa 680 signal in spinal cord (F) and in different parts of the brain (G) was quantified with the help of Quantity One, version 4.6.2 software using the volume contour tool analysis module. Data are expressed as the mean ± SEM of four mice per group. **p < 0.01; ***p < 0.001. H) Spinal cord sections were stained with H & E. Digital images were collected under bright field setting. Infiltration (I) and cuffed vessel (J) were represented quantitatively by using a scale as described in materials and methods. Cerebellum of different groups of mice were analyzed for the mRNA expression of IL-1β and iNOS by semi-quantitative RT-PCR (K) and quantitative real-time PCR (L, IL-1β; M, iNOS). Data are expressed as the mean ± SEM of four mice per group. ***p < 0.001.
Oral administration of HMB inhibits the infiltration of mononuclear cells into the CNS of EAE mice:
Infiltration of mononuclear cells into the CNS is one of the pathological characteristics of EAE and MS (4, 9, 29). Accordingly, we observed widespread infiltration of inflammatory cells into the spinal cord of EAE mice (Fig. 2H). However, consistent to the preservation of the integrity of blood-spinal cord barrier (BSB), oral HMB treatment decreased the invasion of inflammatory cells into the spinal cord of EAE mice (Fig. 2H). Quantitation of relative level of inflammation indicates that HMB dramatically reduced infiltration (Fig. 2I) and the appearance of cuffed vessels (Fig. 2J) in spinal cord of EAE mice. Accordingly, marked expression of proinflammatory molecules like IL-1β and iNOS was observed in cerebellum (Fig. 2K-M), midbrain (Fig. 3A, C, & E) and frontal cortex (Fig. 3B, D, & F) of EAE mice as compared to control mice. However, treatment of EAE mice with HMB resulted in inhibition of pro-inflammatory molecule expression in cerebellum (Fig. 2K-M), midbrain (Fig. 3A, C, & E) and frontal cortex (Fig. 3B, D, & F) of EAE mice.
Figure 3. Oral HMB reduces the expression of proinflammatory cytokines in the CNS of EAE mice.
MOG-induced active EAE mice were treated with HMB (5 mg/kg body wt/d) via gavage starting from 10 dpi. After 10 d of treatment, midbrain (A, C & E) and frontal cortex (B, D & F) of different groups of mice were analyzed for the mRNA expression of IL-1β and iNOS by semi-quantitative RT-PCR (A & B) and quantitative real-time PCR (C & D, IL-1β; E & F, iNOS). Data are expressed as the mean ± SEM of four mice per group. ***p < 0.001; **p < 0.01.
Oral HMB treatment reduces demyelination in mice with EAE:
Since demyelination in the CNS is probably the most important pathological feature of MS and EAE, next, we studied whether HMB could protect EAE mice from demyelination. Upon staining spinal cord sections with Luxol fast blue (LFB) for myelin, we noticed widespread demyelination zones in the white matter of EAE mice compared to that of HBSS-treated control mice (Fig. 4A-B). However, treatment of EAE mice with HMB improved myelin level in spinal cord (Fig. 4A-B). We also analyzed the expression of myelin specific genes and found significant decrease in MOG and PLP mRNAs in cerebellum of EAE mice compared to HBSS-treated control mice by RT-PCR (Fig. 4C) and quantitative real-time PCR (Fig. 4D-E). Similar to LFB staining, treatment of EAE mice with HMB also normalized the mRNA expression of MOG and PLP in cerebellum of EAE mice (Fig. 4C-E).
Figure 4. Effect of HMB on demyelination in EAE mice.
A) Spinal cord sections isolated from normal, EAE (20 dpi) and HMB-treated EAE (20 dpi receiving HMB from 10 dpi) mice were stained with Luxol fast blue. Digital images were collected under bright field setting using an x 40 objective. Demyelination (B) was represented quantitatively by using a scale as described in materials and methods. Cerebellum of different groups of mice were analyzed for the mRNA expression of MOG and PLP by semi-quantitative RT-PCR (C) and quantitative real-time PCR (D, MOG; E, PLP). Data are expressed as the mean ± SEM of four mice per group. ***p < 0.001.
Oral administration of HMB enriches and/or protects Tregs in EAE mice.
It is shown that Tregs, the most important immunomodulatory subtype of T lymphocytes (30, 31), become defective both numerically and functionally during autoimmune insults (32, 33). Therefore, to understand the possible mechanism behind HMB-mediated protection of EAE mice, we examined the effect of HMB treatment on the status of Tregs in EAE mice.
Tregs are characterized by Foxp3 and therefore, EAE mice receiving HMB from 10 dpi were sacrificed on 20 dpi followed by analysis of Tregs in splenocytes. As evident from detailed analysis (Fig. 5A-F) and mean fluorescence intensity (MFI) (Fig. 5I), there was a significant reduction in CD4+Foxp3+ population of T cells in EAE splenocytes as compared to normal splenocytes, which was increased by HMB treatment.
Figure 5. Enrichment of Tregs in EAE mice by HMB treatment.
Splenocytes isolated from normal, EAE (20 dpi) and HMB-treated EAE (20 dpi receiving HMB from 10 dpi) mice (n=4 per group) were gated based on side scatter (SSC-A) versus forward scatter (FSC-A) (A), dead cells excluded with Zombie Aqua™ Fixable Viability Kit (B), and singlets were selected from the FSC-H versus FSC-A dot plot (C), then divided into T cells on the basis of surface expression of CD3 (D). The dot plot on the lower right and upper left show a clear demarcation of CD4 and CD8 cell populations (E). Foxp3+ (F), IFNγ+ (G) and IL-17+ (H) cells were analyzed from CD4+ cells. MFIs of Foxp3 (I), IFNγ (J) and IL-17 (K) in CD4+ population were calculated by using the CellQuest software. Results are mean ± SEM of four mice per group. ***p < 0.001.
Oral HMB suppresses Th1 and Th17 responses in EAE mice.
Next, we examined whether oral HMB could modulate Th1 and Th17 responses in vivo in EAE mice. In this case as well, EAE mice receiving HMB from 10 dpi were sacrificed on 20 dpi followed by analysis of Th1 and Th17 responses in spleen. While induction of EAE by MOG immunization increased the level of CD4+IFNγ+ T cells in spleen, HMB treatment markedly suppressed the upregulation of CD4+IFNγ+ T cells in EAE mice (Fig. 5G & J). Similarly, as expected, induction of EAE also upregulated the level of CD4+IL-17+ T cells in spleen, which was markedly suppressed by oral HMB treatment (Fig. 5H & K).
HMB requires PPARβ, but not PPARα, to suppress EAE:
Next, to investigate mechanism by which HMB ameliorated EAE in mice, we investigated the role of peroxisome proliferator-activated receptors (PPARs), nuclear hormone receptors that are known to be involved in various immunomodulatory mechanisms. To examine the role of PPARα and PPARβ in HMB-mediated inhibition of EAE, we induced EAE in PPARα−/− and PPARβ−/− mice by MOG immunization followed by HMB treatment by gavage from 10 dpi. MOG immunization caused EAE in both PPARα−/− and PPARβ−/− mice with little more severe clinical symptoms seen in PPARβ−/− mice than PPARα−/− mice (Fig. 6A-B). Similar to the inhibition of EAE symptoms in WT mice, HMB treatment reduced clinical symptoms of EAE in PPARα−/− mice (Fig. 6A). On other hand, HMB had no effect on EAE symptoms in PPARβ−/− mice (Fig. 6B). These results clearly suggest that HMB involves PPARβ, but not PPARα, to reduce EAE symptoms in mice. To understand whether HMB is capable of interacting with PPARβ, we employed an in silico approach. We applied Swissdock, a rigid body protein-ligand docking tool, to find out any collaboration between HMB and ligand binding domain (LBD) of PPARβ at a molecular level. We observed that HMB docked in the interface of LBD of PPARβ as shown in Figure 6C-D. The detailed view of that docking showed that HMB formed a strong hydrogen bond with Tyr246 residue of PPARβ LBD at a distance of 2.55 A° (Fig. 6C).
Figure 6. HMB protects mice from EAE via PPARβ, but not PPARα.
EAE was induced in PPARα−/− (A) and PPARβ−/− (B) mice (n=6 per group) by MOG immunization followed by treatment with HMB (5 mg/kg body wt/d) orally via gavage starting from 10 dpi. Clinical scores were recorded until 34 dpi. Data are expressed as the mean ± SEM of six mice per group. ***p < 0.001. (C) A rigid-body in silico docked pose of the PPARβ LBD with HMB. (D) Electrostatic potential surface shows the distribution of charge of PPARβ LBD around the backbone of HMB. Red = a negatively charged surface; blue = a positively charged surface; white = a neutral surface.
HMB upregulates Tregs and suppresses Th1/Th17 responses in EAE mice via PPARβ, but not PPARα:
Since HMB treatment increases Tregs and subdues Th1 and Th17 cells in EAE mice, next, we examined if HMB required PPARα/PPARβ for this function. Therefore, EAE was induced in WT, PPARα−/− and PPARβ−/− mice, and HMB treatment began from 10 dpi for 10 days followed by analysis of Th1 and Th17 responses in spleen. Similar to the enrichment of Tregs in WT-EAE mice, HMB treatment increased Foxp3+CD4+ population of T cells in spleen of PPARα−/− EAE mice as evident from FACS dot plot (Fig. 7A-F) and MFI (Fig. 7I). Accordingly, HMB also inhibited the upregulation of CD4+IFNγ+ (Fig. 7G & J) and CD4+IL-17+ (Fig. 7H & K) T cells in spleen of PPARα−/− EAE mice. In contrast, HMB treatment remained unable to upregulate Foxp3+CD4+ population of T cells in spleen of PPARβ−/− EAE mice (Fig. 8A-F). MFI calculation also supports this finding (Fig. 8I). Similarly, HMB treatment also could not inhibit the upregulation of CD4+IFNγ+ (Fig. 8G & J) and CD4+IL-17+ (Fig. 8H & K) T cells in spleen of PPARβ−/− EAE mice. These results clearly suggest that HMB requires PPARβ, but not PPARα, for immunomodulation in EAE mice.
Figure 7. HMB treatment enriches Tregs in PPARα−/− EAE mice.
Splenocytes isolated from PPARα−/− control, PPARα−/− EAE (20 dpi) and HMB-treated PPARα−/− EAE (20 dpi receiving HMB from 10 dpi) mice (n=4 per group) were gated based on side scatter (SSC-A) versus forward scatter (FSC-A) (A), dead cells excluded with Zombie Aqua™ Fixable Viability Kit (B), and singlets were selected from the FSC-H versus FSC-A dot plot (C), then divided into T cells on the basis of surface expression of CD3 (D). The dot plot on the lower right and upper left show a clear demarcation of CD4 and CD8 cell populations (E). Foxp3+ (F), IFNγ+ (G) and IL-17+ (H) cells were analyzed from CD4+ cells. MFIs of Foxp3 (I), IFNγ (J) and IL-17 (K) in CD4+ population were calculated by using the CellQuest software. Results are mean ± SEM of four mice per group. ***p < 0.001.
Figure 8. HMB treatment does not upregulate Tregs in PPARβ−/− EAE mice.
Splenocytes isolated from PPARβ−/− control, PPARβ−/− EAE (20 dpi) and HMB-treated PPARβ−/− EAE (20 dpi receiving HMB from 10 dpi) mice (n=4 per group) were gated based on side scatter (SSC-A) versus forward scatter (FSC-A) (A), dead cells excluded with Zombie Aqua™ Fixable Viability Kit (B), and singlets were selected from the FSC-H versus FSC-A dot plot (C), then divided into T cells on the basis of surface expression of CD3 (D). The dot plot on the lower right and upper left show a clear demarcation of CD4 and CD8 cell populations (E). Foxp3+ (F), IFNγ+ (G) and IL-17+ (H) cells were analyzed from CD4+ cells. MFIs of Foxp3 (I), IFNγ (J) and IL-17 (K) in CD4+ population were calculated by using the CellQuest software. Results are mean ± SEM of four mice per group. ***p < 0.001.
HMB inhibits the expression of iNOS and the production of NO in MOG-primed splenocytes via PPARβ, but not PPARα:
Now, to understand the mechanism behind HMB-mediated immunomodulation further, we investigated the effect of HMB on the expression of inducible nitric oxide synthase (iNOS) and the production of NO in splenocytes. Earlier we described that CD11b+ macrophages in spleen expressed iNOS and that iNOS-derived NO suppressed the expression of Foxp3 in T cells via soluble guanylate cyclase-cGMP pathway (34, 35). Therefore, splenocytes isolated from MOG-immunized WT, PPARα−/− and PPARβ−/− mice were re-stimulated with MOG in the presence or absence of HMB. MOG re-priming increased the expression of iNOS mRNA in WT (Fig. 9A & D), PPARα−/− (Fig. 9B & E) and PPARβ−/− (Fig. 9C & F) splenocytes as evident by semi-quantitative RT-PCR (Fig. 9A-C) and quantitative real-time PCR (Fig. 9D-F). However, HMB treatment markedly inhibited the expression of iNOS mRNA in WT (Fig. 9A & D) and PPARα−/− (Fig. 9B & E), but not PPARβ−/− (Fig. 9C & F), splenocytes. Western blot analysis of splenocytes with antibodies against iNOS also showed inhibition of iNOS protein in WT (Fig. 9G & J) and PPARα−/− (Fig. 9H & K), but not PPARβ−/− (Fig. 9I & L), splenocytes by HMB. To understand the functional consequences of the induction of iNOS, we also monitored the level of NO in supernatant as nitrite and found HMB-mediated reduction of NO production in WT (Fig. 9M) and PPARα−/− (Fig. 9N), but not PPARβ−/− (Fig. 9O), splenocytes. These results suggest that HMB is capable of inhibiting the induction of iNOS and the production of NO in MOG-primed splenocytes via PPARβ, but not PPARα.
Figure 9. HMB inhibits the expression of iNOS and the production of NO from MOG-primed splenocytes via PPARβ, but not PPARα.
WT, PPARα−/− and PPARβ−/− mice were immunized with MOG35-55 and on 10th day of immunization, splenocytes were isolated followed by incubation with 20 μM HMB. After 1 h, cells were reprimed with MOG35-55 for 6 h followed by monitoring the mRNA expression of iNOS by semi-quantitative RT-PCR (A, WT; B, PPARα−/−; C, PPARβ−/−) and quantitative real-time PCR (D, WT; E, PPARα−/−; F, PPARβ−/−). After 24 h of MOG repriming, the expression of iNOS protein was monitored by Western blot (G, WT; H, PPARα−/−; I, PPARβ−/−). Actin was run as loading control. Bands were scanned and values (iNOS/Actin) presented as relative to respective control (J, WT; K, PPARα−/−; L, PPARβ−/−). Levels of nitrite were measured in supernatants (M, WT; N, PPARα−/−; O, PPARβ−/−) by Griess reagent. Results are mean ± SD of three independent experiments. NS, not significant; ***p < 0.001 by two-sample t-tests.
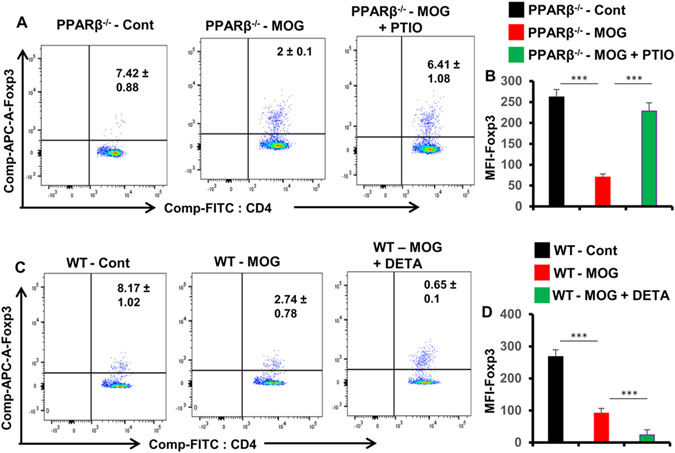
Although HMB treatment remained unable to protect/enhance Tregs in MOG-primed PPARβ−/− splenocytes, consistent to the role of NO in the regulation of Tregs (34, 35), we found that scavenging of NO by PTIO increased CD4+Foxp3+ population of T cells in MOG-primed PPARβ−/− splenocytes (Fig. 10A-B) and that supplementation of NO by DETA-NONOate suppressed CD4+Foxp3+ population of T cells in MOG-primed WT splenocytes (Fig. 10C-D).
Figure 10. While scavenging NO by PTIO upregulates Tregs in MOG-primed PPARβ−/− splenocytes, supplementation of NO by DETA-NONOate suppresses Tregs in MOG-primed WT splenocytes.
A) Splenocytes isolated from MOG-immunized PPARβ−/− mice were reprimed with MOG35-55 in the presence or absence of 50 μM PTIO for 24 h followed by monitoring CD4+Foxp3+ T cells by FACS. B) MFI of Foxp3 in CD4+ population were calculated by using the CellQuest software. C) Splenocytes isolated from MOG-immunized WT mice were reprimed with MOG35-55 in the presence or absence of 25 μM DETA-NONOate for 24 h followed by monitoring CD4+Foxp3+ T cells by FACS. D) MFI of Foxp3 in CD4+ population were calculated by using the CellQuest software. Results are mean ± SD of three independent experiments. ***p < 0.001 by two-sample t-tests.
DISCUSSION
MS is the most common autoimmune demyelinating disease of the CNS and it is important to describe a safe and effective therapeutic option for this debilitating disorder. HMB is a popular nutritional add-on that has become very popular to the health and fitness community for its potential to support muscle growth and exercise routine. According to Nissen et al (36), HMB can be taken safely as an ergogenic aid for exercise. It is reported that the plasma half-life of HMB is approximately 2.5 h and that plasma HMB reaches baseline levels at approximately 9 h following ingestion (37). It is readily available from health food centers, supplement stores, and online. In addition to helping the body-building community, HMB intake is known to improve protein balance and reduce muscle wasting in cancer (38), acquired immunodeficiency syndrome (AIDS) (39), and aging (40). It has been also demonstrated that HMB supplementation results in a net reduction in total cholesterol (5.8%, P < 0.03), a decrease in LDL cholesterol (7.3%, P < 0.01) and a lessening in systolic blood pressure (4.4 mm Hg, P < 0.05) compared with the placebo (36). Here we provide the first evidence that oral administration of HMB is capable of controlling the disease process of EAE in mice. Our results may enhance the possibility of treating MS patients with this easily available muscle-building supplement.
Although there are many drugs for the treatment of MS, most of these display toxic effects and reduced effectiveness over chronic use (41). For example, in some cases, Tysabri (Natalizumab) causes lung infection, breathing problems, wheezing, chest pain, urinary tract infection, vaginitis, nausea, vomiting, and liver damage (41). Tysabri also increases the chance of getting progressive multifocal encephalopathy, a severe brain infection, which may ultimately cause disability and death (42). Another widely-used MS drug IFN-β also exhibits a number of side effects including flu-like symptoms, menstrual disorders in women, reduction in neutrophil and white blood cell count, upregulation of aspartate aminotransferase and alanine aminotransferase levels, and generation of neutralizing antibodies to IFN-β (9, 10, 43). Similarly, common side effects of oral drug fingolimod that is used to reduce relapse are stomach pain, diarrhea, headache, liver injury, flu-like symptoms, etc. (41, 44). On the other hand, such side effects are not known from HMB, which is generally recognized as safe for use as a supplement. Many athletes and body builders are taking HMB on a daily basis without any known toxic effects. Furthermore, different clinical trials (39, 45-48) also did not see any untoward effect of HMB.
The upregulation of Foxp3+ Tregs might be useful for controlling the activation of autoimmune T cells and attenuating autoimmune disorders because it has been shown that there is a significant decrease in the number of CD4+Foxp3+ T cells as well as the expression level of Foxp3 in relapsing-remitting MS and other autoimmune disorders (49-51). Here, we have seen that oral administration of HMB is capable of upregulating and/or protecting Foxp3+ Tregs in vivo in EAE mice. As shown before (34), here, we also found that induction of EAE decreased the population of CD4+Foxp3+ T cells. However, HMB treatment inhibited the loss of CD4+Foxp3+ T cells in EAE mice. As expected, induction of EAE enhanced CD4+IL-17+ Th17 cells. However, consistent to the fact that Tregs secrete IL-35 for governing the propagation of Th17 cells (52), HMB treatment decreased the population of CD4+IL-17+ T cells in EAE mice. Similarly, it is known that Tregs secrete IL-10 to control the proliferation of CD4+IFNγ+ Th1 cells (53). Accordingly, HMB treatment decreased the population of CD4+IFNγ+ T cells in EAE mice. Together, HMB treatment was capable of enhancing anti-autoimmune Tregs and downregulating autoimmune Th17 and Th1 cells in EAE mice.
How does HMB cause immunomodulation? HMB is a derivative of butyric acid, a short-chain fatty acid (SCFA), and SCFAs are known to activate peroxisome proliferator-activated receptors (PPARs) (54, 55), a nuclear hormone receptor family transcription factor that is known to control the metabolism of lipids (56, 57). Since several studies report immunomodulatory properties of PPARs including suppression of EAE (58-61), we investigated the role of PPARα and PPARβ in HMB-mediated inhibition of EAE. Although PPARα plays an important role in EAE (58) as the expression of PPARα in T cells is shown to mediate the gender difference in the development of EAE (59), our results clearly show that HMB inhibits EAE via PPARβ, but not PPARα. Consistent to a critical role of immunomodulation in the protection of EAE, we have also seen that HMB treatment upregulates and/or restores anti-autoimmune Tregs and suppresses autoimmune Th17 and Th1 cells in EAE mice via PPARβ, but not PPARα. Involvement of PPARβ in HMB-mediated downregulation of Th17/Th1 cells is consistent to previous finding that PPARβ/δ serves as an important molecular brake for the control of pathogenic T cells during EAE (60). Drohomyrecky et al (62) have also shown that PPARβ acts within peripheral myeloid cells to reduce Th cell priming during EAE.
How does PPARβ control Tregs? Earlier we have demonstrated that nitric oxide (NO) decreases Foxp3 in naïve T cells (35) as well as MBP-primed T cells (34). Reversal of NO-mediated inhibition of Foxp3 by a specific inhibitor of soluble guanylate cyclase and suppression of Foxp3 by a cell-permeable cGMP derivative (8-Br-cGMP) or an inhibitor of cGMP phosphodiesterase (MY5445) in the absence of NO (34) indicate that NO inhibits the expression of Foxp3 in T cells via soluble guanylate cyclase-cGMP pathway. Since under immune activation, inducible nitric oxide synthase (iNOS) is the major producer of NO, we investigated whether HMB was capable of inhibiting the expression of iNOS and the production of NO in splenocytes. Suppression of iNOS expression and the production of NO by HMB in MOG-primed splenocytes suggests that HMB is anti-inflammatory. Moreover, HMB required PPARβ, but not PPARα, to exhibit this anti-inflammatory effect in MOG-primed splenocytes. Although HMB treatment could not enhance Tregs in the absence of PPARβ, scavenging of NO by PTIO alone upregulated Tregs in MOG-primed PPARβ−/− splenocytes. Alternatively, supplementation of NO by DETA-NONOate suppressed Tregs in MOG-primed WT splenocytes. Together, these results suggest that HMB upregulates Tregs via PPARβ-mediated reduction of NO production.
Although we have described a role of PPARβ in HMB-mediated protection of mice from EAE, involvement of other pathways also could not be ruled out. For example, HMB is a muscle-building drug that is capable of growing the size of muscle and increasing the strength of muscle in order to improve performance during exercise (36, 63). Therefore, by stimulating muscle function, HMB may reduce clinical symptoms (e.g. hind limb weakness, forelimb weakness, etc.) of EAE in mice. Future studies may be planned to address this pathway.
In summary, here, we describe that oral supplementation of HMB augments anti-autoimmune Tregs, diminishes autoimmune Th1 and Th17 cells, reinstates the integrity of BBB and BSB, stabilizes the expression of myelin genes in the CNS, and reduces the clinical symptoms of EAE via PPARβ. Although the disease process of EAE is not exactly the same as MS, our studies may have wonderful implications in the management of MS and other autoimmune disorders by oral HMB.
Key points.
Suppression of EAE by HMB, a body-building supplement in human
Enhancement of Tregs and downregulation of Th17/Th1 by HMB
HMB requires PPARβ, not PPARα, for immunomodulation and protection of EAE
Acknowledgments
This study was supported by a grant (AT010980) from NIH to KP. Moreover, KP is the recipient of a Research Career Scientist Award (1IK6 BX004982) from the Department of Veterans Affairs.
References
- 1.Hafler DA, and Weiner HL. 1989. MS: a CNS and systemic autoimmune disease. Immunol Today 10: 104–107. [DOI] [PubMed] [Google Scholar]
- 2.Leonard JP, Waldburger KE, and Goldman SJ. 1995. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med 181: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin R, McFarland HF, and McFarlin DE. 1992. Immunological aspects of demyelinating diseases. Annu Rev Immunol 10: 153–187. [DOI] [PubMed] [Google Scholar]
- 4.Martino G, and Hartung HP. 1999. Immunopathogenesis of multiple sclerosis: the role of T cells. Curr Opin Neurol 12: 309–321. [DOI] [PubMed] [Google Scholar]
- 5.Brahmachari S, and Pahan K. 2008. Role of cytokine p40 family in multiple sclerosis. Minerva Med 99: 105–118. [PMC free article] [PubMed] [Google Scholar]
- 6.Rudick RA, Schiffer RB, and Herndon RM. 1987. Drug treatment of multiple sclerosis. Semin Neurol 7: 150–159. [DOI] [PubMed] [Google Scholar]
- 7.Weiner HL, and Hafler DA. 1988. Immunotherapy of multiple sclerosis. Ann Neurol 23: 211–222. [DOI] [PubMed] [Google Scholar]
- 8.Ransohoff RM 1989. Multiple sclerosis: new concepts of pathogenesis, diagnosis, and treatment. Compr Ther 15: 39–44. [PubMed] [Google Scholar]
- 9.Pahan K 2010. Neuroimmune pharmacological control of EAE. J Neuroimmune Pharmacol 5: 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller A 1997. Current and investigational therapies used to alter the course of disease in multiple sclerosis. South Med J 90: 367–375. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JA, Reingold SC, Polman CH, Wolinsky JS, and S. International Advisory Committee on Clinical Trials in Multiple. 2012. Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol 11: 467–476. [DOI] [PubMed] [Google Scholar]
- 12.Leroy C, Rigot JM, Leroy M, Decanter C, Le Mapihan K, Parent AS, Le Guillou AC, Yakoub-Agha I, Dharancy S, Noel C, and Vantyghem MC. 2015. Immunosuppressive drugs and fertility. Orphanet J Rare Dis 10: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondal S, and Pahan K. 2015. Cinnamon ameliorates experimental allergic encephalomyelitis in mice via regulatory T cells: implications for multiple sclerosis therapy. PLoS One 10: e0116566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondal S, Kundu M, Jana M, Roy A, Rangasamy SB, Modi KK, Wallace J, Albalawi YA, Balabanov R, and Pahan K. 2020. IL-12 p40 monomer is different from other IL-12 family members to selectively inhibit IL-12Rbeta1 internalization and suppress EAE. Proc Natl Acad Sci U S A 117: 21557–21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mondal S, Dasarathi S, and Pahan K. 2017. Glyceryl Tribenzoate: A Flavoring Ingredient, Inhibits the Adoptive Transfer of Experimental Allergic Encephalomyelitis via TGF-beta: Implications for Multiple Sclerosis Therapy. J Clin Cell Immunol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mondal S, Martinson JA, Ghosh S, Watson R, and Pahan K. 2012. Protection of Tregs, suppression of Th1 and Th17 cells, and amelioration of experimental allergic encephalomyelitis by a physically-modified saline. PLoS One 7: e51869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmachari S, and Pahan K. 2007. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol 179: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, and Pahan K. 2004. Antineuroinflammatory effect of NF-kappaB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol 173: 1344–1354. [DOI] [PubMed] [Google Scholar]
- 19.Dasgupta S, Zhou Y, Jana M, Banik NL, and Pahan K. 2003. Sodium phenylacetate inhibits adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice at multiple steps. J Immunol 170: 3874–3882. [DOI] [PubMed] [Google Scholar]
- 20.Mondal S, Jana M, Dasarathi S, Roy A, and Pahan K. 2018. Aspirin ameliorates experimental autoimmune encephalomyelitis through interleukin-11-mediated protection of regulatory T cells. Sci Signal 11: eaar8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mondal S, Roy A, and Pahan K. 2009. Functional blocking monoclonal antibodies against IL-12p40 homodimer inhibit adoptive transfer of experimental allergic encephalomyelitis. J Immunol 182: 5013–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondal S, Rangasamy SB, Ghosh S, Watson RL, and Pahan K. 2017. Nebulization of RNS60, a Physically-Modified Saline, Attenuates the Adoptive Transfer of Experimental Allergic Encephalomyelitis in Mice: Implications for Multiple Sclerosis Therapy. Neurochem Res 42: 1555–1570. [DOI] [PubMed] [Google Scholar]
- 23.Corbett GT, Gonzalez FJ, and Pahan K. 2015. Activation of peroxisome proliferator-activated receptor alpha stimulates ADAM10-mediated proteolysis of APP. Proc Natl Acad Sci U S A 112: 8445–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy A, Jana M, Kundu M, Corbett GT, Rangaswamy SB, Mishra RK, Luan CH, Gonzalez FJ, and Pahan K. 2015. HMG-CoA Reductase Inhibitors Bind to PPARalpha to Upregulate Neurotrophin Expression in the Brain and Improve Memory in Mice. Cell Metab 22: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kundu M, Raha S, Roy A, and Pahan K. 2022. Regression of Triple-Negative Breast Cancer in a Patient-Derived Xenograft Mouse Model by Monoclonal Antibodies against IL-12 p40 Monomer. Cells 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dasgupta S, Jana M, Liu X, and Pahan K. 2002. Myelin basic protein-primed T cells induce nitric oxide synthase in microglial cells. Implications for multiple sclerosis. J Biol Chem 277: 39327–39333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasgupta S, Jana M, Liu X, and Pahan K. 2003. Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J Biol Chem 278: 22424–22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dasgupta S, Roy A, Jana M, Hartley DM, and Pahan K. 2007. Gemfibrozil ameliorates relapsing-remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferator-activated receptor-alpha. Mol Pharmacol 72: 934–946. [DOI] [PubMed] [Google Scholar]
- 29.Pahan K 2015. Prospects of Cinnamon in Multiple Sclerosis. J Mult Scler (Foster City) 2: 1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Josefowicz SZ, Lu LF, and Rudensky AY. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilate AM, and Lafaille JJ. 2012. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol 30: 733–758. [DOI] [PubMed] [Google Scholar]
- 32.Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H, and Vandenbark AA. 2005. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res 81: 45–52. [DOI] [PubMed] [Google Scholar]
- 33.Viglietta V, Baecher-Allan C, Weiner HL, and Hafler DA. 2004. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 199: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brahmachari S, and Pahan K. 2010. Myelin basic protein priming reduces the expression of Foxp3 in T cells via nitric oxide. J Immunol 184: 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brahmachari S, and Pahan K. 2009. Suppression of regulatory T cells by IL-12p40 homodimer via nitric oxide. J Immunol 183: 2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, and Fuller JC Jr. 2000. beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr 130: 1937–1945. [DOI] [PubMed] [Google Scholar]
- 37.Vukovich MD, Slater G, Macchi MB, Turner MJ, Fallon K, Boston T, and Rathmacher J. 2001. beta-hydroxy-beta-methylbutyrate (HMB) kinetics and the influence of glucose ingestion in humans. J Nutr Biochem 12: 631–639. [DOI] [PubMed] [Google Scholar]
- 38.Molfino A, Gioia G, Rossi Fanelli F, and Muscaritoli M. 2013. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: a systematic review of randomized trials. Amino Acids 45: 1273–1292. [DOI] [PubMed] [Google Scholar]
- 39.Clark RH, Feleke G, Din M, Yasmin T, Singh G, Khan FA, and Rathmacher JA. 2000. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. JPEN J Parenter Enteral Nutr 24: 133–139. [DOI] [PubMed] [Google Scholar]
- 40.Vukovich MD, Stubbs NB, and Bohlken RM. 2001. Body composition in 70-year-old adults responds to dietary beta-hydroxy-beta-methylbutyrate similarly to that of young adults. J Nutr 131: 2049–2052. [DOI] [PubMed] [Google Scholar]
- 41.Callegari I, Derfuss T, and Galli E. 2021. Update on treatment in multiple sclerosis. Presse Med 50: 104068. [DOI] [PubMed] [Google Scholar]
- 42.Clerico M, Artusi CA, Liberto AD, Rolla S, Bardina V, Barbero P, Mercanti SF, and Durelli L. 2017. Natalizumab in Multiple Sclerosis: Long-Term Management. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen JA, Reingold SC, Polman CH, and Wolinsky JS. Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol 11: 467–476. [DOI] [PubMed] [Google Scholar]
- 44.Biolato M, Bianco A, Lucchini M, Gasbarrini A, Mirabella M, and Grieco A. 2021. The Disease-Modifying Therapies of Relapsing-Remitting Multiple Sclerosis and Liver Injury: A Narrative Review. CNS Drugs 35: 861–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhls DA, Rathmacher JA, Musngi MD, Frisch DA, Nielson J, Barber A, MacIntyre AD, Coates JE, and Fildes JJ. 2007. Beta-hydroxy-beta-methylbutyrate supplementation in critically ill trauma patients. J Trauma 62: 125–131; discussion 131–122. [DOI] [PubMed] [Google Scholar]
- 46.May PE, Barber A, D’Olimpio JT, Hourihane A, and Abumrad NN. 2002. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg 183: 471–479. [DOI] [PubMed] [Google Scholar]
- 47.Rahimi MH, Mohammadi H, Eshaghi H, Askari G, and Miraghajani M. 2018. The Effects of Beta-Hydroxy-Beta-Methylbutyrate Supplementation on Recovery Following Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis. J Am Coll Nutr 37: 640–649. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Martinez J, Santos-Lozano A, Garcia-Hermoso A, Sadarangani KP, and Cristi-Montero C. 2018. Effects of beta-hydroxy-beta-methylbutyrate supplementation on strength and body composition in trained and competitive athletes: A meta-analysis of randomized controlled trials. J Sci Med Sport 21: 727–735. [DOI] [PubMed] [Google Scholar]
- 49.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, Medaer R, Hupperts R, and Stinissen P. 2008. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology 123: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paust S, and Cantor H. 2005. Regulatory T cells and autoimmune disease. Immunol Rev 204: 195–207. [DOI] [PubMed] [Google Scholar]
- 51.Ziegler SF 2006. FOXP3: of mice and men. Annu Rev Immunol 24: 209–226. [DOI] [PubMed] [Google Scholar]
- 52.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, and Liew FY. 2007. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 37: 3021–3029. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Gao X, Shen G, Wang W, Li J, Zhao J, Wei YQ, and Edwards CK. 2016. Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity. Sci Rep 6: 24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Georgiadi A, and Kersten S. 2012. Mechanisms of gene regulation by fatty acids. Adv Nutr 3: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alex S, Lange K, Amolo T, Grinstead JS, Haakonsson AK, Szalowska E, Koppen A, Mudde K, Haenen D, Al-Lahham S, Roelofsen H, Houtman R, van der Burg B, Mandrup S, Bonvin AM, Kalkhoven E, Muller M, Hooiveld GJ, and Kersten S. 2013. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor gamma. Mol Cell Biol 33: 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pahan K 2006. Lipid-lowering drugs. Cell Mol Life Sci 63: 1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy A, and Pahan K. 2015. PPARalpha signaling in the hippocampus: crosstalk between fat and memory. J Neuroimmune Pharmacol 10: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Gocke AR, Lovett-Racke A, Drew PD, and Racke MK. 2008. PPAR Alpha Regulation of the Immune Response and Autoimmune Encephalomyelitis. PPAR Res 2008: 546753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, Crowell A, Loh J, Oksenberg J, and Steinman L. 2007. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med 204: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunn SE, Bhat R, Straus DS, Sobel RA, Axtell R, Johnson A, Nguyen K, Mukundan L, Moshkova M, Dugas JC, Chawla A, and Steinman L. 2010. Peroxisome proliferator-activated receptor delta limits the expansion of pathogenic Th cells during central nervous system autoimmunity. J Exp Med 207: 1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanakasabai S, Chearwae W, Walline CC, Iams W, Adams SM, and Bright JJ. 2010. Peroxisome proliferator-activated receptor delta agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis. Immunology 130: 572–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drohomyrecky PC, Doroshenko ER, Akkermann R, Moshkova M, Yi TJ, Zhao FL, Ahn JJ, McGaha TL, Pahan K, and Dunn SE. 2019. Peroxisome Proliferator-Activated Receptor-delta Acts within Peripheral Myeloid Cells to Limit Th Cell Priming during Experimental Autoimmune Encephalomyelitis. J Immunol 203: 2588–2601. [DOI] [PubMed] [Google Scholar]
- 63.Kaczka P, Michalczyk MM, Jastrzab R, Gawelczyk M, and Kubicka K. 2019. Mechanism of Action and the Effect of Beta-Hydroxy-Beta-Methylbutyrate (HMB) Supplementation on Different Types of Physical Performance - A Systematic Review. J Hum Kinet 68: 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]